- 1Breast and Thyroid Surgery, Department of General Surgery, The Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Drug Clinical Trial Institution, The Affiliated Hospital of Zunyi Medical University, Zunyi, China
Background: The mapping method represents a crucial factor affecting the rate of sentinel lymph node detection in breast cancer. We carried out this meta-analysis to assess the clinical utility of carbon nanoparticle suspensions (CNSs) in guiding sentinel lymph node biopsy (SLNB) for breast cancer patients.
Methods: Electronic databases, which comprised the China National Knowledge Infrastructure, the Wanfang electronic database, the Cochrane Library, EMBASE, and PubMed, were explored to identify relevant studies from database inception to July 2021 that studied the detection rate of CNSs-guided SLNB. A meta-analysis was performed to generate pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), a summary receiver operator characteristic curve (SROC), and a diagnostic odds ratio (DOR).
Results: A total of 33 publications that enrolled 2,171 patients were analyzed. The pooled sensitivity, specificity, PLR, and NLR were 0.93 (95% CI: 0.91–0.95, I2 = 0.0%), 0.99 (95% CI: 0.98–0.99, I2 = 56.5%), 42.85 (95% CI: 29.73–61.77, I2 = 47.0%), and 0.09 (95% CI: 0.07–0.11, I2 = 0.0%), respectively. The area under the curve (AUC) of the SROC curve was 0.98. There were no significant differences when analyzed based on the dose and site of CNS injection. There was significant publication bias among the included publications based on Deeks’ funnel plot [Slope (Bias) = −7.35, P = 0.00]. Nonetheless, the sensitivity analysis identified the results to be reliable and stable.
Conclusion: This meta-analysis highlights the accuracy and feasibility of using CNSs for SLNB in patients with breast cancer. Clinically, the identification and predictive values of CNSs as an optimal tracer for SLNB remains undisputed.
Introduction
The modern era of breast cancer surgery is progressing towards the direction of minimally invasive treatment. Previously, axillary lymph node dissection (ALND) represented an indispensable treatment component for breast cancer. However, the current standard of care for axillary staging is SLNB. The sentinel lymph node refers to the first axillary lymph node draining the tumor site and may potentially harbor metastatic deposits (1). SLNB is mainly determined by evaluating the SLN status to determine whether ALND is required. SLNB allows for careful selection of patients who are candidates for ALND. SLNB is as effective as ALNB but has the benefits of lower postoperative complications such as arm lymphedema and sensory loss (2–5). The mapping method is a crucial factor that determines the positive and negative detection rates of SLNB in breast cancer. SLNB techniques incorporate the use of either blue dye (BD) or radioisotopes (RI) (6). The RI method requires specialized equipment, authorized radiation protection areas, and nuclear medicine licensing, thus limiting the widespread use of this approach. BD, on the other hand, is a cost-effective method for SLNB but possesses a lower detection rate (7).
The past decade has seen a surge in research in the field of nanomaterials and nanotechnology. Several novel diagnostic and therapeutic techniques in the field of medicine have begun to incorporate nanobiotechnology. CNSs is a 150 nm nanoparticle lymphatic tracer made up of polymeric carbon granules and has been approved for clinical usage by the Chinese Food and Drug Administration (CFDA). CNSs selectively populate the lymphatic system (diameter: 120–500 nm) over the vascular system (diameter: 20–50 nm), given its permeability and molecular size (8). CNSs have received substantial attention over the recent years, especially with regards to their postulated benefits in lymphatic mapping. Thus, the aim of our analysis was to assess the effectiveness of CNSs for SLN mapping in breast cancer.
Materials and Methods
Literature Search
A systematic literature search was carried out on the China National Knowledge Infrastructure, the Wanfang electronic database, the Cochrane Library, EMBASE, and PubMed to extract all related papers present from database inception until July 2021. The medical subject heading (MESH) terms used were as follows: breast neoplasm, breast carcinoma, breast tumor, breast cancer, carbon nanoparticle, nano-carbon, carbon nanoparticles suspensions, CNSs, sentinel lymph node biopsy, and SLNB.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows:
1. Patients with breast cancer who had clinically negative lymph nodes.
2. The concurrent use of CNSs and other modalities for SLNB mapping.
3. The availability of diagnostic method and clinicopathological data.
4. The SLNB as the main study topic.
5. The reported primary data were sufficient to calculate totals of true negative (TN), false negative (FN), false positive (FP), and true positive (TP).
The exclusion criteria were as follows:
1. Letters, editorials, review articles, and case reports.
2. Overlapping information between studies.
Data Extraction and Quality Assessment
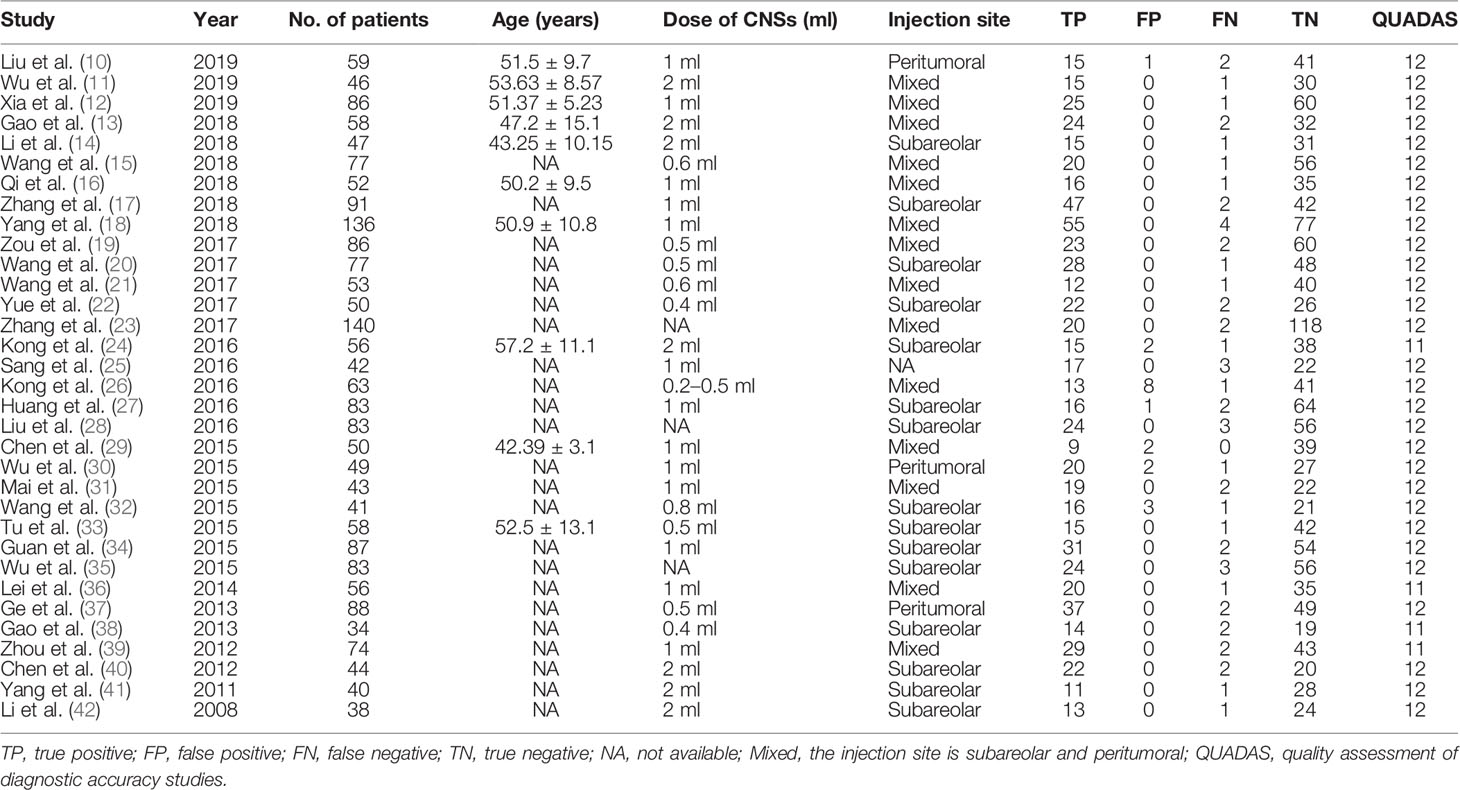
All studies were reviewed by two independent reviewers in order to extract the relevant data. A third reviewer was consulted to reach a consensus in case of a disagreement. A datasheet containing the following information was compiled: year of publication, author, age, dose of CNSs, injection site, TN, FN, FP, and TP values. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) protocols were referenced for quality assessment of the selected studies (9). These guidelines evaluate the degree of biases in the included studies across four major domains that included flow and timing, reference standard, index test, and patient selection. The highest possible score is 14, which indicates high study quality.
Statistical Analysis
The STATA version 15.1 (Stata Corporation, College Station, Texas, USA) and Meta-Disc version 1.4 Software (XI Cochrane Colloquium; Barcelona, Spain) was utilized for this meta-analysis. The degree of heterogeneity among the studies was estimated using I2, while heterogeneity itself was assessed with the Chi-square-based Q statistic test. Heterogeneity was interpreted as being statistically significant when I2 >50% or P <0.05. The fixed-effect model (Mantel–Haenszel) was used in cases of no study heterogeneity. In cases where there was study heterogeneity, a random-effect model (DerSimonian and Laird) was implemented.
