
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 21 March 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.828434
This article is part of the Research Topic Challenges and their Implications for the Clinical Practice of Head and Neck Cancer View all 23 articles
Head and neck cancer (HNC) remains to be a major cause of mortality worldwide because of confounding factors such as late-stage tumor diagnosis, loco-regional aggressiveness and distant metastasis. The current standardized diagnostic regime for HNC is tissue biopsy which fails to determine the thorough tumor dynamics. Therefore, due to the ease of collection, recent studies have focused on the utility of saliva based liquid biopsy approach for serial sampling, early diagnosis, prognosis, longitudinal monitoring of disease progression and treatment response in HNC patients. Saliva collection is convenient, non-invasive, and pain-free and offers repetitive sampling along with real time monitoring of the disease. Moreover, the detection, isolation and analysis of tumor-derived components such as Circulating Tumor Nucleic Acids (CTNAs), Extracellular Vesicles (EVs), Circulating Tumor Cells (CTCs) and metabolites from saliva can be used for genomic and proteomic examination of HNC patients. Although, these circulatory biomarkers have a wide range of applications in clinical settings, no validated data has yet been established for their usage in clinical practice for HNC. Improvements in isolation and detection technologies and next-generation sequencing analysis have resolved many technological hurdles, allowing a wide range of saliva based liquid biopsy application in clinical backgrounds. Thus, in this review, we discussed the rationality of saliva as plausible biofluid and clinical sample for diagnosis, prognosis and therapeutics of HNC. We have described the molecular components of saliva that could mirror the disease status, recent outcomes of salivaomics associated with HNC and current technologies which have the potential to improve the clinical value of saliva in HNC.
Head and Neck Cancer (HNC) is the sixth most prevalent cancer worldwide attributed to etiological factors like tobacco and alcohol consumption, HPV infections and to a certain extent genetic predisposition (1–3). Despite advancements in diagnostic and therapeutic regime, the overall survival of HNC patients has remained dismal for over four decades. Conventional diagnostic strategies comprise of physical examination, imaging techniques such as computed tomography (CT) scan, Ultrasound (US), magnetic resonance imaging (MRI) and tissue biopsies followed by histopathological analysis. Till date, tissue biopsy is the most commonly used method for diagnosis; however, this technique is invasive, quite challenging, painful, time-consuming, and potentially risky for the patient. Moreover, the intra-tumoral and metastatic heterogeneity remains undetected, affecting the specificity, sensitivity and accuracy of assessment (4). Therefore the ‘liquid biopsy approach’ that focuses on detecting tumor-derived components in circulatory fluids for the diagnosis, screening and prognosis of cancer (5) is becoming increasingly important. Liquid biopsies are anticipated to demonstrate high accuracy in terms of representation of tumor genome landscape and mutations. They also provide reproducibility and feasibility of real-time therapeutic monitoring while being minimally invasive and cost effective (6). For HNC cancers, serum, plasma and saliva have been identified as the most frequently used sources for liquid biopsies (7).
Saliva as a potential source for liquid biopsy of HNC patients has several advantages compared to other body fluids as it (i) reflects any genomic, epigenomic, proteomic and physiological/pathological alterations in the oral cavity, larynx and pharynx; (ii) serves as a non-invasive, inexpensive, easier and more accessible screening tool (8); and (iii) provides the opportunity for real-time monitoring of HNC patients by having the flexibility of repetitive sampling and larger volumes for examination without the requirement of trained medical staff for collections (8–10). Despite the potential value in utilizing saliva derived biomarkers as diagnostic tool, its clinical utility is limited due to some challenges. Primarily, the complex composition of saliva comprises of various non-tumorigenic components hampering the ability to detect biomolecules of tumor origin. Moreover, relative contribution of different subsites into the salivary milieu makes the identification of HNC specific markers difficult (11). However, the potential utility of saliva as a liquid biopsy tool for diagnosis, prognosis and therapeutic monitoring of HNC is being extensively explored. Presently, the most common components for liquid biopsy of HNCs comprise cell-free tumor nucleic acids (DNA, mRNA and miRNAs), extracellular vesicles, circulating tumor cells (CTCs) and salivary metabolites (Figure 1). This review encompasses the recent developments, technologies, clinical applications and limitations of saliva derived biomarkers in HNC diagnosis, prognosis, and therapeutics.
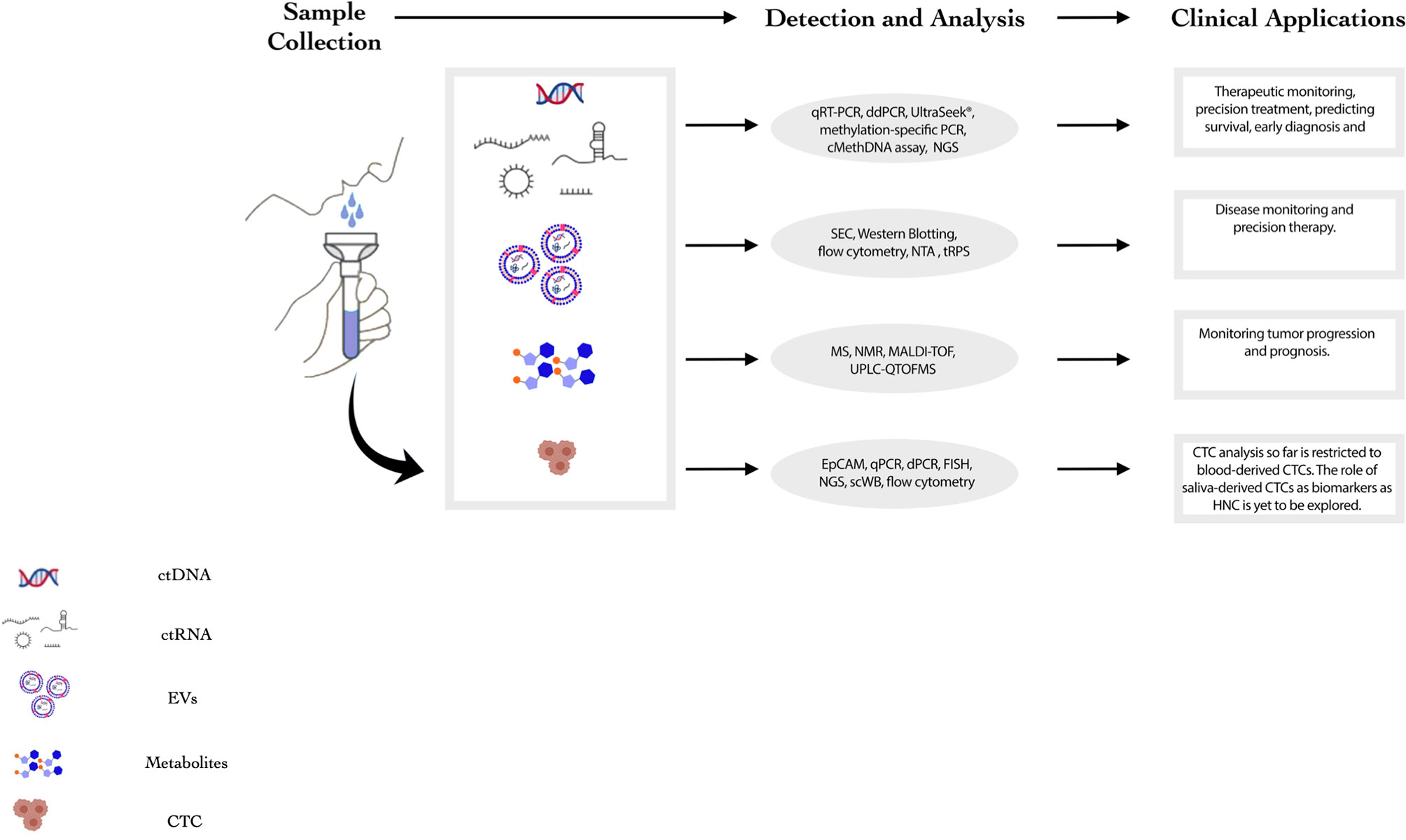
Figure 1 Summary of salivary components that can potentially act as biomarkers for HNC. This figure summarizes the current landscape of salivary components that may act as biomarkers for HNC. The detection and analysis techniques and clinical applications for each component are mentioned in the figure. (NMR: Nuclear Magnetic Resonance, MS: Mass Spectrometry, HNC: Head and Neck Cancer, ddPCR: Droplet Digital Polymerase Chain Reaction, qPCR: quantitative Polymerase Chain Reaction, NGS: Next Generation Sequencing, SEC: Size-Exclusion Chromatography, NTA: Nanoparticle Tracking Analysis).
Circulatory tumor nucleic acids (ctNAs) are fragments of cell-free genomic/viral DNA and/or RNA that are shed by tumor cells through passive processes like necrosis and apoptosis or via active mechanisms like spontaneous release of nucleic acids in systemic circulation (12, 13). These fragments of circulating tumor DNA (ctDNA) and RNA (ctRNA), are found in various body fluids including saliva. They reflect the genetic information of the bulk tumor and reflect clonal heterogeneity and tumor evolution. The rate at which these circulatory nucleic acids release into circulation depends on the tumor’s location, vascularity, scale, resulting in variability across patients (14). Analysis of ctDNA relies more on identifying and targeting certain tumor specific mutations and understanding the epigenetic landscape, whereas ctRNA emphasizes on identifying novel or differential expression patterns of messenger RNA (mRNAs), microRNA (miRNAs), and long ncRNAs (lncRNAs) as a potential salivary biomarker. ctRNA based biomarkers probably gives better dynamic insights about cell-state and regulation as compared to ctDNA biomarkers.
