- 1Department of Cardiac, Thoracic, Vascular Sciences and Public Health, Pathology Unit, University of Padova, Padova, Italy
- 2Department of Emergency and Organ Transplantation, Pathology Unit, University of Bari, Bari, Italy
- 3Department of Interdisciplinary Medicine, Occupational Health Unit, University of Bari, Bari, Italy
Mesothelioma is a rare malignant neoplasm with poor survival. It mainly affects the pleura (90%) but can arise in all serous cavities: peritoneum (5-10%), pericardium and tunica vaginalis testis (<1%). The onset of pleural mesothelioma is strictly related to asbestos exposure with a long latency time. The causal link with asbestos has also been suggested for peritoneal mesothelioma, while the importance of exposure in the onset of pericardial and tunica vaginalis testis mesotheliomas is not well known. Mesothelioma remains an aggressive and fatal disease with a five-year mortality rate higher than 95%. However, new therapeutic approaches based on molecular-targeted and immunomodulatory therapies are being explored but have conflicting results. In this context, the identification of critical targets appears mandatory. Awareness of the molecular and physiological changes leading to the neoplastic degeneration of mesothelial cells and the identification of gene mutations, epigenetic alterations, gene expression profiles and altered pathways could be helpful for selecting targetable mechanisms and molecules. In this review, we aimed to report recent research in the last 20 years focusing on the molecular pathways and prognostic factors in peritoneal mesothelioma and their possible diagnostic and therapeutic implications.
Introduction
Peritoneal mesothelioma (PM) is a rare malignant neoplasm that originates from the mesothelial cells lining the peritoneal serosa. PM was first reported in the early 1900s as a diffuse intraperitoneal neoplastic process associated with ascites in a young woman (1). The neoplasm represents 15-20% (2) of all mesotheliomas and shares some features with the most common pleural counterpart even if several substantial differences make it a separate and definite nosological entity. From an epidemiological point of view, PM more frequently affects females than males, with an earlier median age (52 years) (3) than pleural mesothelioma.
PM usually arises as multiple serosal nodules with thickening of the peritoneum. Adipose tissue invasion and stromal invasion represent indicative features of malignancy (4). Histologically, it is classified into three histotypes: a) epithelioid, with round monomorphic cells arranged into different architectural patterns; b) sarcomatoid, composed of spindle elements; and c) biphasic, with at least 10% of both components. Well-differentiated papillary mesothelioma is included among the mesothelial tumours of the peritoneum, which represents a rare variant with an indolent behaviour that occurs mainly in women of reproductive age (2, 5). The diagnostic algorithm mirrors that of the pleural diagnostic algorithm, with a combination of positivity for mesothelial markers and negativity for BAP1 (BRCA1 associated protein 1) in 40-60% of cases. The histologic variants are clinically relevant, allowing a prognostic stratification of patients and guiding the treatment strategies (6).
The link between asbestos exposure is weaker than that in pleural tumours. Even if asbestos exposure represents the most important risk factor (7), it is found in approximately 33-50% of cases compared to the frequency of over 90% in pleural mesothelioma. Furthermore, the latency period between asbestos exposure and the development of mesothelioma is 20 years for PM, compared to 30-40 years for pleural mesothelioma (8, 9). Asbestos fibres have been identified in the omentum and mesentery of the gastrointestinal tract (10). Various hypotheses have been formulated to explain the mechanisms by which asbestos fibres can reach the peritoneal cavity. A study showed significant incidences of pulmonary asbestosis (17%) and pleural plaques (26%) in a cohort of patients with PM, suggesting a link between pleuropulmonary and peritoneal diseases probably secondary to the migration of asbestos fibres (11). Another hypothesis concerns the migration of asbestos fibres through the female genital system from the uterus to the fallopian tubes up to the peritoneal cavity. Such contamination could take place following sexual intercourse or following the application of talc contaminated with asbestos, as described by some papers that have focused on mesotheliomas that originate from the germinative epithelium of the ovary, which represents a specialization of mesothelial cells (12). Other rare causes related to the onset of PM are those attributable to chronic inflammatory states of the peritoneal serosa, such as chronic peritonitis, recurrent Mediterranean fever, germline mutations of BRCA (BReast CAncer), endometriosis, the period following radiation therapy for other peritoneal or pelvic neoplastic processes, and simian vacuolating virus infection (13–15).
The strict association between asbestos exposure and the development of malignant mesothelioma is indisputable. Our understanding of the mechanisms of action of asbestos fibres and their effects on mesothelial cells has deepened since the middle of the twentieth century. Knowledge about carcinogenesis related to asbestos has increased together with awareness about the molecular changes in this tumour. Mesothelioma appears to be characterized by chromosome rearrangements and gene mutations/deletions (16). More recently, the molecular landscape of mesothelioma has been enriched by the discovery of susceptibility familial factors that influence the impact of asbestos, target mutations in oncogenes and tumour suppressor genes, and epigenetic changes (17, 18).
As treatment options for PM, as well as pleural PM, are currently limited and target therapies are far away from being available, a better understanding of the molecular pathogenesis could suggest further therapeutic opportunities. In this review, we aimed to report recent research in the last 20 years focusing on the most promising molecular pathways and prognostic factors in PM in terms of diagnostic and therapeutic implications.
Discussion
A comprehensive review of the literature of the last decade was conducted in the Medline database, including research with the generic terms “peritoneal mesothelioma” AND “molecular”. All data were further confirmed by examining the list manually. The eligibility of studies was assessed by reading titles, abstracts, and full texts.
