- Zhuhai Precision Medical Center, Guangdong Provincial Key Laboratory of Tumor Interventional Diagnosis and Treatment, Zhuhai People’s Hospital, Zhuhai Hospital Affiliated with Jinan University, Jinan University, Zhuhai, China
Purpose: We performed a systematic review and meta-analysis to compare external beam radiation therapy modalities for hepatocellular carcinoma (HCC) with macrovascular invasion (MVI).
Methods: Studies were selected from online databases from the date of inception to November 2021. The outcomes of interest were overall survival (OS), objective response rate (ORR), and local control rate (LCR).
Results: Forty-four studies (n = 3730) were selected from 1050 articles. The pooled 1-year OS were 60.9%, 45.3%, and 44.9 for particle radiotherapy (PRT) group, conventional radiotherapy (CRT), and stereotactic body radiotherapy (SBRT) group, respectively; p = 0.005 and 0.002 for PRT vs. CRT and SBRT, respectively. Both the PRT group and the SBRT group have the advantage over the CRT group in the pooled ORR. The PRT group showed significantly higher than the CRT group (p = 0.007) in LCR. For combination therapy, CRT plus transarterial chemoembolization can prolong survival than CRT alone (p = 0.006 for 1-year OS; p = 0.014 for 2-year OS). Among grade ≥ 3 complications, the most frequent type of toxicity in CRT, SBRT, PRT group was hematological toxicity, hepatotoxicity, dermatological toxicity, respectively.
Conclusions: Among patients with HCC with MVI, the 1-year OS and the 2-year OS were both higher in the PRT group than in the CRT, SBRT groups. The ORR was similar between the PRT and SBRT groups. The combination therapy based on radiotherapy is expectable. PRT is associated with less complications than photon radiotherapy.
Introduction
According to the Global Cancer Statistics 2020, primary liver cancer is the sixth most common malignancy and the third leading cause of cancer-related death worldwide, with around 906,000 new cases and 830,000 deaths reported in 2020. Approximately 80% of these cases were hepatocellular carcinomas (HCCs) (1). As the clinical manifestations are not evident, most cases of HCCs only detected at the advanced stage. Microvascular invasion (MVI) is common in HCC. Portal vein tumor thrombus (PVTT) occurs in 10–40% of patients with HCC (2, 3). The median survival time is significantly lower in patients with PVTT than in those without (4). Worse outcomes are noted when inferior vena cava thrombi are present (5). There are several treatments for HCC, such as transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), percutaneous ethanol injection (PEIT), and radiofrequency ablation (RFA) (6). However, a tumor thrombus alters the blood supply route to the liver, reduces nutrient supplement, and further reduces the liver function reserve. Therefore, most treatments are no longer effective. Sorafenib is one of the preferred treatments of choice for this condition (6). However, the overall response rate of HCC with MVI to sorafenib is low, and the associated toxicity is severe (7, 8). It is therefore important to consider other effective treatments.
External beam radiation therapy (EBRT) is one of the promising treatments. Previously, the tolerated liver dose was considered to be lower than the tumor killing dose, and therefore, this treatment could not be used for liver cancer (9, 10). However, in recent years, imaging and dose control techniques have made great progress, with reduced toxicity to normal liver tissue. A meta- analysis showed that the 1-year overall survival (OS) and response rate for stereotactic body radiation therapy (SBRT) were 43.8% and 70.7% %, respectively (11). These data objectively reflect the therapeutic advantage of EBRT for HCC with MVI. Recently, several high-quality studies have reported the advantages of EBRT for unresectable HCC, especially for particle radiotherapy (PRT), which shows the preponderance of high response rate, high control and low toxicity (12). However, due to the lack of PRT centers, it is difficult to conduct a head-to-head comparison study with a large sample size for PRT versus other EBRTs for HCC with MVI. Therefore, we conducted this meta-analysis to compare the safety and effectiveness of PRT and photon therapy for HCC with MVI. Meanwhile, it serves to update findings related to EBRT from a previous meta-analysis (11).
Methods
Search and Selection Criteria
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (13). The protocol we designed defined inclusion criteria, search strategy, outcomes of interest, and analysis plan.
We searched Medline (Ovid), Embase, Clinicaltrials, Web of Science, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews, from the date of inception of each database to November 2021. The following keywords or terms were used: “(hepatocellular carcinoma) OR (HCC) OR (hepatoma)” AND “(external beam radiation therapy) OR (stereotactic body radiation therapy) OR (conformal radiotherapy) OR (particle radiotherapy)” AND “(thrombosis)”. Additional references were acquired through manual searches of the reference lists. No filters were used, but only papers written in English were included.
The cohorts in the studies had to meet criteria for inclusion as follows: 1) HCC with macrovascular invasion; 2) treatment with EBRT; 3) reported outcomes of interest (i.e., overall survival, response rate, and adverse events). We excluded case reports with fewer than fifteen patients, reviews, letters, and editorial comments. If more than one available study was conducted from the same treatment center in overlapping timeframes, the study with the biggest group and/or highest quality of article was selected. HCC with microvascular invasion was excluded. The conventional radiotherapy (CRT) included three dimensional conformal radiation therapy (3D-CRT), image-guide radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT). SBRT and CRT are difference type of photon therapies. PRT usually means radiotherapy using beams of protons, carbon ions, or other charged particles. Hematological toxicity includes leukopenia, anemia, thrombocytopenia, etc. Hepatotoxicity includes increased ALT, AST, ALP, bilirubin, GGT level, hypoproteinemia, etc. Dermatological toxicity refers to skin reactions. Gastrointestinal toxicity includes nausea, vomit, anorexia, diarrhea, etc. Objective response rate (ORR) was defined as complete response (CR) plus partial response (PR). Local control rate (LCR) means ORR plus stable disease (SD).
Data Extraction
The details were extracted in a standardized pilot-tested form by two reviewers independently. A third investigator reviewed all data entries. The lists we extracted as follows: study design, country, study period, number of patients, patients’ characteristics (percentage of male patients, age, diameter of lesion, Child-Pugh Class, previous treatment), interventions (radiation dose, modality for EBRT), length of follow-up, median overall survival, and outcomes of interest.
Statistical Analysis
We prespecified the analysis plan for this protocol. We transformed the rates using the variance stabilizing double arcsine transformation. Then, we pooled the transformation rates with random-effect models and assessed heterogeneity. Heterogeneity among studies was tested using Cochran’s Q and the I² statistic. I² values greater than 50% indicating high heterogeneity. Q-test was use in comparisons among groups (11). We performed a subgroup analysis and pooled the rates of interest outcomes for the different types of EBRT. Egger’s test was used to detect publication bias. When textual information in the included study was insufficient, two reviewers independently collected the information from the graphs using Engauge Digitizer 11.1. P < 0.5 was considered as statistical significance. All statistical analyses were conducted using STATA, version 15.1 (Stata Corporation, College Station, TX, USA).
Assessment of Study Quality
Because most of the studies included in our systematic review and meta-analysis were non-comparative studies, we used the modified Newcastle-Ottawa quality assessment scale. The evaluation of quality was independently conducted by two investigators. Any disagreements were resolved by a third investigator.
