
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol., 17 March 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.826040
This article is part of the Research TopicReal-world Evidence in Onco-Hematological PatientsView all 14 articles
 Fabio Efficace1*
Fabio Efficace1* Andrea Patriarca2
Andrea Patriarca2 Mario Luppi3
Mario Luppi3 Leonardo Potenza3
Leonardo Potenza3 Giovanni Caocci4
Giovanni Caocci4 Agostino Tafuri5
Agostino Tafuri5 Francesca Fazio6
Francesca Fazio6 Claudio Cartoni6
Claudio Cartoni6 Maria Teresa Petrucci6
Maria Teresa Petrucci6 Ida Carmosino6
Ida Carmosino6 Riccardo Moia2
Riccardo Moia2 Gloria Margiotta Casaluci2
Gloria Margiotta Casaluci2 Paola Boggione2
Paola Boggione2 Elisabetta Colaci3
Elisabetta Colaci3 Davide Giusti3
Davide Giusti3 Valeria Pioli3
Valeria Pioli3 Francesco Sparano1
Francesco Sparano1 Francesco Cottone1
Francesco Cottone1 Paolo De Fabritiis7
Paolo De Fabritiis7 Nicolina Rita Ardu7
Nicolina Rita Ardu7 Pasquale Niscola7
Pasquale Niscola7 Isabella Capodanno8
Isabella Capodanno8 Anna Paola Leporace5
Anna Paola Leporace5 Sabrina Pelliccia5
Sabrina Pelliccia5 Elisabetta Lugli8
Elisabetta Lugli8 Edoardo La Sala1
Edoardo La Sala1 Luigi Rigacci9
Luigi Rigacci9 Michelina Santopietro9
Michelina Santopietro9 Claudio Fozza10
Claudio Fozza10 Sergio Siragusa11
Sergio Siragusa11 Massimo Breccia6
Massimo Breccia6 Paola Fazi1
Paola Fazi1 Marco Vignetti1
Marco Vignetti1Digital health tools are increasingly being used in cancer care and may include electronic patient-reported outcome (ePRO) monitoring systems. We examined physicians’ perceptions of usability and clinical utility of a digital health tool (GIMEMA-ALLIANCE platform) for ePRO monitoring in the real-life practice of patients with hematologic malignancies. This tool allows for the collection and assessment of ePROs with real-time graphical presentation of results to medical staff. Based on a predefined algorithm, automated alerts are sent to medical staff. Participating hematologists completed an online survey on their experience with the platform. Of the 201 patients invited to participate between December 2020 and June 2021 (cut-off date for current analysis), 180 (90%) agreed to enter the platform and had a median age of 57 years. Twenty-three hematologists with a median age of 42 years and an average of 17 years of experience in clinical practice were surveyed. All hematologists agreed or strongly agreed that the platform was easy to use, and 87%, agreed or strongly agreed that ePROs data were useful to enhance communication with their patients. The majority of physicians (78%) accessed the platform at least once per month to consult the symptom and health status profile of their patients. The frequency of access was independent of physician sex (p=0.393) and years of experience in clinical practice (p=0.404). In conclusion, our preliminary results support the clinical utility, from the perspective of the treating hematologist, of integrating ePROs into the routine cancer care of patients with hematologic malignancies.
Patients with cancer typically experience disease- and treatment-related symptoms that affect their health-related quality of life (HRQoL). Therefore, it is critical to capture the patient experience via validated patient-reported outcome (PRO) measures that provide unique information, unobtainable by other sources of more traditional clinical and laboratory measures. For example, PROs, such as functional aspects or symptoms reported by patients themselves, provide independent prognostic information for survival (1, 2). Additionally, there is ample literature documenting that clinicians often underestimate the severity of their patients’ symptoms (3–6).
The assessment of PROs has been historically confined to clinical research settings; however, in recent years, we have seen a greater interest in using PROs in clinical practice in an effort to improve the quality of patient care. Indeed, systematic evaluation of PROs in routine practice has been found to be associated with several benefits, including improved symptom control, HRQoL, patient satisfaction, as well as improved physician-patient communication and decreased hospitalizations and emergency department visits (7–10).