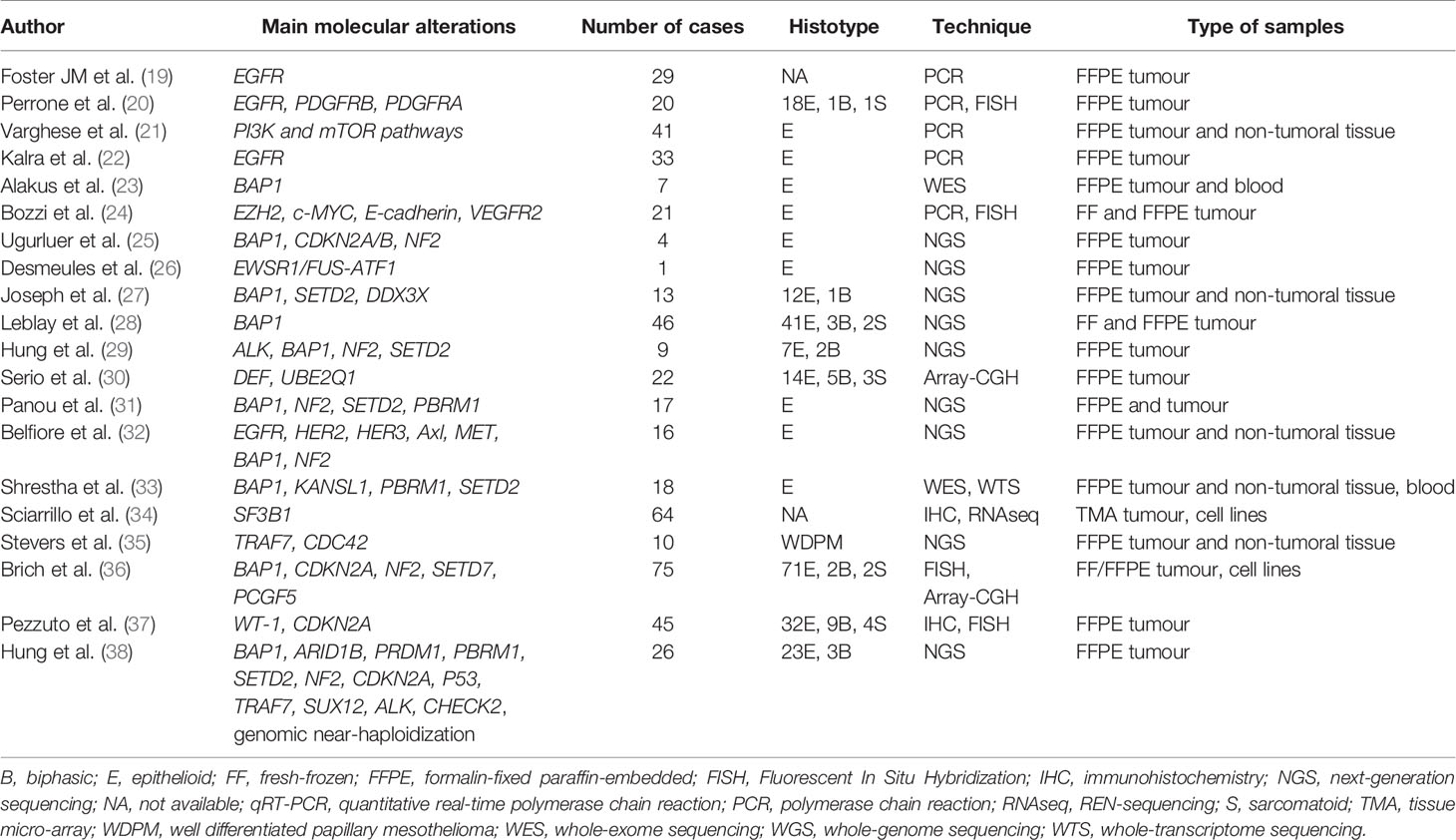
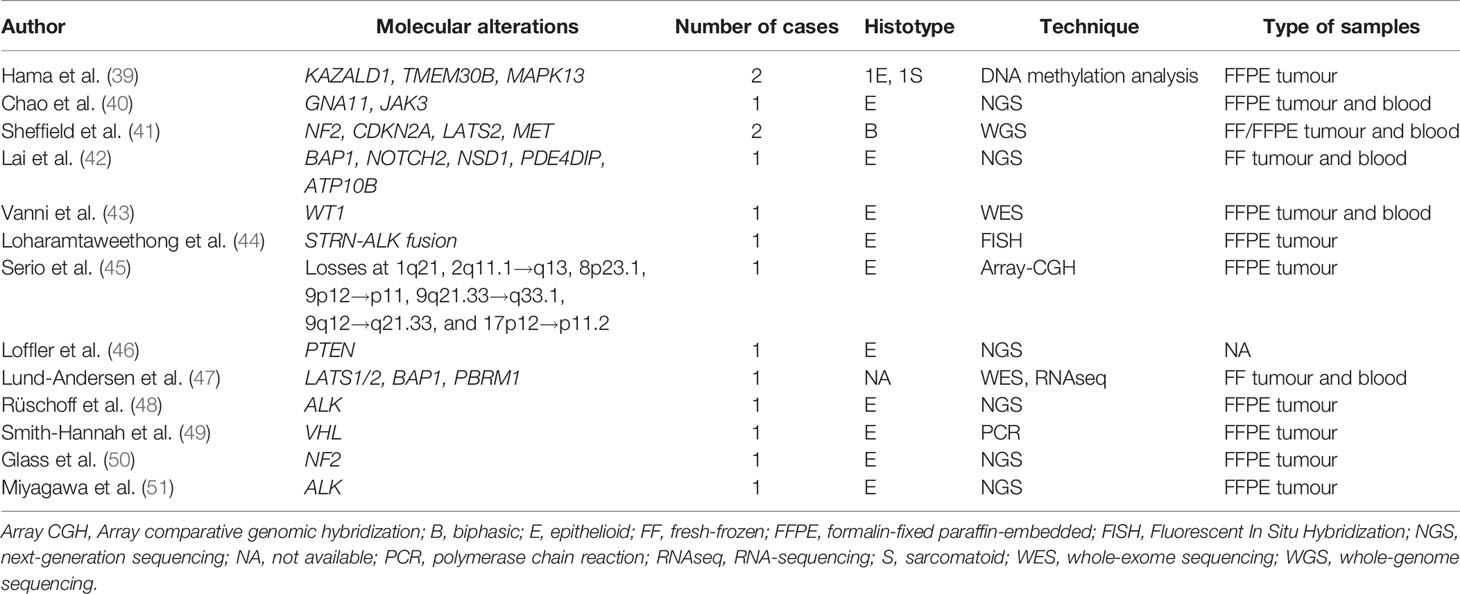
In Tables 1 and 2 are respectively listed in chronological order the research studies and the case reports in which molecular analyses of PM were performed. Below, the most important molecular alterations that have emerged are discussed and grouped into “Oncogenes”, “Tumour suppressor genes” and “Post-transcriptional alterations” sections. Two additional sections are dedicated to the description of the role of the tumour microenvironment and on therapeutic approaches in PM.