Results
Study Selection and Quality Assessment
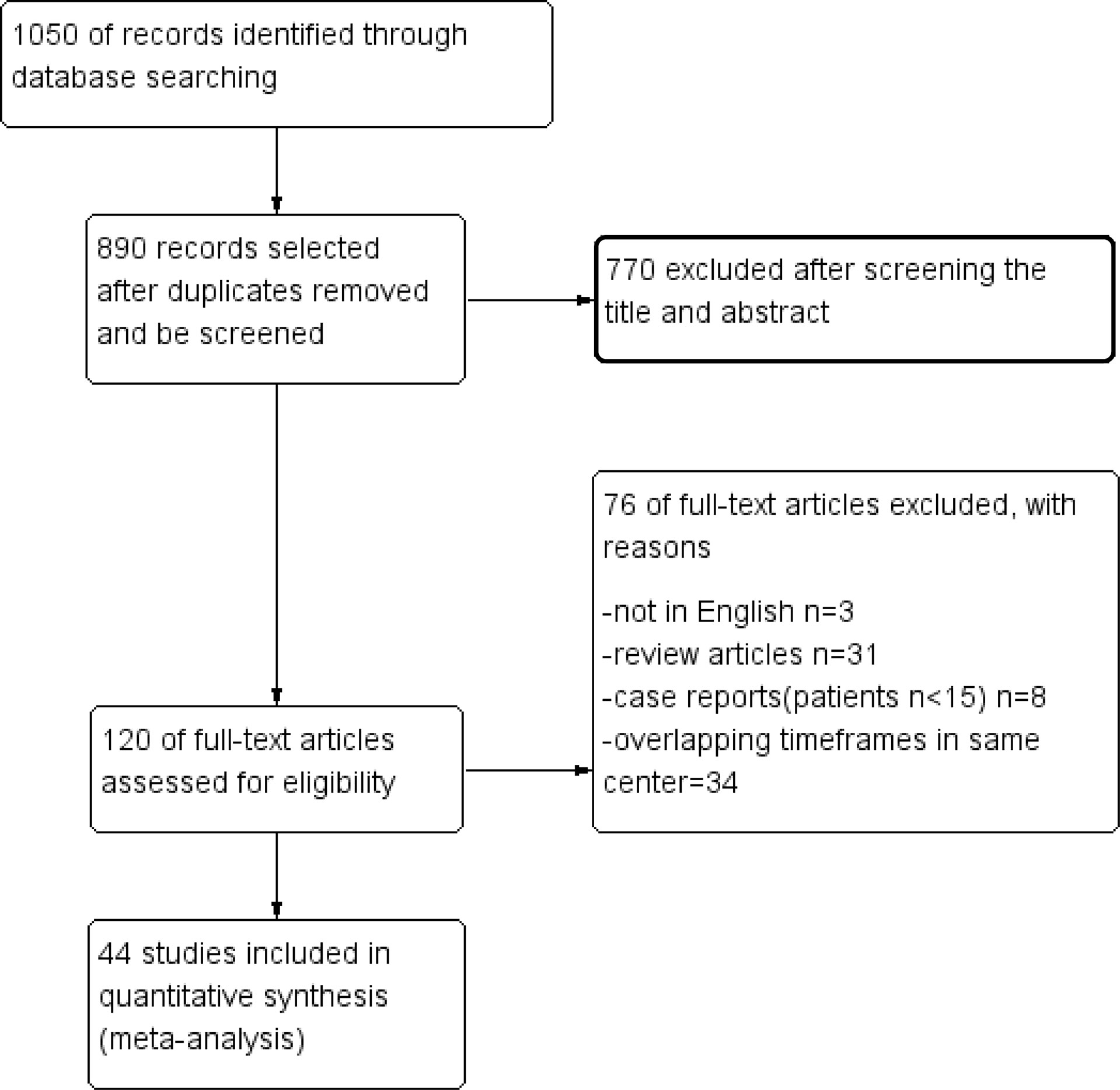
The selection process is shown in detail in the PRISMA flowchart (Figure 1). According to a previous search strategy, 1050 results were initially identified from the online databases. After removing duplicates, 890 records remained. Then, 770 records were excluded after screening the titles and abstracts. Then, 76 reports were removed for various reasons, of which 34 were excluded because of overlapping timeframes in the same center. Finally, 44 studies were included in this meta-analysis (14–57). The quality assessment is shown in the Supplementary Material.
Study Characteristics
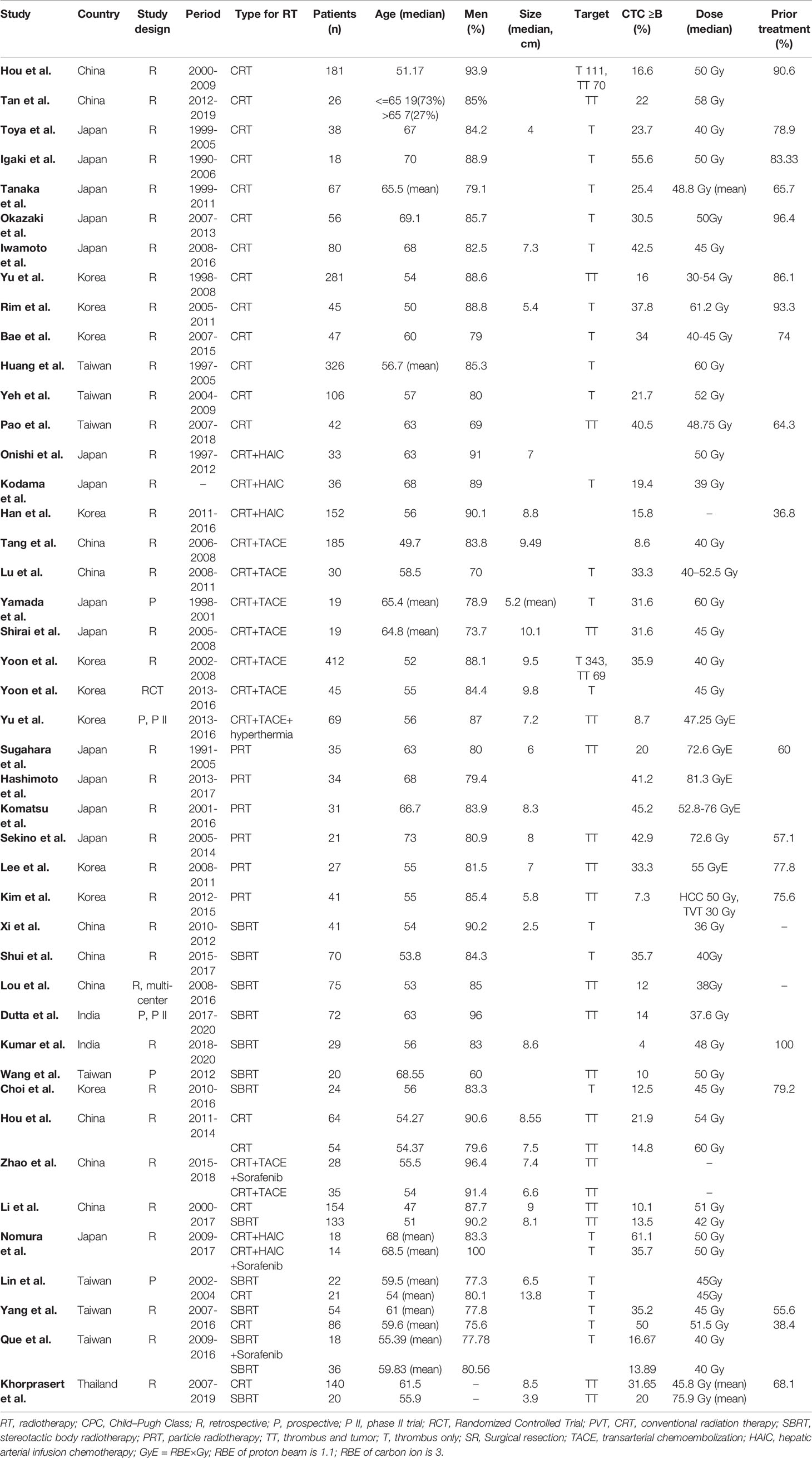
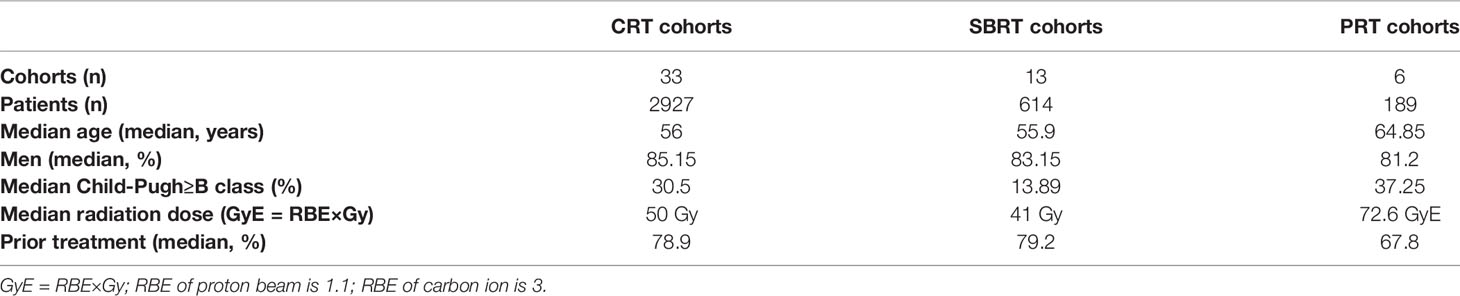
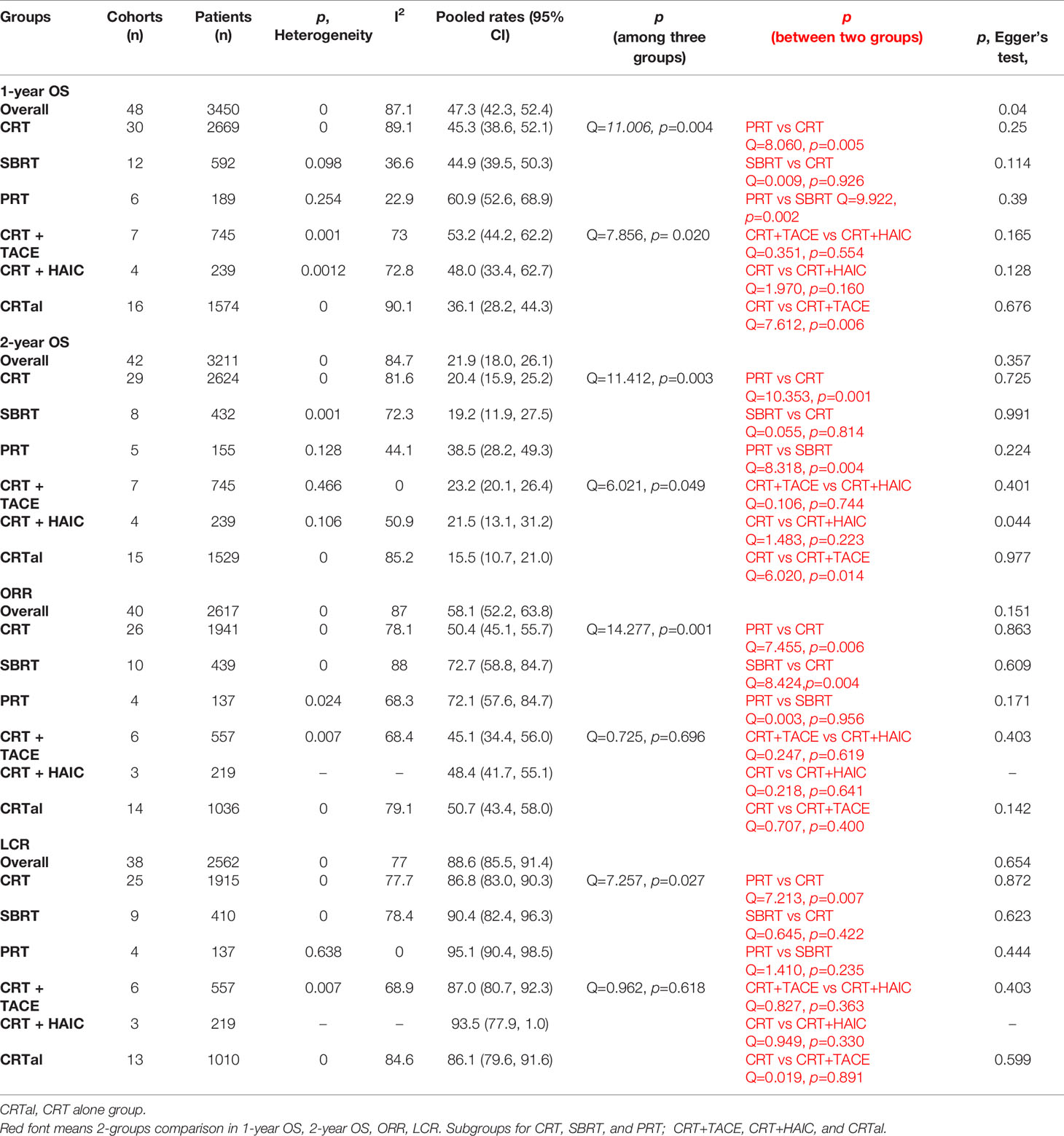
The characteristics of the cohorts in the included studies were summarized in Tables 1 and 2. Overall, 44 studies involving 3730 patients were included. 1 was Randomized Controlled Trial, 5 were prospective studies, and 38 were retrospective studies. There are 2927 patients in 33 cohorts for CRT group; 614 patients in 13 cohorts in SBRT group; 189 patients in 6 cohorts in PRT group. The median age of the patients was 56 years (range, 47-73 years) in the overall studies, 56 for CRT group, 55.9 for SBRT group, 64.85 for PRT group. The median lesion size was 8 cm (range, 2.5–13.8 cm). The median percentage of previous-treatment patients was 75.6% (range 36.8%-100%) in the overall studies, 78.8% for CRT group, 79.2 for SBRT group, 67.8% for PRT group. The median percentage of patients with a class of no less than B was 20.23% (range 0%-41.18%) in the overall studies, 30.5% for CRT group, 13.89% for SBRT group, 37.25% for PRT group. The median dose was 48 Gy in the overall studies, 50 Gy for CRT group, 41 Gy for SBRT group, 72.6 GyE for PRT group. GyE is equal to the RBE multiplication with Gy; RBE of proton beam is 1.1; RBE of carbon ion is 3.
