- 1Oral Maxillofacial Surgery, Far Eastern Memorial Hospital, Taipei, Taiwan
- 2Head and Neck Cancer Surveillance and Research Group, Far Eastern Memorial Hospital, New Taipei, Taiwan
- 3Otolaryngology, Far Eastern Memorial Hospital, Taipei, Taiwan
- 4Genomics Research Center, Academia Sinica, Taipei, Taiwan
- 5Division of Radiation Oncology, Department of Radiology, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 6Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan
- 7Institute of Traditional Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan
- 8Department of Electrical Engineering, Yuan Ze University, Taoyuan, Taiwan
Background: The relative risk for cerebrovascular disease (CVD) is increased in patients with head and neck cancer (HNC) treated with radiotherapy (RT). However, the current relative risk for CVD following RT has not been well clarified. The purpose of this study was to analyze the effect of RT and update the risk of CVD following RT in HNC patients through a systematic review and meta-analysis.
Material and Methods: We conducted an online database search and systematic review of observational studies that reported on CVD and extracranial carotid stenosis in patients with HNC who had undergone RT. Articles published in Medline and PubMed from 1980 to 2021 were identified and collected.
Results: Of the forty-seven articles identified from PubMed and forty-four articles identified from 3 systematic reviews, twenty-two studies were included. We found that neck RT was a significant risk factor for CVD (HR 3.97, 95% CI: 2.89-5.45). Patients with HNC treated by RT had an increased OR (7.36, 95% CI: 4.13-13.11) for CVD, and approximately 26% (95% CI: 22%-31%) of HNC patients treated with RT were at risk for CVD with more than 50% reduction in carotid diameter.
Conclusion: The risk of CVD is increased in patients with HNC treated by RT, and recent improvements in RT techniques may have contributed to the decreased risk of CVD. These results suggest that regular follow-up and appropriate screening for CVD should be required for patients with HNC.
Background
In the United States, cancer-related mortality has declined with improved treatment, and consequently, the number of cancer survivors increased to 17 million in 2019 (1). Due to the increasing number of head and neck cancer survivors, cancer-therapy-related cardiovascular complications impact both morbidity and mortality (2). Among these complications, radiation-induced cerebrovascular disease (CVD) is one of the most important issues.
Radiotherapy (RT) or concurrent with chemoradiation therapy (CCRT) is an essential therapeutic modality for patients with head and neck cancer (HNC). However, CVD in patients with HNC is under-identified and undertreated (3). The increased risk in ischemic CVD following RT has been reported in several cohort studies (4–8). Although previous systematic reviews have been reported, the quantitative method has not been updated, and there are limitations in the study design. Because of the risk of RT-related CVD, we organized a task force to conduct a comprehensive review on the risk of RT-related CVD in HNC survivors.
In the current study, a quantitative meta-analysis of the risk of CVD in post-RT/ CCRT HNC patients was designed and studied. Moreover, the assessment/screening for CVD in post-RT/CCRT HNC patients and the prevention/treatment of CVD in post-RT/CCRT HNC patients were investigated to provide potential clinical applications.
Material and Methods
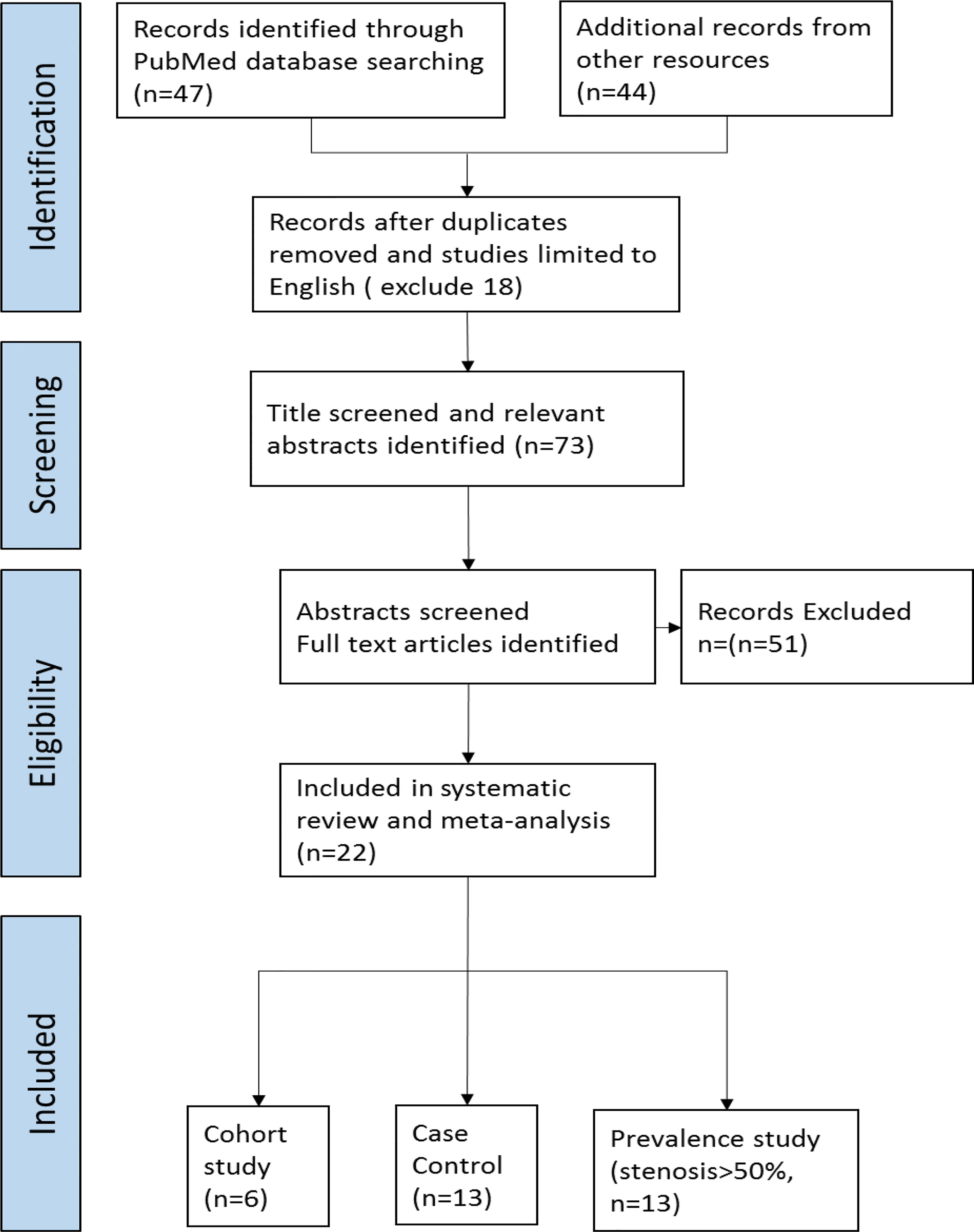
We conducted a search on Medline and PubMed with the MeSH terms “Cerebrovascular disease AND head and neck cancer AND Radiotherapy (((head and neck cancer) AND radiotherapy [MeSH Terms]) AND Cerebrovascular disease [MeSH Terms] in the PubMed database)” in October 2021 following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Figure 1) to identify relevant studies in the published literature. The search was performed for articles published from 1980 to 2021. Additional records from other review articles were also extracted (9–11).
Literature Inclusion Criteria