Study sensitivity, specificity, PLR, NLR, and DOR were evaluated using a bivariate meta-analysis model. A suitable statistical analysis model was first used to calculate the estimates with the corresponding 95% CI. The AUC and SROC of these models were also determined. A higher diagnostic effect was recognized in results that had an AUC closer to 1.0. Publication bias was determined with the Deek test for funnel plot asymmetry.
Results
Characteristics of Identified Studies
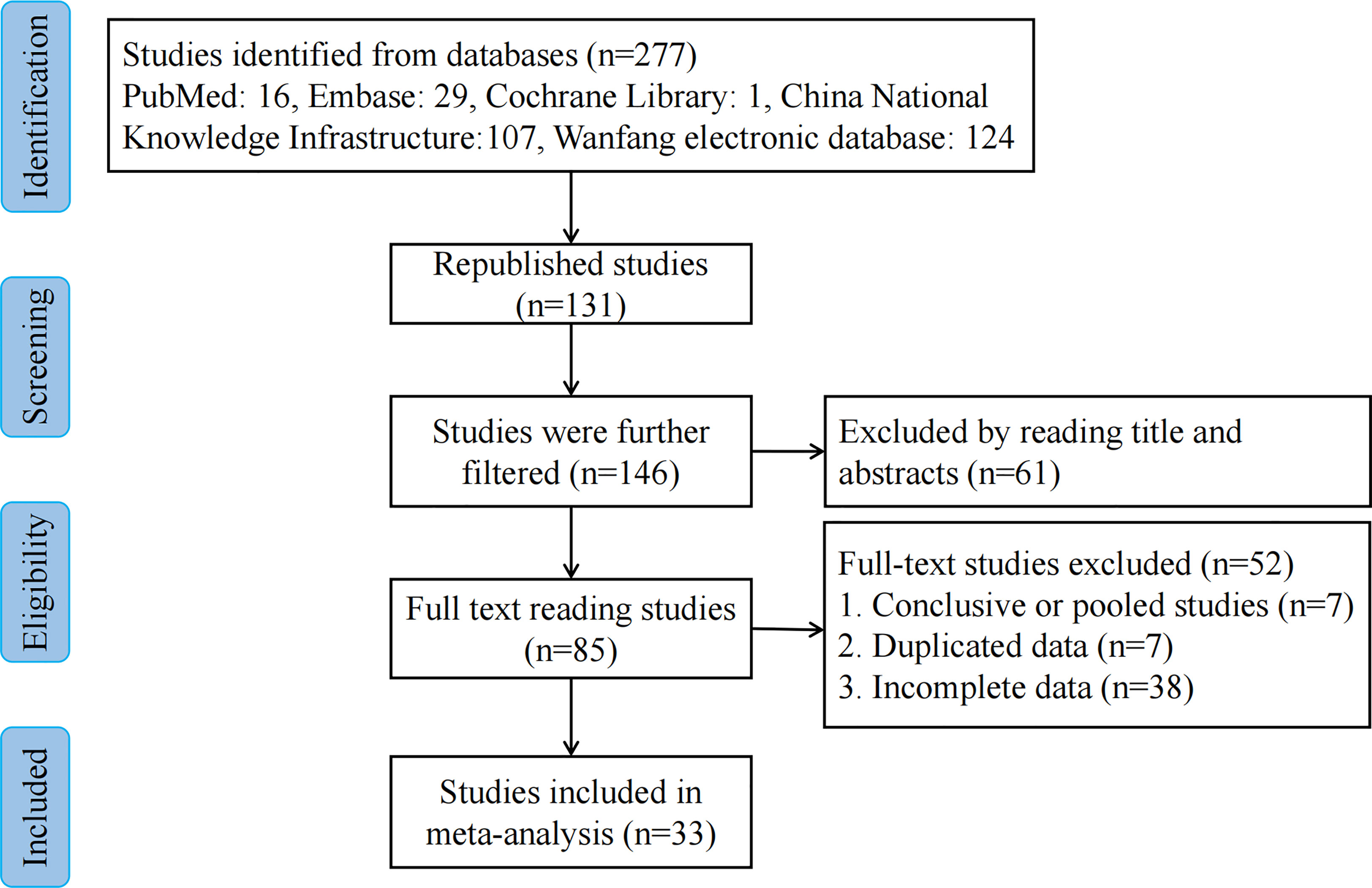
We extracted 277 potentially relevant publications. Of these, 131 duplicates were removed, and 61 were deemed irrelevant based on screening titles and abstracts. A total of 85 remaining full-text articles were then scrutinized for eligibility (Figure 1). Another 52 articles were additionally excluded: 7 articles were excluded due to duplicate use of the same data, 7 articles were summary and summary data, while 38 articles contained incomplete data. Finally, 33 studies (10–42) including 2,171 patients were included in our meta-analysis. The amount of CNSs injected ranged from 0.2 to 2 ml. Peritumoral CNSs injection for SLNB was used in 3 studies, subareolar CNSs injection was used in 15 studies, and both peritumoral and subareolar CNSs injection were used in 14 studies. Table 1 depicts the characteristics of the identified papers.
Diagnostic Accuracy
Figures 2–6 demonstrate the forest plot of sensitivity, specificity, PLR, NLR, and DOR for CNS in SLNB. The overall pooled sensitivity and specificity of all studies were 0.93 (95% CI: 0.91–0.95, I2 = 0.0%) and 0.99 (95% CI: 0.98–0.99, I2 = 56.5%). The overall pooled PLR and NLR were 42.85 (95% CI: 29.73–61.77, I2 = 47.0%) and 0.09 (95% CI: 0.07–0.11, I2 = 0.0%), respectively. The pooled DOR was 530.19 (95% CI: 314.70–893.22, I2 = 0.0%). The SROC curve demonstrated an AUC of 0.98, which indicated excellent diagnostic accuracy (Figure 7). Additionally, the left upper quadrant (LUQ) in the likelihood ratio scatter diagram was occupied by summary PLR and NLR, indicating that CNSs was useful in improving the diagnostic accuracy of SLNB in breast cancer (Figure 8).
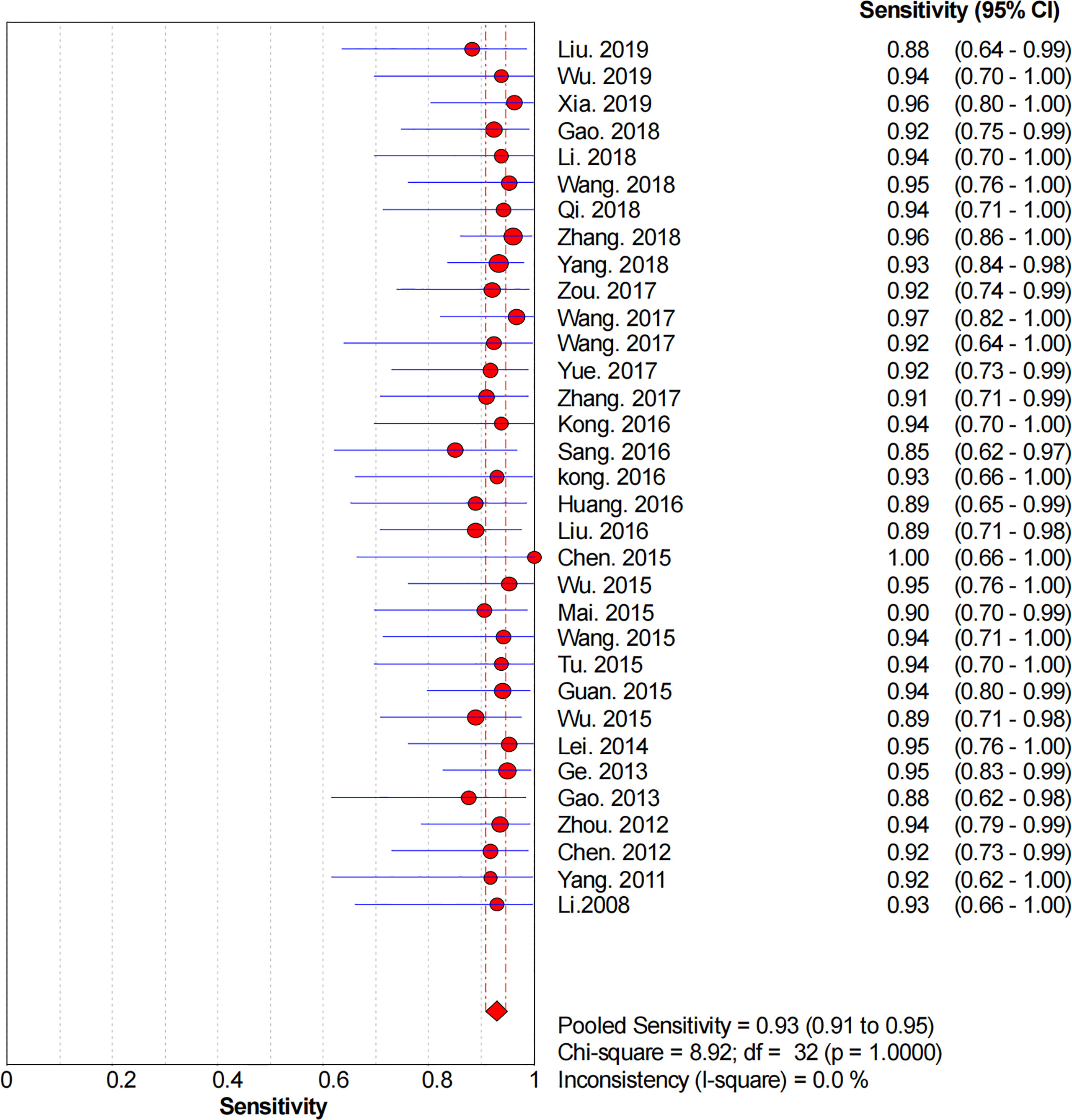
Figure 2 Forest plot of pooled sensitivity of the diagnosis value of CNSs in SLNB of breast cancer. 95% CI, 95% confidence interval.
