- 1Department of Oncology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 3Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Traditional medicine preparations (TMPs) combined with chemotherapy is widely used for patients with advanced pancreatic cancer (APC); however, its efficacy and safety are still unclear. The purpose of this meta-analysis was to evaluate the clinical efficacy and safety of TMPs combined with chemotherapy for the treatment of APC.
Methods: A systematic search of eight electronic databases for randomized controlled trials (RCTs) was conducted from inception to October 15, 2021. Tumor response was identified as primary outcome, whereas quality of life (QoL), cancer biomarkers, and adverse drug reactions (ADRs) were identified as secondary outcomes. Quality of the evidence for each outcome was evaluated by GRADE profiler.
Results: In total, 31 RCTs involving 1,989 individuals were included. This meta-analysis showed that TMPs combined with chemotherapy significantly improved the objective response rate (ORR) (RR=1.64, 95% CI [1.43 to 1.88], p <0.00001), disease control rate (DCR) (RR=1.29, 95% CI [1.21 to 1.38], p <0.00001), and QoL (continuous data: SMD=0.81, 95% CI [0.44 to 1.18], p <0.0001, dichotomous data: RR=1.44, 95% CI [1.22 to 1.70], p<0.0001), compared to those with chemotherapy alone. In addition, the combined treatment group also had lower levels of CA19-9 (SMD=-0.46, 95% CI [-0.90 to -0.02], p=0.04) and CEA (SMD=-0.55, 95% CI [-0.93 to -0.17], p=0.004). Moreover, TMPs reduced the ADRs during chemotherapy.
Conclusion: This systematic review suggests that TMPs combined with chemotherapy might be a potential option to enhance therapeutic effects and reduce ADRs during the treatment of APC. However, more high-quality randomized controlled trials with more participants are needed.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=209825, identifier PROSPERO Number: CRD42021264938.
1 Introduction
Pancreatic cancer is recognized as a highly deadly malignant tumor with approximately equivalent number of new cases (496,000) and deaths (466,000 cases) annually (1). Remarkably, the incidence of pancreatic cancer has increased significantly by 39.3% between 2007 and 2017, thus ranking among top five actively growing cancers worldwide (2). Despite its 5-year survival rates are only 10-25% (3, 4), and surgery remains the only possible cure for pancreatic cancer. Unfortunately, the sobering reality is that only less than 20% of patients have a chance of undergoing surgery due to the lack of prominent symptoms at early stages of this disease (5) and the most patients are often diagnosed with local vessel involvement or distant metastases. Systemic chemotherapy plays an important role for the management of advanced pancreatic cancer (APC) and can aid to prolong survival (6). However, the median total survival of APC is only approximately 6 to 11 months (7, 8). Meanwhile, several adverse drug reactions (ADRs) of chemotherapy (neutropenia, anemia, neurotoxicity etc.) have severely affected the treatment outcome of patients with APC (9, 10). Thus, APC contributes to substantial burden to individuals and society.
In order to prolong the long-term survival while preserving the quality of life (QoL) of patients, the search for novel complementary treatment combined with chemotherapy becomes crucial.
Traditional medicine preparations (TMPs) are defined as any formulation of medicinal herbs including extracts of herbs, herbal injection, Chinese proprietary medicine, or self-prepared herbal decoctions prescribed by practitioners, with the advantages of easy availability, low price, and generally exhibit few ADRs. A number of experimental studies have shown that several plant extracts such as curcumin (11), bitter melon juice (12), elemene (13) etc. can exhibit significant efficacy against different cancers. These natural compounds function as potent anti-neoplastic agents by interfering with multiple cellular processes, but limited chemical stability and oral bioavailability have hampered their rapid clinical translation which might be improved by nanoformulations (11). There are several reports in literature describing the beneficial effects of many TMPs in cancer treatment for improving clinical efficacy and safety (14–16).
Over the past 20 years, some clinical studies have been published describing the application of TMPs for the treatment of APC but their findings are relatively less convincing because of the potential use of small sample size. Therefore, we have performed a meta-analysis to systematically analyze the results of these prior studies, which aimed to evaluate the efficacy and safety of TMPs combined with chemotherapy for APC, thus hoping to provide an important reference for the clinicians.
2 Methods
2.1 Study Design
This systemic review and meta-analysis strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guidelines (17). Its registered number in PROSPERO is CRD42020209825.
2.2 Inclusion and Exclusion Criteria
2.2.1 Inclusion Criteria
2.2.1.1 Patients
Patients diagnosed with unresectable (locally advanced and/or metastatic) or stage III–IV pancreatic cancer through the histological and cytological diagnostic criteria, and TNM staging systems were included. The baseline data of patients in the two groups were comparable. There were no restrictions on age or sex.
2.2.1.2 Interventions
The experimental group received TMPs combined with chemotherapy. The TMPs included extracts of herbs, patented herbal products, or self-prepared herbal decoctions prescribed by practitioners. The administration or formulation of TMPs including decoction, granule, capsule, or injection were not limited. The control group received the same chemotherapy regimen alone.
2.2.1.3 Primary Outcome
The primary outcome was tumor response assessed using the objective response rate (ORR) and disease control rate (DCR), measured separately before the start of each trial and at the end of the follow-up time, according to the WHO (18) and RECIST (19) criteria. Trials not stating evaluation criteria were also included and subgroup analysis was carried out thereafter.
2.2.1.4 Secondary Outcomes
The secondary outcomes were defined as the QoL, cancer biomarkers, and ADRs. The interventions were considered to be effective for QoL when the Karnofsky Performance Status (KPS) score was no more than 10 points lower after treatment. Comparisons were also made for the mean ± standardized difference (SD) of KPS scores before and after treatment was also allowed. Cancer biomarker levels were assessed by measuring the CA19-9 and CEA levels separately before the start of each trial and at the end of the follow-up time. The mean ± SD changes in CA19-9 and CEA levels were synthesized to evaluate the differences between the two groups. ADRs were evaluated by calculating the number of people at stage 0-IV cancer experiencing gastrointestinal toxicity (nausea, vomiting, and diarrhea), myelosuppression (leukopenia, decreased hemoglobin, and thrombopenia), hair loss, liver dysfunction, and renal dysfunction, according to the WHO or NCI recommendations for grading acute and subacute toxicity. The interventions were considered to lead to ADRs when patients had levels III-IV.
2.2.1.5 Types of Studies
All published randomized controlled trials (RCTs) published were included. Quasi-randomized trials were excluded. Only full journal publications with sufficient data for analysis were included. The language was not restricted.
2.2.2 Exclusion Criteria
The exclusion criteria were the following: (1) simultaneous other types of primary tumors; (2) the TMPs were not fixed within 1 study; (3) unspecified or inconsistent observation nodes between two groups within 1 study; (4) insufficient data; (5) irregular outcome evaluation criteria; and (6) duplicated data.
2.3 Search Strategy for the Identification of Studies
RCTs were searched from inception to October 15, 2021, in the following electronic databases: PubMed, EMBASE, the Cochrane Library, clinicaltrials.gov, Trip Database, Allied and Complementary Medicine Database (AMED), Latin American and Caribbean Health Sciences Literature (LILACS), China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP database), Wangfang Data Knowledge Service Platform, and Chinese Biomedical Literature Database (CBM). The following terms were used in the English databases: “neoplasms”, “ carcinoma”, “ adenocarcinoma”, “cancer*”, “carcin*”, “neoplas*”, “tumo*”, “adenocarcinoma*”, “pancreas”, “pancreatic”, “complementary therapies”, “drugs, Chinese herbal”, “medicine, traditional”, “herbal medicine”, “medicine, east asian traditional”, “plant extracts”, “phytotherapy”, “alternative medicine”, “complementary therap*”, “chinese medicine”, “herb*”, “herbalism”, “plant extract*”, “medicinal plant*”, “oriental medicine”. Equivalent search words were used in the Chinese databases (the detailed search strategy is available in Supplement 1). We searched for additional trials by reviewing the reference lists of studies related to TMPs combined with chemotherapy for APC. All studies were independently searched by two reviewers (J. Hu and J. Jiang). Any disagreement arising from this process was resolved by consensus or by a third reviewer (M. Cheng).
2.4 Data Extraction
Two reviewers (G. Zhu and S. He) independently imported the studies into Endnote X9 software. After the exclusion of duplicate studies, the remaining studies were determined independently by two reviewers (H. Yu and B. Shi) by reading the title, abstract, and full text. Any disagreement arising during this process was discussed or decided by a third reviewer (J. Hu). Two reviewers (Y. Li and Z. Yao) imported the relevant data from the included studies into EpiData 3.1. The extracted data included the basic information (title, first author, year of publication, sample size ratio, sex ratio, age range, etc.), methods (blind methods, random methods, interventions, etc.), and outcomes. When relevant data were incomplete, we contacted the author or included an explanation in our article.
2.5 Assessment of Risk of Bias
Three reviewers (J. Hu, J. Jiang and X. Zhang) independently evaluated the included studies using the Cochrane risk of bias tool for RCTs according to the guidance of the Cochrane Handbook for Systematic Review of Interventions (version 5.1.0), which includes the following seven bias domains: selection bias due to random sequence generation, selection bias due to allocation concealment, performance bias due to blinding of participants and personnel, detection bias due to blinding of outcome assessment, attrition bias due to incomplete outcome data, reporting bias due to selective reporting, and other biases. The overall judgment on the risk of bias for each domain had three response options (low/high/unclear) (20). Any disagreement arising from this process was resolved by consensus or by a third reviewer (R. Liu).
2.6 Statistical Analysis
2.6.1 Strategy for Data Synthesis
Two reviewers (J. Hu and J. Jiang) conducted a meta-analysis on the included studies using Review Manager 5.3. The risk ratio (RR) was used to present the dichotomous data, whereas the standardized mean difference (SMD) was used to present continuous data. The 95% confidence intervals (CIs) were defined, and statistical significance was set at p<0.05. Cochran’s Q test and the I² statistic were used to assess heterogeneity. The heterogeneity among different trials was described by the I² index, indicating a high statistical heterogeneity at > 50%. If heterogeneity (p ≥ 0.10, I² ≤ 50%) was rejected, a fixed-effects model (FEM) was used to synthesize the RR, SMD, and their 95% CI. Otherwise, a random-effects model (REM) was utilized. Sensitivity analysis was performed by sequentially excluding each trial to examine the robustness of the results. Publication bias was evaluated according to the non-parametric trim-and-fill analysis of publication bias and Egger’s test when there were more than 10 included studies.
2.6.2 Analysis of Subgroups or Subsets
According to the KPS score, drug delivery of TMPs, the number of chemotherapy drug, chemotherapy regimen, follow-up time, and different herbs or combination of herbs, subgroup analysis was performed to reveal the clinical heterogeneity and its influence on outcomes.
2.7 Assessment of Evidence Quality
Two reviewers (M. Cheng and J. Hu) independently evaluated the quality of evidence for each outcome by GRADE profiler, which included the following five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall judgment on the quality of the evidence for each outcome had four response options (high/moderate/low/very low) (21). Any disagreement arising from this process was resolved by consensus or by a third reviewer (R. Liu).
3 Results
3.1 Literature Screening Results
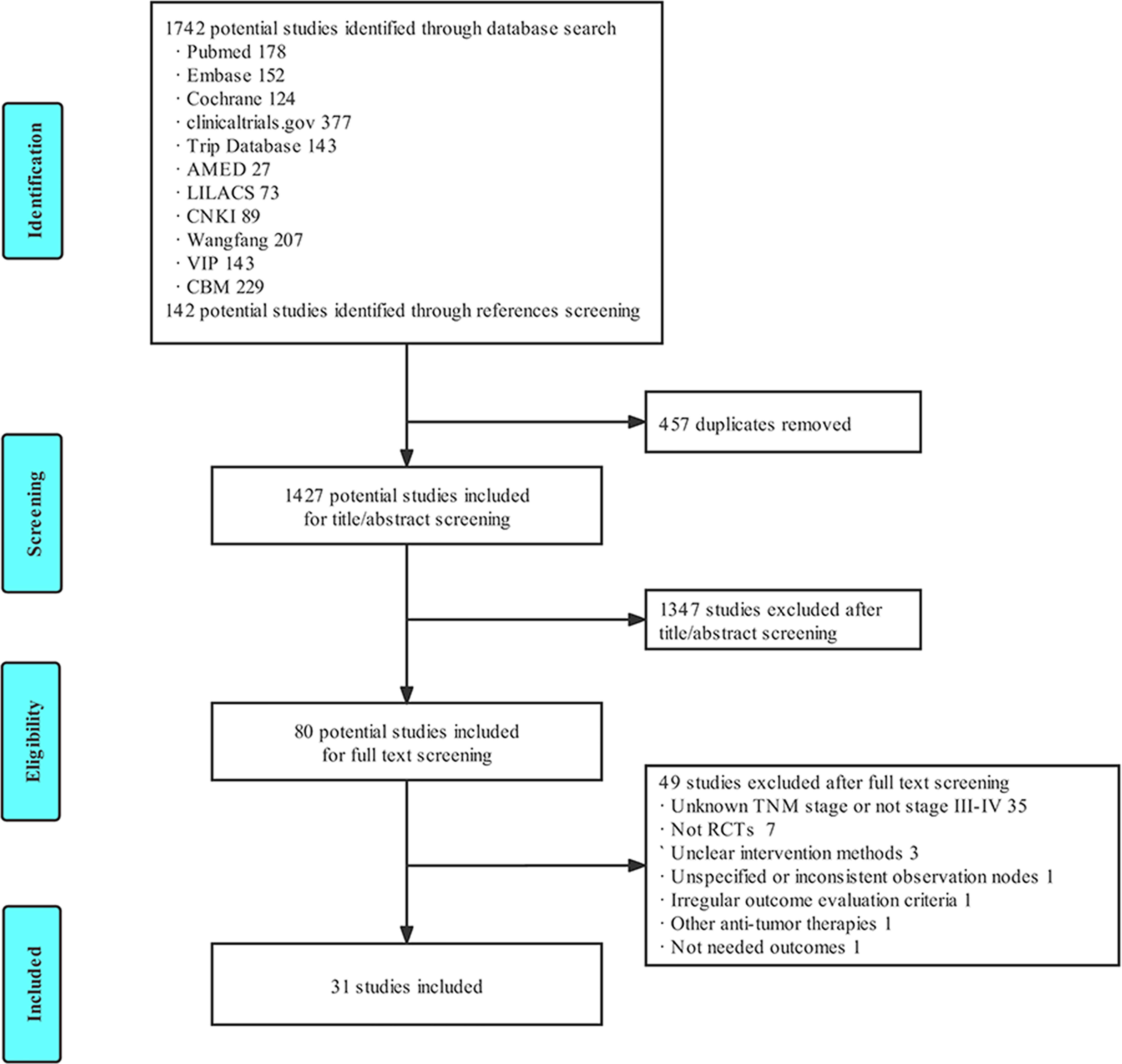
A total of 1,884 studies were obtained in the primary search and references screening, and 1,427 studies were included after the elimination of 457 duplicated studies. A total of 80 studies were selected after screening the titles, abstracts. Ultimately, 31 eligible studies were included in the final meta-analysis after reading the full text, according to the inclusion and exclusion criteria. The literature screening process is illustrated in Figure 1.
