- 1Department of Pathophysiology, School of Basic Medical Sciences, Zhengzhou University, Zhengzhou, China
- 2Collaborative Innovation Center of Henan Province for Cancer Chemoprevention, Zhengzhou, China
- 3State Key Laboratory of Esophageal Cancer Prevention and Treatment, Zhengzhou, China
- 4Department of Special Service, No. 988 Hospital of the Joint Service Support Force of People’s Liberation Army of China (PLA), Zhengzhou, China
Background: Perineural invasion (PNI) is a malignant metastatic mode of tumors and has been reported in many tumors including esophageal cancer (EC). However, the role of PNI in EC has been reported differently. This systematic review and meta-analysis aims to focus on the role of PNI in EC.
Methods: Eight databases of CNKI, VIP, Wanfang, Scopus, Wiley, ISI, PubMed, and EBSCO are used for literature search. The association of PNI with gender, pathological stages of T and N (pT and pN), lymphovascular invasion (LVI), lymph node metastasis, 5-year overall survival (OS), and 5-year disease-free survival (DFS) was examined in the meta-analysis by Revman5.0 Software. The pooled OR/HR and 95% CI were used to assess the risk and prognostic value.
Results: Sixty-nine published studies were screened for analysis of PNI in EC. The incidence of PNI in esophageal squamous carcinoma (ESCC) and esophageal adenocarcinoma (EAC) was different, but not statistically significant (p > 0.05). The PNI-positive patients had a significantly higher risk of pT stage (OR = 3.85, 95% CI = 2.45–6.05, p < 0.00001), pN stage (OR = 1.86, 95% CI = 1.52–2.28, p < 0.00001), LVI (OR = 2.44, 95% CI = 1.55–3.85, p = 0.0001), and lymph node metastasis (OR = 2.87, 95% CI = 1.56–5.29, p = 0.0007). Furthermore, the cumulative analysis revealed a significant correlation between PNI and poor OS (HR = 1.37, 95% CI = 1.24–1.51, p < 0.0001), as well as poor DFS (HR = 1.55, 95% CI = 1.38–1.74, p < 0.0001).
Conclusion: PNI occurrence is significantly related to tumor stage, LVI, lymph node metastasis, OS, and DFS. These results indicate that PNI can serve as an indicator of high malignant degree and poor prognosis in EC.
Introduction
Esophageal cancer (EC) is one of the top ten malignant tumors. According to global cancer statistics in 2020, EC ranks seventh in terms of incidence and sixth in mortality overall (1). The histological types of EC mainly contain esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). ESCC is the most common histological type in China, accounting for more than 90%. In EC treatment, surgical excision is the best treatment for the early stage, and radiotherapy and chemotherapy are often used for the middle and late stage. Recently, neoadjuvant chemoradiation (nCRT) followed by esophagectomy is increasingly applied to locally advanced EC. However, the prognosis of EC is still poor after these treatments due to its insidious and highly invasive nature in the early stage. Most EC patients are prone to relapse and metastasis. In recent years, researchers found that there is a new metastasis pathway, perineural invasion (PNI), often happening in EC patients.
PNI refers to the phenomenon of cancer cells surrounding nerve fibers and entering the surrounding nerve, spreading local infiltration and metastasis. Now, the definition of PNI is that the tumor cells are in close contact with the nerve and surround at least 33% of the nerve periphery or invade any of the three layers of the nerve sheath, which is also taken as the current pathological diagnostic criteria for PNI (2, 3). The occurrence of PNI not only is with incomplete resection of the tumor and recurrence of prognosis, but also often leads to pain in many cancers, such as prostate cancer, pancreatic cancer, and head and neck cancer (2–5). However, the role of PNI in EC is differently reported. For example, PNI is associated with poor overall survival (OS) and can serve as an independent factor for OS in multivariate analysis (6–8), while Lee et al. thought PNI was not an important prognostic parameter in EC (9). These inconsistent conclusions may be due to the insufficient sample size. Hence, we collected a larger number of data from EC patients and used a systematic review and meta-analysis to obtain more accurate conclusions of PNI. The study determined the association of PNI with pathological parameters, OS and DFS, and then evaluated the role and effect of PNI on EC.
Materials and Methods
Literature Search
A literature search was performed by using the CNKI, VIP, Wanfang, Scopus, Wiley, ISI, PubMed, and EBSCO databases from January 1, 1990 to March 30, 2022. The main keywords in the abstract were “perineural invasion”, “esophageal”, and “cancer”. The articles in CNKI, VIP, and Wanfang databases only come from the Chinese Core Journal. Duplicate articles were deleted and full articles were used for analysis. The articles with patient samples from the same institution in the repeated recruitment period, reviews, and case reports were excluded. The literatures of esophageal neuroendocrine carcinoma were also excluded. The quality of all studies was assessed by using the Newcastle–Ottawa Scale and was scored from 6 to 8 (full score = 9).
Literature Extraction
To analyze the positive rate of PNI in different pathological types of EC, the following information in the articles was extracted: first author, year of publication, country of study, patient samples of recruitment period, pathological types of EC (ESCC and EAC), and the number of samples who are PNI positive.
For meta-analysis to examine the association of PNI with gender, pT stage, pN stage, lymphovascular invasion (LVI), lymph node metastasis, 5-year OS, and 5-year disease-free survival (DFS), the extracted information contains the following: first author, year of publication, odds ratio (OR), hazard ratio (HR), and the corresponding 95% confidence interval (CI). The pooled HR and 95% CI were calculated using the method of inverse variance and the p-value threshold was set at 0.05. Some articles do not directly provide HR data, but provide RR data. HR and RR can be combined because without considering the time factor in the paper, they represent the same meaning.
Statistical Analysis
Statistical calculation was completed with SPSS21.0 software and statistical heterogeneity was tested using the Chi-square test. p < 0.05 indicated statistical significance. The forest and funnel plots of the meta-analysis were made using Review Manager 5.0 software (Revman5.0). p < 0.10 or/and I² > 50% were used to indicate heterogeneity.
