
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 April 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.818613
This article is part of the Research Topic Women in Gynecological Oncology: 2021 View all 34 articles
 Antonella Ravaggi1,2,3*†
Antonella Ravaggi1,2,3*† Angela Gambino1,2†
Angela Gambino1,2† Federico Ferrari2
Federico Ferrari2 Alessandro Olivari1
Alessandro Olivari1 Laura Zanotti1,3
Laura Zanotti1,3 Chiara Romani3
Chiara Romani3 Laura Ardighieri4
Laura Ardighieri4 Paolo Antonelli5
Paolo Antonelli5 Giorgia Garganese6,7
Giorgia Garganese6,7 Daniela Gallo7,8
Daniela Gallo7,8 Giovanni Scambia7,8
Giovanni Scambia7,8 Eliana Bignotti2,3
Eliana Bignotti2,3 Enrico Sartori1,2
Enrico Sartori1,2 Stefano Calza5,9‡
Stefano Calza5,9‡ Franco Odicino1,2‡
Franco Odicino1,2‡Background: Radical surgical resection of the primary tumor with mono/bilateral inguinofemoral lymph node dissection is the standard treatment for invasive vulvar squamous cell carcinoma (VSCC) and is frequently related to severe morbidity. Tailoring surgical treatment is of paramount importance, and a comprehensive preoperative evaluation is mandatory. Vascular endothelial growth factor D (VEGF-D) is considered a regulator of lymphangiogenesis involved in tumor spread via lymphatic vessels. The aim of this study was to evaluate the potential of VEGF-D in the prediction of inguinofemoral lymph node metastasis.
Methods: We analyzed the preoperative levels of serum VEGF-D (sVEGF-D) from two independent cohorts of patients with VSCC by enzyme-linked immunosorbent assay and its protein expression on tumor tissue by immunohistochemistry. Logistic regression was performed to identify the independent risk factors for lymph node metastasis, and Cox proportional hazard model was used for survival analysis.
Results: High levels of sVEGF-D, but not tissue VEGF-D, significantly correlated with positive groin nodes and a more advanced International Federation of Gynecologists and Obstetricians (FIGO) stage. In multivariable analysis, a high sVEGF-D level was an independent predictor of lymph node metastasis and worse prognosis. A prediction model based on sVEGF-D, tumor grade assessed on biopsy, tumor diameter, and lymph node clinical evaluation was able to predict lymph node metastasis, reaching C-index values of 0.79 and 0.73 in the training and validation cohorts, respectively.
Conclusions: The preoperative sVEGF-D level might be a reliable biomarker for the prediction of lymph node metastasis and prognosis in patients with VSCC, supporting better clinical/surgical decision. Multicenter prospective studies are required to confirm our findings.
Vulvar carcinoma is a rare gynecologic cancer with an annual incidence of 2.4 every 100,000 women based on cases in 2014–2018 (1). In 2021, approximately 6,120 new cases of vulvar cancer have been predicted in the United States, and about 1,550 women are expected to die of this cause (2). The incidence is higher among women aged 70 years or older; however, recently, an increasing frequency of vulvar cancer in young women has been observed (3, 4).
For patients with invasive vulvar squamous cell carcinoma (VSCC), the most common type of vulvar cancer, the standard treatment in clinical early-stage disease is radical resection of the tumor with bilateral inguinofemoral lymphadenectomy or sentinel lymph node (SLN) biopsy, when appropriate. In the absence of clinical suspicious lymph nodes (cN0), the SLN procedure should be considered the preferred procedure instead of radical dissection only within narrow selection criteria, including: unifocal lesions of <4 cm, not completely excised at diagnostic biopsy, and never undergone previous vulvar/groin surgery or neoadjuvant treatments (5–7). Furthermore, the SLN procedure should be performed only in centers with high-level experience in order to minimize false-negative rates. Therefore, a large cohort of cN0 patients is currently not submitted to the SLN procedure and instead referred to radical lymphadenectomy. This procedure showed no evidence of lymph node metastasis at final histology in 70% of cases, with increased postoperative physical and psychological morbidity rates (8). For this reason, many efforts are being made to optimize clinical management in order to avoid overtreatment of patients without lymph node metastasis and to ensure a high diagnostic sensitivity in identifying patients with positive groin nodes (9).
In this context, the identification of circulating or tissue biomarkers involved in the aggressiveness of tumor cells and in the metastasis process could be an important tool to guide and/or refine the surgical decision.
One of the most studied molecular systems in the regulation of tumor lymphangiogenesis is based on the interaction between vascular endothelial growth factor D (VEGF-D) and the corresponding VEGF receptor 3 (VEGFR-3), commonly expressed on the surface of lymphatic endothelial cells (10, 11). VEGF-D is a member of the VEGF family, which also includes VEGF-A, VEGF-B, VEGF-C, and placental growth factor (PlGF), and it is an important key regulator of both physiological and pathological angiogenesis and lymphangiogenesis (11). VEGF-D promotes lymphatic metastasis by inducing tumor-associated lymphangiogenesis in a mouse tumor model (12), and its over expression was associated with lymphatic tumor spread and poor patient prognosis in several human cancers (13–16). To the best of our knowledge, only one study evaluated the expressions of VEGF-D and VEGFR-3 using immunohistochemistry (IHC) in VSCC (17). Further investigations are required to clarify the clinical significance of these markers in patients with VSCC.
In the present study, we investigated the role of VEGF-D in predicting inguinofemoral lymph node metastasis. For the first time, the preoperative levels of serum VEGF-D (sVEGF-D) were quantified and correlated with the clinicopathological characteristics and prognosis of patients affected by VSCC.
This retrospective study aimed to investigate the significance of VEGF-D in the clinical setting of VSCC patients and, in particular, in the preoperative prediction of lymph node metastasis. To this intent, two groups of patients with VSCC were recruited. Cohort A was used to analyze the correlations between sVEGF-D levels or the tissue expressions of VEGF-D and VEGFR-3 with the clinicopathological features and prognosis. Moreover, more importantly, cohort A was used as a training set to design a predictive algorithm for lymph node metastases. Three different models for the preoperative prediction of lymph node involvement were built using logistic regression: base model, including only clinical/radiological lymph node status; clinical model, including the clinicopathological characteristics available before surgery (e.g., tumor diameter, tumor grade from biopsy, and clinical lymph node status); and extended model, built with the addition of the sVEGF-D level to the clinical model.
Cohort B was kept external and independent and was used as a validation set to evaluate the performance of the extended model in the prediction of lymph node metastasis.
A retrospective study was performed on a total of 135 patients with VSCC divided into two independent cohorts, hereafter called A and B, comprising 80 and 55 women, respectively.
The inclusion criteria were as follows: histologically confirmed VSCC, tumor depth of invasion of at least 1 mm, surgical treatment performed in the enrolling centers, availability of frozen serum samples collected prior to surgery, and/or availability of formalin-fixed paraffin-embedded (FFPE) tumor tissue samples surgically obtained from the primary tumor site. For cohort B, only serum samples were required. Patients with synchronous cancer or with a history of malignancy in the 5 years prior to the VSCC diagnosis were excluded from the study of circulating sVEGF-D.
