- Department of Orthopedics, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, China
Taxanes (paclitaxel and docetaxel) play an important role in the treatment of advanced sarcomas. Albumin-bound paclitaxel (nab-paclitaxel) is a new kind of taxane and has many advantages compared with paclitaxel and docetaxel. Nab-paclitaxel is currently approved for the treatment of advanced breast, non-small cell lung, and pancreatic cancers. However, the efficacy of nab-paclitaxel in sarcomas has not been reviewed. In this review, we first compare the similarities and differences among nab-paclitaxel, paclitaxel, and docetaxel and then summarize the efficacy of nab-paclitaxel against various non-sarcoma malignancies based on clinical trials with reported results. The efficacy and clinical research progress on nab-paclitaxel in sarcomas are also summarized. This review will serve as a good reference for the application of nab-paclitaxel in clinical sarcoma treatment studies and the design of clinical trials.
Introduction
Sarcomas (sarcomas in this review refer only to bone and soft tissue sarcomas, excluding gastrointestinal stromal tumors and Kaposi sarcoma) are malignant tumors derived from mesenchymal tissue (1). Sarcomas have low incidence, accounting for only about 2% of all newly diagnosed malignancies in humans each year (1, 2), yielding approximately 200,000 new cases (3, 4). A small number of these patients have metastases at initial diagnosis. Approximately 50% of newly diagnosed nonmetastatic cases eventually progress to an advanced stage (2, 4–6). The systemic treatment for advanced sarcomas is the same as that for other malignancies, including chemotherapy, targeted therapy, and immunotherapy (1, 7).
As chemotherapy drugs, paclitaxel and docetaxel play an important role in the treatment of advanced sarcomas (7–12). Paclitaxel and docetaxel are taxanes (13). Taxanes represent an important class of antineoplastic agents that interfere with microtubule function, leading to altered mitosis and cellular death in vitro (14); in vivo, intratumoral concentrations of taxanes cause cell death due to chromosome