Results
Literature Search Results
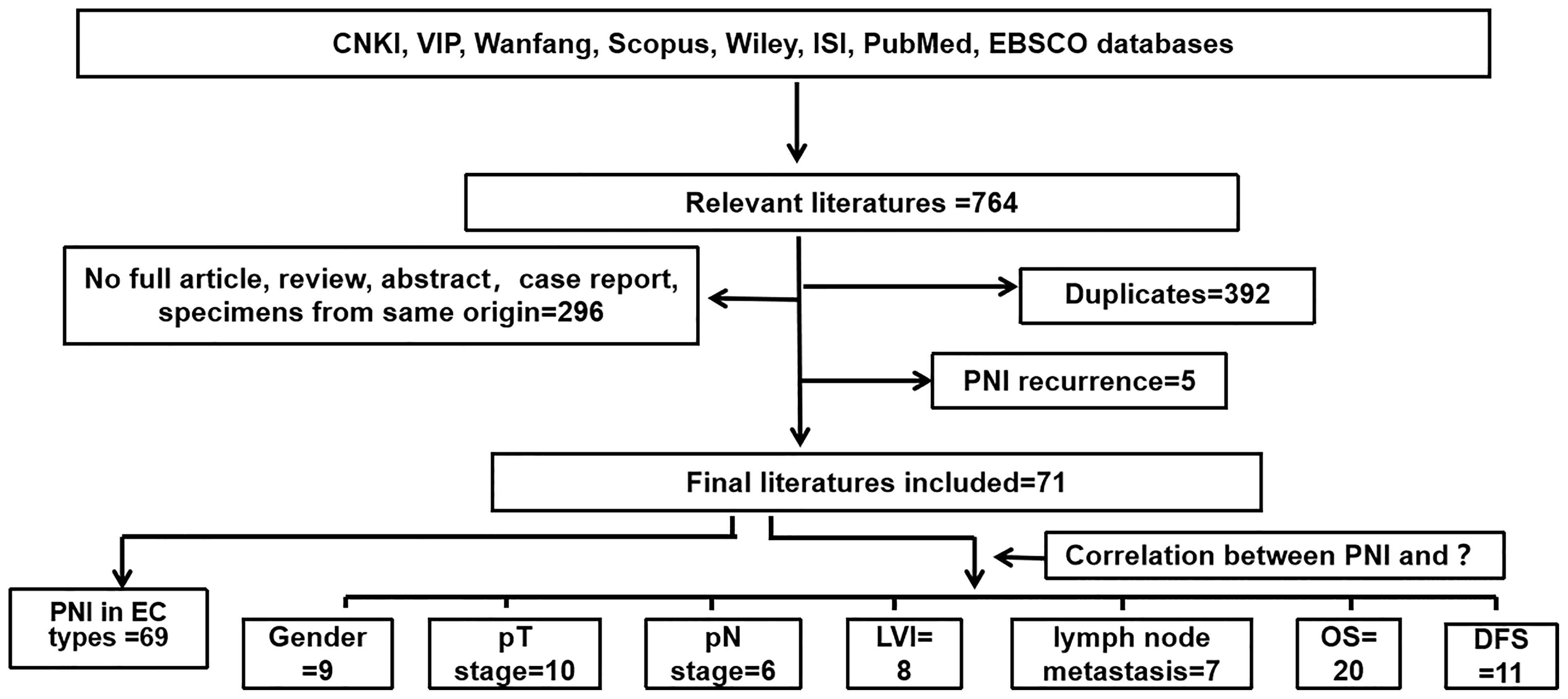
The systematic search identified 764 potentially eligible articles, 392 of which were excluded due to duplication. Of the remaining 372 studies, 296 were excluded because they were reviews or specimens of the same origin. Five studies were excluded for PNI recurrence after neoadjuvant therapy or chemotherapy. Finally, 71 studies were finally included in this study: 69 studies were used to analyze the positive rate of PNI in different pathological types of EC; 9, 10, 6, 8, 7, 20, and 11 studies were used to analyze the correlation between PNI and gender, pT stage, pN stage, LVI, lymph node metastasis, OS, and DFS, respectively. The detailed screening process is shown in Figure 1.
The PNI Occurrence Is Different Between ESCC and EAC
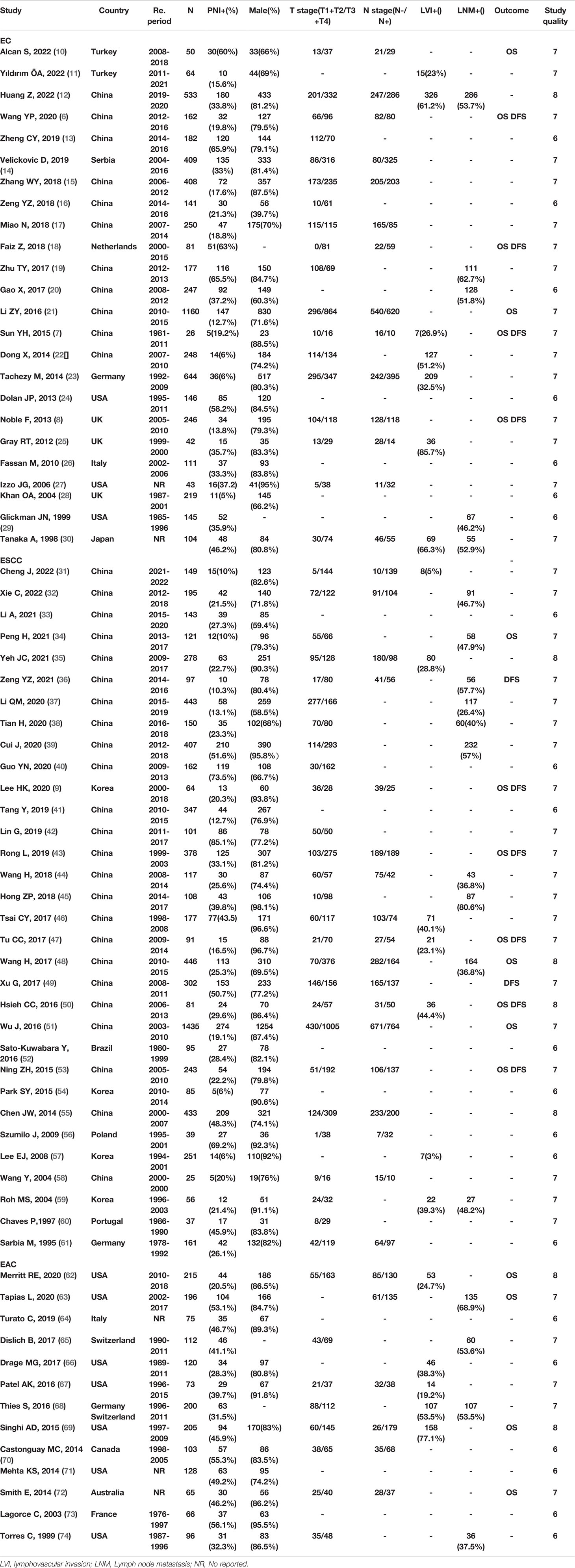
A total of 69 studies were used to analyze the distribution of PNI in different pathological types of EC, including 24 studies on EC (ESCC, EAC, and other types including esophageal small cell undifferentiated carcinoma and esophagus carcinosarcoma) (6–8, 10–30), 32 studies on ESCC (9, 31–61), and 13 studies on EAC (62–74). The detailed information is shown in Table 1. The median of PNI incidence of EC, ESCC, and EAC was 33% (range from 5% to 66%), 24% (range from 6% to 85%), and 46% (range from 20% to 56%), respectively. Data analysis showed that the PNI occurrence rate has no significant difference between ESCC and EAC (Figure S1A).
The Significant Correlation Between PNI and Pathological Parameters of EC
Nine, 10, and 6 studies published from 1995 to 2022 were used to analyze the relationship between PNI and gender (6, 21, 23, 30, 46, 49, 53, 55, 75), T stage (6, 21, 23, 30, 46, 49, 53, 55, 61, 75), and N stage (6, 21, 23, 49, 53, 55), respectively.
The occurrence of PNI has no correlation with gender (OR = 1.22, 95% CI: 0.98–1.52, I² = 0, p = 0.08) (Figure 2A), while the PNI-positive patients have a significantly higher risk of pT stage (OR = 3.85, 95% CI = 2.45–6.05, p < 0.00001) (Figure 2B) and pN stage (OR = 1.86, 95% CI = 1.52–2.28, p < 0.00001) (Figure 2C). In addition, in the meta-analysis for the relationship between PNI and pT stage, no obvious publication bias was observed in the entire funnel plots (Figure S1B).