3.2 Study Characteristics
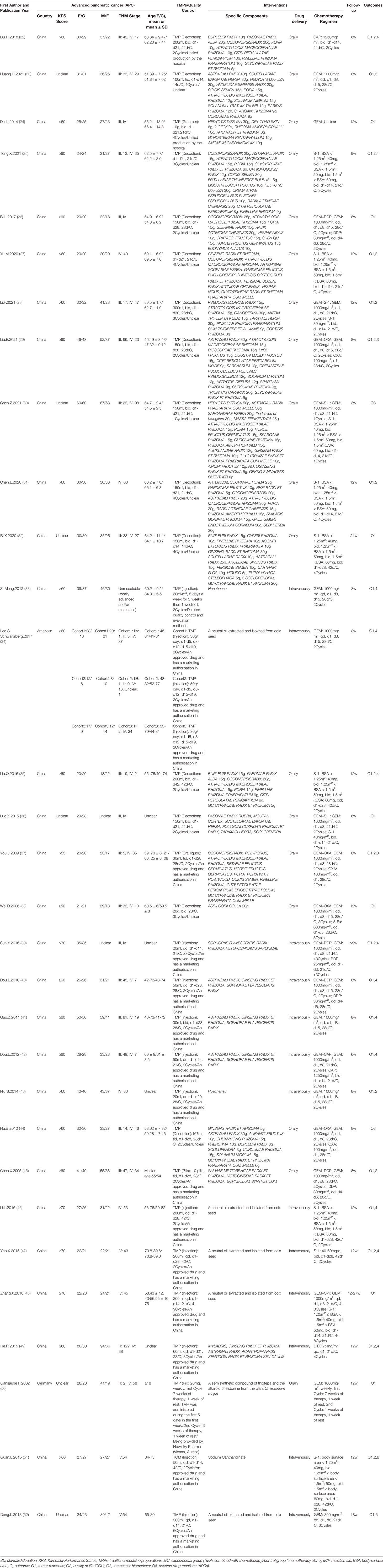
A total of 1,989 individuals (1,014 subjects in the experimental group and 975 subjects in the control group) with APC were included in 31 RCTs whose basic characteristics are listed in Table 1. Studies were conducted in China, America or Germany and published in Chinese or English between 2002 and 2021. 21 trials (22, 24–29, 31, 33–35, 37, 38, 40–45, 49, 51) included individuals with KPS score < 70, 4 trials (39, 46–48) included individuals with KPS score ≥70, and 6 trials (23, 30, 32, 36, 50, 52) included individuals with unclear KPS score. Of the different drug delivery, 18 trials (22–32, 35–38, 44, 45, 50) used oral TMPs, whereas 13 trials (33, 34, 39–43, 46–49, 51, 52) used intravenous TMPs. Regarding the chemotherapy regimens, 18 trials (22–25, 27, 31–35, 41, 43, 46, 47, 49–52) used single-drug chemotherapy, whereas 13 trials (26, 28–30, 36–40, 42, 44, 45, 48) used double-drugs chemotherapy. Furthermore, 21 trials (23, 24, 26, 28–30, 33, 34, 36–45, 48, 50, 52) used GEM-based chemotherapy, 12 trials (25, 27, 28, 30–32, 35, 36, 46–48, 51) used S-1-based chemotherapy, and 2 trials (22, 49) used others. The follow-up time for all trials was between 3 and 27 weeks. Moreover, 25 trials reported tumor response including ORR or DCR according to the WHO or RECIST guidelines, and 3 trials did not state evaluation criteria. 14 trials reported QoL according to the KPS, 5 trials reported the level of cancer biomarkers, and 14 trials reported ADRs according to the WHO or NCI chemotherapy toxicity response grading criteria.
3.3 Assessment of Methodological Bias Risk
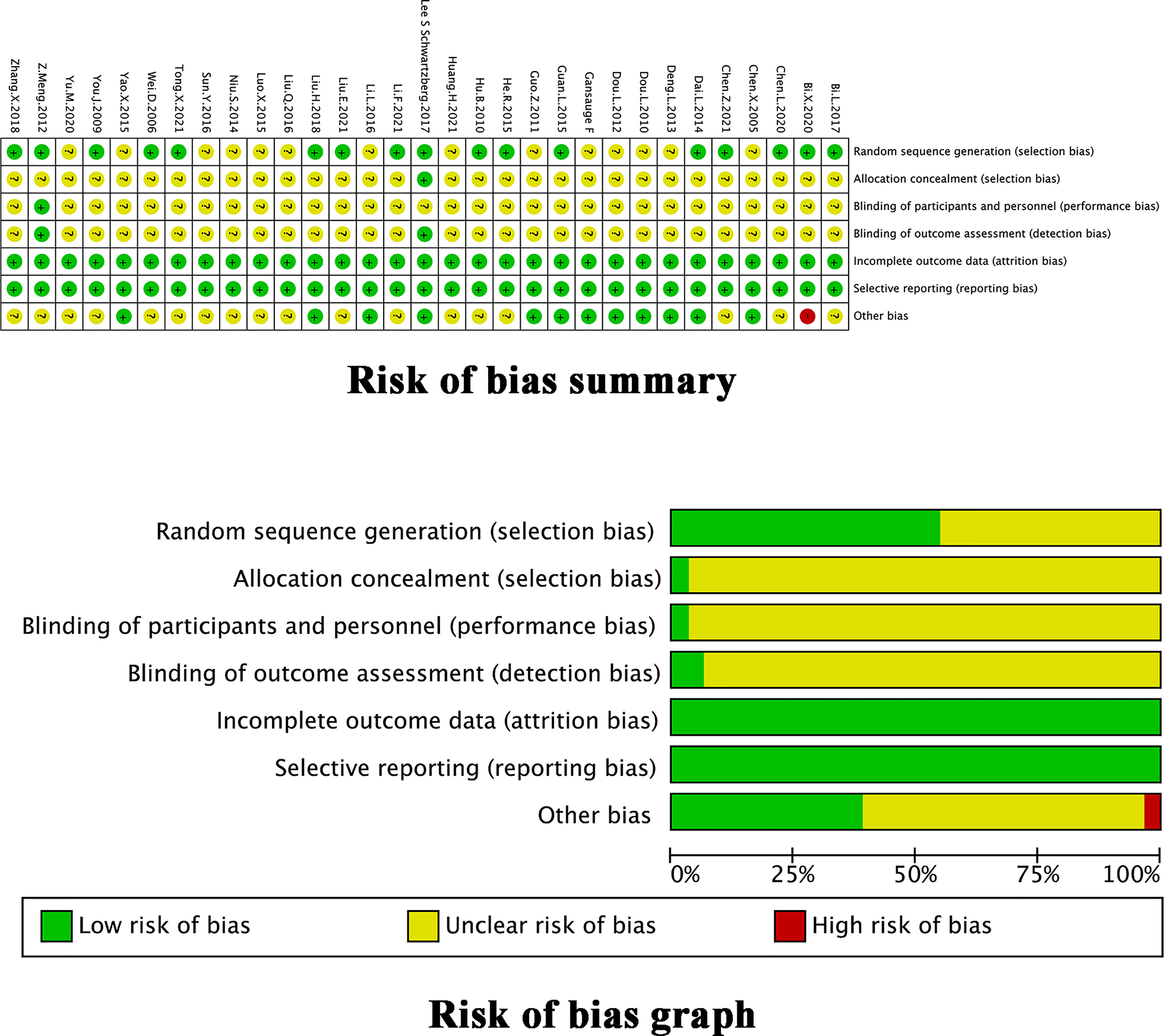
The assessment of the methodological bias risk of each trial included is shown in Figure 2. Only 17 trials (22, 24–26, 28–34, 37, 38, 44, 48, 49, 51) reportedly used a random sequence generation including random number table, envelope, bayesian algorithm and centralized interactive voice response system. Unclear selection bias existed because 14 trials did not describe random sequence generation. Just 1 trial (34) reported allocation concealment. Except for 2 trials (33, 34), studies failed to report the blinding method, which led to unclear performance and detection biases. None of the trials reported any loss to follow-up. All trials had low risk on attrition and reporting bias. The ORR and DCR evaluation criteria in 1 trial (32) did not coincide with our study which might influence results. Some unclear information, including KPS score, gender, age at the time of inclusion, and evaluation criteria of outcomes, in 10 trials (23, 30, 32, 33, 36, 37, 39, 43, 44, 48, 49) might lead to other potential bias. The quality of the TMPs was shown in Table 1, 14 trials (34, 37, 39–43, 45–52) described an approved TMPs having clear manufacturer, production batch number and marketing authorisation in China. 1 trial (33) described in detail the product quality control was assured and monitored by acquiring raw material from designated source provinces, establishing fingerprinting, measuring the concentrations of certain compounds in the extract, and comparing the high performance liquid chromatography fingerprinting. 1 trial (50) only described the provider of TMPs. The rest 15 trials (22–32, 35, 36, 38, 44) used self-prepared herbal decoctions prescribed by practitioners but did not describe the origin, processing method or dosage of herbs and none of them described a quality control method.
3.4 Tumor Response
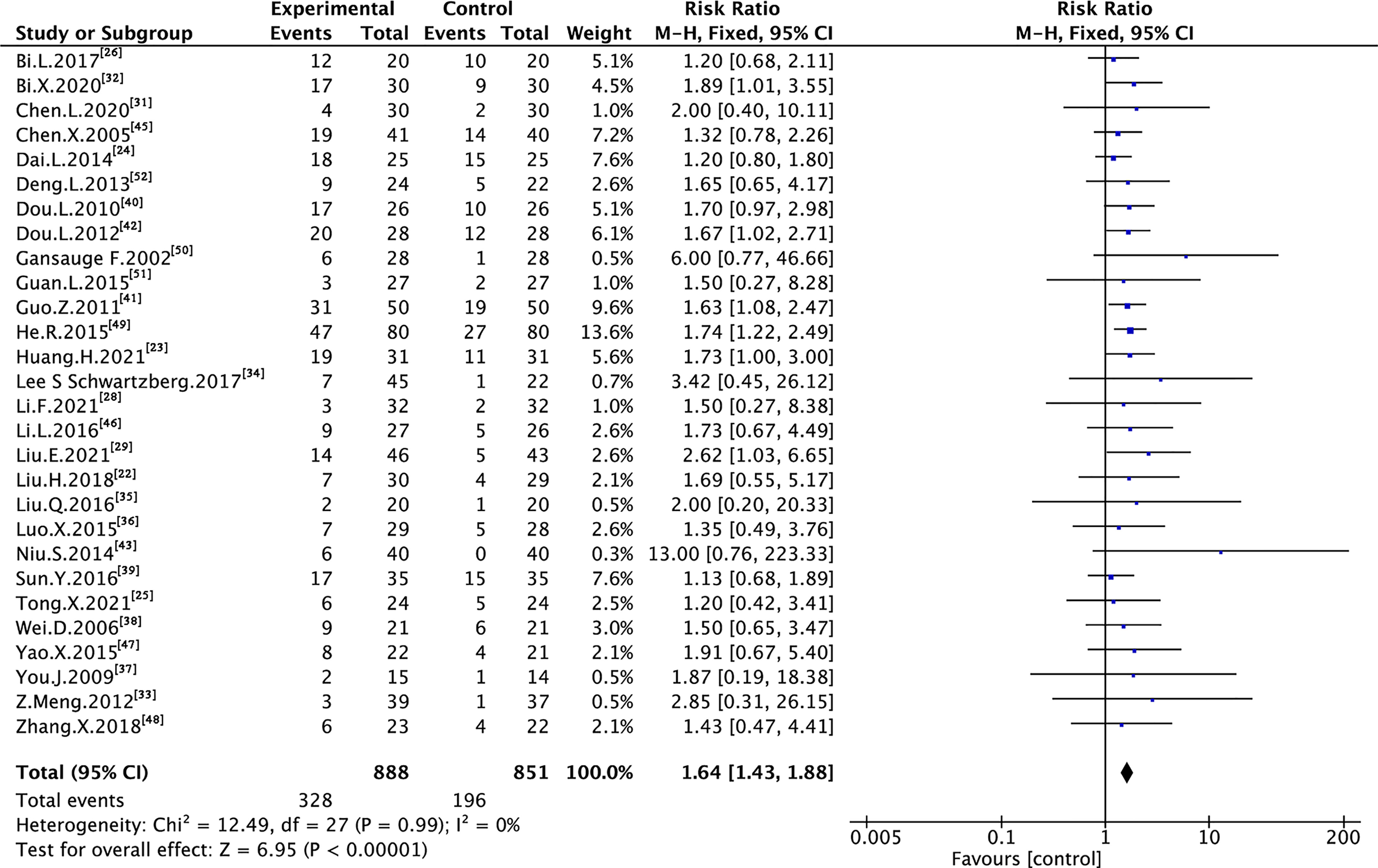
A total of 29 trials assessing 1,739 and 1,703 cases reported ORR and DCR, respectively (Figure 3 and Figure 4). As shown in the figures, there was low heterogeneity between trials as per Cochran’s Q test and Higgins’s I² (I² = 0%, I² = 22%); therefore, the FEM was used to synthesize data from different trials. The results of the meta-analysis showed that TMPs combined with chemotherapy increased ORR (RR=1.64, 95% CI [1.43 to 1.88], p <0.00001) and DCR (RR=1.29, 95% CI [1.21 to 1.38], p <0.00001), compared to chemotherapy alone.