Detection and analysis of ctNAs is quite challenging. Currently, real-time PCR (qRT-PCR), digital droplet PCR (ddPCR) and UltraSeek® (Agena Bioscience) mass-spectrometry-based PCR method are the most widely used techniques, as it helps in optimizing samples with low ctNA concentration in HNCs. ddPCR is still the most preferred method demonstrating higher sensitivity, specificity and multiplexing capacity (15, 16). Further, techniques such as methylation-specific PCR (17, 18), methylation on beads (19), and cMethDNA assay (20, 21) are used to detect the difference in methylation patterns on promoter of ctDNA in HNC patient samples. PCR based techniques are preferred when there are low number of target regions (≤ 20 targets), limited sample input and when there is limited assessment of tumor heterogeneity or identification of known variants. Next Generation Sequencing (NGS) methods such as CAPP-Seq (cancer personalized profiling by deep sequencing), TAm-Seq (tagged amplicon deep sequencing), Safe-Seq (safe sequencing system), and AmpliSeq are being used to isolate and capture ctNAs; each with relatively higher strengths in sensitivity, specificity and scalability (22–24). These NGS techniques can detect both known and unknown tumor-specific mutations and analyze differential expression patterns of single markers or a panel of markers. Targeted NGS methods are less time-consuming, result in fewer wastage of resources and offer a higher discovery rate, thus aiding in identification of novel variants. Despite the current limitations, these techniques have demonstrated potential to detect and isolate smaller concentrations of ctDNA from saliva, thus opening new avenues for clinical applications (25). With technological advancements, higher specificity and sensitivity of ctDNA detection could effectively increase their clinical applications. Nonetheless development of cost-effective NGS assays is crucial for their widespread clinical utility (26, 27).
ctDNA represents a trivial fraction (<1%) of whole cfDNA shed from tumor cells into the circulation. However, this small subpopulation is believed to reflect the somatic mutations and genomic landscape from primary tumors that can be useful in early diagnosis and risk prediction of HNC. Recently, few studies have emphasized utilizing ctDNA derived from saliva in early detection of cancer. Wang et al., conducted a comprehensive analysis of somatic mutations (TP53, CDKN2A, NRAS, NOTCH1, PIK3CA, FBXW7, and HRAS) and HPV (HPV16 and 18) genes in saliva and plasma of 93 HNC patient samples comprising of oral cavity, oropharynx, larynx, and hypopharynx subsites. The study demonstrated detection of ctDNA at 96% rate irrespective of the tumor size, stage and location. Moreover, recurrence post-surgery was observed in majority of patients having these somatic mutations. In OSCC patients, the detection rate of ctDNA was higher in saliva as compared to plasma, indicating that salivary ctDNA can be used for OSCC detection (28).. Similarly, p53 mutation in exon 4 codon 63 was detected in saliva of early stage OSCC patients (93.33% of cases, p<0.05) with a similar detection rate as patient tumor samples (29). However, Perdomo et al. reported that, targeted mutation detection approach failed to demonstrate significant concordance in detecting TP53 mutations from tumor and saliva derived ctDNA. El Naggar et al. and Spafford et al., detected microsatellite instability and loss of heterozygosity at certain chromosomes in oral mucosal cells from HNC patients and saliva (p < 0.001) with different sensitivity and specificity based on sample size and sampling subsites (30, 31). Moreover, genetic alterations in PMAIP1 and PTPN1 genes had the potential to discern HNC patients from healthy individuals (32). Collectively, these studies suggest that assessment of somatic mutations from salivary ctDNA can be an effective non-invasive substitute to tissue biopsy for early diagnosis, disease surveillance and prognosis of HNC patients. However, multiple mutation detection-based studies with standardized protocols and larger cohort of patients will be required for clinical translation (33) of this approach. Low yields of ctDNA after purification from saliva is also a key limiting factor. To increase the efficacy and sensitivity of salivary ctDNA as a biomarker, specific ctDNA panels need to be designed that can help detect and monitor HNC cases in real-time and a cost-effective manner.
Several studies have highlighted the importance and feasibility of detecting epigenetic alterations in ctDNA from body fluids and its immense diagnostic potential. Promoter hyper-methylation of genes such as EDNRB (κ = 0.60), KIF1A (κ = 0.64), NID2(κ = 0.60), and HOXA9 (κ = 0.60) in salivary DNA have shown potential utility for early detection of oral cancer patients (34–36). Few studies have demonstrated a significant clinical correlation between hypermethylation in promoter region of salivary ctDNA with prognosis and risk prediction in HNC patients. Specifically, methylated gene loci were identified in both tissue and preoperative saliva samples and could serve as a classifier to differentiate between preoperative and postoperative samples for HNC patients (37). Analogous to this, Carvalho et al. indicated that detection of promoter hypermethylation of either or all genes (TIMP-3, CCNA1, DCC, MGMT, MINT-31, DAPK p16) in pre-treated salivary DNA could effectively predict poor survival (HR=2.8; 95% CI=1.2–6.5; p=0.016) and recurrence (HR=12.2; 95% CI=1.8–80.6; p= 0.010) of HNC patients (38).These findings suggest that elevated ctDNA hypermethylation patterns have the potential to predict disease aggressiveness, overall survival rate and therapeutic monitoring and surveillance of HNC patients (Table 1). As we thrive towards the development of epigenetic-based diagnostic tests, we need to consider the challenges that come along with it. One of the major challenges: Given the epigenetic plasticity in non-cancerous cells, we need to develop tools that can filter the false positive signals and enhance the specificity and sensitivity of these assays making them more translatable.
Collectively, somatic mutations and methylation patterns of salivary DNA could be utilized as potential biomarkers and prognosticators in HNC. This approach can accelerate the diagnosis and risk prediction of HNC and pave the path for improved patient outcomes by monitoring their therapeutic response.
Analysis of transcriptomic profiles of circulating body fluids is a widely explored method for early cancer detection and several studies have shown significant association of the transcriptome with disease progression. Several studies have demonstrated the association of salivary mRNAs with development and detection of HNC. Li et al. demonstrated a significant 3.5-fold elevation in OSCC saliva with significant sensitivity (91%) and specificity (91%) (P < 0.01) of transcripts of salivary SAT, IL8, S100P, IL1B, OAZ1, DUSP1 and HA3, in oral cancer patients as compared to their healthy counterparts (41). David Elashoff and colleagues (42) substantiated the effectiveness of these biomarkers in a larger patient cohort (382 patients), suggesting the potential role of salivary mRNA markers in oral cancer detection. With respect to individual marker performance across the five cohorts, the increase in IL8 and SAT was statistically significant(p<0.02). The validation of these biomarkers in larger patient cohorts shows their feasibility in the discrimination of OSCCs from healthy controls (42). Transcript level expression of tumor suppressor gene transgelin was observed to be significantly elevated in saliva of OSCC patients as compared to the normal counterparts. The salivary gene expression levels were in concordance with the tumor tissue and associated with overall survival (p=0.011) of patients, demonstrating its immense potential as a promising biomarker and an independent prognosticator in OSCC (43). HPV-16 has also been identified as a major etiological factor responsible for HNSCC tumorigenesis. HPV-16 mRNA showed a significantly altered expression in salivary rinses of HNSCC patients with a simultaneous effect on p16(INK4a), a known tumor suppressor having a vital role in regulating the cell cycle(p<0.05) (44). Thus, the expression pattern of different salivary mRNAs correlates with various important clinical parameters including tumor progression, differentiation, and overall survival. More importantly, the expression of salivary mRNA depicts an independent prognosis factor for HNC, suggesting that salivary mRNA might be a potential biomarker for early detection of HNC and predicting the prognosis for HNC patients.
Tumor derived circulating RNA profile is complex as it comprises of distinctive components such as noncoding RNAs (e.g., lncRNA and piwi-interacting RNAs) and microRNAs (miRNAs). Alterations in miRNA and lncRNAs expression can be exploited to investigate their potential in differentiating HNC patients from healthy volunteers (45, 46), given the fact that substantial research has been conducted in exploring the diagnostic and prognostic potential of ncRNAs derived from saliva of HNC patients (Table 2) (55). Various saliva-derived circulatory miRNAs such as miR-139-5p in TSCC (49) miR-3612, miR-650, miR-4259, miR-937-5p and miR-4478 in NPC (51) and miR-125a, miR-200a, and miR-21 have been identified as plausible biomarkers for different subsites of HNC (46, 48). In a preliminary study, expression of 314 salivary miRNAs was assessed in OSCC patients in comparison to their healthy counterparts. miR-200a and miR-125 were observed to be significantly down regulated (p<0.05) in the patient cohort as compared to the healthy volunteers. This study emphasized that salivary miRNAs were stable in saliva and could be utilized in early detection of oral cancers (46). These findings were validated by Wiklund and colleagues demonstrating that differential expression of miR-200a and miR-375 along with promoter methylation of miR-200c-141 in oral rinses and saliva of OSCC patients can be utilized for early detection of oral cancers (56). The potential role of circulatory miRNAs in effectively monitoring tumor progression, therapeutic response and recurrence have been reported in several studies – (i) Salivary miR-21 is associated with T-stage classification (p=0.02) (54) (ii) and miR-136 expression showed significant correlation with complete remission cases (AUC=0.904 CI=0.75-1 P<0.05) (25) Moreover, a preliminary study conducted by Greither et al. demonstrated differential expression of salivary miR-200a (p=0.036) and miR-93 (p=0.047) in HNSCC patients post-radiotherapy (50). Similarly, another study identified significant correlation between increased expression of salivary miR-15a-5p and disease-free survival in post-intensity modulated radiotherapy patients (HR=0.25; 95% CI=0.05-0.78; p<0.016) (57). These studies highlighted the utility and efficacy of saliva-based miRNA biomarkers in predicting therapeutic response despite the significant alterations in salivary components post-radiation.
