
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 08 December 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.821626
This article is part of the Research Topic Clinical and Basic Research of Radiotherapy for Esophageal Cancer View all 20 articles
Objective: To systematically evaluate the safety and adverse event profiles of immune checkpoint inhibitors (ICIs) in patients with esophageal cancer (EPC) or gastroesophageal junction cancer (GEJC).
Methods: PubMed, Web of Science, Cochrane Library, and major conference proceedings were systematically searched for all phase II or phase III randomized controlled trials (RCTs) in EPC or GEJC using ICIs. Safety outcomes including treatment-related adverse events (trAEs), immune-related adverse events (irAEs), and serious trAEs were evaluated by network meta-analysis or dichotomous meta-analysis based on the random-effects model.
Results: Eleven RCTs involving EPC (five RCTs) and GEJC (six RCTs) were included in the final meta-analysis. NMA showed that placebo was associated with the best safety ranking for grade 3–5 trAEs (SUCRA = 96.0%), followed by avelumab (78.6%), nivolumab (73.9%), ipilimumab (57.0%), and pembrolizumab (56.6%). Conventional pairwise meta-analysis (CPM) showed that ICIs have similar grade 3–5 trAE risk compared with chemotherapy (RR = 0.764, 95% CI: 0.574 to 1.016, I2 = 95.7%, Z = 1.85, P = 0.065). NMA showed that the general safety of grade 3–5 irAEs ranked from high to low is as follows: ChT (85.1%), placebo (76.5%), ipilimumab (56.0%), nivolumab (48.5%), avelumab (48.4%), camrelizumab (41.8%), pembrolizumab (36.4%), and nivolumab + ipilimumab (21.6%). CPM showed that the rates of grade 3–5 irAEs in the ICI group and the chemotherapy group were 7.35% (154/2,095, 95% CI: [6.23%, 8.47%]) versus 2.25% (42/1,869, 95% CI: [1.58%, 2.92%]), with statistical significance (RR = 3.151, 95% CI = 2.175 to 4.563, Z = 6.07, P = 0.000). The most common irAEs in the ICI group were skin reaction (15.76%, 95% CI: [13.67%, 17.84%]), followed by hypothyroidism (9.73%, 95% CI: [8.07%, 11.39%]), infusion-related reactions (5.93%, 95% CI: [4.29%, 7.58%]), hepatitis (5.25%, 95% CI: [4.28%, 6.22%]), and pneumonitis (4.45%, 95% CI: [3.5%, 5.4%]).
Conclusion: Different ICIs had different toxicity manifestations and should not be considered as an entity. Compared with chemotherapy, ICIs were more prone to irAEs, but the overall rates remained low and acceptable. For clinicians, it is important to recognize and monitor the adverse events caused by ICIs for patients with EPC or GEJC.
Worldwide, esophageal cancer (EPC) still remains one of the most commonly diagnosed cancers and the leading cause of cancer-related death (1), particularly with the highest rates and mortality occurring in China (2). According to the GLOBOCAN report, an estimated 604,100 new cases were diagnosed in 2020 globally, among which Chinese cases accounted for 53.5%, which was up to 324,000 cases (3). An estimated 544,000 new deaths occurred in 2020 globally, while Chinese cases accounted for 55.3%, which was up to 301,000 cases (3). Patients with EPC are most commonly diagnosed with locally advanced cancer stages, and more than 50.4% of cases suffered from distant metastases and irreversible diseases at the time of diagnosis, which led to a frustrating overall 5-year survival rate of less than 20% (4). Generally, fluoropyrimidine and platinum-based regimens are recommended and accepted as standard first-line treatment regimen (5). Although chemotherapy has improved the overall 5-year survival rate to a certain extent, the prognosis of esophageal cancer is still poor (6). In particular, after first-line chemotherapy, there is no accepted and satisfactory standard treatment for advanced or metastatic esophageal cancer (7).
In recent years, cancer immunotherapies based on immune checkpoint inhibitors (ICIs) have become the fifth largest tumor treatment after surgery, chemotherapy, radiotherapy, and small molecules targeted therapy in oncology and have revolutionized the treatment landscape and made major breakthroughs in the treatment of tumors, especially for advanced or metastatic cancer (8). Clinical applications or trials on ICIs had been carried out in the field of various types of tumors, and more and more cancer patients had benefited from this innovative treatment (9). Compared with that of the four existing traditional treatment regimens, the scope of application of cancer immunotherapies is appropriately enlarged, and the number of patients receiving immunotherapies is increasing (10). ICIs directly and selectively killed cancer cells through immunogenic cell death by activating the immune system of cancer patients (11). ICIs had improved the survival rate of many refractory tumors and the quality of life of patients with advanced cancer (12). However, the emergence of a new treatment model and drugs is also accompanied by the emergence of new medication regimens and adverse reactions (11). Although ICIs have shown significant clinical benefits in improving the survival prognosis for most cancer patients, immune-related adverse events (irAEs) that affect body organs are one of the major hindrances when these drugs are applied (13). Although a large number of studies have confirmed the efficacy and safety of ICIs in esophageal cancer, there is a lack of direct head-to-head comparison of evidence for different types of ICIs (9, 14). Therefore, it is not clear if different ICIs have different toxicity profiles in the immunotherapy of esophageal cancer (15). Generally speaking, it is difficult to carry out special randomized controlled trials to compare the differences in the adverse event spectrum of different ICIs, because the occurrence of these adverse events is difficult to predict, and the rates of grade 3–5 adverse events are very low (8). Therefore, meta-analysis is an effective research method for studies focusing on adverse events of ICIs. Network meta-analysis (NMA) may be applied to integrate all available evidence from phase II or phase III RCTs to get direct or indirect comparisons of different ICIs, especially when head-to-head RCTs among regimens are lacking (16). To the best of our knowledge, no NMA of ICI regimens that explored the spectrum of adverse events of immunotherapy is available yet in advanced esophageal or gastroesophageal junction cancer. Therefore, we conducted a systematic review to investigate the safety and adverse event profiles of ICIs for advanced esophageal or gastroesophageal junction cancer using NMA.
The current study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (17), and the quality control and quality assurance (QC and QA) of the manuscript were instructed by the corresponding authors (JL and JZ).
Relevant clinical trials published in various databases such as PubMed, Web of Science, and Cochrane Library were searched. Major conference proceedings including the Clinicaltrial.gov, American Society of Clinical Oncology (ASCO), and European Society for Medical Oncology (ESMO) databases were also searched for recent conference abstracts.
Relevant search terms relating to the present study were composed of various combinations of the medical subject headings (MeSH) and free-text terms. Search terms were combined by the Boolean operator “AND” or “OR” if necessary. A PubMed search was conducted using the following search terms: 1) search terms related to disease were “esophageal neoplasm,” “esophagus cancer,” “esophageal cancer,” “gastro-oesophageal junction cancer,” “gastro-esophageal junction cancer,” “cancer of the gastroesophageal junction,” “adenocarcinoma of the esophagus and gastroesophageal junction,” etc. (2) Search terms related to drugs or immunotherapy were “ipilimumab,” “pembrolizumab,” “nivolumab,” “atezolizumab,” “durvalumab,” “camrelizumab,” and other ICIs. The trade name of the drug includes “Yervoy,” “Keytruda,” “Opdivo,” “Tecentriq,” “Imfinzi,” etc. 3) Other search terms included “anti-CTLA-4 mab,” “anti-PDL1,” “anti-PD1,” “PD1 receptor,” “programmed cell death 1 protein,” “PD-1,” “PD-L1,” etc.
The selection criteria for clinical trials were organized according to the guidelines of the participants, interventions, comparisons, outcomes, and study design (PICOS) recommended by the Cochrane Collaboration. The inclusion criteria were as follows: i) the included patients were all pathologically diagnosed esophageal cancer or gastroesophageal junction cancer (GEJC) patients (P); ii) interventions of concern referred to immunotherapy with ICIs alone or in combination with chemotherapy (I); iii) controlled treatment regimens included chemotherapy alone (ChT) or best supportive treatment (BST), but there were no restrictions related to the chemotherapy regimens and chemotherapy cycles (C); iv) five safety outcomes included rates of treatment-related adverse events (trAEs), immune-related adverse events (irAEs), death, discontinuation of therapy, and grades 3–5 organ-special adverse events (O); and v) all randomized, open-label, controlled clinical trials with efficacy and safety data of ICIs were included. Although priority was given to phase III clinical trials, phase II clinical trials with a control group would be also included.
The exclusion criteria were as follows: i) phase I clinical trials and non-RCT studies, ii) participants with other tumors, iii) case reports and reviews, iv) incomplete data or non-original research, and v) repeated publications.
Articles were only included if they were published in English, but there was no restriction related to publication year. Two researchers (JZ and BH) were assigned to independently review all the data. If there were repeated articles in the selected clinical trials, only the latest published articles will be used for the final analysis. After the discussion according to the inclusion criteria and reaching a consensus, a decision was made to finally include or exclude the eligible articles. If a consensus cannot be reached, the corresponding author (JL) of this article is responsible for the final ruling.
After reading the full text, two researchers (JZ and Tingting Li) extracted and cross-checked the data, including the following: 1) basic information: such as the title of the trial, author’s name, year of publication, source of literature, etc.; 2) methodological information of the trial: the sample size of the study included, the basic information of the study population, including the entry time and number of participants, disease stages, etc.; the randomization method of the trial, the evaluation method of important outcome indicators; median follow-up duration, death, and withdrawal, etc.; 3) detailed information on intervention measures: ICI medication, medication in the control group, etc.; and 4) detailed information for safety outcome indicators mentioned above. Disagreements were resolved by consensus.
Two independent researchers evaluated the included RCTs according to the bias risk assessment method recommended by the Cochrane Assistance Network. The evaluation methodological criteria and items were as follows: 1) generation of random allocation sequence, 2) the method of allocation concealment, 3) the method of blinding the patient, 4) the method of blinding the doctor or the therapist, 5) the method of blinding the data collection and analysis personnel, 6) the incomplete data reported, 7) selective reporting bias, and 8) other potential bias affecting authenticity.
We evaluate the risk of bias for each RCT according to the following criteria: “Yes” indicates a low risk of bias, “No” indicates a high risk of bias, and “Unclear” indicates that the literature does not provide sufficient information for bias assessment. The two researchers discussed according to the above standards and methods and, if necessary, reached a consensus according to the opinions of the third researcher.