1) Studies that were original research; 2) studies that evaluated patients with histopathologically proven head and neck cancer who underwent radiotherapy; 3) studies that provided data about cerebrovascular events, such as carotid stenosis, carotid intima-media thickness or ischemia stroke; 4) studies published between 1980 and 2021; an 5) studies published in English.
Literature Exclusion Criteria
1) Studies that did not meet our inclusion criteria, 2) studies for which the data had already been published or were duplicate data and 3) studies with incomplete raw data.
Extracted Information, Excel Spreadsheet, and Information Retrieval
1) The general information extracted included the title, first author, and publication date. 2) The relative risk (RR) or hazard ratio (HR) and 95% CI were extracted for cohort studies; the number of patients with RT-related treatment and the number of patients in the control group were extracted for case–control studies; and the number of cases of CVD among the total number of RT patients was extracted for prevalence studies.
Statistical Synthesis and Analysis
The hazard ratio (HR) with 95% confidence interval (CI) was calculated to evaluate the risk of CVD in the general population and in those receiving different treatment modalities by using a random-effects model meta-analysis. The odds ratio (OR) with the corresponding 95% CI was used to compare the clinical characteristics of the post-RT vs. non-RT groups. The cumulative incidence of carotid stenosis and the 95% confidence interval (CI) were computed to estimate the prevalence of CVD (more than 50% of carotid artery diameter stenosis). The I-squared statistic was used to assess heterogeneity. An I-squared greater than 50% indicated significant heterogeneity. A random-effects model was used to pool the effect size of significant heterogeneity. A forest plot was used to graphically display the effect size in each study and the pooled estimates. A p value < 0.05 was considered significant. We performed the meta-analysis with two R software packages: “meta” (12) was used for pooling the hazard ratio and OR, while the package “metaphor” (13) was used for meta-regression to elucidate the possible etiology of heterogeneity.
Results
Search Results
The search process is shown in Figure 1. The initial literature search yielded 91 potentially relevant records after duplicates were removed, 47 from a PubMed search (N=47) and 44 from 3 other systematic reviews (9–11). After screening the titles and abstracts, 73 articles were retrieved for full-text evaluation. Twenty-two studies met the predetermined eligibility criteria and were included in the meta-analysis, as shown in the PRISMA flow diagram.
Twenty-two studies were included (4–7, 14–31). Within the 22 studies, there were six cohort studies, of which two studies reported RR (6, 14), and another four studies reported HR (4, 5, 15, 16). Moreover, there were 13 studies with case–control study designs (7, 17–28), and another three studies (29–31) reported the number of patients with carotid stenosis after neck radiation. A total of 35,160 patients had a history of head and neck cancer treated with radiation therapy. Most patients were diagnosed with laryngeal carcinoma (32%), followed by undesignated head and neck squamous cell carcinoma (18%), oral cancer (17%), nasopharyngeal cancer (14%), oropharyngeal cancer (12%), hypopharyngeal cancer (3%), salivary gland cancer (3%), and nasal cavity or sinus cancer (1%). The imaging modalities used for the detection of carotid stenosis were Doppler ultrasound (most of the included studies) and magnetic resonance angiography (one study) (28). In the cohort study reporting the RR of CVD following radiation, Dorresteijn et al. (2002) (6) reported that radiation to the neck significantly increased the RR (5.6, 95% CI: 3.1-9.4) of stroke compared to the general population. Haynes et al. (2002) (14) also reported that radiation to the neck with/without surgery increased the relative risk of stroke (RR 2.09, 95% CI: 1.28-3.22) compared to that of the general population (Table 1).