The other arm of ncRNAs are long non-coding RNAs (lncRNAs) which are approximately more than 200 nucleotides long and are not translated into protein. Considering their inevitable role in tumor progression and metastasis, signatures of saliva derived lncRNAs have been explored as probable biomarkers for monitoring disease progression of OSCC. A pilot study has reported measurable levels of HOTAIR and MALAT lncRNAs in the saliva of OSCC patients (58). Furthermore, these elevated levels were associated with nodal metastasis ascertaining its potential as a predictive marker.
Recently, circular RNAs (circRNAs) have attracted attention globally, because of their stability (owing to the circular structure) in comparison to lncRNAs and miRNAs (45). Various circRNAs secreted into the saliva of HNC patients regulate several biological and physical processes (59). A study found differential expression of 32 salivary circRNAs in OSCC patients as compared to matched controls. The upregulation of hsa-circ-0001874 clinically correlated with tumor grade and staging. Expression level of hsa-circ-0001971 was associated with TNM stage. Further, these circRNAs could also differentiate OSCC from oral leucoplakias (AUC of 0.895) (60). These findings prompt towards their potential role as diagnostic biomarkers for OSCC; however, additional investigation on circRNAs as probable non-invasive biomarkers for HNCs will be needed to assess their prognostic and diagnostic value.
The use of salivary ctRNAs as biomarkers for detection, disease surveillance, therapy response, and prognosis sound promising but a major limitation of salivary RNA quantification is the risk of RNA degradation due to the presence of enzymes including RNases in the saliva. This in turn affects the quality of RNA extracted thereby increasing the false-positive and false-negative detection rates. Moreover, the risk of sample contamination with blood from the oral mucosa and lesions due to inflammation are other limiting factors. Multicentric preclinical/clinical studies with standardized protocols are required to verify the existing findings before establishing the clinical utility of circulatory RNAs.
Extracellular vesicles (EVs) are 30-200 nm membrane encapsulated organelles that are secreted by cells into the extracellular space in response to various physiological conditions such as proteases, growth factors, apoptotic signals, biomechanical shear and stress conditions (61, 62). Developing evidence suggests that tumor-derived EVs enable the tumor bulk to manipulate its microenvironment as they have the potential to mediate intercellular communication by transporting their molecular cargo (DNA, RNA and protein) to local or distant sites through circulatory fluids (63).
Conventional EV isolation techniques are dependent on their physical and biological properties such as size, density and surface marker expression (64). Conventionally utilized techniques for EV isolation and purification based on size include filtration and size-exclusion chromatography (SEC) whereas immune-affinity capture method identifies the EV population based on surface markers. Currently, the widely used methods for EV isolation are ultracentrifugation and/or differential centrifugation and polymer precipitation method which is commonly used in commercially available kits. Recently developed microfluidics-based technologies for EV isolation comprise of antibody-functionalized microfluidic channels (65), nanoscale size-based filtration (66) and spiral inertial microfluidic devices (67). After isolation, western blotting and flow cytometry using surface protein markers CD9, CD63, CD81, Alix, TSG101, are the most conventionally used analytical methodologies for characterisation of EVs (68–71). Nanoparticle Tracking Analysis (NTA) that works on the principle of determining Brownian motion of the particles is another extensively used technique and has higher resolution as compared to flow cytometry (72). Similar to NTA, Tunable Resistive Pulse Sensing (tRPS) is an emerging technology that estimates the EV concentration based on the particle movement and flow rates in fluid cells corresponding to the pulses/voltage applied (73, 74). However, the clinical applicability of tRPS remains to be challenging considering the heterogenous size of the EV population. Several techniques have been explored for isolation and characterisation of EVs using various patient samples; however, sensitivity and specificity of these techniques in terms of clinical utility for liquid biopsies requires comprehensive standardization of protocols and larger patient cohort studies.
Various findings have revealed that saliva harbors ample numbers of EVs, the components of which differ based on the physiological or pathological state of an individual (75). Some of the advantages of salivary EVs as compared to serum and plasma derived EVs are – (i) the collection process is non-invasive; (ii) they contain less protein content that makes their identification and quantification simpler (76, 77); and (iii) they do not undergo coagulation which stimulates a persistent secretion of EVs from platelets, thus altering the composition of circulating EVs (78). Recently, the possibility of potential biomarkers from circulatory EVs derived from saliva of HNC patients is gaining interest (Table 3). On comparing plasma and salivary EVs derived from oral cancer patients it was found that salivary EVs were concomitantly elevated as the plasma derived EVs and demonstrated a clinical association with tumor staging (p<0.01) and loco-regional aggressiveness (p<0.01) (81). These results are in corroboration with previous studies showing that salivary EVs from oral cancer patients have an irregular morphology, are greater in size and formed more aggregates as compared to EVs from normal controls (82–84).
Recent studies have found a significant role of salivary EV derived non-coding miRNAs as potential biomarkers for early diagnosis, prognosis and therapeutic targets in HNC patients given their stability within the EV and ability to regulate both oncogenes and tumor suppressor genes. Significantly, elevated levels of miR-21, miR-494-3p, miR-412-3p, miR-184, miR-27a-3p, and miR-512-3p (p<0.05) were observed in salivary exosomes derived from OSCC patients compared to the control cohort (9, 85). A recent study demonstrated that salivary miR-24-3p was enriched in OSCC and tongue cancer patients and could significantly increase the proliferation of these cells (53). Collectively these findings suggest that salivary exosomal miRNAs can be an asset for convenient and non-invasive sampling as well as pave way for early diagnosis, disease monitoring and therapeutic response evaluation in various HNC subsites (86–88).
Recent studies have reported that EVs contain long non coding RNAs (lncRNAs), however their expression has not been explored extensively in salivary EVs. High expression of a subset of lncRNAs, including HOTAIR, has been reported in the saliva of metastatic HNC patients. Thus, besides miRNAs, lncRNAs in salivary EVs could be a valued prognostic and diagnostic asset for HNC (89, 90).
The discovery of tumor associated proteins in saliva is accredited to high-throughput mass spectrometry screening of patient samples. From these studies, a series of protein biomarkers has been detected in salivary EVs for OSCC, such as LGALS3BP, PKM1/M2, A2M, MUC5B, IGHA1, HPa, and PIP (80, 91). Moreover, these tumor-associated proteins have been reported to be involved in multiple signaling pathways, including metal transport, cell proliferation, and tumor immune responses (80). Additionally, exosomal EGFR, ANXA1 and programmed cell death (PD)-1/PD-ligand 1 (PD-L1) pathway (tumor suppressor in HNSCC) have been identified as potential biomarkers for predicting prognosis and therapeutic monitoring in tumor derived exosomes of HNSCC patients (92).
There are several benefits of EVs as compared to ctDNA and CTCs. However, a wide range of isolation and analysis techniques for EVs and lack of universally accepted EV reference standards are some of the major hurdles for developing diagnostic assays to enumerate EVs from patient samples. Moreover, interference from hemolytic, lipaemic and platelet contaminated samples and issues with sample stability compromise the reproducibility of EV detection, modify EV’s physical and biological properties and affect their purity and recovery rate (93). Hence, developing a consistent external quality assessment (EQA) scheme involving application of strict but attainable sample requirements for assays, establishing standardized collection and storage environments that can minimize EV degradation and applying standard methods of EV characterization and enumeration is needed.
Salivary EVs have enormous potential for future diagnostic and therapeutic modalities, but this potential needs to be underpinned with solid scientific groundwork. A comprehensive understanding about the mechanism of how cancer cells utilize EVs to promote carcinogenesis may direct the advancement of novel therapies for HNC.
Metabolomics focusses on identification and quantification of small metabolites produced during the process of metabolism from biological samples including body fluids, cells, and tissues. Increasing evidence has highlighted the importance and potential clinical utility of metabolomics in differentiating between HNC patients and controls using bio-fluids such as saliva, plasma and serum of HNC patients.
Currently, mass spectrometry (MS) and nuclear magnetic resonance (NMR) are the most frequently used procedures for screening salivary metabolites for early diagnosis and therapeutic monitoring of HNC patients (94). For salivary metabolite-based analysis, solution state NMR is the most preferred technique and protons (1H) are the most commonly analyzed NMR-active nuclei (95). One of the major advantages of this technique is that simple steps such as centrifugation are sufficient and no other pre-processing is required for sample preparation (96). The utility of MS techniques such as matrix-assisted laser desorption ionization (MALDI) in combination with time-of-flight (TOF) is being explored in salivary metabolomics as it can provide a high-throughput profile from a small sample volume without the requirement of a separation step (97, 98). Apart from this, liquid chromatography MS (LC-MS) is a frequently used technique for screening saliva samples for metabolites. Capillary electrophoresis MS (CE-MS) is an emerging technique that utilizes high voltages to induce an electrophoretic flow of ions through a capillary (20–200 µm i.d.) using very small sample volumes (10-100 nanolitre). The unique advantage of CE-MS is its ability to boost the range of detectable polar metabolites; however complex assembly and the high possibility of capillary blockage are confounders (99, 100). Therefore, it is crucial to develop a standard protocol for processing saliva samples for metabolomic analysis for successful clinical translation.