The eligible patients of cohort A were consecutively enrolled between January 2003 and August 2019 at the Division of Obstetrics and Gynecology of “ASST-Spedali Civili di Brescia” (Brescia, Italy). The eligible patients of cohort B were consecutively enrolled between May 2012 and October 2018 at the Division of Gynecologic Oncology of “Fondazione Policlinico Universitario A. Gemelli IRCCS” (Rome, Italy).
All patients were preoperatively assessed by expert gynecologic oncologists and dedicated specialists for primary tumor histology, obtained by incisional biopsy, and lymph node status evaluation, obtained by clinical exam and dedicated imaging (ultrasound and/or PET). A positive clinical lymph node status was defined in the presence of at least one suspicious lymph node at imaging.
All patients were surgically treated by complete surgical tumor resection (partial or radical vulvectomy) and inguinal lymph node dissection, performed mono- or bilaterally, as appropriate, on the base of the distance of the primary tumor from the midline, according to international guidelines (5, 6) at the time of the study.
All 80 patients from cohort A underwent radical lymphadenectomy. Among the 55 patients in cohort B, 12 underwent exclusive SLN, 9 underwent combined SLN and inguinofemoral lymphadenectomy, and 34 underwent inguinofemoral lymphadenectomy.
The stage of disease was assessed in accordance with the International Federation of Gynecologists and Obstetricians (FIGO) revised staging system of 2009, in use during the enrollment period.
Adjuvant treatment was administered to 24 out of 80 patients in cohort A (radiotherapy, chemotherapy, or chemoradiotherapy to 21, 2, and 1 patient, respectively). Fifty-one patients did not receive adjuvant therapies, and for 5 patients, this information was missing. All patients in cohort A were followed from the time of their confirmed diagnosis until death or March 2021. Follow-up data were not required for the patients in cohort B. Clinical and histopathological data were acquired from the original reports.
The characteristics of the patients in the two cohorts are summarized in Table 1.
The study was performed following the set of principles in the Declaration of Helsinki and was approved by the Research Review Board–Ethics Committee of the ASST Spedali Civili, Brescia, Italy (study reference no. NP3512) and of the Fondazione Policlinico Universitario “A. Gemelli” IRCCS—Università Cattolica del Sacro Cuore, Rome, Italy (study reference no. ID2844). Written informed consent for the collection and use of personal records and biological material for health research was obtained from all patients enrolled. All data were collected in an electronic database and managed in accordance with privacy regulations.
In both cohorts, fasting blood samples were drawn from patients strictly before surgery. Serum was separated by centrifugation at 1,500 × g for 10 min within 1 h, frozen in liquid nitrogen, and then stored at −80°C until analysis. Sample analysis was centralized in Brescia Hospital laboratory and carried out without any prior knowledge of the patients’ clinical status.
The sVEGF-D levels were analyzed with enzyme-linked immunosorbent assay (ELISA) using the immunoassay Human VEGF-D Quantikine ELISA (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions. Briefly, 50 ml of standards, controls, and serum samples were analyzed in duplicate, and plates were read at 450 nm, setting a wavelength correction to 570 nm, on an automatic plate reader (Spectramax 340 PC; Molecular Devices Corporation, Sunnyvale, CA, USA). As declared by the manufacturer, the dynamic range of VEGF-D detection goes from 125 to 4,000 pg/ml, with intra-assay and inter-assay imprecision (coefficient of variation, CV) values ranging from 2.4% to 6.2% and from 7.2% to 8.0%, respectively.
To evaluate the protein expression levels of VEGF-D and VEGFR-3, IHC staining was performed on VSCC tissue samples from cohort A at the Department of Pathology, “ASST-Spedali Civili of Brescia.” Whole tissue sections (2 μm) were obtained from FFPE blocks, stained with hematoxylin and eosin, and analyzed by a staff surgical pathologist. IHC analyses were performed on 4-μm tissue sections using the Leica Bond III fully automated IHC Stainer (Leica Biosystems, New Castle upon Tyne, UK). No antigen retrieval was carried out. The sections were incubated with the primary antibodies anti-VEGF-D diluted 1:200 (clone 78923; R&D Systems) for 15 min and anti-VEGFR-3 diluted 1:50 (clone NCL-L-VEGFR-3; Leica Biosystems) for 30 min. The reaction was revealed using the automated Leica BOND system by the Bond Polymer Refine Detection Kit (DS9800; Leica Biosystems), which consisted of sequential incubation with post-primary and horseradish peroxidase (HRP)–polymer for 8 min each, followed by diaminobenzidine as chromogen and by hematoxylin as nuclear counterstain.
Cellular staining was graded for intensity (0, negative staining; 1, weak staining; 2, moderate staining; and 3, strong staining) and percentage of positive cells (0, 0%; 1, 1%–20%; 2, 11%–50%; and 3, 51%–100%). A single IHC scale with scores 0–9 was calculated by multiplying the intensity and the percentage staining scores. Then, four total scores (0, 1, 2, 3) were obtained, grouping score 0 in total score 0, scores 1–3 in total score 1, scores 4 and 6 in total score 2, and score 9 in total score 3.
The association between the levels of sVEGF-D and the clinicopathological characteristics in cohort A was evaluated using univariable linear models after transforming the sVEGF-D levels on a log2 scale.
The role of sVEGF-D as a predictor of lymph node involvement was evaluated using a logistic regression model including the clinicopathological characteristics available before radical vulvectomy: estimated tumor diameter, tumor grade assessed on biopsy, and lymph node status from clinical/radiological evaluation. The results were reported as odds ratios (ORs) and 95% confidence intervals (95%CIs).
The discrimination performance of the models was quantified with the concordance index (C-index), which is equivalent to the area under the receiver operating characteristic (ROC) curve (AUC). The 95%CI for the C-index was obtained after 200 bootstrap resampling (18). Model calibration was evaluated graphically and reporting scaled Brier scores (the bigger the better) (19) and the Hosmer–Lemeshow test (20). Unreliability test (testing the H0 for a calibration line with slope = 1 and intercept = 0) was performed using a chi-squared test with 2 df (21).
Penalized maximum likelihood was used to obtain more stable coefficients through shrinkage in order to achieve better performance for prediction (22, 23). Selection of the optimal penalization parameter was performed based on Hurvich and Tsai’s corrected Akaike’s information criterion (AIC) (24).
Progression-free survival (PFS) was defined as the time from surgery to progression or recurrence, while disease-specific survival (DSS) was defined as the time from surgery to cancer-related death. Univariable and multivariable Cox proportional hazard models were used for modeling PFS and DSS.
For display purposes, the sVEGF-D values were dichotomized using maximally selected rank statistics (25), both for DSS and PFS. Briefly, this procedure searches for the optimal threshold that maximizes the log-rank statistic, accounting for test multiplicity. Binary-coded sVEGF-D values were used in the multivariable models accounting for stage, grade, and vascular and perineural invasion, and the corresponding survival curves were displayed using the Kaplan–Meier method.