Adverse events including trAEs and irAEs were evaluated from two different perspectives: overview and detail. An overview analysis involved all kinds of AEs observed in ≥ grade 3–5 or all grade of the study population, and a detailed analysis involved some prespecified AEs of interest observed in ≥ grade 3–5 or all grades of the study population. The detailed information of related safety was extracted from the original literature and recorded as the number of events reported and no events for each specific treatment, respectively. If enough data were available to achieve network meta-analysis, a random-effects NMA was conducted in the frequency framework, using the command of “network” in Stata 16.0. Direct or indirect safety effects were combined into some summary statistics, that is, risk ratios (RRs) and 95% credibility intervals, to quantify the effect of adverse events in the network meta-analysis. Risk ratios less than 1 represented a beneficial effect favoring the ICI group. Two-sided P <0.05 indicated that the comparison was statistically significant. If the data were unavailable for the NMA, a conventional pairwise meta-analysis based on the random-effects model or the fixed-effects model was conducted depending on the size of heterogeneity. In this case, the outcome of interest may be grouped by whether ICIs were given. Heterogeneity was assessed using the chi-square test and I2 statistics. I2 ≥50% indicated obvious heterogeneity, and a random-effects model should be applied for pooled analysis. The classic half-integer continuity correction, that is adding 0.5 to each cell, was used in the data preprocessing stage if zero adverse events in any arm were reported.
The pooled rates of grade 3–5 or all-grade adverse events for treatments were meta-analyzed by the command of “metan” in STATA 16.0. Subgroup analyses for RRs between the ICI-treated group and the control group were performed based on the panoramic analysis, and prespecified, exploratory stratification factors for subgroup analyses involved the phase of the study (phase II versus phase III), treatment lines (first line, second line, and third line), ICI drug type (anti-PD-1, anti-CTLA-4, anti-PD-L1), treatment mode (ICIs alone versus ICIs combined with ChT), sample size (<500 versus ≥500), etc.
In the literature retrieval stage, a total of 459 articles were obtained through preliminary screening. After reading the titles and abstracts, 422 articles including duplicate reports, irrelevant articles, non-randomized controlled trials, review articles, and phase I trials were excluded. The remaining 20 articles were excluded based on the selection criteria after reading the full text. Finally, a total of 11 trials reported in 17 articles met the inclusion criteria, of which 6 articles were updated or subgroup reports (18–30). Therefore, 11 articles were included in the meta-analysis, all of which were published in English (18, 20–25, 27–30). Updated reports or subgroup reports included three clinical trials, which were ATTRACTION−3 (19), ATTRACTION-4 (26), and ATTRACTION-2 (31–34).
Figure 1 shows the flowchart of the study selection and design procedure. The baseline characteristics of the 11 studies are summarized and shown in Table 1. All 11 studies included 7,089 patients, and the number of analysis population for AEs was 6,992. Most patients included came from an international multicenter. The cancer types included in the study were esophageal carcinoma (18, 20–23) and gastroesophageal junction cancer (24, 25, 27–30). Only one trial was a phase II study (28). First-line ICIs were applied in four clinical trials (22–25), second-line ICIs were applied in five clinical trials (18, 20, 21, 27, 28), and third-line ICIs were applied in two clinical trials (29, 30). Monotherapy with ICIs was found in six clinical trials (18, 20, 21, 27–30). In particular, anti-PD-L1 ICIs were used in only one trial (29), and anti-CTLA-4 ICIs were used in only two trials (23, 28).
The risk of bias assessment for the included studies involving the 11 articles is summarized and shown in Figures 2A, B. Four clinical trials were judged to be at high risk of bias mainly due to incomplete outcome data for major results of irAEs (18, 23–25). One clinical trial was judged to be at unclear risk of bias because of the lack of total results of grade 3–5 irAEs (21). The remaining studies had a low risk of bias and can be considered high quality.
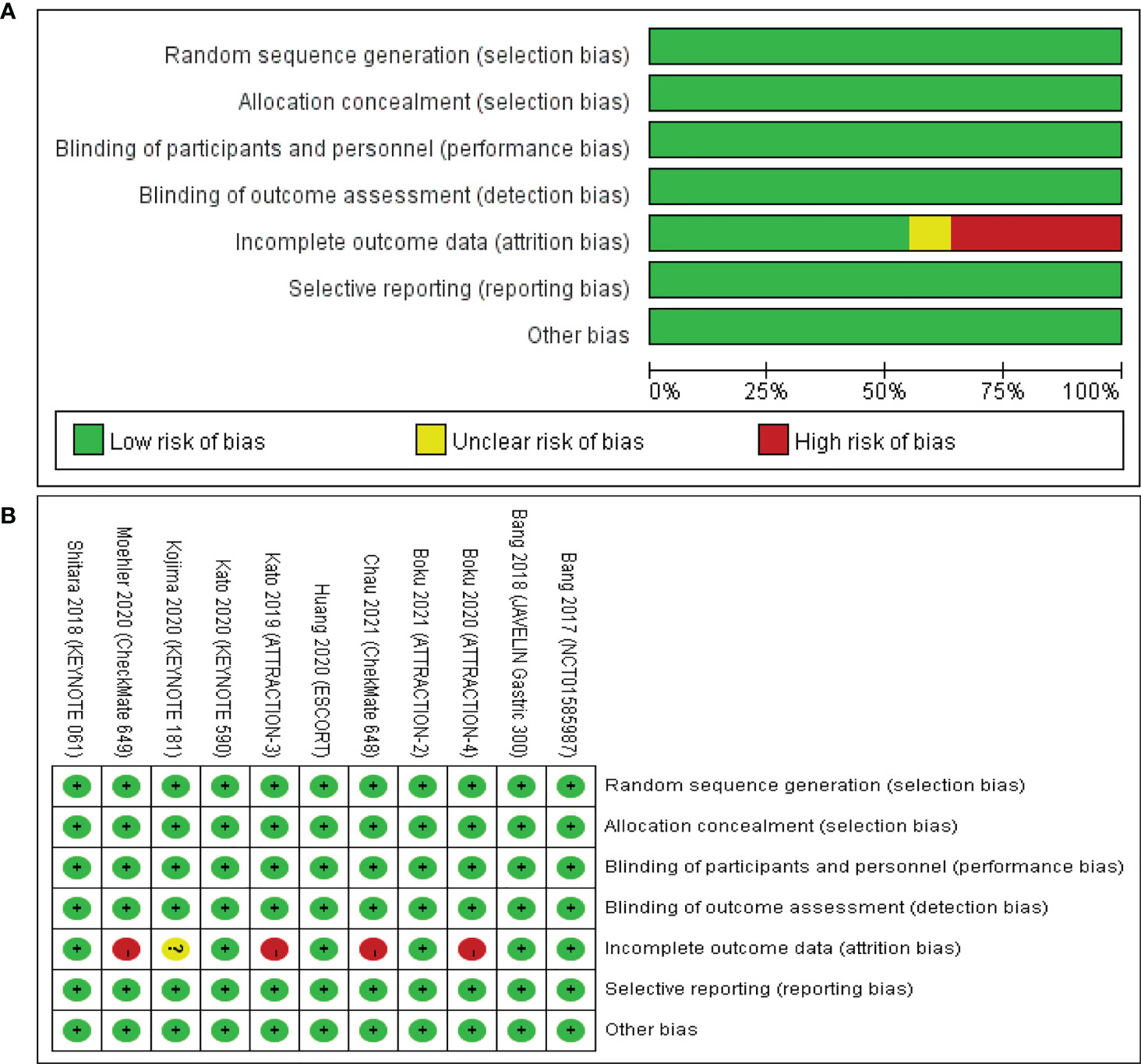
Figure 2 Risk of bias assessment for the included studies. Green for low risk of bias, yellow for unclear risk of bias, and red for high risk of bias. (A) The risk of bias graph shows an overall risk of bias for each item. (B) The risk of bias summary shows the detailed risk of bias of each item for each study.
Only one trial had not reported the results of grade 3–5 trAE (25). Therefore, data from the other studies can be successfully applied to implement NMA. A total of 3,005 patients (nine trials) were assigned to ChT therapy, 184 patients (one trial) to avelumab therapy, 228 patients (one trial) to camrelizumab therapy, 57 patients (one trial) to ipilimumab therapy, 539 patients (two trials) to nivolumab therapy, 1,461 patients (three trials) to nivolumab plus ChT therapy, 322 patients (one trial) to nivolumab + ipilimumab + ChT therapy, 314 patients (one trial) to pembrolizumab therapy, 664 patients (one trial) to pembrolizumab plus ChT therapy, and 218 patients (two trials) to placebo therapy. The network plot is shown in Figures 3A, B.
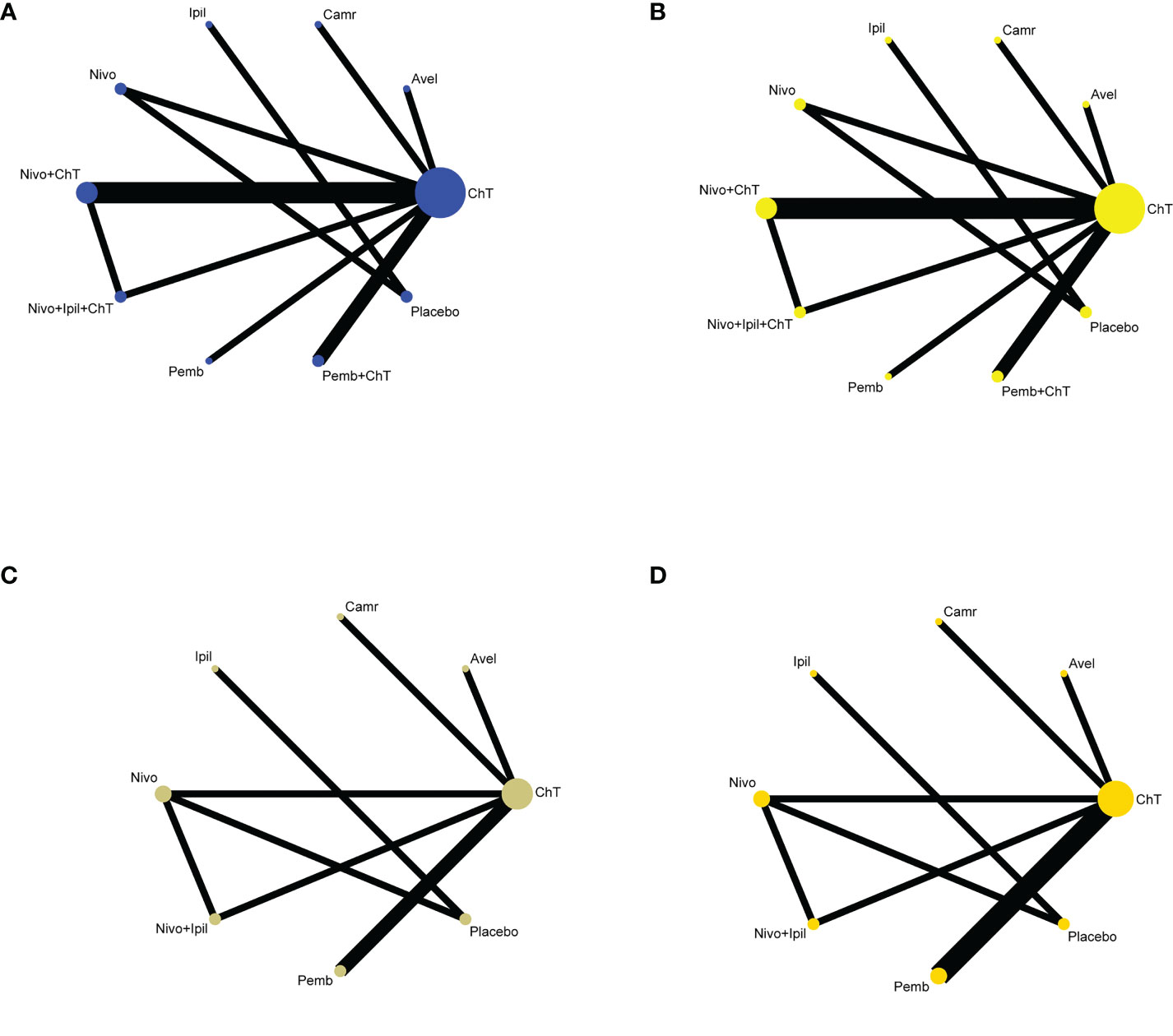
Figure 3 Network plots of comparisons with (A) grade 3–5 trAEs, (B) all-grade trAEs, (C) grade 3–5 irAEs, and (D) all-grade irAEs based on the network meta-analyses. ChT, chemotherapy; Avel, avelumab; Camr, camrelizumab; Ipil, ipilimumab; Nivo, nivolumab; Pemb, pembrolizumab.