Identification of salivary metabolites such as d-glycerate-2-phosphate, pseudouridine, norcocaine nitroxide, 1-methylhistidine, 2-oxoarginine, inositol 1,3,4-triphosphate, sphinganine-1-phosphate, and 4-nitroquinoline-1-oxide demonstrated the potential of this technique to differentiate between malignant and precancerous lesions (94). Wei et al. used ultra-performance liquid chromatography combined with quadrupole/time-of-flight spectrometry (UPLC-QTOFMS) analysis to identify a signature panel of salivary metabolites (valine, lactic acid, γ-aminobutyric acid, n-eicosanoic acid, and phenylalanine) in 37 OSCC patient samples that could distinguish between OSCC from their normal counterparts with 86.5% sensitivity and 82.4% specificity. Furthermore, lactic acid and valine were significantly elevated in OSCC with respect to oral leucoplakia (OLK) with a fold change of 2.97 (p = 0.0032) and 1.60 (p = 0.0034) respectively (101). Similarly, Sugimoto et al., and Ishikawa et al. analyzed the salivary metabolomic profiles in oral cancer patients in two independent studies. These studies identified several metabolites such as cadaverine, glutamic acid, pyrrolinehydrocarboxylic acid, choline, threonine, beta-alanine, piperidine, carnitine, tryptophan, glutamine, taurine, leucine plus isoleucine, pipecolic acid, alanine, valine, and histidine that were consistently elevated in the saliva and tumor tissues of the patient samples as compared to controls (102, 103). Sugimoto’s group identified taurine and piperdine as the key oral cancer-specific markers (p < 0.05) in a pool of 69 OSCC saliva fluid samples, suggesting that metabolites in saliva can be used as biomarkers for HNC screening. Ishikawa et al. reported a high fold change value for kynurenine (FC = 38.1, p < 0.0001) (a metabolite associated with reactive oxygen species mediated stress) in tumor samples from oral cancer patients. Collectively these findings suggest that salivary metabolites reflect changes in metabolites found in tumor tissues and thus could be used for diagnosis and prognosis of oral cancers (101, 102).
Among these differentially expressed metabolites, several studies observed significantly higher levels of salivary polyamine in oral cancer patients which showed a clinical association with tumor invasion and metastasis (102). A study conducted by Hsu et al. confirmed the elevation of polyamine along with its intermediate metabolites and demonstrated a vital involvement of polyamine pathway in oral cancer progression (103, 104). These findings highlight the importance of polyamine homeostasis and its clinical utility in identifying and understanding tumor progression.
Although many studies have successfully utilized salivary metabolomics to detect HNC, inconsistency in saliva/serum derived metabolite profiles hampers the clinical utility of this approach (105). To resolve this, more evidence using larger patient cohorts is warranted. Additionally, establishing standardized protocols, analyzing intracellular metabolites and their role in HNC and understanding the underlying mechanisms behind metabolomic alterations are required in order to identify genes or proteins affected by metabolomic changes. The salivary metabolites profile tends to fluctuate as it is highly responsive to various conditions including stress. Such factors need to be accounted for as they directly impact the reproducibility of the results as well as the sample collection protocol. Salivary metabolomics is still at a nascent stage and may develop into a diagnostic tool for early detection of oral cancer.
Recent studies have highlighted the role of oral microbiome in the development, progression and treatment monitoring of HNC (106). Moreover, oral microbiota has also been reported to influence salivary metabolomic profiles of HNC patients (107). Studies based on identification of bacterial spectra on the surface of OSCC mucosa in comparison to normal oral mucosa of patients revealed that there was a predominance of anaerobic pathogens in OSCC patients, compared to normal oral mucosa (108, 109). However, very little is known about the relationship between the oral microbiota and disease progression in HNC patients.
The past approaches for identification of bacterial taxa were culture dependent. However the diversity of the oral microbiome cannot be completely identified by these approaches. PCR technology and DNA-DNA hybridization methods are commonly used to describe oral microflora. However this experimental design can only identify limited changes in the microflora of a tissue (110–112). With the emergence of NGS technology, rRNA sequencing is promoted to discover the associations between microbial flora and HNC.
Pushalkar et al. examined the saliva microbiome of OSCC patients and suggested its potential application as a diagnostic tool (113). A 16S rRNA gene sequencing study on Caucasian participants found that a panel of Capnocytophaga, Corynebacterium, Porphyromonas, Haemophilus, Oribacterium, Rothia, and Paludibacter could discriminate between patients with oropharyngeal cancers and oral cavity cancers from age-matched controls (p<0.05) (114). A recent study demonstrated that an elevated presence of Capnocytophaga (AUC= 0.81 p<0.05) in saliva could be used as a probable screening tool for prognosis and diagnosis of HNC patients (115). Similarly, abundance of Dialister (p<0.05) in HNC patients correlated with aggressive laryngeal and oral tumors (116). Collectively, these studies suggest that salivary microbiota maybe useful in diagnosis and early detection of HNC.
The comprehensive role of oral microbiome in HNC development and progression is still at a nascent stage, but has been explored considerably in the last decade. However, it is still difficult to understand the exact mechanisms by which the oral microbiome contributes to HNC pathogenesis. Recently, data that links specific microbiome species to HNC aetiopathogenesis has been reported (106); however, studies based on longitudinal time frames with larger patient cohorts are needed. Longitudinal studies are critical in evaluating the dynamic nature of salivary oral microbiome before, during and after HNC development. Further research along these lines for identifying microbial biomarkers involved in tumor progression may assist in better understanding of the process of tumorigenesis and development of personalized treatments for better patient management in HNC.
The tumor mass tends to shed a large number of cells through the process of apoptosis/necrosis. These cells are known as Circulating Tumor Cells (CTCs) that have the potential to create metastatic niches (117) by migrating to adjacent or distant tissues through the blood or lymphatic system. Thus, these cells are considered as seeds of metastasis or risk predictors of disease aggressiveness. CTCs have a promising role in early risk prediction, disease progression and therapeutic monitoring, and as potential drug targets (118).
CTC detection is a two-step process that involves an initial enrichment step followed by a detection step. The enrichment process comprises of two alternative approaches namely –(i) negative depletion: which focuses on removal of undesired cells (RBCs and lymphocytes) either via lysis or by immuno-magnetic bead-based depletion of CD45+ leukocytes; and (ii) positive selection: that involves isolation of epithelial cells using surface markers like epithelial cell adhesion molecule (EpCAM) or cytokeratins in order to distinguish the CTCs from contaminating leukocytes. The subsequent detection step is carried out using techniques ranging from quantitative PCR (qPCR) and digital PCR (dPCR) for mutational profiling to whole-genome sequencing, fluorescence in situ hybridization (FISH) based cytogenetic analysis and targeted NGS (119, 120). Targeted NGS-based detection of CTCs is a relatively recent advancement and is being explored for various types of cancers, including HNC (121). Immunocytochemistry (122) and flow cytometry (123) are used for single-CTC analysis but a major drawback of these two techniques is their poor multiplexing capacity. To overcome this limitation new technologies are emerging such as single-cell Western Blotting (scWB), a microfluidics-based technique used to evaluate protein levels in metastatic cancers (124). In addition, CellSearch® is an EpCAM-based CTC detection system that is the only system clinically approved by the FDA for enumerating epithelial CTCs. Recent studies have highlighted the heterogeneity of CTC populations and CellSearch® fails to detect CTCs with low or no expression of EpCAM and is unable to detect non epithelial tumors like sarcomas or other mesenchymal tumors. This shortcoming is overcome by using antigen-independent systems that identify CTCs based on their biophysical characteristics like density, size, and electrical properties.
CTCs can predict the risk of metastasis in HNC patients even before clinical examination (125). Hence, they may be useful for risk prediction in HNC. The presence of CTCs has been detected in saliva, however, the current landscape of CTC-based studies in HNSCC have utilized blood/plasma/serum-derived samples. Moreover, CTC evaluation is a challenge in saliva due to their limited numbers which makes isolation and detection difficult (126). The feasibility of EpCAM markers in salivary detection of CTCs, remains uncertain because of the shedding of normal epithelial cells along with cancerous cells in saliva. Nonetheless, existing studies have shown promising potential of circulatory CTCs for diagnosis, prognosis, and therapeutic monitoring in HNSCC, which suggests that further research can lead to better prospects for salivary CTCs in HNC (127–130).
Several studies conducted in the last decade demonstrated the plausibility of identification of potential biomarkers from biofluids and their relevance in clinical settings. Liquid biopsy has paved the way for early diagnosis and prognosis, recurrence and therapy monitoring as well as screening of high-risk populations. Although blood-based liquid biopsies have been the utmost common avenue of research, the use of salivary or oral rinse-based liquid biopsies for HNC offer a unique opportunity, as these cancers are of upper aerodigestive mucosal origin and can shed tumor cells, tumor DNA, and EVs directly into saliva. Moreover, this biopsy approach is minimally invasive, entails analysis of various circulating biomarkers and enables real time monitoring of tumor progression using repetitive testing. Such real time monitoring is simply not possible with traditional biopsies. As cancer treatment moves toward an attention on targeted precision medicine, liquid biopsy has the potential to guide such treatments based on real time monitoring of patients. The current review highlights new technological advancements and potential clinical applications of saliva as a liquid biopsy tool in HNC. CTCs, ctNAs, EVs, and salivary metabolome can yield useful biomarkers using non-invasive techniques. These biomarkers could reflect actual tumor biomarkers. The copious work, involving an extensive variety of assays based on diverse principles, has been quite productive in terms of utility of these biomarkers in diagnosis and disease monitoring of head and neck cancers. However, a major obstacle for all biomolecules in liquid biopsy remains the relatively low and fluctuating concentration derived from a tumor against the background of normal counterparts; in most patient samples. Such hurdles are tackled using the approaches highlighted in the technologies addressed above. These methods are presently sensitive enough to detect and analyze very rare mutation events. Nevertheless, it is crucial that laboratories working with such techniques must be consistent in their methodologies to avoid inaccurate results. Though passé, the association of a needle in a haystack relates and is fitting for each of these practices.
The investigation of ctNAs and EVs has benefitted from advances in the field of enrichment former to the analytical procedures. While at a nascent stage, reports have revealed that isolation and enrichment techniques will be an important asset in refining nucleic acid-based assays and as an individual diagnostic in the future.