All tests were two-sided and assumed a 5% significance level. All statistical analyses were performed with the program R Core Team (version 4.1.1).
Preoperative serum samples from 62 out of 80 patients in cohort A were suitable to analyze the sVEGF-D levels using ELISA. The median and the mean values of sVEGF-D were 456.0 pg/ml (range = 370.9–573.5) and 483.4 pg/ml (SD = 178.5), respectively. As shown in Table 2, high levels of sVEGF-D significantly correlated with lymph node metastasis (p = 0.023) and higher FIGO stage (III–IV vs. I–II, p = 0.023). No other significant correlations with the clinicopathological factors were evident.
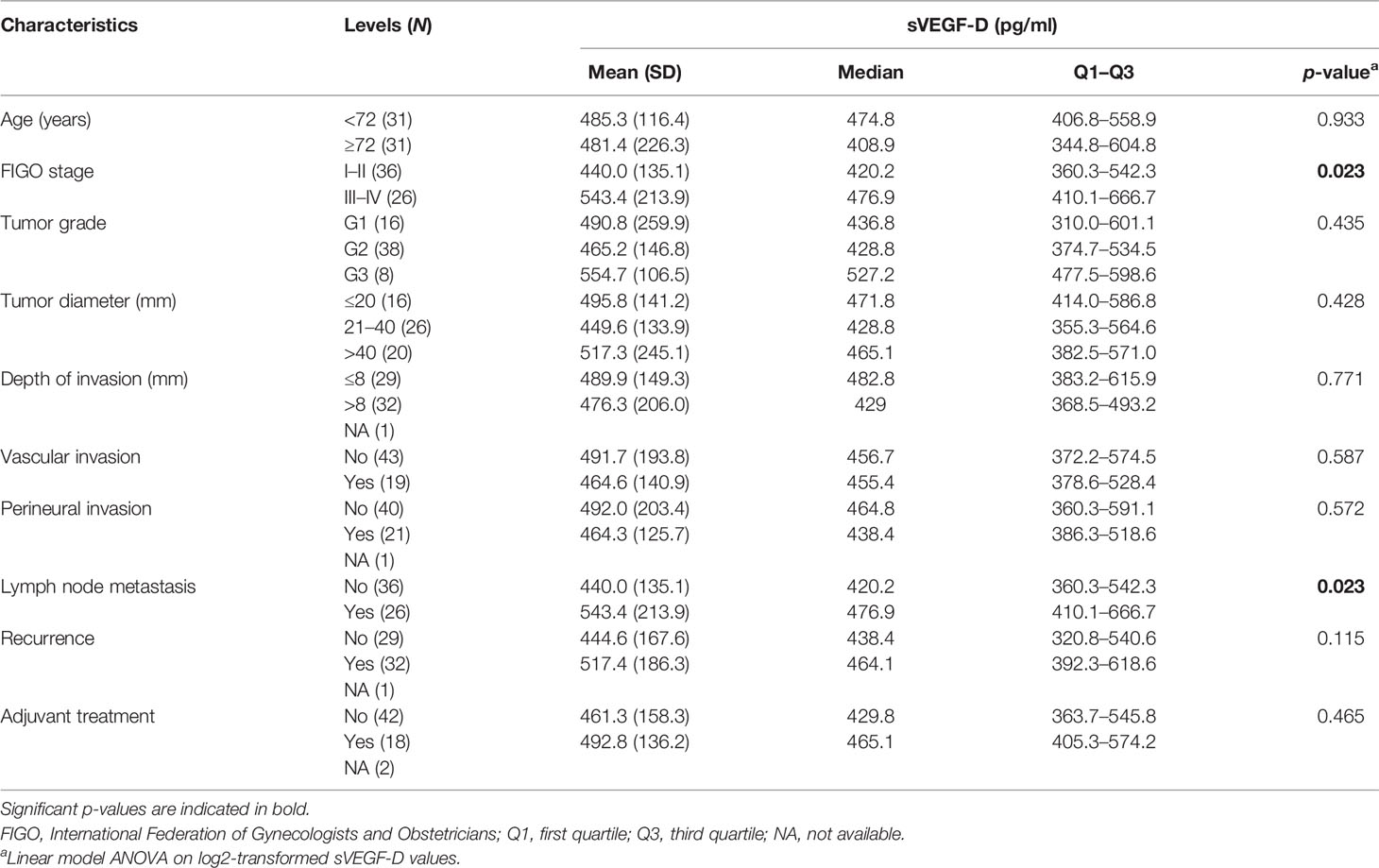
Table 2 Vascular endothelial growth factor D (VEGF-D) serum levels and correlation with the clinicopathological characteristics of vulvar squamous cell carcinoma (VSCC) patients from cohort A (N = 62).
In 49 out of 80 patients, FFPE tumor tissue samples surgically obtained from the primary tumor site were available to evaluate the tissue protein expression of VEGF-D and its receptor VEGFR-3. A pattern of positive cytoplasmic expression for VEGF-D and VEGFR-3 was found in 92% and 94% of the VSCC patients, respectively (Supplementary Figure S1). No correlation was found between the expression of VEGF-D or VEGFR-3 and the clinicopathological characteristics of the tumor (Supplementary Table S1); therefore, the next steps of the study were conducted considering exclusively serum VEGF-D.
For 31 out of 80 patients, matched tissue and serum samples were available. The serum VEGF-D level was only partially correlated with the tissue VEGF-D protein score, with a borderline statistical significance (rs = 0.317, p = 0.082).
In cohort A, 79 out of 80 patients were considered for survival analysis (median follow-up = 101.1 months, IQR = 58.0–150.5 months). Forty-one (51.9%) had disease recurrence or progression. At the time of the last follow-up, 36 patients (45.6%) were alive, 30 (38.0%) were dead of disease, and 13 (16.4%) died of other causes.
In the univariable analysis for DSS, higher preoperative sVEGF-D levels (log scale) were significantly associated with worse prognosis [hazard ratio (HR) = 2.70, p = 0.02] and with other traditional prognostic factors, such as advanced FIGO stage (HR = 9.43, p < 0.01), perineural invasion (HR = 2.17, p = 0.03), lymphovascular invasion (HR = 2.55, p = 0.01), and lymph node metastasis (HR = 9.43, p < 0.01) (Figure 1A and Supplementary Table S2).