missaggregation in multipolar spindles (15). Paclitaxel was originally extracted from the Pacific yew tree, a small slow-growing evergreen, coniferous tree (16). Owing to the initial scarcity of paclitaxel, docetaxel, a semisynthetic analog of paclitaxel produced from the needles of the European yew tree, was developed (17, 18). Docetaxel differs from paclitaxel in its chemical structure in two positions (19). These small alterations make docetaxel different from paclitaxel in terms of water solubility, cellular effects, pharmacology, and other aspects (19–22) (Table 1).
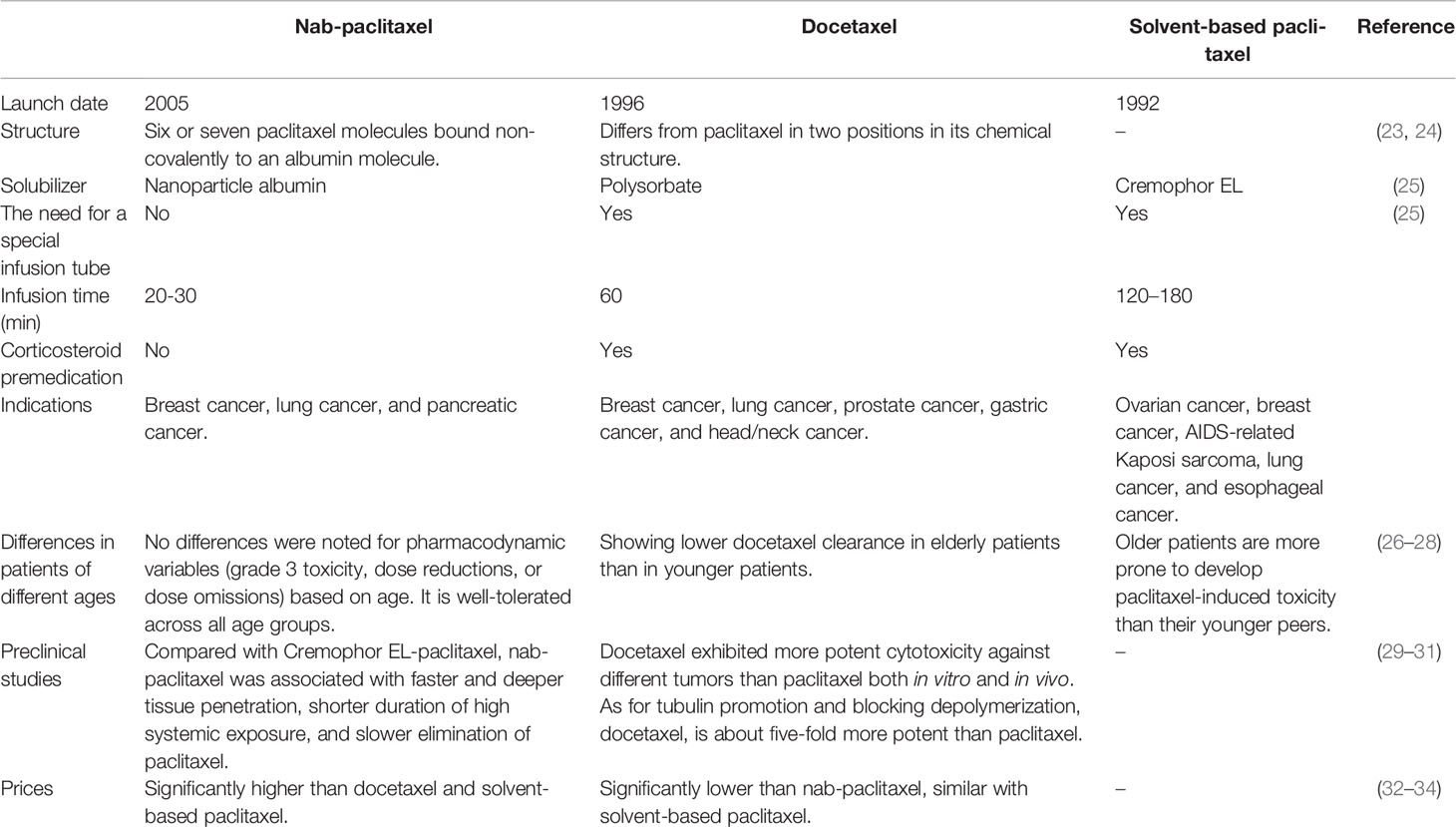
Table 1 Comparison of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) with solvent-based paclitaxel and docetaxel.
Paclitaxel is currently one of the widely used antineoplastic agents with broad activity in several cancers, including breast cancer, ovarian cancer, endometrial cancer, squamous cell carcinoma, adenocarcinoma, non-small cell lung cancer (NSCLC), bladder cancer, cervical carcinoma, and esophageal cancer (35, 36). Paclitaxel is also used in the treatment of some sarcomas (8), especially angiosarcoma (11, 37). Docetaxel is a widely prescribed antineoplastic agent for a broad range of malignancies including breast cancer, lung cancer, head/neck cancer, prostate cancer, and gastric cancer (18, 21, 29, 38). The efficacy of docetaxel-based chemotherapy in the treatment of soft tissue sarcomas is comparable to that of first-line treatment with doxorubicin, but is considered inferior to doxorubicin chemotherapy because of its toxicity and administration complexity (39). Docetaxel-based chemotherapy is also considered a second-line treatment for advanced osteosarcoma (40). Nevertheless, paclitaxel and docetaxel have several clinical problems, including poor drug solubility and serious dose-limiting toxicities such as peripheral sensory neuropathy, myelosuppression, and allergic reactions. A number of these clinical problems have been associated with the solvents used for diluting these antineoplastic agents: Cremophor EL for paclitaxel and polysorbate 80 for docetaxel (23, 29, 41–43).