Evidently, EVs have various advantages for prognosis and diagnosis. They aid in extraction of high-quality RNA from fresh or frozen saliva, thus enhancing the scope of detectable mutations that comprise of splice variants, mutations, fusions along with expression-based assays for mRNA, microRNA, lncRNA and other non-coding RNAs. ctDNA contains all genes at an equal level, while RNA originating from a highly expressed gene could be present in thousands of copies/cells. Nevertheless, as mutations exist on both ctRNA (dying/apoptotic process) and exosome RNA (living process), developing a platform that can aid in both will have palpable advantages for detecting rare mutations. This can be of great help in the case of patients who do not have an ample quantity of mutated nucleic acid in circulatory fluid. Moreover, as DNA mutations will only notify limited information of the disease, investigating RNA expression in biofluids such as saliva can further help in understanding the processes within the HNC patient. Although saliva is a promising source of all these biomolecules it is currently unclear which one of these (ctNAs, EVs or metabolites) will eventually be useful in early diagnosis, tumor prognosis and real time therapeutic monitoring. It is entirely possible that each of these end points require monitoring different biomolecule levels. Advances in technologies for sensitive, robust and inexpensive detection of such biomolecules will enable the use of saliva based liquid biopsies in routine clinical use.
Cancer is a multifaceted and dynamic disease that can undergo quick changes. To copiously deliver on the assurance and surety of personalized medicine, development of reliable non-invasive avenues for the diagnosis, prognosis, patient stratification and treatment response monitoring are paramount. Further studies in clinical settings and in large patient cohorts with well-annotated data are needed to validate the salivary transcriptomic, genomic and proteomic data. The several liquid biopsy platforms explained in this review have the ability to add immense value to the care of cancer patients.
AP and SP participated in the literature analysis. AP and PP searched the literature, drafted the manuscript and created the figures and tables. SP and VT designed, conceptualized, finalized and contributed to the critical review of the manuscript. All authors contributed to the article and approved the submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Financial support to SP from the DBT-RA Program in Biotechnology and Life Sciences from the Department of Biotechnology, Government of India is gratefully acknowledged.
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
2. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and Neck Squamous Cell Carcinoma. Nat Rev Dis Primer (2020) 6(1):1–22. doi: 10.1038/s41572-020-00224-3
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
4. Yates LR, Gerstung M, Knappskog S, Desmedt C, Gundem G, Van Loo P, et al. Subclonal Diversification of Primary Breast Cancer Revealed by Multiregion Sequencing. Nat Med (2015) 21(7):751–9. doi: 10.1038/nm.3886
5. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating Liquid Biopsies Into the Management of Cancer. Nat Rev Clin Oncol (2017) 14(9):531–48. doi: 10.1038/nrclinonc.2017.14
6. Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid Biopsy: Monitoring Cancer-Genetics in the Blood. Nat Rev Clin Oncol (2013) 10(8):472–84. doi: 10.1038/nrclinonc.2013.110
7. Spector ME, Farlow JL, Haring CT, Brenner JC, Birkeland AC. The Potential for Liquid Biopsies in Head and Neck Cancer. Discovery Med (2018) 125(139):251.
8. Kaczor-Urbanowicz KE, Carreras-Presas CM, Aro K, Tu M, Garcia-Godoy F, Wong DT. Saliva Diagnostics - Current Views and Directions. Exp Biol Med Maywood NJ (2017) 242(5):459–72. doi: 10.1177/1535370216681550
9. Gai C, Camussi F, Broccoletti R, Gambino A, Cabras M, Molinaro L, et al. Salivary Extracellular Vesicle-Associated miRNAs as Potential Biomarkers in Oral Squamous Cell Carcinoma. BMC Cancer (2018) 1818(1):1–11. doi: 10.1186/s12885-018-4364-z
10. Khurshid Z, Zafar MS, Khan RS, Najeeb S, Slowey PD, Rehman IU. Role of Salivary Biomarkers in Oral Cancer Detection. Adv Clin Chem (2018) 86:23–70. doi: 10.1016/bs.acc.2018.05.002
11. Miller SM. Saliva Testing–a Nontraditional Diagnostic Tool. Clin Lab Sci J Am Soc Med Technol (1994) 7(1):39–44.
12. Schwarzenbach H, Hoon DSB, Pantel K. Cell-Free Nucleic Acids as Biomarkers in Cancer Patients. Nat Rev Cancer (2011) 11(6):426–37. doi: 10.1038/nrc3066
13. Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the Possible Origin and Mechanism of Circulating DNA Apoptosis and Active DNA Release. Clin Chim Acta Int J Clin Chem (2001) 313(1–2):139–42. doi: 10.1016/S0009-8981(01)00665-9
14. Haber DA, Velculescu VE. Blood-Based Analyses of Cancer: Circulating Tumor Cells and Circulating Tumor DNA. Cancer Discovery (2014) 4(6):650–61. doi: 10.1158/2159-8290.CD-13-1014
15. Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B. Transforming Single DNA Molecules Into Fluorescent Magnetic Particles for Detection and Enumeration of Genetic Variations. Proc Natl Acad Sci (2003) 100(15):8817–22. doi: 10.1073/pnas.1133470100
16. Sorber L, Zwaenepoel K, Deschoolmeester V, Van Schil PEY, Van Meerbeeck J, Lardon F, et al. Circulating Cell-Free Nucleic Acids and Platelets as a Liquid Biopsy in the Provision of Personalized Therapy for Lung Cancer Patients. Lung Cancer Amst Neth (2017) 107:100–7. doi: 10.1016/j.lungcan.2016.04.026
17. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-Specific PCR: A Novel PCR Assay for Methylation Status of CpG Islands. Proc Natl Acad Sci (1996) 93(18):9821–6. doi: 10.1073/pnas.93.18.9821
18. Licchesi JDF, Herman JG. Methylation-Specific PCR. Methods Mol Biol Clifton NJ (2009) 507:305–23. doi: 10.1007/978-1-59745-522-0_22
19. Bailey VJ, Zhang Y, Keeley BP, Yin C, Pelosky KL, Brock M, et al. Single-Tube Analysis of DNA Methylation With Silica Superparamagnetic Beads. Clin Chem (2010) 56(6):1022–5. doi: 10.1373/clinchem.2009.140244
20. Fackler MJ, Sukumar S. Quantitation of DNA Methylation by Quantitative Multiplex Methylation-Specific PCR (QM-MSP) Assay. Methods Mol Biol (2018) 1708:473–96. doi: 10.1007/978-1-4939-7481-8_24
21. Fackler MJ, Lopez Bujanda Z, Umbricht C, Teo WW, Cho S, Zhang Z, et al. Novel Methylated Biomarkers and a Robust Assay to Detect Circulating Tumor DNA in Metastatic Breast Cancer. Cancer Res (2014) 74(8):2160–70. doi: 10.1158/0008-5472.CAN-13-3392
22. Liu Q, Sommer SS. Pyrophosphorolysis-Activated Polymerization (PAP): Application to Allele-Specific Amplification. BioTechniques (2000) 29(5):1072–83. doi: 10.2144/00295rr03
23. Diaz LA, Bardelli A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J Clin Oncol (2014) 32(6):579–86. doi: 10.1200/JCO.2012.45.2011
24. Han X, Wang J, Sun Y. Circulating Tumor DNA as Biomarkers for Cancer Detection. Genomics Proteomics Bioinf (2017) 15(2):59–72. doi: 10.1016/j.gpb.2016.12.004
25. Momen-Heravi F, Trachtenberg AJ, Kuo WP, Cheng YS. Genomewide Study of Salivary MicroRNAs for Detection of Oral Cancer. J Dent Res (2014) 93(7_suppl):86S–93S. doi: 10.1177/0022034514531018
26. Zaporozhchenko IA, Ponomaryova AA, Rykova EY, Laktionov PP. The Potential of Circulating Cell-Free RNA as a Cancer Biomarker: Challenges and Opportunities. Expert Rev Mol Diagn (2018) 18(2):133–45. doi: 10.1080/14737159.2018.1425143
27. Zhang H, Liu R, Yan C, Liu L, Tong Z, Jiang W, et al. Advantage of Next-Generation Sequencing in Dynamic Monitoring of Circulating Tumor DNA Over Droplet Digital PCR in Cetuximab Treated Colorectal Cancer Patients. Transl Oncol (2019) 12(3):426–31. doi: 10.1016/j.tranon.2018.11.015
28. Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of Somatic Mutations and HPV in the Saliva and Plasma of Patients With Head and Neck Squamous Cell Carcinomas. Sci Transl Med (2015) 7(293):293ra104. doi: 10.1126/scitranslmed.aaa8507
29. Mewara A, Gadbail AR, Patil S, Chaudary M, Chavhan SD. C-Deletion Mutation of the P53 Gene at Exon 4 of Codon 63 in the Saliva of Oral Squamous Cell Carcinoma in Central India: A Preliminary Study. J Investig Clin Dent (2010) 1(2):108–13. doi: 10.1111/j.2041-1626.2010.00014.x
30. El-Naggar AK, Mao L, Staerkel G, Coombes MM, Tucker SL, Luna MA, et al. Genetic Heterogeneity in Saliva From Patients With Oral Squamous Carcinomas: Implications in Molecular Diagnosis and Screening. J Mol Diagn (2001) 3(4):164–70. doi: 10.1016/S1525-1578(10)60668-X
31. Spafford MF, Koch WM, Reed AL, Califano JA, Xu LH, Eisenberger CF, et al. Detection of Head and Neck Squamous Cell Carcinoma Among Exfoliated Oral Mucosal Cells by Microsatellite Analysis. Clin Cancer Res (2001) 7(3):607–12.