The inclusion of PROs in routine practice settings has been facilitated by advances in digital health technology, which now allows the implementation of PROs into electronic formats that can be administered remotely via online platforms (11). Two recent randomized controlled trials (RCTs), including patients with several types of cancer during chemotherapy, showed that remote symptom monitoring with electronic PROs (ePROs) was associated with reduced symptom burden and improved HRQoL outcomes (12, 13). Remarkably, the systematic monitoring of PROs via web-based platforms has also been found to be associated with improved overall survival in patients with advanced cancers (14–17).
The recent coronavirus disease pandemic has further boosted the adoption of digital health tools that could facilitate remote patient monitoring during emergencies, making ePROs even more critical in enhancing patient-centered care. However, implementation of ePRO monitoring in the routine care of patients with hematologic malignancies has been less documented in the literature (18), and only recently have we seen valuable evidence in this area (19, 20). In any case, there is a paucity of information about users’ perceptions of the clinical utility of digital health tools in routine care.
Late in 2020, the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) developed a digital health tool for adult patients with hematological malignancies (GIMEMA-ALLIANCE platform) (21) with the main goal of facilitating patient-centered care in routine practice.
We herein report a survey conducted to better understand the hematologists’ perceptions of usability and clinical utility of this platform in real-life practice.
Adult patients with a diagnosis of any hematologic malignancies according to the 2016 World Health Organization classification (22), who signed a written informed consent form, were eligible for enrollment in the GIMEMA-ALLIANCE platform. For the purpose of this project, patients could be included regardless of their type of therapy or individual characteristics, including age, level of education, or presence of comorbidities. After registration, patients were given (by their treating hematologist) a personal password to access the patient portal and complete a PRO survey that assessed aspects related to HRQoL, symptoms, and medication adherence. PRO measures include the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core (EORTC QLQ-C30) (23), four items from the EORTC Item Library (24), and the shortened 7-item Adherence to Refills and Medications Scale (ARMS-7) (25). These measures were selected based on their clinical relevance for the population under consideration. Indeed, the PRO questionnaires and items included in the platform, cover several aspects which are of importance across various hematological malignancies and have been widely used in previous studies. Each patient entering the platform has to be followed up for two years from the date of registration. As of January 2022, the platform includes 420 patients with hematologic malignancies, and 23 centers have obtained ethical approval to participate to this study. PRO results are available for both patients and physicians and are displayed graphically (in real time) with colored bars indicating the presence or absence of a clinically important problem or symptom. An example of the interfaces of the platform with the clinician with regard to display of functional aspects and symptoms is reported in the Appendix (Supplementary Figures 1, 2). Treating hematologists were required to collect clinical and socio-demographic information at baseline (e.g., patients’ and physicians’ characteristics, disease status at study entry) and every 3 months at follow-up (e.g., disease progression and survival status). Given the real-life nature of this study, no specific time-points were preplanned for the completion of the PRO survey. However, the platform is currently designed to send automated reminders to patients for completing the Survey after one week from registration (if this has not been completed within the first week from study inclusion), and thereafter every two weeks from the first PRO survey completion. In addition, physicians are encouraged (by the GIMEMA-ALLIANCE management team) to emphasize to their patients the importance of possibly completing the survey on a regular basis and, in any case, just few days before a planned clinical visit. The rationale for this latter aspect is that of providing a basis (updated information on patient’s HRQoL and symptoms) for further discussion during the clinical consultation. This study was registered at ClinicalTrials.gov (NCT04581187).
The GIMEMA-ALLIANCE platform is hosted in the Computer-based Health Evaluation System (CHES) infrastructure, a software used worldwide for the electronic collection, analysis, and presentation of ePROs (26). Full details of the development process and architecture of the GIMEMA-ALLIANCE platform, including the study rationale and the implementation of ePRO measures, as well as clinical data collected, have been described previously (21). Briefly, the platform consists of two dedicated secure portals, the patient (https://alliance.gimema.it) and physician (https://physician-alliance.gimema.it) portals. Based on a predefined algorithm, the treating hematologists and medical staff receive automated email alerts following the presence of clinically important problems, symptoms, or problems with adherence to therapy. The definition of clinically important problems and symptoms is based on previously defined evidence-based thresholds for the EORTC QLQ-C30 (27). Once the alert is received, and depending on the types and frequencies of the alerts received, the physician may decide to contact the patient by phone, schedule a face-to-face visit, or arrange a video-consultation within the GIMEMA-ALLIANCE platform. Indeed, the possibility of video consultations is an additional feature of this tool. A specific standard operating procedure (SOP) on “how to handle e-mail alerts” was not developed because the platform is open to patients with any hematologic malignancy, hence representing a wide range of patients with different clinical conditions and different needs. Therefore, the protocol stipulated that physicians are free to decide which action they feel most appropriate for their specific patients.