In the consistency model, for the rates of grade 3–5 trAEs, the results with significant benefits for different pairwise comparisons could be found in avelumab versus ChT, nivolumab versus ChT, pembrolizumab versus ChT, placebo versus ChT, placebo versus nivolumab + ChT, and placebo versus pembrolizumab + ChT. The results with significant increasing risk could be found in nivolumab + ChT versus avelumab, nivolumab + ipilimumab + ChT versus avelumab, pembrolizumab + ChT versus avelumab, nivolumab + ChT versus camrelizumab, nivolumab + ChT versus nivolumab, and nivolumab + ipilimumab + ChT versus nivolumab.
For the rates of all-grade trAEs, the results with significant benefits for different pairwise comparisons could be found in ipilimumab versus ChT, placebo versus ChT, ipilimumab versus avelumab, placebo versus avelumab, ipilimumab versus camrelizumab, placebo versus camrelizumab, placebo versus nivolumab, placebo versus nivolumab + ChT, placebo versus nivolumab + ipilimumab + ChT, placebo versus pembrolizumab, and placebo versus pembrolizumab + ChT. The results with significant increasing risk could be found in nivolumab versus ipilimumab, nivolumab + ChT versus ipilimumab, nivolumab + ipilimumab + ChT versus ipilimumab, pembrolizumab versus ipilimumab, and pembrolizumab + ChT versus ipilimumab. The details of all comparisons are indicated in Figure 4A. The ranking of benefits for different treatment regimens was assessed by the surface under the cumulative ranking curves (SUCRAs) and is shown in Figure 4B.
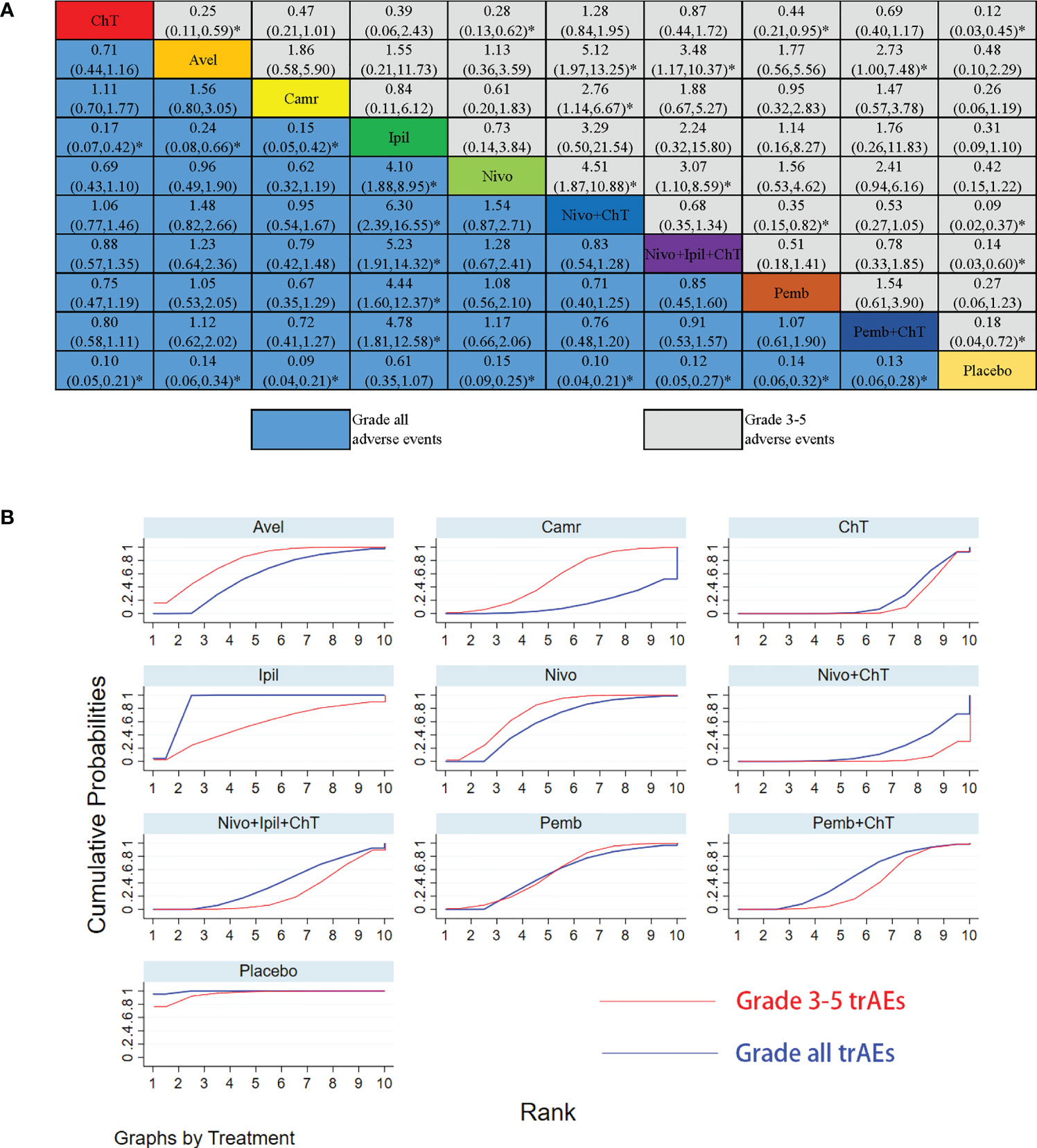
Figure 4 Results of the network meta-analysis for 10 treatment regimens in terms of treatment-related adverse events(trAEs) with grade 3–5 trAEs and all-grade trAEs. (A) League table for different treatment regimens. Relative effects (RRs) with 95% confidence intervals are shown for different treatment regimens compared with each other. The RR for a given comparison could be read in the intersection of two treatments. All Z-tests to compare two treatments were performed two-sided. *P < 0.05. ChT, chemotherapy; Avel, avelumab; Camr, camrelizumab; Ipil, ipilimumab; Nivo, nivolumab; Pemb, pembrolizumab. Placebo also involves the best supportive care. (B) The surface under the cumulative ranking curves (SUCRAs) for grade 3–5 trAEs and all-grade trAEs.
As shown in Figure 4B, placebo was associated with the best safety ranking for grade 3–5 trAEs (SUCRA = 96.0%), followed by avelumab (78.6%), nivolumab (73.9%), ipilimumab (57.0%), and pembrolizumab (56.6%); placebo was associated with the best safety ranking for all-grade trAEs (99.5%), followed by ipilimumab (89.3%), nivolumab (60.1%), avelumab (56.8%), and pembrolizumab (53.5%). The relevant SUCRA values for the different treatments are detailed in Supplementary Material Table S1. Forest plots for pairwise comparisons of all individual regimens and their combinations are shown in Figures 5A, B.
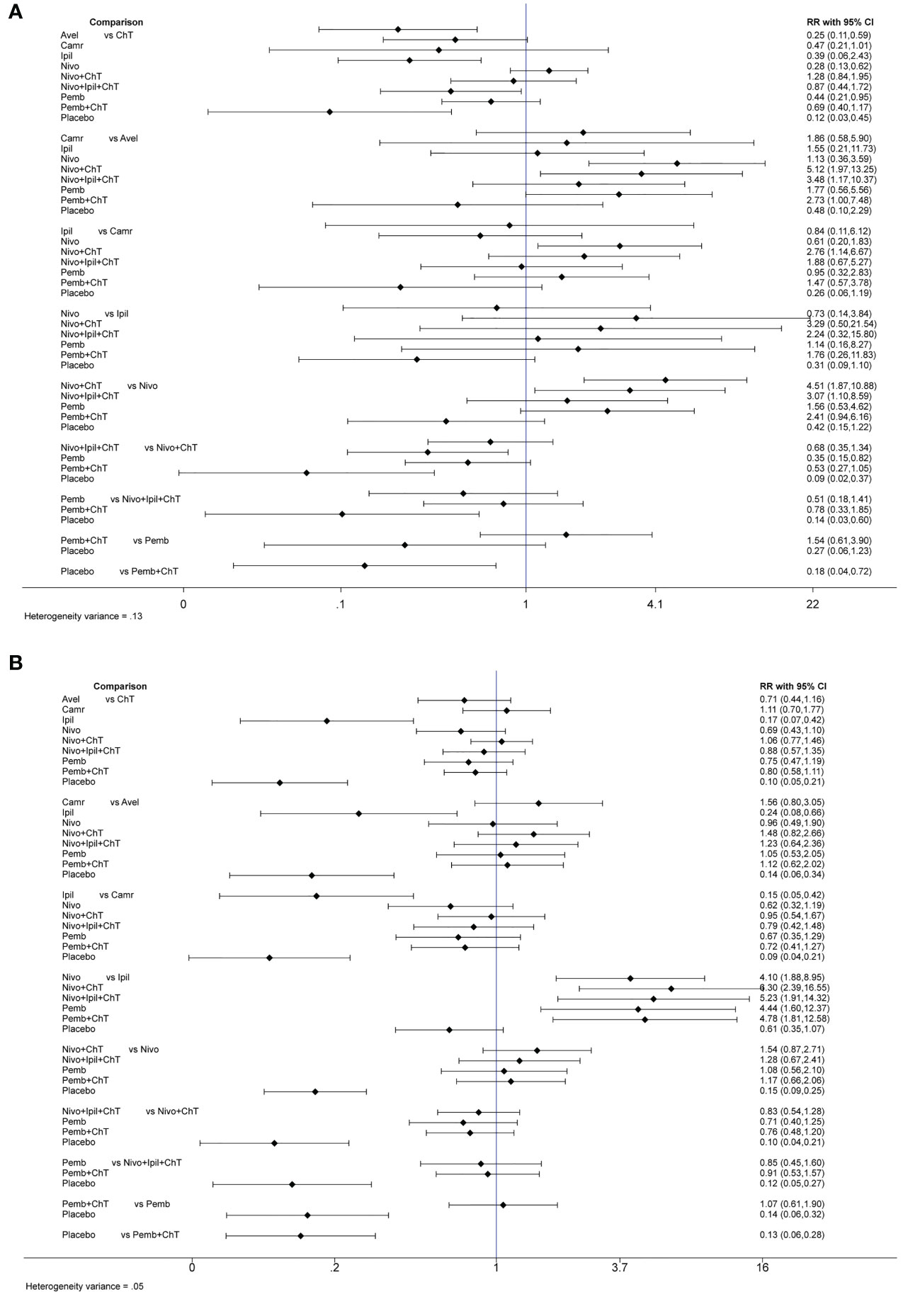
Figure 5 Forest plots for pairwise comparisons of all individual regimens with each other with (A) forest plots for grade 3–5 trAEs and (B) forest plots for all-grade trAEs.