Oncogenes
Receptor tyrosine kinases are surface receptors that link growth factors, cytokines, and hormones, functioning as key regulators of normal cellular processes. Mutations in receptor tyrosine kinases alter signalling cascades, leading to dysregulation of protein expression.
EGFR has been investigated in the pathogenesis of PM. Foster et al. (19) found EGFR (Epidermal Growth Factor Receptor) mutations, both in L858R and other catalytic domains, in a high percentage of PM patients. In different studies, ligand-dependent activation (e.g., HER2- Human epidermal growth factor receptor 2, HER3, Axl, and MET-mesenchymal epithelial transition factor) and coactivation of EGFR and PDGFRB (Platelet Derived Growth Factor Receptor Beta) were shown, together with cooperation of these receptors with the mTOR (mammalian target of rapamycin) pathway, suggesting the potential efficacy of the combined inhibition of these cascades in PM (20, 32). In this direction, a patient with multicystic PM (52) and two patients with papillary PM (53) were successfully treated with rapamycin, an mTOR inhibitor. The mTOR signalling pathway closely interacts with PI3K (Phosphoinositide 3-kinases). Varghese et al. (21) detected the overexpression of genes in these pathways in patients with the shortest survival in a group of 41 PM patients treated with surgical cytoreduction and regional intraoperative chemotherapy perfusion. In subsequent studies, EGFR alterations emerged as more complex than somatic mutations, with the detection of silent polymorphisms and cooperation with other receptors, such as the formation of heterodimers, showing that PM do not harbour somatic mutations in the EGFR tyrosine kinase domain that would make them sensitive to molecularly targeted therapy (22).
In 2016, Loharamtaweethong et al. (44) described an anecdotic case of ALK (Anaplastic lymphoma kinase)-rearranged PM in a child, thus opening a new perspective in the molecular dissection of this entity. The presence of ALK alteration was the topic of investigation for the group of Hung et al. (29), who detected this new promising pathogenetic mechanism in 3% of patients, mostly young women. This epidemiologic distribution of ALK rearrangement was confirmed in more recent studies (38, 48, 51), all representing by striatin (STRN)-ALK fusion, an extremely rare ALK rearrangement reported in only 56 cancers of different organs (51). This evidence suggests the need to explore the alteration in such groups, for the possible use of molecular target therapies.
In the complex scenario of hidden alterations, some studies focused on alterations that could also be present in mesothelioma, translating the experience of other better characterized neoplasms. This is the case of EWSR1 (EWS RNA Binding Protein 1) rearrangements. In 2013, Panagopoulos et al. (54) detected a specific fusion gene in mesothelioma. These results were further confirmed by Desmeules et al. (26), who associated EWSR1 alteration with a unique subset of mesothelioma arising in young, nonexposed, BAP1-retaining patients, resulting in an undirect activation of c-MET gene.
WT1 (Wilm’s tumor 1) gene mutations have been rarely described in PM, especially in those non-asbestos related (43). The immunohistochemical expression of WT1, which represents a useful diagnostic tool to assess the mesothelial origin of neoplastic cells, could also have a prognostic role associated with a better prognosis, as described by Pezzuto et al. (37).
Tumour Suppressor Genes
Tumour suppressor genes regulate cell division and replication, thus leading to growth abnormalities when mutated, and their function is lost or reduced. This event seems to occur frequently in the development of PM. BAP1 plays a key role in PM susceptibility and oncogenesis. Alakus et al. (23) described for the first time that the loss of BAP1 occurred in PM in the absence of any other oncogenic drivers, such as NF2 (neurofibromatosis type 2) and CDKN2A (Cyclin Dependent Kinase Inhibitor 2A). Although these genes have also been detected in the pleural form, some differences have been found between the two entities (25). This is mainly true for the prevalence of these alterations. In PM, a higher frequency of BAP1 and a lower frequency of CDKN2A and NF2 have been described (27, 55, 56). This could be related to the lower frequency in the peritoneum of biphasic and sarcomatoid mesothelioma that typically harbour these mutations (38). While BAP1 was independent of the clinical outcome, the latter two have a negative prognostic significance, thus becoming interesting targets for therapeutic approaches. In contrast, Leblay et al. found that BAP1 protein nuclear expression mirrored molecular status, and its detection was a good and reliable prognostic marker for the complete loss of BAP1 activity in PM (28). Particularly, the authors found a better overall survival for patients with BAP1 mutations, protein expression loss, or at least one of these alterations independently of tumour histological subtype, age, and sex. In terms of biomarker discovery, Lai et al. (42) identified a tumour-specific neoantigen for BAP1 following insertion of a frameshift mutation translated into a truncated protein which was predicted to be presented by the patient’s HLA-B molecule as a tumour-specific neo-antigen. A separate issue is represented by BAP1 germline mutations. Together with somatic mutations, a BAP1-related cancer syndrome characterized by mesothelioma, uveal melanoma, and possibly other cancer types has been identified. This possibility should be taken into account when identifying patients at high risk. One group described a significant proportion of patients with mesothelioma carry germline mutations in cancer susceptibility genes, especially those with PM, absent asbestos exposure, second cancer diagnosis, and young age (31). As for the germline mutations of NF2, only very rare reports described the onset of a PM in the context of a type 2 neurofibromatosis (50). Two cases of PM have been described respectively associated with Cowden (46) and Li-Fraumeni syndrome (40). Von Hippel-Lindau (VHL) disease tumour suppressor gene VHL was found mutated in a unique case of clear cell epithelioid PM in a non-exposed women (49). Two tumour suppressor genes, TRAF7 and CDC42, respectively involved in activation of mitogen‐ activated protein kinases (MAPKs) and in Rho GTPase signalling, were found mutually exclusively mutated in a series of well-differentiated papillary mesothelioma of the peritoneum (35), in absence of the typical mutations of the malignant counterpart, as those involving BAP1, NF2, CDKN2A, ALK, contributing to a clear-cut separation between the two entities. These last exceptional cases show how a specific molecular signature could correspond an unusual morphological variant and this should be kept in mind especially for diagnostic purposes to avoid misinterpretations.