32. Sethi S, Benninger MS, Lu M, Havard S, Worsham MJ. Noninvasive Molecular Detection of Head and Neck Squamous Cell Carcinoma: An Exploratory Analysis. Diagn Mol Pathol (2009) 18(2):81–7. doi: 10.1097/PDM.0b013e3181804b82
33. Liao P-H, Chang Y-C, Huang M-F, Tai K-W, Chou M-Y. Mutation of P53 Gene Codon 63 in Saliva as a Molecular Marker for Oral Squamous Cell Carcinomas. Oral Oncol (2000) 36(3):272–6. doi: 10.1016/S1368-8375(00)00005-1
34. Guerrero-Preston R, Soudry E, Acero J, Orera M, Moreno-López L, Macía-Colón G, et al. NID2 and HOXA9 Promoter Hypermethylation as Biomarkers for Prevention and Early Detection in Oral Cavity Squamous Cell Carcinoma Tissues and Saliva. Cancer Prev Res (Phila Pa) (2011) 4(7):1061–72. doi: 10.1158/1940-6207.CAPR-11-0006
35. Demokan S, Chang X, Chuang A, Mydlarz WK, Kaur J, Huang P, et al. KIF1A and EDNRB are Differentially Methylated in Primary HNSCC and Salivary Rinses. Int J Cancer (2010) 127(10):2351–9. doi: 10.1002/ijc.25248
36. Ovchinnikov DA, Cooper MA, Pandit P, Coman WB, Cooper-White JJ, Keith P, et al. Tumor-Suppressor Gene Promoter Hypermethylation in Saliva of Head and Neck Cancer Patients. Transl Oncol (2012) 5(5):321–6. doi: 10.1593/tlo.12232
37. Viet CT, Schmidt BL. Methylation Array Analysis of Preoperative and Postoperative Saliva DNA in Oral Cancer Patients. Cancer Epidemiol Biomarkers Prev (2008) 17(12):3603–11. doi: 10.1158/1055-9965.EPI-08-0507
38. Carvalho AL, Henrique R, Jeronimo C, Nayak CS, Reddy AN, Hoque MO, et al. Detection of Promoter Hypermethylation in Salivary Rinses as a Biomarker for Head and Neck Squamous Cell Carcinoma Surveillance. Clin Cancer Res Off J Am Assoc Cancer Res (2011) 17(14):4782–9. doi: 10.1158/1078-0432.CCR-11-0324
39. Lacombe J, Brooks C, Hu C, Menashi E, Korn R, Yang F, et al. Analysis of Saliva Gene Expression During Head and Neck Cancer Radiotherapy: A Pilot Study. Radiat Res (2017) 188(1):75–81. doi: 10.1667/RR14707.1
40. Hanna GJ, Lau CJ, Mahmood U, Supplee JG, Mogili AR, Haddad RI, et al. Salivary HPV DNA Informs Locoregional Disease Status in Advanced HPV-Associated Oropharyngeal Cancer. Oral Oncol (2019) 95:120–6. doi: 10.1016/j.oraloncology.2019.06.019
41. Li Y, St. John MAR, Zhou X, Kim Y, Sinha U, Jordan RCK, et al. Salivary Transcriptome Diagnostics for Oral Cancer Detection. Clin Cancer Res (2004) 10(24):8442–50. doi: 10.1158/1078-0432.CCR-04-1167
42. Elashoff D, Zhou H, Reiss J, Wang J, Xiao H, Henson B, et al. Prevalidation of Salivary Biomarkers for Oral Cancer Detection. Cancer Epidemiol Biomarkers Prev (2012) 21(4):664–72. doi: 10.1158/1055-9965.EPI-11-1093
43. Bu J, Bu X, Liu B, Chen F, Chen P. Increased Expression of Tissue/Salivary Transgelin mRNA Predicts Poor Prognosis in Patients With Oral Squamous Cell Carcinoma (OSCC). Med Sci Monit (2015) 21:2275–81. doi: 10.12659/msm.893925
44. Chai RC, Lim Y, Frazer IH, Wan Y, Perry C, Jones L, et al. A Pilot Study to Compare the Detection of HPV-16 Biomarkers in Salivary Oral Rinses with Tumour p16(INK4a) Expression in Head and Neck Squamous Cell Carcinoma Patients. BMC Cancer (2016) 16(1):178. doi: 10.1186/s12885-016-2217-1
45. Han B, Chao J, Yao H. Circular RNA and its Mechanisms in Disease: From the Bench to the Clinic. Pharmacol Ther (2018) 187:31–44. doi: 10.1016/j.pharmthera.2018.01.010
46. Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, et al. Salivary microRNA: Discovery, Characterization, and Clinical Utility for Oral Cancer Detection. Clin Cancer Res Off J Am Assoc Cancer Res (2009) 15(17):5473–7. doi: 10.1158/1078-0432.CCR-09-0736
47. Al-Malkey MK, Abbas AA, Khalaf NF, Mubarak IA, Jasim IA. Expression Analysis of Salivary Microrna-31 in Oral Cancer Patients. Int J Curr Microbiol App Sci (2015) 4(12):375–82.
48. Zahran F, Ghalwash D, Shaker O, Al-Johani K, Scully C. Salivary microRNAs in Oral Cancer. Oral Dis (2015) 21(6):739–47. doi: 10.1111/odi.12340
49. Duz MB, Karatas OF, Guzel E, Turgut NF, Yilmaz M, Creighton CJ, et al. Identification of miR-139-5p as a Saliva Biomarker for Tongue Squamous Cell Carcinoma: A Pilot Study. Cell Oncol Dordr (2016) 39(2):187–93. doi: 10.1007/s13402-015-0259-z
50. Greither T, Vorwerk F, Kappler M, Bache M, Taubert H, Kuhnt T, et al. Salivary miR-93 and miR-200a as Post-Radiotherapy Biomarkers in Head and Neck Squamous Cell Carcinoma. Oncol Rep (2017) 38(2):1268–75. doi: 10.3892/or.2017.5764
51. Wu L, Zheng K, Yan C, Pan X, Liu Y, Liu J, et al. Genome-Wide Study of Salivary microRNAs as Potential Noninvasive Biomarkers for Detection of Nasopharyngeal Carcinoma. BMC Cancer (2019) 19(1):843. doi: 10.1186/s12885-019-6037-y.
52. Fadhil RS, Wei MQ, Nikolarakos D, Good D, Nair RG. Salivary microRNA miR-Let-7a-5p and miR-3928 Could be Used as Potential Diagnostic Bio-Markers for Head and Neck Squamous Cell Carcinoma. PloS One (2020) 15(3):e0221779. Ahmad A, editor. doi: 10.1371/journal.pone.0221779
53. He L, Ping F, Fan Z, Zhang C, Deng M, Cheng B, et al. Salivary Exosomal miR-24-3p Serves as a Potential Detective Biomarker for Oral Squamous Cell Carcinoma Screening. BioMed Pharmacother (2020) 121:109553. doi: 10.1016/j.biopha.2019.109553
54. Uma Maheswari TN, Nivedhitha MS, Ramani P. Expression Profile of Salivary Micro RNA-21 and 31 in Oral Potentially Malignant Disorders. Braz Oral Res (2020) 34:e002. doi: 10.1590/1807-3107bor-2020.vol34.0002
55. Guo Y, Yang J, Huang Q, Hsueh C, Zheng J, Wu C, et al. Circular RNAs and Their Roles in Head and Neck Cancers. Mol Cancer (2019) 18(1):1–18. doi: 10.1186/s12943-019-1003-5
56. Wiklund ED, Gao S, Hulf T, Sibbritt T, Nair S, Costea DE, et al. MicroRNA Alterations and Associated Aberrant DNA Methylation Patterns Across Multiple Sample Types in Oral Squamous Cell Carcinoma. Zhang B editor PloS One (2011) 6(11):e27840. doi: 10.1371/journal.pone.0027840
57. Ahmad P, Sana J, Slavik M, Gurin D, Radova L, Gablo N, et al. MicroRNA-15b-5p Predicts Locoregional Relapse in Head and Neck Carcinoma Patients Treated With Intensity-Modulated Radiotherapy. Cancer Genomics Proteomics (2019) 16(2):139–46. doi: 10.21873/cgp.20119
58. Tang H, Wu Z, Zhang J, Su B. Salivary lncRNA as a Potential Marker for Oral Squamous Cell Carcinoma Diagnosis. Mol Med Rep (2013) 7(3):761–6. doi: 10.3892/mmr.2012.1254
59. Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, et al. The Landscape of microRNA, Piwi-Interacting RNA, and Circular RNA in Human Saliva. Clin Chem (2015) 61(1):221–30. doi: 10.1373/clinchem.2014.230433
60. Zhao S-Y, Wang J, Ouyang S-B, Huang Z-K, Liao L. Salivary Circular RNAs Hsa_Circ_0001874 and Hsa_Circ_0001971 as Novel Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Cell Physiol Biochem (2018) 47(6):2511–21. doi: 10.1159/000491624
61. Liu J, Chen Y, Pei F, Zeng C, Yao Y, Liao W, et al. Extracellular Vesicles in Liquid Biopsies: Potential for Disease Diagnosis. BioMed Res Int (2021) 2021:6611244. doi: 10.1155/2021/6611244
62. Taylor J, Bebawy M. Proteins Regulating Microvesicle Biogenesis and Multidrug Resistance in Cancer. Proteomics (2019) 19(1–2):e1800165. doi: 10.1002/pmic.201800165
63. van Niel G, D’Angelo G, Raposo G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat Rev Mol Cell Biol (2018) 19(4):213–28. doi: 10.1038/nrm.2017.125
64. Armstrong D, Wildman DE. Extracellular Vesicles and the Promise of Continuous Liquid Biopsies. J Pathol Transl Med (2018) 52(1):1. doi: 10.4132/jptm.2017.05.21
65. Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic Device (ExoChip) for on-Chip Isolation, Quantification and Characterization of Circulating Exosomes. Lab Chip (2014) 14(11):1891–900. doi: 10.1039/C4LC00136B