For the rates of grade 1–2 trAEs, nivolumab + ChT was associated with the best safety ranking for grade 1–2 trAEs (88.2%), followed by pembrolizumab + ChT (85.1%), nivolumab + ipilimumab + ChT (82.4%), ChT (53.5%), and placebo (53.3%). The results of NMA are indicated in Supplementary Figures S1, S2A, B, and S3 and Table S1.
Stratification factors used for subgroup analyses included treatment lines (first line, second line, and third line), ICI drug type (anti-PD-1, anti-CTLA-4, anti-PD-L1), treatment mode (ICIs alone versus ICIs combined with ChT), and sample size (<500 versus ≥500). Based on the panoramic analysis of whether ICI treatment was applied, although the overall rates of grade 3–5 and all-grade trAEs were similar between the two groups, there were statistical differences in the rates of trAEs in some subgroups. For first-line treatment, ICIs were usually applied in combination with chemotherapy; consequently, the additional ICIs had significantly increased the rates of grade 3–5 trAEs (RR = 1.159, 95% CI = 1.012 to 1.327). However, for second-line treatment, ICIs had significantly decreased the rates of grade 3–5 trAEs (RR = 0.395, 95% CI = 0.317 to 0.491). In the case of ICIs alone, compared with chemotherapy, ICIs significantly reduced the rates of grade 3–5 trAEs (RR = 0.584, 95% CI = 0.350 to 0.974). The detailed results for subgroup analyses are listed in Table 2. Forest plots for subgroup analyses are indicated in Supplementary Figures S4A–E and S5A–S5E.
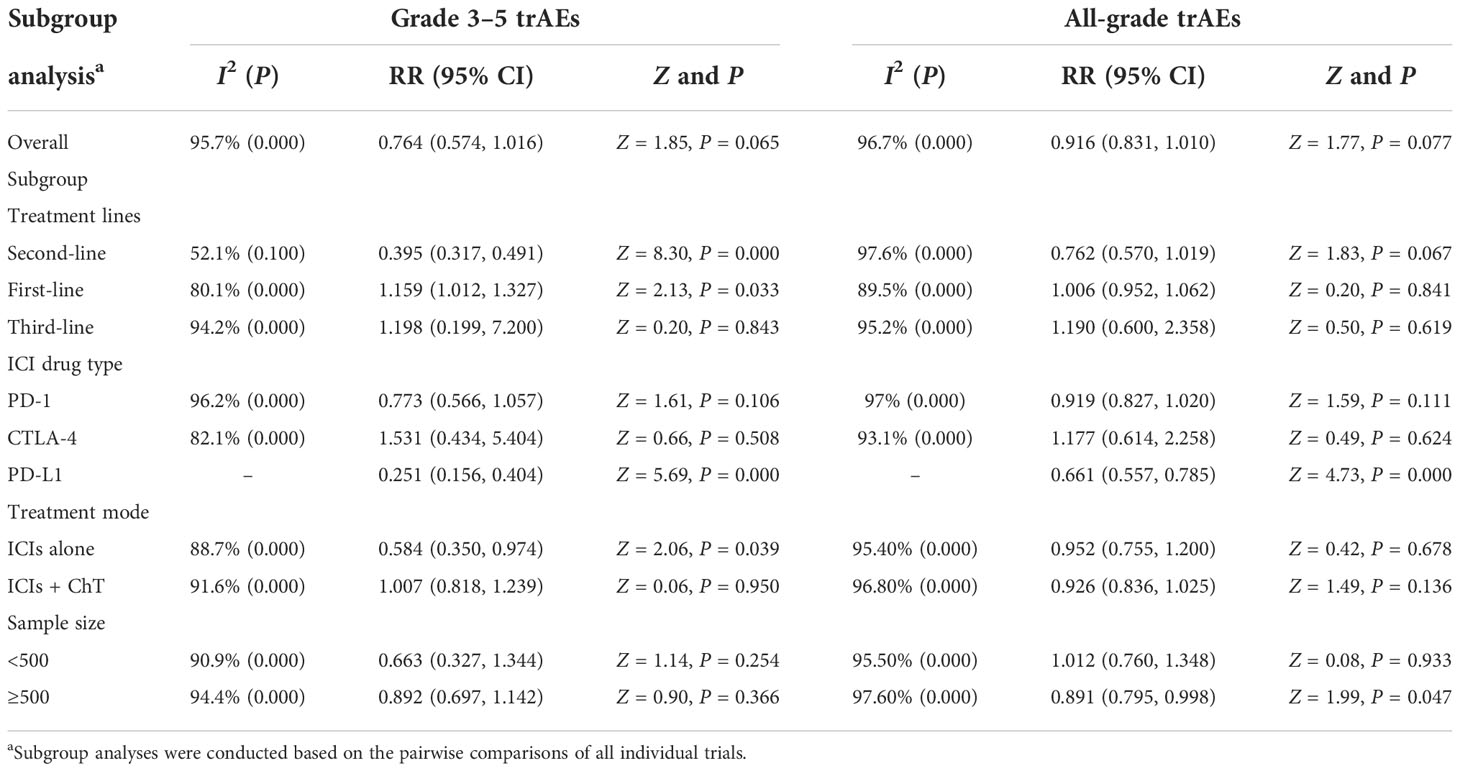
Table 2 Subgroup analysis of risk ratios for treatment-related adverse events (trAEs) comparing ICI therapy with chemotherapy.
Only five clinical trials had provided detailed data comparing the rates of serious trAEs between ICIs and chemotherapy (18, 20, 23, 24, 28). The meta-analysis shows that the rates of serious trAEs in the ICI group and the chemotherapy group were 22.66% (434/1,915) and 11.46% (216/1,885), respectively. However, no significant difference between the two groups was found (RR = 1.786, 95% CI = 0.978 to 3.262, Z = 1.89, P = 0.059). Six clinical trials had provided detailed data comparing the rates of events leading to discontinuation (18, 20–24). The
meta-analysis shows that the rates of events leading to discontinuation in the ICI group and the chemotherapy group were 22.42% (570/2542) and 11.59% (289/2,494), respectively, without statistical significance (RR = 1.447, 95% CI = 0.908 to 2.307, Z = 1.55, P = 0.120). Five clinical trials had provided detailed data comparing the rates of treatment-related death (18, 20–23). The meta-analysis shows that the rates of treatment-related death in the ICI group and the chemotherapy group were 1.88% (33/1,753) and 1.41% (24/1,702), respectively, without statistical significance (RR = 1.335, 95% CI = 0.793 to 2.249, Z = 1.09, P = 0.277). The corresponding forest plots are shown in Supplementary Figures S6A–C.
The meta-analysis for some specific treatment-related adverse events of interest is listed in Figures 6A, B. Compared with chemotherapy, in the ICI group, the rates for grade 3–5 trAEs of the following had been significantly reduced: decreased neutrophil count, decreased white blood cell count, neutropenia, anemia, febrile neutropenia, vomiting, and nausea. Moreover, the rates of the following in the ICI group were similar: asthenia, fatigue, decreased appetite, diarrhea, alopecia, peripheral sensory neuropathy, and rash. The highest rates of adverse events in the chemotherapy group were decreased neutrophil count (14.9%), followed by decreased white blood cell count (13.65%), neutropenia (11.75%), anemia (5.88%), and febrile neutropenia (5.45%). However, the most common adverse events in the ICI group were anemia (1.67%), followed by diarrhea (1.42%), fatigue (1.18%), and asthenia (1.11%).
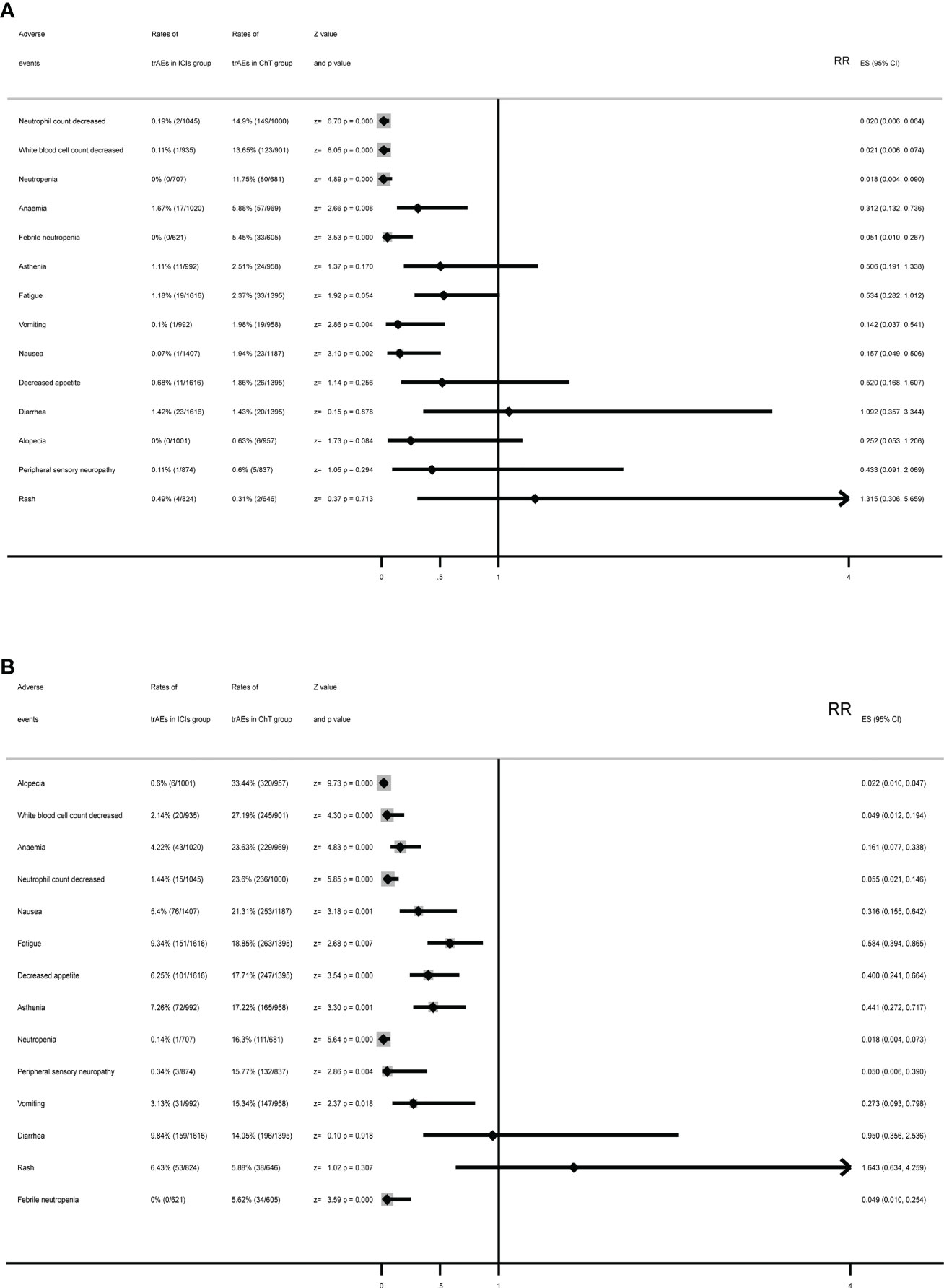
Figure 6 Summary forest plots for specific treatment-related adverse events with (A) forest plots for grade 3–5 trAEs and (B) forest plots for all-grade trAEs.
