
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 09 May 2022
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.830003
Teratomas are very rare, originating from embryonal germ layers. The majority of them are mature, most common in the gonads, and with only 15% out of gonads. In particular, primary adrenal teratomas are extremely rare. The present study reported a case of a young female patient with right adrenal tumor who underwent intermittent pain in the right waist and abdomen and whose CT of adrenal gland showed an 88 mm × 79 mm × 69 mm mass. Besides, her adrenal gland-related hormones are not abnormal. Laparoscopic adrenal tumor resection was performed on her and the histopathological results confirmed that the mass was mature adrenal teratomas. As a newly diagnosed case, strict and regular follow-up is needed, and it is also necessary to detect her AFP and check her adrenal CT in the future. In addition, we have reviewed the literature from 1952 to the present, and a total of 49 cases of adrenal teratoma have been identified and analyzed.
Teratomas are tumor stemming from embryonal germ layers that are mainly composed of pluripotent stem cells. Teratomas are very rare and uncommon, whose incidence is 0.9/100,000 among the population (1); thus, only few cases were published in literature. The majority of teratomas are mature and contain multiple layers of embryonic germ cell layer, with the ectoderm accounting for the majority and the endoderm constituting the least part; therefore, teratomas may contain skin, hair, teeth, brain tissue, nerves, adipose tissue, cartilage, and so forth (2). Based on the original locations of a teratoma, the most common sites of occurrence are the gonads (3), with extragonadal sites including the anterior mediastinum, retroperitoneum, sacrococcygeal region, pineal gland, and suprasellar region (4). Among the retroperitoneal teratomas, primary adrenal teratomas are extremely rare.
According to the differentiation degree of teratomas tissue, teratoma can be divided into two types, namely, mature teratoma and malignant teratoma, which consists of tissue derived from more than one kind of embryonic germ cell layer germ cell layers, including ectoderm, mesoderm, and endoderm (5). Although most teratomas are mature teratomas, which are benign, they have the potential to become malignant and, as they increase in size, can compress surrounding organs and even rupture bleeding and infection. Therefore, once diagnosed, surgery should be performed as soon as possible (6). The surgeon may choose open surgery or laparoscopic surgery, based on the patient’s specific condition and the surgeon’s own experience, and the key to treatment is complete removal of the tumor (7). Although the prognosis after resection of mature teratomas is good with an overall 5-year survival rate of nearly 100% (8), regular post-operative follow-up is necessary because of the risk of malignant regression. Postoperatively, malignant teratomas are prone to recurrence and require adjuvant radiotherapy and chemotherapy with a lifelong follow-up (9). The present study reported a case of a primary right adrenal mature teratoma and aimed to further raise awareness of this rare disease by elucidating the clinical and pathological characteristics of primary adrenal teratoma and by supplementing relevant treatment experience.
A 19-year-old female patient presented to the outpatient department of our hospital with the symptoms of intermittent pain in the right waist and abdomen for 4 months; she had no other symptoms. The patient had received no specific treatment prior to this, she follows a healthy daily diet and routine, and no relatives in her family had similar illnesses. After hospitalization, the patient’s temperature, heart rate, breath frequency, blood pressure, and oxygen saturation were normal. A mass was palpated on the upper pole of the right kidney area, about 10 cm × 8 cm in size, with mild percussion pain, but no bulge, no pressure, or percussion pain in both sides of the ureteral region.
Cortisol (COR): 36.5 nmol/L, β-HCG < 0.1 MIU/ml (non-pregnant women 0–5 MIU/ml), AFP: 3.1 ng/ml (<9 ng/ml), CEA: 0.6 μg/L (non-pregnant women <3 μg/L, smoking <5 μg/L), CA19-9: 4 U/ml (<25 U/ml), renin: 1.09 ng/ml, angiotensin I: 1.62 ng/ml, and angiotensin II: 38.77 pg/ml; other indexes were normal and are shown in Table 1.