To overcome the clinical problems associated with the solvents used for diluting paclitaxel and docetaxel, albumin-bound paclitaxel (nab-paclitaxel) was developed (44). Nab-paclitaxel is an albumin-bound, 130-nm particle size formulation of paclitaxel that is devoid of any solvents or ethanol (14, 44). The lyophilized formulation, comprised of albumin and paclitaxel, is reconstituted in 0.9% NaCl to form a colloidal suspension (45). Compared with paclitaxel, despite having the same active ingredient (paclitaxel), the two different formulations exhibit unique efficacy and safety profiles (14, 30). The development of albumin-bound nanotechnology as a delivery system for paclitaxel neutralized its hydrophobicity and provided better pharmacokinetic and pharmacodynamic characteristics (45, 46). Nab-paclitaxel showed increased endothelial cell transport, intratumor paclitaxel concentrations, and antitumor activity compared with cremophor-based paclitaxel (31, 44) (Table 1). To date, nab-paclitaxel has been indicated for the treatment of metastatic breast cancer, locally advanced or metastatic NSCLC, and metastatic pancreatic cancer in the USA (47). It also showed promising efficacy in many other solid tumors (48–50). Currently, a number of clinical trials on the efficacy of nab-paclitaxel in various malignancies are being conducted (https://clinicaltrials.gov). The available evidence shows that the efficacy and safety of nab-paclitaxel are superior to those of paclitaxel and docetaxel in a variety of malignancies (22, 51–53). Studies have also confirmed the effectiveness of nab-paclitaxel in the treatment of sarcomas (54–57). However, it is not clear whether this formulation is more effective than conventional paclitaxel and docetaxel. In this review, we first considered the efficacy of nab-paclitaxel in the treatment of various non-sarcoma malignancies, and then focused on related studies of nab-paclitaxel in the treatment of sarcomas, with the aim of providing a reference to establish a clinical study design and to support clinical treatment of sarcomas.
Efficacy of Nab-Paclitaxel in Non-Sarcoma Malignancies
To fully evaluate the efficacy of nab-paclitaxel in the treatment of malignancies, systematic hand and online searches were conducted. The inclusion criteria for literature and clinical trials were as follows: 1) published in 2011 and later; 2) prospective phase II or III clinical trials; 3) trials of nab-paclitaxel monotherapy or combination therapy of malignancies; 4) trials with a registration number; and 5) trials with complete data published in open-access journals. The retrieved clinical trials and literature were classified according to the histopathological type.
Breast Cancer
Breast cancer was the first indication for which nab-paclitaxel was approved by the US Food and Drug Administration (FDA). The results of the first phase III trial in women with metastatic breast cancer showed that nab-paclitaxel demonstrated significantly higher response rates than standard paclitaxel (33% vs. 19%, respectively) and significantly longer progression-free survival (23.0 vs. 16.9 weeks, respectively) (58). This led to FDA approval of nab-paclitaxel in 2005 for second-line and above-line treatment of metastatic breast cancer. Since then, nab-paclitaxel has been widely studied in breast cancer, including attempts to use this formulation as a first-line treatment for metastatic breast cancer. However, these studies have not yielded sufficiently positive results to enable replacement of traditional first-line treatment (59–61). In a multicenter, randomized, comparative phase III study reported in 2018, atezolizumab plus nab-paclitaxel treatment prolonged progression-free survival among patients with metastatic triple-negative breast cancer in both the intention-to-treat population and the programmed death ligand 1-positive subgroup (62). This led to FDA approval of nab-paclitaxel in combination with atezolizumab for first-line treatment of metastatic breast cancer in 2019.
Several recent clinical trials have shown that nab-paclitaxel is effective for the treatment of non-metastatic breast cancer in the neoadjuvant setting. Nab-paclitaxel improved the pathological complete response rate and event-free survival compared with traditional taxanes, and with acceptable toxicities (63). Current studies of nab-paclitaxel in breast cancer have focused on dose adjustment and combination regimens with other drugs (64–68). In conclusion, the application of nab-paclitaxel in breast cancer has gradually improved from second-line and above- to first-line and neoadjuvant therapy. The application regimen has gradually evolved from a single drug to combinations with other chemotherapy drugs and/or with immunotherapy. Nab-paclitaxel is now a basic drug in the treatment of breast cancer. With the invention and application of new anti-tumor drugs, the combination of nab-paclitaxel with other drugs, and different application scenarios in the treatment of breast cancer, the role of nab-paclitaxel will be further studied.