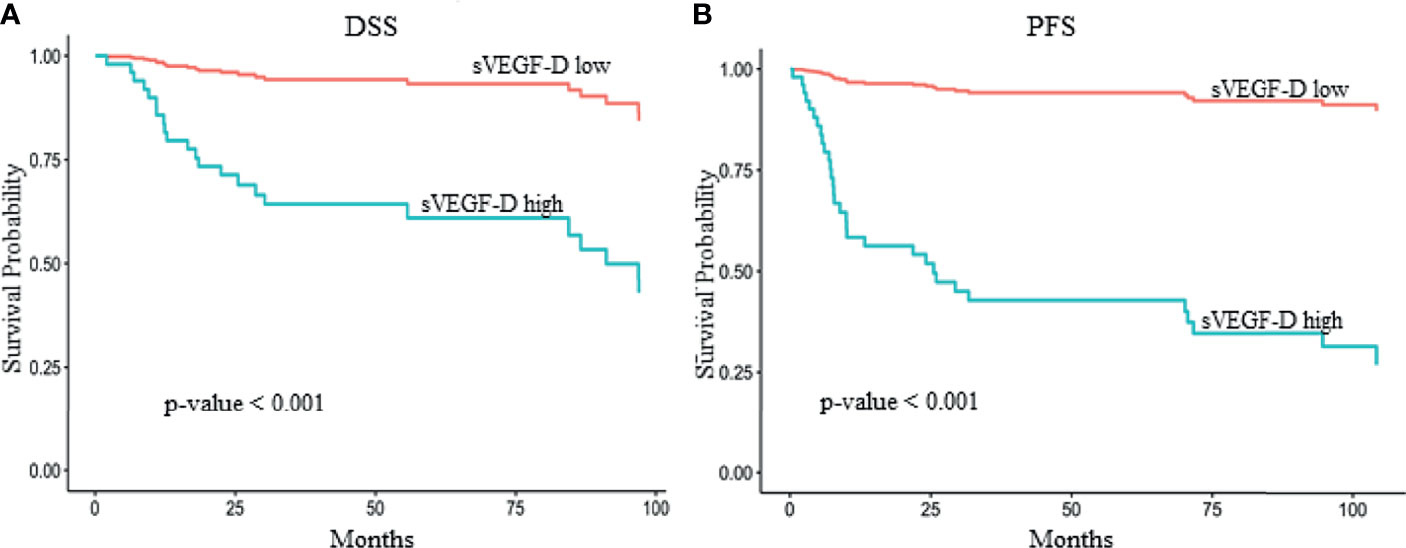
Figure 1 Kaplan–Meier survival curves showing the effect of serum vascular endothelial growth factor D (sVEGF-D) level in multivariate models adjusted for stage, grade, and vascular and perineural invasion. The optimal threshold for sVEGF-D categorization was determined by maximally selected rank statistics. The reported p-values were adjusted for multiple testing. Higher sVEGF-D levels [>393 and >329 pg/ml for disease-specific survival (DSS) and progression-free survival (PFS), respectively] exhibited a significant association with reduced DSS (A) (p < 0.001) and PFS (B) (p < 0.001) in 61 patients with vulvar squamous cell carcinoma (VSCC) from cohort A.
In addition, in the univariable analysis for PFS, higher preoperative sVEGF-D levels (HR = 2.15, p = 0.036), lymph node metastasis (HR = 3.20, p < 0.01), lymphovascular invasion (HR = 1.95, p = 0.04), and advanced FIGO stage (HR = 3.20, p < 0.01) were significantly associated with a high risk of recurrence (Figure 1B and Supplementary Table S2).
In the multivariable analysis, the preoperative sVEGF-D levels (entered as a restricted cubic spline with 3 knots, i.e., a quadratic trend) were demonstrated to be independent prognostic factors for poor DSS (likelihood ratio test: p = 0.026) (Table 3 and Figure 1A). Similarly, in the multivariable analysis for PFS, sVEGF-D (linear trend) was marginally significant (p = 0.047) (Table 3 and Figure 1B).
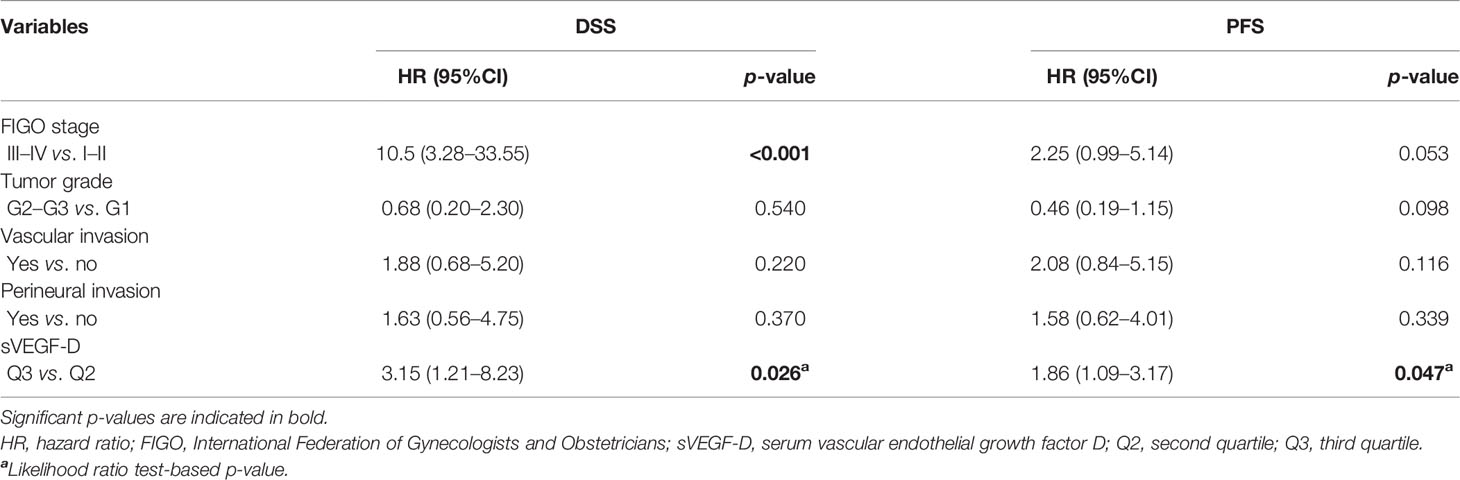
Table 3 Multivariable survival analysis for both disease-specific survival (DSS) and progression-free survival (PFS) on 61 VSCC patients from cohort A.
In order to assess the factors associated with lymph node metastasis and to build a predictive algorithm, pathological lymph node status was compared with the clinicopathological variables available before and after surgery. As reported in Supplementary Table S3, lymph node metastasis was significantly associated with positive/suspicious clinical lymph node status (p = 0.002), increased tumor diameter (p = 0.029), higher sVEGF-D levels (p = 0.027), greater depth of stromal invasion (p = 0.024), and the presence of vascular (p = 0.012) and perineural (p = 0.005) invasion.
As described above in the Study Design, three different models for preoperative prediction of lymph node involvement were built using logistic regression (Table 4).