Effectiveness Outcomes
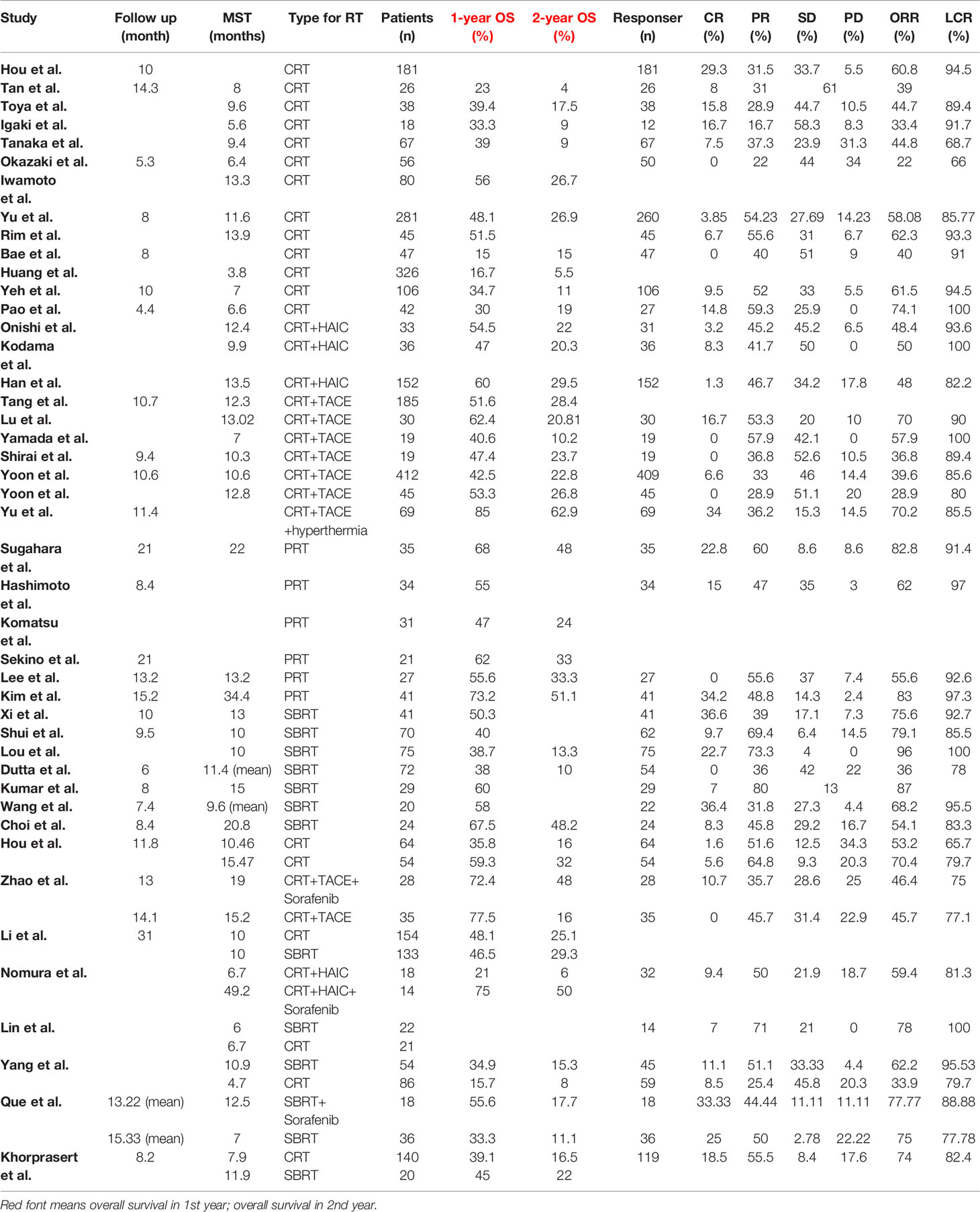
A total of 52 cohorts in 44 studies were included in the data synthesis. All valid data were extracted and are displayed in Table 3. Pooled data shown in Table 4 and in Supporting Information. The 1-year pooled OS for CRT, SBRT, PRT were 45.3% (n = 2669, study = 30), 44.9% (n = 592, study = 12), 60.9% (n = 189, study = 6), respectively. The 2-year OS for CRT, SBRT, PRT were 20.4% (n = 2624, study = 29), 19.2% (n = 432, study = 8), 38.5% (n = 155, study = 5), respectively. Except pooled 1-year OS for SBRT group, PRT group; 2-year OS for PRT group with low heterogeneity, other pooled rates with high heterogeneity, respectively. The PRT group showed significantly higher than the CRT group and the SBRT group in OS (PRT vs. CRT: p = 0.005 for 1-year OS, p = 0.001 for 2-year OS; PRT vs. SBRT: p = 0.002 for 1-year OS, p = 0.004 for 2-year OS. Compared with previous meta-analysis, the results were stable for the CRT group and SBRT group as the increasing number of patients and studies (11).
The ORR for CRT, SBRT, PRT were 50.4% (n = 1941, study = 26), 72.7% (n = 439, study = 10), 72.1% (n = 137, study = 4), respectively. The LCR for CRT, SBRT, PRT were 86.8% (n = 1915, study = 25), 90.4% (n = 410, study = 9), 95.1% (n = 137, study = 4), respectively. Except pooled LCR for PRT group; other 5 pooled rates with high heterogeneity. The CRT group showed significantly lower than the PRT group (p = 0.006) and SBRT group (p = 0.004) in ORR. There was no statistical significance between PRT group and SBRT group in ORR (p = 0.956). The PRT group showed significantly higher than the CRT group (p = 0.007) in LCR.
In recent years, several studies have shown advantage in the combination of RT. We further compared the effects between CRT + TACE, CRT + HAIC, and CRTal groups (CRTal represents CRT alone). CRT + CATE group showed statistically significant advantage in survival prolongation than the CRT alone group (p = 0.006 for 1-year OS; p = 0.014 for 2-year OS). Pooled ORR and LCR was not statistically significant between three groups. Except pooled 2-year OS for CRT+TACE group; other pooled rates with high heterogeneity.
Safety
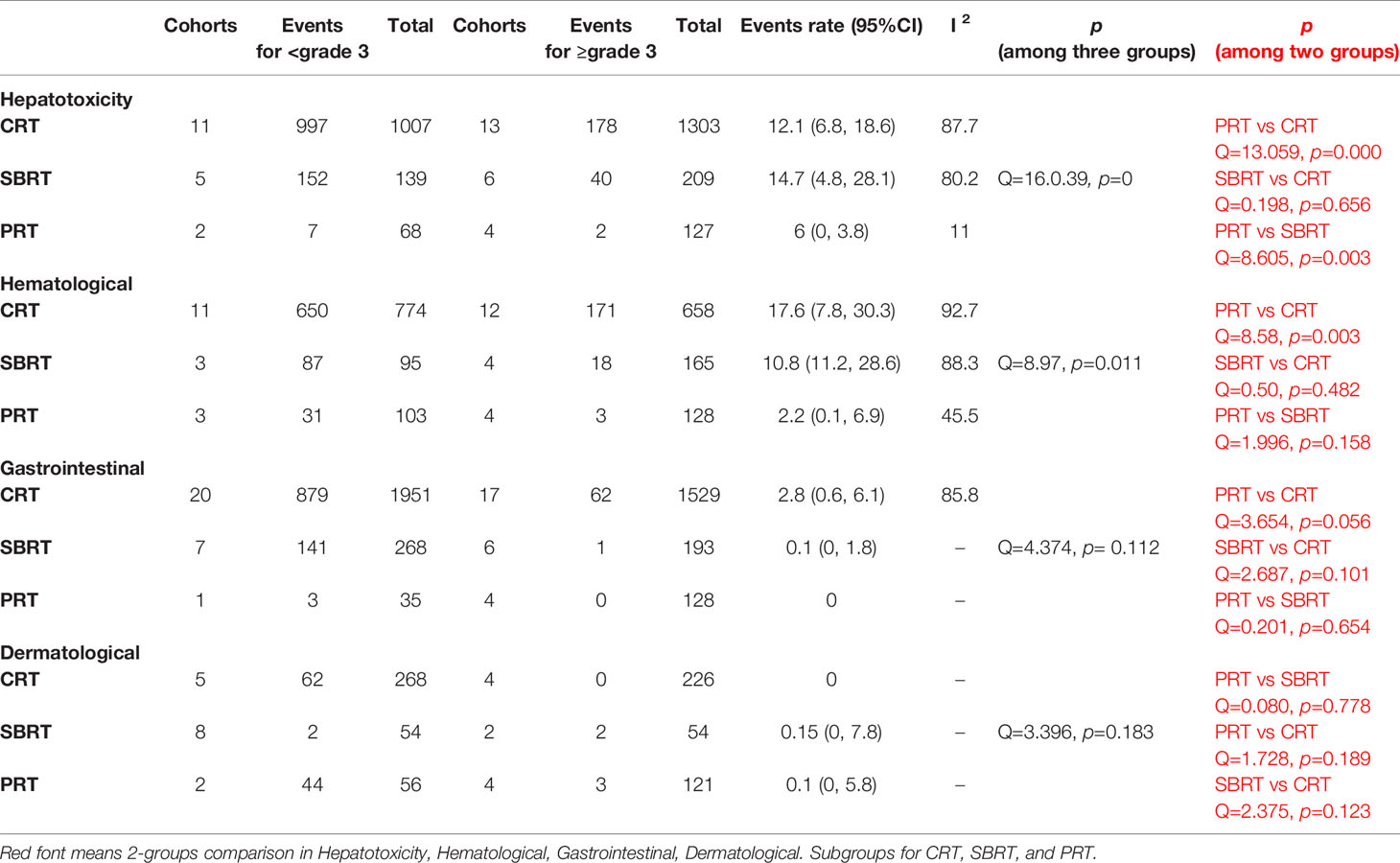
Toxic effect events for groups showed in Table 5. For grade < 3 toxicity, the most common type of toxicity in CRT group was hepatotoxicity (977 events in 1007 patients), in SBRT group was hepatotoxicity as well (152 events in 139 patients), in PRT group was dermatological toxicity (44 events in 56 patients). For grade ≥ 3, the most frequent type of toxicity in CRT, SBRT, PRT group was hematological toxicity, hepatotoxicity, dermatological toxicity, respectively. PRT group showed advantage in avoiding hepatotoxicity than SBRT group (p = 0.003) and CRT group (p = 0.000); in avoiding hematological toxicity than CRT group (p = 0.003). There were no statistical difference among three groups in gastrointestinal toxicity (p = 0.112) and dermatological toxicity (p = 0.183). Five studies definitively reported late toxic events with total of 27 cases, 16 about gastrointestinal toxicity, 11 for dermatological toxicity.