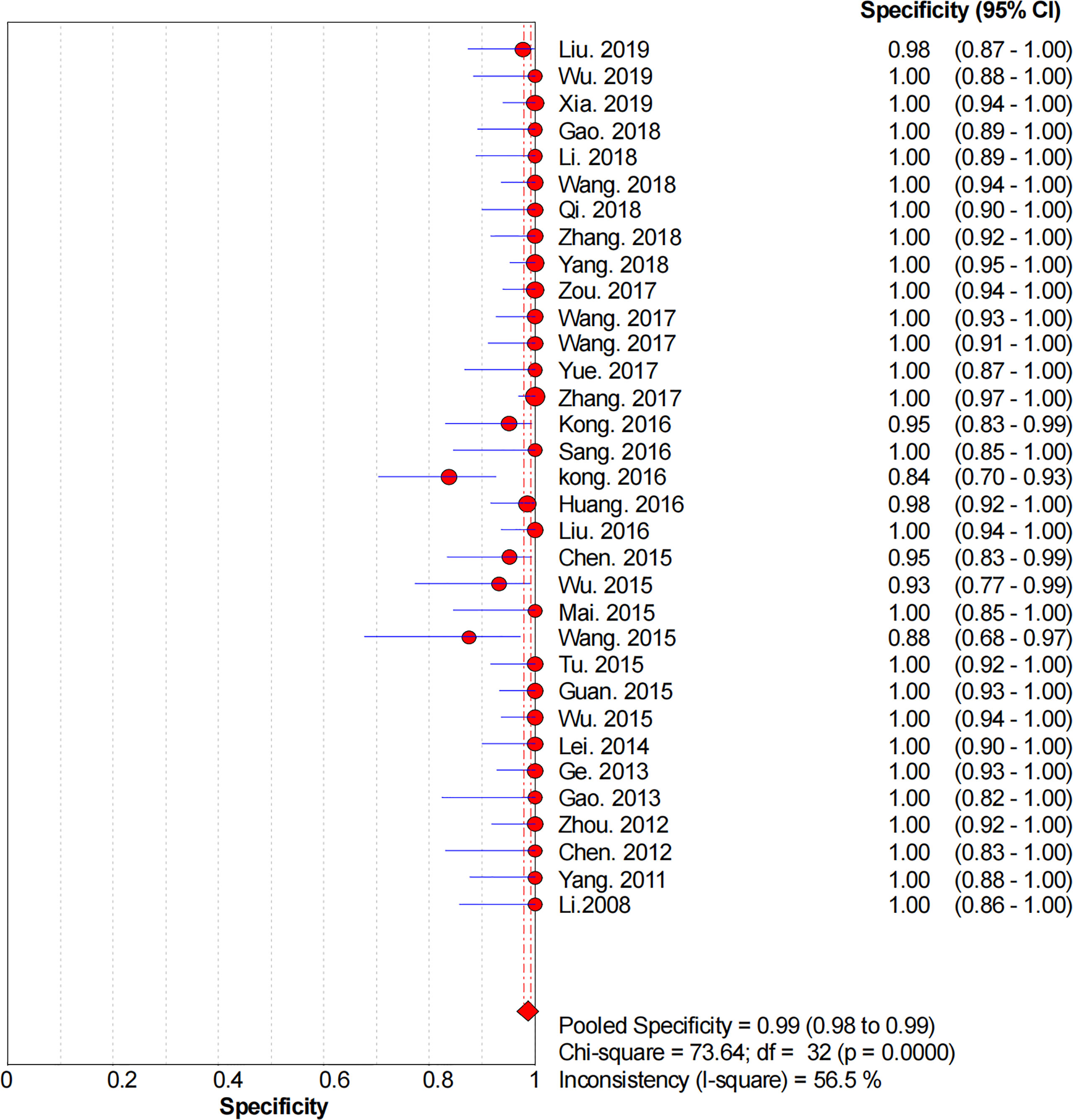
Figure 3 Forest plot of pooled specificity of the diagnosis value of CNSs in SLNB of breast cancer. 95% CI, 95% confidence interval.
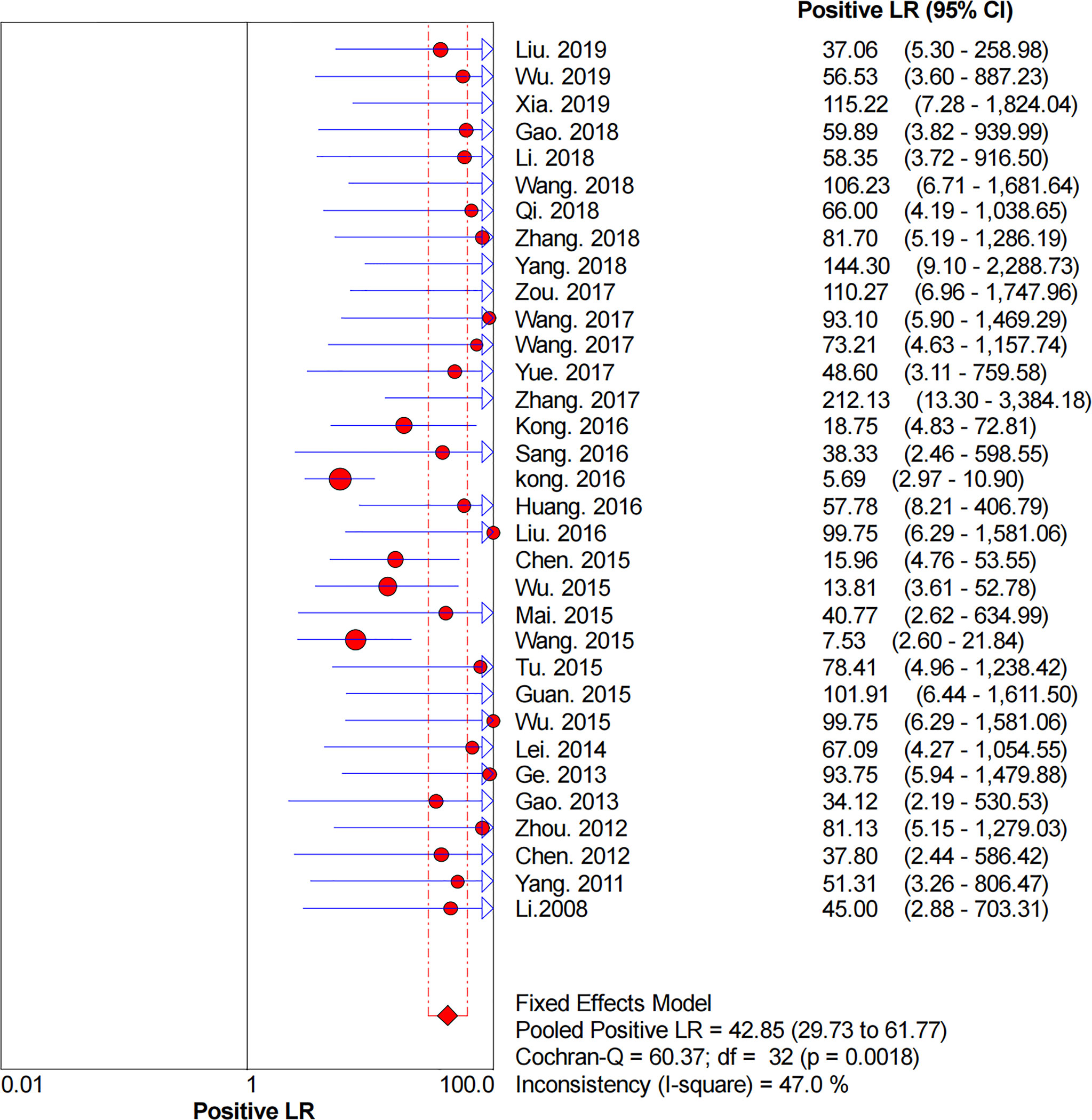
Figure 4 Forest plot of pooled PLR of the diagnosis value of CNSs in SLNB of breast cancer. 95% CI, 95% confidence interval; PLR, positive likelihood ratio.