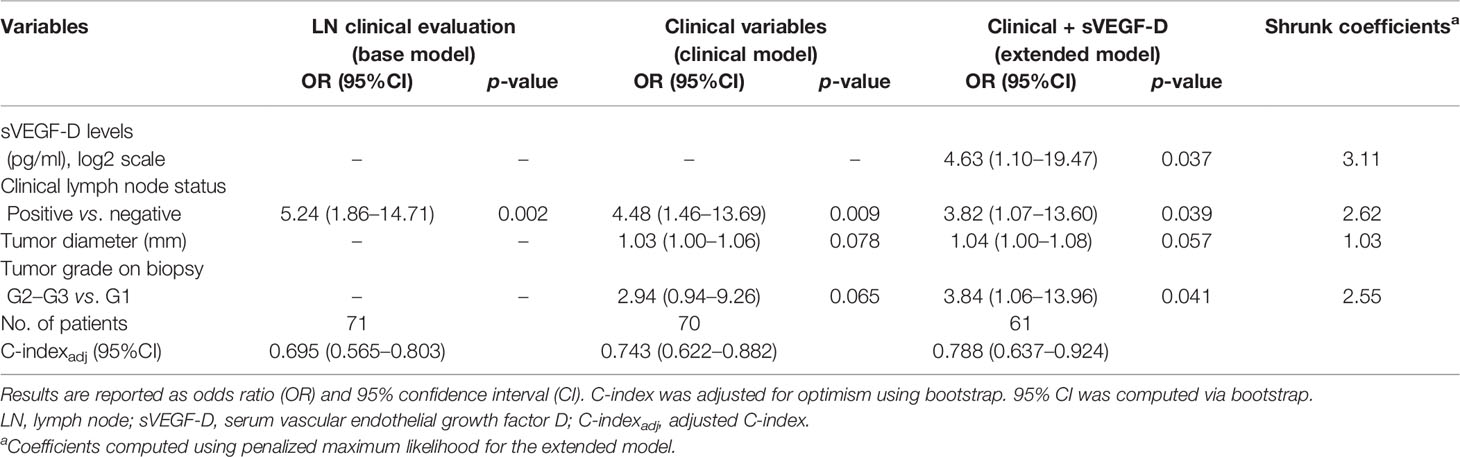
Table 4 Logistic regression model estimates for association with prediction of lymph node in cohort A.
The base model, including only the clinical/radiological lymph node status, showed a moderate adjusted C-index (C-indexadj) of 0.70 (95%CI = 0.57–0.80); the clinical model, including the clinicopathological characteristics available before surgery (e.g., tumor diameter, tumor grade evaluated on biopsy, and clinical/radiological lymph node status), showed good discrimination, with C-indexadj of 0.74 (95%CI = 0.62–0.88), and good calibration (scaled Brier score = 0.23) (Supplementary Figure S2A). The extended model, built by adding the sVEGF-D level to the clinical model, resulted in a significant improvement of the fit (likelihood ratio test: p = 0.037), suggesting an independent association between sVEGF-D and lymph node involvement. Both discrimination and calibration showed a slight improvement, with higher C-indexadj (0.79, 95%CI = 0.64–0.92) and scaled Brier score (0.34) (Supplementary Figure S2B).
The extended model applied to cohort B (validation set) showed a reasonably high C-index (0.73, 95%CI = 0.54–0.87). Comparison of the probabilities predicted by the extended model between the two groups of patients divided according to the presence of positive lymph nodes showed good separation with a discrimination slope of 0.78 and an acceptable agreement between the observed and predicted proportions of lymph node metastasis (Hosmer–Lemeshow test, p = 0.08) (Supplementary Figure S3).
Finally, we evaluated the performance of the extended model on the entire cohort of patients (A + B) in comparison with the base model. The model with sVEGF-D showed a significantly superior performance compared to the clinical/radiological evaluation of the lymph nodes (ROC-AUCs of 0.792 and 0.685, respectively, p = 0.008) (Table 5). At the optimal cutoff value (Youden), the specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), false-negative rate (FNR), and false-positive rate (FPR) of the extended model were 83.0%, 74.4%, 76.3%, 80.4%, 25.6%, and 17.0%, respectively.
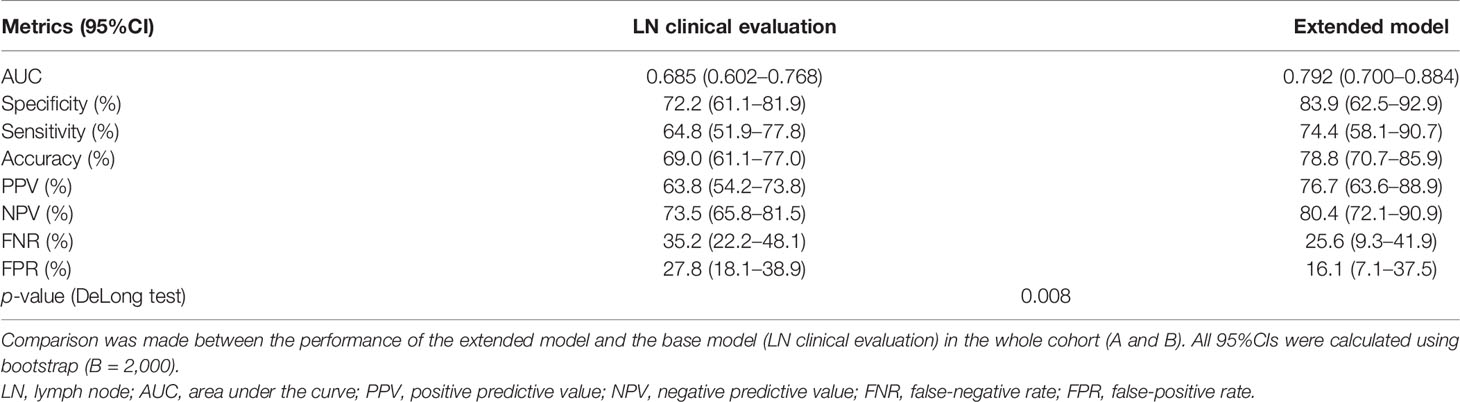
Table 5 Metrics assessing the performance of the proposed methods for the prediction of lymph node metastasis.
Lymph node status is the most important prognostic factor for patients with VSCC, and failure to remove metastatic lymph nodes has serious consequences due to its high mortality (26). On the other hand, removal of lymph nodes can cause both short- and long-term serious side effects (8). For these reasons, a diagnostic test with an optimal sensitivity and specificity would be needed to safely avoid lymphadenectomy. Special attention seems to be focused on high-performance imaging modalities in the prediction of lymph node status, such as ultrasound (27, 28) and 18F-FDG-PET/CT (PET) (29–31), in order to better select cN0 patients and identify before surgery those with nodal metastases, even with not palpable nodes. However, to date, imaging cannot diagnose small metastases prior to surgery when their size is below the resolution limits of the available imaging techniques (32).
In this context, circulating biomarkers could offer a valid contribution in the design of a predictive algorithm. For several different tumors such as breast (13), colon (14), ovarian (15), endometrial (16), cervical (33), bladder (34), thyroid (35), gastric (36), esophageal (37), and gallbladder (38) cancers, a strong association between an increased expression of VEGF-D and the presence of lymph node metastases has already been demonstrated by IHC (13–16, 33) or at serum level (34–38); however, investigations on this biomarker regarding VSCC are lacking. To the best of our knowledge, this is the first study investigating the role of sVEGF in VSCC.