The results of chest CT scan and ECG were normal. An 88 mm × 72 mm mixed-echoic mass in the space between right kidney and liver were observed by color ultrasound of the urinary system, and CDFI showed no blood flow in the mass. CT scan + contrast-enhancement + 3D of adrenal gland showed an 88 mm × 79 mm × 69 mm mass with mixed density shadow, multiple nodular calcareous density, flake-like high density, and adipose density shadows but no obvious enhancement signals were monitored. Besides, the upper pole of right kidney was extruded by the huge mass, and there were no enlarged lymph nodes in the local area. The tumor was suspected to be a teratoma in the right adrenal gland area (Figures 1A–C). No abnormalities in bilateral renal arteries were discovered in the CT image of renal arteries, and the inferior vena cava below the lower border of the liver was compressed by the mass of the right adrenal gland, resulting in localized stenosis of the vena cava (Figures 2A–C). Through the ultrasound examination of inferior vena cava, inferior vena cava ultrasound: the inferior vena cava was visible on the tangent plane, the normal vein wall was thick, and no tumor thrombus and embolus were not found in the lumen. In addition, the CDFI discovered continuous blood flow signal without obvious filling defect, and the blood flow velocity in the liver segment of the inferior vena cava was 74 cm/s.
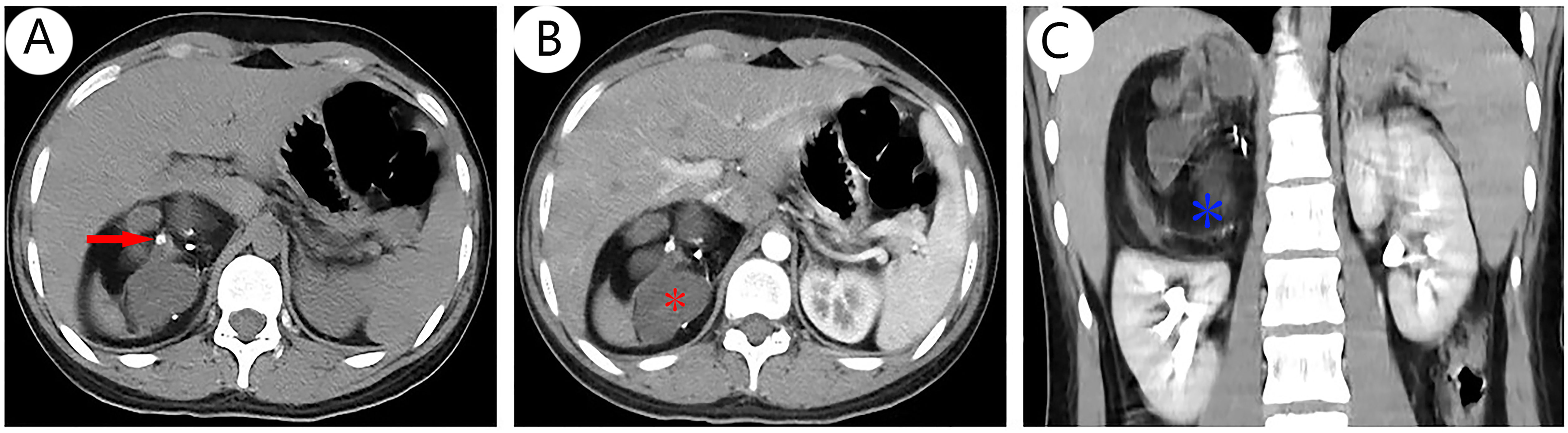
Figure 1 CT scan + contrast-enhanced ultrasound + 3D of adrenal gland revealed a spherical mass arising from the right adrenal gland with mixed density that was suspected to malignant tumor, a solid with multiple high-density nodules [(A), red arrow], some cystic-like lesions [(B), red *], and fat density shadows [(C), blue *], and no obvious enhancement signals (B, C). Other abdominal structures were normal and there was no evidence of distant metastasis although the right kidney was slightly displaced by the mass.