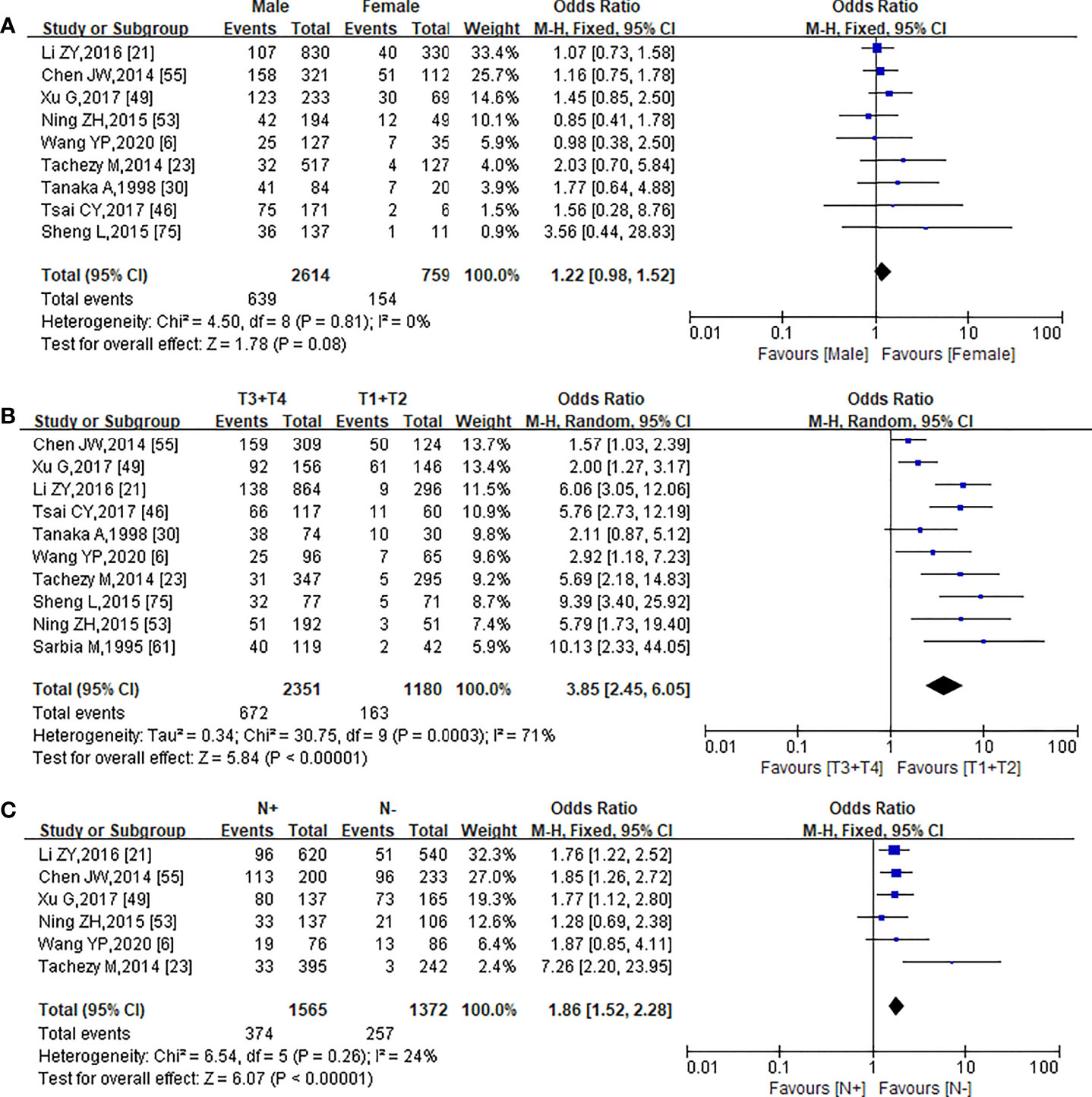
Figure 2 Forest plot of the pooled OR for the association of PNI with Gender (A), pTstage (B), and pN stage (C).
A total of 2,332 patients from 8 studies were included in the meta-analysis of the correlation between PNI and LVI (6, 18, 21, 30, 46, 49, 53, 70). In PNI-positive (+) patients, the positive rate of LVI was 47.85% (323/675) and the negative rate of LVI was 52.15% (352/675), while in PNI-negative (−) patients, the positive rate of LVI was 21.85% (362/1,657) and the negative rate of LVI was 78.15% (1,295/1,657). The Chi-square test showed that PNI was significantly correlated with LVI (χ2 = 156.347, p = 0.000, r = 0.259). The forest plot was also statistically significant (OR = 2.44, 95% CI = 1.55–3.85, p = 0.0001) using a random-effect model for calculation (heterogeneity: I² = 72%, p = 0.0001) (Figure 3A).
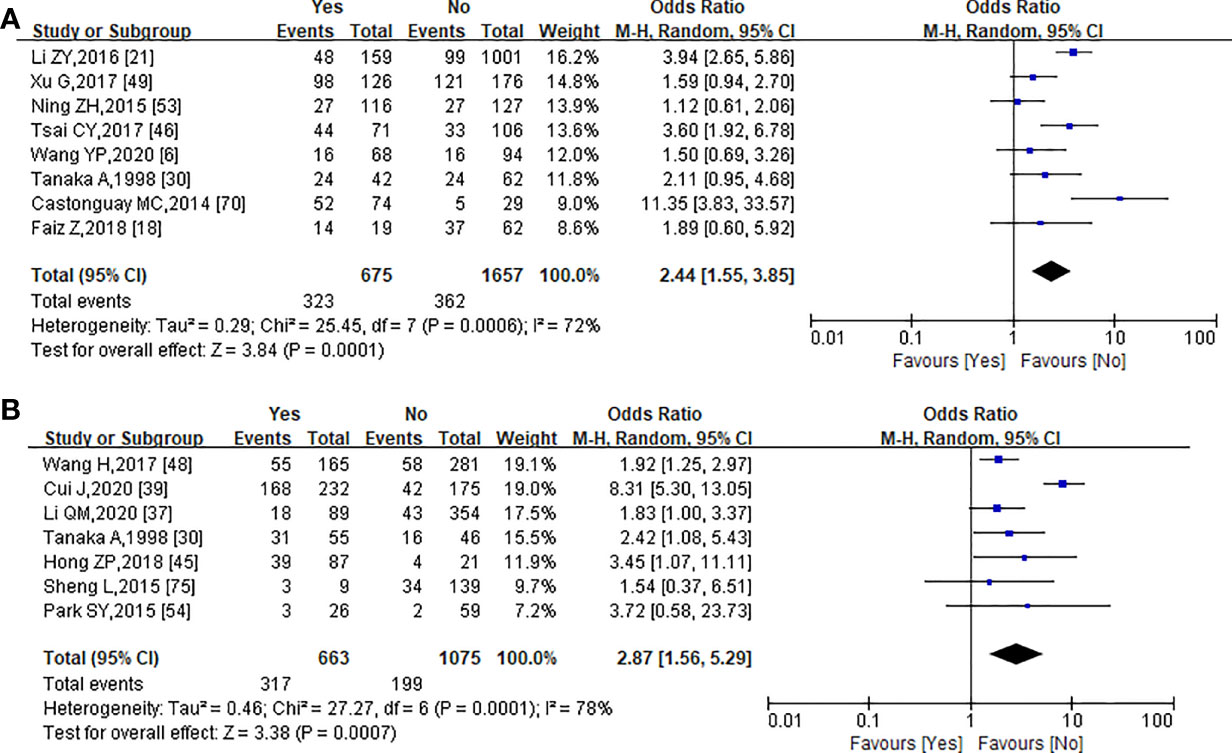
Figure 3 Forest plot analysis of the relationship between PNI and lymphovascularinvasion (A), and lymph node metastasis (B).