3.5 Quality of Life
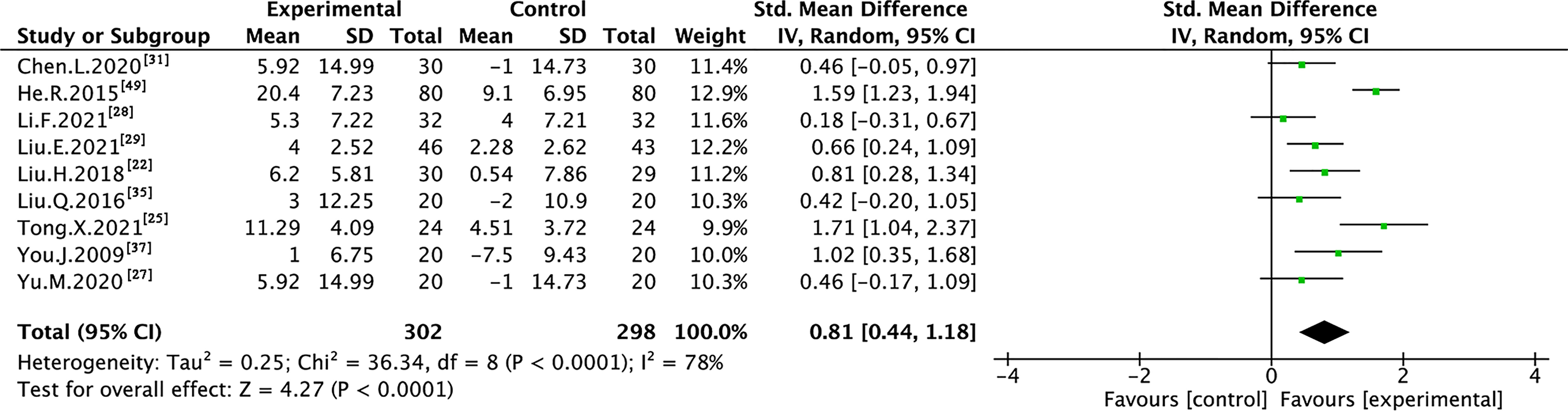
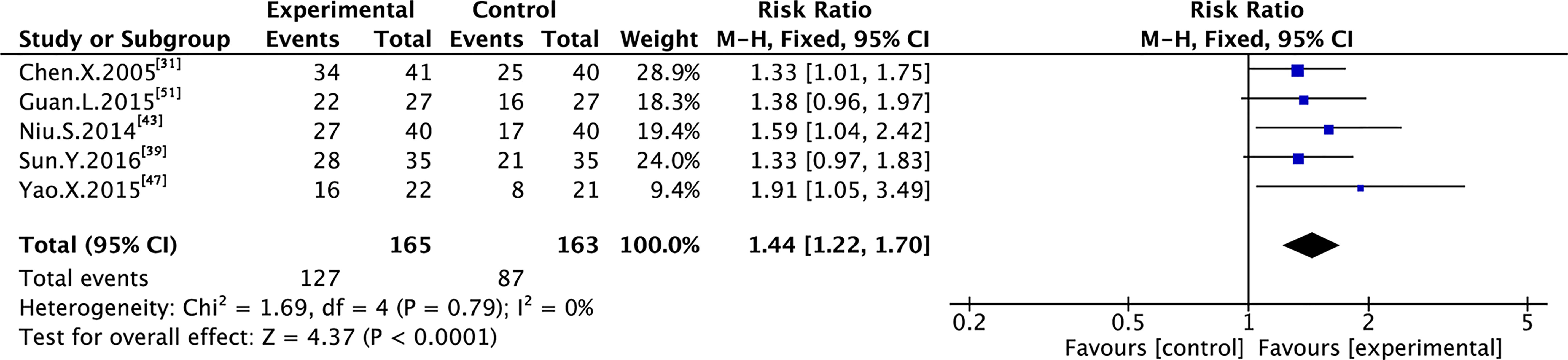
Nine trials with 600 individuals reported the QoL by using continuous data (Figure 5), whereas four trials with 274 individuals reported it using dichotomous data (Figure 6) according to the KPS scale.
In the continuous data, high heterogeneity was observed in QoL (I² = 78%); therefore, REM was used to synthesize data from different trials. The results of the meta-analysis showed that TMPs combined with chemotherapy increased QoL (SMD=0.81, 95% CI [0.44 to 1.18], p <0.0001), compared to chemotherapy alone. To demonstrate the reason for the statistical heterogeneity of the results, subgroup analysis was performed (Table S1 and Figures S1–S5). The drug delivery of TMPs might be the reason for the heterogeneity in QoL (Figure S2).
In the dichotomous data, no heterogeneity was observed in QoL (I² = 0%); therefore, FEM was used to synthesize data from different trials. The results of the meta-analysis showed that TMPs combined with chemotherapy increased QoL (RR=1.44, 95% CI [1.22 to 1.70], p<0.0001), compared to chemotherapy alone.
3.6 Cancer Biomarkers
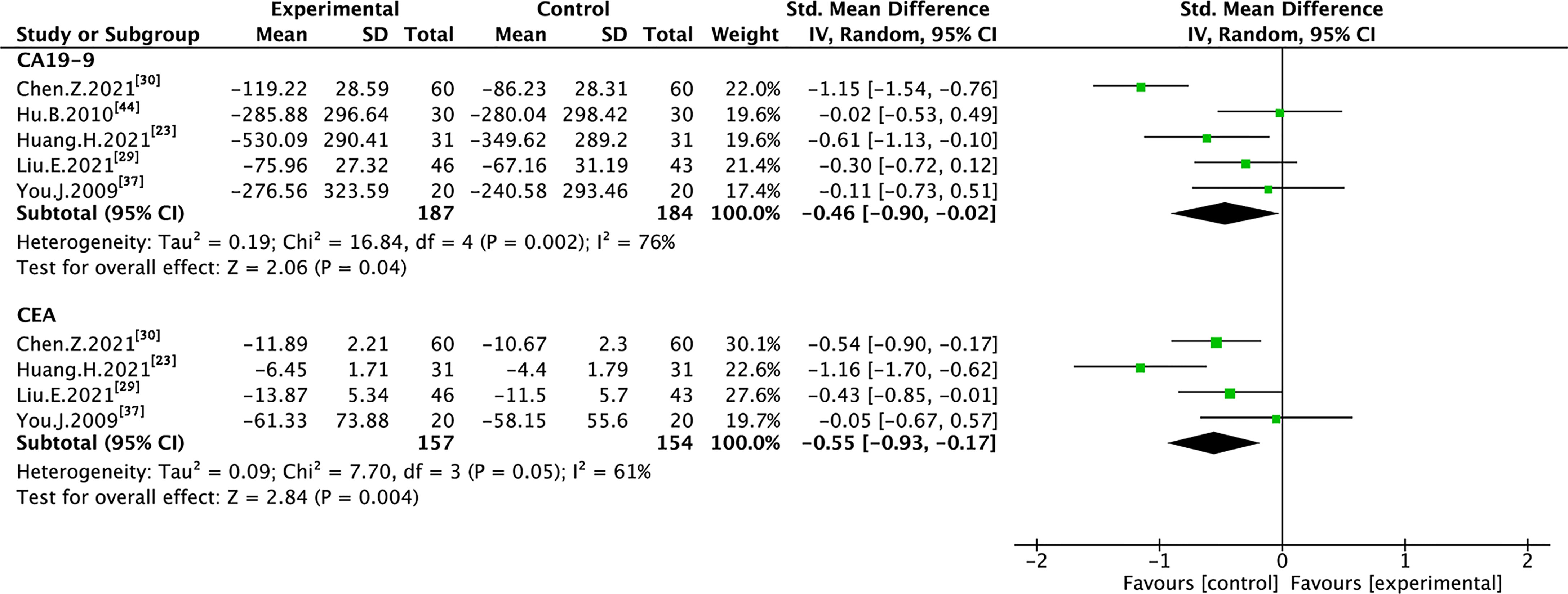
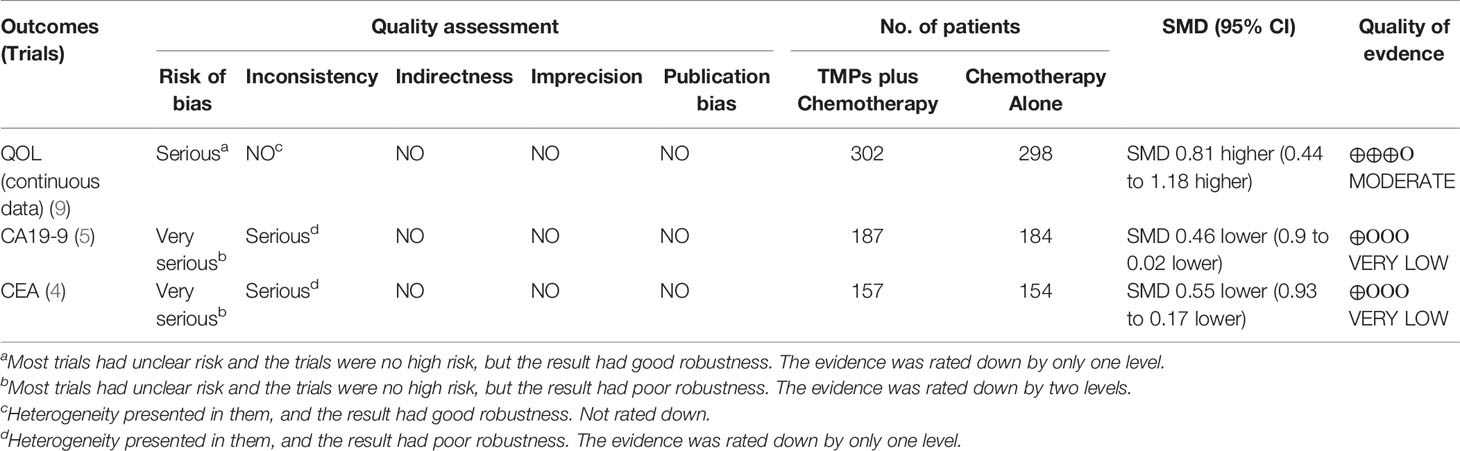
Five trials with 371 individuals reported cancer biomarkers (Figure 7). Statistical heterogeneity was demonstrated in CA19-9 (I² = 76%), and CEA (I² = 61%); therefore, REM was used to synthesize SMD. The results of the meta-analysis showed that TMPs combined with chemotherapy reduced the level of CA19-9 (SMD=-0.46, 95% CI [-0.90 to -0.02], p=0.04), and CEA (SMD=-0.55, 95% CI [-0.93 to -0.17], p=0.004), compared to chemotherapy alone. To demonstrate the reason for the statistical heterogeneity of the results, a subgroup analysis was performed (Tables S2, S3 and Figures S6–S9). The follow-up time might be the reason for the heterogeneity in CA19-9 (Figure S7) and the number of chemotherapy drug might be the reason for the heterogeneity in CEA (Figure S8).