Comparing the HR of CVD in the general population (Pop) with that of patients receiving different treatment modalities, RT alone for head and neck patients indeed increased the risk of CVD [HR 3.97(2.89-5.45)] compared with that in the general population in the random-effects model (Figure 2). Additionally, concurrent chemoradiation therapy also increased the HR [3.26 (2.43-4.38)] for CVD. Interestingly, compared to RT with surgery, RT alone significantly increased the risk of CVD (HR: 1.42, 1.14-1.77) (Figure 2).
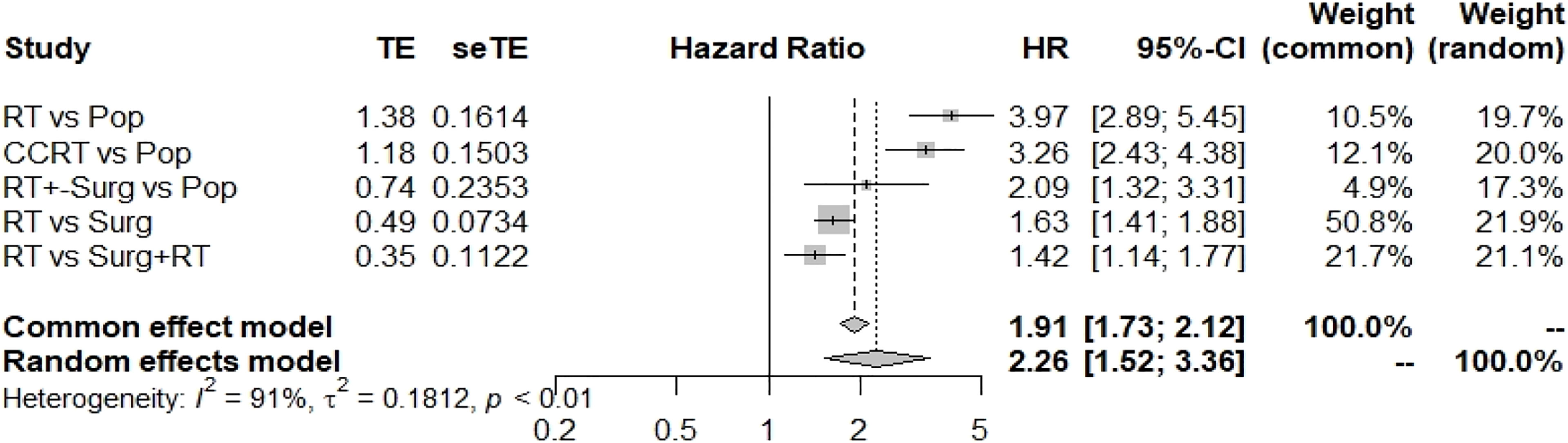
Figure 2 Summary of the hazard ratios for CVD for different treatment methods. Pop, population; RT, radiotherapy; Surg, surgery.
Thirteen case–control studies reported carotid stenosis in patients with HNC (Table 1). The RT-related CCA vasculopathy results are shown in Figure 3. The pooled OR (odds ratio) for an increased risk of CVD was 7.36 (4.13-13.11) using a cutoff point of 50% carotid artery stenosis in the random-effects model. However, there was significant heterogeneity among studies.
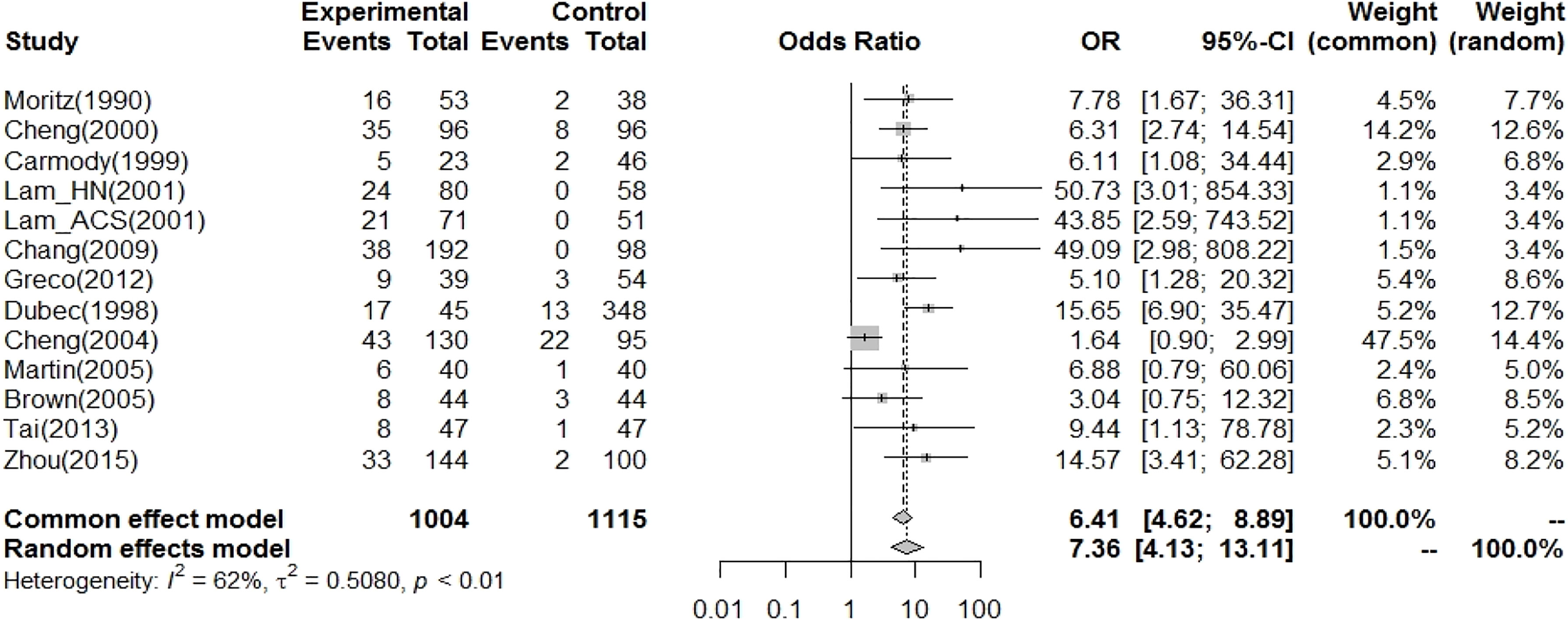
Figure 3 In case–control studies, the pooled OR for radiation-related CA vasculopathy (carotid artery stenosis>50%~%70 as risk) was 7.36 (95% CI: 4.13-13.11).