A brief schematic workflow of the data process is shown in Figure 1. After obtaining approval from the local ethics committee, and before being officially opened for recruitment, a start-up training session was organized by the GIMEMA-ALLIANCE management team. This session aimed to instruct the clinical staff of the participating hospital in using the platform and interpreting PRO data. SOPs developed for using the platform were illustrated during this online training session and also sent to the clinical staff just afterwards.
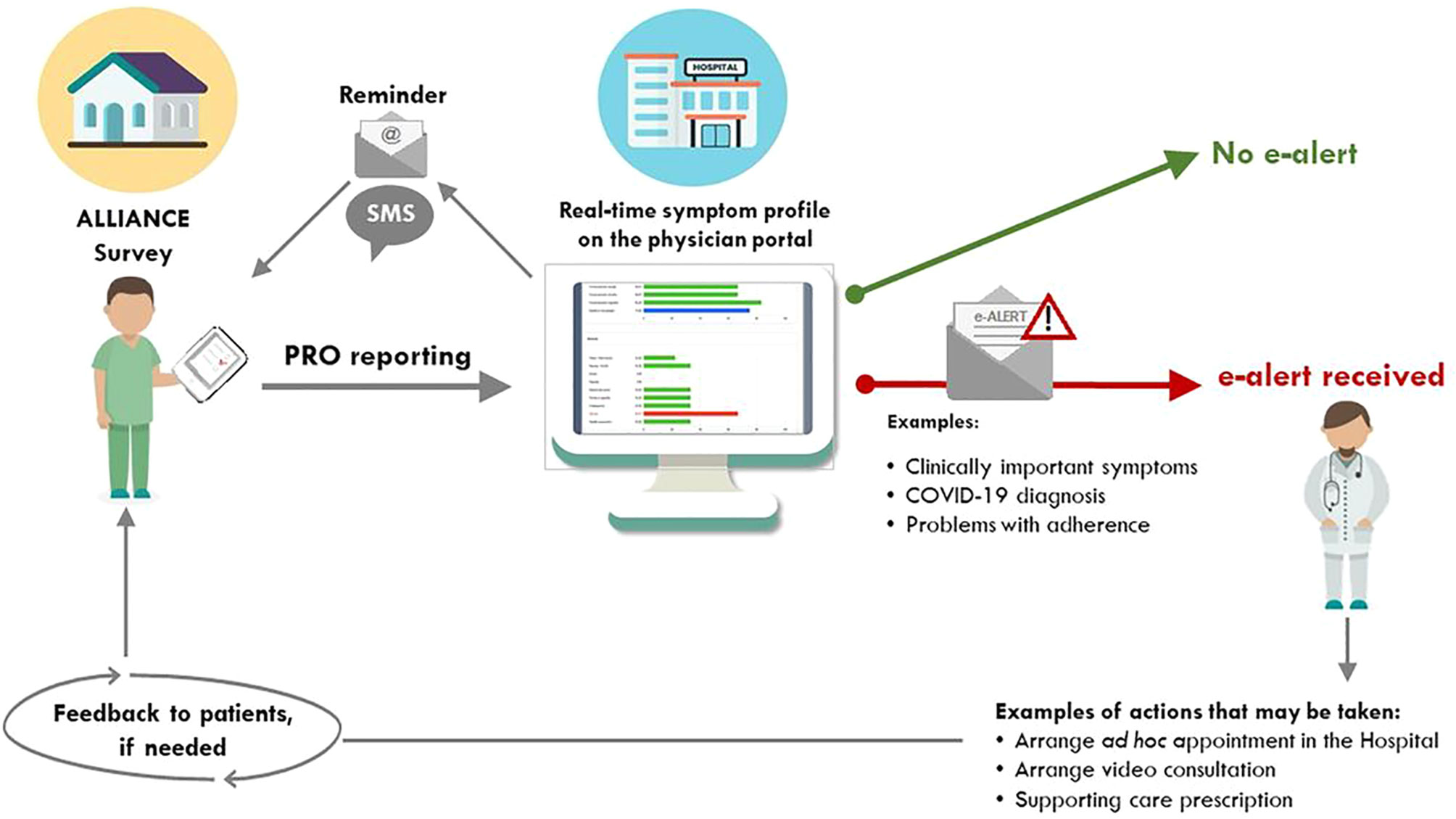
Figure 1 Schematic workflow of the patient-generated alerts to the medical team. PRO, patient-reported outcomes. This Figure was first published under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) in JMIR Research Protocols: Efficace F, Breccia M, Fazi P, Cottone F, Holzner B, Vignetti M. The GIMEMA-ALLIANCE Digital Health Platform for Patients With Hematologic Malignancies in the COVID-19 Pandemic and Postpandemic Era: Protocol for a Multicenter, Prospective, Observational Study JMIR Res Protoc 2021;10(6):e25271 (21). URL: https://www.researchprotocols.org/2021/6/e25271.