Post-Transcriptional Alterations
Although the genomics of PM has deepened, much less is known about the epigenomic landscape of this tumour. Starting from the evidence of the pleural form, epigenetic alterations in PM have also been suggested to contribute to carcinogenesis. Hama et al. (39) quantitatively analysed the methylation of KAZALD1 (Kazal Type Serine Peptidase Inhibitor Domain 1), TMEM308 (Tumor Microenvironment of Metastasis 308), and MAPK13 (Mitogen-Activated Protein Kinase 13) and reported hypermethylation of these genes in PM. The authors found a correlation between KAZALD1 and the sarcomatoid variant, showing a certain histotype distribution of molecular alterations. Bozzi et al. focused on epithelioid PM, where tumour cells were characterized by stemness and plasticity supported by epigenetic reprogramming in the context of mesenchymal epithelial reverse transcription. Thus, the authors suggesting that the PM is likely to be responsive to epigenetic regulator inhibitors, basing also on the inverse correlation between strong EZH2 expression and the loss of the BAP1 (24). One group studied splicing alterations in PM reporting an upregulation of spliceosomal genes with a high expression of SF3B1 (Splicing factor 3B subunit 1) which correlated with a worse prognosis (34). An important posttranslational modification to target for the development of new therapeutic approaches for cancer treatment is ubiquitination. Recent studies have observed that ubiquitination is involved in the metabolic reprogramming of cancer cells. In a recent study, Serio et al. found several losses among which loss of function of ubiquitination and defensins in PM (30, 45, 57), suggesting an important role in the initial development and progression of neoplasia or in combination with other mechanisms.
Role of the Tumour Immune Microenvironment (TME)
The interactions between tumour cells and immune cells are complex. This strict association is well recognized in several thoracic malignancies, such as lung cancer (58, 59). An increasing emphasis has been attributed to the immune milieu and thus to the employment of immunotherapy against mesothelioma. Most research has been conducted on the pleural form (60, 61), while much less is known about the characterization of the TME in the peritoneal form. It seems that the role of the TME is not detached from MM carcinogenesis. Shrestha et al. (33) found high checkpoint receptor activation in BAP1 haploinsufficient PM, thus suggesting predictive value for tumour response to this marker. Similarly, White et al. (62) demonstrated a significant increase in PD-L1 (Programmed death-ligand 1) expression in PM patients with a high mutational burden and germline mutations.
Advances in PM Treatment and Future Perspectives
Strictly connected to the topic of molecular alterations in PM is the development of new treatment strategies. To date, MM remains a rare cancer with only a few promising changes in treatment. This is mainly true for PM, as most of the knowledge is extrapolated from the pleural form. Together with the deeper awareness of the genomic alterations, therapeutic strategies targeting new pathways have been explored in the pleura with multiple trials available. As previously described, BAP1 inactivating mutations are frequently detected, with itself or its multiple downstream pathways considered fascinating targets. In this scenario, PARP (63) and EZH2 (Enhancer of zeste homolog 2) (64) inhibitors have been investigated in BAP1-negative tumours, with disappointing results. Similarly, CDKN2A, commonly deleted in mesothelioma with the loss of p16 protein expression, has been targeted in a clinical trial (65) with only limited success. Another well-known inactivation concerns NF2, leading to the loss of merlin protein and the dysregulation of several streams, among which the Hippo-YAP/TAZ pathways (66), potentially blocked by mTOR inhibitors (52, 53), warrant further analysis. An attractive target has also been found in mesothelin, a membrane glycoprotein that has been blocked in epithelioid pleural mesothelioma (67–69). Regarding rare alterations, anecdotal reports about ALK fusions in PM need to be reported in terms of the response to ALK inhibitors, such as in lung cancers (48). Immunotherapy deserves a separate discussion and is widely studied in MM, for which the best results have been obtained (70) with different administration strategies, alone or in combination, which represents a considerable goal.
Conclusions
The molecular events that cause PM have not been clearly defined, and comprehensive genetic characterization remains challenging. Important steps have been made towards the definition of the molecular signature of neoplasia to identify therapeutic targets, along with the goals of other tumours. The recent published studies, particularly those based on next-generation or other high-throughput sequencing methodologies, show heterogenous molecular alterations, mostly involving BAP1 and other DNA repair, chromatin, and cell cycle regulators. Awareness of the molecular and physiological changes leading to the neoplastic degeneration of mesothelial cells and the identification of gene mutations, epigenetic alterations, gene expression profiles and altered pathways could be helpful for selecting targetable mechanisms and molecules.