Publication Bias
Egger’s test showed publication biases as follows: 1-year OS in the CRT, SBRT PRT groups (p = 0.25, 0.114, 0.390, respectively); 2-year OS in the CRT, SBRT PRT groups (p = 0.725, 0.991, 0.224); ORR in the CRT, SBRT PRT groups (p = 0.863, 0.609, 0.171). LCR in the CRT, SBRT PRT groups (p = 0.872, 0.623, 0.444). 1-year OS in the CRT+TACE, CRT+HAIC, CRTal groups (p = 0.165, 0.128, 0.676); 2-year OS in the CRT+TACE, CRT+HAIC, CRTal groups (p = 0.401, 0.044, 0.977, respectively); ORR in the CRT+TACE, CRTal groups (p = 0.403, 0.142). LCR in the CRT+TACE, CRTal groups (p = 0.403, 0.599).
Discussion
There are 44 studies about external beam radiotherapy for HCC with MVI included in our study. The results showed PRT yields survival prolongation compared with SBRT and CRT. Meanwhile, PRT and SBRT both provide a higher ORR than CRT. In addition, radiotherapy based combination therapies are beneficial to prolong the survival of patients, especially for RT combined with TACE.
In cases of microvascular tumor invasion, especially to the main portal vein, the prognosis is poor. The reasons are as follows: (1) an extensive intrahepatic metastatic spread may result from shedding of HCC cells along the portal vein thrombosis; (2) when the main portal vein is completely blocked, liver function continues to deteriorate leading to liver failure occurs; and (3) exacerbation of portal hypertension causes refractory ascites and bleeding in the esophagus (58). Such physiological changes not only reduce patient survival, but also limit the choice of treatment. TACE is one of the standard treatments for unresectable liver cancer, especially for BCLC stage B tumors. However, it is contraindicated for portal vein tumor thrombus because post-operative ischemia may cause liver failure. At present, sorafenib is one of the first choices for HCC with MVI (59), but it has a slow-acting effect and is unable effectively alleviate the metastasis of liver cancer cells induced by PVTT. Kim et al. (60) reported that the median duration of efficacy of sorafenib alone in PVTT for liver tumor was less than five months.
Due to the rapid thrombosis of HCC, immediate reduction of macrovascular is important for follow-up treatment of the primary tumor. In our study, radiotherapy achieved a high ORR in a short time, especially SBRT and PRT. EBRT is a promising treatment and can recanalize the portal vein in a short time, improve nutrient supply to the liver, delay liver decompensation, and even reduce the Child–Pugh score, improving the survival rate. In addition, radiotherapy has a synergistic effect with mainstream treatments for HCC. TACE plus RT is an effective combination treatments. Radiotherapy targets vascular invasion and re-opens the portal vein, to facilitate conditions for TACE treatment. TACE can effectively inhibit the intrahepatic primary tumor and prevent recurrence of MVI. In our study, the CRT plus TACE group and the CRT plus HAIC group are superior to CRT group in survival (1-year OS: 53.2%, 48.0% vs 36.1%, p = 0.020; 2-year OS: 23.2%, 21.5% vs 15.5%, p = 0.049). Sorafenib, an inhibitor of RAF kinase and VEGFR, can limit tumor cell proliferation and tumor angiogenesis, decrease radiation-activated NF-κB and increase radiation-induced apoptosis (61–63). RT plus sorafenib displayed clinical benefit and safety for patients with macrovascular invasion (23, 27). A meta-analysis showed concurrent Sorafenib and RT significantly greater benefit in OS than did the non-concurrent treatment, and they recommend vascular tumor involvement as the only target of EBRT to avoid excessive toxicities (64). It illustrated the potential of radiotherapy in combination therapy.
Hepatocellular carcinoma (HCC) is a radiosensitive tumor with a dose-response relationship (65). Some large clinical studies showed that a high cumulative and per fraction dose can significantly improve the response rate, local control rate, and prolong survival in patients with HCC (66, 67). Dose of 40 to 45 Gy in 3 fractions or 40 to 50 Gy in 5 fractions (53 to 84 GyE) have been demonstrated to be safe with good therapeutic effect (65). Recently, conformal radiotherapy technique is converting from 3D to IMRT, which can improve curative effect. IMRT achieved higher biologically effective dose within fewer fractions and a shorter duration of therapeutic method than 3D-CRT. Compared with 3D-CRT, IMRT provides a survival benefit in HCC with MVI (29). Meanwhile, a study showed median OS and LCR in the IMRT group were similar to those of the SBRT group for HCC with MVI (47). However, study about IMRT for HCC with MVI is scarce, and the clinical efficacy requires more clinical data to support. SBRT and PRT have a dose advantage over conformal radiotherapy by delivering large doses of radiation to the target tumor volume in a small fraction. The treatments can be completed in a short time because of a higher biologically effective dose. A short course of treatment is conducive due to less interference with other therapeutic methods, reducing toxicity. The outcomes in our study are consistent with prevailing views about the dose-response. SBRT and PRT are associated with higher response rates than CRT. PRT show higher survival rates than CRT.
SBRT has made excellent progress in the field of radiation therapy. However, due to the inherent physical characteristics of photons, SBRT has limited advantage with respect to side effects and liver toxicity. Based on the findings, SBRT is inferior to PRT in avoiding hepatotoxicity. Due to its excellent physical properties, PRT can significantly reduce dose exposure to normal tissues when high doses are used to treat target tumors. PRT is expected to be an ideal treatment for HCCs with high Child-Pugh score. The dosimetric superiority of PRT was correlated with the tumor location. A study by Gandhi et al. showed that PRT can reduce radiation toxicity to target tumor located in the dome and of a size >3 cm (68). Some clinical studies have also proven the safety and efficiency of PRT in the treatment of inferior vena cava tumor thrombi (39, 40). In our study, PRT showed an advantage over SBRT and CRT with respect to hepatotoxicity and hematological toxicity in ≥ grade 3 toxic effect events.
This meta-analysis has several limitations. On one side, meta-analysis is controversial for observational studies. It has been known that RCTs are the most effective means of reducing bias, and meta-analyses of RCTs provide the strongest evidence support (69). However, randomized controlled trial of radiation oncology is difficult to carry out. Radiation therapy competes with other treatments. 60% of all patients with cancer have received primarily treatments in other disciplines before receiving radiotherapy (70). Results from RCTs cannot always be feasible to answer clinical questions, especially in oncology. Meta-analysis of observational studies is an effective method to overcome the information gaps resulting from the insufficient RCT-based data (71). Meta-analysis of observational studies with high-quality did not show significantly different effect sizes from those of RCTs (72). On the other side, heterogeneity is inevitable because of the integrated information in studies with the diversities of designs and populations. The radiotherapy standard of HCC with MVI has not reached a consensus. Too strict inclusion criteria can reduce heterogeneity among studies, but cannot help to address clinical challenges in the real world. Heterogeneity should not be seen as an obstacle to the conclusion. Heterogeneity in meta-analysis requires statistical evaluation and interpretation of clinical phenomena to guide clinical decision-making and solve real-world problems (73).