For the purpose of this work, approximately after six months from the implementation of the platform, we asked the participating hematologists for structured feedback on their experience with its use, with a focus on their perception of its usability, clinical utility in their daily practice, and impact on quality of care. Only the hematologists who had registered at least one patient (also from the same center), were invited to complete the survey. Only one of the respondents was involved in the development process of the platform.
We developed an ad hoc web survey covering the following three broad domains: 1) usability and potential benefits; 2) monitoring of symptoms and health status; and 3) aspects related to physician-patient communication. Selection of items included in the Survey was based on consensus among the management Team and it was aimed at capturing the physicians’ perception of the specific features of the Platform.
The survey was implemented and administered online to physicians via REDCap (28). Each treating hematologist received a personal link through which they could enter and complete the online survey. Every two days, automatic reminders were sent to hematologists who had not yet completed the survey. Once the hematologists completed their survey, REDCap automatically saved the answers into a secure online database. Of note, REDCap was only used for the purpose of capturing physicians’ answers to the Survey and it had no role in the development or management of the GIMEMA-ALLIANCE Platform. The invited hematologists had two weeks to respond, and after this deadline, the survey was taken offline. The database with all the responses was closed and downloaded for statistical analyses. The characteristics of the enrolled patients and treating hematologists were summarized by proportions, mean, median, and range. Additionally, in order to check the possible association of the characteristics of hematologists with survey results, we performed a multivariable logistic regression analysis including the sex of the treating hematologists (male=1 vs. female=0) and the corresponding years of experience in dealing with hematologic patients as independent variables. The statistical tests we performed were bilateral, with α=0.05, set as the threshold for statistical significance. All analyses were performed using SAS software v.4 (SAS Institute Inc., Cary, NC, USA).
Between December 2020 and June 2021 (cut-off date for current analysis), 201 patients were invited to participate, and 180 (90%) accepted to enter the ALLIANCE platform. The median age of the patients was 57 years (range 21-91). The majority were diagnosed with chronic myeloid leukemia (n=32, 18%) or multiple myeloma (n=31, 17%). Overall, there were 89 (49%) of patients in stable disease. Twenty-three hematologists (44% males and 56% females) from 11 centers, with a median age of 42 years (range 31-63) and an average of 17 years (range 5-34) of experience in clinical practice completed the online survey.
All the treating hematologists agreed or strongly agreed that the platform was easy to use, and the majority agreed or strongly agreed (91.3%, n=21) that it is useful in the clinical management of their patients. Regardless of receiving the alerts when clinically important problems and symptoms occurred, 30.4% (n=7) of physicians entered the portal at least once a week to monitor their patients’ health status, while 30.4% did so at least once every two weeks. Only 21.7% (n=5) entered the portal less than once per month. The frequency of access on a regular basis was also independent of physician sex (p=0.393) and years of experience in clinical practice (p=0.404). After receiving the alert, the majority of physicians entered the portal the same day (60.9%, n=14) and made a phone call to their patients (69.6%, n=16). The hematologists often (30.4%, n=7) or very often (26.1%, n=6) used the ePRO information from the platform for their discussion with the patients, but this was not the case within their team. The same information was sometimes (30.4%, n=7), rarely (34.8%, n=8), or never (17.4%, n=4) used for discussion with other colleagues. Further details are presented in Table 1.