Author Contributions
Conceptualization, FF, FP, GS, and LV. Methodology, AM and FP. Data curation DC, DR, and Ad’A. Writing—original draft preparation FF and FP. Writing—review and editing GS. Supervision, GS and LV. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miller J, Wynn HW. A Malignant Tumour Arising From the Endothelium of the Peritoneum, and Producing a Mucoid Ascitic Fluid. J Pathol Bacter (1908) 12:267–78. doi: 10.1002/path.1700120212
2. Carbone M, Adusumilli PS, Alexander HR Jr, Baas P, Bardelli F, Bononi A, et al. Mesothelioma: Scientific Clues for Prevention, Diagnosis, and Therapy [Published Correction Appears in CA Cancer J Clin. 2020 Jul;70(4):313-314]. CA Cancer J Clin (2019) 69(5):402–29. doi: 10.3322/caac.21572
3. Pavlisko EN, Liu B, Green C, Sporn TA, Roggli VL. Malignant Diffuse Mesothelioma in Women: A Study of 354 Cases. Am J Surg Pathol (2020) 44(3):293–304. doi: 10.1097/PAS.0000000000001418
4. Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch Pathol Lab Med (2018) 142(1):89–108. doi: 10.5858/arpa.2017-0124-RA
5. Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma: 2012 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch Pathol Lab Med (2013) 137(5):647–67. doi: 10.5858/arpa.2012-0214-OA
6. Kusamura S, Kepenekian V, Villeneuve L, Lurvink RJ, Govaerts K, De Hingh IHJT, et al. Peritoneal Mesothelioma: PSOGI/EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Eur J Surg Oncol (2021) 47(1):36–59. doi: 10.1016/j.ejso.2020.02.011
7. Consonni D, Calvi C, De Matteis S, Mirabelli D, Landi MT, Caporaso NE, et al. Peritoneal Mesothelioma and Asbestos Exposure: A Population-Based Case-Control Study in Lombardy, Italy. Occup Environ Med (2019) 76(8):545–53. doi: 10.1136/oemed-2019-105826
8. Spirtas R, Heineman EF, Bernstein L, Beebe GW, Keehn RJ, Stark A, et al. Malignant Mesothelioma: Attributable Risk of Asbestos Exposure. Occup Environ Med (1994) 51(12):804–11. doi: 10.1136/oem.51.12.804
9. Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard AC, Isaac S, Gilly FN, et al. Peritoneal Mesothelioma Treated by Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy: Results of a Prospective Study. Ann Surg Oncol (2006) 13(3):405–12. doi: 10.1245/ASO.2006.05.041
10. Dodson RF, O’Sullivan MF, Huang J, Holiday DB, Hammar SP. Asbestos in Extrapulmonary Sites: Omentum and Mesentery. Chest (2000) 117(2):486–93. doi: 10.1378/chest.117.2.486
11. de Ridder GG, Kraynie A, Pavlisko EN, Oury TD, Roggli VL. Asbestos Content of Lung Tissue in Patients With Malignant Peritoneal Mesothelioma: A Study of 42 Cases. Ultrastruct Pathol (2016) 40(3):134–41. doi: 10.3109/01913123.2016.1170085
12. Vimercati L, Cavone D, Delfino MC, Bruni B, De Maria L, Caputi A, et al. Primary Ovarian Mesothelioma: A Case Series With Electron Microscopy Examination and Review of the Literature. Cancers (Basel) (2021) 13(9):2278. doi: 10.3390/cancers13092278
13. Sekido Y. Molecular Pathogenesis of Malignant Mesothelioma. Carcinogenesis (2013) 34(7):1413–9. doi: 10.1093/carcin/bgt166
14. Malpica A, Euscher ED, Marques-Piubelli ML, Miranda RN, Fournier KF, Raghav KP, et al. Malignant Peritoneal Mesothelioma Associated With Endometriosis: A Clinicopathologic Study of 15 Cases. Int J Gynecol Pathol (2021) 41(1):59–67. doi: 10.1097/PGP.0000000000000762
15. Farioli A, Ottone M, Morganti AG, Compagnone G, Romani F, Cammelli S, et al. Radiation-Induced Mesothelioma Among Long-Term Solid Cancer Survivors: A Longitudinal Analysis of SEER Database. Cancer Med (2016) 5(5):950–9. doi: 10.1002/cam4.656
16. Cakiroglu E, Senturk S. Genomics and Functional Genomics of Malignant Pleural Mesothelioma. Int J Mol Sci (2020) 21(17):6342. doi: 10.3390/ijms21176342
17. Jaurand MC, Didier Jean CM. Asbestos and Mesothelioma: What Is Recent Advance in Research on Asbestos-Induced Molecular Carcinogenesis? Malignant Pleural Mesothelioma. Advances in Pathogenesis, Diagnosis, and Treatments (2021) 17–31. doi: 10.1007/978-981-15-9158-7_2
18. Mahfuz A, Zubair-Bin-Mahfuj AM, Podder DJ. A Network-Biology Approach for Identification of Key Genes and Pathways Involved in Malignant Peritoneal Mesothelioma. Genomics Inform (2021) 19(2):e16. doi: 10.5808/gi.21019
19. Foster JM, Radhakrishna U, Govindarajan V, Carreau JH, Gatalica Z, Sharma P, et al. Clinical Implications of Novel Activating EGFR Mutations in Malignant Peritoneal Mesothelioma. World J Surg Oncol (2010) 8:88. doi: 10.1186/1477-7819-8-88
20. Perrone F, Jocollè G, Pennati M, Deraco M, Baratti D, Brich S, et al. Receptor Tyrosine Kinase and Downstream Signalling Analysis in Diffuse Malignant Peritoneal Mesothelioma. Eur J Cancer (2010) 46(15):2837–48. doi: 10.1016/j.ejca.2010.06.130
21. Varghese S, Chen Z, Bartlett DL, Pingpank JF, Libutti SK, Steinberg SM, et al. Activation of the Phosphoinositide-3-Kinase and Mammalian Target of Rapamycin Signaling Pathways are Associated With Shortened Survival in Patients With Malignant Peritoneal Mesothelioma. Cancer (2011) 117(2):361–71. doi: 10.1002/cncr.25555
22. Kalra N, Ashai A, Xi L, Pingpank JF, Libutti SK, Steinberg SM, et al. Patients With Peritoneal Mesothelioma Lack Epidermal Growth Factor Receptor Tyrosine Kinase Mutations That Would Make Them Sensitive to Tyrosine Kinase Inhibitors. Oncol Rep (2012) 27(6):1794–800. doi: 10.3892/or.2012.1725
23. Alakus H, Yost SE, Woo B, French R, Lin GY, Jepsen K, et al. BAP1 Mutation is a Frequent Somatic Event in Peritoneal Malignant Mesothelioma. J Transl Med (2015) 13:122. doi: 10.1186/s12967-015-0485-1
24. Bozzi F, Brich S, Dagrada GP, Negri T, Conca E, Cortelazzi B, et al. Epithelioid Peritoneal Mesothelioma: A Hybrid Phenotype Within a Mesenchymal-Epithelial/Epithelial-Mesenchymal Transition Framework. Oncotarget (2016) 7(46):75503–17. doi: 10.18632/oncotarget.12262
25. Ugurluer G, Chang K, Gamez ME, Arnett AL, Jayakrishnan R, Miller RC, et al. Genome-Based Mutational Analysis by Next Generation Sequencing in Patients With Malignant Pleural and Peritoneal Mesothelioma. Anticancer Res (2016) 36(5):2331–8.