Conclusion
When compared with SBRT and CRT groups, PRT can prolong survival and reduces the occurrence of hepatotoxic events in patients with HCC and MVI. PRT and SBRT have advantages over CRT with respect to the ORR. A combination treatment based on radiotherapy can provide survival benefits to these patients. Since some of the included studies were observational studies, high-quality comparative studies are needed to provide reliable conclusions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
GW designed the study, acquired data, analyzed data, drafted the manuscript and accepted final version. GH designed the study, acquired the data, reviewed the manuscript and accepted final version. JH acquired the data, reviewed the manuscript and accepted final version. LL designed the study, reviewed the manuscript and accepted final version. SP designed the study, reviewed the manuscript and accepted final version. YL designed the study, analyzed data, reviewed the manuscript and accepted final version. WZ designed the study, analyzed data, reviewed the manuscript and accepted final version. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the National Key Research and Development Program of China (2017YFA0205200), the National Natural Science Foundation of China (81901857, 81771957), Guangdong Provincial Key Laboratory of Tumor Interventional Diagnosis and Treatment (2021B1212040004), and the Science and Technology Development Fund, Macau SAR (0011/2019/AKP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.829708/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural History of Untreated Nonsurgical Hepatocellular Carcinoma: Rationale for the Design and Evaluation of Therapeutic Trials. Hepatology (1999) 29:62–7. doi: 10.1002/hep.510290145
3. Minagawa M, Makuuchi M. Treatment of Hepatocellular Carcinoma Accompanied by Portal Vein Tumor Thrombus. World J Gastroenterol (2006) 12:7561–7. doi: 10.3748/wjg.v12.i47.7561
4. Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A. Cammà C. A Meta-Analysis of Survival Rates of Untreated Patients in Randomized Clinical Trials of Hepatocellular Carcinoma. Hepatology (2010) 51:1274–83. doi: 10.1002/hep.23485
5. Kokudo T, Hasegawa K, Yamamoto S, Shindoh J, Takemura N, Aoki T, et al. Surgical Treatment of Hepatocellular Carcinoma Associated With Hepatic Vein Tumor Thrombosis. J Hepatol (2014) 61:583–8. doi: 10.1016/j.jhep.2014.04.032
6. Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular Carcinoma With Macrovascular Invasion: Defining the Optimal Treatment Strategy. Liver Cancer (2017) 6:360–74. doi: 10.1159/000481315
7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
8. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and Safety of Sorafenib in Patients With Advanced Hepatocellular Carcinoma: Subanalyses of a Phase III Trial. J Hepatol (2012) 57:821–9. doi: 10.1016/j.jhep.2012.06.014
9. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic Toxicity Resulting From Cancer Treatment. Int J Radiat Oncol Biol Phys (1995) 31:1237–48. doi: 10.1016/0360-3016(94)00418-k
10. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of Normal Tissue to Therapeutic Irradiation. Int J Radiat Oncol Biol Phys (1991) 21:109–22. doi: 10.1016/0360-3016(91)90171-y
11. Rim CH, Kim CY, Yang DS, Yoon WS. Comparison of Radiation Therapy Modalities for Hepatocellular Carcinoma With Portal Vein Thrombosis: A Meta-Analysis and Systematic Review. Radiother Oncol (2018) 129:112–22. doi: 10.1016/j.radonc.2017.11.013
12. Qi WX, Fu S, Zhang Q, Guo XM. Charged Particle Therapy Versus Photon Therapy for Patients With Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Radiother Oncol (2015) 114:289–95. doi: 10.1016/j.radonc.2014.11.033
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
14. Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Risk-Adapted Simultaneous Integrated Boost-Proton Beam Therapy (SIB-PBT) for Advanced Hepatocellular Carcinoma With Tumour Vascular Thrombosis. Radiother Oncol (2017) 122:122–9. doi: 10.1016/j.radonc.2016.12.014
15. Hashimoto S, Ogino H, Iwata H, Hattori Y, Nakajima K, Nakanishi M, et al. Efficacy of Proton Beam Therapy for Hepatocellular Carcinoma With Portal Vein or Inferior Vena Cava Tumor Thrombosis. In: ASTRO 59th Annual Meeting - American Society for Radiation Oncology, Vol. 2099. San Diego, CA United States: Elsevier Science Inc, International Journal of Radiation Oncology*Biology*Physics (2017). pp. E2152–3.
16. Dutta D, Tatineni T, Yarlagadda S, Gupte A, Reddy SK, Madhavan R, et al. Hepatocellular Carcinoma Patients With Portal Vein Thrombosis Treated With Robotic Radiosurgery: Interim Results of a Prospective Study. Indian J Gastroenterol (2021) 40:389–401. doi: 10.1007/s12664-021-01172-w
17. Khorprasert C, Thonglert K, Alisanant P, Amornwichet N. Advanced Radiotherapy Technique in Hepatocellular Carcinoma With Portal Vein Thrombosis: Feasibility and Clinical Outcomes. PloS One (2021) 16:e0257556. doi: 10.1371/journal.pone.0257556
18. Tan Z, Lu J, Zhu G, Chen L, Wang Y, Zhang Q, et al. Portal Vein Irradiation Stent Plus Chemoembolization Versus External Radiotherapy Plus Chemoembolization in Hepatocellular Carcinoma With Portal Vein Tumour Thrombus: A Retrospective Study. Cardiovasc Intervent Radiol (1414) 44:1414–22. doi: 10.1007/s00270-021-02889-z
19. Onishi H, Nouso K, Nakamura S, Katsui K, Wada N, Morimoto Y, et al. Efficacy of Hepatic Arterial Infusion Chemotherapy in Combination With Irradiation for Advanced Hepatocellular Carcinoma With Portal Vein Invasion. Hepatol Int (2015) 9:105–12. doi: 10.1007/s12072-014-9592-y
20. Han S, Lee HW, Park JY, Kim SU, Kim DY, Ahn SH, et al. Appraisal of Long-Term Outcomes of Liver-Directed Concurrent Chemoradiotherapy for Hepatocellular Carcinoma With Major Portal Vein Invasion. J Hepatocell Carcinoma (2020) 7:403–12. doi: 10.2147/JHC.S276528
21. Que J, Wu HC, Lin CH, Huang CI, Li LC, Ho CH. Comparison of Stereotactic Body Radiation Therapy With and Without Sorafenib as Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis. Medicine (2020) 99:1–9. doi: 10.1097/MD.0000000000019660
22. Yang JF, Lo CH, Lee MS, Lin CS, Dai YH, Shen PC, et al. Stereotactic Ablative Radiotherapy Versus Conventionally Fractionated Radiotherapy in the Treatment of Hepatocellular Carcinoma With Portal Vein Invasion: A Retrospective Analysis. Radiat Oncol (2019) 14:1–10. doi: 10.1186/s13014-019-1382-1
23. Zhao Y, Wang H, Dong D, Gao S, Zhu X, Wang W. Safety and Efficacy of Transcatheter Arterial Chemoembolization Plus Radiotherapy Combined With Sorafenib in Hepatocellular Carcinoma Showing Macrovascular Invasion. Front Oncol (2019) 9:1065. doi: 10.3389/fonc.2019.01065