The current study demonstrated that the prevalence of CVD with more than 50% carotid stenosis in post-RT HNC patients was 26% (95% CI: 22%-31%, Table 2 and Figure 4). In meta-regression analysis to clarify the possible factors contributing to the heterogeneity among studies, we found that the publication year was a significant factor that contributed to the heterogeneity (p-value < 0.001, Table 2). In studies published before 2004, the prevalence of CVD with more than 50% carotid stenosis in post-RT HNC patients was 33% (95% CI: 29%-38%).

Table 2 Results of meta-regression analysis with the R package metafor, showing that the year of publication and subsites of cancer were significant contributing factors to the heterogeneity.
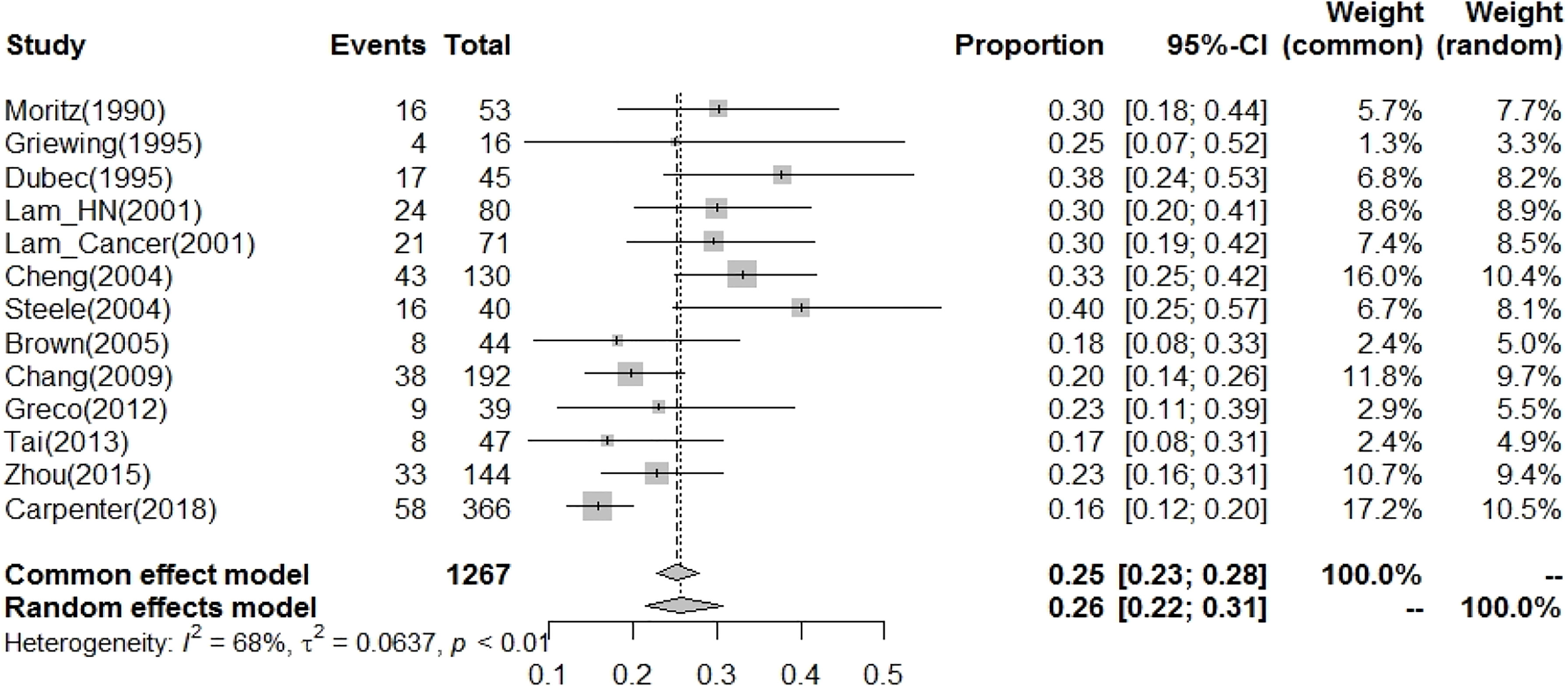
Figure 4 The prevalence of CVD risk (CA stenosis>50% as increasing risk for CVD) for patients after radiotherapy to the neck was 26% (95% CI: 22%-31%).