26. Desmeules P, Joubert P, Zhang L, Al-Ahmadie HA, Fletcher CD. A Subset of Malignant Mesotheliomas in Young Adults Are Associated With Recurrent EWSR1/FUS-ATF1 Fusions. Am J Surg Pathol (2017) 41(7):980–8. doi: 10.1097/PAS.0000000000000864
27. Joseph NM, Chen YY, Nasr A, Yeh I, Talevich E, Onodera C, et al. Genomic Profiling of Malignant Peritoneal Mesothelioma Reveals Recurrent Alterations in Epigenetic Regulatory Genes BAP1, SETD2, and DDX3X. Mod Pathol (2017) 30(2):246–54. doi: 10.1038/modpathol.2016.188
28. Leblay N, Leprêtre F, Le Stang N, Gautier-Stein A, Villeneuve L. BAP1 Is Altered by Copy Number Loss, Mutation, and/or Loss of Protein Expression in More Than 70% of Malignant Peritoneal Mesotheliomas. J Thorac Oncol (2017) 12(4):724–33. doi: 10.1016/j.jtho.2016.12.019
29. Hung YP, Dong F, Watkins JC, Nardi V, Bueno R, Dal Cin P, et al. Identification of ALK Rearrangements in Malignant Peritoneal Mesothelioma. JAMA Oncol (2018) 4(2):235–8. doi: 10.1001/jamaoncol.2017.2918
30. Serio G, Vimercati L, Pennella A, Gentile M, Cavone D, Buonadonna AL, et al. Genomic Changes of Chromosomes 8p23.1 and 1q21: Novel Mutations in Malignant Mesothelioma. Lung Cancer (2018) 126:106–11. doi: 10.1016/j.lungcan.2018.10.012
31. Panou V, Gadiraju M, Wolin A, Weipert CM, Skarda E, Husain AN, et al. Frequency of Germline Mutations in Cancer Susceptibility Genes in Malignant Mesothelioma. J Clin Oncol (2018) 36(28):2863–71. doi: 10.1200/JCO.2018.78.5204
32. Belfiore A, Busico A, Bozzi F, Brich S, Dallera E, Conca E, et al. Molecular Signatures for Combined Targeted Treatments in Diffuse Malignant Peritoneal Mesothelioma. Int J Mol Sci (2019) 20(22):5817. doi: 10.3390/ijms20225817
33. Shrestha R, Nabavi N, Lin YY, Mo F, Anderson S, Volik S, et al. BAP1 Haploinsufficiency Predicts a Distinct Immunogenic Class of Malignant Peritoneal Mesothelioma. Genome Med (2019) 11(1):8. doi: 10.1186/s13073-019-0620-3
34. Sciarrillo R, Wojtuszkiewicz A, El Hassouni B, Funel N, Gandellini P, Lagerweij T, et al. Splicing Modulation as Novel Therapeutic Strategy Against Diffuse Malignant Peritoneal Mesothelioma. EBioMed (2019) 39:215–25. doi: 10.1016/j.ebiom.2018.12.025
35. Stevers M, Rabban JT, Garg K, Van Ziffle J, Onodera C, Grenert JP, et al. Well-Differentiated Papillary Mesothelioma of the Peritoneum Is Genetically Defined by Mutually Exclusive Mutations in TRAF7 and CDC42. Mod Pathol (2019) 32(1):88–99. doi: 10.1038/s41379-018-0127-2
36. Brich S, Bozzi F, Perrone F, Tamborini E, Cabras AD, Deraco M, et al. Fluorescence in Situ Hybridization (FISH) Provides Estimates of Minute and Interstitial BAP1, CDKN2A, and NF2 Gene Deletions in Peritoneal Mesothelioma. Mod Pathol (2020) 33(2):217–27. doi: 10.1038/s41379-019-0371-0
37. Pezzuto F, Serio G, Fortarezza F, Scattone A, Caporusso C, Punzi A, et al. Prognostic Value of Ki67 Percentage, WT-1 Expression and P16/CDKN2A Deletion in Diffuse Malignant Peritoneal Mesothelioma: A Single-Centre Cohort Study. Diagnostics (Basel) (2020) 10(6):386. doi: 10.3390/diagnostics10060386
38. Hung YP, Dong F, Torre M, Crum CP, Bueno R, Chirieac LR. Molecular Characterization of Diffuse Malignant Peritoneal Mesothelioma. Mod Pathol (2020) 33(11):2269–79. doi: 10.1038/s41379-020-0588-y
39. Hama R, Watanabe Y, Shinada K, Yamada Y, Ogata Y, Yoshida Y, et al. Characterization of DNA Hypermethylation in Two Cases of Peritoneal Mesothelioma. Tumour Biol (2012) 33(6):2031–40. doi: 10.1007/s13277-012-0462-8
40. Chao A, Lai CH, Lee YS, Ueng SH, Lin CY, Wang TH. Molecular Characteristics of Endometrial Cancer Coexisting With Peritoneal Malignant Mesothelioma in Li-Fraumeni-Like Syndrome. BMC Cancer (2015) 15:8. doi: 10.1186/s12885-015-1010-x
41. Sheffield BS, Tinker AV, Shen Y, Hwang H, Li-Chang HH, Pleasance E, et al. Personalized Oncogenomics: Clinical Experience With Malignant Peritoneal Mesothelioma Using Whole Genome Sequencing. PloS One (2015) 10(3):e0119689. doi: 10.1371/journal.pone.0119689