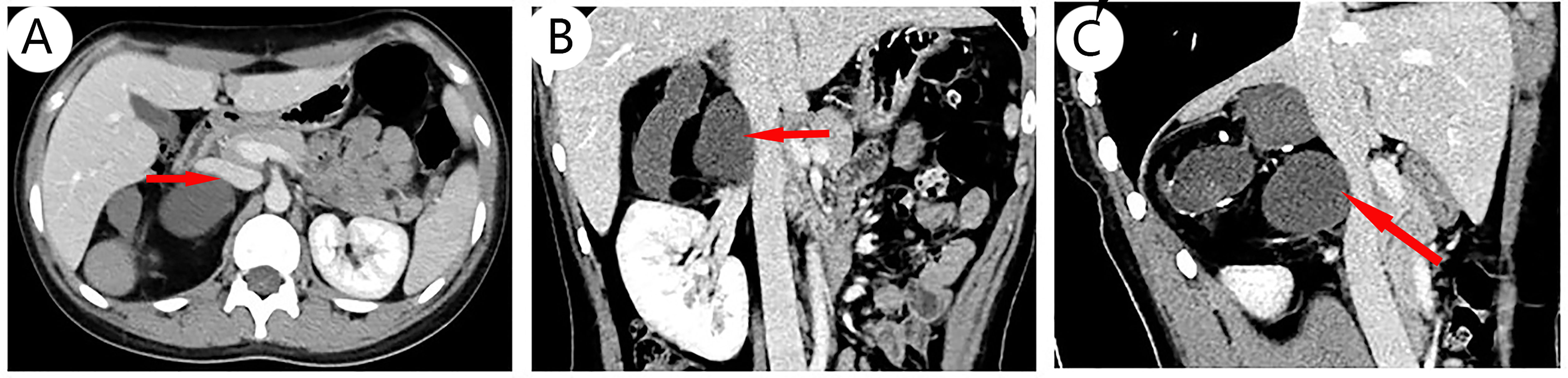
Figure 2 CT scan of blood vessels: No abnormalities in bilateral renal arteries were discovered in CT imaging of renal arteries. A part of inferior vena cava below the lower border of the liver segment is confined by the mass of the right adrenal gland, and the venous lumen is a local stenosis; different layers show that the vena cava is compressed, including transverse [(A), arrow], coronal [(B), arrow], and sagittal planes [(C), arrow].
It is difficult for the surgeon to determine preoperatively whether the right adrenal tumor is an occult functional disease. The patient was regularly administered phenoxybenzamine 10 mg orally twice a day for 2 weeks, the blood volume was supplemented 3 days before operation, and the heart rate and blood pressure were normal. Laparoscopic right adrenal tumor resection was performed, lasting 2 h and 55 min. The size of gross tumor was about 9 cm × 7 cm × 7 cm, and its envelope was intact (Figure 3A). The cut surface was gray-yellow and cystic solid with gray necrotic fluid, and sebum and hair were found in part of the cyst cavity (Figure 3B). After the operation, the patient stopped using phenoxybenzamine tablets. Subsequently, postoperative plasma aldosterone (QKT) was 16.10 ng/dl, and cortisol (COR) was 138.3 nmol/L. Other indexes were normal. The tumor of right adrenal gland was diagnosed as a mature teratoma by pathologists (Figure 3C); therefore, we did not do any further genetic diagnosis. The symptoms were relieved and there were no complications when the patient was ready to leave the hospital.
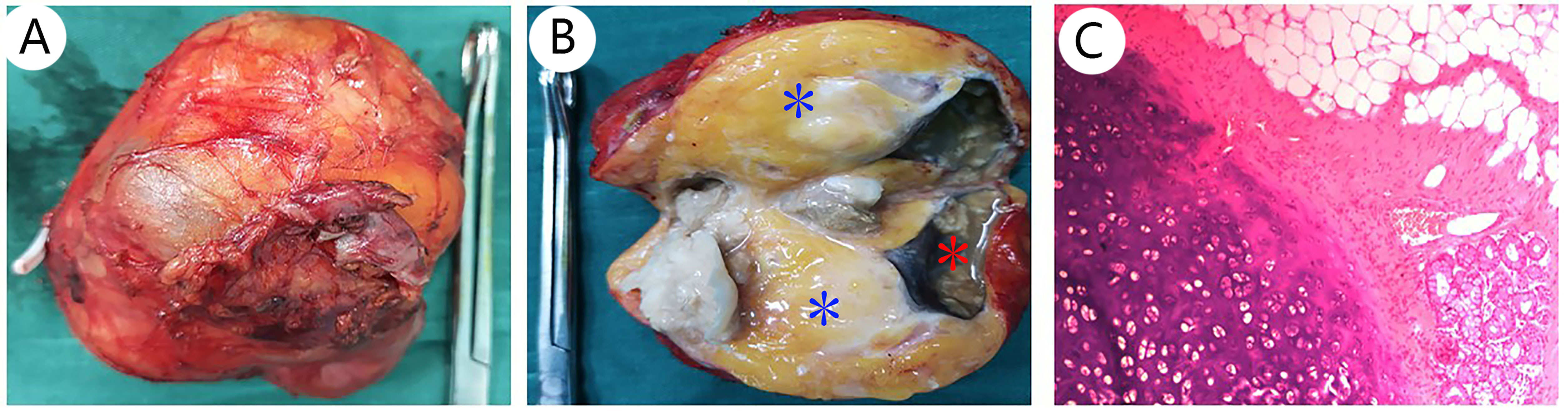
Figure 3 Pathological features of adrenal teratoma: (A) shows the gross appearance of the adrenal teratoma; the size was about 9 cm × 7 cm × 7 cm, and its envelope is intact. The cut surface is gray-yellow, namely, adipose tissue [(B), blue *], cystic solid, gray necrotic fluid [(B), red *], and sebum were found in part of the cyst cavity (B). H&E stain, 10× (C).