Lung Cancer
The results of a multicenter, randomized, controlled phase III clinical trial showed that nab-paclitaxel demonstrated a significantly higher objective response rate than solvent-based paclitaxel (33% vs. 25%) as first-line therapy in patients with advanced NSCLC (43). This led to FDA approval of nab-paclitaxel in combination with carboplatin as first-line treatment for advanced NSCLC in 2012. Since then, nab-paclitaxel has been widely studied in lung cancer. Subsequent clinical trials demonstrated the good activity of nab-paclitaxel combined with carboplatin in different patients with advanced NSCLC (69–72); however, nab-paclitaxel-based therapy had unsatisfactory efficacy in advanced small cell lung cancer (73, 74). Other clinical trials have shown that nab-paclitaxel-based chemotherapy worked as well as, or better than, other conventional drugs and regimens in advanced NSCLC (75–78). Recent clinical trial results show that nab-paclitaxel-based chemoradiotherapy has promising efficacy in the treatment of locally advanced NSCLC (79–81). Furthermore, the combination of nab-paclitaxel-based chemotherapy and immunotherapy can also achieve good efficacy (82–85). In conclusion, the clinical study of nab-paclitaxel in lung cancer has deepened our understanding of the treatment of advanced NSCLC patients who failed multi-line treatment by showing efficacy in multi-drug combination therapy, first-line, or neoadjuvant therapy in different subgroups. Clinical studies of nab-paclitaxel in lung cancer will become increasingly detailed and complex.
Pancreatic Cancer
The results of an international, multicenter, open label, randomized phase III study showed that treating metastatic pancreatic cancer patients with gemcitabine plus nab-paclitaxel (GnP) as first-line therapy resulted in a significantly higher median overall survival time than treatment with gemcitabine alone (8.5 vs. 6.7 months) (86). This led to FDA approval of nab-paclitaxel in combination with gemcitabine for first-line treatment of metastatic pancreatic adenocarcinoma in 2013. Since then, nab-paclitaxel has been widely studied for treating pancreatic cancer. These clinical trials and studies can be grouped into four categories: 1. Continued testing of GnP in different groups of patients with advanced pancreatic cancer, such as elderly patients and patients with poor physical performance (87–89); 2. Comparing the efficacy and safety of GnP with FOLFIRINOX (a conventional chemotherapy regimen) for pancreatic cancer (90–93); 3. Testing new multidrug combination regimens based on nab-paclitaxel (94–97); and 4. Preoperative adjuvant therapy in patients with locally advanced pancreatic cancer (98–100). In conclusion, the research and application of nab-paclitaxel in pancreatic cancer are also a complex process from single to multi-drug combination therapy, from an ordinary to a special patient group, and from advanced first-line therapy to neoadjuvant therapy. According to various trial results, nab-paclitaxel has clear efficacy in the treatment of pancreatic cancer and has been considered a basic drug for adjuvant chemotherapy in this disease.
Other Cancers
As the price of nab-paclitaxel has decreased, the numbers of studies and trials on this agent in various cancers have increased significantly. At present, clinical trial results have shown that nab-paclitaxel has promising efficacy in gastric cancer, melanoma, gynecological malignancy, urothelial carcinoma, head and neck squamous cell carcinoma, biliary tract carcinoma, and nasopharyngeal carcinoma (50, 52, 101–104). The research and application of nab-paclitaxel in every kind of malignant tumor have gradually evolved toward the direction of combined and perioperative treatment, similar with its path in breast cancer, lung cancer, and pancreatic cancer (105–108). It is believed that, with further research, the status of nab-paclitaxel in various malignant tumors will gradually be equal to or even exceed that of traditional taxanes.
Efficacy of Traditional Taxanes and Nab-Paclitaxel in Sarcomas
Efficacy of Traditional Taxanes in Sarcomas
Taxanes are one of the important class of drugs in the treatment of sarcomas and are recommended as systemic chemotherapy drugs in the National Comprehensive Cancer Network guidelines for the treatment of sarcomas (109, 110). Recent data demonstrate that paclitaxel causes cell death due to chromosome missegregation on multipolar spindles (15). As the first taxanes, paclitaxel was proved to be ineffective in most soft tissue sarcomas, except angiosarcoma, in the 1990s (111). At present, paclitaxel is the first choice of chemotherapy for vasogenic malignancies (112, 113). With the application of targeted drugs in the treatment of sarcoma, the combination of paclitaxel and targeted drugs also shows promising activity (114). However, because of the simultaneous use of steroids, paclitaxel is obviously not suitable for use in combination with immunotherapeutic drugs.
As described earlier in this review, docetaxel, an improved and invented drug based on paclitaxel, is as effective as or better than paclitaxel in a variety of malignancies (Table 1) (19). Therefore, researchers initially concluded that the drug should also be more effective in sarcoma (17). However, the results of initial clinical trial have shown that docetaxel monotherapy has limited activity in sarcoma (115). Fortunately, subsequent clinical trials have shown that gemcitabine in combination with docetaxel is more effective (116, 117). This has led to extensive research and application of docetaxel and gemcitabine combination therapy in sarcomas. Currently, gemcitabine plus docetaxel is considered to have similar efficacy to doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (39). It is important to note that docetaxel plus gemcitabine combined with pazopanib is too toxic to be used. This has limited the application of this approach in the era of targeted therapy (118). In addition, docetaxel is also clearly not suitable for use in combination with immunotherapy drugs, since steroid use is also required.