42. Lai J, Zhou Z, Tang XJ, Gao ZB, Zhou J, Chen SQ. A Tumor-Specific Neo-Antigen Caused by a Frameshift Mutation in BAP1 Is a Potential Personalized Biomarker in Malignant Peritoneal Mesothelioma. Int J Mol Sci (2016) 17(5):739. doi: 10.3390/ijms17050739
43. Vanni I, Coco S, Bonfiglio S, Cittaro D, Genova C, Biello F, et al. Whole Exome Sequencing of Independent Lung Adenocarcinoma, Lung Squamous Cell Carcinoma, and Malignant Peritoneal Mesothelioma: A Case Report. Med (Baltimore) (2016) 95(48):e5447. doi: 10.1097/MD.0000000000005447
44. Loharamtaweethong K, Puripat N, Aoonjai N, Sutepvarnon A, Bandidwattanawong C. Anaplastic Lymphoma Kinase (ALK) Translocation in Paediatric Malignant Peritoneal Mesothelioma: A Case Report of Novel ALK-Related Tumour Spectrum. Histopathol (2016) 68(4):603–7. doi: 10.1111/his.12779
45. Serio G, Pezzuto F, Marzullo A, Scattone A, Cavone D, Punzi A, et al. Peritoneal Mesothelioma With Residential Asbestos Exposure. Report of a Case With Long Survival (Seventeen Years) Analyzed by Cgh-Array. Int J Mol Sci (2017) 18(8):1818. doi: 10.3390/ijms18081818
46. Löffler MW, Steinhilber J, Hilke FJ, Haen SP, Bösmüller H, Montes-Mojarro IA, et al. First Case Report of Malignant Peritoneal Mesothelioma and Oral Verrucous Carcinoma in a Patient With a Germline PTEN Mutation: A Combination of Extremely Rare Diseases With Probable Further Implications. BMC Med Genet (2018) 19(1):144. doi: 10.1186/s12881-018-0651-4
47. Lund-Andersen C, Nakken S, Nygård S, Fromm B, Aasheim LB, Davidson B, et al. Integrative Genomic Analysis of Peritoneal Malignant Mesothelioma: Understanding a Case With Extraordinary Chemotherapy Response. Cold Spring Harb Mol Case Stud (2019) 5(2):a003566. doi: 10.1101/mcs.a003566
48. Rüschoff JH, Gradhand E, Kahraman A, Rees H, Ferguson JL, Curioni-Fontecedro A, et al. STRN-ALK Rearranged Malignant Peritoneal Mesothelioma With Dramatic Response Following Ceritinib Treatment. JCO Precis Oncol (2019) 3:PO.19.00048. doi: 10.1200/PO.19.00048
49. Smith-Hannah A, Naous R. Primary Peritoneal Epithelioid Mesothelioma of Clear Cell Type With a Novel VHL Gene Mutation: A Case Report. Hum Pathol (2019) 83:199–203. doi: 10.1016/j.humpath.2018.07.033
50. Glass C, Sholl LM, Landgraf JR, Chirieac L, Roggli VL. Molecular Analysis of a Patient With Neurofibromatosis 2 (NF2) and Peritoneal Malignant Mesothelioma. Am J Surg Pathol (2020) 44(2):288–92. doi: 10.1097/PAS.0000000000001359
51. Miyagawa C, Takaya H, Sakai K, Nishio K, Konishi M, Minamiguchi S, et al. A Novel Malignant Peritoneal Mesothelioma With STRN Exon 2 and ALK Exon 20: A Case Report and Literature Review. Oncologist (2021) 26(5):356–61. doi: 10.1002/onco.13714
52. Stallone G, Infante B, Cormio L, Macarini L, Grandaliano G. Rapamycin Treatment for Benign Multicystic Peritoneal Mesothelioma: A Rare Disease With a Difficult Management. Am J Case Rep (2017) 18:632–6. doi: 10.12659/AJCR.903548
53. Dolly SO, Migali C, Tunariu N, Della-Pepa C, Khakoo S, Hazell S, et al. Indolent Peritoneal Mesothelioma: PI3K-mTOR Inhibitors as a Novel Therapeutic Strategy. ESMO Open (2017) 2(1):e000101. doi: 10.1136/esmoopen-2016-000101
54. Panagopoulos I, Thorsen J, Gorunova L, Micci F, Haugom L, Davidson B, et al. RNA Sequencing Identifies Fusion of the EWSR1 and YY1 Genes in Mesothelioma With T(14;22)(Q32;Q12). Genes Chromosomes Cancer (2013) 52(8):733–40. doi: 10.1002/gcc.22068
55. Singhi AD, Krasinskas AM, Choudry HA, Bartlett DL, Pingpank JF, Zeh HJ, et al. The Prognostic Significance of BAP1, NF2, and CDKN2A in Malignant Peritoneal Mesothelioma. Mod Pathol (2016) 29(1):14–24. doi: 10.1038/modpathol.2015.121
56. Borczuk AC, Pei J, Taub RN, Levy B, Nahum O, Chen J, et al. Genome-Wide Analysis of Abdominal and Pleural Malignant Mesothelioma With DNA Arrays Reveals Both Common and Distinct Regions of Copy Number Alteration. Cancer Biol Ther (2016) 17(3):328–35. doi: 10.1080/15384047.2016.1145850
57. Serio G, Pagliarulo V, Marzullo, Punzi A, Pezzuto F, Gentile M, et al. Case Report Molecular Changes of Malignant Mesothelioma in the Testis and Their Impact on Prognosis: Analyses of Two Cases. Int J Clin Exp Pathol (2016) 9(7):7658–67.