Except for diarrhea and rash, ICIs had significantly reduced the rates of specific treatment-related adverse events. The most common all-grade trAEs were alopecia (33.44%), followed by decreased white blood cell count (27.19%), anemia (23.63%), decreased neutrophil count (23.6%), and nausea (21.31%) in the chemotherapy group and diarrhea (9.84%) in the ICI group, followed by fatigue (9.34%), asthenia (7.26%), rash (6.43%), and decreased appetite (6.25%).
Only seven and eight trials had provided data on the rates of grade 3–5 irAEs (20, 22, 23, 27–30) and all-grade irAEs (20–23, 27–30), respectively. The network plot is shown in Figures 3C, D.
The NMA results of the consistency model for the rates of grade 3–5 irAEs and all-grade irAEs are indicated in Figures 7A, B. The general safety of grade 3–5 irAEs assessed by SUCRA for different ICI drugs or ChT ranked from high to low is as follows: ChT (85.1%), placebo (76.5%), ipilimumab (56.0%), nivolumab (48.5%), avelumab (48.4%), camrelizumab (41.8%), pembrolizumab (36.4%), and nivolumab + ipilimumab (21.6%). In terms of all-grade irAEs, the general safety of different ICI drugs or ChT ranked from high to low is as follows: ChT (98.7%), placebo (82.0%), pembrolizumab (73.6%), nivolumab (54.6%), nivolumab + ipilimumab (39.9%), camrelizumab (23.3%), avelumab (17.6%), and ipilimumab (10.3%). The SUCRA values are detailed in Supplementary Table S2.
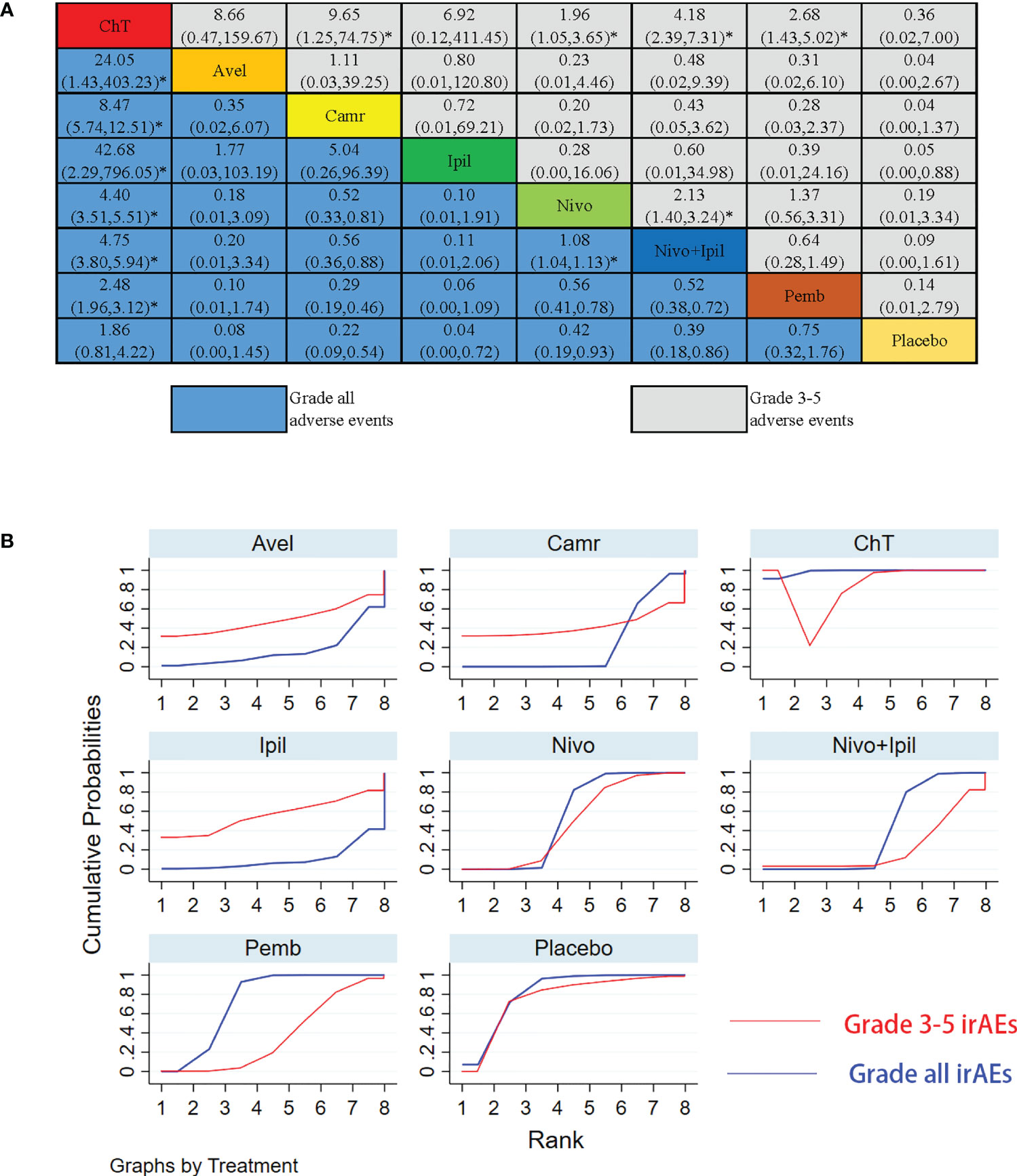
Figure 7 Results of the network meta-analysis for 10 treatment regimens in terms of immune-related adverse events (irAEs) with grade 3–5 irAEs and all-grade irAEs. (A) League table for different treatment regimens. (B) The surface under the cumulative ranking curves (SUCRAs) for grade 3–5 irAEs and all-grade irAEs. *: P<0.05.
Conventional pairwise meta-analysis was used to integrate all available data of irAEs. Seven clinical trials had provided detailed data comparing the rates of grade 3–5 irAEs between ICIs and chemotherapy (20, 22, 23, 27–30). The meta-analysis shows that the rates of grade 3–5 irAEs in the ICI group and the chemotherapy group were 7.35% (154/2,095, 95% CI: [6.23%, 8.47%]) and 2.25% (42/1,869, 95% CI: [1.58%, 2.92%]), respectively, with statistical significance (RR = 3.151, 95% CI = 2.175 to 4.563, Z = 6.07, P = 0.000). Eight clinical trials had provided detailed data comparing the rates of all-grade irAEs between ICIs and chemotherapy (20–23, 27–30). The meta-analysis shows that the rates of all-grade irAEs in the ICI group and the chemotherapy group were 44.46% (1,071/2,409) and 11.09% (240/2,165), respectively, with statistical significance (RR = 3.851, 95% CI = 2.767 to 5.359, Z = 8.00, P = 0.000). Therefore, immunotherapy not only increased immune-related adverse events of grades 3–5 but also increased immune-related adverse events of all grades. The corresponding forest plots are shown in Figures 8A, B.