Efficacy of Nab-Paclitaxel in Sarcomas
As a new and improved taxane chemotherapeutic drug, nab-paclitaxel has many advantages compared with paclitaxel and docetaxel (Table 1) (46). Like other drugs, the inhibitory effect of paclitaxel on tumor tissue is dose-dependent (15). Mechanistically, because it is less toxic and more penetrating, a higher tumor/plasma paclitaxel drug ratio in favor of nab-paclitaxel was observed (119). Because secreted protein acidic and rich in cysteine (SPARC) shows high binding affinity to albumin, a study has proved that pediatric sarcoma xenografts expressing SPARC would show enhanced uptake and accumulation of nab- paclitaxel (120). We therefore infer that nab-paclitaxel is likely to be more effective in sarcomas. However, as a new taxane drug, nab-paclitaxel has not been studied extensively in sarcomas. We systematically searched all relevant literature on the treatment of bone and soft tissue sarcomas with nab-paclitaxel, and the results are shown in Table 2. Current evidence shows that nab-paclitaxel has promising effects in the treatment of angiosarcoma, epithelioid sarcoma, leiomyosarcoma, and other subtypes of soft tissue sarcomas (Table 2). GnP showed mild activity in Ewing sarcoma (Table 2). However, the evidence level in these studies was not high. Compared with research on nab-paclitaxel in other malignant tumors and that on solvent-based paclitaxel in sarcomas, research on nab-paclitaxel in sarcomas is in its infancy.
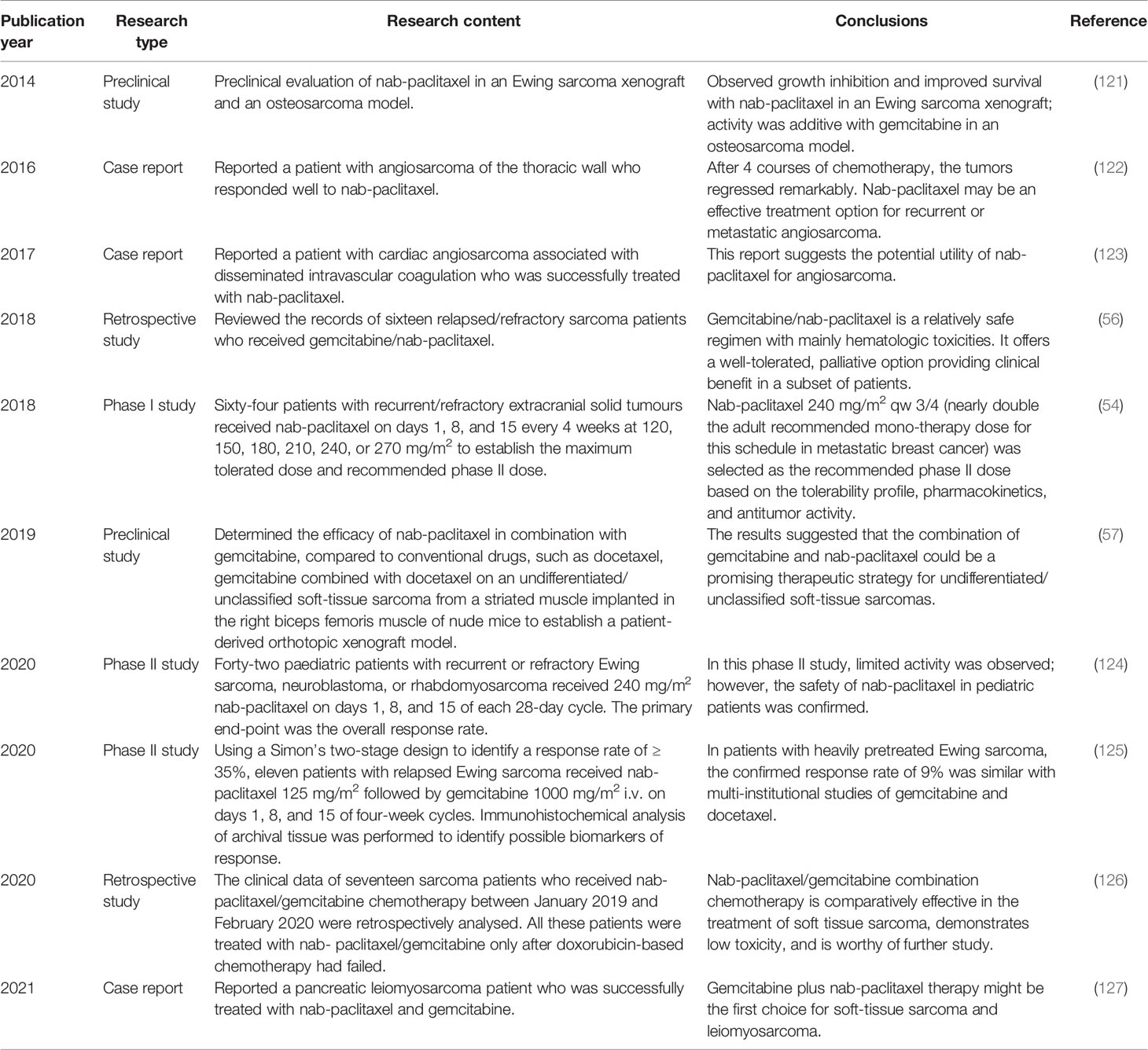
Table 2 Studies on the treatment of sarcomas with nanoparticle albumin-bound paclitaxel (nab-paclitaxel).
Discussion
In this review, we first determined the similarities and differences between nab-paclitaxel and other taxanes. We found that nab‐paclitaxel has many advantages over traditional solvent-based taxanes in terms of structure and performance. We then summarized the therapeutic efficacy and clinical trial process of nab-paclitaxel in non-sarcoma malignancies. The