Seven studies with a total of 1,738 patients provided data about lymph node metastasis (30, 37, 39, 45, 48, 54, 75). Lymph node metastasis occurred in 317 of the 516 PNI(+) patients (61.43%) and in 346 of the 1,222 PNI(−) patients (28.31%). There was a significant correlation between lymph node metastasis and PNI in EC patients (χ2 = 168.665, p = 0.000, r = 0.312). Of these seven studies, there was a significant association between PNI with lymph node metastasis (OR = 2.87, 95% CI = 1.56–5.29, p = 0.0007; Figure 3B) using a random-effect model for calculation (heterogeneity: I² = 85%, p = 0.0007).
The Effect of PNI on 5-Year Overall Survival and 5-Year Disease-Free Survival in EC
We also studied the effect of PNI on 5-year OS and 5-year DFS of EC patients. HR and 95% CI of OS and DFS were directly reported in 20 (6–10, 18, 21, 34, 43, 47, 48, 50, 51, 53, 62, 63, 69, 72, 75, 76) and 11 (6–9, 18, 36, 43, 47, 49, 50, 53) articles, respectively. Since some studies did not provide HR directly, we used fixed-effect models for the prognostic analysis regardless of the heterogeneity. There was a significant association between PNI and OS (HR = 1.37, 95% CI = 1.24–1.51, p < 0.0001; Figure 4A) using a fixed-effect model for calculation (heterogeneity: I2 = 83%, p < 0.0001), and the entire funnel plots had obvious publication bias (Figure S1C). A meta-analysis of 10 studies on DFS showed that PNI was associated with poor prognosis in EC patients (HR = 1.55, 95% CI = 1.38–1.74, p < 0.0001; Figure 4B) using a fixed-effect model for calculation (heterogeneity: I² = 71%, p < 0.0001). In this meta-analysis, a funnel plot was used to assess the publication bias. The entire funnel plots had no obvious publication bias (Figure S1D).
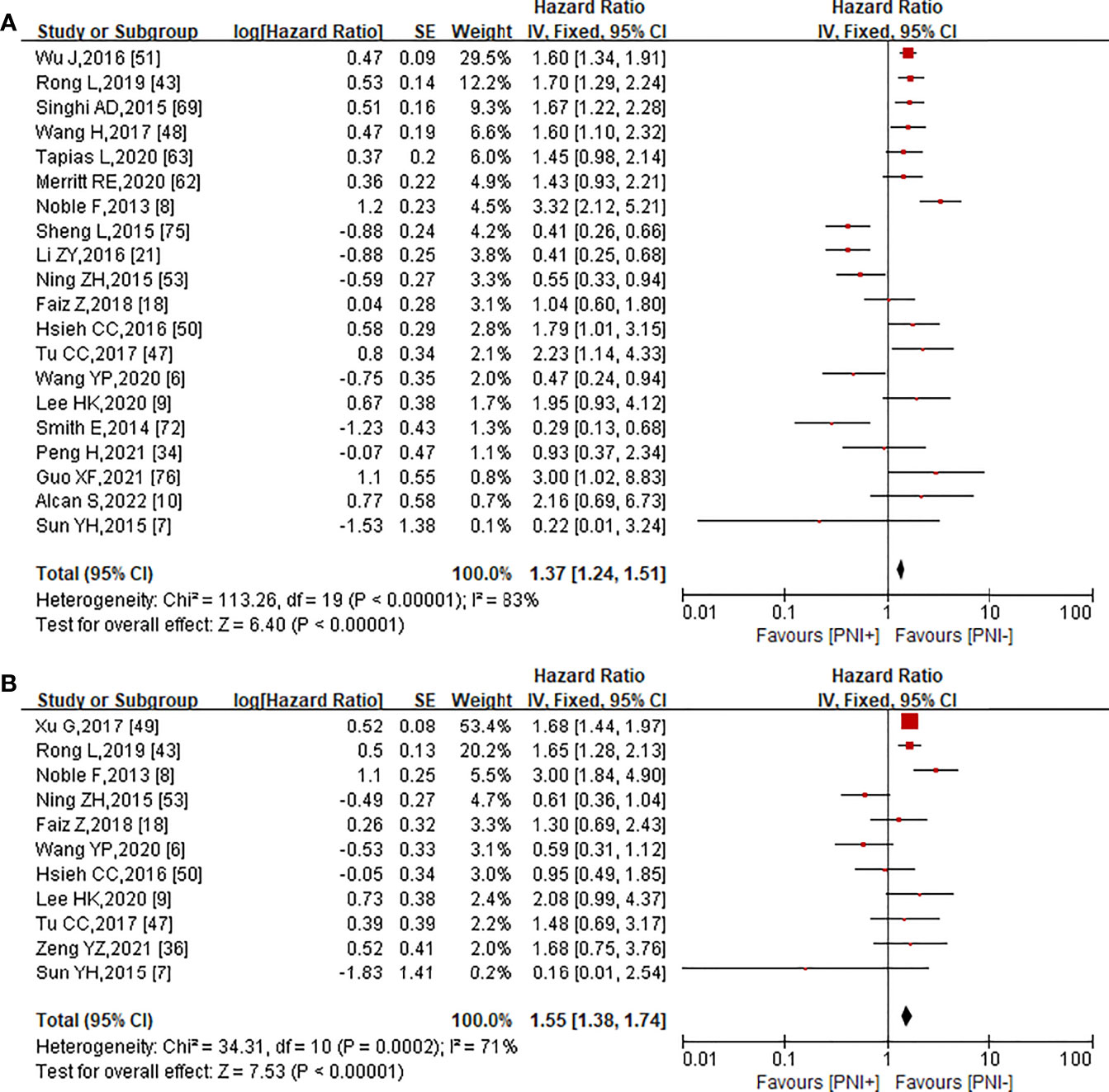
Figure 4 Forest plot analysis for significant correlation between the presence of PNI and5-year OS (A), 5-year DFS (B).
Discussion
PNI was identified in a variety of malignant tumors, such as prostate cancer, pancreatic cancer, and head and neck cancer (2–5). The percentage of patients with PNI in pancreatic ductal adenocarcinoma is 70%–100%, and is closely related to the occurrence of ache (3). In colorectal cancer, PNI is an independent risk factor of recurrence, which indicates a worse phenotype of tumor (3). EC is one of the common malignant tumors with high invasion. PNI often occurs in EC, but there are conflicting reports about the effects on EC of PNI (13, 16, 25, 28). This review and meta-analysis was conducted to better understand the relationship of PNI with the development process and prognosis of EC.
The esophageal nerve includes the vagal nerve and sympathetic nerve. The abundant nerve plexus is mainly distributed in the submucosa and smooth muscle layer and is often accompanied by blood vessels and lymphatic vessels. The development of PNI implies advanced tumor staging, the depth and range of LVI, and lymph node metastasis, as reported by studies (18, 30). However, other studies indicated that there was no relation between PNI and tumor staging, LVI, and lymph node metastasis (6, 54). According to our study, we found that the incidence of PNI in ESCC and EAC was different, but not statistically significant. PNI had a significant association with pT stage, pN stage, LVI, and lymph node metastasis in EC, which are well-known malignant characteristics of EC (77, 78). Moreover, it is worth noting that in cancer tissues with PNI, researchers not only found abundant blood vessels and lymphatic vessels, but also found angiogenesis and lymphangiogenesis, further promoting the development and metastasis of tumor (79, 80). Thus, these results further suggested that PNI was an important feature for the malignant degree of cancer.