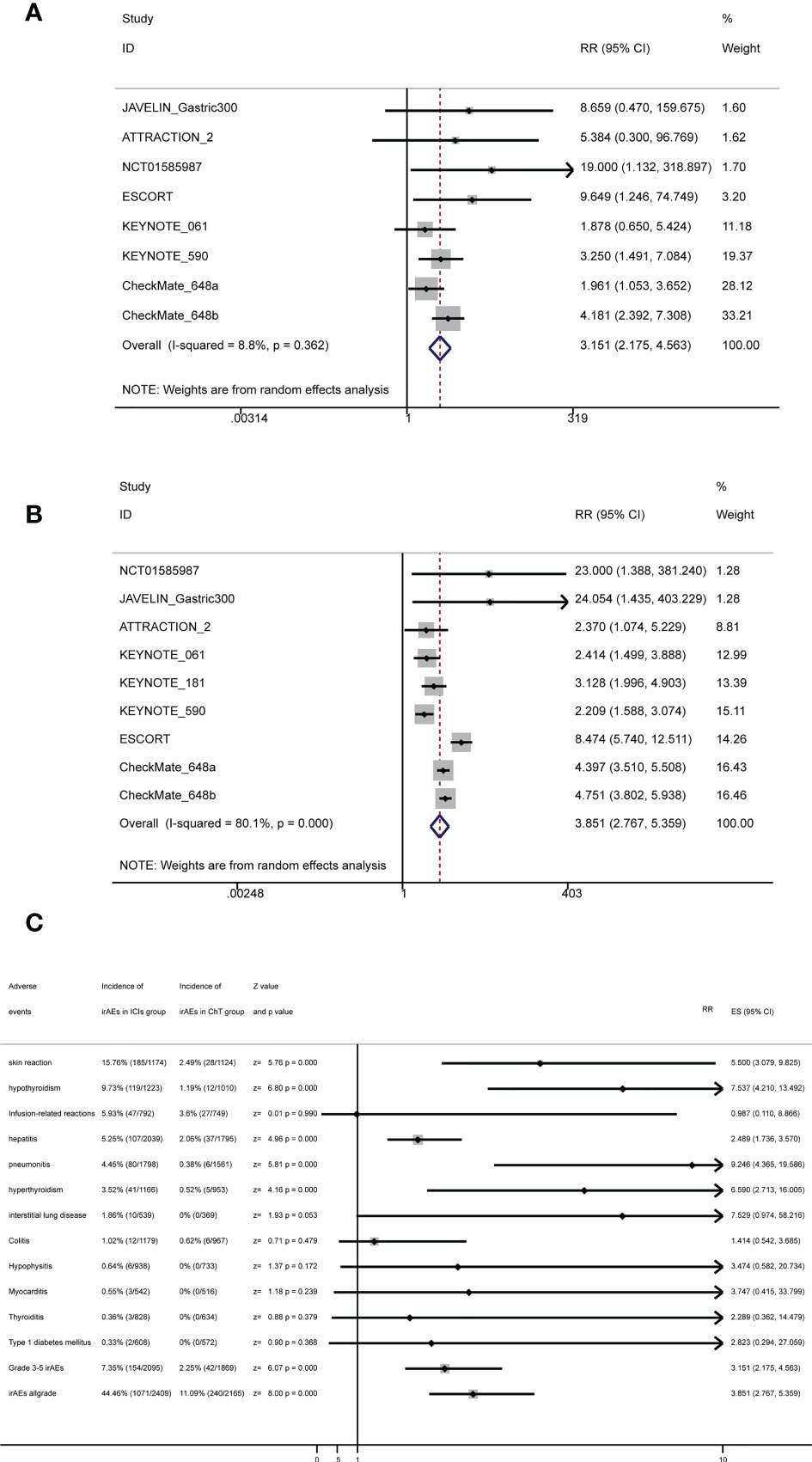
Figure 8 Forest plots for irAEs with (A) forest plots for grade 3–5 irAEs, (B) forest plots for all-grade irAEs, and (C) summary forest plots for irAEs.
The stratification factors of irAEs were the same as those of trAEs. The detailed results for the subgroup analyses are listed in Table 3. Forest plots for subgroup analyses are indicated in Supplementary Figures S7A–E and S8A–E. In terms of grade 3–5 irAEs, except for the second-line treatment subgroup and the PD-L1 subgroup, it can be observed that ICIs had significantly increased the adverse events in almost all of the other subgroups. Moreover, it can be observed that ICIs had significantly increased all-grade irAEs in all subgroups.
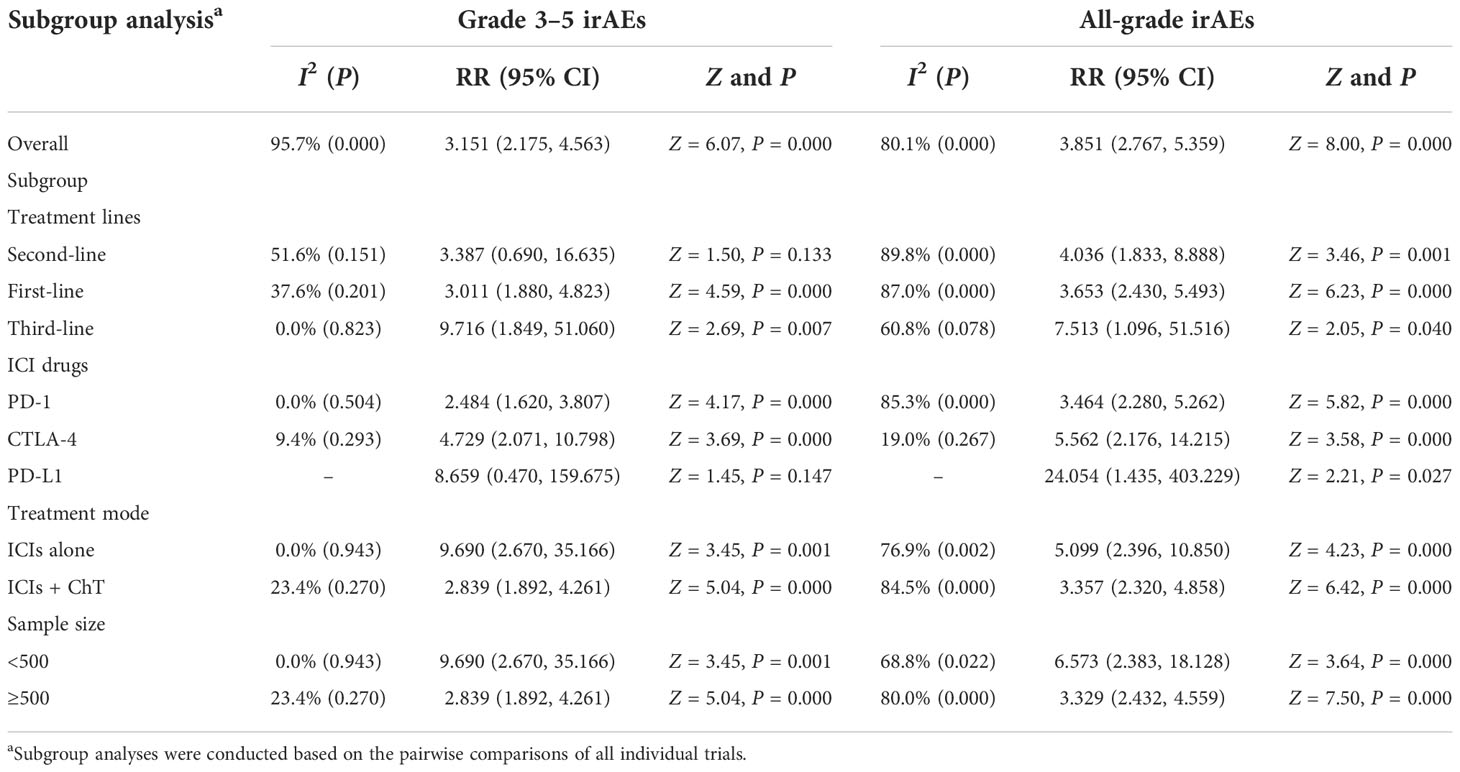
Table 3 Subgroup analysis of risk ratios for immune-related adverse events (irAEs) comparing ICI therapy with chemotherapy.
Some specific immune-related adverse events of interest are listed in Figure 8C and Supplementary Table S3. The most common irAEs in the ICI group were skin reaction (15.76%, 95% CI: [13.67%, 17.84%]), followed by hypothyroidism (9.73%, 95% CI: [8.07%, 11.39%]), infusion-related reactions (5.93%, 95% CI: [4.29%, 7.58%]), hepatitis (5.25%, 95% CI: [4.28%, 6.22%]), and pneumonitis (4.45%, 95% CI: [3.5%, 5.4%]).
Immunotherapy based on ICIs has currently become one of the most promising treatment regimens for cancer, which plays an encouraging role in the treatment of advanced cancer (35). Under normal physiological conditions, immune checkpoints help in maintaining self-tolerance and protecting host tissues from damage by the immune system when the immune system responds to specific physiological and pathological conditions (36). Tumor cells take full advantage of this feature to escape the attack of immune cells (37). Currently, the CTLA-4/B7-1/2 and PD-1/PD-L1 pathways has become the most popular in the field of cancer research on immunotherapy, both of which are the key pathways for immune T-cell activation (38). Most ICIs change the activity of immune checkpoints by targeting the inhibitory receptors (IRs) CTLA-4, PD-1, or PD-L1 and reactivate the immune response of T cells to tumor cells, thereby achieving antitumor effects (39). As immunotherapeutics have made substantial clinical progress in a variety of solid tumors, many PD-1/PD-L1 and CTLA-4 inhibitors have been approved by the FDA and can be used alone or combined with surgery, chemotherapy, radiotherapy, targeted therapy, and other therapeutic methods for many tumors (40). Due to the lack of effective treatment strategies, patients with advanced esophageal cancer generally have poor long-term survival and quality of life (41). Chemotherapy has been the main treatment strategy for patients with advanced esophageal cancer, but there is a serious lack of effective systemic chemotherapy regimens (42). The ATTRACTION-1 (43), KEYNOTE-028 (44), and KEYNOTE-180 (45) studies confirmed the efficacy and safety of immunotherapy in the second-line and third-line treatment of advanced esophageal cancer. The KEYNOTE-590 and CheckMate-648 studies further established the fundamental status of ICIs in the first-line treatment of advanced or resectable esophageal squamous cell carcinoma (22, 23). Although immunotherapeutics have special antitumor effects compared with cytotoxic chemotherapy and molecular targeted therapy, treatment- and immune-related adverse events should deserve attention and research (46). Some studies had shown that the toxicity of ICI drugs is generally lower than that of standard chemotherapy, but serious irAEs of ICI drugs will still be reported in clinical trials from time to time (47). In this review, we focused on the rates of trAEs and irAEs for ICIs in different treatment lines for advanced esophageal cancer. Meta-analysis was conducted based on 11 published RCTs to evaluate the safety of ICIs. In this review, we systematically describe the rates and influencing factors of various adverse events caused by ICIs in patients with advanced esophageal cancer or gastroesophageal junction cancer. Now, we discuss the problems discovered during the study process as follows.
In this review, we have included a total of 11 studies, including 7,089 patients, of which 6,992 cases can be used for adverse event analysis. As far as we know, the current meta-analysis may be the study with the largest sample size to explore the possible adverse events of immunotherapy in esophageal cancer and gastroesophageal junction cancer. Based on the results of our NMA analysis of different lines of immunotherapy for esophageal/gastroesophageal junction cancer, we can draw five main conclusions that may affect clinical practice.