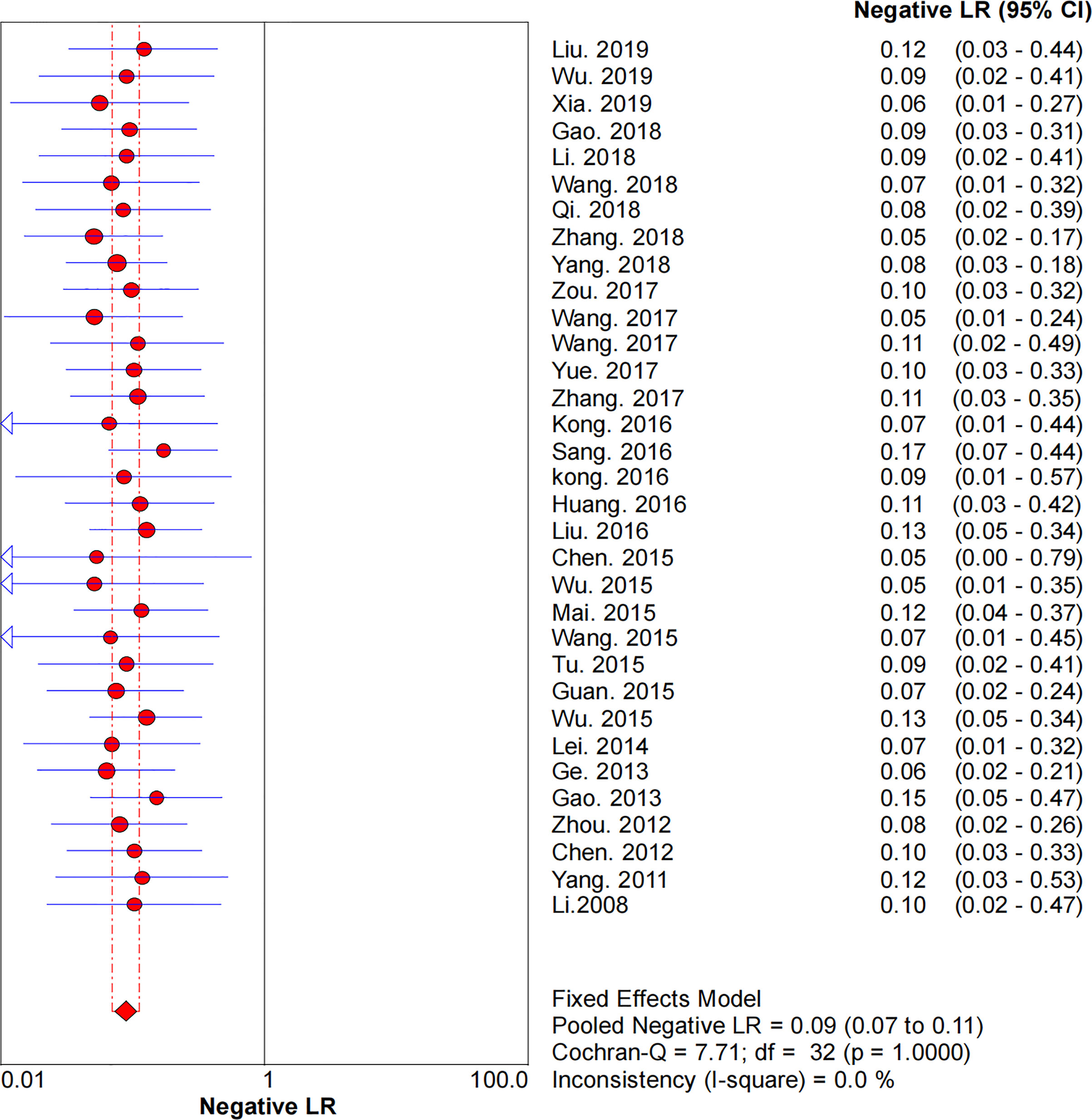
Figure 5 Forest plot of pooled NLR of the diagnosis value of CNSs in SLNB of breast cancer. 95% CI, 95% confidence interval; NLR, negative likelihood ratio.
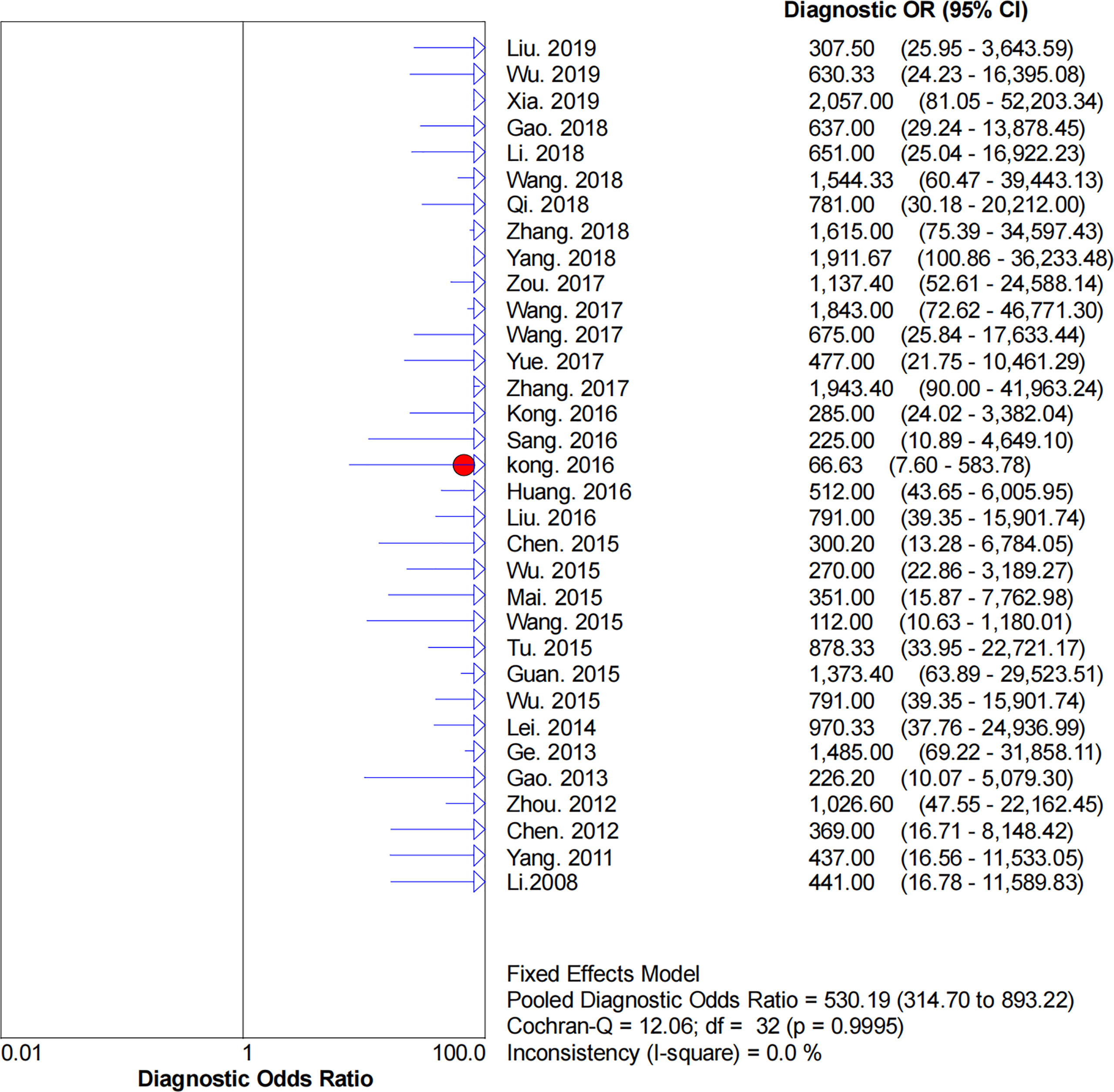
Figure 6 Forest plot of pooled DOR of the diagnosis value of CNSs in SLNB of breast cancer. 95% CI, 95% confidence interval. DOR, diagnostic odds ratio.