Almost all the treating hematologists agreed or strongly agreed (95.6%, n=22) that the graphics about patients’ health status displayed on the platform were easy to understand and interpret. Sixteen physicians (69.6%) agreed and 3 (13.0%) strongly agreed that the platform helped them to better understand the patients’ general health status. Sixteen physicians (69.6%) agreed and 4 (17.4%) strongly agreed that the platform helped them to better understand the patients’ symptoms. Overall, 91.3% of physicians (n=21) agreed or strongly agreed that ePRO is useful to more accurately document patients’ symptomatic adverse events (AEs). In addition, 82.6% and 60.9% of physicians deemed ePRO information helpful to better identify low-grade and high-grade symptomatic adverse events, respectively. Further details are presented in Table 2.
Overall, 91.3% of physicians (n=21) deemed ePRO information useful to favor shared decision-making, and all of them considered this information helpful in suggesting supportive care strategies. Twenty hematologists (87.0%) deemed the information reported in the GIMEMA-ALLIANCE platform helpful in setting up unplanned visits with their patients and to enhance physician-patient communication. Only 13% of the treating physicians (n=3) did not agree with these statements. The details are presented in Table 3.
In this study, we explored the physicians’ perception of the usability and clinical utility of a digital health tool for ePRO monitoring in real-life hematology practice. While the clinical value of eHealth platforms has been well studied and documented in the context of solid tumors, less is known about their value in the context of hematologic malignancies.
Overall, our findings indicated a positive feedback from the hematologists interviewed, as most of them used the platform routinely, regardless of receiving automated alerts informing them about patients’ clinically relevant problems or symptoms. Additionally, graphically displayed ePRO results were found to be useful in enhancing patient-physician communication and in improving the detection of low-grade symptomatic AEs, by a large majority of respondents. This latter aspect may be of special relevance in routine practice across several hematologic cancer populations, such as those receiving long-term oral anticancer therapies. Indeed, it was previously observed that in these settings, patient-reported symptoms are typically of low to mild intensity and are therefore most likely to be unrecognized by the treating hematologist (5). Therefore, a better understanding of these chronic low-to-mild symptomatic AEs experienced by patients may have important clinical implications, for example, the adoption of more timely supportive care interventions. Results from the survey suggest that our platform may play a role in this respect, as all the physicians found it helpful in suggesting supportive care strategies. However, it should also be observed that there were 39% of physicians who did not find it useful to detect high-grade symptomatic AEs.
Recently, two studies evaluated the clinical utility and patient and staff feedback of ePRO systems (29, 30) in routine cancer care. In a non-randomized prospective cohort feasibility study, Kennedy et al. (29) explored the acceptability of an electronic system for collecting patient-self-reported AEs and quality of life. Staff feedback was positive, and 64% emphasized the benefits of receiving regular symptom reporting. In the PRO-TECT trial (30), 91% of the oncologists who responded to the survey found ePRO information useful, and this finding is consistent with that observed in our survey, where 87% of hematologists declared to have better understood patients’ symptoms by using the platform.
The clinical utility of ePRO systems is also linked to their ability to enhance patient-physician communication. In the PRO-TECT trial, 65% of the oncologists declared that they use PROs to often or sometimes guide discussions with patients (30), and this data is similar to our findings indicating that 74% of hematologists used (sometimes, often or very often) PRO information during clinical visits with their patients.
The active participation of clinicians is critical to enhance patients’ involvement and facilitate patient-centered care in routine practice. A recent study showed that the more clinicians looked at ePRO information from an online eHealth system (i.e., the eRAPID) before or during an appointment, the higher the patient engagement was with this system (13). One of the main challenges in implementing ePRO systems is clinicians’ reluctance to take on additional responsibility as well as perceived disruptions of the workflow (31). To minimize this risk, one solution may be to find physicians willing to engage their colleagues by demonstrating the flexibility of the tool, highlighting efficiencies in the overall work process, and convincing them of the value of the ePROs (31). It is also important to keep training physicians in the use of PROs with specialized training programs (32).