PubMed databases were searched for case reports and case series of adrenal teratoma published from 1952 to 2022. The following keywords were used: (adrenal glands or adrenal gland or adrenal or glands adrenal) and (teratoma or teratoma), and 296 results were retrieved. After removing duplicates and irrelevant studies, finally, 24 publications describing 49 cases were identified (Table 2). In addition, primary retroperitoneal teratomas involving the adrenal glands were excluded.
The series of review comprised 49 patients (14 men and 35 women) with a mean age of 30.3 years. Adrenal-related hormones were normal in all patients and 27 (55%) patients were clinically asymptomatic. The majority of adrenal teratomas were unilateral, with only one case of bilateral adrenal involvement. In contrast to previous reports, the current data suggest that right-sided adrenal teratomas are more common. In most cases, mature fat and calcified tissue were observed. All cases were surgically resected and histopathologically confirmed as teratomas. Most of the adrenal teratomas were mature teratomas, two of which were within focal carcinomas and neuroblastomas, respectively, and one was a malignant transformation of a teratoma into an adenocarcinoma. Only one case was an undifferentiated malignant teratoma. Thirty-four of the 49 patients were followed up for 3 months to 10 years, and all had a good prognosis with no tumor recurrence or other discomfort during the follow-up period.
The teratoma is a rare neoplasm; according to different original sites, teratomas can be categorized into gonadal and extragonadal teratomas, and retroperitoneal space is a site of the extragonadal location. When primary retroperitoneal neoplasms in adults were found that represent an infrequent mass (4), the primary adrenal teratomas are extremely rare. Previous reports demonstrated that adrenal teratomas tend to occur more often in women than in men and involve predominantly the left adrenal gland (29), which is consistent with the results of our systematic review in terms of gender, but the reviews suggest that teratomas occur mainly in the right adrenal gland. However, Zhong et al. found an almost identical distribution on both sides (33). In this case, it occurred in the right adrenal gland in a female patient.
Primary adrenal teratoma has no distinctive clinical presentation with normal adrenal-related hormones, presenting as “incidentalomas”, and they are not clearly distinguishable from other adrenal tumor with no endocrine function. In clinical practices, primary adrenal teratoma is usually found in medical examination and diagnosis of other functional adrenal tumors, such as adrenal cortical adenoma or pheochromocytoma (34). Therefore, adrenal teratomas should be differentiated from adrenal incidentalomas, non-functioning adrenal lesions and adrenal cystic lesions (35). Adrenal teratomas are usually large because there is enough retroperitoneal space for its growth. However, as the tumor grows, about half of the patients may suffer from abdominal distension, abdominal pain, back pain, or even intestinal obstruction due to the tumor pressing on the surrounding organs and blood vessels (36). A previous data indicated that the median primary adrenal teratomas mean diameter was 8.25 cm (37). In this case, the complaint of the patient was intermittent pain in the right waist and abdomen and the tumor size was 9 cm × 7 cm × 7 cm (Figure 1).