Discussion
We collected multiple studies and combined different study designs to clarify the effects of radiation effect to the neck. We concluded that radiation is a significant risk factor for CVD (HR 3.97, 95% CI: 2.89-5.45). Post-RT head and neck cancer patients had an increased OR (7.36, 95% CI: 4.13-13.11) for the risk of CVD, and approximately 26% of patients were at risk for CVD, defined as having more than 50% carotid diameter reduction. Our findings provide scientific evidence and are helpful for the development of protocols for the diagnosis and prevention of CVD.
Another meta-analysis of eight studies reported the pooled relative risk (9) and RR (7.54, 95% CI: 3.65-15.59) for high-grade carotid stenosis. Because the total number of patients at risk was not followed prospectively, the effect size should be calculated as the OR (32). Our OR for carotid stenosis more than 50% results is similar (7.36, 95% CI: 4.13-13.11).
The cost-effectiveness of carotid artery stenosis screening depends on the prevalence. One study revealed that the prevalence of carotid stenosis in the general population was 0% to 7.5% for moderate stenosis (carotid stenosis >50%) and 0% to 3.1% for severe stenosis (carotid stenosis >70%) (33). CVD screening is recommended if the prevalence of carotid artery stenosis is more than 20% (34). Previous meta-analysis reported that the prevalence of carotid stenosis in post-RT HNC patients was 25% (95% CI: 19%-32%) for moderate stenosis, 12% (95% CI: 7%-17%) for severe stenosis, and 4% (95% CI: 2%-8%) (11) for carotid occlusion. In our study, we estimated that the pooled prevalence for carotid stenosis (>50% luminal stenosis) was 26% (95% CI: 22%-31%). This result indicates that screening in post-RT HNC patients is necessary.
CVD is an underestimated condition for head and neck cancer patients (3). Okoye et al. reported that approximately 23% (27/115) of head and neck cancer patients have cardiovascular disease at diagnosis. Among these patients, 15% (17/115) had coronary artery disease and 9% (10/115) had carotid artery disease (35). A high prevalence of cardiovascular disease risk factors at HNC diagnosis requires personalized lifestyle changes and risk factor modifications to achieve LDL, blood pressure and blood sugar targets as early as possible (36).
Radiation-related carotid vasculopathy is a dynamic and progressive process that can result in the depletion of parenchymal and vascular endothelial cells, with both macro- and microvascular effects (37). Oxidative stress caused by reactive oxygen species promotes endothelial dysfunction and inflammatory changes in the radiation field (38). Accordingly, RT induces the release of thromboxane (39) and increases the level of von Willebrand factor, which causes platelet adhesion to endothelial cells and predisposes patients to arterial thrombosis (40). Simonetto et al. reported an increase in carotid intima media thickness (CIMT) one year after radiation for hypopharyngeal cancers (41). Therefore, it is necessary to screen the carotid artery one year after neck radiation. The late effects of radiation to the carotid artery will progress (42); therefore, regular extracranial color-coded duplex sonography examination is reasonable.
Neck irritation will induce inflammation in the arteries; however, the mechanism through which this occurs is still poorly understood. To date, there are no guidelines for medication in the prevention of radiation-associated CVD. In radiotherapy-induced carotid artery vasculopathy, CIMT was reported to be related to LDL cholesterol levels (43). According to a retrospective study, statin use was associated with a significant reduction in the incidence of stroke of 32% among cancer patients after radiation to the thorax, head and neck (44). There is growing evidence of anti-inflammatory medication to prevent radiation-associated CVDs, such as statins, colchicine and aspirin (45). More evidence is necessary for anti-inflammatory medication to prevent radiation-associated CVD.
The treatment of head and neck cancer requires a multidisciplinary team, including head and neck surgeons, radiation oncologists, hemato-oncologists and cardio-oncologists. Novel models for comprehensive head and neck cancer survival are necessary to provide a multidisciplinary approach to the prevention, screening and treatment of radiation-related CVD.
Due to technical innovations, the prevalence of radiation-related carotid vasculopathy may be decreased. In our studies, we found that publication year was an important factor in the heterogeneity among studies. One study reported that IMRT can reduce the risk for CVD compared to 2D RT (46). However, Addison et al. reported that patients with HPV-related head and neck cancer who underwent radiation had an increased risk for CVD (HR 4.4, 95% CI: 1.5-13.2) compared to HPV-negative patients (47). In addition, advances in head and neck cancer treatment have led to increased survival. Radiation-related CVD will still be an important issue in the future due to the emergence of HPV-related HNC.
There are limitations in the current study. First, there was significant heterogeneity among the collected studies, which may be due to various radiation dosages, radiation protocols, radiation techniques, and follow-up times. However, there were no sufficient information about the radiation dosages, protocols, and techniques from the included studies. The follow-up duration of the included studies was varying. The radiation dosages were either recorded as main tumor, neck or carotid region. The radiation protocols and techniques were mostly not mentioned. Thus, we cannot achieve further analysis. Second, the enrolled studies were nonrandomized and were observation studies. Only four of the included reports were prospective cohort studies, and others were retrospective studies. Third, there are still no solid guidelines for screening and treatment, and further studies are necessary to develop cost-effective methods in the management of radiation-related CVD. Fourth, the timeframe of the included articles is very large, the CVD risk may be change by radiation technique, HPV status and patients’ survival condition.