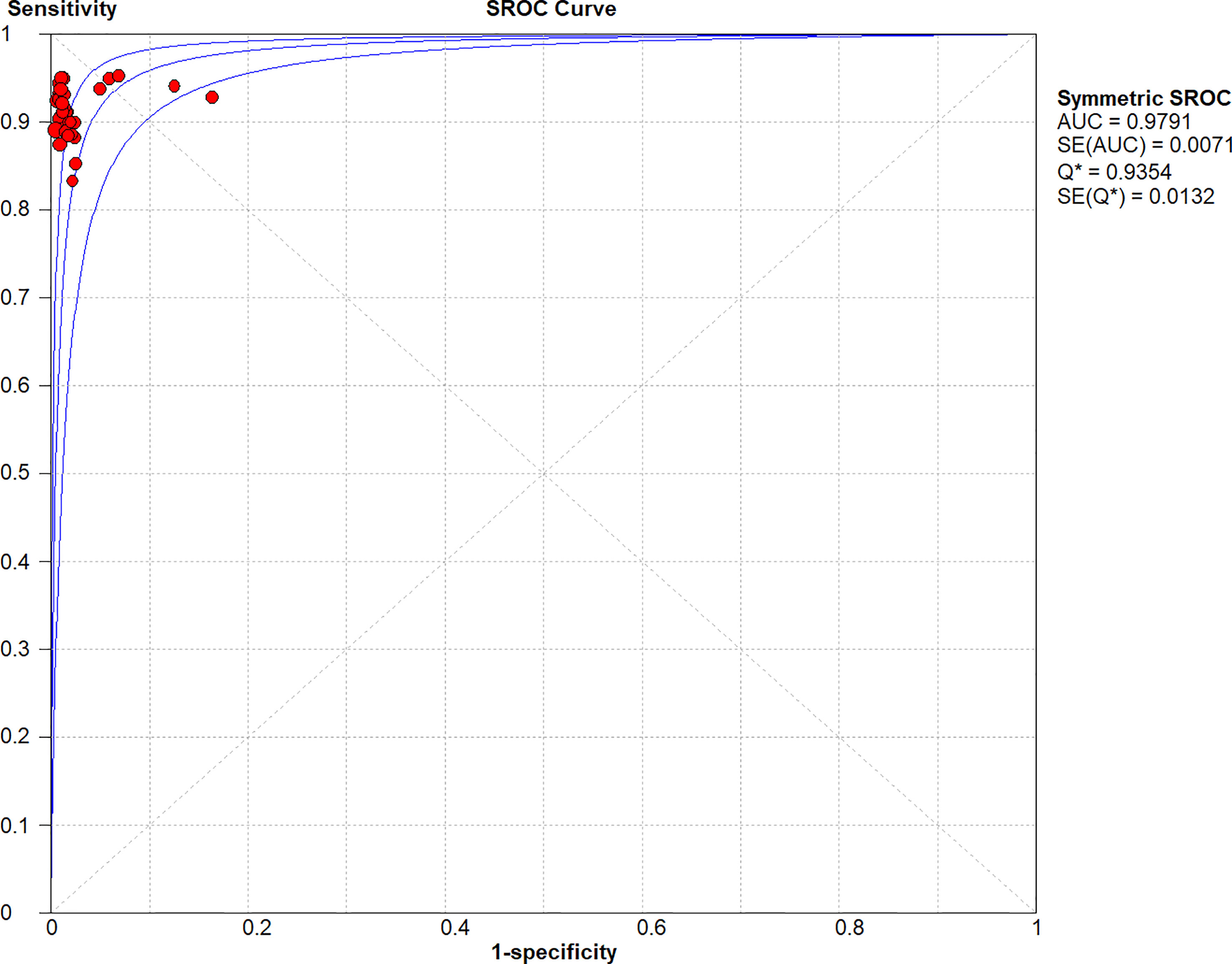
Figure 7 Symmetric SROC curve of the diagnosis value of CNSs in SLNB of breast cancer. SROC, summary receiver operating characteristic curve; AUC, the area under the receiver-operator characteristic curve.
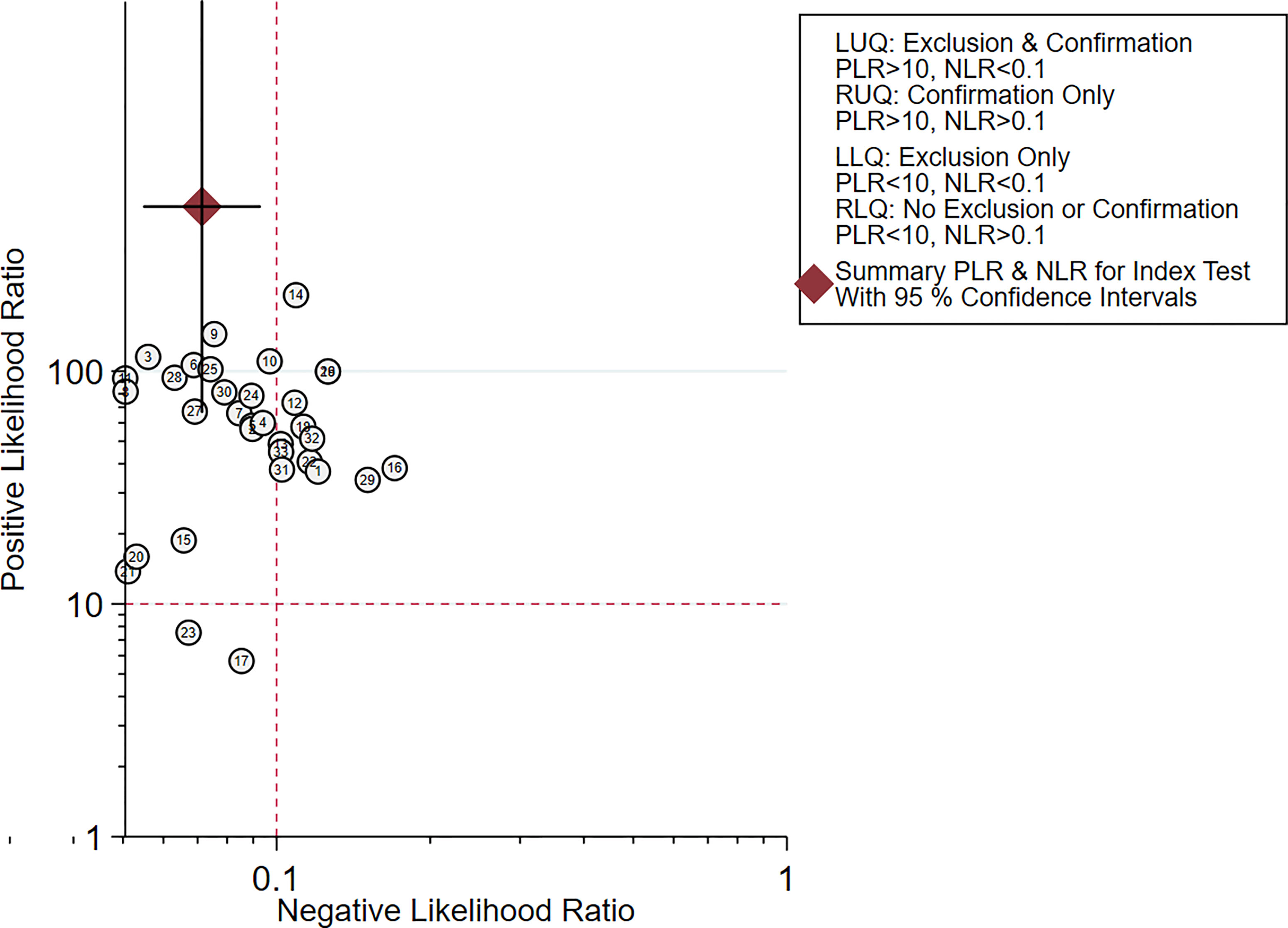
Figure 8 Scattergram of the PLR and NLR of the diagnosis value of CNSs in SLNB of breast cancer. PLR, positive likelihood ratio; NLR, negative likelihood ratio; LLQ, left lower quadrant; LUQ, left upper quadrant; RLQ, right lower quadrant; RUQ, right upper quadrant.
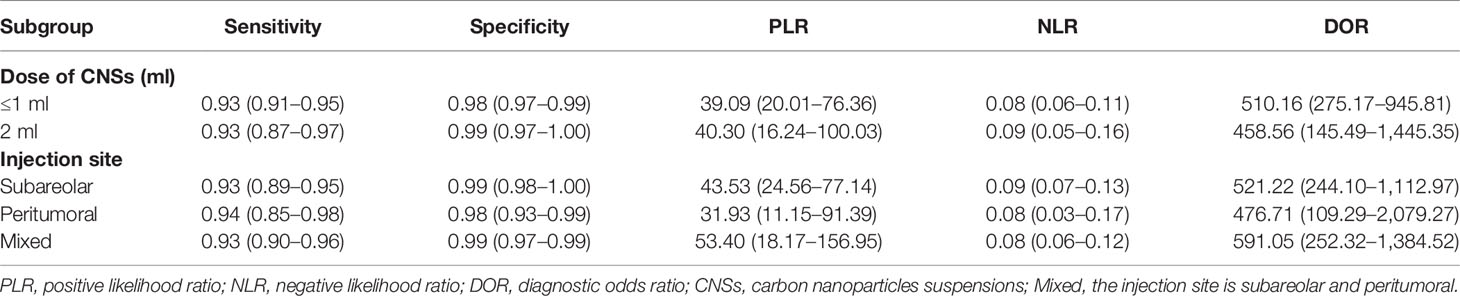
There is controversy over the optimal dose and site of injection for the tracking agents. We compared the combined sensitivity and specificity of SLNB according to different CNSs doses (Table 2). For the studies that used a less than or equal to 1 ml injection of CNSs, the combined sensitivity was 0.93 (95% CI: 0.91–0.95, I2 = 0.0%) and specificity was 0.98 (95% CI: 0.97–0.99, I2 = 63.0%) (Figure S1). For the studies that used a 2 ml injection of CNSs, the combined sensitivity and specificity was 0.93 (95% CI: 0.87–0.97, I2 = 0.0%) and 0.99 (95% CI: 0.97–1.00, I2 = 9.3%) (Figure S2). The results suggested that the diagnostic value of CNSs was not dose-dependent over the range of doses tested.