In the present study, our findings indicated that an increased serum VEGF-D level is an independent predictor for the presence of metastatic disease in the lymph nodes and an independent risk factor of poor outcomes in terms of both DSS and PFS. In addition, a predictive model adding sVEGF-D to other clinicopathological parameters significantly improved the prediction of nodal metastasis.
Higher levels of sVEGF-D in cancer patients compared to healthy controls or benign pathologies have been detected for some tumor types (36–38) and not for others (34, 35), but regardless of this, a positive correlation with lymph node metastasis has been demonstrated in all studies.
VEGF-C and VEGF-D are key stimulators of both angiogenesis, via the activation of VEGFR-2, and lymphangiogenesis, via the activation of VEGFR-3, constitutively expressed by lymphatic endothelial cells, promoting growth and remodeling of lymphatic vessels (10, 39, 40). In animal models, VEGF-D-driven lymphatic metastasis has been attributed to both the growth of new lymphatics adjacent to the tumor and the enlargement of preexisting collecting lymphatic vessels (41). In addition to VEGF-D and VEGF-C, VEGF-A, fibroblast growth factor-2, and hepatocyte growth factor have been reported to exert lymphangiogenic activity either directly or indirectly, mediated by the VEGF-C/VEGF-D/VEGFR-3 signaling pathway (42–44).
Tumor spread through the lymphatics is known to be a negative prognosticator, but the molecular mechanisms of VEGF-D as an independent prognostic factor in many types of cancers are still unclear (13–16, 38). One possible hypothesis is that VEGF-D is a mitogenic and morphogenic effector of the proto-oncogene c-Fos and, consequently, may be involved in c-Fos-induced tumor transformation and progression (45, 46).
We found, for the first time, that high circulating levels of sVEGF-D correlate with lymph node metastasis and advanced stage in VSCC patients, as already shown for other malignancies (34–38). On the other hand, the tissue expressions of VEGF-D and VEGFR-3 by IHC did not correlate with any clinicopathological characteristics, as demonstrated by a previous study showing a positive IHC staining for VEGF-D and VEGFR-3 in 100% and 90% of VSCC cases, respectively, and no correlation with the presence of lymph node metastases (17).
According to previous literature (47–49), our study showed that clinical lymph node status, tumor diameter, vascular and perineural invasion, and depth of invasion significantly correlated with inguinofemoral lymph node metastasis. With the aim of providing a useful tool for a tailored surgical approach, we focused our attention on the variables available before surgery: tumor diameter, tumor grade evaluated on biopsy, clinical lymph node status, and sVEGF-D level. The extended model based on these four covariates showed good performance, demonstrated by the C-index of discrimination equal to 0.788. Notably, the performance of the extended model was significantly superior compared to that of the clinical model including only clinical predictors, but excluding sVEGF-D, and to that of the base model based on clinical/radiological evaluation of lymph nodes. The results, validated on an external independent cohort of VSCC patients, confirmed the ability of the extended model to classify patients with respect to lymph node positivity. It is well known that the performance of a predictive model is always lower in the validation group compared to that in the cohort of samples used for its development. The slight difference in the C-index values between our training and validation sets could be explained by the non-homogeneity of the two cohorts as they differed in the surgical approach used to assess lymph nodal status.
Only one previous study proposed two predictive models for groin node metastases based on four parameters (depth of infiltration, grade of differentiation, tumor diameter, and EGFR), but only the first two were independent predictors (50). The two models have not been validated in independent cohorts of patients and their performance is unclear.
Our study provided encouraging preliminary evidence for further investigations on sVEGF-D in association with other clinicopathological variables available preoperatively in the prediction of lymph node metastasis in patients with VSCC. The limits of the present study, which can be mainly attributed to the rarity of this tumor, are mainly related to the relatively small number of cases, the retrospective design, and the prolonged time interval of enrollment, during which many things have changed. The most important evolution concerned the improvement of imaging performance in the prediction of lymph node status due to technological development, the availability of more experienced examiners, and the increased knowledge of this rare pathology obtained by dedicated studies. Currently, imaging provides higher NPV, which in fact is the favored predictive driver since failing to recognize a metastasis and missing the surgical removal could significantly impair prognosis. On the other hand, sVEGF-D could potentially support a higher PPV with other diagnostic tools, being associated with the presence of lymph node metastasis.
In conclusion, it will be essential to validate these retrospective results in larger multicenter prospective studies to identify a threshold value that can achieve FNR and NPV values in accordance with the guidelines of the Gynecologic Cancer Group of the European Organization for Research and Treatment of Cancer (EORTC) (5) and to update the sVEGF-D predictive model integrated with current high-performance imaging methods.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comitato Etico di Brescia, ASST Spedali Civili, Brescia, Italy, and Comitato Etico Policlinico Universitario “A. Gemelli” IRCCS—Università Cattolica del Sacro Cuore, Rome, Italy. The patients/participants provided written informed consent to participate in this study.
AR, AG, and FO conceived and designed the study. AR and LA performed the experiments. PA and SC executed the statistical analysis. AO, EB, LZ, CR, and DG collected data. FO, ES, EB, FF, GG, and GS contributed to the analyses and interpretation of data. AR and SC wrote the first drafts of the manuscript. All authors contributed to the article and approved the submitted version.
AG and FO were supported by local research grants from the University of Brescia. SC was supported by a research grant from the Italian Ministry of Education, University and Research (PRIN project no. 20178S4EK9).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LT declared a shared affiliation with one of the authors GG to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We wish to thank all the physicians and the nurses working in the Department of Obstetrics and Gynecology, ASST Spedali Civili, University of Brescia, particularly to Mrs. Maria Teresa Pedretti for the assistance in collecting blood samples. This paper is dedicated to the memory of our colleague, Dr. Anna Benetti, who recently passed away.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.818613/full#supplementary-material
Supplementary Table 1 | Protein expression of VEGF-D and VEGFR-3 on vulvar cancer tissues and correlation with clinicopathological characteristics (49 VSCC patients from Cohort A).
Supplementary Table 2 | Univariable survival analysis for both disease specific survival (DSS) and progression free survival (PFS) on VSCC patients from Cohort A.
Supplementary Table 3 | Clinicopathologic characteristics of VSCC patients from Cohort A in relation to pathologic lymph node status.
Supplementary Figure 1 | Representative immunohistochemical staining for VEGF-D and VEGFR-3 in vulvar invasive squamous cell carcinoma. Magnification 20x.
Supplementary Figure 2 | Calibration curves for prediction models. (A) Calibration curve for the clinical model. (B) Calibration curve for the extended model.
Supplementary Figure 3 | Calibration curve for the penalized extended model applied to the validation cohort B.
1. National cancer Institute. Cancer Stat Facts: Vulvar Cancer. (2021). Available at: http://www.seer.cancer.gov/statfacts/html/vulva.html.