While we have documented a positive uptake of the use of this platform from the physicians’ standpoint, we cannot speculate on the patients’ perception of using this platform. However, a recent study that specifically examined the value of ePRO collection in the hematologic setting (including 102 patients with multiple myeloma and chronic lymphocytic leukemia) focused on the patients’ perception of the use of the portal and provided some reassuring data (19). The authors found that the majority of patients (84%) were willing to use the portal; however, they also observed that the completion of ePROs decreased over time, mainly because of the patient’s forgetfulness, and suggested ways to increase long-term participation rates (19). In another recent study, 227 lymphoma and chronic lymphocytic leukemia patients who completed web-based PRO questionnaires were randomized to care as usual (CAU), or to CAU plus return of PRO results (with or without a web-based self-management intervention) (20). No negative effects, for example in terms of psychological distress, were observed when individual PRO results were returned to patients, and authors concluded that this approach can be safely implemented in routine care practice (20).
The findings of our survey should be interpreted considering several limitations. It is possible that the positive results might be partly influenced by the characteristics of the sample, which consisted of physicians accepting to participate in the GIMEMA-ALLIANCE project. Hence, they are more likely to be enthusiastic about its use and reflect this positive perception in the rating of the survey. In addition, these findings should be regarded as preliminary, as the survey was performed approximately six months after the implementation of this tool and involved a small sample of hematologists. Additionally, our findings cannot be contextualized for a specific hematologic population or type of therapy. A key strength of our study is that it is one of very few reports documenting hematologists’ perception of the use of ePROs in real-life practice. In addition, we were able to document the feasibility of using the platform across several different institutions, each with different IT infrastructures and logistic support.
In conclusion, our results support the clinical utility, from the perspective of the treating hematologist, of integrating ePROs into the routine cancer care of patients with hematologic malignancies. Efforts are currently being made to put in place further educational and training activities for the use of PROs for hematologists involved and to implement novel IT functionalities that can further enhance its use in daily busy clinical practice.
The raw data supporting the conclusions of this article are available upon reasonable request to the corresponding author.
The study was reviewed and approved by Comitato Etico dell’Università “Sapienza”. Also, each participating center obtained approval from its local ethics committee. The patients/participants provided their written informed consent to participate in this study.
FE and MV designed the study. FC and FE performed statistical analysis. FE and FS wrote the first draft. All the authors interpreted results and validated the manuscript’s content. All authors contributed to the article and approved the submitted version.
This study was partly supported by the Associazione Italiana contro le leucemie linfomi e mieloma, Sezione di Roma (ROMAIL “Vanessa Verdecchia” Onlus) and by an unconditional contribution of AbbVie.
FE: Consultancy or Advisory Board: Amgen, AbbVie, Janssen, Takeda and Novartis. Research support (to his Institution) from AbbVie, Amgen and Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to all patients and physicians participating in the GIMEMA-ALLIANCE platform.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.826040/full#supplementary-material
1. Efficace F, Collins GS, Cottone F, Giesinger JM, Sommer K, Anota A, et al. Patient-Reported Outcomes as Independent Prognostic Factors for Survival in Oncology: Systematic Review and Meta-Analysis. Value Health (2021) 24(2):250–67. doi: 10.1016/j.jval.2020.10.017
2. Husson O, de Rooij BH, Kieffer J, Oerlemans S, Mols F, Aaronson NK, et al. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients With Cancer in the “Real-World”: Results From the Population-Based PROFILES Registry. Oncologist (2020) 25(4):e722–e32. doi: 10.1634/theoncologist.2019-0348