66. Wang Z, Wu H, Fine D, Schmulen J, Hu Y, Godin B, et al. Ciliated Micropillars for the Microfluidic-Based Isolation of Nanoscale Lipid Vesicles. Lab Chip (2013) 13(15):2879. doi: 10.1039/c3lc41343h
67. Tay HM, Kharel S, Dalan R, Chen ZJ, Tan KK, Boehm BO, et al. Rapid Purification of Sub-Micrometer Particles for Enhanced Drug Release and Microvesicles Isolation. NPG Asia Mater (2017) 9(9):e434–e434. doi: 10.1038/am.2017.175
68. Liu C, Xu X, Li B, Situ B, Pan W, Hu Y, et al. Single-Exosome-Counting Immunoassays for Cancer Diagnostics. Nano Lett (2018) 18(7):4226–32. doi: 10.1021/acs.nanolett.8b01184
69. van Niel G, Charin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The Tetraspanin CD63 Regulates ESCRT-Independent and -Dependent Endosomal Sorting During Melanogenesis. Dev Cell (2011) 21(4):708–21. doi: 10.1016/j.devcel.2011.08.019
70. Verweij FJ, van Eijndhoven MA, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, et al. LMP1 Association With CD63 in Endosomes and Secretion via Exosomes Limits Constitutive NF-κb Activation. EMBO J (2011) 30(11):2115–29. doi: 10.1038/emboj.2011.123
71. Ma C, Jiang F, Ma Y, Wang J, Li H, Zhang J. Isolation and Detection Technologies of Extracellular Vesicles and Application on Cancer Diagnostic. Dose-Response (2019) 17(4):1559325819891004. doi: 10.1177/1559325819891004
72. Carnell-Morris P, Tannetta D, Siupa A, Hole P, Dragovic R. Analysis of Extracellular Vesicles Using Fluorescence Nanoparticle Tracking Analysis. Methods Mol Biol Clifton NJ (2017) 1660:153–73. doi: 10.1007/978-1-4939-7253-1_13
73. Rupert DLM, Lässer C, Eldh M, Block S, Zhdanov VP, Lotvall JO, et al. Determination of Exosome Concentration in Solution Using Surface Plasmon Resonance Spectroscopy. Anal Chem (2014) 86(12):5929–36. doi: 10.1021/ac500931f
74. Grasso L, Wyss R, Weidenauer L, Thampi A, Demurtas D, Prudent M, et al. Molecular Screening of Cancer-Derived Exosomes by Surface Plasmon Resonance Spectroscopy. Anal Bioanal Chem (2015) 407(18):5425–32. doi: 10.1007/s00216-015-8711-5
75. Michael A, Bajracharya S, Yuen P, Zhou H, Star R, Illei G, et al. Exosomes From Human Saliva as a Source of microRNA Biomarkers: microRNA Biomarkers in Salivary Exosomes. Oral Dis (2010) 16(1):34–8. doi: 10.1111/j.1601-0825.2009.01604.x
76. Topkas E, Keith P, Dimeski G, Cooper-White J, Punyadeera C. Evaluation of Saliva Collection Devices for the Analysis of Proteins. Clin Chim Acta (2012) 413(13–14):1066–70. doi: 10.1016/j.cca.2012.02.020
77. Schulz BL, Cooper-White J, Punyadeera CK. Saliva Proteome Research: Current Status and Future Outlook. Crit Rev Biotechnol (2013) 33(3):246–59. doi: 10.3109/07388551.2012.687361
78. Gemmell CH, Sefton MV, Yeo EL. Platelet-Derived Microparticle Formation Involves Glycoprotein IIb-IIIa. Inhibition by RGDS and a Glanzmann’s Thrombasthenia Defect. J Biol Chem (1993) 268(20):14586–9. doi: 10.1016/S0021-9258(18)82371-7
79. Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, et al. Exosomes Derived From Hypoxic Oral Squamous Cell Carcinoma Cells Deliver miR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res (2016) 76(7):1770–80. doi: 10.1158/0008-5472.CAN-15-1625
80. Winck FV, Prado Ribeiro AC, Ramos Domingues R, Ling LY, Riaño-Pachón DM, Rivera C, et al. Insights Into Immune Responses in Oral Cancer Through Proteomic Analysis of Saliva and Salivary Extracellular Vesicles. Sci Rep (2015) 5(1):16305. doi: 10.1038/srep16305
81. Zhong W, Ren J, Xiong X, Man Q, Zhang W, Gao L, et al. Increased Salivary Microvesicles are Associated With the Prognosis of Patients With Oral Squamous Cell Carcinoma. J Cell Mol Med (2019) 23(6):4054. doi: 10.1111/jcmm.14291
82. Ogawa Y, Miura Y, Harazono A, Kanai-Azuma M, Akimoto Y, Kawakami H, et al. Proteomic Analysis of Two Types of Exosomes in Human Whole Saliva. Biol Pharm Bull (2011) 34(1):13–23. doi: 10.1248/bpb.34.13
83. Sharma S, Gillespie BM, Palanisamy V, Gimzewski JK. Quantitative Nanostructural and Single-Molecule Force Spectroscopy Biomolecular Analysis of Human-Saliva-Derived Exosomes. Langmuir ACS J Surf Colloids (2011) 27(23):14394–400. doi: 10.1021/la2038763
84. Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Salo T, Vered M. Morphological and Molecular Features of Oral Fluid-Derived Exosomes: Oral Cancer Patients Versus Healthy Individuals. J Cancer Res Clin Oncol (2016) 142(1):101–10. doi: 10.1007/s00432-015-2005-3
85. Xiao C, Song F, Zheng YL, Lv J, Wang QF, Xu N. Exosomes in Head and Neck Squamous Cell Carcinoma. Front Oncol (2019) 9:894. doi: 10.3389/fonc.2019.00894
86. Hadavand M, Hasni S. Exosomal Biomarkers in Oral Diseases. Oral Dis (2019) 25(1):10–5. doi: 10.1111/odi.12878
87. Wang X, Kaczor-Urbanowicz KE, Wong DTW. Salivary Biomarkers in Cancer Detection. Med Oncol Northwood Lond Engl (2017) 34(1):7. doi: 10.1007/s12032-016-0863-4
88. Xie C, Ji N, Tang Z, Li J, Chen Q. The Role of Extracellular Vesicles From Different Origin in the Microenvironment of Head and Neck Cancers. Mol Cancer (2019) 18(1):1–15. doi: 10.1186/s12943-019-0985-3
89. Arantes L, De Carvalho AC, Melendez ME, Lopes Carvalho A. Serum, Plasma and Saliva Biomarkers for Head and Neck Cancer. Expert Rev Mol Diagn (2018) 18(1):85–112. doi: 10.1080/14737159.2017.1404906
90. Chiabotto, Gai, Deregibus, Camussi. Salivary Extracellular Vesicle-Associated exRNA as Cancer Biomarker. Cancers (2019) 11(7):891. doi: 10.3390/cancers11070891
91. Cheng J, Nonaka T, Wong D. Salivary Exosomes as Nanocarriers for Cancer Biomarker Delivery. Mater (2019) 12(4):654. doi: 10.3390/ma12040654
92. Hofmann L, Ludwig S, Vahl JM, Brunner C, Hoffmann TK, Theodoraki M-N. The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. Int J Mol Sci (2020) 21(11):4072. doi: 10.3390/ijms21114072
93. Clayton A, Buschmann D, Byrd JB, Carter DRF, Cheng L, Compton C, et al. Summary of the ISEV Workshop on Extracellular Vesicles as Disease Biomarkers, Held in Birmingham, UK, During December 2017. J Extracell Vesicles (2018) 7(1):1473707. doi: 10.1080/20013078.2018.1473707
94. Yan SK, Wei BJ, Lin ZY, Yang Y, Zhou ZT, Zhang WD. A Metabonomic Approach to the Diagnosis of Oral Squamous Cell Carcinoma, Oral Lichen Planus and Oral Leukoplakia. Oral Oncol (2008) 44(5):477–83. doi: 10.1016/j.oraloncology.2007.06.007
95. Silwood CJL, Lynch E, Claxson AWD, Grootveld MC. 1 H and 13 C NMR Spectroscopic Analysis of Human Saliva. J Dent Res (2002) 81(6):422–7. doi: 10.1177/154405910208100613
96. Gardner A, Carpenter G, So P-W. Salivary Metabolomics: From Diagnostic Biomarker Discovery to Investigating Biological Function. Metabolites (2020) 10(2):47. doi: 10.3390/metabo10020047
97. Schipper R, Loof A, de Groot J, Harthoorn L, Dransfield E, van Heerde W. SELDI-TOF-MS of Saliva: Methodology and Pre-Treatment Effects. J Chromatogr B (2007) 847(1):45–53. doi: 10.1016/j.jchromb.2006.10.005
98. Ploypetch S, Roytrakul S, Jaresitthikunchai J, Phaonakrop N, Krobthong S, Suriyaphol G. Salivary Proteomics of Canine Oral Tumors Using MALDI-TOF Mass Spectrometry and LC-Tandem Mass Spectrometry. PloS One (2019) 14(7):e0219390. Bencharit S, editor. doi: 10.1371/journal.pone.0219390
99. Buko A. Capillary Electrophoresis Mass Spectrometry Based Metabolomics. J Appl Bioanal (2017) 3(1):5–20. doi: 10.17145/jab.17.002
100. Beale DJ, Jones OAH, Karpe AV, Dayalan S, Oh DY, Kouremenos KA, et al. A Review of Analytical Techniques and Their Application in Disease Diagnosis in Breathomics and Salivaomics Research. Int J Mol Sci (2016) 18(1):E24. doi: 10.3390/ijms18010024
101. Wei J, Xie G, Zhou Z, Shi P, Qiu Y, Zheng X, et al. Salivary Metabolite Signatures of Oral Cancer and Leukoplakia. Int J Cancer (2011) 129(9):2207–17. doi: 10.1002/ijc.25881
102. Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. Capillary Electrophoresis Mass Spectrometry-Based Saliva Metabolomics Identified Oral, Breast and Pancreatic Cancer-Specific Profiles. Metabolomics (2010) 6(1):78–95. doi: 10.1007/s11306-009-0178-y
103. Ishikawa S, Sugimoto M, Kitabatake K, Sugano A, Nakamura M, Kaneko M, et al. Identification of Salivary Metabolomic Biomarkers for Oral Cancer Screening. Sci Rep (2016) 6(1):31520. doi: 10.1038/srep31520
104. Hsu C-W, Chen Y-T, Hsieh Y-J, Chang K-P, Hsueh P-C, Chen T-W, et al. Integrated Analyses Utilizing Metabolomics and Transcriptomics Reveal Perturbation of the Polyamine Pathway in Oral Cavity Squamous Cell Carcinoma. Anal Chim Acta (2019) 1050:113–22. doi: 10.1016/j.aca.2018.10.070
105. Sridharan G, Ramani P, Patankar S, Vijayaraghavan R. Evaluation of Salivary Metabolomics in Oral Leukoplakia and Oral Squamous Cell Carcinoma. J Oral Pathol Med (2019) 48(4):299–306. doi: 10.1111/jop.12835
106. Sami A, Elimairi I, Stanton C, Ross RP, Ryan CA. The Role of the Microbiome in Oral Squamous Cell Carcinoma With Insight Into the Microbiome–Treatment Axis. IJMS (2020) 21(21):8061. doi: 10.3390/ijms21218061
107. Chattopadhyay I, Verma M, Panda M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol Cancer Res Treat (2019) 18:153303381986735. doi: 10.1177/1533033819867354
108. Nagy KN, Sonkodi I, Szöke I, Nagy E, Newman HN. The Microflora Associated With Human Oral Carcinomas. Oral Oncol (1998) 34(4):304–8. doi: 10.1016/S1368-8375(98)80012-2
109. Bolz J, Dosá E, Schubert J, Eckert AW. Bacterial Colonization of Microbial Biofilms in Oral Squamous Cell Carcinoma. Clin Oral Invest (2014) 18(2):409–14. doi: 10.1007/s00784-013-1007-2
110. Tateda M, Shiga K, Saijo S, Sone M, Hori T, Yokoyama J, et al. Streptococcus Anginosus in Head and Neck Squamous Cell Carcinoma: Implication in Carcinogenesis. Int J Mol Med (2000) 6(6):699–703. doi: 10.3892/ijmm.6.6.699
111. Morita E, Narikiyo M, Yano A, Nishimura E, Igaki H, Sasaki H, et al. Different Frequencies of Streptococcus Anginosus Infection in Oral Cancer and Esophageal Cancer. Cancer Sci (2003) 94(6):492–6. doi: 10.1111/j.1349-7006.2003.tb01471.x
112. Mager D, Haffajee A, Devlin P, Norris C, Posner M, Goodson J. The Salivary Microbiota as a Diagnostic Indicator of Oral Cancer: A Descriptive, non-Randomized Study of Cancer-Free and Oral Squamous Cell Carcinoma Subjects. J Transl Med (2005) 3(1):27. doi: 10.1186/1479-5876-3-27
113. Pushalkar S, Mane SP, Ji X, Li Y, Evans C, Crasta OR, et al. Microbial Diversity in Saliva of Oral Squamous Cell Carcinoma. FEMS Immunol Med Microbiol (2011) 61(3):269–77. doi: 10.1111/j.1574-695X.2010.00773.x
114. Lim Y, Fukuma N, Totsika M, Kenny L, Morrison M, Punyadeera C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front Cell Infect Microbiol (2018) 8(AUG):267. doi: 10.3389/fcimb.2018.00267
115. Zuo H-J, Fu MR, Zhao H-L, Du X-W, Hu Z-Y, Zhao X-Y, et al. Study on the Salivary Microbial Alteration of Men With Head and Neck Cancer and Its Relationship With Symptoms in Southwest China. Front Cell Infect Microbiol (2020) 0:690. doi: 10.3389/fcimb.2020.514943
116. Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, Rodríguez-Hilario A, González H, Bondy J, et al. 16s rRNA Amplicon Sequencing Identifies Microbiota Associated With Oral Cancer, Human Papilloma Virus Infection and Surgical Treatment. Oncotarget (2016) 7(32):51320–34. doi: 10.18632/oncotarget.9710
117. Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Sci (2011) 331(6024):1559–64. doi: 10.1126/science.1203543
118. Sharma S, Zhuang R, Long M, Pavlovic M, Kang Y, Ilyas A, et al. Circulating Tumor Cell Isolation, Culture, and Downstream Molecular Analysis. Biotechnol Adv (2018) 36(4):1063–78. doi: 10.1016/j.biotechadv.2018.03.007
119. Catelain C, Pailler E, Oulhen M, Faugeroux V, Pommier A-L, Farace F. Detection of Gene Rearrangements in Circulating Tumor Cells: Examples of ALK-, ROS1-, RET-Rearrangements in Non-Small-Cell Lung Cancer and ERG-Rearrangements in Prostate Cancer. In: Magbanua MJM, Park JW, editors. Isolation and Molecular Characterization of Circulating Tumor Cells [Internet], vol. 994 . Cham: Springer International Publishing (2017). p. 169–79. Available at: http://link.springer.com/10.1007/978-3-319-55947-6_9.
120. Liu K, Chen N, Wei J, Ma L, Yang S, Zhang X. Clinical Significance of Circulating Tumor Cells in Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma. Oncol Rep (2020) 43(5):1525–35. doi: 10.3892/or.2020.7536
121. Onidani K, Shoji H, Kakizaki T, Yoshimoto S, Okaya S, Miura N, et al. Monitoring of Cancer Patients via Next-Generation Sequencing of Patient-Derived Circulating Tumor Cells and Tumor DNA. Cancer Sci (2019) 110(8):2590–9. doi: 10.1111/cas.14092
122. Jatana KR, Balasubramanian P, Lang JC, Yang L, Jatana CA, White E, et al. Significance of Circulating Tumor Cells in Patients With Squamous Cell Carcinoma of the Head and Neck: Initial Results. Arch Otolaryngol Head Neck Surg (2010) 136(12):1274–9. doi: 10.1001/archoto.2010.223
123. Hristozova T, Konschak R, Stromberger C, Fusi A, Liu Z, Weichert W, et al. The Presence of Circulating Tumor Cells (CTCs) Correlates With Lymph Node Metastasis in Nonresectable Squamous Cell Carcinoma of the Head and Neck Region (SCCHN). Ann Oncol (2011) 22(8):1878–85. doi: 10.1093/annonc/mdr130
124. Sinkala E, Sollier-Christen E, Renier C, Rosàs-Canyelles E, Che J, Heirich K, et al. Profiling Protein Expression in Circulating Tumour Cells Using Microfluidic Western Blotting. Nat Commun (2017) 8(1):14622. doi: 10.1038/ncomms14622
125. Schmidt H, Kulasinghe A, Perry C, Nelson C, Punyadeera C, liquid biopsy for head A. And Neck Cancers. Expert Rev Mol Diagn (2016) 16(2):165–72. doi: 10.1586/14737159.2016.1127758
126. Heitzer E, Auer M, Ulz P, Geigl JB, Speicher MR. Circulating Tumor Cells and DNA as Liquid Biopsies. Genome Med (2013) 5(8):73. doi: 10.1186/gm477
127. Grisanti S, Almici C, Consoli F, Buglione M, Verardi R, Bolzoni-Villaret A, et al. Circulating Tumor Cells in Patients With Recurrent or Metastatic Head and Neck Carcinoma: Prognostic and Predictive Significance. PloS One (2014) 9(8):e103918. doi: 10.1371/journal.pone.0103918
128. Inhestern J, Oertel K, Stemmann V, Schmalenberg H, Dietz A, Rotter N, et al. Prognostic Role of Circulating Tumor Cells During Induction Chemotherapy Followed by Curative Surgery Combined With Postoperative Radiotherapy in Patients With Locally Advanced Oral and Oropharyngeal Squamous Cell Cancer. PloS One (2015) 10(7):e0132901. doi: 10.1371/journal.pone.0132901
129. Economopoulou P, Kladi-Skandali A, Strati A, Koytsodontis G, Kirodimos E, Giotakis E, et al. Prognostic Impact of Indoleamine 2,3-Dioxygenase 1 (IDO1) mRNA Expression on Circulating Tumour Cells of Patients With Head and Neck Squamous Cell Carcinoma. ESMO Open (2020) 5(3):e000646. doi: 10.1136/esmoopen-2019-000646
Keywords: head and neck cancer, liquid biopsy, saliva, biomarker, circulating tumor nucleic acids, extracellular vesicles, metabolomics
Citation: Patel A, Patel S, Patel P and Tanavde V (2022) Saliva Based Liquid Biopsies in Head and Neck Cancer: How Far Are We From the Clinic? Front. Oncol. 12:828434. doi: 10.3389/fonc.2022.828434
Received: 03 December 2021; Accepted: 25 February 2022;
Published: 21 March 2022.
Edited by:
Markus Wirth, Klinikum rechts der Isar, GermanyReviewed by:
Vito Carlo Alberto Caponio, University of Foggia, ItalyCopyright © 2022 Patel, Patel, Patel and Tanavde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vivek Tanavde, dml2ZWsudGFuYXZkZUBhaGR1bmkuZWR1Lmlu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.