Conclusion
The included studies demonstrated that the prevalence of CVD with more than 50% carotid stenosis in post-RT HNC patients was 26%. Based on our analysis, RT for HNC patients can increase the risk of CVD. To combat this complication, close follow-up studies and appropriate screenings for CVD are recommended for HNC patients who receive RT
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
1. Conceived and designed the study: P-CC, W-LH, and L-JL; 2. Collected the data: P-YL, P-CC, W-LH, W-CL, C-HH, P-WS, and L-JL; 3. Performed the analysis P-CC, W-LH, and L-JL; 4. Wrote the paper: P-YL, P-CC, W-LH, W-CL, C-HH, P-WS, and L-JL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Far Eastern Memorial Hospital (FEMH -2021-C-011, PI20190002) and National Science Council of the Republic of China (MOST-109-2314-B-418-004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We also thank our colleagues in the Head and Neck Cancer Surveillance & Research Group, Far Eastern Memorial Hospital.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.820808/full#supplementary-material
Supplementary Figure 1 | The prevalence of CVD risk (CA stenosis>50% as increasing risk for CVD) for patients after radiotherapy to the neck was 33% (95% CI: 29%-38%) among studies published before 2004.
Supplementary Figure 2 | The prevalence of CVD risk (CA stenosis>50% as increasing risk for CVD) for patients after radiotherapy to the neck was 19% (95% CI: 16%-22%) among studies published after 2004.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
2. Wang L, Wang F, Chen L, Geng Y, Yu S, Chen Z. Long-Term Cardiovascular Disease Mortality Among 160 834 5-Year Survivors of Adolescent and Young Adult Cancer: An American Population-Based Cohort Study. Eur Heart J (2021) 42(1):101–9. doi: 10.1093/eurheartj/ehaa779
3. Yeh YC, Fang KM, Hsu W-L, Liao L-J. It is Time to Take Action to Prevent Cardiovascular Disease in Postirradiation Head and Neck Cancer Patients. Eur Arch Oto-Rhino-Laryngol (2019) 276(8):2361–2. doi: 10.1007/s00405-019-05492-8
4. Smith GL, Smith BD, Buchholz TA, Giordano SH, Garden AS, Woodward WA, et al. Cerebrovascular Disease Risk in Older Head and Neck Cancer Patients After Radiotherapy. J Clin Oncol (2008) 26(31):5119–25. doi: 10.1200/JCO.2008.16.6546
5. Arthurs E, Hanna TP, Zaza K, Peng Y, Hall SF. Stroke After Radiation Therapy for Head and Neck Cancer: What Is the Risk? Int J Radiat Oncol Biol Phys (2016) 96(3):589–96. doi: 10.1016/j.ijrobp.2016.07.007
6. Dorresteijn LD, Kappelle AC, Boogerd W, Klokman WJ, Balm AJ, Keus RB, et al. Increased Risk of Ischemic Stroke After Radiotherapy on the Neck in Patients Younger Than 60 Years. J Clin Oncol (2002) 20(1):282–8. doi: 10.1200/JCO.2002.20.1.282
7. Brown PD, Foote RL, McLaughlin MP, Halyard MY, Ballman KV, Collie AC, et al. A Historical Prospective Cohort Study of Carotid Artery Stenosis After Radiotherapy for Head and Neck Malignancies. Int J Radiat Oncol Biol Phys (2005) 63(5):1361–7. doi: 10.1016/j.ijrobp.2005.05.046
8. Lee JY, Kim YA, Kim HS, Back JH, Jung YH, Lee DH, et al. Radiotherapy can Increase the Risk of Ischemic Cerebrovascular Disease in Head and Neck Cancer Patients: A Korean Population-Based Cohort Study. Radiother Oncol (2020) 142:85–91. doi: 10.1016/j.radonc.2019.09.025
9. Bashar K, Healy D, Clarke-Moloney M, Burke P, Kavanagh E, Walsh SR. Effects of Neck Radiation Therapy on Extra-Cranial Carotid Arteries Atherosclerosis Disease Prevalence: Systematic Review and a Meta-Analysis. PloS One (2014) 9(10):e110389. doi: 10.1371/journal.pone.0110389
10. Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic Stroke and Transient Ischemic Attack After Head and Neck Radiotherapy: A Review. Stroke (2011) 42(9):2410–8. doi: 10.1161/STROKEAHA.111.615203
11. Texakalidis P, Giannopoulos S, Tsouknidas I, Song S, Rivet DJ, Reiter ER. Prevalence of Carotid Stenosis Following Radiotherapy for Head and Neck Cancer: A Systematic Review and Meta-Analysis. Head Neck (2020) 42(5):1077–88. doi: 10.1002/hed.26102
12. Balduzzi S, Rücker G, Schwarzer G. How to Perform a Meta-Analysis With R: A Practical Tutorial. Evidence-Based Ment Health (2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117
13. Viechtbauer W. Conducting Meta-Analyses in R With the Metafor Package. J Stat Softw 36(3):1–48. doi: jss.v036.i03/jss.v036.i03