3. Di Maio M, Gallo C, Leighl NB, Piccirillo MC, Daniele G, Nuzzo F, et al. Symptomatic Toxicities Experienced During Anticancer Treatment: Agreement Between Patient and Physician Reporting in Three Randomized Trials. J Clin Oncol (2015) 33(8):910–5. doi: 10.1200/JCO.2014.57.9334
4. Sparano F, Aaronson NK, Cottone F, Piciocchi A, La Sala E, Anota A, et al. Clinician-Reported Symptomatic Adverse Events in Cancer Trials: Are They Concordant With Patient-Reported Outcomes? J Comp Eff Res (2019) 8(5):279–88. doi: 10.2217/cer-2018-0092
5. Efficace F, Rosti G, Aaronson N, Cottone F, Angelucci E, Molica S, et al. Patient- Versus Physician-Reporting of Symptoms and Health Status in Chronic Myeloid Leukemia. Haematologica (2014) 99(4):788–93. doi: 10.3324/haematol.2013.093724
6. Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How Accurate is Clinician Reporting of Chemotherapy Adverse Effects? A Comparison With Patient-Reported Symptoms From the Quality-Of-Life Questionnaire C30. J Clin Oncol (2004) 22(17):3485–90. doi: 10.1200/JCO.2004.03.025
7. Basch E, Barbera L, Kerrigan CL, Velikova G. Implementation of Patient-Reported Outcomes in Routine Medical Care. Am Soc Clin Oncol Educ Book (2018) 38):122–34. doi: 10.1200/EDBK_200383
8. Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring Quality of Life in Routine Oncology Practice Improves Communication and Patient Well-Being: A Randomized Controlled Trial. J Clin Oncol (2004) 22(4):714–24. doi: 10.1200/JCO.2004.06.078
9. Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-Related Quality-of-Life Assessments and Patient-Physician Communication: A Randomized Controlled Trial. JAMA (2002) 288(23):3027–34. doi: 10.1001/jama.288.23.3027
10. Barbera L, Sutradhar R, Seow H, Earle CC, Howell D, Mittmann N, et al. Impact of Standardized Edmonton Symptom Assessment System Use on Emergency Department Visits and Hospitalization: Results of a Population-Based Retrospective Matched Cohort Analysis. JCO Oncol Pract (2020) 16(9):e958–e65. doi: 10.1200/JOP.19.00660
11. Bennett AV, Jensen RE, Basch E. Electronic Patient-Reported Outcome Systems in Oncology Clinical Practice. CA Cancer J Clin (2012) 62(5):337–47. doi: 10.3322/caac.21150
12. Maguire R, McCann L, Kotronoulas G, Kearney N, Ream E, Armes J, et al. Real Time Remote Symptom Monitoring During Chemotherapy for Cancer: European Multicentre Randomised Controlled Trial (eSMART). Bmj (2021) 374:n1647. doi: 10.1136/bmj.n1647
13. Absolom K, Warrington L, Hudson E, Hewison J, Morris C, Holch P, et al. Phase III Randomized Controlled Trial of eRAPID: Ehealth Intervention During Chemotherapy. J Clin Oncol (2021) 39(7):734–47. doi: 10.1200/JCO.20.02015
14. Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol (2016) 34(6):557–65. doi: 10.1200/JCO.2015.63.0830
15. Denis F, Basch E, Septans AL, Bennouna J, Urban T, Dueck AC, et al. Two-Year Survival Comparing Web-Based Symptom Monitoring vs Routine Surveillance Following Treatment for Lung Cancer. Jama (2019) 321(3):306–7. doi: 10.1001/jama.2018.18085
16. Denis F, Lethrosne C, Pourel N, Molinier O, Pointreau Y, Domont J, et al. Randomized Trial Comparing a Web-Mediated Follow-Up With Routine Surveillance in Lung Cancer Patients. J Natl Cancer Inst (2017) 109(9). doi: 10.1093/jnci/djx029
17. Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA (2017) 318(2):197–8. doi: 10.1001/jama.2017.7156
18. Cannella L, Efficace F, Giesinger J. How Should We Assess Patient-Reported Outcomes in the Onco-Hematology Clinic? Curr Opin Support Palliat Care (2018) 12(4):522–9. doi: 10.1097/SPC.0000000000000386
19. Lehmann J, Buhl P, Giesinger JM, Wintner LM, Sztankay M, Neppl L, et al. Using the Computer-Based Health Evaluation System (CHES) to Support Self-Management of Symptoms and Functional Health: Evaluation of Hematological Patient Use of a Web-Based Patient Portal. J Med Internet Res (2021) 23(6):e26022. doi: 10.2196/preprints.26022
20. Oerlemans S, Arts LPJ, Kieffer JM, Prins J, Hoogendoorn M, van der Poel M, et al. Web-Based Return of Individual Patient-Reported Outcome Results Among Patients With Lymphoma: Randomized Controlled Trial. J Med Internet Res (2021) 23(12):e27886. doi: 10.2196/27886
21. Efficace F, Breccia M, Fazi P, Cottone F, Holzner B, Vignetti M. The GIMEMA-ALLIANCE Digital Health Platform for Patients With Hematologic Malignancies in the COVID-19 Pandemic and Postpandemic Era: Protocol for a Multicenter, Prospective, Observational Study. JMIR Res Protoc (2021) 10(6):e25271. doi: 10.2196/25271
22. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. WHO Classification of Tumours. Available at: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017.
23. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J Natl Cancer Inst (1993) 85(5):365–76. doi: 10.1093/jnci/85.5.365
24. EORTC Item Library. Available at: https://www.eortc.be/itemlibrary/ (Accessed May 11, 2020).
25. Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and Evaluation of the Adherence to Refills and Medications Scale (ARMS) Among Low-Literacy Patients With Chronic Disease. Value Health (2009) 12(1):118–23. doi: 10.1111/j.1524-4733.2008.00400.x
26. Holzner B, Giesinger JM, Pinggera J, Zugal S, Schopf F, Oberguggenberger AS, et al. The Computer-Based Health Evaluation Software (CHES): A Software for Electronic Patient-Reported Outcome Monitoring. BMC Med Inform Decis Mak (2012) 12:126. doi: 10.1186/1472-6947-12-126
27. Giesinger JM, Loth FLC, Aaronson NK, Arraras JI, Caocci G, Efficace F, et al. Thresholds for Clinical Importance Were Established to Improve Interpretation of the EORTC QLQ-C30 in Clinical Practice and Research. J Clin Epidemiol (2020) 118:1–8. doi: 10.1016/j.jclinepi.2019.10.003
28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J BioMed Inform (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010
29. Kennedy F, Absolom K, Clayton B, Rogers Z, Gordon K, Francischetto EOC, et al. Electronic Patient Reporting of Adverse Events and Quality of Life: A Prospective Feasibility Study in General Oncology. JCO Oncol Pract (2021) 17(3):e386–e96. doi: 10.1200/OP.20.00118
30. Basch E, Stover AM, Schrag D, Chung A, Jansen J, Henson S, et al. Clinical Utility and User Perceptions of a Digital System for Electronic Patient-Reported Symptom Monitoring During Routine Cancer Care: Findings From the PRO-TECT Trial. JCO Clin Cancer Inform (2020) 4:947–57. doi: 10.1200/CCI.20.00081
31. Nordan L, Blanchfield L, Niazi S, Sattar J, Coakes CE, Uitti R, et al. Implementing Electronic Patient-Reported Outcomes Measurements: Challenges and Success Factors. BMJ Qual Saf (2018) 27(10):852–6. doi: 10.1136/bmjqs-2018-008426
Keywords: digital health, symptoms, quality of life, hematology, patient-reported outcomes (PROs), leukemia, multiple myeloma, lymphoma
Citation: Efficace F, Patriarca A, Luppi M, Potenza L, Caocci G, Tafuri A, Fazio F, Cartoni C, Petrucci MT, Carmosino I, Moia R, Margiotta Casaluci G, Boggione P, Colaci E, Giusti D, Pioli V, Sparano F, Cottone F, De Fabritiis P, Ardu NR, Niscola P, Capodanno I, Leporace AP, Pelliccia S, Lugli E, La Sala E, Rigacci L, Santopietro M, Fozza C, Siragusa S, Breccia M, Fazi P and Vignetti M (2022) Physicians’ Perceptions of Clinical Utility of a Digital Health Tool for Electronic Patient-Reported Outcome Monitoring in Real-Life Hematology Practice. Evidence From the GIMEMA-ALLIANCE Platform. Front. Oncol. 12:826040. doi: 10.3389/fonc.2022.826040
Received: 30 November 2021; Accepted: 03 February 2022;
Published: 17 March 2022.
Edited by:
Claudia Vener, University of Milan, ItalyReviewed by:
Albrecht Reichle, University Medical Center Regensburg, GermanyCopyright © 2022 Efficace, Patriarca, Luppi, Potenza, Caocci, Tafuri, Fazio, Cartoni, Petrucci, Carmosino, Moia, Margiotta Casaluci, Boggione, Colaci, Giusti, Pioli, Sparano, Cottone, De Fabritiis, Ardu, Niscola, Capodanno, Leporace, Pelliccia, Lugli, La Sala, Rigacci, Santopietro, Fozza, Siragusa, Breccia, Fazi and Vignetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Efficace, Zi5lZmZpY2FjZUBnaW1lbWEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.