It is well known that the malignant degree of EC has a significant association with poor prognosis of EC. Faiz et al. and Noble et al. reported that PNI is positively related to poor prognosis (8, 18), while Li et al. and Dong et al. identified that it was negatively related to poor outcome (21, 22). We evaluated the effect of PNI on 5-year OS and 5-year DFS of EC and found that there was a statically significant association between PNI and OS and DFS. These results indicated that PNI was an independent risk factor for the prognosis of EC. However, no matter what the treatment is, PNI is also significantly associated with worse OS and DFS, and can be evaluated as a prognostic predictor (42, 81).
At present, it is believed that PNI is the result of the interaction between tumor cells and nerves. The occurrence of PNI is not only closely related to the distribution of nerves in tissues and tumor progression, but also associated with the regulation at the molecular level. In ESCC PNI, studies indicated that several genes, such as NF-KB (27), P53 (60), nuclear programmed cell death 4 (PDCD4) (82), and NK1R (83), were significantly positively associated with PNI development, while an inverse correlation was found between platelet counts and PNI (84). The expression of nuclear PDCD4 can predict the prognosis of EC. Moreover, nuclear PDCD4 expression was negatively correlated with PNI (82). Substance P (SP) plays an important role in several types of cancer promotion and progression by binding to its preferential neurokinin 1 receptor (NK1R). NK1R was upregulated, and its overexpression correlated with larger tumor size, deeper tumor invasion, more PNI, and eventually caused poorer OS (83). However, the exact molecular mechanism of PNI in EC remains unclear and is worth further exploring.
In brief, PNI is a dynamic pathological process, and its underlying molecular mechanisms need to be further investigated. Our study only proves that PNI plays an important role in EC. Moreover, our results suggested that PNI can be incorporated into patient stratification factors to make more accurate surgical or treatment plans. This not only greatly improves the survival rate and prognosis of patients, but also enables the further development of precision medicine.
Conclusion
This review and meta-analysis was conducted to better understand the relationship of PNI with the development process and prognosis of EC. The results indicated that the effect of PNI on poor prognosis is not isolated and associated with gene expression, especially the presence of a number of adverse prognostic factors, such as depth of invasion, clinical stage, LVI, and lymph node metastasis. In general, PNI is a significant indicator of high malignant degree and poor prognosis in EC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LB provided the idea and logic of the article, and was responsible for the data collection and statistical processing of it. LY assisted LB to complete the production of pictures and tables, and put forward other ideas. The other authors put forward valuable suggestions and revised and polished the manuscript in the process of writing the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant no. 81101686), the Foundation of Henan Educational Committee (Grant no. 21A310030 and Grant no. 22A310024), and the Natural Science Foundation for Young Teachers’ Basic Research of Zhengzhou University (Grant no. JC202035025).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.816270/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Canc J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural Invasion in Cancer: A Review of the Literature. Cancer (2009) 115:3379–91. doi: 10.1002/cncr.24396
3. Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ. Perineural Invasion of Cancer: A Complex Crosstalk Between Cells and Molecules in the Perineural Niche. Am J Canc Res (2019) 9:1–21.
4. Hechler B, Carlson ER, Heidel RE, Fahmy MD, McCoy JM. Are Oral Pain and Otalgia Predictive of Perineural Invasion in Squamous Cell Carcinoma of the Oral Tongue? J Oral Maxillofac Surg (2020) 78:1418–26. doi: 10.1016/j.joms.2020.03.029
5. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural Invasion and Associated Pain in Pancreatic Cancer. Nat Rev Canc (2011) 11:695–707. doi: 10.1038/nrc3131
6. Wang YP, Wu LY, Min YL. The Effect of Nerve Invasion on the Prognosis of Patients With Esophageal Carcinoma. J Clin Res (2020) 37:311–4. doi:10.3969/j.issn.1671-7171.2020.02.04
7. Sun YH, Lin SW, Chen CH, Liang WY, Hsieh CC. Adenosquamous Carcinoma of the Esophagus and Esophagogastric Junction: Clinical Manifestations and Treatment Outcomes. J Gastrointest Surg (2015) 19:1216–22. doi: 10.1007/s11605-015-2852-x
8. Noble F, Hopkins J, Curtis N, Kelly JJ, Bailey IS, Byrne JP, et al. The Role of Systemic Inflammatory and Nutritional Blood-Borne Markers in Predicting Response to Neoadjuvant Chemotherapy and Survival in Oesophagogastric Cancer. Med Oncol (2013) 30:596. doi: 10.1007/s12032-013-0596-6
9. Lee HK, Kwon MJ, Ra YJ, Lee HS, Kim HS, Nam ES, et al. Significance of Druggable Targets (PD-L1, KRAS, BRAF, PIK3CA, MSI, and HPV) on Curatively Resected Esophageal Squamous Cell Carcinoma. Diagn Pathol (2020) 15:126. doi: 10.1186/s13000-020-01045-4
10. Alcan S, Ergin M, Keskin H, Erdoğan A. What are the Independent Prognostic Factors in Patients Undergoing Esophagectomy for Esophageal Cancer? Turk Gogus Kalp Damar Cerrahisi Derg (2022) 30(1):83–91. doi: 10.5606/tgkdc.dergisi.2022.20969
11. Yıldırım ÖA, Erdur E. Medical Oncology or Surgical Oncology: Which Branch Should Be Started in Esophageal Cancer Diagnostic Evaluation? Cureus (2022) 14:e22286. doi: 10.7759/cureus.2228.
12. Huang Z, Jin Y, Cai X, Chen L, Shen X, Li B, et al. Association of the Programmed Death Ligand-1 Combined Positive Score in Tumors and Clinicopathological Features in Esophageal Cancer. Thorac Canc (2022) 13(4):523–32. doi: 10.1111/1759-7714.14285
13. Zheng CY, Nie RH, Wang ZY. The Study of Impacts of Perioperative Complications on the Long-Term Survival of Patients With Primary Esophageal Cancer. Pract J Canc (2019) 34:242–4. doi: 10.3969/j.issn.1001-5930.2019.02.018
14. Velickovic D, Sabljak P, Stojakov D, Velickovic J, Ebrahimi K, Sljukic V, et al. Prognostic Impact of Allogenic Blood Transfusion Following Surgical Treatment of Esophageal Canc Vol. 51. . Vienna: Springer (2019).