3.7 Adverse Drug Reactions
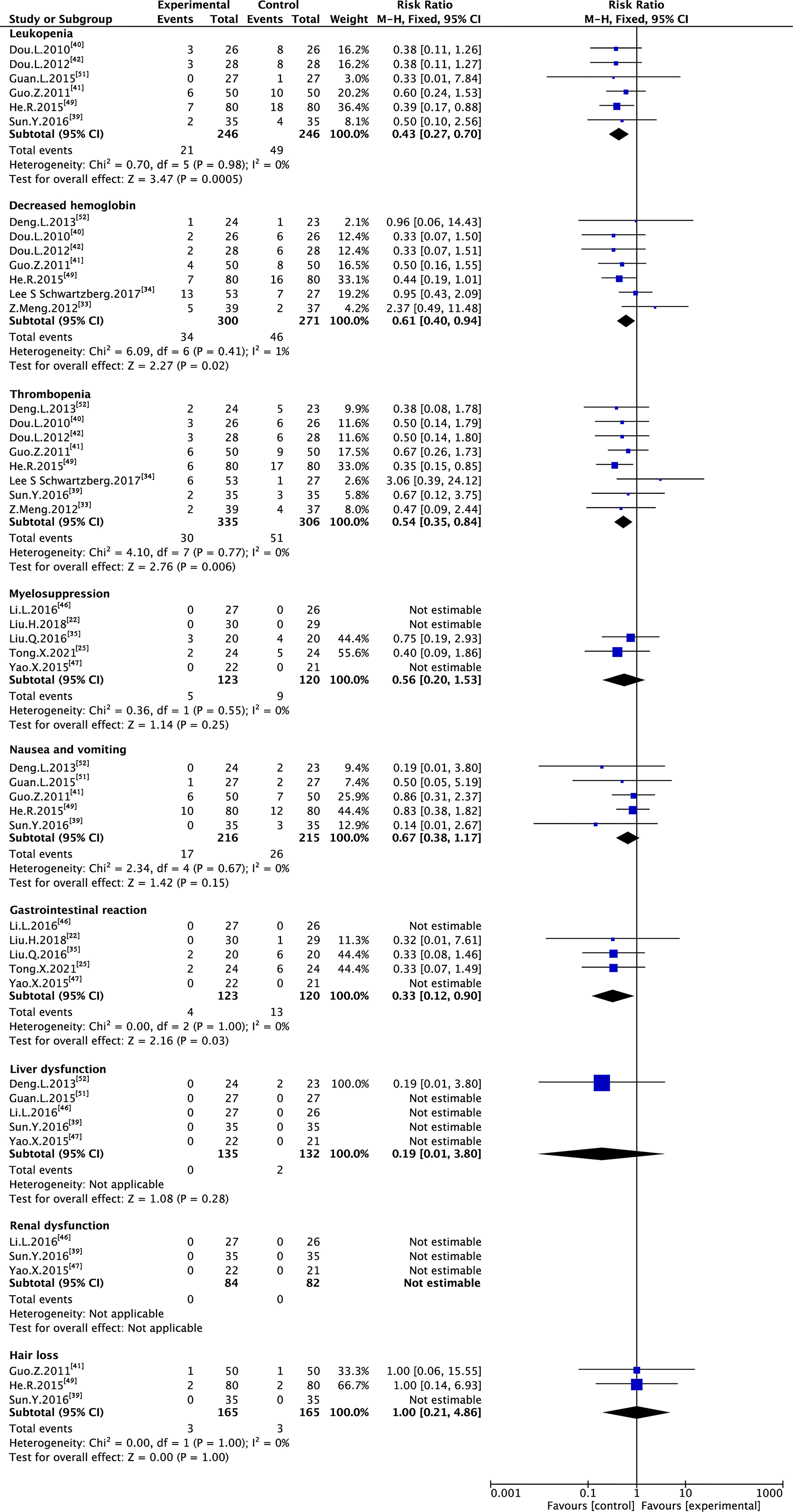
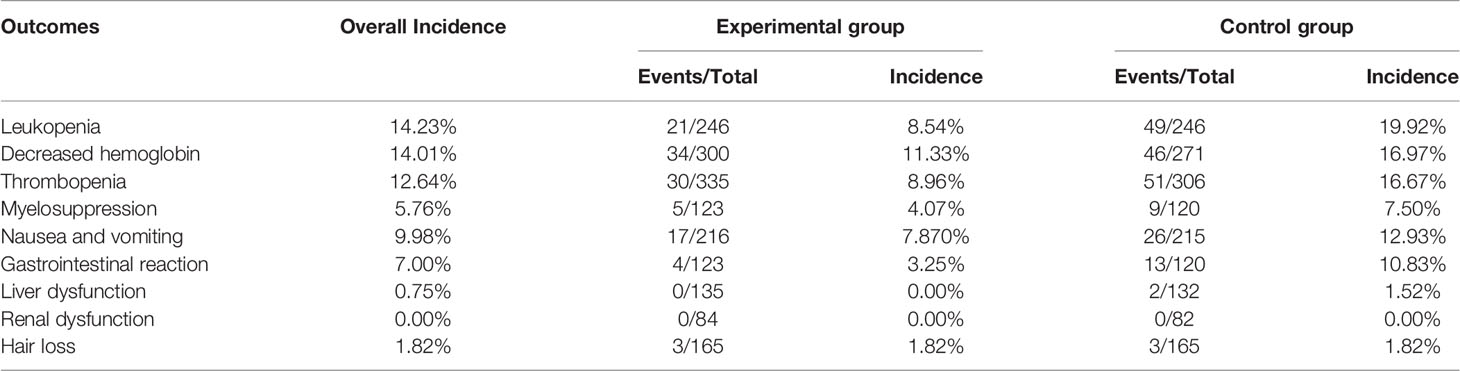
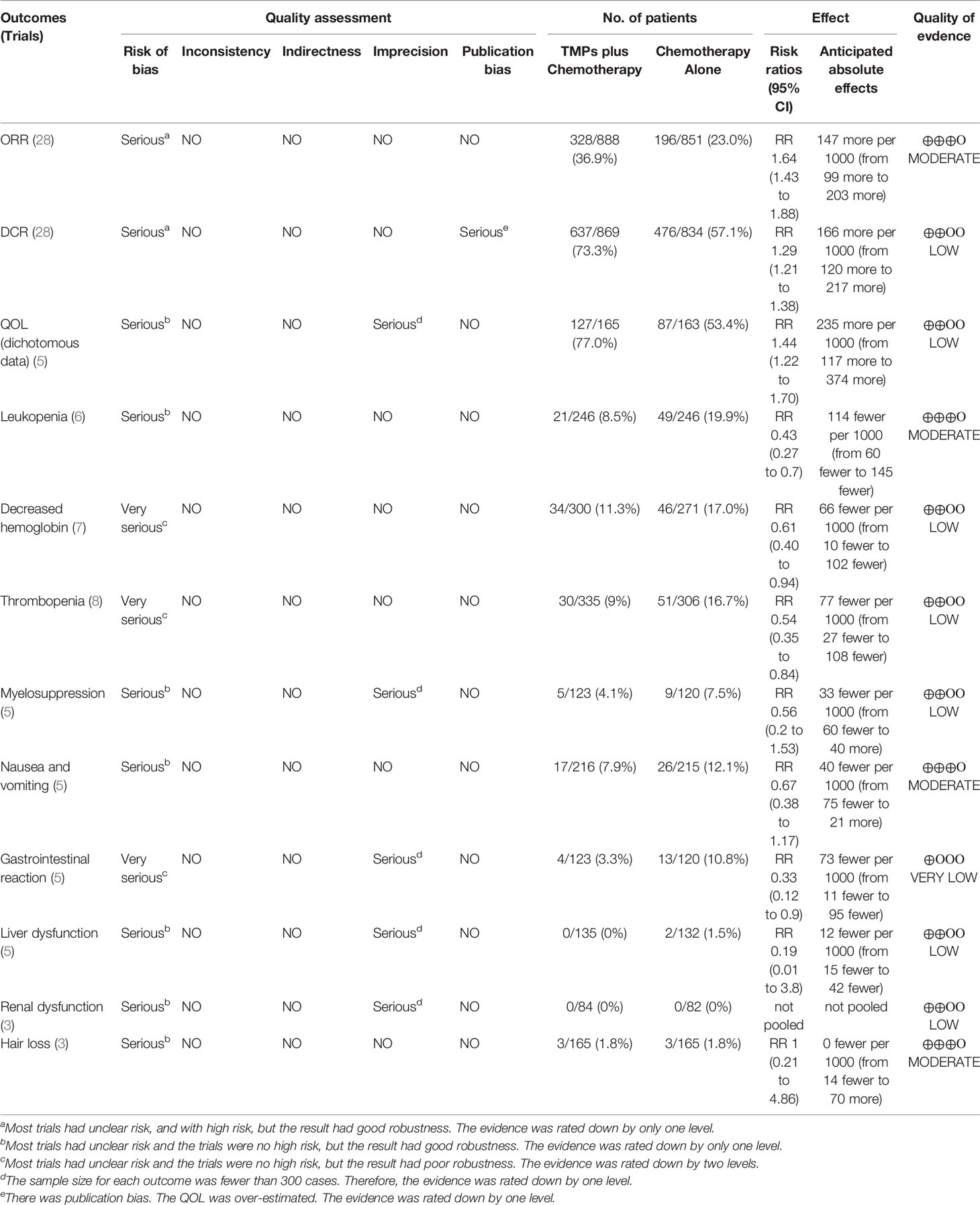
Six trials with 492 individuals reported leukopenia, seven trials with 571 individuals reported decreased hemoglobin, eight trials with 641 individuals reported thrombopenia, five trials with 243 individuals reported myelosuppression, five trials with 431 individuals reported nausea and vomiting, five trials with 243 individuals reported gastrointestinal reaction, five trials with 220 individuals reported liver dysfunction, three trials with 166 individuals reported renal dysfunction, and three trials with 330 individuals reported hair loss (Table 2 and Figure 8).
Minimal heterogeneity was observed in decreased hemoglobin (I² = 1%), whereas no heterogeneity (I² = 0%) was observed in others. FEM was used to synthesize data from different trials. The results of the meta-analysis showed that TMPs combined with chemotherapy reduced the risk of leukopenia (RR=0.43, 95% CI [0.27-0.70], p =0.0005), decreased hemoglobin (RR=0.61, 95% CI [0.40-0.94], p =0.02), thrombopenia (RR=0.54, 95% CI [0.35-0.84], p =0.006), and gastrointestinal reaction (RR=0.33, 95% CI [0.12-0.90], p =0.03), compared to chemotherapy alone. However, there was no difference between two groups in myelosuppression (RR=0.56, 95% CI [0.20-1.53], p =0.25), nausea and vomiting (RR=0.67, 95% CI [0.38-1.17], p =0.15), liver dysfunction (RR=0.19, 95% CI [0.01-3.80], p =0.28) and hair loss (RR=1.00, 95% CI [0.21-4.86], p =1.00). Besides leukopenia, decreased hemoglobin and thrombopenia were common ADRs during treatment while kidney dysfunction did not occur in either group (Table 3).
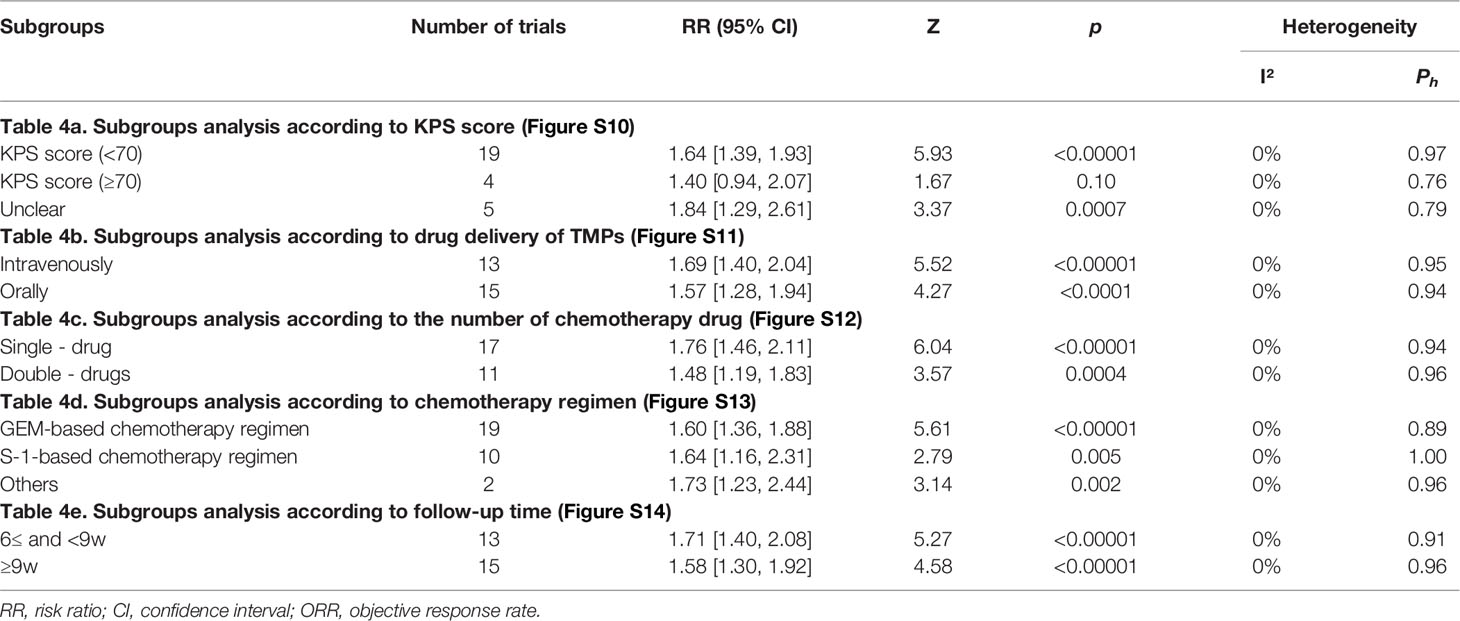
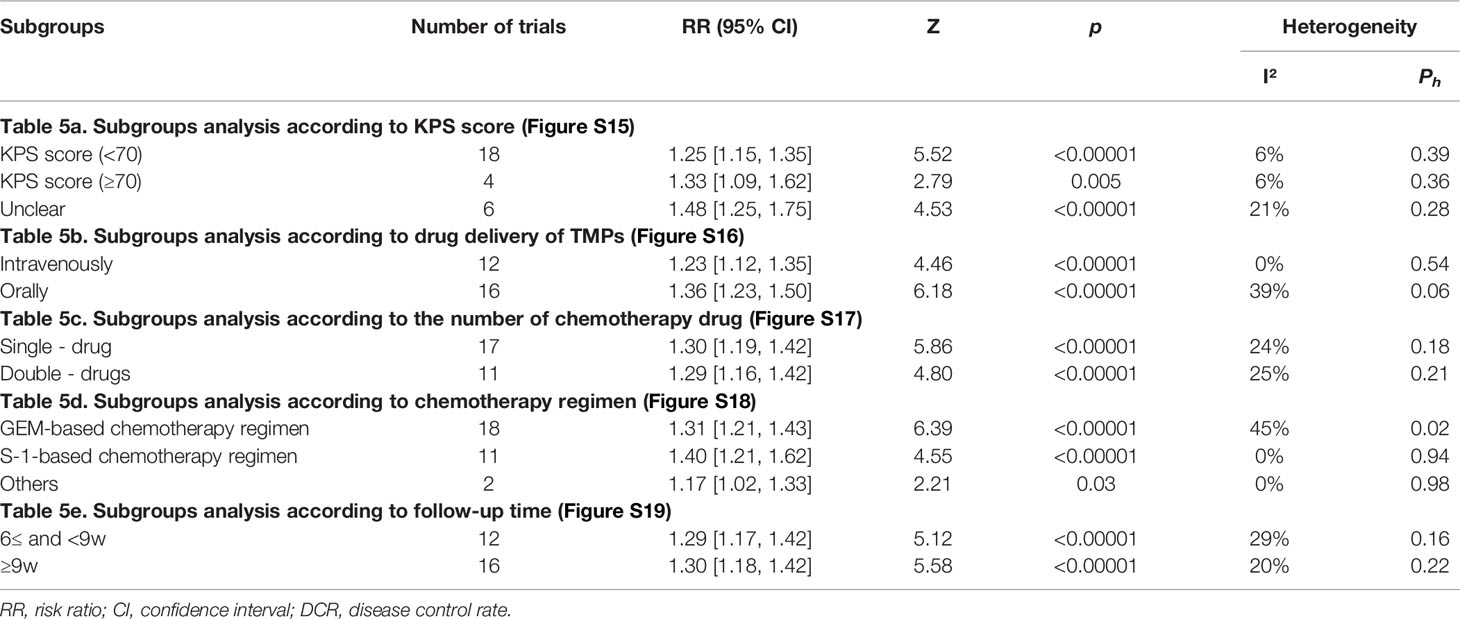
3.8 Subgroup Analysis of ORR and DCR
Subgroup analysis was performed on ORR and DCR according to the KPS score, drug delivery of TMPs, the number of chemotherapy drug, chemotherapy regimen, and follow-up time (Tables 4, 5, and Figures S10–S19). The KPS score was divided into three parts: <70, ≥70, and unclear. Subgroup analysis showed that TMPs increased ORR when KPS score <70 and unclear and DCR in every part (Figures S10, S15). The drug delivery of TMPs was either intravenously or orally. Subgroup analysis showed that TMPs increased ORR and DCR regardless of whether it was administered intravenously or orally (Figures S11, S16). Based on the number of chemotherapy drug, individuals were divided into those who used single-drug and those who used double-drugs. Subgroup analysis showed that TMPs increased ORR and DCR regardless of whether the number of chemotherapy drug used (Figures S12, S17). The chemotherapy regimen was divided into three categories: GEM-based, S-1-based, and other chemotherapy regimens. Subgroup analysis showed that TMPs increased ORR and DCR regardless of the above chemotherapy regimen used (Figures S13, S18). The follow-up time was divided into two parts: 6w≤ and <9w, and ≥9w. Subgroup analysis showed that TMPs increased ORR and DCR in every part of the follow-up time (Figures S14, S19).