We further compared the effect of different injection sites, peritumoral or subareolar, on the SLNB (Table 2). The pooled sensitivity for studies that used subareolar injection was 0.93 (95% CI: 0.89–0.95, I2 = 0.0%), while in studies using peritumoral and mixed injection, the pooled sensitivity was 0.94 (95% CI: 0.85–0.98, I2 = 0.0%) and 0.93 (95% CI: 0.90–0.96, I2 = 0.0%). The combined specificity for studies using subareolar, peritumoral and mixed injection was 0.99 (95% CI: 0.98–1.00, I2 = 37.5%), 0.98 (95% CI: 0.93–0.99, I2 = 50.6%) and 0.99 (95% CI: 0.97–0.99, I2 = 71.2%), respectively. All groups were not significantly different from each other (Figures S3–5).
Publication Bias and Sensitivity Analysis
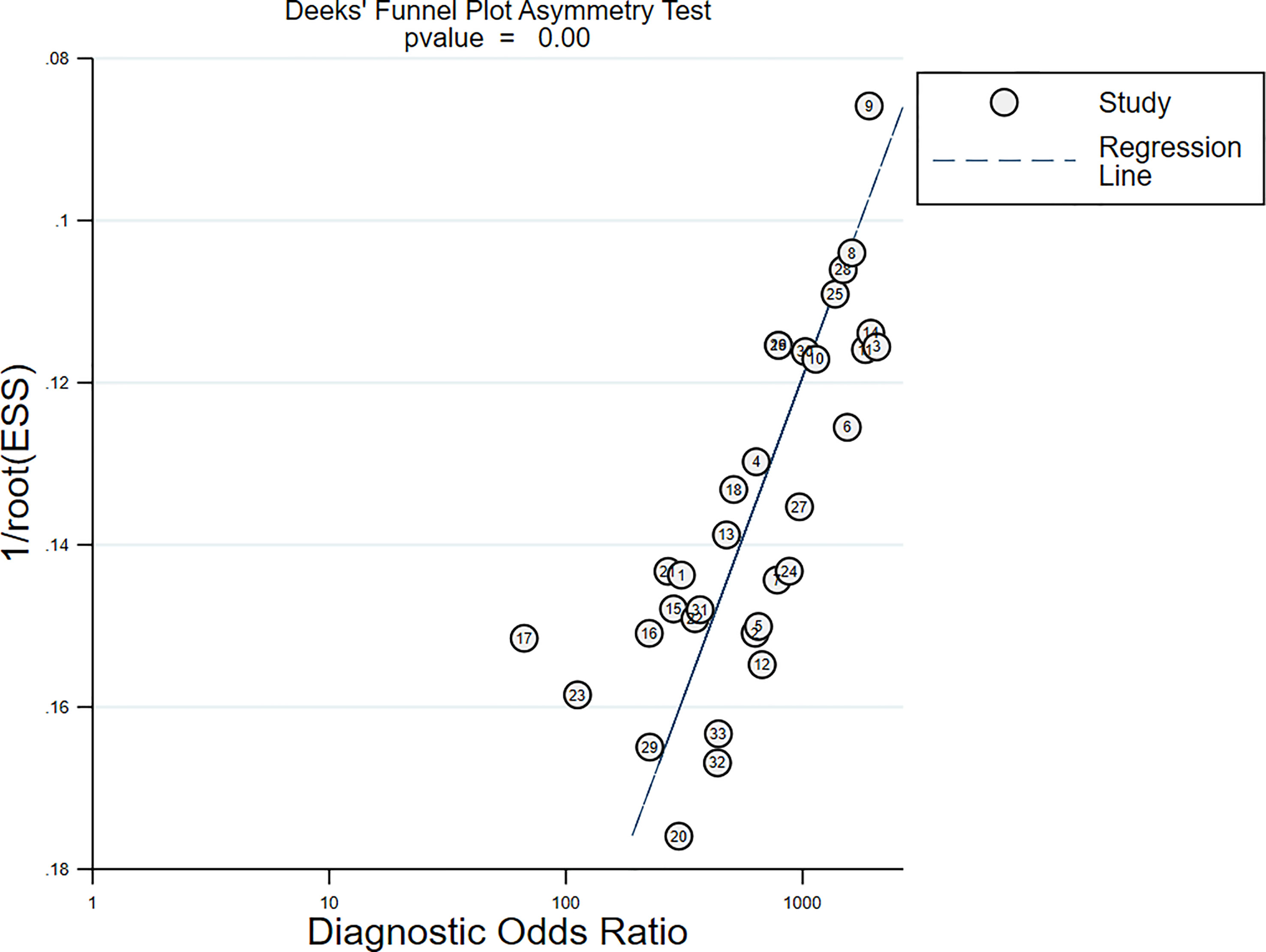
All studies harbored significant publication bias, as indicated by the Deeks’ funnel plot [Slope (Bias) = −7.35, P = 0.00; Figure 9]. Nonetheless, the sensitivity analysis showed that the results were reliable and stable (Table S1).
Discussion
SLNB was first reported in cutaneous melanoma by Morton et al. in 1992 (43). The SLNB concept was soon accepted for use in patients with breast cancer and led to better, less debilitating, axillary management (44). Both ALND and SLNB are not significantly different in terms of patient survival and tumor recurrence, thus further popularizing the widespread use of SLNB. SLNB carries the significant benefits of lower morbidity, especially with regards to arm lymphedema, paresthesia, and overall dysfunction (2–5). Currently, SLNB represents the standard surgical approach for axillary management in early breast cancer.
The mapping method is a decisive factor that determines the identification rate of SLN in breast cancer. RI technetium-99m was first used for SLNB mapping in 1993, followed by the use of blue dye (44, 45). The NSABP B-32 trial found that a combination of BD and radiocolloid resulted in a 97.1% detection rate for SNLB, compared with a 70.2% for BD and 89.4% for radiocolloid when used alone (46). Similar findings were noted in the ALMANAC study that demonstrated that a combination of isotope and BD had a 96.1% detection rate, but the use of either isotope or BD alone was 85.6% (47). Therefore, the method of combining BD and RI is currently regarded as the gold standard. Nevertheless, there are also disadvantages associated with this approach, namely, BD allergic reactions, the need for highly specialized nuclear medicine units, and the risk of radiation exposure to healthcare professionals and patients. New methods of lymphatic mapping that offer equal accuracy without the risks of allergies or irradiation are currently being trialed. A network meta-analysis showed that in contrast to using BD alone, superparamagnetic iron oxide nanoparticles or indocyanine green fluorescence alone are superior. The use of these novel agents alone is even comparable to the standard dual-modality technique. However, their use still mandates specialized equipment that may not be widely available (48).
CNSs is a new method that requires no specialized medical facilities for SLNB. This meta-analysis aimed to evaluate the diagnostic performance of CNSs for SLNB in breast cancer. The pooled sensitivity, specificity, and AUC of the SROC were 0.93, 0.99, and 0.98, respectively. The pooled DOR, a diagnostic performance index that takes into consideration specificity and sensitivity, in the current analysis was 530.19. Higher DOR values indicate a stronger discriminating power. The results suggest CNSs could be utilized to identify true positive patients with SLN metastases while also ruling out false negatives.
The optimal dose and injection site of CNSs for SLNB is controversial. The most regularly used doses are 1 and 2 ml. In the 33 studies analyzed, the volume of CNSs varied from 0.2 to 2 ml (Table 1). Subgroup analysis highlighted that there was no difference in specificity or sensitivity between the studies that used ≤1 ml versus 2 ml injections of CNSs (Table 2), which indicated that 1 ml volume of CNSs is sufficient. In this meta-analysis, peritumoral CNSs injection for SLNB was used in 3 studies, subareolar CNSs injection was used in 15 studies, and 14 studies were used in both approaches. No significant difference in the sensitivity and specificity of SLNB was detected between studies using peritumoral and subareolar CNSs injection. Therefore, both peritumoral and subareolar are appropriate injection sites for SLNB with CNSs (Figure 10) (49).