15. Zhang WY, Chen XX, Chen WH, Zhang H, Zou CL. Nomograms for Predicting Risk of Locoregional Recurrence and Distant Metastases for Esophageal Cancer Patients After Radical Esophagectomy. BMC Canc (2018) 18:879. doi: 10.1186/s12885-018-4796-5
16. Zeng YZ, Zhang YQ, Zhang LY, Wei XL. Relationship Between Expression of TRPC1 Protein and Clinicopathological Features in Esophageal Cancer and Its Correlation With Ki-67. J Shantou Univ Med Coll (2018) 31:197–200+255. doi: 10.13401/j.cnki.jsumc.2018.04.00217
17. Miao N, JZ LI, Wang ZQ, Liu T. Expression of Wnt11 and ROCK2 in Esophageal Squamous Cell Carcinoma and its and Clinical Significance. J Clin Pathol Res (2018) 38:724–30. doi: 10.1016/j.prp.2016.07.008
18. Faiz Z, Huijgen LJW, Alqethami HJ, Burgerhof JGM, Kats-Ugurlu G, Plukker JTM. Prevalence and Prognostic Significance of Extramural Venous Invasion in Patients With Locally Advanced Esophageal Cancer. Ann Surg Oncol (2018) 25:1588–97. doi: 10.1245/s10434-018-6448-z
19. Zhu TY, Fan GH, Wang TS, Huang J. Impacts of Postoperative Complications on the Long-Term Outcome of Esophageal Cancer Patients. Med J Wuhan Univ (2017) 38:100–3+111. doi: 10.14188/j.1671-8852.2017.01.024
20. Gao X, Tian W, Tian YL. Pathological Characteristics of Esophageal Cancer and Influencing Factors on Prognosis. Chin J Med (2017) 52:45–8. doi: 10.3969/j.issn.1008-1070.2017.02.015
21. Li ZY, Wang HF, Lu YR. Iskandara Abulimiti. Analysis of Prognostic Effect of Perineural Invasion and Related Factors in Esophageal Cancer. ChinaJ Modern Med (2016) 26:48–53. doi: 10.4103/0366-6999.172570
22. Dong X. Study on Pathological Characteristics and Survival Prognosis of in Female Patients With Esophageal Cancer. Chin J Med Guide (2014) 16:1278–80.
23. Tachezy M, Tiebel AK, Gebauer F, Kutup A, Tharun L, Pantel K, et al. Prognostic Impact of Perineural, Blood and Lymph Vessel Invasion for Esophageal Cancer. Histol Histopathol (2014) 29:1467–75. doi: 10.14670/HH-29.1467
24. Dolan JP, Kaur T, Diggs BS, Luna RA, Schipper PH, Tieu BH, et al. Impact of Comorbidity on Outcomes and Overall Survival After Open and Minimally Invasive Esophagectomy for Locally Advanced Esophageal Cancer. Surg Endosc (2013) 27:4094–103. doi: 10.1007/s00464-013-3066-5
25. Gray RT, O'Donnell ME, Verghis RM, McCluggage WG, Maxwell P, McGuigan JA, et al. Bone Marrow Micrometastases in Esophageal Carcinoma: A 10-Year Follow-Up Study. Dis Esophagus (2012) 25:709–15. doi: 10.1111/j.1442-2050.2011.01307.x
26. Fassan M, Cagol M, Pennelli G, Rizzetto C, Giacomelli L, Battaglia G, et al. Programmed Cell Death 4 Protein in Esophageal Cancer. Oncol Rep (2010) 24:135–9. doi: 10.3892/or_00000838
27. Izzo JG, Malhotra U, Wu TT, Ensor J, Luthra R, Lee JH, et al. Association of Activated Transcription Factor Nuclear Factor Kappab With Chemoradiation Resistance and Poor Outcome in Esophageal Carcinoma. J Clin Oncol (2006) 24:748–54. doi: 10.1200/JCO.2005.03.8810
28. Khan OA, Alexiou C, Soomro I, Duffy JP, Morgan WE, Beggs FD. Pathological Determinants of Survival in Node-Negative Oesophageal Cancer. Br J Surg (2004) 91:1586–91. doi: 10.1002/bjs.4778
29. Glickman JN, Torres C, Wang HH, Turner JR, Shahsafaei A, Richards WG, et al. The Prognostic Significance of Lymph Node Micrometastasis in Patients With Esophageal Carcinoma. Cancer (1999) 85:769–78. doi: 10.1002/(SICI)1097-0142(19990215)85:4<769::AID-CNCR3>3.0.CO;2-I
30. Tanaka A, Matsumura E, Yosikawa H, Uchida T, Machidera N, Kubo R, et al. An Evaluation of Neural Invasion in Esophageal Cancer. Surg Today (1998) 28:873–8. doi: 10.1007/s005950050245
31. Cheng J, Guo M, Yang Y, Liu Y, Hu W, Shang Q, et al. Perioperative Outcomes of Minimally Invasive Esophagectomy After Neoadjuvant Immunotherapy for Patients With Locally Advanced Esophageal Squamous Cell Carcinoma. Front Immunol (2022) 13:848881. doi: 10.3389/fimmu.2022.848881
32. Xie C, Chen Z, Xu J, Meng Z, Huang Z, Lin J. Influence of Lymphangio Vascular (V) and Perineural (N) Invasion on Survival of Patients With Resected Esophageal Squamous Cell Carcinoma (ESCC): A Single-Center Retrospective Study. PeerJ (2022) 10:e12974. doi: 10.7717/peerj.12974
33. Ang L, Zhongyou J, Han J, Lili L. Prediction of Vascular and Neural Invasion in Esophageal Cancer Based on PET Radiomics. Mod Med Imageol (2021) 30(12):2216–9.
34. Peng H, Tan X. The Prognostic Significance of Sarcopenia and the Neutrophil-To-Lymphocyte Ratio in Elderly Patients With Esophageal Squamous Cell Carcinoma. Canc Manag Res (2021) 13:3209–18. doi: 10.2147/CMAR.S302274
35. Yeh JC, Yu WH, Yang CK, Chien LI, Lin KH, Huang WS, et al. Predicting Aggressive Histopathological Features in Esophageal Cancer With Positron Emission Tomography Using a Deep Convolutional Neural Network. Ann Transl Med (2021) 9(1):37. doi: 10.21037/atm-20-1419
36. Zeng YZ, Zhang YQ, Lin XQ, Chen JY, Zhang F, Zhu JL, et al. Co-Expression of VEGF-C and Survivin Predicts Poor Prognosis in Esophageal Squamous Cell Carcinoma. Transl Canc Res (2021) 10(1):210–22. doi: 10.21037/tcr-20-2498
37. Li QM, Zhang GQ, Hou ZC, Liu XD, Liu TY, Zhao S, et al. Construction of Prediction Model of Celiac Lymph Node Metastasis in Thoracic Esophageal Squamous Cell Carcinoma and Risk Subgroup Analysis of Celiac Lymph Node Metasis Probability. Chin J Dig Surg (2020) 19:637–43. doi: 10.3760/cma.j.cn115610-20200427-00331