First of all, from the point of view of different treatment modalities, different combinations of treatment modalities had obviously distinct safety outcomes in trAEs and irAEs. Similar to the results of practice in lung cancer, ICIs were generally less toxic in monotherapy than in chemotherapy, and the combination of ICIs and chemotherapy would increase the rates of grade 3–5 trAEs and grade 3–5 irAEs (48, 49). Although the overall survival and progression-free survival of combination therapy were significantly longer than those of chemotherapy alone, the treatment- and immuno-related toxicities had also been increased, which should not be underestimated (50). From the results of our network meta-analysis, compared with chemotherapy alone or ICIs alone, almost all combination treatments of ICIs and chemotherapy had increased the rates of treatment-related adverse events. Nivolumab + ChT, ChT, nivolumab + ipilimumab + ChT, and pembrolizumab + ChT had the smallest area under the SUCRA curve (see Supplementary Table S1), which means that these treatment modalities have the highest probability of grade 3–5 trAEs. From the perspective of monotherapy, the general safety of grade 3–5 trAEs for different ICI drugs ranked from high to low is as follows: avelumab, nivolumab, ipilimumab, pembrolizumab, and camrelizumab. However, camrelizumab had the highest rates of all-grade trAEs. Further analysis showed that increased all-grade trAEs are mainly caused by the increased occurrence of reactive capillary endothelial proliferation (RCEP), which was a skin reaction that rarely occurred in other ICIs but commonly manifested in camrelizumab. RCEP mostly appeared within 2 to 4 weeks after medication, most of which were grade 1 to 2 with rare grade 3–4 events occurring. The data showed that the rates of RECP were about 66.8%–70% in solid tumors (51, 52) and 80% in the ESCORT trial (20). In our meta-analysis, as only the ESCORT trial reported the data of RECP, the pooled analysis was not carried out. Our meta-results showed that the risk of all-grade trAEs for different treatment modalities ranked from high to low is as follows: camrelizumab, nivolumab + ChT, ChT, nivolumab + ipilimumab + ChT, and pembrolizumab + ChT. From the perspective of monotherapy, the general safety of all-grade trAEs for different ICI drugs ranked from high to low is as follows: ipilimumab, nivolumab, avelumab, and pembrolizumab (see Supplementary Table S1). Similar to the previous observations reported in related meta-analysis investigating the safety of ICIs, we confirmed that anti-programmed cell death ligand 1 ICI drugs (PD-L1) were safer than ChT in the subgroup analysis (49, 53, 54). A meta-analysis reported that 46% (95% CI 40–53) of patients who received the combination of immunotherapy and chemotherapy encountered grade ≥3 AEs, which was significantly higher than immunotherapy alone or chemotherapy alone (55).
Secondly, the application of ICI drugs in esophageal cancer involved first-line, second-line, third-line, or later-line treatment (56). In most cases, second-line treatment or later-line treatment would be dominated by single-agent therapy, including single-agent chemotherapy or single-agent immunotherapy (56). Monotherapy tended to be better tolerated, especially for patients with advanced tumors with poor ECOG score. In the first-line treatment, ICIs are usually used in combination with chemotherapy in the hope that the efficacy can be further improved (22–25). In our subgroup analysis, we found that ICIs applied in second-line treatment significantly reduced the rates of grade 3–4 trAEs, while ICIs applied in first-line treatment had the opposite performance, which further indicated that adding ICIs to chemotherapy will increase the treatment-related adverse events. Although the influence of treatment line on the rates of adverse events was largely due to different treatment combinations, our data showed that ICIs should be avoided as much as possible in combination with chemotherapy in the second- or third-line treatment for esophageal cancer to reduce the risk of adverse events (57).
Third, previous studies had shown that different types of ICIs have different toxicity profiles because of their different mechanisms of action (58). Anti-CTLA-4 drugs work by enhancing T-cell priming, while PD-1/PD-L1 inhibitors are thought to work by reactivating the pre-existing CD8 T-cell response (59). CTLA-4 inhibitors were generally considered to be more toxic, while PD-L1 inhibitors were considered to be more tolerable (60). A previous meta-analysis showed that 34% (95% CI 27–42) of patients treated with CTLA-4 inhibitors encountered grade ≥3 AEs, but only 14% (95% CI 12–16) of patients treated with PD-1/PD-L1 inhibitors suffered grade ≥3 AEs (55). In our meta-analysis, only two trials published the safety data on CTLA-4 inhibitors for esophageal cancer (23, 28). In terms of grade 3–4 trAEs, the risk of AEs caused by ipilimumab was lower than that of pembrolizumab and camrelizumab but higher than that of avelumab and nivolumab. This finding is different from previous reports and should be noted. On the other hand, from the perspective of immune-related adverse events, CTLA-4 inhibitors in our NMA had the highest risk of grade 1–5 irAEs, while the risk of grade 3–5 adverse events was relatively low. Compared with monotherapy, combined immunotherapy of nivolumab and ipilimumab had the highest risk of grade 3–5 irAEs. This finding was similar to the previously reported results (55), and the same findings have been found in clithe nical practice of lung cancer (35). Whether using CTLA-4 or PD-1/PD-L1 inhibitors, the application of ICIs significantly increased both the rates of grade 3–5 irAEs and grade 1–5 irAEs in our subgroup analyses. Therefore, a careful balance between toxicity and efficacy should be evaluated when ICIs need to be applied (55).
Fourth, the spectrum of trAEs caused by ICIs was also significantly different from that caused by ChT. Our meta-analysis based on specific treatment-related adverse events showed that ICIs were safer and had a significantly different spectrum of grade 3–5 trAEs and all-grade trAEs from chemotherapy. Hematological toxicity was the main adverse event for chemotherapy, while systemic symptoms such as fatigue, asthenia, and decreased appetite were the main adverse events for ICIs (50). For patients with poor bone marrow function, immunotherapy may be a better treatment option (61). For specific immune-related adverse events, our meta-analysis results showed that the most common irAEs in the ICI group were skin reaction (15.76%, 95% CI: [13.67%, 17.84%]), followed by hypothyroidism (9.73%, 95% CI: [8.07%, 11.39%]), infusion-related reactions (5.93%, 95% CI: [4.29%, 7.58%]), hepatitis (5.25%, 95% CI: [4.28%, 6.22%]), and pneumonitis (4.45%, 95% CI: [3.5%, 5.4%]). Due to the limited data obtained, we cannot further analyze the detailed rates of 3–5 grade irAEs and cannot further analyze the difference in the rates of specific trAEs and irAEs between CTLA-4 inhibitors and PD-1/PD-L1 inhibitors. However, the rates of all-grade-specific irAEs were close to the results of the previous meta-analysis reported. More specifically, colitis and hypophysitis seem to be more common with CTLA-4 inhibitors, whereas pneumonitis, hypothyroidism, and arthralgia appear to be more commonly associated with PD-1/PD-L1 inhibitors (55, 62, 63).
Finally, there was no consensus on whether the rates of irAEs were related to the primary site of the tumor. One review found that the rates of several specific AEs of interest varied among different cancer types (64). However, another review found that the overall rates of all-grade and grade 3–5 irAEs did not differ among different tumor sites (62). In our systematic review, both patients with esophageal cancer and gastroesophageal junction cancer were selected as the research subjects. Two reasons for this were the limited number of randomized controlled trials of immunotherapy for esophageal cancer and that some patients with esophageal adenocarcinoma had to be enrolled in some trials on GEJC. We did not further investigate whether specific irAEs differed between EPC and GEJC, which may be a potential focus for future analyses. In our view, the occurrence and severity of adverse events would be influenced by many factors, including the patients’ characteristics (disease stage, physical condition, age, gender, basic diseases, etc.). However, the rates were low in some special adverse events, especially for irAEs (47, 65). It is difficult to analyze the impact of these factors on the rates of AEs through the available data extracted from the literature, so we had to ignore the potential impact.
It should be pointed out from the results of our meta-analysis that, although ICIs increased the adverse events, the rates were actually low and acceptable. Although immunotherapy had increased the rates of irAEs, to a certain extent, the occurrence of immune-related events may be positively correlated with the therapy’s efficacy and the patient’s prognosis (66, 67). When focusing on the anti-tumor effects of ICIs, we should also pay attention to the occurrence of irAEs when ICIs are applied (68). However, we should not stop eating for fear of choking; after all, the current evidence showed that the benefits of ICIs outweigh the potential risks. For clinicians, the task we have to do is to achieve the best balance between the antitumor effects and the related adverse events of ICIs based on the best evidence-based medical practice.
There are some limitations in our review that need to be mentioned. First, the network meta-analysis assumes that the estimates of the study effects between the various trials have commonality, transferability, and exchangeability, which means that the similarities of population characteristics, interventions, chemotherapy regimen, and other features among different trials are required. However, as the conditions of the trials may affect the study results, this assumption is very unrealistic. In our meta-analysis, heterogeneity was detected in the results of grade 3–5 trAEs and all-grade trAEs. Subgroup meta-analyses revealed that trials with treatment line = second line, treatment line = first line, treatment mode, and a sample size ≥500 patients were potential sources of heterogeneity. Second, some specific irAEs and trAEs may be selectively reported in most trials because the rates of these adverse events were lower than a preset threshold, such as 1% or 5%. In this case, we cannot obtain the pooled estimates of rates for these rare adverse events, so it is inevitable to underestimate the overall mean rates of some adverse events. Third, in order to catch the latest data from newly published trials, some recent conference abstracts were enrolled in our meta-analysis, from which some summary data were extracted. However, this may lead to another selection bias because the comprehensive toxicity data might not be reported in these abstracts. Furthermore, some previous meta-analyses on this topic had shown the influence of different drug doses on the occurrence of adverse events (54). In our study, the related data on the influence of doses were not available. Therefore, we had to ignore this point. Finally, sometimes, serious adverse effects are either rare or not encountered. In this case, the confidence interval of the calculated effect estimate is too wide, which will affect the accuracy of the pooled effect size. This was extremely common in the evaluations of adverse events. In our meta-analysis, the rates of irAEs in arms without ICIs were very low, so a large number of wide-ranging estimates of RR appeared. Therefore, one should be cautious when interpreting the results of the meta-analysis and drawing conclusions.
Monotherapy with immune checkpoint inhibitors displayed better safety profiles in terms of trAEs than chemotherapy alone; however, combinational treatment regimens involving ICIs increased the risk of trAEs. Different ICIs had different toxicity manifestations and should not be considered as an entity. Compared with chemotherapy, ICIs were more prone to irAEs, but the overall rates remained low and acceptable. For clinicians, it is important to recognize and monitor the adverse events caused by ICIs for patients with esophageal cancer or gastroesophageal junction cancer.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
JZ and LX collected the data. JZ and MW performed data cleaning and analysis. JZ and BH performed the systematic review. JZ and BH evaluated the data. JL drafted and reviewed the manuscript for scientific soundness. All authors contributed to the article and approved the submitted version.