24. Lou J, Li Y, Liang K, Guo Y, Song C, Chen L, et al. Hypofractionated Radiotherapy as a Salvage Treatment for Recurrent Hepatocellular Carcinoma With Inferior Vena Cava/Right Atrium Tumor Thrombus: A Multi-Center Analysis. BMC Cancer (2019) 19:1–7. doi: 10.1186/s12885-019-5870-3
25. Iwamoto H, Nomiyama M, Niizeki T, Shimose S, Shirono T, Nakano M, et al. Dose and Location of Irradiation Determine Survival for Patients With Hepatocellular Carcinoma With Macrovascular Invasion in External Beam Radiation Therapy. Oncology (2019) 96:192–9. doi: 10.1159/000495568
26. Shui Y, Yu W, Ren X, Guo Y, Xu J, Ma T, et al. Stereotactic Body Radiotherapy Based Treatment for Hepatocellular Carcinoma With Extensive Portal Vein Tumor Thrombosis. Radiat Oncol (2018) 13:1–9. doi: 10.1186/s13014-018-1136-5
27. Kodama K, Kawaoka T, Aikata H, Uchikawa S, Nishida Y, Inagaki Y, et al. Comparison of Outcome of Hepatic Arterial Infusion Chemotherapy Combined With Radiotherapy and Sorafenib for Advanced Hepatocellular Carcinoma Patients With Major Portal Vein Tumor Thrombosis. Oncology (2018) 94:215–22. doi: 10.1159/000486483
28. Yu JI, Park HC, Jung SH, Choi C, Shin SW, Cho SK, et al. Combination Treatment With Transarterial Chemoembolization, Radiotherapy, and Hyperthermia (CERT) for Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis: Final Results of a Prospective Phase II Trial. Oncotarget (2017) 8:52651–64. doi: 10.18632/oncotarget.17072
29. Hou JZ, Zeng ZC, Wang BL, Yang P, Zhang JY, Mo HF. High Dose Radiotherapy With Image-Guided Hypo-IMRT for Hepatocellular Carcinoma With Portal Vein and/or Inferior Vena Cava Tumor Thrombi Is More Feasible and Efficacious Than Conventional 3D-CRT. Jpn J Clin Oncol (2016) 46:357–62. doi: 10.1093/jjco/hyv205
30. Okazaki E, Yamamoto A, Nishida N, Hamuro M, Ogino R, Hosono M, et al. Three-Dimensional Conformal Radiotherapy for Locally Advanced Hepatocellular Carcinoma With Portal Vein Tumour Thrombosis: Evaluating Effectiveness of the Model for End-Stage Liver Disease (MELD) Score Compared With the Child-Pugh Classification. Br J Radiol (1063) 89:1–8. doi: 10.1259/bjr.20150945
31. Bae BK, Kim JC. The Response of Thrombosis in the Portal Vein or Hepatic Vein in Hepatocellular Carcinoma to Radiation Therapy. Radiat Oncol J (2016) 34:168–76. doi: 10.3857/roj.2016.01669
32. Yeh SA, Chen YS, Perng DS. The Role of Radiotherapy in the Treatment of Hepatocellular Carcinoma With Portal Vein Tumor Thrombus. J Radiat Res (2015) 56:325–31. doi: 10.1093/jrr/rru104
33. Wang PM, Hsu WC, Chung NN, Chang FL, Jang CJ, Fogliata A, et al. Feasibility of Stereotactic Body Radiation Therapy With Volumetric Modulated Arc Therapy and High Intensity Photon Beams for Hepatocellular Carcinoma Patients. Radiat Oncol (2014) 9:1–9. doi: 10.1186/1748-717X-9-18
34. Tanaka Y, Nakazawa T, Komori S, Hidaka H, Okuwaki Y, Takada J, et al. Radiotherapy for Patients With Unresectable Advanced Hepatocellular Carcinoma With Invasion to Intrahepatic Large Vessels: Efficacy and Outcomes. J Gastroenterol Hepatol (2014) 29:352–7. doi: 10.1111/jgh.12333
35. Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, et al. Effectiveness of Stereotactic Body Radiotherapy for Hepatocellular Carcinoma With Portal Vein and/or Inferior Vena Cava Tumor Thrombosis. PloS One (2013) 8:1–7. doi: 10.1371/journal.pone.0063864
36. Tang Q, Li A, Yang G, Lai EC, Zhou W, Jiang Z, et al. Surgical Resection Versus Conformal Radiotherapy Combined With TACE for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Comparative Study. World J Surg (1362) 37:1362–70. doi: 10.1007/s00268-013-1969-x
37. Toya R, Murakami R, Baba Y, Nishimura R, Morishita S, Ikeda O, et al. Conformal Radiation Therapy for Portal Vein Tumor Thrombosis of Hepatocellular Carcinoma. Radiother Oncol (2007) 84:266–71. doi: 10.1016/j.radonc.2007.07.005
38. Lin CS, Jen YM, Chiu SY, Hwang JM, Chao HL, Lin HY, et al. Treatment of Portal Vein Tumor Thrombosis of Hepatoma Patients With Either Stereotactic Radiotherapy or Three-Dimensional Conformal Radiotherapy. Jpn J Clin Oncol (2006) 36:212–7. doi: 10.1093/jjco/hyl006
39. Sekino Y, Okumura T, Fukumitsu N, Iizumi T, Numajiri H, Mizumoto M, et al. Proton Beam Therapy for Hepatocellular Carcinoma Associated With Inferior Vena Cava Tumor Thrombus. J Cancer Res Clin Oncol (2020) 146:711–20. doi: 10.1007/s00432-019-03096-7
40. Komatsu S, Kido M, Asari S, Toyama H, Ajiki T, Demizu Y, et al. Particle Radiotherapy, a Novel External Radiation Therapy, Versus Liver Resection for Hepatocellular Carcinoma Accompanied With Inferior Vena Cava Tumor Thrombus: A Matched-Pair Analysis. Surgery (2017) 162:1241–9. doi: 10.1016/j.surg.2017.08.006
41. Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, et al. Effectiveness and Safety of Proton Beam Therapy for Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis. Strahlenther u Onkol (2014) 190:806–14. doi: 10.1007/s00066-014-0604-6
42. Choi HS, Kang KM, Jeong BK, Jeong H, Lee YH, Ha IB, et al. Effectiveness of Stereotactic Body Radiotherapy for Portal Vein Tumor Thrombosis in Patients With Hepatocellular Carcinoma and Underlying Chronic Liver Disease. Asia-Pac J Clin Oncol (2021) 17:209–15. doi: 10.1111/ajco.13361
43. Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of High-Dose Three-Dimensional Conformal Radiotherapy in Hepatocellular Carcinoma With Portal Vein Thrombosis. Jpn J Clin Oncol (2012) 42:721–9. doi: 10.1093/jjco/hys082
44. Shirai S, Sato M, Suwa K, Kishi K, Shimono C, Sonomura T, et al. Feasibility and Efficacy of Single Photon Emission Computed Tomography-Based Three-Dimensional Conformal Radiotherapy for Hepatocellular Carcinoma 8 Cm or More With Portal Vein Tumor Thrombus in Combination With Transcatheter Arterial Chemoembolization. Int J Radiat Oncol Biol Phys (2010) 76:1037–44. doi: 10.1016/j.ijrobp.2009.03.023
45. Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, et al. Prospective Trial of Combined Transcatheter Arterial Chemoembolization and Three-Dimensional Conformal Radiotherapy for Portal Vein Tumor Thrombus in Patients With Unresectable Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys (2003) 57:113–9. doi: 10.1016/S0360-3016(03)00434-6
46. Kumar R, Yadav HP, Thaper D, Kamal R, Gupta A, Kirti S. Efficacy and Toxicity of SBRT in Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis - A Retrospective Study. Rep Pract Oncol Radiother (2021) 26:573–81. doi: 10.5603/RPOR.a2021.0048
47. Li L-Q, Zhou Y, Huang Y, Liang P, Liang S-X, Su T-S. Stereotactic Body Radiotherapy Versus Intensity-Modulated Radiotherapy for Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis. Hepatol Int (2021) 15:630–41. doi: 10.1007/s12072-021-10173-y