38. Tian H, Yang YG, Mei X. Expression and Clinicopathologic Significance of Podoplanin of Tumor Cells and Stromal Cells in Esophageal Squamous Cell Carcinoma. Chin J Clin Exp Pathol (2020) 36:547–51. doi: 10.13315/j.cnki.cjcep.2020.05.010
39. Cui J, Sun X, Sui TY, Liu A, Jiao WJ. Characteristics of Lymph Node Metastasis in Thoracic Esophageal Squamous Cell Carcinoma: A Study of 407 Patients. Chin J Clin Thorac Cardiovasc Surg (2020) 27:1063–9. doi: 10.7507/1007-4848.202003151
40. Guo YN, Tian DP, Gong QY, Huang H, Yang P, Chen SB, et al. Perineural Invasion is a Better Prognostic Indicator Than Lymphovascular Invasion and a Potential Adjuvant Therapy Indicator for Pn0m0 Esophageal Squamous Cell Carcinoma. Ann Surg Oncol (2020) 27:4371–81. doi: 10.1245/s10434-020-08667-4
41. Tang Y, Liu JY, Zhou Y, Ding MQ, Luo JH. Therapeutic Effect of Different Treatment Modes for Esophageal Squamous Cell Carcinoma. China J Mod Med (2019) 32:743–6. doi: 10.13429/j.cnki.cjcr.2019.06.006
42. Lin G, Liu H, Li J. Pattern of Recurrence and Prognostic Factors in Patients With Pt1-3 N0 Esophageal Squamous Cell Carcinoma After Surgery: Analysis of a Single Center Experience. J Cardiothorac Surg (2019) 14:58. doi: 10.1186/s13019-019-0883-1
43. Rong L, Liu Y, Hui Z, Zhao Z, Zhang Y, Wang B, et al. PD-L1 Expression and its Clinicopathological Correlation in Advanced Esophageal Squamous Cell Carcinoma in a Chinese Population. Diagn Pathol (2019) 14:6. doi: 10.1186/s13000-019-0778-4
44. Wang H, Zhou Y, Liu Q, Xu J, Ma Y. Prognostic Value of SOX2, Cyclin D1, P53, and Ki-67 in Patients With Esophageal Squamous Cell Carcinoma. Onco Targets Ther (2018) 11:5171–81. doi: 10.2147/OTT.S160066
45. Hong ZP, Wang XZ, Zhang RQ. Clinical Research of Lymph Node Metastasis on the Middle and Lower Segment of 108 Patients With Esophageal Squamous Cell Carinoma of Mongolian Nationality. Chin J Primary Med Pharm (2018) 25:748–53. doi: 10.3760/cma.j.issn.10086706.2018.06.018
46. Tsai CY, Yeh CJ, Chao YK, Chang HK, Tseng CK, Liu YH. Perineural Invasion Through the Sheath in Posttherapy Esophagectomy Specimens Predicts Poor Survival in Patients With Esophageal Squamous Cell Carcinoma. Eur J Surg Oncol (2017) 43:1970–6. doi: 10.1016/j.ejso.2017.07.014
47. Tu CC, Hsu PK, Chien LI, Liu WC, Huang CS, Hsieh CC, et al. Prognostic Histological Factors in Patients With Esophageal Squamous Cell Carcinoma After Preoperative Chemoradiation Followed by Surgery. BMC Canc (2017) 17:62. doi: 10.1186/s12885-017-3063-5
48. Wang H, Deng F, Liu Q, Ma Y. Prognostic Significance of Lymph Node Metastasis in Esophageal Squamous Cell Carcinoma. Pathol Res Pract (2017) 213:842–7. doi: 10.1016/j.prp.2017.01.023
49. Xu G, Feng F, Liu Z, Liu S, Zheng G, Xiao S, et al. Prognosis and Progression of ESCC Patients With Perineural Invasion. Sci Rep (2017) 7:43828. doi: 10.1038/srep43828
50. Hsieh CC, Hsu HS, Chang SC, Chen YJ. Circulating Cell-Free DNA Levels Could Predict Oncological Outcomes of Patients Undergoing Esophagectomy for Esophageal Squamous Cell Carcinoma. Int J Mol Sci (2016) 17:2131. doi: 10.3390/ijms17122131
51. Wu J, Chen QX. Prognostic and Predictive Significance of Tumor Length in Patients With Esophageal Squamous Cell Carcinoma Undergoing Radical Resection. BMC Canc (2016) 16:394. doi: 10.1186/s12885-016-2417-8
52. Sato-Kuwabara Y, Fregnani JH, Jampietro J, Carvalho KC, Franco CP, da Costa WL Jr, et al. Comparative Analysis of Basaloid and Conventional Squamous Cell Carcinomas of the Esophagus: Prognostic Relevance of Clinicopathological Features and Protein Expression. Tumour Biol (2016) 37:6691–9. doi: 10.1007/s13277-015-4551-3
53. Ning ZH, Zhao W, Li XD, Chen LJ, Xu B, Gu WD, et al. The Status of Perineural Invasion Predicts the Outcomes of Postoperative Radiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Int J Clin Exp Pathol (2015) 8:6881–90.
54. Park SY, Kim DJ, Jung HS, Yun MJ, Lee JW, Park CK. Relationship Between the Size of Metastatic Lymph Nodes and Positron Emission Tomographic/Computer Tomographic Findings in Patients With Esophageal Squamous Cell Carcinoma. World J Surg (2015) 39:2948–54. doi: 10.1007/s00268-015-3221-3
55. Chen JW, Xie JD, Ling YH, Li P, Yan SM, Xi SY, et al. The Prognostic Effect of Perineural Invasion in Esophageal Squamous Cell Carcinoma. BMC Canc (2014) 14:313. doi: 10.1186/1471-2407-14-313
56. Szumilo J, Burdan F, Zinkiewicz K, Dudka J, Klepacz R, Dabrowski A, et al. Expression of Syndecan-1 and Cathepsins D and K in Advanced Esophageal Squamous Cell Carcinoma. Folia Histochem Cytobiol (2009) 47:571–8. doi: 10.2478/v10042-008-0012-8
57. Lee EJ, Lee BB, Han J, Cho EY, Shim YM, Park J, et al. CpG Island Hypermethylation of E-Cadherin (CDH1) and Integrin Alpha4 Is Associated With Recurrence of Early Stage Esophageal Squamous Cell Carcinoma. Int J Canc (2008) 123:2073–9. doi: 10.1002/ijc.23598
58. Wang Y, He SQ, Shi XH, Shen ZZ, Luo JM, LI XQ. Expression of Vascular Endothelial Growth Factor mRNA in Esophageal Carcinoma and Preliminary Inquiry Into its Clinical Significance. Chin J Radiat Oncol (2004), 1:50–3.