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Lai J, Elleray R, Nordin A, Hirschowitz L, Rous B, Gildea C, et al. Vulval Cancer Incidence, Mortality and Survival in England: Age-Related Trends. BJOG (2014) 121(6):728–38. doi: 10.1111/1471-0528.12459
4. Meltzer-Gunnes CJ, Smastuen MC, Kristensen GB, Trope CG, Lie AK, Vistad I. Vulvar Carcinoma in Norway: A 50-Year Perspective on Trends in Incidence, Treatment and Survival. Gynecol Oncol (2017) 145(3):543–8. doi: 10.1016/j.ygyno.2017.03.008
5. Oonk MHM, Planchamp F, Baldwin P, Bidzinski M, Brannstrom M, Landoni F, et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients With Vulvar Cancer. Int J Gynecol Cancer (2017) 27(4):832–7. doi: 10.1097/IGC.0000000000000975
6. Koh WJ, Greer BE, Abu-Rustum NR, Campos SM, Cho KR, Chon HS, et al. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2017) 15(1):92–120. doi: 10.6004/jnccn.2017.0008
7. Collarino A, Fuoco V, Garganese G, Pereira Arias-Bouda LM, Perotti G, Manca G, et al. Lymphoscintigraphy and Sentinel Lymph Node Biopsy in Vulvar Carcinoma: Update From a European Expert Panel. Eur J Nucl Med Mol Imaging (2020) 47(5):1261–74. doi: 10.1007/s00259-019-04650-8
8. Hinten F, van den Einden LC, Hendriks JC, van der Zee AG, Bulten J, Massuger LF, et al. Risk Factors for Short- and Long-Term Complications After Groin Surgery in Vulvar Cancer. Br J Cancer (2011) 105(9):1279–87. doi: 10.1038/bjc.2011.407
9. Garganese G, Collarino A, Fragomeni SM, Rufini V, Perotti G, Gentileschi S, et al. Groin Sentinel Node Biopsy and (18)F-FDG PET/CT-Supported Preoperative Lymph Node Assessment in Cn0 Patients With Vulvar Cancer Currently Unfit for Minimally Invasive Inguinal Surgery: The GroSNaPET Study. Eur J Surg Oncol (2017) 43(9):1776–83. doi: 10.1016/j.ejso.2017.06.018
10. Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, et al. Vascular Endothelial Growth Factor D (VEGF-D) is a Ligand for the Tyrosine Kinases VEGF Receptor 2 (Flk1) and VEGF Receptor 3 (Flt4). Proc Natl Acad Sci USA (1998) 95(2):548–53. doi: 10.1073/pnas.95.2.548
11. Achen MG, Stacker SA. Vascular Endothelial Growth Factor-D: Signaling Mechanisms, Biology, and Clinical Relevance. Growth Factors (2012) 30(5):283–96. doi: 10.3109/08977194.2012.704917
12. Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D Promotes the Metastatic Spread of Tumor Cells via the Lymphatics. Nat Med (2001) 7(2):186–91. doi: 10.1038/84635
13. Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, et al. Prognostic Significance of Vascular Endothelial Growth Factor D in Breast Carcinoma With Long-Term Follow-Up. Clin Cancer Res (2003) 9(2):716–21.
14. White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, et al. Vascular Endothelial Growth Factor-D Expression is an Independent Prognostic Marker for Survival in Colorectal Carcinoma. Cancer Res (2002) 62(6):1669–75.
15. Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Vascular Endothelial Growth Factor-D is an Independent Prognostic Factor in Epithelial Ovarian Carcinoma. Br J Cancer (2003) 88(2):237–44. doi: 10.1038/sj.bjc.6600701
16. Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Expression of Vascular Endothelial Growth Factor (VEGF)-D and its Receptor, VEGF Receptor 3, as a Prognostic Factor in Endometrial Carcinoma. Clin Cancer Res (2003) 9(4):1361–9.
17. Jach R, Dyduch G, Radon-Pokracka M, Przybylska P, Mika M, Dulinska-Litewka J, et al. Expression of Vascular Endothelial Growth Factors VEGF- C and D, VEGFR-3, and Comparison of Lymphatic Vessels Density Labeled With D2-40 Antibodies as a Prognostic Factors in Vulvar Intraepithelial Neoplasia (VIN) and Invasive Vulvar Cancer. Neuro Endocrinol Lett (2011) 32(4):530–9.
18. McGeechan K, Macaskill P, Irwig L, Liew G, Wong TY. Assessing New Biomarkers and Predictive Models for Use in Clinical Practice: A Clinician’s Guide. Arch Intern Med (2008) 168(21):2304–10. doi: 10.1001/archinte.168.21.2304
19. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the Performance of Prediction Models: A Framework for Traditional and Novel Measures. Epidemiology (2010) 21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2
20. Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A Comparison of Goodness-of-Fit Tests for the Logistic Regression Model. Stat Med (1997) 16(9):965–80. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o
21. Harrell FE, Lee KL. Using Logistic Model Calibration to Assess the Quality of Probability of Predictions. (1991). pp. 1–24. Available from: https://biostat.app.vumc.org/wiki/pub/Main/FrankHarrell/logistCal.pdf.
22. Copas JB. Regression, Prediction and Shrinkage. J R Stat Soc B (1983) 45(3):311–54. doi: 10.1111/j.2517-6161.1983.tb01258.x