application of nab-paclitaxel in various malignant tumors is a simple, but complex, process. Finally, we reviewed the application of traditional taxanes and nab-paclitaxel in treating sarcomas. At present, research on nab-paclitaxel in sarcomas is in its infancy. Current evidence shows that nab-paclitaxel may have better activity than traditional taxanes in some subtypes, such as angiosarcoma, epithelioid sarcoma, and leiomyosarcoma.
Based on the above review, we believe that the use of nab-paclitaxel in treating sarcomas is worthy of further study. These studies can be conducted in at least four different directions (Figure 1). 1) Sarcomas are divided into dozens of histological subtypes, and each subtype has a different sensitivity to taxanes. Therefore, the efficacy of nab-paclitaxel therapy should be evaluated for each subtype. 2) Combined application of nab-paclitaxel with other drugs (such as chemotherapy drugs, targeted drugs, and immunotherapy drugs), or radiotherapy and interventional therapy, should be studied further. For example, traditional taxanes cannot be effectively combined with immunotherapy because they require concurrent administration of steroids. However, studies have shown that nab‐paclitaxel can regulate the immune microenvironment and sensitize cancers to immunotherapy (128–130). This suggests that nab‐paclitaxel combined with immunotherapy for sarcomas may have a better curative effect. 3) Expanding the application of nab-paclitaxel to patients of different ages and patients with poor physical fitness should be explored. Compared with traditional taxanes, nab-paclitaxel has fewer toxic side effects and lower requirements for infusion conditions. Thus, it may have significant advantages in both young and elderly patients, or those with poor physical fitness. 4) Expand the application of nab-paclitaxel to different application scenarios of sarcoma treatment, such as neoadjuvant therapy and first-line treatment of advanced cancer patients.