59. Roh MS, Lee JI, Choi PJ. Tumor Budding as a Useful Prognostic Marker in Esophageal Squamous Cell Carcinoma. Dis Esophagus (2004) 17:333–7. doi: 10.1111/j.1442-2050.2004.00436.x
60. Chaves P, Pereira AD, Pinto A, Oliveira AG, Queimado L, Gloria L, et al. P53 Protein Immunoexpression in Esophageal Squamous Cell Carcinoma and Adjacent Epithelium. J Surg Oncol (1997) 65:3–9. doi: 10.1002/(SICI)1096-9098(199705)65:1<3::AID-JSO2>3.0.CO;2-C
61. Sarbia M, Porschen R, Borchard F, Horstmann O, Willers R, Gabbert HE. Incidence and Prognostic Significance of Vascular and Neural Invasion in Squamous Cell Carcinomas of the Esophagus. Int J Canc (1995) 61:333–6. doi: 10.1002/ijc.2910610310
62. Merritt RE, Abdel-Rasoul M, Souza DM, Kneuertz PJ. Nomograms for Predicting Overall and Recurrence-Free Survival After Trimodality Therapy for Esophageal Adenocarcinoma. J Surg Oncol (2021) 123:881–90. doi: 10.1002/jso.26349
63. Tapias L, Tapias LF, Moonsamy P, Lanuti M, Gaissert HA, Wright CD, et al. Impact of Radial Margin Status After Esophagectomy for Adenocarcinoma. J Gastrointest Surg (2020) 24:983–90. doi: 10.1007/s11605-019-04258-1
64. Turato C, Scarpa M, Kotsafti A, Cappon A, Quarta S, Biasiolo A, et al. Squamous Cell Carcinoma Antigen 1 is Associated to Poor Prognosis in Esophageal Cancer Through Immune Surveillance Impairment and Reduced Chemosensitivity. Canc Sci (2019) 110:1552–63. doi: 10.1111/cas.13986
65. Dislich B, Stein A, Seiler CA, Kröll D, Berezowska S, Zlobec I, et al. Expression Patterns of Programmed Death-Ligand 1 in Esophageal Adenocarcinomas: Comparison Between Primary Tumors and Metastases. Cancer Immunol Immunother (2017) 66:777–86. doi: 10.1007/s00262-017-1982-2
66. Drage MG, Tippayawong M, Agoston AT, Zheng Y, Bueno R, Hornick JL, et al. Morphological Features and Prognostic Significance of ARID1A-Deficient Esophageal Adenocarcinomas. Arch Pathol Lab Med (2017) 141:970–7. doi: 10.5858/arpa.2016-0318-OA
67. Patel AK, Pan X, Vila DM, Frankel WL, Chen W, Perry KA, et al. Perineural Invasion Predicts for Locoregional Failure in Patients With Oesophageal Adenocarcinoma Treated With Neoadjuvant Chemoradiotherapy. J Clin Pathol (2021) 74:228–33. doi: 10.1136/jclinpath-2020-206424
68. Thies S, Guldener L, Slotta-Huspenina J, Zlobec I, Koelzer VH, Lugli A, et al. Impact of Peritumoral and Intratumoral Budding in Esophageal Adenocarcinomas. Hum Pathol (2016) 52:1–8. doi: 10.1016/j.humpath.2016.01.016
69. Singhi AD, Foxwell TJ, Nason K, Cressman KL, McGrath KM, Sun W, et al. Smad4 Loss in Esophageal Adenocarcinoma is Associated With an Increased Propensity for Disease Recurrence and Poor Survival. Am J Surg Pathol (2015) 39:487–95. doi: 10.1097/PAS.0000000000000356
70. Castonguay MC, Li-Chang HH, Driman DK. Venous Invasion in Oesophageal Adenocarcinoma: Enhanced Detection Using Elastic Stain and Association With Adverse Histological Features and Clinical Outcomes. Histopathology (2014) 64:693–700. doi: 10.1111/his.12308
71. Mehta KS, Bianco V, Hamilton A, Gooding E, Luketich JD, Pennathur A.. The Impact of Perineural Invasion in Patients With Esophageal Adenocarcinoma Treated With Esophagectomy. J Surg Res (2014) 186:601–1. doi: 10.1016/j.jss.2013.11.569
72. Smith E, Ruszkiewicz AR, Jamieson GG, Drew PA. IGFBP7 is Associated With Poor Prognosis in Oesophageal Adenocarcinoma and is Regulated by Promoter DNA Methylation. Br J Canc (2014) 110:775–82. doi: 10.1038/bjc.2013.783
73. Lagorce C, Paraf F, Vidaud D, Couvelard A, Wendum D, Martin A, et al. Cyclooxygenase-2 is Expressed Frequently and Early in Barrett's Oesophagus and Associated Adenocarcinoma. Histopathology (2003) 42:457–65. doi: 10.1046/j.1365-2559.2003.01627.x
74. Torres C, Turner JR, Wang HH, Richards W, Sugarbaker D, Shahsafaei A, et al. Pathologic Prognostic Factors in Barrett's Associated Adenocarcinoma: A Follow-Up Study of 96 Patients. Cancer (1999) 85:520–8. doi: 10.1002/(SICI)1097-0142(19990201)85:3<520::AID-CNCR2>3.0.CO;2-L
75. Sheng L, Ji Y, Du X. Perineural Invasion Correlates With Postoperative Distant Metastasis and Poor Overall Survival in Patients With PT1-3N0M0 Esophageal Squamous Cell Carcinoma. Onco Targets Ther (2015) 8:3153–7. doi: 10.2147/OTT.S90909
76. Guo XF, Zhu M, Hao AL. Value of Tumor Markers in Prognosis of Adenocarcinoma of Esophageal Junction. China Med Eng (2021) 29(04):63–6. doi: 10.3892/or_00000838
77. Arigami T, Uchikado Y, Omoto I, Sasaki K, Kita Y, Owaki T, et al. Primary Tumor Score Based on Tumor Depth and Length Predicts Prognosis in Esophageal Squamous Cell Carcinoma. Anticancer Res (2018) 38:5447–52. doi: 10.21873/anticanres.12876
78. Bhatt A, Kamath S, Murthy SC, Raja S. Multidisciplinary Evaluation and Management of Early Stage Esophageal Cancer. Surg Oncol Clin N Am (2020) 29:613–30. doi: 10.1016/j.soc.2020.06.011
79. Takahashi H, Katsuta E, Yan L, Tokumaru Y, Katz MHG, Takabe K. Transcriptomic Profile of Lymphovascular Invasion, a Known Risk Factor of Pancreatic Ductal Adenocarcinoma Metastasis. Cancers (Basel) (2020) 12:2033. doi: 10.3390/cancers12082033
80. Freeman A. Perineural and Lymphovascular Invasion on Prostatic Biopsy: Pathological Assessment and Significance. Surg Oncol (2009) 18:200–2. doi: 10.1016/j.suronc.2009.02.010
81. Blackham AU, H Naqvi SM, Schell MJ, Jin W, Gangi A, Almhanna K, et al. Recurrence Patterns and Associated Factors of Locoregional Failure Following Neoadjuvant Chemoradiation and Surgery for Esophageal Cancer. J Surg Oncol (2018) 117:150–9. doi: 10.1002/jso.24808
82. Fassan M, Cagol M, Pennelli G, Rizzetto C, Giacomelli L, Battaglia G, et al. Programmed Cell Death 4 Protein in Esophageal Cancer. Oncol Rep (2010) 24:135–9. doi: 10.3892/or_00000838.
83. Wang F, Liu SS, Liu JQ, Feng F, Guo Y, Zhang W, et al. SP Promotes Cell Proliferation in Esophageal Squamous Cell Carcinoma Through the NK1R/Hes1 Axis. Biochem BIOPH Res CO (2019) 514:1210–6. doi: 10.1016/j.bbrc.2019.05.092
Keywords: esophageal cancer, perineural invasion, lymphovascular invasion, lymph node metastasis, prognosis
Citation: Bai L, Yan L, Guo Y, He L, Sun Z, Cao W, Lu J and Mo S (2022) Perineural Invasion Is a Significant Indicator of High Malignant Degree and Poor Prognosis in Esophageal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 12:816270. doi: 10.3389/fonc.2022.816270
Received: 16 November 2021; Accepted: 10 May 2022;
Published: 08 June 2022.
Edited by:
Mark Doherty, University of Toronto, CanadaReviewed by:
Yan Qi, Central People’s Hospital of Zhanjiang, ChinaMarco Scarpa, University Hospital of Padua, Italy
Copyright © 2022 Bai, Yan, Guo, He, Sun, Cao, Lu and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saijun Mo, c2ptb0B6enUuZWR1LmNu; orcid.org/0000-0001-7375-3562
†These authors have contributed equally to this work
 Liuyang Bai
Liuyang Bai Liangying Yan1†
Liangying Yan1† Yaping Guo
Yaping Guo Jing Lu
Jing Lu