Adrenal teratomas are usually asymptomatic or have non-specific complaints, and laboratory tests are generally normal, thus posing a significant preoperative diagnostic challenge and relying primarily on imaging for diagnosis. Ultrasound usually shows a heterogeneously echogenic mass including hyperechoic calcifications and ossifications, as well as hypoechoic fatty and cystic areas (38). CT indicates a mixed density lesion with hypointense cystic and fatty areas and hyperintense calcifications (39). MRI presents as mixed signals on both T1- and T2-weighted images, with a high fat content. In addition, CT and MRI can also clarify the location of tumor growth and its relationship to surrounding organs, facilitating the assessment of surgical approaches and risks. However, other adrenal tumors with a fatty component are considered for differentiation from adrenal teratomas, including myelolipoma, lipoma, angiomyolipoma, and liposarcoma (12). In previously reported cases, a number of adrenal teratomas have been misdiagnosed preoperatively. Therefore, postoperative pathological examination is usually required to confirm the diagnosis. In this case, a mixed-echoic mass near the adrenal gland were observed by color ultrasound, and CT showed an 88 mm × 79 mm × 69 mm mass with mixed density shadow, multiple nodular calcareous density, flake-like high density, and adipose density shadows, which is consistent with the imaging features of adrenal teratoma. The patient was diagnosed preoperatively with a right adrenal teratoma.
Most mature teratomas are benign tumors, and the surrounding organs can be compressed by the growing tumor, even with the possibility of tumor rupture, bleeding, and infection. Once diagnosed, surgery should be performed as soon as possible. Laparoscopic surgery has become the “gold standard” for the treatment of adrenal tumors smaller than 6 cm (40). For adrenal tumors larger than 6 cm, open surgery is the main approach to resection of the tumor. However, scholars have pointed out that laparoscopic surgery is safe and feasible for benign adrenal tumors, as long as there is no local invasion on preoperative imaging or intraoperatively (23), and laparoscopic treatment of adrenal tumors larger than 6 cm has become feasible (29). In our systematic review, at least 12 patients with a tumor larger than 6 cm in diameter were successfully resected by laparoscopic adrenalectomy without complications, which further confirms the feasibility of this view. In this case, it is difficult to determine whether the right adrenal tumor is an occult functional disease. The patient was regularly administered phenoxybenzamine 10 mg orally twice a day for 2 weeks, and the blood volume was supplemented 3 days before operation, with these being the strengths of our case, the heart rate and blood pressure were normal. Because a part of the inferior vena cava below the lower border of the liver segment is confined by the mass of the right adrenal gland, the venous lumen is a local stenosis. After general anesthesia, transabdominal right adrenal tumor resection under the modified left lateral position was performed with no complications.
The etiology of teratomas is not known and the pathological classification includes mature teratomas and immature teratomas, and the pathological criteria for benign lesions include the following four aspects: (I) absence of malignant or immature components in the tumor, (II) absence of other similar lesions elsewhere in the body, (III) normal levels of AFP and HCG, and (IV) absence of recurrence at follow-up (17). Although the prognosis of mature teratoma is good, it has been reported that about 1.46% of mature teratomas will have malignant potential (13). It is necessary to regularly follow up, and radiotherapy and chemotherapy are not necessary. For immature teratoma, the rate of recurrence and metastasis is high, and radiotherapy and chemotherapy are still required postoperatively with a lifelong follow-up (9). For the cases in this report, resected tumors were identified as mature cystic teratomas by pathologists (Figure 3C). As the cases were recent, there was not enough time for follow-up, which was a disadvantage in this case. However, our reviews showed that 34 cases had no recurrence or other discomfort at the 6-month to 10-year follow-up.
Elevated AFP, β-HCG, and CEA were found in some immature teratomas, which were of great significance for preoperative diagnosis and prognosis (41). For the immature adrenal teratomas, chemotherapy should be supplemented after surgery, but there is currently no standard chemotherapy regimen. BEP chemotherapy regimen (bleomycin, etoposide, cisplatin) was used for a patient with immature adrenal teratomas in a review, and no recurrence or metastasis was found after 13 months of follow-up (13). At present, there is no sensitive indicators for monitoring relapse of adrenal teratoma. The level of AFP was correlated with the recurrence of teratoma, and it can be used as a predictive index for curative effect (42). The patient in this report is a mature teratoma; now, we do not know the long-term prognosis for only 1 month post-operation. Strict regular follow-up is needed, and it is necessary to detect AFP and adrenal CT in the future.
My intermittent pain in my right lower back and abdomen was affecting my daily life and work, causing me great distress. After the doctor’s consultation, he helped me to make a correct diagnosis and opted for minimally invasive laparoscopic surgery to completely remove the tumor from my body and eliminate the pain in my upper right abdomen. My fears and worries about the tumor disappeared, especially after the pathology confirmed the diagnosis of a mature teratoma. I was able to achieve both physical and psychological healing. I consider that I have received a very successful treatment. I agreed to share my medical history and signed an informed consent form.