14. Haynes JC, Machtay M, Weber RS, Weinstein GS, Chalian AA, Rosenthal DI. Relative Risk of Stroke in Head and Neck Carcinoma Patients Treated With External Cervical Irradiation. Laryngoscope (2002) 112(10):1883–7. doi: 10.1097/00005537-200210000-00034
15. Chen MC, Kuan FC, Huang SF, Lu CH, Chen PT, Huang CE, et al. Accelerated Risk of Premature Ischemic Stroke in 5-Year Survivors of Nasopharyngeal Carcinoma. Oncologist (2019) 24(9):e891–7. doi: 10.1634/theoncologist.2018-0747
16. Swisher-McClure S, Mitra N, Lin A, Ahn P, Wan F, O'Malley B. Risk of Fatal Cerebrovascular Accidents After External Beam Radiation Therapy for Early-Stage Glottic Laryngeal Cancer. Head Neck (2014) 36(5):611–6. doi: 10.1002/hed.23342
17. Moritz MW, Higgins RF, Jacobs JR. Duplex Imaging and Incidence of Carotid Radiation Injury After High-Dose Radiotherapy for Tumors of the Head and Neck. Arch Surg (1990) 125(9):1181–3. doi: 10.1001/archsurg.1990.01410210107017
18. Cheng SW, Ting AC, Lam LK, Wei WI. Carotid Stenosis After Radiotherapy for Nasopharyngeal Carcinoma. Arch Otolaryngol Head Neck Surg (2000) 126(4):517–21. doi: 10.1001/archotol.126.4.517
19. Carmody BJ, Arora S, Avena R, Curry KM, Simpkins J, Cosby K, et al. Accelerated Carotid Artery Disease After High-Dose Head and Neck Radiotherapy: Is There a Role for Routine Carotid Duplex Surveillance? J Vasc Surg (1999) 30(6):1045–51. doi: 10.1016/S0741-5214(99)70042-X
20. Lam WW, Yuen HY, Wong KS, Leung SF, Liu KH, Metreweli C. Clinically Underdetected Asymptomatic and Symptomatic Carotid Stenosis as a Late Complication of Radiotherapy in Chinese Nasopharyngeal Carcinoma Patients. Head Neck (2001) 23(9):780–4. doi: 10.1002/hed.1111
21. Lam WW, Leung SF, So NM, Wong KS, Liu KH, Ku PK, et al. Incidence of Carotid Stenosis in Nasopharyngeal Carcinoma Patients After Radiotherapy. Cancer (2001) 92(9):2357–63. doi: 10.1002/1097-0142(20011101)92:9<2357::AID-CNCR1583>3.0.CO;2-K
22. Chang YJ, Chang TC, Lee TH, Ryu SJ. Predictors of Carotid Artery Stenosis After Radiotherapy for Head and Neck Cancers. J Vasc Surg (2009) 50(2):280–5. doi: 10.1016/j.jvs.2009.01.033
23. Greco A, Gallo A, De Virgilio A, Marinelli C, Macri GF, Fusconi M, et al. Carotid Stenosis After Adjuvant Cervical Radiotherapy in Patients With Head and Neck Cancers: A Prospective Controlled Study. Clin Otolaryngol (2012) 37(5):376–81. doi: 10.1111/coa.12007
24. Dubec JJ, Munk PL, Tsang V, Lee MJ, Janzen DL, Buckley J, et al. Carotid Artery Stenosis in Patients Who Have Undergone Radiation Therapy for Head and Neck Malignancy. Br J Radiol (1998) 71(848):872–5. doi: 10.1259/bjr.71.848.9828801
25. Cheng SW, Ting AC, Ho P, Wu LL. Accelerated Progression of Carotid Stenosis in Patients With Previous External Neck Irradiation. J Vasc Surg (2004) 39(2):409–15. doi: 10.1016/j.jvs.2003.08.031
26. Martin JD, Buckley AR, Graeb D, Walman B, Salvian A, Hay JH. Carotid Artery Stenosis in Asymptomatic Patients Who Have Received Unilateral Head-and-Neck Irradiation. Int J Radiat Oncol Biol Phys (2005) 63(4):1197–205. doi: 10.1016/j.ijrobp.2005.04.017
27. Tai SM-L, Niyaz M, Ng C-G, Govindasamy GK, Tan C-T. Extracranial Carotid Stenosis After Radiotherapy in Nasopharyngeal Carcinoma, a Malaysian Study. Neurol Asia (2013) 18(2): 143–51.
28. Zhou L, Xing P, Chen Y, Xu X, Shen J, Lu X. Carotid and Vertebral Artery Stenosis Evaluated by Contrast-Enhanced MR Angiography in Nasopharyngeal Carcinoma Patients After Radiotherapy: A Prospective Cohort Study. Br J Radiol (2015) 88(1050):20150175. doi: 10.1259/bjr.20150175
29. Griewing B, Guo Y, Doherty C, Feyerabend M, Wessel K, Kessler C. Radiation-Induced Injury to the Carotid Artery: A Longitudinal Study. Eur J Neurol (1995) 2(4):379–83. doi: 10.1111/j.1468-1331.1995.tb00143.x
30. Steele SR, Martin MJ, Mullenix PS, Crawford JV, Cuadrado DS, Andersen CA. Focused High-Risk Population Screening for Carotid Arterial Stenosis After Radiation Therapy for Head and Neck Cancer. Am J Surg (2004) 187(5):594–8. doi: 10.1016/j.amjsurg.2004.01.014
31. Carpenter DJ, Mowery YM, Broadwater G, Rodrigues A, Wisdom AJ, Dorth JA, et al. The Risk of Carotid Stenosis in Head and Neck Cancer Patients After Radiation Therapy. Oral Oncol (2018) 80:9–15. doi: 10.1016/j.oraloncology.2018.02.021