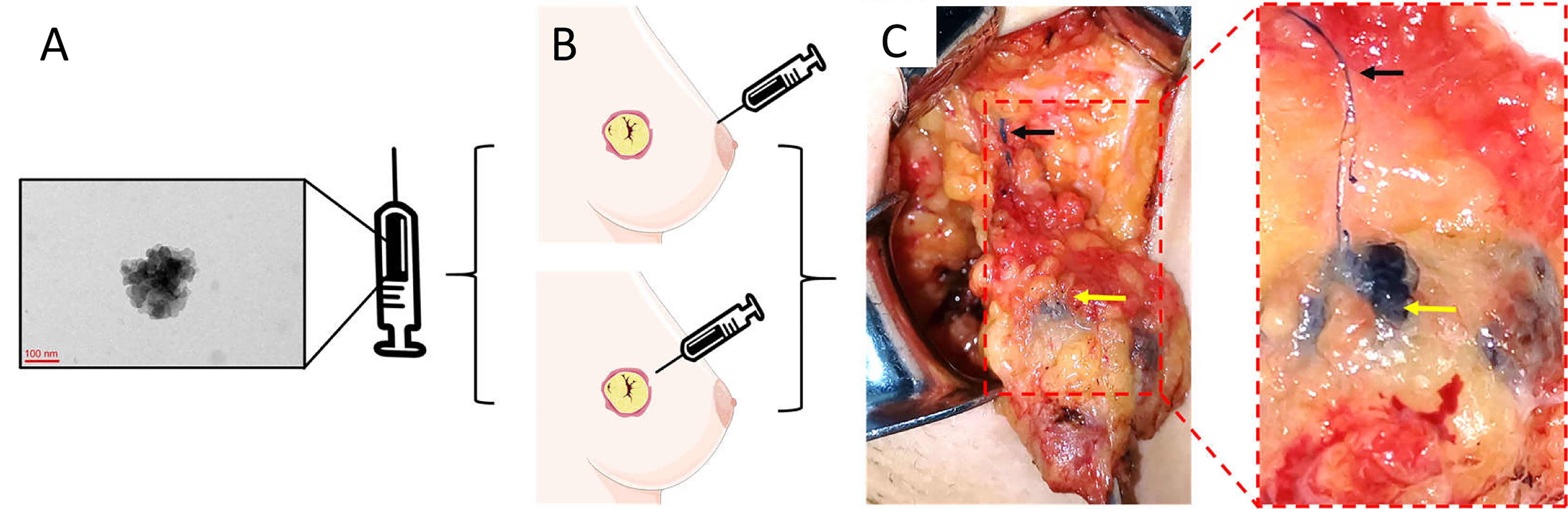
Figure 10 The specific operation steps of CNSs as lymphatic tracer in SLNB. (A) The morphology of CNSs; (B) Injection site of CNSs; (C) Color rendering of CNSs in SLNB (Black arrow: Lymphatic vessel; Yellow arrow: Lymph node). (A) is the image of CNSs under transmission electron microscopy. Republished with permission of SAGE Publications, Inc., from Liu X, Chang S, Jiang XL, Huang P, Yuan ZT. Identifying parathyroid glands with carbon nanoparticle suspension does not help protect parathyroid function in thyroid surgery: a prospective, randomized control clinical study. Surg Innov (2016) 23(4):381–9. doi: 10.1177/1553350615624787. © The Author(s) 2016; permission conveyed through Copyright Clearance Center, Inc (49).
In terms of adverse effects, none of the 2,171 included patients in this analysis developed a local inflammatory response, fat or skin necrosis, or an anaphylactic reaction. Nevertheless, the use of CNSs does have some limitations, with skin staining being the most frequently encountered side effect of CNSs (18, 35). This complication appears to be linked to the depth of injection based on our empirical observations. Therefore, a subcutaneous injection should be used instead of an intradermal injection. Another disadvantage of CNSs is that they cannot be seen through the skin and fatty tissue, therefore possessing lower visualization clarity compared to a fluorescent tracer (e.g., indocyanine green). Interestingly, a recent study suggests that CNSs have not only been employed as lymph node tracers but may also be useful as a carrier for antitumor therapy (50).
Conclusions
This meta-analysis highlights the accuracy and feasibility of using CNSs for SLNB mapping in breast cancer patients. The CNSs mapping method would be especially helpful in institutions without access to fluorescence imaging systems or RI. CNSs may be incorporated in a wide range of clinical applications, namely, theranostics and in breast cancer therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
All authors read and approved the final manuscript prior to submission. YJ, JiL, BC, YB, CL, YL, and TL: data curation, software, writing—original draft. JuL and XC: supervision, writing—review and editing. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81860715) and the Doctor Foundation of Affiliated Hospital of Zunyi Medical University (No. 201712).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.818812/full#supplementary-material
References
1. Cabanas RM. An Approach for the Treatment of Penile Carcinoma. Cancer (1977) 39(2):456–66. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i
2. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-Lymph-Node Resection Compared With Conventional Axillary-Lymph-Node Dissection in Clinically Node-Negative Patients With Breast Cancer: Overall Survival Findings From the NSABP B-32 Randomised Phase 3 Trial. Lancet Oncol (2010) 11(10):927–33. doi: 10.1016/s1470-2045(10)70207-2
3. Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity Results From the NSABP B-32 Trial Comparing Sentinel Lymph Node Dissection Versus Axillary Dissection. J Surg Oncol (2010) 102(2):111–8. doi: 10.1002/jso.21535
4. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized Multicenter Trial of Sentinel Node Biopsy Versus Standard Axillary Treatment in Operable Breast Cancer: The ALMANAC Trial. J Natl Cancer Inst (2006) 98(9):599–609. doi: 10.1093/jnci/djj158
5. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA (2017) 318(10):918–26. doi: 10.1001/jama.2017.11470
6. Benson JR, della Rovere GQ. Management of the Axilla in Women With Breast Cancer. Lancet Oncol (2007) 8(4):331–48. doi: 10.1016/s1470-2045(07)70103-1
7. Yang J, Xu L, Liu P, Du Z, Chen J, Liang F, et al. Accuracy of Sentinel Lymph Node Biopsy in Breast Cancer: Pitfalls in the Application of Single Tracers. Cancer Manag Res (2020) 12:3045–51. doi: 10.2147/CMAR.S244806
8. Modugno G, Menard-Moyon C, Prato M, Bianco A. Carbon Nanomaterials Combined With Metal Nanoparticles for Theranostic Applications. Br J Pharmacol (2015) 172(4):975–91. doi: 10.1111/bph.12984
9. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
10. Liu XM, Bao GQ, Qiu B, Fan D, Tang H, Yang XJ, et al. Application of Nano-Carbon Suspension in Tracing Sentinel Lymph Node in the Diagnosis and Treatment of Early Breast Cancer. Oncol Prog (2019) 17(18):2134–6. doi: 10.11877/j.issn.1672-1535.2019.17.18.08
11. Wu PY, Chen YJ. Application Study of Nano-Carbon Staining Method in Sentinel Lymph Node Biopsy of Early Breast Cancer. China Modern Med (2019) 26(25):82–5. doi: 10.3969/j.issn.1674-4721.2019.25.024
12. Xia GH, Cao B, Yang Y, Tang J, Zhu JY. Application of Carbon Nanoparticles and Methylene Blue in Sentinel Lymph Node Biopsy of Breast Cancer. Clin Educ Gen Pract (2019) 17(6):554–5. doi: 10.13558/j.cnki.issn1672-3686.2019.06.024
13. Gao HY, Dai JB, Xue J. Carbon Nanoparticle Suspension With Methy Blue Injection in Sentinel Lymph Node Biopsy for Breast Cancer Patients. J Chin Oncol (2018) 24(9):892–5. doi: 10.11735/j.issn.1671-170X.2018.09.B010
14. Li DH, Kong Y. Application Value of Nano-Carbon and Methylene Blue in Sentinel Lymph Node Biopsy of Breast Cancer. J Prev Med Chin PLA (2018) 36(5):689–90. doi: 10.13704/j.cnki.jyyx.2018.05.039
15. Wang M, Yao F. Carbon Nanoparticle Suspension in Sentinel Lymph Node Biopsy in Patients With Breast Cancer. J Chin Pract Diagn Ther (2018) 32(4):358–61. doi: 10.13507/j.issn.1674-3474.2018.04.015
16. Qi X. The Clinical Value of the Use of Carbon Nanoparticle Suspension Injection for Sentinel Lymph Node Biopsy in Breast Cancer. [Master’s Thesis]. Jilin, China: Jilin University (2018).
17. Yang SX, Wei WS, Jiang QH, Zhou YF, Qu W, Tu JH, et al. Analysis of 246 Sentinel Lymph Node Biopsies of Patients With Clinical Primary Breast Cancer by Application of Carbon Nanoparticle Suspension. J Obstet Gynaecol Res (2018) 44(6):1150–7. doi: 10.1111/jog.13635
18. Zhang L, Huang Y, Yang C, Zhu T, Lin Y, Gao H, et al. Application of a Carbon Nanoparticle Suspension for Sentinel Lymph Node Mapping in Patients With Early Breast Cancer: A Retrospective Cohort Study. World J Surg Oncol (2018) 16(1):112. doi: 10.1186/s12957-018-1414-6
19. Zou WW, Bai Y, Wang XL, Cheng K, Sun HG, Wu MM, et al. Comparison Between Indocyanine Green Fluorescence Imaging Plus Methylene Blue and Plus Carbon Nanoparticles Suspension Injection for Sentinel Lymph Node Biopsy in Breast Cancer Patients. J Pract Med (2017) 33(11):1857–60. doi: 10.3969/j.issn.1006-5725.2017.11.036
20. Wang XW. Application of Nano-Carbon and Methylene Blue in Sentinel Lymph Node Biopsy of Breast Cancer. [Master’s Thesis]. Beijing, China: Peking Union Medical College (2017). doi: 10.7666/d.Y3277830
21. Wang L, Chen X, Zhao CG, Peng Q, Chen L, Yu HJ. Application of Nano-Carbon to the Sentinel Lymph Node Biopsy for Breast Cancer. Chin J Clin Oncol Rehabil (2017) 24(1):49–51. doi: 10.13455/j.cnki.cjcor.2017.01.14
22. Yue J, Pei J, Ren M, Zhang JJ, Yang F, Zhang ZS, et al. Application of Fluorescence Combined With Nano-Carbon in Sentinel Lymph Node Biopsy of Breast Cancer. Acta Universitatis Medicinalis Anhui (2017) 52(2):236–9. doi: 10.19405/j.cnki.issn1000-1492.2017.02.019
23. Zhang JW. Comparison of the Value of Methylene Blue and Carbon Nano Partical Suspension in Sentinel Lymph Node Biopsy of Breast Cancer. [Master’s Thesis]. Shenyang, China: China Medical University (2017).