We have described the features of imaging, pathology, and the treatment of the typical primary adrenal teratoma to further raise people’ awareness of this rare disease, and primary adrenal teratomas should be included in the differential diagnosis of adrenal masses.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XW and XGL were the patient’s urologists, reviewed the literature, and contributed to manuscript drafting. HC, WX, XH and PS were responsible for the revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (grant no. 81860524) and grants from the Department of Science and Technology of Guizhou Province (grant no. 386, in 2021-year).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AFP, Alpha-fetoprotein; CT, Computed tomography; CA19-9, Carbohydrate antigen 19-9; CA15-3, Carbohydrate antigen 15-3; CA125, Carbohydrate antigen 125; CDFI, Color Doppler flow image; CEA, Carcinoembryonic antigen; ECG, Electrocardiograph; β-HCG, Human chorionic gonadotrophin; MRI, Magnetic resonance imaging.
1. Taori K, Rathod J, Deshmukh A, Sheorain VS, Jawale R, Sanyal R, et al. Primary Extragonadal Retroperitoneal Teratoma in an Adult. Br J Radiol (2006) 79(946):e120–2. doi: 10.1259/bjr/33507627
2. Scott AL, Abbassi-Ghadi N, Archer CM, Swamy R, Gupta S. Neuroendocrine Carcinoma Arising Within a Retroperitoneal Mature Teratoma. Ann R Coll Surg Engl (2010) 92(6):W5–8. doi: 10.1308/147870810X12699662980952
3. Shintani Y, Funaki S, Nakagiri T, Inoue M, Sawabata N, Minami M, et al. Experience With Thoracoscopic Resection for Mediastinal Mature Teratoma: A Retrospective Analysis of 15 Patients. Interact Cardiovasc Thorac Surg (2013) 16(4):441–4. doi: 10.1093/icvts/ivs543
4. Bedri S, Erfanian K, Schwaitzberg S, Tischler AS. Mature Cystic Teratoma Involving Adrenal Gland. Endocr Pathol (2002) 13(1):59–64. doi: 10.1385/ep:13:1:59
5. Saba L, Guerriero S, Sulcis R, Virgilio B, Melis G, Mallarini G. Mature and Immature Ovarian Teratomas: CT, US and MR Imaging Characteristics. Eur J Radiol (2009) 72(3):454–63. doi: 10.1016/j.ejrad.2008.07.044
6. Pandit N, Awale L, Jaiswal LS. Giant Calcified Retroperitoneal Teratoma. Indian J Surg Oncol (2018) 9(3):436–7. doi: 10.1007/s13193-018-0789-8
7. Chowdhury W, Lodhi MU, Syed IA, Rahim U, Rahim M. Mature Testicular Teratoma With a Focus of Embryonal Carcinoma: A Case Report and Review of Literature. Cureus (2018) 10(3):e2329. doi: 10.7759/cureus.2329
8. Pinson CW, ReMine SG, Fletcher WS, Braasch JW. Long-Term Results With Primary Retroperitoneal Tumors. Arch Surg (1989) 124(10):1168–73. doi: 10.1001/archsurg.1989.01410100070012
9. Sharma S, Dawson L, Mandal AK. Primary Retroperitoneal Teratoma With Predominant Neurogenic Elements Masquerading as Adrenal Tumor. Turk Patoloji Derg (2019) 35(1):69–73. doi: 10.5146/tjpath.2016.01365
10. Li Y, Wu H, Yao G, Zhao X. Diagnosis and Treatment of Mature Adrenal Teratoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2011) 36(2):174–7. doi: 10.3969/j.issn.1672-7347.2011.02.015
11. Zhao J, Sun F, Jing X, Zhou W, Huang X, Wang H, et al. The Diagnosis and Treatment of Primary Adrenal Lipomatous Tumours in Chinese Patients: A 31-Year Follow-Up Study. Can Urol Assoc J (2014) 8(3-4):E132–6. doi: 10.5489/cuaj.977
12. He C, Yang Y, Yang Y, Wang F, Hu J, Zhang J, et al. Teratoma of the Adrenal Gland: Clinical Experience and Literature Review. Gland Surg (2020) 9(4):1056–64. doi: 10.21037/gs-20-648
13. Ban A, Satapara J, Rathod K, Bahri N. Teratoma Involving Adrenal Gland - A Case Report and Review of Literature. Indian J Radiol Imaging (2019) 29(4):472–6. doi: 10.4103/ijri.IJRI_452_18