58. Pezzuto F, Fortarezza F, Lunardi F, Calabrese F. Are There Any Theranostic Biomarkers in Small Cell Lung Carcinoma? J Thorac Dis (2019) 11(Suppl 1):S102–12. doi: 10.21037/jtd.2018.12.14
59. Calabrese F, Lunardi F, Pezzuto F, Fortarezza F, Vuljan SE, Marquette C, et al. Are There New Biomarkers in Tissue and Liquid Biopsies for the Early Detection of Non-Small Cell Lung Cancer? J Clin Med (2019) 8(3):414. doi: 10.3390/jcm8030414
60. Pezzuto F, Lunardi F, Vedovelli L, Fortarezza F, Urso L, Grosso F, et al. P14/ARF-Positive Malignant Pleural Mesothelioma: A Phenotype With Distinct Immune Microenvironment. Front Oncol (2021) 11:653497. doi: 10.3389/fonc.2021.653497
61. Pasello G, Zago G, Lunardi F, Urso L, Kern I, Vlacic G, et al. Malignant Pleural Mesothelioma Immune Microenvironment and Checkpoint Expression: Correlation With Clinical-Pathological Features and Intratumor Heterogeneity Over Time. Ann Oncol (2018) 29(5):1258–65. doi: 10.1093/annonc/mdy086
62. White MG, Schulte JJ, Xue L, Berger Y, Schuitevoerder D, Vining CC, et al. Heterogeneity in PD-L1 Expression in Malignant Peritoneal Mesothelioma With Systemic or Intraperitoneal Chemotherapy. Br J Cancer (2021) 124(3):564–6. doi: 10.1038/s41416-020-01130-x
63. Fennell DA, King A, Mohammed S, Branson A, Brookes C, Darlison L, et al. Rucaparib in Patients With BAP1-Deficient or BRCA1-Deficient Mesothelioma (MiST1): An Open-Label, Single-Arm, Phase 2a Clinical Trial. Lancet Respir Med (2021) 9(6):593–600. doi: 10.1016/S2213-2600(20)30390-8
64. Glass Zauderer M, Wojciech Szlosarek P, Le Moulec S, Popat S, Taylor P, Planchard D, et al. Safety and Efficacy of Tazemetostat, an Enhancer of Zeste-Homolog 2 Inhibitor, in Patients With Relapsed or Refractory Malignant Mesothelioma. J Clin Onc (2020) 38:15_suppl:9058–8. doi: 10.1200/JCO.2020.38.15_suppl.9058
65. Fennell DA, King A, Mohammed S, Branson A, Greystoke A, Moody S, et al. A Phase II Trial of Abemaciclib in Patients With P16ink4a Negative, Relapsed Mesothelioma. J Clin Onc (2021) 39(15_suppl):8558–8. doi: 10.1200/JCO.2021.39.15_suppl.8558
66. Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, et al. YAP Induces Malignant Mesothelioma Cell Proliferation by Upregulating Transcription of Cell Cycle-Promoting Genes. Oncogene (2012) 31(49):5117–22. doi: 10.1038/onc.2012.5
67. Hassan R, Alewine C, Mian I, Spreafico A, Siu LL, Gomez-Roca C, et al. Phase 1 Study of the Immunotoxin LMB-100 in Patients With Mesothelioma and Other Solid Tumors Expressing Mesothelin. Cancer (2020) 126(22):4936–47. doi: 10.1002/cncr.33145
68. Hassan R, Sharon E, Thomas A, Zhang J, Ling A, Miettinen M, et al. Phase 1 Study of the Antimesothelin Immunotoxin SS1P in Combination With Pemetrexed and Cisplatin for Front-Line Therapy of Pleural Mesothelioma and Correlation of Tumor Response With Serum Mesothelin, Megakaryocyte Potentiating Factor, and Cancer Antigen 125. Cancer (2014) 120(21):3311–9. doi: 10.1002/cncr.28875
69. Hassan R, Blumenschein GR Jr, Moore KN, Santin AD, Kindler HL, Nemunaitis JJ, et al. First-In-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody-Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J Clin Oncol (2020) 38(16):1824–35. doi: 10.1200/JCO.19.02085
Keywords: malignant mesothelioma, peritoneal mesothelioma, molecular pathways, prognostic factors, asbestos
Citation: Fortarezza F, Pezzuto F, Marzullo A, Cavone D, Romano DE, d’Amati A, Serio G and Vimercati L (2022) Molecular Pathways in Peritoneal Mesothelioma: A Minireview of New Insights. Front. Oncol. 12:823839. doi: 10.3389/fonc.2022.823839
Received: 28 November 2021; Accepted: 17 January 2022;
Published: 10 February 2022.
Edited by:
Dario Palmieri, The Ohio State University, United StatesReviewed by:
Sabahattin Cömertpay, Kahramanmaras Sütçü Imam University, TurkeyCopyright © 2022 Fortarezza, Pezzuto, Marzullo, Cavone, Romano, d’Amati, Serio and Vimercati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Fortarezza, ZnJhbmNlc2NvLmZvcnRhcmV6emFAdW5pcGQuaXQ=; ZnJhbmNlc2NvZm9ydGFyZXp6YS5tZEBnbWFpbC5jb20=
†These authors share first authorship
 Francesco Fortarezza
Francesco Fortarezza Federica Pezzuto
Federica Pezzuto Andrea Marzullo
Andrea Marzullo Domenica Cavone
Domenica Cavone Daniele Egidio Romano
Daniele Egidio Romano Antonio d’Amati
Antonio d’Amati Gabriella Serio
Gabriella Serio Luigi Vimercati
Luigi Vimercati