24. Kong ZH, Huang YF, Li JJ, Mo CS, Liang LC. Comparison of Nano-Carbon and Methylene Blue Tracer in Axillary Sentinel Lymph Node Biopsy of Breast Cancer. Guangxi Med J (2016) 38(6):852–3+8. doi: 10.11675/j.issn.0253-4304.2016.06.28
25. Sang JH. Clinical Observation of Methylene Blue Combined With Carbon Nano-Tracers of Early Breast Cancer SLNB. [Master’s Thesis]. Zhengzhou, China: Zhengzhou University (2016).
26. Kong C, Xia Q, Yu H, Zhang CJ. A Clinical Comparative Study Between Carbon Nanoparticles and Methylene-Blue for the Application of Sentinel Lymph Node Biopsy in Early Breast Cancer. Med Philosophy (B) (2016) 37(3):26–8+85. doi: 10.12014/j.issn.1002-0772.2016.03b.08
27. Huang K, Zeng Y. Feasibility of Applying Nano-Carbon in Sentinel Lymph Node Biopsy in Early Breast Cancer. IMHGN (2016) 22(20):3097–9. doi: 10.3760/cma.j.issn.1007-1245.2016.20.013
28. Liu B, Li F, Wang SL, Zhang FF, Long HL. Study on the Application of Nano-Carbon Sentinel Lymph Node Biopsy in Patients With Early Breast Cancer Under Color Doppler Ultrasonography. Psychologist (2016) 22(35):176–8.
29. Chen PZ. Clinical Study of Nano-Carbon Tracer in Sentinel Lymph Node Biopsy of Breast Cancer. Strait Pharm J (2015) 27(12):186–7. doi: 10.3969/j.issn.1006-3765.2015.12.100
30. Wu Y, Zhou Y, Yang GS, Liu X, Huang H. Application Anulysis of Carbon Nanoparticles Suspension in Sentinel Lymph Node Biopsy for Breast Cancer. J Clin Surg (2015) 23(7):511–3. doi: 10.3969/j.issn.1005-6483.2015.07.013
31. Mai F. Clinical Comparison Between Carbon Nanoparticles and Methylene Blue Staining on Sentinel Lymph Lymph Node Biopsy for Breast Cancer. [Master’s Thesis]. Dalian, China: Dalian Medical University (2015).
32. Wang LC, Li LJ, Li YQ, Li XQ, Gao YF, Li JY, et al. Application Value of Carbon Nanoparticle in Axillary Sentinel Lymph Node Biopsy for Breast Cancer. Hubei J TCM (2015) 37(2):19–20.
33. Tu JH, Zhang H, Lu YH, Wang S, Ling LJ. Feasibility of Nanogate Carbon as Agent for Sentinel Lymph Node Biospy in Early Breast Cancer Patients. Chin J Exp Surg (2015) 32(12):3147–50. doi: 10.3760/cma.j.issn.1001-9030.2015.12.073
34. Guan HT, Ma XB, Diao Y, Shan CY, Zhao Y, Min WL, et al. Sentinel Lymph Node Biopsy in Breast Cancer Patients by Using Nanometer Carbon Suspension Liquid. J North Sichuan Med Coll (2015) 30(4):439–41+446. doi: 10.3969/j.issn.1005-3697.2015.04.04
35. Wu XF, Lin QZ, Chen G, Lu JP, Zeng Y, Chen X, et al. Sentinel Lymph Node Detection Using Carbon Nanoparticles in Patients With Early Breast Cancer. PloS One (2015) 10(8). doi: 10.1371/journal.pone.0135714
36. Lei SG, Yu XF, Xie CW, Ji MY, Liu QM. Application of Carbon Nanoparficle in the Axillary Sentinel Lymph Node Biopsy for Breast Cancer. Chin J Postgrad Med (2014) 37(26):1–3. doi: 10.3760/cma.j.issn.1673-4904.2014.26.001
37. Ge J, Yan B, Cao XC. Comparison of Sentinel Lymph Node Detection by Methylene Blue and Carbon Nanoparticle Suspension Injection in Early Breast Cancer. Chin J Oncol (2013) 41(8):828–9. doi: 10.3969/j.issn.0253-9896.2013.08.032
38. Gao W, Guo WB. Application of Carbon Nanoparticle Suspension Injection Into the Mammary Gland in the Sentinel Lymph Node Biopsy for Breast Cancer. Pract Pharm And Clin Remedies (2013) 16(3):187–9. doi: 10.3969/j.issn.1673-0070.2013.03.003
39. Zhou Y, Du CH, Chen ZM, Li Y, Lin QM, Wang QT. Comparison Between Carbon Nanoparticles and Methylene Blue on the Sentinel Lymph Node Biopsy for Breast Cancer. Lingnan Modern Clinics Surg (2012) 12(2):118–20. doi: 10.3969/j.issn.1009-976X.2012.02.012
40. Chen ZY, Hu XC, Wang HJ, Liu DS, Hong W. Application of Nano-Carbon Suspension Injection in Sentinel Lymph Node Biopsy of Middle and Early Stage Breast Cancer. Inner Mongol J Tradit Chin Med (2012) 31(18):78–9. doi: 10.3969/j.issn.1006-0979.2012.18.092
41. Yang LJ, Li Z. Clinical Research of Sentinel Lymph Nodes Biopsy in Breast Cancer by Methylene Blue and Nanometres Carbon Suspension Liquid. Modern Med Health (2011) 27(15):2255–6.
42. Li X, Duan XQ. Comparative Study of Methylene Blue, Isosulfide Blue and Nano-Carbon Suspension in Sentinel Lymph Node Biopsy of Breast Cancer. J Qiqihar Med Coll (2008) 29(9):1051–3. doi: 10.3969/j.issn.1002-1256.2008.09.013
43. Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch Surg (1992) 127(4):392–9. doi: 10.1001/archsurg.1992.01420040034005
44. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann Surg (1994) 220(3):391–401. doi: 10.1097/00000658-199409000-00015
45. Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical Resection and Radiolocalization of the Sentinel Lymph Node in Breast Cancer Using a Gamma Probe. Surg Oncol (1993) 2(6):335–40. doi: 10.1016/0960-7404(93)90064-6
46. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical Outcomes of Sentinel-Lymph-Node Resection and Conventional Axillary-Lymph-Node Dissection in Patients With Clinically Node-Negative Breast Cancer: Results From the NSABP B-32 Randomised Phase III Trial. Lancet Oncol (2007) 8(10):881–8. doi: 10.1016/s1470-2045(07)70278-4
47. Goyal A, Newcombe RG, Chhabra A, Mansel RE. Factors Affecting Failed Localisation and False-Negative Rates of Sentinel Node Biopsy in Breast Cancer–Results of the ALMANAC Validation Phase. Breast Cancer Res Treat (2006) 99(2):203–8. doi: 10.1007/s10549-006-9192-1
48. Mok CW, Tan SM, Zheng Q, Shi L. Network Meta-Analysis of Novel and Conventional Sentinel Lymph Node Biopsy Techniques in Breast Cancer. BJS Open (2019) 3(4):445–52. doi: 10.1002/bjs5.50157
49. Liu X, Chang S, Jiang XL, Huang P, Yuan ZT. Identifying Parathyroid Glands With Carbon Nanoparticle Suspension Does Not Help Protect Parathyroid Function in Thyroid Surgery: A Prospective, Randomized Control Clinical Study. Surg Innov (2016) 23(4):381–9. doi: 10.1177/1553350615624787
Keywords: meta-analysis, diagnosis, sentinel lymph node biopsy, carbon nanoparticle suspensions, breast cancer
Citation: Jiang Y, Li J, Chen B, Bao Y, Luo C, Luo Y, Li T, Lv J and Cheng X (2022) Sentinel Lymph Node Biopsy Mapped With Carbon Nanoparticle Suspensions in Patients With Breast Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 12:818812. doi: 10.3389/fonc.2022.818812
Received: 20 November 2021; Accepted: 21 February 2022;
Published: 28 March 2022.
Edited by:
Abhishek Mahajan, Tata Memorial Hospital, IndiaReviewed by:
Stephanie Kacerovsky-Strobl, Medical University of Vienna, AustriaZhenggui Du, Sichuan University, China
Copyright © 2022 Jiang, Li, Chen, Bao, Luo, Luo, Li, Lv and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyuan Lv, anVueXVhbmx2QHptdS5lZHUuY24=; Xiaoming Cheng, Y3htMTY4OEBzaW5hLmNvbQ==
 Yan Jiang
Yan Jiang Jiayang Li2
Jiayang Li2 Baolin Chen
Baolin Chen Taolang Li
Taolang Li