This study was supported in part by the National Clinical Key Specialty Construction Program (Grant No. 2021 to JL), the Fujian Provincial Clinical Research Center for Cancer Radiotherapy and Immunotherapy (Grant No. 2020Y2012 to JL), the Fujian Provincial Health Technology Project (Youth Scientific Research Project, 2019-1-50 to JZ), and the Nursery Fund Project of the Second Affiliated Hospital of Fujian Medical University (Grant No. 2021MP05 to JZ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.821626/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Fan J, Liu Z, Mao X, Tong X, Zhang T, Suo C, et al. Global trends in the incidence and mortality of esophageal cancer from 1990 to 2017. Cancer Med (2020) 9(18):e03338. doi: 10.1002/cam4.3338
5. Stahl M, Budach W, Meyer HJ, Cervantes A, Group EGW. Esophageal cancer: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2010) 21(Suppl 5):v46–9. doi: 10.1093/annonc/mdq163
6. Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol (2015) 21(26):7933–43. doi: 10.3748/wjg.v21.i26.7933
7. Ter Veer E, Haj Mohammad N, van Valkenhoef G, Ngai LL, Mali RMA, Anderegg MC, et al. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: A network meta-analysis. J Natl Cancer Inst (2016) 108(10):1–13. doi: 10.1093/jnci/djw166
8. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
9. Ku GY. The current status of immunotherapies in esophagogastric cancer. Hematol Oncol Clin North Am (2019) 33(2):323–38. doi: 10.1016/j.hoc.2018.12.007
10. Wu DW, Huang HY, Tang Y, Zhao Y, Yang ZM, Wang J, et al. Clinical development of immuno-oncology in China. Lancet Oncol (2020) 21(8):1013–6. doi: 10.1016/S1470-2045(20)30329-6
11. Zayac A, Almhanna K. Esophageal, gastric cancer and immunotherapy: small steps in the right direction? Transl Gastroenterol Hepatol (2020) 5(2020):9. doi: 10.21037/tgh.2019.09.05
12. Kakeji Y, Oshikiri T, Takiguchi G, Kanaji S, Matsuda T, Nakamura T, et al. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus (2021) 18(1):25–32. doi: 10.1007/s10388-020-00782-1
13. Fan Y, Xie W, Huang H, Wang Y, Li G, Geng Y, et al. Association of immune related adverse events with efficacy of immune checkpoint inhibitors and overall survival in cancers: A systemic review and meta-analysis. Front Oncol (2021) 11:633032. doi: 10.3389/fonc.2021.633032
14. Mimura K, Yamada L, Ujiie D, Hayase S, Tada T, Hanayama H, et al. Immunotherapy for esophageal squamous cell carcinoma: a review. Fukushima J Med Sci (2018) 64(2):46–53. doi: 10.5387/fms.2018-09
15. Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J Gastrointest Oncol (2015) 6(5):561–9. doi: 10.3978/j.issn.2078-6891.2015.037
16. Cipriani A, Barbui C, Rizzo C, Salanti G. What is a multiple treatments meta-analysis? Epidemiol Psychiatr Sci (2012) 21(2):151–3. doi: 10.1017/s2045796011000837
17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol (2009) 62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005
18. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
19. Takahashi M, Kato K, Okada M, Chin K, Kadowaki S, Hamamoto Y, et al. Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: a subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3). Esophagus (2021) 18(1):90–9. doi: 10.1007/s10388-020-00794-x
20. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8
21. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
22. Kato K, Sun JM, Shah MA, Enzinger PC, Shen L. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol (2020) 31(2020):S1192–S3. doi: 10.1016/j.annonc.2020.08.2298
23. Chau I, Doki Y, A.Ajani J, Xu J, Wyrwicz L, Motoyama S. Nivolumab plus ipilimumab or nivolumab plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma: first results of the ChekMate 648 study. In: Oncology ASoC, editor. 2021 ASCO annual meeting; (Alexandria: American society of clinical oncology) (2021).
24. Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol (2020) 31:S1191. doi: 10.1016/j.annonc.2020.08.2296
25. Boku N, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, et al. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann Oncol (2020) 31(2020):S1192. doi: 10.1016/j.annonc.2020.08.2297
26. Boku N, Ryu M, Kato K, Chung H, Minashi K, Lee K, et al. Safety and efficacy of nivolumab in combination with s-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann oncology: Off J Eur Soc Med Oncol (2019) 30(2):250–8. doi: 10.1093/annonc/mdy540
27. Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (2018) 392(10142):123–33. doi: 10.1016/S0140-6736(18)31257-1
28. Bang YJ, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, et al. Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally Advanced/Metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res (2017) 23(19):5671–8. doi: 10.1158/1078-0432.CCR-17-0025
29. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN gastric 300. Ann Oncol (2018) 29(10):2052–60. doi: 10.1093/annonc/mdy264
30. Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer (2021) 24(1):946–58. doi: 10.1007/s10120-021-01173-w
31. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017) 390(10111):2461–71. doi: 10.1016/S0140-6736(17)31827-5
32. Kato K, Satoh T, Muro K, Yoshikawa T, Tamura T, Hamamoto Y, et al. A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer (2019) 22(2):344–54. doi: 10.1007/s10120-018-0899-6
33. Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer (2020) 23(3):510–9. doi: 10.1007/s10120-019-01034-7
34. Satoh T, Kang YK, Chao Y, Ryu MH, Kato K, Cheol Chung H, et al. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer (2020) 23(1):143–53. doi: 10.1007/s10120-019-00970-8
35. Wang M, Liang H, Wang W, Zhao S, Cai X, Zhao Y, et al. Immune-related adverse events of a PD-L1 inhibitor plus chemotherapy versus a PD-L1 inhibitor alone in first-line treatment for advanced non-small cell lung cancer: A meta-analysis of randomized control trials. Cancer (2021) 127(5):777–86. doi: 10.1002/cncr.33270
36. De Sousa Linhares A, Leitner J, Grabmeier-Pfistershammer K, Steinberger P. Not all immune checkpoints are created equal. Front Immunol (2018) 9:1909(1909). doi: 10.3389/fimmu.2018.01909
37. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
38. Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J BioMed Sci (2017) 24(1):26. doi: 10.1186/s12929-017-0329-9
39. Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol (2005) 25(21):9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005
40. Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol (2018) 96(1):21–33. doi: 10.1111/imcb.1003
41. Davidson M, Chau I. Immunotherapy for oesophagogastric cancer. Expert Opin Biol Ther (2016) 16(10):1197–207. doi: 10.1080/14712598.2016.1213233
42. Shankaran V, Xiao H, Bertwistle D, Zhang Y, You M, Abraham P, et al. A comparison of real-world treatment patterns and clinical outcomes in patients receiving first-line therapy for unresectable advanced gastric or gastroesophageal junction cancer versus esophageal adenocarcinomas. Adv Ther (2021) 38(1):707–20. doi: 10.1007/s12325-020-01567-9
43. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol (2017) 18(5):631–9. doi: 10.1016/S1470-2045(17)30181-X
44. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol (2018) 36(1):61–7. doi: 10.1200/JCO.2017.74.9846
45. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 KEYNOTE-180 study. JAMA Oncol (2019) 5(4):546–50. doi: 10.1001/jamaoncol.2018.5441
46. Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline summary. J Oncol Pract (2018) 14(4):247–9. doi: 10.1200/jop.18.00005
47. Stelmachowska-Banaś M, Czajka-Oraniec I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: an updated review. Endocrine Connections (2020) 9(10):R207–R28. doi: 10.1530/ec-20-0342
48. Zhou Y, Chen C, Zhang X, Fu S, Xue C, Ma Y, et al. Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J Immunother Cancer (2018) 6(1):155. doi: 10.1186/s40425-018-0477-9
49. Wu Y, Shi H, Jiang M, Qiu M, Jia K, Cao T, et al. The clinical value of combination of immune checkpoint inhibitors in cancer patients: A meta-analysis of efficacy and safety. Int J Cancer (2017) 141(12):2562–70. doi: 10.1002/ijc.31012
50. Shao J, Wang C, Ren P, Jiang Y, Tian P, Li W. Treatment- and immune-related adverse events of immune checkpoint inhibitors in advanced lung cancer. Biosci Rep (2020) 40(5):1–11. doi: 10.1042/BSR20192347
51. Teng Y, Guo R, Sun J, Jiang Y, Liu Y. Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent. Acta Oncol (2019) 58(3):388–9. doi: 10.1080/0284186X.2019.1567935
52. Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen Z, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol (2020) 13(1):47. doi: 10.1186/s13045-020-00886-2
53. Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol (2017) 35(34):3815–22. doi: 10.1200/JCO.2016.72.1167
54. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ (2018) 363:k4226. doi: 10.1136/bmj.k4226
55. Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer (2019) 145(3):639–48. doi: 10.1002/ijc.32132
56. da Silva L, Aguiar P, Park R, Edelman Saul E, Haaland B, de Lima Lopes G. Comparative efficacy and safety of programmed death-1 pathway inhibitors in advanced gastroesophageal cancers: A systematic review and network meta-analysis of phase III clinical trials. Cancers (Basel) (2021) 13(11):1–14. doi: 10.3390/cancers13112614
57. Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
58. Khan Z, Hammer C, Guardino E, Chandler GS, Albert ML. Mechanisms of immune-related adverse events associated with immune checkpoint blockade: using germline genetics to develop a personalized approach. Genome Med (2019) 11(1):39. doi: 10.1186/s13073-019-0652-8
59. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
60. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
61. Cybulska-Stopa B, Ługowska I, Jagodzińska-Mucha P, Koseła-Paterczyk H, Kozak K, Klimczak A, et al. Immune checkpoint inhibitors therapy in older patients (≥ 70 years) with metastatic melanoma: a multicentre study. Postepy Dermatol Alergol (2019) 36(5):566–71. doi: 10.5114/ada.2018.79940
62. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol (2019) 5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393
63. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol (2017) 28(10):2377–85. doi: 10.1093/annonc/mdx286
64. Si X, Song P, Ni J, Di M, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related adverse events: A review of case reports. Thorac Cancer (2020) 11(3):498–504. doi: 10.1111/1759-7714.13315
65. Kottschade LA. Incidence and management of immune-related adverse events in patients undergoing treatment with immune checkpoint inhibitors. Curr Oncol Rep (2018) 20(3):24. doi: 10.1007/s11912-018-0671-4
66. Dupont R, Berard E, Puisset F, Comont T, Delord JP, Guimbaud R, et al. The prognostic impact of immune-related adverse events during anti-PD1 treatment in melanoma and non-small-cell lung cancer: a real-life retrospective study. Oncoimmunology (2020) 9(1):1682383. doi: 10.1080/2162402X.2019.1682383
67. Kobayashi K, Iikura Y, Hiraide M, Yokokawa T, Aoyama T, Shikibu S, et al. Association between immune-related adverse events and clinical outcome following nivolumab treatment in patients with metastatic renal cell carcinoma. In Vivo (2020) 34(5):2647–52. doi: 10.21873/invivo.12083
Keywords: immune checkpoint inhibitors, esophageal cancer, gastro-esophageal junction cancer, network meta-analysis, safety assessment
Citation: Zheng J, Huang B, Xiao L, Wu M and Li J (2022) Treatment- and immune-related adverse events of immune checkpoint inhibitors in esophageal or gastroesophageal junction cancer: A network meta-analysis of randomized controlled trials. Front. Oncol. 12:821626. doi: 10.3389/fonc.2022.821626
Received: 24 November 2021; Accepted: 14 November 2022;
Published: 08 December 2022.
Edited by:
Tao Li, Sichuan Cancer Hospital, ChinaReviewed by:
Chunyan Hua, Wenzhou Medical University, ChinaCopyright © 2022 Zheng, Huang, Xiao, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiancheng Li, amlhbmNoZW5nbGlfamFja0AxMjYuY29t; Jianqing Zheng, MTgwNjAxMDgyNjhAMTg5LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.