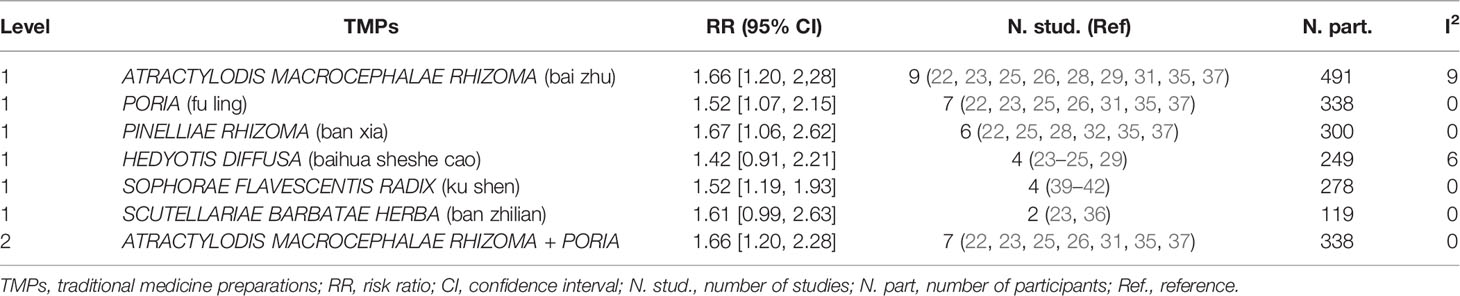
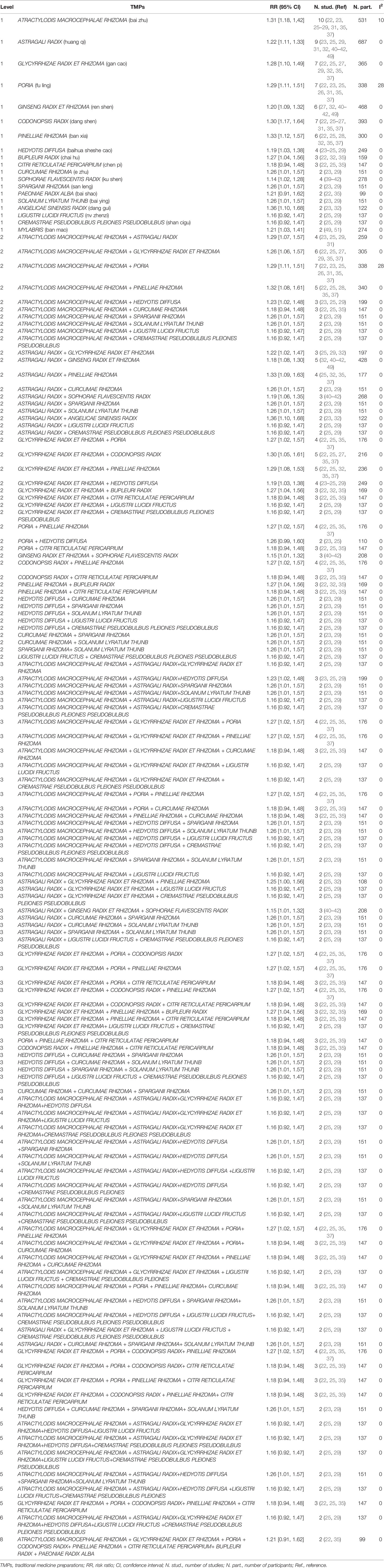
TMPs are different combinations of multiple herbs. To determine which herbs or combination of herbs combined with chemotherapy contributed the most to APC, subgroup analysis was conducted based on the specific ingredients of TMPs from each study listed in Table 1 according to the method described in Chen MH, et al. (53) and Chen Y et al. (54). All significant RR results were shown in Tables 6A and 6B, and only the RRs with low heterogeneity (I2 < 30%) that were not greater than the total pooled RR were shown in the text. A total of 82 herbs were involved in the included trials, and the more frequently used herbs in treating APC were: ATRACTYLODIS MACROCEPHALAE RHIZOMA (bai zhu), ASTRAGALI RADIX (huang qi), GLYCYRRHIZAE RADIX ET RHIZOMA (gan cao), PORIA (fu ling), GINSENG RADIX ET RHIZOMA (ren shen), CODONOPSIS RADIX (dang shen), and PINELLIAE RHIZOMA (ban xia). As shown in Table 6A, six herbs had significant RRs with low heterogeneity in benefit for ORR. These single herbs were paired with each other and 15 pairs were generated. Only one herb pair named ATRACTYLODIS MACROCEPHALAE RHIZOMA + PORIA (n=7) (RR 1.66 [1.20, 2.28], I2 = 0%) had lower RR when compared with the total pool RR. As shown in Table 6B, nineteen herbs had significant RRs with low heterogeneity in benefit for ORR. These single herbs were paired with each other and forty-five pairs had lower RRs when compared with the total pool RR. The most frequent combinations were: ATRACTYLODIS MACROCEPHALAE RHIZOMA + PORIAATRACTYLODIS (n=7) (RR 1.29 [1.11, 1.51], I2 = 28%), and MACROCEPHALAE RHIZOMA + GLYCYRRHIZAE RADIX ET RHIZOMA (n=6) (RR 1.26 [1.06, 1.57], I2 = 0%). The combination of GINSENG RADIX ET RHIZOMA + SOPHORAE FLAVESCENTIS RADIX (n = 3) had the lowest RR (1.15 [1.01, 1.32], I2 = 0%). Compared with the total pool RR, 43 combinations of three plants presented lower RRs. The most frequent combinations were: ATRACTYLODIS MACROCEPHALAE RHIZOMA + GLYCYRRHIZAE RADIX ET RHIZOMA + PORIA (n=4) (RR 1.27 [1.02, 1.57], I2 = 0%), ATRACTYLODIS MACROCEPHALAE RHIZOMA + GLYCYRRHIZAE RADIX ET RHIZOMA + PINELLIAE RHIZOMA (n=4) (RR 1.27 [1.02, 1.57], I2 = 0%), ATRACTYLODIS MACROCEPHALAE RHIZOMA + PORIA + PINELLIAE RHIZOMA (n=4) (RR 1.27 [1.02, 1.57], I2 = 0%), and GLYCYRRHIZAE RADIX ET RHIZOMA + PORIA + CODONOPSIS RADIX (n=4) (RR 1.27 [1.02, 1.57], I2 = 0%). The combination of ASTRAGALI RADIX + GINSENG RADIX ET RHIZOMA + SOPHORAE FLAVESCENTIS RADIX (n = 3) had the lowest RR (1.15 [1.01, 1.32], I2 = 0%). Compared with the total pool RR, 23 combinations of four plants presented lower RRs. The most frequent combinations were: ATRACTYLODIS MACROCEPHALAE RHIZOMA + GLYCYRRHIZAE RADIX ET RHIZOMA + PORIA+ PINELLIAE RHIZOMA (n=4) (RR 1.27 [1.02, 1.57], I2 = 0%), and GLYCYRRHIZAE RADIX ET RHIZOMA + PORIA + CODONOPSIS RADIX+ PINELLIAE RHIZOMA (n=4) (RR 1.27 [1.02, 1.57], I2 = 0%). Compared with the total pool RR, 6 combinations of five plants presented lower RRs. The most frequent combinations were: GLYCYRRHIZAE RADIX ET RHIZOMA + PORIA + CODONOPSIS RADIX+ PINELLIAE RHIZOMA + CITRI RETICULATAE PERICARPIUM (n=3) (RR 1.18 [0.94, 1.48], I2 = 0%). Compared with the total pool RR, 1 combination of six plants presented lower RRs. The combination was: ATRACTYLODIS MACROCEPHALAE RHIZOMA + ASTRAGALI RADIX+GLYCYRRHIZAE RADIX ET RHIZOMA+HEDYOTIS DIFFUSA+LIGUSTRI LUCIDI FRUCTUS +CREMASTRAE PSEUDOBULBUS PLEIONES PSEUDOBULBUS (n=2) (RR 1.16 [0.92, 1.47], I2 = 0%). Liu.H.2018 (22) and Liu.Q.2016 (35) has the same ingredients of TMPs and their combination of herbs was therefore directly generalized to level 8. The combination was: ATRACTYLODIS MACROCEPHALAE RHIZOMA + GLYCYRRHIZAE RADIX ET RHIZOMA + PORIA + CODONOPSIS RADIX+ PINELLIAE RHIZOMA + CITRI RETICULATAE PERICARPIUM+ BUPLEURI RADIX + PAEONIAE RADIX ALBA (n=2) (RR 1.21 [0.91, 1.62], I2 = 0%).
3.9 Sensitivity Analysis
We analyzed the sensitivity of the main outcome indicators, including ORR and DCR, by excluding each trial to check the robustness of the results. The results showed that the pooled RR values of the ORR and DCR were stable.
3.10 Publication Bias
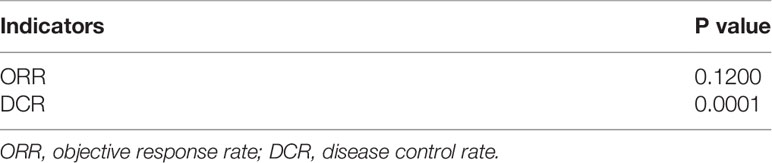
According to the contour-enhanced plot of ORR (Figure S20) and DCR (Figure S21), some trim-and-fill data fell in the area of no statistical significance, indicating that some negative results were not published, possibly leading to publication bias. Further Egger’s test (Table 7) showed no significant publication bias in the meta-analysis of ORR (p = 0.1200), whereas significant publication bias existed in DCR (p = 0.0001).
3.11 Quality of Evidence
As shown in Tables 8A and 8B, the quality of evidence was moderate for ORR, leukopenia, nausea and vomiting, hair loss, and QoL (continuous data); low for DCR, QoL (dichotomous data), decreased hemoglobin, thrombopenia, myelosuppression, liver dysfunction, and renal dysfunction and very low for gastrointestinal reaction, CA19-9, and CEA.
4 Discussion
Natural products can serve as an important source of drug discovery. Many prescription medicines approved by the Food and Drug Administration for cancer treatment have been obtained from the natural products (55), and more than 50% of newly approved drugs between 1946 and 2019 were natural small molecules or their derivatives (56). TMPs are the products derived from the combination of natural products and traditional medicine theories. They have a complex chemical diversity that enables them to act on a variety of biological targets (enzymes, receptors, pathways, etc.) to achieve maximal efficacy in cancer therapy with minimal adverse reactions (57). Numerous studies have described the clinical efficacy and safety of TMPs for colorectal cancer (58), non-small cell lung cancer (59), and liver cancer (60), as well as for some cancer-related symptoms such as insomnia (61), pain (62), and anemia (63). Thus, mining TMPs with scientific and systematic methods can serve as an important strategy for cancer treatment. Pancreatic cancer is a fatal malignant tumor of the digestive system, and patients are usually in their advanced stages when diagnosed. TMPs combined with chemotherapy have been widely used in patients with APC to achieve greater survival benefit and QoL, but there is no reported systematic evaluation of whether these therapeutic regimens are significantly effective. Therefore, we have conducted this meta-analysis. As far as we know, this is the first systematic review and meta-analysis of RCTs describing the potential efficacy and safety of TMPs combined with chemotherapy in treating APC. The various outcomes of this meta-analysis include tumor response, QoL, cancer biomarkers and ADRs. A total of 28 different RCTs involving 1,832 APC individuals were included in this review.