23. Draper NR, Smith H. Applied Regression Analysis. 3rd Ed Vol. xvii. . New York: Wiley (1998). p. 706.
24. Hurvich CM, Tsai CL. Regression and Time-Series Model Selection in Small Samples. Biometrika (1989) 76(2):297–307. doi: 10.2307/2336663
25. Hothorn T, Zeileis A. Generalized Maximally Selected Statistics. Biometrics (2008) 64(4):1263–9. doi: 10.1111/j.1541-0420.2008.00995.x
26. Raspagliesi F, Hanozet F, Ditto A, Solima E, Zanaboni F, Vecchione F, et al. Clinical and Pathological Prognostic Factors in Squamous Cell Carcinoma of the Vulva. Gynecol Oncol (2006) 102(2):333–7. doi: 10.1016/j.ygyno.2005.12.027
27. Garganese G, Fragomeni SM, Pasciuto T, Leombroni M, Moro F, Evangelista MT, et al. Ultrasound Morphometric and Cytologic Preoperative Assessment of Inguinal Lymph-Node Status in Women With Vulvar Cancer: MorphoNode Study. Ultrasound Obstet Gynecol (2020) 55(3):401–10. doi: 10.1002/uog.20378
28. Garganese G, Bove S, Fragomeni S, Moro F, Triumbari EKA, Collarino A, et al. Real-Time Ultrasound Virtual Navigation in 3D PET/CT Volumes for Superficial Lymph-Node Evaluation: Innovative Fusion Examination. Ultrasound Obstet Gynecol (2021) 58(5):766–72. doi: 10.1002/uog.23613
29. Collarino A, Garganese G, Fragomeni SM, Pereira Arias-Bouda LM, Ieria FP, Boellaard R, et al. Radiomics in Vulvar Cancer: First Clinical Experience Using (18)F-FDG PET/CT Images. J Nucl Med (2018) 60(2):199–206. doi: 10.2967/jnumed.118.215889
30. Rufini V, Garganese G, Ieria FP, Pasciuto T, Fragomeni SM, Gui B, et al. Diagnostic Performance of Preoperative [(18)F]FDG-PET/CT for Lymph Node Staging in Vulvar Cancer: A Large Single-Centre Study. Eur J Nucl Med Mol Imaging (2021) 48(10):3303–14. doi: 10.1007/s00259-021-05257-8
31. Triumbari EKA, de Koster EJ, Rufini V, Fragomeni SM, Garganese G, Collarino A. 18f-FDG PET and 18F-FDG PET/CT in Vulvar Cancer: A Systematic Review and Meta-Analysis. Clin Nucl Med (2021) 46(2):125–32. doi: 10.1097/RLU.0000000000003411
32. Fischerova D, Garganese G, Reina H, Fragomeni SM, Cibula D, Nanka O, et al. Terms, Definitions and Measurements to Describe Sonographic Features of Lymph Nodes: Consensus Opinion From the Vulvar International Tumor Analysis (VITA) Group. Ultrasound Obstet Gynecol (2021) 57(6):861–79. doi: 10.1002/uog.23617
33. Dai Y, Tong R, Guo H, Yu T, Wang C. Association of CXCR4, CCR7, VEGF-C and VEGF-D Expression With Lymph Node Metastasis in Patients With Cervical Cancer. Eur J Obstet Gynecol Reprod Biol (2017) 214:178–83. doi: 10.1016/j.ejogrb.2017.04.043
34. Benoit T, Keller EX, Wolfsgruber P, Hermanns T, Gunthart M, Banzola I, et al. High VEGF-D and Low MMP-2 Serum Levels Predict Nodal-Positive Disease in Invasive Bladder Cancer. Med Sci Monit (2015) 21:2266–74. doi: 10.12659/MSM.894383
35. Lai CW, Duh QY, Chen CW, Chuang FJ, Chang YJ, Lin MT, et al. VEGF-D and A Preoperative Serum Levels Predict Nodal and Distant Metastases in Differentiated Thyroid Cancer Patients. World J Surg (2015) 39(7):1742–9. doi: 10.1007/s00268-015-3016-6
36. Tsirlis TD, Kostakis A, Papastratis G, Masselou K, Vlachos I, Papachristodoulou A, et al. Predictive Significance of Preoperative Serum VEGF-C and VEGF-D, Independently and Combined With Ca19-9, for the Presence of Malignancy and Lymph Node Metastasis in Patients With Gastric Cancer. J Surg Oncol (2010) 102(6):699–703. doi: 10.1002/jso.21677
37. Kozlowski M, Kowalczuk O, Milewski R, Chyczewski L, Niklinski J, Laudanski J. Serum Vascular Endothelial Growth Factors C and D in Patients With Oesophageal Cancer. Eur J Cardiothorac Surg (2010) 38(3):260–7. doi: 10.1016/j.ejcts.2010.01.061
38. Liu MC, Jiang L, Hong HJ, Meng ZW, Du Q, Zhou LY, et al. Serum Vascular Endothelial Growth Factors C and D as Forecast Tools for Patients With Gallbladder Carcinoma. Tumour Biol (2015) 36(8):6305–12. doi: 10.1007/s13277-015-3316-3
39. Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A Novel Vascular Endothelial Growth Factor, VEGF-C, is a Ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) Receptor Tyrosine Kinases. EMBO J (1996) 15(2):290–98. doi: 10.1002/j.1460-2075.1996.tb00359.x
40. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and Lymphatic Vessel Remodelling in Cancer. Nat Rev Cancer (2014) 14(3):159–72. doi: 10.1038/nrc3677
41. Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D Promotes Tumor Metastasis by Regulating Prostaglandins Produced by the Collecting Lymphatic Endothelium. Cancer Cell (2012) 21(2):181–95. doi: 10.1016/j.ccr.2011.12.026
42. Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, et al. Hepatocyte Growth Factor is a Lymphangiogenic Factor With an Indirect Mechanism of Action. Blood (2006) 107(9):3531–6. doi: 10.1182/blood-2005-06-2538
43. Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, et al. Dose-Dependent Response of FGF-2 for Lymphangiogenesis. Proc Natl Acad Sci USA (2004) 101(32):11658–63. doi: 10.1073/pnas.0404272101
44. Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, et al. VEGF-A Stimulates Lymphangiogenesis and Hemangiogenesis in Inflammatory Neovascularization via Macrophage Recruitment. J Clin Invest (2004) 113(7):1040–50. doi: 10.1172/JCI20465
45. Orlandini M, Marconcini L, Ferruzzi R, Oliviero S. Identification of a C-Fos-Induced Gene That is Related to the Platelet-Derived Growth Factor/Vascular Endothelial Growth Factor Family. Proc Natl Acad Sci USA (1996) 93(21):11675–80. doi: 10.1073/pnas.93.21.11675
46. Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, et al. C-Fos-Induced Growth Factor/Vascular Endothelial Growth Factor D Induces Angiogenesis in vivo and in vitro. Proc Natl Acad Sci USA (1999) 96(17):9671–6. doi: 10.1073/pnas.96.17.9671
47. Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A, et al. Prognostic Factors for Groin Node Metastasis in Squamous Cell Carcinoma of the Vulva (a Gynecologic Oncology Group Study). Gynecol Oncol (1993) 49(3):279–83. doi: 10.1006/gyno.1993.1127
48. Smyczek-Gargya B, Volz B, Geppert M, Dietl J. A Multivariate Analysis of Clinical and Morphological Prognostic Factors in Squamous Cell Carcinoma of the Vulva. Gynecol Obstet Invest (1997) 43(4):261–7. doi: 10.1159/000291869
49. Ferrari F, Forte S, Ardighieri L, Bonetti E, Fernando B, Sartori E, et al. Multivariate Analysis of Prognostic Factors in Primary Squamous Cell Vulvar Cancer: The Role of Perineural Invasion in Recurrence and Survival. Eur J Surg Oncol (2019) 45(11):2115–9. doi: 10.1016/j.ejso.2019.07.029
Keywords: vulvar squamous cell carcinoma, VEGF-D, lymph node metastasis, prognosis, serum
Citation: Ravaggi A, Gambino A, Ferrari F, Olivari A, Zanotti L, Romani C, Ardighieri L, Antonelli P, Garganese G, Gallo D, Scambia G, Bignotti E, Sartori E, Calza S and Odicino F (2022) VEGF-D Serum Level as a Potential Predictor of Lymph Node Metastasis and Prognosis in Vulvar Squamous Cell Carcinoma Patients. Front. Oncol. 12:818613. doi: 10.3389/fonc.2022.818613
Received: 19 November 2021; Accepted: 14 March 2022;
Published: 08 April 2022.
Edited by:
Chiara Mazziotta, University of Ferrara, ItalyReviewed by:
Matteo Morotti, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandCopyright © 2022 Ravaggi, Gambino, Ferrari, Olivari, Zanotti, Romani, Ardighieri, Antonelli, Garganese, Gallo, Scambia, Bignotti, Sartori, Calza and Odicino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonella Ravaggi, YW50b25lbGxhLnJhdmFnZ2lAdW5pYnMuaXQ=; orcid.org/0000-0003-4661-9979
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.