48. Igaki H, Nakagawa K, Shiraishi K, Shiina S, Kokudo N, Terahara A, et al. Three-Dimensional Conformal Radiotherapy for Hepatocellular Carcinoma With Inferior Vena Cava Invasion. Jpn J Clin Oncol (2008) 38:438–44. doi: 10.1093/jjco/hyn038
49. Pao T-H, Hsueh W-T, Chang W-L, Chiang N-J, Lin Y-J, Liu Y-S, et al. Radiotherapy for Inferior Vena Cava Tumor Thrombus in Patients With Hepatocellular Carcinoma. BMC Cancer (2019) 19:560. doi: 10.1186/s12885-019-5654-9
50. Lu D-H, Fei Z-L, Zhou J-P, Hu Z-T, Hao W-S. A Comparison Between Three-Dimensional Conformal Radiotherapy Combined With Interventional Treatment and Interventional Treatment Alone for Hepatocellular Carcinoma With Portal Vein Tumour Thrombosis. J Med Imaging Radiat Oncol (2015) 59:109–14. doi: 10.1111/1754-9485.12207
51. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol (2018) 4:661–9. doi: 10.1001/jamaoncol.2017.5847
52. Yoon S, Lim Y-S, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy Plus Transarterial Chemoembolization for Hepatocellular Carcinoma Invading the Portal Vein: Long-Term Patient Outcomes. Int J Radiat Oncol Biol Phys (2012) 82:2004–11. doi: 10.1016/j.ijrobp.2011.03.019
53. Yu JI, Park HC, Lim DH, Park W, Yoo BC, Paik SW, et al. Prognostic Index for Portal Vein Tumor Thrombosis in Patients With Hepatocellular Carcinoma Treated With Radiation Therapy. J Korean Med Sci (2011) 26:1014–22. doi: 10.3346/jkms.2011.26.8.1014
54. Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen HC, Huang EY, et al. The Treatment Responses in Cases of Radiation Therapy to Portal Vein Thrombosis in Advanced Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys (2009) 73:1155–63. doi: 10.1016/j.ijrobp.2008.06.1486
55. Nomura T, Tani J, Deguchi A, Nakahara M, Oura K, Tadokoro T, et al. Efficacy of Combined Modality Therapy With Sorafenib Following Hepatic Arterial Injection Chemotherapy and Three-Dimensional Conformal Radiotherapy for Advanced Hepatocellular Carcinoma With Major Vascular Invasion. Mol Clin Oncol (2019) 11:447–54. doi: 10.3892/mco.2019.1920
56. Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, et al. Proton-Beam Therapy for Hepatocellular Carcinoma Associated With Portal Vein Tumor Thrombosis. Strahlenther Und Onkol (2009) 185:782–8. doi: 10.1007/s00066-009-2020-x
57. Hou J-Z, Zeng Z-C, Zhang J-Y, Fan J, Zhou J, Zeng M-S. Influence of Tumor Thrombus Location on the Outcome of External-Beam Radiation Therapy in Advanced Hepatocellular Carcinoma With Macrovascular Invasion. Int J Radiat Oncol Biol Phys (2012) 84:362–8. doi: 10.1016/j.ijrobp.2011.12.024
58. Lau WY LE, Yu SC. Management of Portal Vein Tumor Thrombus. In: Lau WY, editor. Hepatocellular Carcinoma. Singapore: World Scientific Publishing (2008). p. 739–60.
59. Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology (2011) 53:1020–2. doi: 10.1002/hep.24199
60. Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, et al. Comparison of Chemoembolization With and Without Radiation Therapy and Sorafenib for Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis: A Propensity Score Analysis. J Vasc Interv Radiol (2015) 26:320–9.e326. doi: 10.1016/j.jvir.2014.10.019
61. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res (2004) 64:7099–109. doi: 10.1158/0008-5472.Can-04-1443
62. Huang CY, Lin CS, Tai WT, Hsieh CY, Shiau CW, Cheng AL, et al. Sorafenib Enhances Radiation-Induced Apoptosis in Hepatocellular Carcinoma by Inhibiting STAT3. Int J Radiat Oncol Biol Phys (2013) 86:456–62. doi: 10.1016/j.ijrobp.2013.01.025
63. Yu W, Gu K, Yu Z, Yuan D, He M, Ma N, et al. Sorafenib Potentiates Irradiation Effect in Hepatocellular Carcinoma In Vitro and In Vivo. Cancer Lett (2013) 329:109–17. doi: 10.1016/j.canlet.2012.10.024
64. Rim CH, Park S, Shin IS, Yoon WS. Is the Concurrent Use of Sorafenib and External Radiotherapy Feasible for Advanced Hepatocellular Carcinoma? A Meta-Analysis. Cancers (Basel) (2021) 13:1–14. doi: 10.3390/cancers13122912
65. Schaub SK, Hartvigson PE, Lock MI, Høyer M, Brunner TB, Cardenes HR, et al. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Current Trends and Controversies. Technol Cancer Res Treat (2018) 17:1533033818790217. doi: 10.1177/1533033818790217
66. Jang WI, Kim MS, Bae SH, Cho CK, Yoo HJ, Seo YS, et al. High-Dose Stereotactic Body Radiotherapy Correlates Increased Local Control and Overall Survival in Patients With Inoperable Hepatocellular Carcinoma. Radiat Oncol (2013) 8:250. doi: 10.1186/1748-717x-8-250
67. Seong J, Lee IJ, Shim SJ, Lim DH, Kim TH, Kim JH, et al. A Multicenter Retrospective Cohort Study of Practice Patterns and Clinical Outcome on Radiotherapy for Hepatocellular Carcinoma in Korea. Liver Int (2009) 29:147–52. doi: 10.1111/j.1478-3231.2008.01873.x
68. Gandhi SJ, Liang X, Ding X, Zhu TC, Ben-Josef E, Plastaras JP, et al. Clinical Decision Tool for Optimal Delivery of Liver Stereotactic Body Radiation Therapy: Photons Versus Protons. Pract Radiat Oncol (2015) 5:209–18. doi: 10.1016/j.prro.2015.01.004
69. Berlin JA, Golub RM. Meta-Analysis as Evidence: Building a Better Pyramid. Jama (2014) 312:603–5. doi: 10.1001/jama.2014.8167
70. Benzies KM, Premji S, Hayden KA, Serrett K. State-Of-the-Evidence Reviews: Advantages and Challenges of Including Grey Literature. Worldviews Evid Based Nurs (2006) 3:55–61. doi: 10.1111/j.1741-6787.2006.00051.x
71. Frieden TR. Evidence for Health Decision Making - Beyond Randomized, Controlled Trials. N Engl J Med (2017) 377:465–75. doi: 10.1056/NEJMra1614394
72. Concato J, Shah N, Horwitz RI. Randomized, Controlled Trials, Observational Studies, and the Hierarchy of Research Designs. N Engl J Med (2000) 342:1887–92. doi: 10.1056/nejm200006223422507
Keywords: radiation therapy, hepatocellular carcinoma, macrovascular invasion, portal vein tumor thrombosis, conformal radiation therapy, stereotactic body radiotherapy, particle therapy
Citation: Wu G, Huang G, Huang J, Lu L, Peng S, Li Y and Zhao W (2022) Comparison of External Beam Radiation Therapy Modalities for Hepatocellular Carcinoma With Macrovascular Invasion: A Meta-Analysis and Systematic Review. Front. Oncol. 12:829708. doi: 10.3389/fonc.2022.829708
Received: 06 December 2021; Accepted: 24 January 2022;
Published: 15 February 2022.
Edited by:
An Liu, City of Hope National Medical Center, United StatesReviewed by:
Bin-Yan Zhong, The First Affiliated Hospital of Soochow University, ChinaChai Hong Rim, Korea University, South Korea
Copyright © 2022 Wu, Huang, Huang, Lu, Peng, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Li, bG9ycnk1MTYwQDE2My5jb20=; Wei Zhao, emhhb3dlaXNtdUBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Guanheng Wu
Guanheng Wu Guomin Huang†
Guomin Huang† Shaojun Peng
Shaojun Peng