32. Ranganathan P, Aggarwal R, Pramesh CS. Common Pitfalls in Statistical Analysis: Odds Versus Risk. Perspect Clin Res (2015) 6(4):222–4. doi: 10.4103/2229-3485.167092
33. de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, et al. Prevalence of Asymptomatic Carotid Artery Stenosis in the General Population: An Individual Participant Data Meta-Analysis. Stroke (2010) 41(6):1294–7. doi: 10.1161/STROKEAHA.110.581058
34. Yin D, Carpenter JP. Cost-Effectiveness of Screening for Asymptomatic Carotid Stenosis. J Vasc Surg (1998) 27(2):245–55. doi: 10.1016/S0741-5214(98)70355-6
35. Okoye CC, Bucher J, Tatsuoka C, Parikh SA, Oliveira GH, Gibson MK, et al. Cardiovascular Risk and Prevention in Patients With Head and Neck Cancer Treated With Radiotherapy. Head Neck (2017) 39(3):527–32. doi: 10.1002/hed.24646
36. Yang EH, Marmagkiolis K, Balanescu DV, Hakeem A, Donisan T, Finch W, et al. Radiation-Induced Vascular Disease-A State-Of-the-Art Review. Front Cardiovasc Med (2021) 8:652761. doi: 10.3389/fcvm.2021.652761
37. Kim JH, Jenrow KA, Brown SL. Mechanisms of Radiation-Induced Normal Tissue Toxicity and Implications for Future Clinical Trials. Radiat Oncol J (2014) 32(3):103–15. doi: 10.3857/roj.2014.32.3.103
38. Ping Z, Peng Y, Lang H, Xinyong C, Zhiyi Z, Xiaocheng W, et al. Oxidative Stress in Radiation-Induced Cardiotoxicity. Oxid Med Cell Longev (2020) 2020:3579143. doi: 10.1155/2020/3579143
39. Virmani R, Farb A, Carter AJ, Jones RM. Pathology of Radiation-Induced Coronary Artery Disease in Human and Pig. Cardiovasc Radiat Med (1999) 1(1):98–101. doi: 10.1016/S1522-1865(98)00010-9
40. Verheij M, Dewit LG, Boomgaard MN, Brinkman HJ, van Mourik JA. Ionizing Radiation Enhances Platelet Adhesion to the Extracellular Matrix of Human Endothelial Cells by an Increase in the Release of Von Willebrand Factor. Radiat Res (1994) 137(2):202–7. doi: 10.2307/3578813
41. Simonetto C, Mayinger M, Ahmed T, Borm K, Kundrát P, Pigorsch S, et al. Longitudinal Atherosclerotic Changes After Radio(chemo)therapy of Hypopharyngeal Carcinoma. Radiat Oncol (2020) 15:102. doi: 10.1186/s13014-020-01541-3
42. Huang TL, Hsu HC, Chen HC, Lin HC, Chien CY, Fang FM, et al. Long-Term Effects on Carotid Intima-Media Thickness After Radiotherapy in Patients With Nasopharyngeal Carcinoma. Radiat Oncol (2013) 8:261. doi: 10.1186/1748-717X-8-261
43. Pereira EB, Gemignani T, Sposito AC, Matos-Souza JR, Nadruz W Jr. Low-Density Lipoprotein Cholesterol and Radiotherapy-Induced Carotid Atherosclerosis in Subjects With Head and Neck Cancer. Radiat Oncol (2014) 9:134. doi: 10.1186/1748-717X-9-134
44. Boulet J, Peña J, Hulten EA, Neilan TG, Dragomir A, Freeman C, et al. Statin Use and Risk of Vascular Events Among Cancer Patients After Radiotherapy to the Thorax, Head, and Neck. J Am Heart Assoc (2019) 8(13):e005996. doi: 10.1161/JAHA.117.005996
45. Camara Planek MI, Silver AJ, Volgman AS, Okwuosa TM. Exploratory Review of the Role of Statins, Colchicine, and Aspirin for the Prevention of Radiation-Associated Cardiovascular Disease and Mortality. J Am Heart Assoc (2020) 9(2):e014668. doi: 10.1161/JAHA.119.014668
46. Liao W, Zhou H, Fan S, Zheng Y, Zhang B, Zhao Z, et al. Comparison of Significant Carotid Stenosis for Nasopharyngeal Carcinoma Between Intensity-Modulated Radiotherapy and Conventional Two-Dimensional Radiotherapy. Sci Rep (2018) 8(1):13899. doi: 10.1038/s41598-018-32398-y
Keywords: cerebrovascular disease, head and neck cancer, radiotherapy, radiotherapy - adverse effects, systematic review and meta-analysis
Citation: Lin P-Y, Cheng P-C, Hsu W-L, Lo W-C, Hsieh C-H, Shueng P-W and Liao L-J (2022) Risk of CVD Following Radiotherapy for Head and Neck Cancer: An Updated Systematic Review and Meta-Analysis. Front. Oncol. 12:820808. doi: 10.3389/fonc.2022.820808
Received: 23 November 2021; Accepted: 02 May 2022;
Published: 01 June 2022.
Edited by:
Markus Wirth, Klinikum rechts der Isar, GermanyReviewed by:
Francesco Ricchetti, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyChristoph Straube, Technical University of Munich, Germany
Copyright © 2022 Lin, Cheng, Hsu, Lo, Hsieh, Shueng and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Jen Liao, bGlhb2xqQG50dS5lZHUudHc=
†These authors have contributed equally to this work
 Ping-Yi Lin1,2†
Ping-Yi Lin1,2† Ping-Chia Cheng
Ping-Chia Cheng Chen-Hsi Hsieh
Chen-Hsi Hsieh Pei-Wei Shueng
Pei-Wei Shueng Li-Jen Liao
Li-Jen Liao