14. Lam KY, Lo CY. Teratoma in the Region of Adrenal Gland: A Unique Entity Masquerading as Lipomatous Adrenal Tumor. Surgery (1999) 126(1):90–4. doi: 10.1067/msy.1999.98924
15. Narla SL, Jacob S, Kurian A, Parameswaran A. Primary Mature Cystic Teratoma With Carcinoid Mimicking an Adrenal Tumor: Report of a Rare Association and Review of Literature. Indian J Pathol Microbiol (2016) 59(2):200–2. doi: 10.4103/0377-4929.182012
16. Haddad SE, Hessissen L, Kababri ME, Lamalmi N, Kisra M, Allali N, et al. Primary Mature Adrenal Teratoma in Infant. Pan Afr Med J (2020) 37:27. doi: 10.11604/pamj.2020.37.27.24016
17. Li S, Li H, Ji Z, Yan W, Zhang Y. Primary Adrenal Teratoma: Clinical Characteristics and Retroperitoneal Laparoscopic Resection in Five Adults. Oncol Lett (2015) 10(5):2865–70. doi: 10.3892/ol.2015.3701
18. Zhou L, Pan X, He T, Lai Y, Li W, Hu Y, et al. Primary Adrenal Teratoma: A Case Series and Review of the Literature. Mol Clin Oncol (2018) 9(4):437–42. doi: 10.3892/mco.2018.1687
19. Ramakant P, Rana C, Singh KR, Mishra A. Primary Adrenal Teratoma: An Unusual Tumor - Challenges in Diagnosis and Surgical Management. J Postgrad Med (2018) 64(2):112–4. doi: 10.4103/jpgm.JPGM_588_16
20. Ersoz S, Kucuk H, Mungan S, Turgutalp H, Imamoglu M, Kosucu P. Neurocytoma Arising in an Adrenal Gland Mature Teratoma. Fetal Pediatr Pathol (2011) 30(5):275–9. doi: 10.3109/15513815.2011.572955
21. Niu M, Liu A, Zhao Y, Feng L. Malignant Transformation of a Mature Teratoma of the Adrenal Gland: A Rare Case Report and Literature Review. Med (Baltimore) (2017) 96(45):e8333. doi: 10.1097/MD.0000000000008333
22. McMillan A, Horwich A. Malignant Teratoma Presenting With an Adrenal Mass. Clin Radiol (1987) 38(3):327–8. doi: 10.1016/s0009-9260(87)80088-0
23. Wang J, Zhang J, Xiao C, Fan C. Laparoscopic Simultaneous Resection of Bilateral Giant Primary Mature Retroperitoneal Teratoma of the Adrenal Region: A Case Report. Med (Baltimore) (2019) 98(44):e17836. doi: 10.1097/MD.0000000000017836
24. Castillo OA, Vitagliano G, Villeta M, Arellano L, Santis O. Laparoscopic Resection of Adrenal Teratoma. JSLS (2006) 10(4):522–4.
25. Li H, Zhao T, Wei Q, Yuan H, Cao D, Shen P, et al. Laparoscopic Resection of a Huge Mature Cystic Teratoma of the Right Adrenal Gland Through Retroperitoneal Approach: A Case Report and Literature Review. World J Surg Oncol (2015) 13:318. doi: 10.1186/s12957-015-0734-z
26. Ciftci I, Cihan T, Koksal Y, Ugras S, Erol C. Giant Mature Adrenal Cystic Teratoma in an Infant. Acta Inform Med (2013) 21(2):140–1. doi: 10.5455/aim.2013.21
27. Zhong W, Ma R, Cheng S, Tian J, Wang H, Wang T, et al. Clinical Characteristics and Surgical Management of Adult Adrenal Teratoma: A 15-Year Experience and Systematic Review of the Literature. Urology (2020) 135:71–5. doi: 10.1016/j.urology.2019.05.032
28. Bhatia V, Sharma S, Sood S, Mardi K, Venkat B. Case 231: Retroperitoneal Adrenal Teratoma Presenting as Trichoptysis. Radiology (2016) 280(1):317–21. doi: 10.1148/radiol.2016140459
29. Kuo EJ, Sisk AE, Yang Z, Huang J, Yeh MW, Livhits MJ. Adrenal Teratoma: A Case Series and Review of the Literature. Endocr Pathol (2017) 28(2):152–8. doi: 10.1007/s12022-017-9468-5
30. Garg A, Pollak-Christian E. Unnikrishnan N. A Rare Adrenal Mass in a 3-Month-Old: A Case Report and Literature Review. Case Rep Pediatr (2017) 2017:4542321. doi: 10.1155/2017/4542321
31. Tang DD, Zhang XS, Hao ZY, Zhou J, Liang CZ. A Giant Primary Retroperitoneal Mature Cystic Teratoma in Right Adrenal Region in a 39-Year-Old Female. Int J Clin Exp Med (2014) 7(6):1611–3.