At present, various TMPs containing diverse bioactive molecules have been shown to exhibit multiple anti-pancreatic cancer effects. These compounds, including quercetin (64), baicalein (65), honokiol (66), luteolin (67), and silibinin (68), have been found to be present in numerous TMPs. They can suppress pancreatic cancer cell proliferation or induce apoptotic and autophagic by modulating various oncogenic pathways including Wnt, phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin, mitogen-activated protein kinases and Nuclear factor-kappa B pathways (69). For instance, fraxetin isolated from the bark of Fraxinus bungeana A.DC., piperlongumine isolated from the fruit of the pepper Piper longum, and curcumin extracted from Curcuma longa were found to significantly enhance the anti- pancreatic cancer activity of gemcitabine (70–72). Therefore, the combination of TMPs and chemotherapy as therapeutic regimens has potential clinical value in APC treatment. This view has also been confirmed by various clinical trials in recent years (29, 34, 49). Our results showed that TMPs combined with chemotherapy can significantly enhance the tumor response, which was consistent with the previous experimental and clinical studies.
In addition, previous studies published have shown that ginkgo biloba extract (GBE 761 ONC) (73), Phytosome complex of curcumin (74), a Chinese botanical formula (PHY906) (75) were not only safe but also efficiently translate in a good response rate in the treatment of APC when combined with chemotherapy. In addition, the use of a modified supercritical carbon dioxide extract of Nerium oleander leaves (PBI-05204) (76) and Viscum album [L.] extract (77) alone has been reported to be beneficial for prolonging overall survival of patients with APC. However, none of them were RCTs about TMPs combined with chemotherapy in treating APC. These TMPs have the potential to treat APC, but it is unclear whether they effective and safet when combined with chemotherapy. The purpose of this study is to make more researchers pay attention to the good clinical value of TMPs combined with chemotherapy in treating APC.
TMPs consist of single or multiple herbs and are widely used in the clinical treatment for APC. The results of subgroup analysis showed that the following 6 herbs had significant combined RRs and no heterogeneity at multiple combined levels: ATRACTYLODIS MACROCEPHALAE RHIZOMA, ASTRAGALI RADIX, GLYCYRRHIZAE RADIX ET RHIZOMA, PORIA, CODONOPSIS RADIX, and PINELLIAE RHIZOMA. Therefore, these herbs were considered to have a consistent effect on enhancing the tumor response in multiple combinations which might be especially effective for treating APC when combined with chemotherapy and were more instructive to researchers. A study reported by Zhang et al. showed that calycosin, a bioactive isoflavonoid of ASTRAGALI RADIX, inhibited the growth of pancreatic cancer cells by inducing p21Waf1/Cip1-induced cell cycle arrest and caspase-dependent apoptosis (78). Cheng et al. concluded that a triterpene mixture extracted from PORIA inhibited the migration of pancreatic cancer cells associated with CDC20 (79). Moreover, Zhao C. found that licocoumarone, the extracts from GLYCYRRHIZAE RADIX ET RHIZOMA, suppressed human pancreatic adenocarcinoma BxPC-3 cell proliferation and induces cell apoptotic (80). However, there are less studies about the other three herbs and combinations of the six herbs in treating APC.
CA19-9 is a characteristic tumor biomarker of pancreatic cancer. The level of CA19-9 has been associated with tumor size (81), stage, and survival (82), as it is often used for diagnosis, prognosis and monitoring of patients with pancreatic cancer (83, 84). It has been observed that particularly, if the duration of the decline in CA19-9 levels was greater than 3 months during the 6 months period after initiation of the treatment, it could be significantly related to the good prognosis of APC (85). The previous studies have also shown that CA19-9 can effectively accelerate the process of pancreatic cancer by causing protein modification (86), binding to E-selectin, as well as by promoting angiogenesis, and is therefore considered as a potential target and an important research area for the treatment of APC (87). Our analysis results showed that TMPs combined with chemotherapy significantly reduced the levels of CA19-9, compared with chemotherapy alone, thereby indicating a positive effect of TMPs on the treatment of APC.
It has been established that ADRs during the treatment duration can influence the progress of treatment and the QoL of patients. Therefore, reducing the occurrence of ADRs is also an important task of clinicians. Our results suggested that patients treated with TMPs had a relatively lower incidence of leukopenia, decreased hemoglobin, thrombopenia, and gastrointestinal reaction, compared to the chemotherapy alone. Instead of increasing ADRs, addition of various TMPs as adjuvant and alternative drugs was found to markedly reduce ADRs which reflected the better safety profile of TMPs.
The included trials did not report TMPs-related adverse reactions. As the main compositions of TMPs in the treatment of APC, a number of previous studies have confirmed that ATRACTYLODIS MACROCEPHALAE RHIZOMA (88), ASTRAGALI RADIX (89), PORIA (90) and CODONOPSIS RADIX (91) do not exhibit significant toxicity and are safe for the clinical application. However, adverse reactions of some compositions of TMPs in included trials have been reported. For example, the most important side effects of GLYCYRRHIZAE RADIX ET RHIZOMA have been found to be hypertension and hypokalemic-induced secondary disorders and which need to be used with caution during pregnancy (92). Large-scale consumption of GINSENG RADIX ET RHIZOMA may cause anaphylaxis, palpitations, hypertension, skin hypersensitivity reactions and headache (93, 94). The toxicity of PINELLIAE RHIZOMA includes mucosal irritation, hepatorenal and gestational toxicity (95). While this does not mean that TMPs necessarily cause these adverse reactions, it must be used with caution before analyzing their safety through strict evaluation. It is very common to use TMPs for treatment of cancer patients. For instance, one study showed that herbal and supplementary medicine was used by 78% of patients undergoing chemotherapy, but 27% of them were assessed as at risk of adverse herb-chemotherapy interaction (96) which has become an important consideration in pharmacotherapy. Therefore, TMPs pose potential risks for interactions with chemotherapy drugs (97, 98), which are often caused by TMPs-related induction or inhibition of the drug metabolizing enzyme system cytochrome P-450 (CYP) and/or the P-glycoprotein drug efflux transport system (99). Herbal products that have shown clinical interactions with chemotherapeutic drugs include ECHINACEA, ALLIUM SATIVUM, GINSENG, CITRUS PARADISI, SILYBUM MARIANUM and HYPERICUM PERFORATUM. GINSENG is a commonly used herbal products used in the treatment of patients with APC, but the findings on its inductive effect on drug-metabolizing enzymes are relatively mixed (100, 101). In one case report, ginseng was observed to cause hepatotoxicity when used in combination with imatinib (102). Moreover, other herbal products commonly applied in APC, including DIOSCOREA VILLOSA, RHODIOLA ROSEA and GANODERMA, might also display strong potential for herb-chemotherapy interactions although there are no clinically relevant data (103). Considering the significant application of various TMPs for treating APC, clinicians and researchers should document in detail TMPs use during chemotherapy in patients with APC and be vigilant in monitoring for any potential interactions as well as adverse effects while administering TMPs- chemotherapy combination to the patients.
Our study has some potential limitations. First, we have only searched the English and Chinese databases which might miss some key trials published in other language. Second, the assessment of the methodological bias risk showed some bias in included trials thereby leading to low or very low quality of some potential outcomes. Regardless, the primary outcome was robust and reliable. We will include high-quality RCTs to update this study regularly. Third, there is currently some debate about whether TMPs combined with chemotherapy can significantly prolong the survival time in APC patients (34, 103–105). However, this study did not define it as an observational outcome due to presence of only few reports in the published trials. Therefore, further research is needed in the future. Fourth, the time elapsed from the chemotherapy termination until the measurements of outcomes were different because of the diverse chemotherapy regimens employed in each study. Although we observed the same trend of outcomes in the included trials, this factor might also substantially influence the interpretation of the results. Fifth, the specific mechanisms of action of TMPs are not clear although great progress has been made in the study of the effects of TMPs in treating APC. Sixth, according to the CONSORT Extension for Chinese Herbal Medicine Formulas (106), the name, provenance, dosage form, preparation method, dosage and route of administration of herbal medicine formulas should be reported in detail in RCT. Besides, the name, origin, processing method and dosage of all herbs in the formula should be reported. The certification method, quality control method and safety monitoring data for herbs and formulas should also be described. However, included trials using self-prepared herbal decoctions did not report the information above which might lead to potential bias. This indicated irregular reports in RCTs about self-prepared herbal decoctions in treating APC which need to be improved. Overall, we are hopeful that this study can provide relevant clinical evidences and experimental research direction for researchers. Finally, we expect that more attention will be paid to the potential therapeutic applications of TMPs in the treatment of APC and better deigned clinical trials will be conducted in future.
5 Conclusion
Our study confirmed the clinical efficacy and safety of TMPs combined with chemotherapy for APC. This combination regimen might benefit for the prognosis of patients with APC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
BH and HZ designed the research. JH, JJ, and MC performed literature search. GZ, SH, HY, BS, and JH performed article selection. JH, XZ, and RL assessed methodological bias risk. JH, JJ, and MC conducted a meta-analysis and assessed study quality. JH finished the manuscript draft. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Special training of scientific and technological talents, China Academy of Chinese Medical Sciences (Grant No. ZZ13-YQ-023), the National Natural Science Foundation of China (Grant No. 82174465), the Beijing Municipal Science and Technology Commission (Grant No. Z181100001618006), the Fundamental Research Funds for the Central Public Welfare Research Institutes (Grant No. ZZ13-YQ-028), the Youth project of National Natural Science Foundation of China (Grant No. 82104961) and the CACMS Innovation Fund (Grant No. CI2021A01814).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to Yue Li and Ziang Yao for helping us perform data extraction.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.828450/full#supplementary-material
The detailed search strategy is available in Supplementary 1; the subgroup analysis of the QoL is available in Supplementary 2; the subgroup analysis of the cancer biomarkers is available in Supplementary 3; the figures of subgroup analysis of the ORR and DCR is available in Supplementary 4; the contour-enhanced plot is available in Supplementary 5.
Abbreviations
APC, advanced pancreatic cancer; ADRs, adverse drug reactions; QoL, quality of life; TMPs, traditional medicine preparations; ORR, objective response rate; DCR, disease control rate; KPS, Karnofsky Performance Status; SD, standardized difference; RCTs, randomized controlled trials; RR, risk ratio; SMD, standardized mean difference; CIs, confidence intervals; FEM, fixed-effects model; REM, random-effects model.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol (2019) 5:1749–68. doi: 10.1001/jamaoncol.2019.2996
3. Millikan KW, Deziel DJ, Silverstein JC, Kanjo TM, Christein JD, Doolas A, et al. Prognostic Factors Associated With Resectable Adenocarcinoma of the Head of the Pancreas. Am Surg (1999) 65:618–23.
4. Okasha H, Elkholy S, El-Sayed R, Wifi MN, El-Nady M, El-Nabawi W, et al. Real Time Endoscopic Ultrasound Elastography and Strain Ratio in the Diagnosis of Solid Pancreatic Lesions. World J Gastroenterol (2017) 23:5962–8. doi: 10.3748/wjg.v23.i32.5962
5. Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients With Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol (2017) 24:2023–30. doi: 10.1245/s10434-017-5810-x
6. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic Cancer. Lancet (2020) 395:2008–20. doi: 10.1016/S0140-6736(20)30974-0
7. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX Versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
8. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased Survival in Pancreatic Cancer With Nab-Paclitaxel Plus Gemcitabine. N Engl J Med (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
9. Rehman H, Chi J, Hakim N, Goyal SP, Olazagasti C, Jose J, et al. Attenuated Regimen of Biweekly Gemcitabine/Nab-Paclitaxel in Patients Aged 65 Years or Older With Advanced Pancreatic Cancer. Therap Adv Gastroenterol (2020) 13:1756284820974912. doi: 10.1177/1756284820974912
10. Go SI, Lee SC, Bae WK, Zang DY, Lee HW, Jang JS, et al. Modified FOLFIRINOX Versus S-1 as Second-Line Chemotherapy in Gemcitabine-Failed Metastatic Pancreatic Cancer Patients: A Randomised Controlled Trial (MPACA-3). Eur J Cancer (2021) 157:21–30. doi: 10.1016/j.ejca.2021.08.002
11. Kabir MT, Rahman MH, Akter R, Behl T, Kaushik D, Mittal V, et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules (2021) 11:392. doi: 10.3390/biom11030392
12. Dhar D, Raina K, Kumar D, Wempe MF, Bagby SM, Pitts TM, et al. Bitter Melon Juice Intake With Gemcitabine Intervention Circumvents Resistance to Gemcitabine in Pancreatic Patient-Derived Xenograft Tumors. Mol Carcinog (2020) 59:1227–40. doi: 10.1002/mc.23251
13. Long J, Liu Z, Hui L. Anti-Tumor Effect and Mechanistic Study of Elemene on Pancreatic Carcinoma. BMC Complement Altern Med (2019) 19:133. doi: 10.1186/s12906-019-2544-2
14. Chen S, Bao Y, Xu J, Zhang X, He S, Zhang Z, et al. Efficacy and Safety of TCM Combined With Chemotherapy for SCLC: A Systematic Review and Meta-Analysis. J Cancer Res Clin Oncol (2020) 146:2913–35. doi: 10.1007/s00432-020-03353-0
15. Lin Z, Chen J, Han S. The Efficacy of Heat-Clearing (Qingre) and Detoxifying (Jiedu) Traditional Chinese Medicine Gargle for Chemotherapy-Induced Oral Mucositis: A Systematic Review and Meta-Analysis. Front Pharmacol (2021) 12:627628:627628. doi: 10.3389/fphar.2021.627628
16. Saif MW. Is There A Role for Herbal Medicine in the Treatment of Pancreatic Cancer?. Highlights From the “44th ASCO Annual Meeting”. Chicago, IL, USA. May 30 - June 3, 2008. JOP (2008) 9:403–7.
17. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the PRISMA-DTA Group, et al. Preferred Reporting Items for a Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA (2018) 319:388–96. doi: 10.1001/jama.2017.19163
18. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting Results of Cancer Treatment. Cancer (1981) 47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
19. Park JO, Lee SI, Song SY, Kim K, Kim WS, Jung CW, et al. Measuring Response in Solid Tumors: Comparison of RECIST and WHO Response Criteria. Jpn J Clin Oncol (2003) 33:533–7. doi: 10.1093/jjco/hyg093
20. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 . Available at: www.training.cochrane.org/handbook (Accessed June 13, 2021).
21. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ (2008) 336:924–6. doi: 10.1136/bmj.39-489.470347.AD
22. Liu H, Wu J, Sun T. Clinical Study on Chaishao Liujunzi Decoction Combined With Chemotherapy in Treating Advanced Pancreatic Cancer. Chin J Inf Tradit Chin Med (2018) 25:26–9. doi: 10.3969/j.issn.1005-5304.2018.02.006
23. Huang H, Lan X. Effect of Modified Fuzheng Yiliu Decoction Adjuvant Chemotherapy on Immune Function, Serum Tumor Markers and Toxic and Side Effects of Pancreatic Cancer. Chin J Integr Tradit West Med Dig (2021) 29:720–724+730. doi: 10.3969/j.issn.1671-038X.2021.10.09
24. Dai L. Evaluation of Efficacy of Jiedu Huayu Tongfu Granule on Advanced Pancreatic Cancer Patients With Damp-Heat and Stasis-Toxic Syndrome. J Tianjin Univ Tradit Chin Med (2014) 33:270–3. doi: 10.11656/j.issn.1673-9043.2014.05.05
25. Tong X, Ma J, Hu Q. The Clinical Efficacy Observation of S-1 Combined With Fuzhengxiaoji Decoction in the Treatment of Advanced Pancreatic Cancer. Zhejiang Med J (2021) 43:669–71. doi: 10.12056/j.issn.1006-2785.2021.43.6.2020-4604
26. Bi L. Clinical Observation of the Xiaoji Decoction Combined With Chemotherapy in the Treatment of Pancreatic Cancer. Chin Med Guide (2017) 15:1–2. doi: 10.15912/j.cnki.gocm.2017.14.002
27. Yu M. Observation of Curative Effect of Xiaoliu Jian Combined With Tiogio on Patients With Advanced Pancreatic Cancer. Chin Med Guide (2020) 18:24 + 27. doi: 10.15912/j.cnki.gocm.2020.07.013
28. Li F, Li Y, Gao F, Qin Y, Deng Y, Tang J, et al. Effect of Modified Yi Yan Xiao Formula Combined With Chemotherapy on Quality of Life and Survival Time in Patients With Advanced Pancreatic Cancer. J Trad Chin Med (2021) 62:887–92. doi: 10.13288/j.11-2166/r.2021.10.012
29. Liu E, Zheng G, Ding S. The Yiqihuoxue Formula Combined With GEMOX Regimen to Treat 46 Cases of Advanced Pancreatic Cancer. Trad Chin Med Res (2021) 34:32–7. doi: 10.3969/j.issn.1001-6910.2021.04.11
30. Chen Z. Clinical Effect of Yiqi Jianpi Huayu Decoction in Adjuvant Treatment of Advanced Pancreatic Cancer. China Mod Med (2021) 28:195–7. doi: 10.3969/j.issn.1674-4721.2021.25.053
31. Chen L. Randomized Parallel Controlled Experiment of Yinchenhao Decoction Combined With Tegafur, Gimeracil and Oteracil Potassium Capsules on Advanced Pancreatic Cancer. Guangming J Chin Med (2020) 35:1891–3. doi: 10.3969/j.issn.1003-8914.2020.12.044
32. Bi X. Observation on the Clinical Effect of Self-Made Traditional Chinese Medicine Compound Chaihu Kuijian Decoction Combined With Chemotherapy on Postoperative Patients With Advanced Pancreatic Cancer. Chin J Mod Drug Appl (2020) 14:211–3. doi: 10.14164/j.cnki.cn11-5581/r.2020.17.096
33. Meng Z, Garrett CR, Shen Y, Liu L, Yang P, Huo Y, et al. Prospective Randomised Evaluation of Traditional Chinese Medicine Combined With Chemotherapy: A Randomised Phase II Study of Wild Toad Extract Plus Gemcitabine in Patients With Advanced Pancreatic Adenocarcinomas. Br J Cancer (2012) 107:411–6. doi: 10.1038/bjc.2012.283
34. Schwartzberg LS, Arena FP, Bienvenu BJ, Kaplan EH, Camacho LH, Campos LT, et al. A Randomized, Open-Label, Safety and Exploratory Efficacy Study of Kanglaite Injection (KLTi) Plus Gemcitabine Versus Gemcitabine in Patients With Advanced Pancreatic Cancer. J Cancer (2017) 8:1872–83. doi: 10.7150/jca.15407
35. Liu Q, Liu H, Xie J. Clinical Observation of 20 Cases of Advanced Pancreatic Cancer Combined With Chinese and Western Medicine. Hunan J Trad Chin Med (2016) 32:53–5. doi: 10.16808/j.cnki.issn1003-7705.2016.12.023
36. Luo X, Hong G. Chinese Medicine Combined With Chemotherapy to Treat 29 Cases of Advanced Pancreatic Cancer. Zhejiang J Trad Chin Med (2015) 50:520. doi: 10.13633/j.cnki.zjtcm.2015.07.037
37. You J, Yao J. The Efficacy Observation of 40 Cases With Middle-Advanced Pancreatic Cancer Treated With Fuzheng Hewei Oral Liquor and GEMOX. Liaoning J Trad Chin Med (2009) 36:2135–8. doi: 10.13192/j.ljtcm.2009.12.124.youjl.079
38. Wei D, Tan Y, Liu H, Su X, Peng J. 21 Cases of Myelosuppression After Chemotherapy for Advanced Pancreatic Cancer Treated With Donkey-Hide Gelatin. Chin J Integr Tradit West Med (2006) 26:659–60. doi: 10.3321/j.issn:1003-5370.2006.07.024
39. Sun Y. Effect and Attenuation of Compound Kushen Injection Combined With Gemcitabine and Cisplatin in Treating Pancreatic Cancer. China Mod Med (2016) 23:66–8.
40. Dou L. Kangai Injections in Combination With GP Regimen to Treat Local Advanced Pancreatic Cancer. Chin J Exp Tradit Med Formulae (2010) 16:207–9. doi: 10.3969/j.issn.1005-9903.2010.18.062
41. Guo Z, Dou L. Observation of the Efficacy of Kangai Injections Combined With Gemcitabine in the Treatment of Advanced Pancreatic Cancer. China Pharm (2011) 22:699–701.
42. Dou L. The Synergy and Detoxification of Kangai Injection With Chemotherapy in Patients With Advanced Pancreatic Cancer. Chin J Gerontol (2012) 32:140–1.
43. Niu S. Observation of the Efficacy of Huchansu Injection Combined With Gemcitabine in the Treatment of Advanced Pancreatic Cancer. Chin J Clin Ration Drug Use (2014) 7:61–2. doi: 10.15887/j.cnki.13-1389/r.2014.11.125
44. Hu B, Zhou Z, Qiu X, Zhang L. Clinical Observation of Middle-Advanced Pancreatic Cancer Treated by Buqi Tongluo Jiedu Formula Combined With GEMOX Regimen. Beijing J Trad Chin Med (2010) 29:770–2. doi: 10.16025/j.1674-1307.2010.10.032
45. Chen X, Liang Q, Li X, Zhang Y, Li J, Liang Z, et al. Effect of Composite Salviae Dropping Pill Combined With Chemotherapy in 41 Cases With Pancreatic Carcinoma. J Chin Oncol (2005) 11:46–8. doi: 10.3969/j.issn.1671-170X.2005.01.017
46. Li L, Chen J, Zhang D, Zhang R. Efficacy of Tegafur Gimeracil Oteracil Potassium Capsule Combined With Kanglaite Injection for Advanced Pancreatic Carcinoma. China Mod Doct (2016) 54:67–9.
47. Yao X. Clinical Observation of Tegafur Gimeracil Oteracil Potassium Capsule Combined With Kanglaite Injection for Advanced Pancreatic Carcinoma in Old Patients. J Basic Clin Oncol (2015) 28:350–2. doi: 10.3969/j.issn.1673-5412.2015.04.029
48. Zhang X, Qiao C, Cheng X, Liu Q, Gao Q, Yang X, et al. Clinical Trial of Kanglaite Injection Combined With Gemcitabine Injection and Tegafur-Gimeracil-Oteracil Potassium Capsule in the Treatment of Advanced Pancreatic Cancer. Chin J Clin Pharmacol (2018) 34:111–3. doi: 10.13699/j.cnki.1001-6821.2018.02.005
49. He R, Tan X. Curative Effect of Ai’di Injection Combined With Docetaxel in the Treatment of Middle-Advanced Pancreatic Carcinoma. Chin J Clin Oncol Rehabil (2015) 22:1054–6. doi: 10.13455/j.cnki.cjcor.2015.09.09
50. Gansauge F, Ramadani M, Pressmar J, Gansauge S, Muehling B, Stecker K, et al. NSC-631570 (Ukrain) in the Palliative Treatment of Pancreatic Cancer. Results of A Phase II Trial. Langenbecks Arch Surg (2002) 386:570–4. doi: 10.1007/s00423-001-0267-5
51. Guan L, Ma J, Yuan S, Qu J. Disodium Cantharidinate and Vitamin B6 Combined With Tegafur, Gimeracil and Oteracil Potassium for Treating Stage IV Pancreatic Cancer in 27 Cases. China Pharm (2015) 24:124–5.
52. Deng L, Shen W, Zhang Y, Shu Z, Sheng H, Xi L, et al. Clinical Observation on Coicis Oil Injection Combined With Gemcitabine in Old Patients With Advanced Pancreatic Cancer. Mod J Integr Tradit Chin West Med (2013) 22:2281–3.
53. Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative Medicine for Relief of Nausea and Vomiting in the Treatment of Colorectal Cancer Using Oxaliplatin-Based Chemotherapy: A Systematic Review and Meta-Analysis. Phytother Res (2016) 30:741–53. doi: 10.1002/ptr.5586
54. Chen Y, Cheng CS, Tan HY, Tam CW, Wang N, Feng Y. Efficacy of Herbal Medicines Intervention for Colorectal Cancer Patients With Chemotherapy-Induced Gastrointestinal Toxicity - A Systematic Review and Meta-Analysis. Front Oncol (2021) 11:629132:629132. doi: 10.3389/fonc.2021.629132
55. Yang Y, Li N, Wang TM, Di L. Natural Products With Activity Against Lung Cancer: A Review Focusing on the Tumor Microenvironment. Int J Mol Sci (2021) 22:10827. doi: 10.3390/ijms221910827
56. Newman DJ, Cragg GM. Natural Products as Sources of New Drugs Over the Nearly Four Decades From 01/1981 to 09/2019. J Nat Prod (2020) 83:770–803. doi: 10.1021/acs.jnatprod.9b01285
57. Man S, Luo C, Yan M, Zhao G, Ma L, Gao W. Treatment for Liver Cancer: From Sorafenib to Natural Products. Eur J Med Chem (2021) 224:113690. doi: 10.1016/j.ejmech.2021.113690
58. Chen M, May BH, Zhou IW, Xue CC, Zhang AL. Meta-Analysis of Oxaliplatin-Based Chemotherapy Combined With Traditional Medicines for Colorectal Cancer: Contributions of Specific Plants to Tumor Response. Integr Cancer Ther (2016) 15:40–59. doi: 10.1177/1534735415596424
59. Yu ZY, Peng RY, Han M, Grant S, Yang GY, Liu JP, et al. Adjunctive Effect of Compound Kushen Injection to Chemotherapy for Non-Small Cell Lung Cancer: An Evidence Map and Overview of Systematic Reviews. J Ethnopharmacol (2021) 281:114538. doi: 10.1016/j.jep.2021.114538
60. She Y, Huang Q, Ye Z, Hu Y, Wu M, Qin K, et al. The Therapeutic Principle of Combined Strengthening Qi and Eliminating Pathogens in Treating Middle-Advanced Primary Liver Cancer: A Systematic Review and Meta-Analysis. Front Pharmacol (2021) 12:714287:714287. doi: 10.3389/fphar.2021.714287
61. Yoon JH, Kim EH, Park SB, Lee JY, Yoon SW. Traditional Herbal Medicine for Insomnia in Patients With Cancer: A Systematic Review and Meta-Analysis. Front Pharmacol (2021) 12:753140:753140. doi: 10.3389/fphar.2021.753140
62. Xiangyong Y, Zhongsheng Y, Wenchao L, Hui D, Shuzhou Q, Gang C, et al. External Application of Traditional Chinese Medicine in the Treatment of Bone Cancer Pain: A Meta-Analysis. Support Care Cancer (2016) 24:11–7. doi: 10.1007/s00520-015-2737-2
63. Dang Z, Liu X, Wang X, Li M, Jiang Y, Wang X, et al. Comparative Effectiveness and Safety of Traditional Chinese Medicine Supporting Qi and Enriching Blood for Cancer Related Anemia in Patients Not Receiving Chemoradiotherapy: A Meta-Analysis and Systematic Review. Drug Des Devel Ther (2018) 13:221–30. doi: 10.2147/DDDT.S181182
64. Asgharian P, Tazehkand AP, Soofiyani SR, Hosseini K, Martorell M, Tarhriz V, et al. Quercetin Impact in Pancreatic Cancer: An Overview on Its Therapeutic Effects. Oxid Med Cell Longev (2021) 2021:4393266. doi: 10.1155/2021/4393266
65. Ma D, Chen S, Wang H, Wei J, Wu H, Gao H, et al. Baicalein Induces Apoptosis of Pancreatic Cancer Cells by Regulating the Expression of miR-139-3p and miR-196b-5p. Front Oncol (2021) 11:653061:653061. doi: 10.3389/fonc.2021.653061
66. Qin T, Li J, Xiao Y, Wang X, Gong M, Wang Q, et al. Honokiol Suppresses Perineural Invasion of Pancreatic Cancer by Inhibiting SMAD2/3 Signaling. Front Oncol (2021) 11:728583:728583. doi: 10.3389/fonc.2021.728583
67. Huang X, Dai S, Dai J, Xiao Y, Bai Y, Chen B, et al. Luteolin Decreases Invasiveness, Deactivates STAT3 Signaling, and Reverses Interleukin-6 Induced Epithelial-Mesenchymal Transition and Matrix Metalloproteinase Secretion of Pancreatic Cancer Cells. Onco Targets Ther (2015) 8:2989–3001. doi: 10.2147/OTT.S91511
68. Zhang X, Jiang J, Chen Z, Cao M. Silibinin Inhibited Autophagy and Mitochondrial Apoptosis in Pancreatic Carcinoma by Activating JNK/SAPK Signaling. Pathol Res Pract (2019) 215:152530. doi: 10.1016/j.prp.2019.152530
69. Gao Y, Chen S, Sun J, Su S, Yang D, Xiang L, et al. Traditional Chinese Medicine May Be Further Explored as Candidate Drugs for Pancreatic Cancer: A Review. Phytother Res (2021) 35:603–28. doi: 10.1002/ptr.6847
70. Guo Y, Xiao Y, Guo H, Zhu H, Chen D, Wang J, et al. The Anti-Dysenteric Drug Fraxetin Enhances Anti-Tumor Efficacy of Gemcitabine and Suppresses Pancreatic Cancer Development by Antagonizing STAT3 Activation. Aging (Albany NY) (2021) 13:18545–63. doi: 10.18632/aging.203301
71. Wang Y, Wu X, Zhou Y, Jiang H, Pan S, Sun B. Piperlongumine Suppresses Growth and Sensitizes Pancreatic Tumors to Gemcitabine in a Xenograft Mouse Model by Modulating the NF-Kappa B Pathway. Cancer Prev Res (Phila) (2016) 9:234–44. doi: 10.1158/1940-6207.CAPR-15-0306
72. Liu P, Ying Q, Liu H, Yu SQ, Bu LP, Shao L, et al. Curcumin Enhances Anti-Cancer Efficacy of Either Gemcitabine or Docetaxel on Pancreatic Cancer Cells. Oncol Rep (2020) 44:1393–402. doi: 10.3892/or.2020.7713
73. Hauns B, Häring B, Köhler S, Mross K, Robben-Bathe P, Unger C. Phase II Study With 5-Fluorouracil and Ginkgo Biloba Extract (GBE 761 ONC) in Patients With Pancreatic Cancer. Arzneimittelforschung (1999) 49:1030–4. doi: 10.1055/s-0031-1300546
74. Pastorelli D, Fabricio ASC, Giovanis P, D’Ippolito S, Fiduccia P, Soldà C, et al. Phytosome Complex of Curcumin as Complementary Therapy of Advanced Pancreatic Cancer Improves Safety and Efficacy of Gemcitabine: Results of A Prospective Phase II Trial. Pharmacol Res (2018) 132:72–9. doi: 10.1016/j.phrs.2018.03.013
75. Saif MW, Li J, Lamb L, Kaley K, Elligers K, Jiang Z, et al. First-In-Human Phase II Trial of the Botanical Formulation PHY906 With Capecitabine as Second-Line Therapy in Patients With Advanced Pancreatic Cancer. Cancer Chemother Pharmacol (2014) 73:373–80. doi: 10.1007/s00280-013-2359-7
76. Roth MT, Cardin DB, Borazanci EH, Steinbach M, Picozzi VJ, Rosemury A, et al. A Phase II, Single-Arm, Open-Label, Bayesian Adaptive Efficacy and Safety Study of PBI-05204 in Patients With Stage IV Metastatic Pancreatic Adenocarcinoma. Oncologist (2020) 25:e1446–50. doi: 10.1634/theoncologist.2020-0440
77. Tröger W, Galun D, Reif M, Schumann A, Stanković N, Milićević M. Viscum Album [L.] Extract Therapy in Patients With Locally Advanced or Metastatic Pancreatic Cancer: A Randomised Clinical Trial on Overall Survival. Eur J Cancer (2013) 49:3788–97. doi: 10.1016/j.ejca.2013.06.043
78. Zhang Z, Auyeung KK, Sze SC, Zhang S, Yung KK, Ko JK. The Dual Roles of Calycosin in Growth Inhibition and Metastatic Progression During Pancreatic Cancer Development: A “TGF-β Paradox”. Phytomedicine (2020) 68:153177. doi: 10.1016/j.phymed.2020.153177
79. Cheng S, Castillo V, Sliva D. CDC20 Associated With Cancer Metastasis and Novel Mushroom−Derived CDC20 Inhibitors With Antimetastatic Activity. Int J Oncol (2019) 54(6):2250–6. doi: 10.3892/ijo.2019.4791
80. Marchegiani G, Andrianello S, Malleo G, De Gregorio L, Scarpa A, Mino-Kenudson M, et al. Does Size Matter in Pancreatic Cancer?: Reappraisal of Tumour Dimension as a Predictor of Outcome Beyond the TNM. Ann Surg (2017) 266:142–8. doi: 10.1097/SLA.0000000000001837
81. Zhao C, Wang D, Gao Z, Kan H, Qiu F, Chen L, et al. Licocoumarone Induces BxPC-3 Pancreatic Adenocarcinoma Cell Death by Inhibiting DYRK1A. Chem Biol Interact (2020) 316:108913. doi: 10.1016/j.cbi.2019.108913
82. Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 Levels can Predict Stage and Survival in Patients With Resectable Pancreatic Adenocarcinoma. J Clin Oncol (2006) 24:2897–902. doi: 10.1200/JCO.2005.05.3934
83. Yu J, Yang X, Wu H, Li J. Clinical Significance of Color Ultrasound, MRI, miR-21, and CA199 in the Diagnosis of Pancreatic Cancer. J Oncol (2021) 2021:2380958. doi: 10.1155/2021/2380958
84. Rhodes JM. Usefulness of Novel Tumour Markers. Ann Oncol (1999) 10:118–21. doi: 10.1093/annonc/10.suppl_4.S118
85. Tomishima K, Ishii S, Fujisawa T, Ikemura M, Ota H, Kabemura D, et al. Duration of Reduced CA19-9 Levels Is a Better Prognostic Factor Than Its Rate of Reduction for Unresectable Locally Advanced Pancreatic Cancer. Cancers (Basel) (2021) 13:4224. doi: 10.3390/cancers13164224
86. Engle DD, Tiriac H, Rivera KD, Pommier A, Whalen S, Oni TE, et al. The Glycan CA19-9 Promotes Pancreatitis and Pancreatic Cancer in Mice. Science (2019) 364:1156–62. doi: 10.1126/science.aaw3145
87. Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, et al. Roles of CA19-9 in Pancreatic Cancer: Biomarker, Predictor and Promoter. Biochim Biophys Acta Rev Cancer (2021) 1875:188409. doi: 10.1016/j.bbcan.2020.188409
88. Zhu B, Zhang QL, Hua JW, Cheng WL, Qin LP. The Traditional Uses, Phytochemistry, and Pharmacology of Atractylodes Macrocephala Koidz.: A Review. J Ethnopharmacol (2018) 226:143–67. doi: 10.1016/j.jep.2018.08.023
89. Zhang CH, Yang X, Wei JR, Chen NM, Xu JP, Bi YQ, et al. Ethnopharmacology, Phytochemistry, Pharmacology, Toxicology and Clinical Applications of Radix Astragali. Chin J Integr Med (2021) 27:229–40. doi: 10.1007/s11655-019-3032-8
90. Nie A, Chao Y, Zhang X, Jia W, Zhou Z, Zhu C. Phytochemistry and Pharmacological Activities of Wolfiporia Cocos (F.A. Wolf) Ryvarden & Gilb. Front Pharmacol (2020) 11:505249:505249. doi: 10.3389/fphar.2020.505249
91. Gao SM, Liu JS, Wang M, Cao TT, Qi YD, Zhang BG, et al. Traditional Uses, Phytochemistry, Pharmacology and Toxicology of Codonopsis: A Review. J Ethnopharmacol (2018) 219:50–70. doi: 10.1016/j.jep.2018.02.039
92. Nazari S, Rameshrad M, Hosseinzadeh H. Toxicological Effects of Glycyrrhiza Glabra (Licorice): A Review. Phytother Res (2017) 31:1635–50. doi: 10.1002/ptr.5893
93. Paik DJ, Lee CH. Review of Cases of Patient Risk Associated With Ginseng Abuse and Misuse. J Ginseng Res (2015) 39:89–93. doi: 10.1016/j.jgr.2014.11.005
94. Liu Y, Zhang H, Dai X, Zhu R, Chen B, Xia B, et al. A Comprehensive Review on the Phytochemistry, Pharmacokinetics, and Antidiabetic Effect of Ginseng. Phytomedicine (2021) 92:153717. doi: 10.1016/j.phymed.2021.153717
95. Wang Y. Wang Q. Research Progress on Chemical Composition, Pharmacological Action and Toxicity of Pinellia Ternate. China Pharm (2020) 31:2676–82. doi: 10.6039/j.issn.1001-0408.2020.21.20
96. McCune JS, Hatfield AJ, Blackburn AA, Leith PO, Livingston RB, Ellis GK. Potential of Chemotherapy-Herb Interactions in Adult Cancer Patients. Support Care Cancer (2004) 12:454–62. doi: 10.1007/s00520-004-0598-1
97. Wolf CPJG, Rachow T, Ernst T, Hochhaus A, Zomorodbakhsch B, Foller S, et al. Complementary and Alternative Medicine (CAM) Supplements in Cancer Outpatients: Analyses of Usage and of Interaction Risks With Cancer Treatment. J Cancer Res Clin Oncol (2021). doi: 10.1007/s00432-021-03675-7
98. Zeller T, Muenstedt K, Stoll C, Schweder J, Senf B, Ruckhaeberle E, et al. Potential Interactions of Complementary and Alternative Medicine With Cancer Therapy in Outpatients With Gynecological Cancer in A Comprehensive Cancer Center. J Cancer Res Clin Oncol (2013) 139:357–65. doi: 10.1007/s00432-012-1336-6
99. Engdal S, Klepp O, Nilsen OG. Identification and Exploration of Herb-Drug Combinations Used by Cancer Patients. Integr Cancer Ther (2009) 8:29–36. doi: 10.1177/1534735408330202
100. Fasinu PS, Rapp GK. Herbal Interaction With Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front Oncol (2019) 9:1356. doi: 10.3389/fonc.2019.01356
101. Malati CY, Robertson SM, Hunt JD, Chairez C, Alfaro RM, Kovacs JA, et al. Influence of Panax Ginseng on Cytochrome P450 (CYP)3A and P-Glycoprotein (P-Gp) Activity in Healthy Participants. J Clin Pharmacol (2012) 52:932–9. doi: 10.1177/0091270011407194
102. Bilgi N, Bell K, Ananthakrishnan AN, Atallah E. Imatinib and Panax Ginseng: A Potential Interaction Resulting in Liver Toxicity. Ann Pharmacother (2010) 44:926–8. doi: 10.1345/aph.1M715
103. Alsanad SM, Williamson EM, Howard RL. Cancer Patients at Risk of Herb/Food Supplement-Drug Interactions: A Systematic Review. Phytother Res (2014) 28:1749–55. doi: 10.1002/ptr.5213
104. Kim EH, Yoon JH, Yoon SS, Lee JY, Yoon SW. Efficacy of Chemotherapy Integrated With Traditional Korean Medicine in Patients With Metastatic Pancreatic Cancer: A Single-Center Retrospective Study. Integr Cancer Ther (2020) 19:1534735420983457. doi: 10.1177/1534735420983457
105. Cao N, Zhao A, Zhao G, Wang X, Han B, Lin R, et al. Survival Analysis of 272 Patients With Pancreatic Cancer Undergoing Combined Treatment. Integr Cancer Ther (2015) 14:133–9. doi: 10.1177/1534735414564185
Keywords: advanced pancreatic cancer, traditional medicine preparations, chemotherapeutic therapy, systematic review, meta-analysis
Citation: Hu J, Jiang J, Liu R, Cheng M, Zhu G, He S, Shi B, Zhao Y, He Z, Yu H, Zhang X, Zheng H and Hua B (2022) Clinical Efficacy and Safety of Traditional Medicine Preparations Combined With Chemotherapy for Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 12:828450. doi: 10.3389/fonc.2022.828450
Received: 03 December 2021; Accepted: 20 January 2022;
Published: 23 February 2022.
Edited by:
Gil Bar-Sela, Ha’Emek Medical Center, IsraelReviewed by:
Elad Schiff, Technion Israel Institute of Technology, IsraelMoshe Frenkel, Rambam Health Care Campus, Israel
Ilana Levy, Technion Israel Institute of Technology, Israel
Copyright © 2022 Hu, Jiang, Liu, Cheng, Zhu, He, Shi, Zhao, He, Yu, Zhang, Zheng and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Honggang Zheng, aG9uZ2dhbmd6aGVuZ0AxMjYuY29t; Baojin Hua, aHVhYmFvamlueHNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jiaqi Hu
Jiaqi Hu Juling Jiang
Juling Jiang Rui Liu1†
Rui Liu1† Guanghui Zhu
Guanghui Zhu Huibo Yu
Huibo Yu Xing Zhang
Xing Zhang Baojin Hua
Baojin Hua