32. Nadeem M, Ather MH, Sulaiman MN, Pervez S. "Looks Can Be Deceiving": Adrenal Teratoma Causing Diagnostic Difficulty. Case Urol (2015) 2015:232591. doi: 10.1155/2015/232591
33. Zhong W, Ma R, Cheng S, Tian J, Wang H, Wang T, et al. Clinical Characteristics and Surgical Management of Adult Adrenal Teratoma: A 15-Year Experience and Systematic Review of the Literature. Urology (2020) 135:71–5. doi: 10.1016/j.urology.2019.05.032
34. Lam KY. Adrenal Tumours in Chinese. Virchows Arch A Pathol Anat Histopathol (1992) 421(1):13–6. doi: 10.1007/BF01607133
35. Cavallaro G, Crocetti D, Paliotta A, De Gori A, Tarallo MR, Letizia C, et al. Cystic Adrenal Lesions: Clinical and Surgical Management. The Experience of a Referral Centre. Int J Surg (2015) 13:23–6. doi: 10.1016/j.ijsu.2014.11.023
36. Ratan SK, Ratan J, Kalra R. Large Benign Cystic Teratoma of the Mesosigmoid Causing Intestinal Obstruction: Report of a Case. Surg Today (2002) 32(10):922–4. doi: 10.1007/s005950200183
37. Ramakant P, Rana C, Singh KR, Mishra A. Primary Adrenal Teratoma: An Unusual Tumor - Challenges in Diagnosis and Surgical Management. J Postgrad Med (2018) 64(2):112–4. doi: 10.4103/jpgm.JPGM_588_16
38. Resnick EL, Talmadge JM, Winn SS. Mediastinal Teratoma Diagnosed via Ultrasound-Guided Biopsy. Ultrasound Q (2013) 29(3):245–6. doi: 10.1097/RUQ.0b013e3182a0acb7
39. Park SB, Cho KS, Kim JK. CT Findings of Mature Cystic Teratoma With Malignant Transformation: Comparison With Mature Cystic Teratoma. Clin Imaging (2011) 35(4):294–300. doi: 10.1016/j.clinimag.2010.08.016
40. Conzo G, Gambardella C, Candela G, Sanguinetti A, Polistena A, Clarizia G, et al. Single Center Experience With Laparoscopic Adrenalectomy on a Large Clinical Series. BMC Surg (2018) 18(1):2. doi: 10.1186/s12893-017-0333-8
41. d’Amuri FV, Maestroni U, Pagnini F, Russo U, Melani E, Ziglioli F, et al. Magnetic Resonance Imaging of Adrenal Gland: State of the Art. Gland Surg (2019) 8(Suppl 3):S223–S32. doi: 10.21037/gs.2019.06.02
Keywords: teratoma, adrenal gland, imaging and pathological features, case report, laparoscopic resection
Citation: Wang X, Li X, Cai H, Xiao W, Su P, Huang X, Luo X, Zhang N and Fu N (2022) Rare Primary Adrenal Tumor: A Case Report of Teratomas and Literatures Review. Front. Oncol. 12:830003. doi: 10.3389/fonc.2022.830003
Received: 06 December 2021; Accepted: 14 March 2022;
Published: 09 May 2022.
Edited by:
Damiano Caruso, Sapienza University of Rome, ItalyReviewed by:
Mariarita Tarallo, Sapienza University of Rome, ItalyCopyright © 2022 Wang, Li, Cai, Xiao, Su, Huang, Luo, Zhang and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neng Zhang, ZW5lcmd5MjAxNzAxMThAaG90bWFpbC5jb20=; Ni Fu, MzEyNDY0NTcwQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.