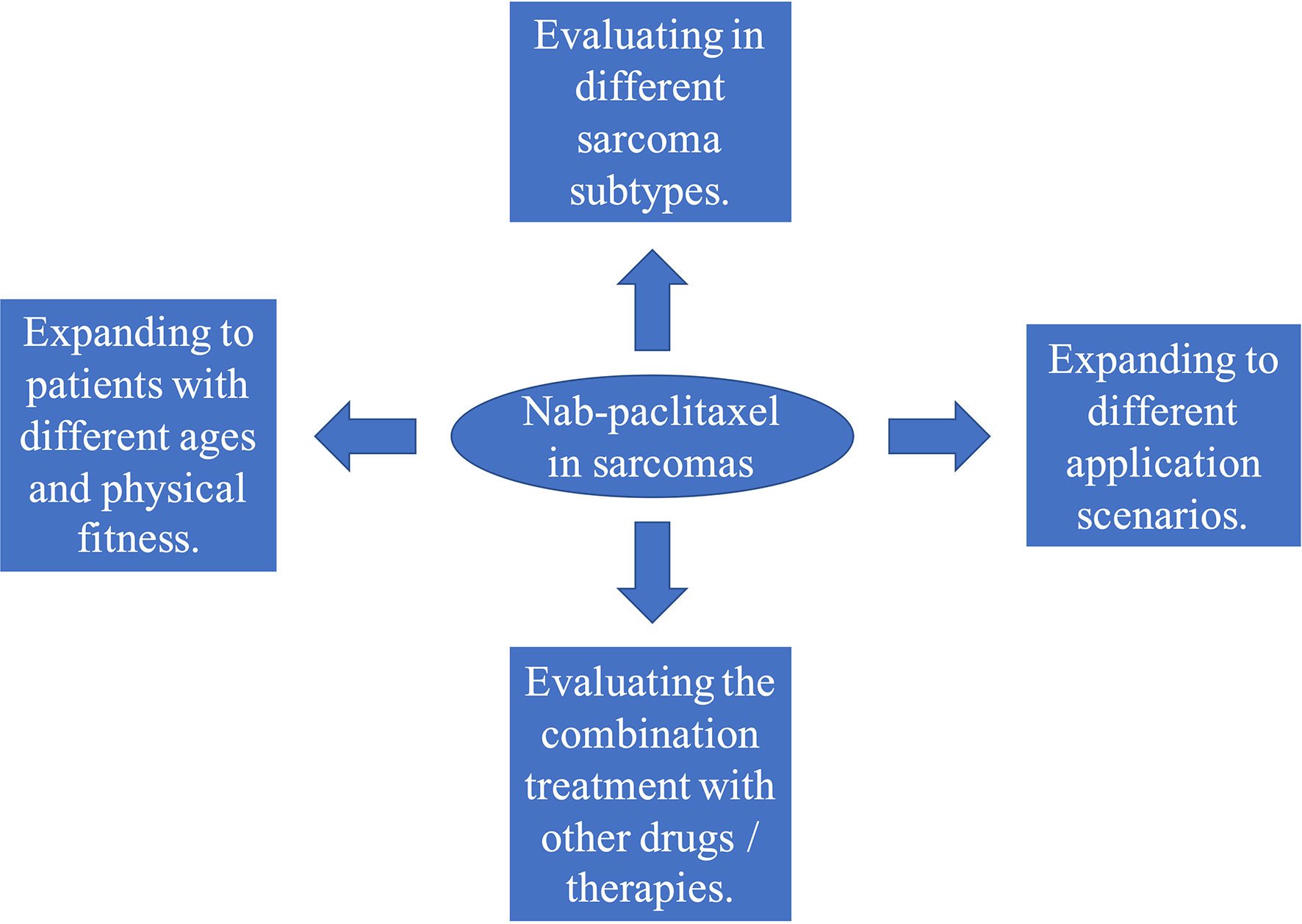
Figure 1 Four directions for clinical studies on albumin-bound paclitaxel (nab-paclitaxel) in sarcomas.
Over the past decade, the main reason for restricting the wide use of nab-paclitaxel has been its high price. At present, nab-paclitaxel has successfully entered the list of essential drugs in China’s national medical insurance system, and its price has been greatly reduced. This indicates that the application and research of nab-paclitaxel in various malignant tumors, including sarcomas, will expand.
In conclusion, nab‐paclitaxel has been shown to have better activity than paclitaxel and docetaxel in many types of malignant tumors. Although research on its efficacy in sarcomas is in its infancy, it has been shown to have promising efficacy in some sarcoma subtypes. Overall, nab-paclitaxel is expected to become one of the important basic drugs in the field of sarcoma treatment.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2018) 16:536–63. doi: 10.6004/jnccn.2018.0025
2. Sarcoma Committee of Chinese Anti-Cancer Association, Chinese Society of Clinical Oncology. Chinese Expert Consensus on Diagnosis and Treatment of Soft Tissue Sarcomas (Version 2015). Zhonghua Zhong Liu Za Zhi (2016) 38:310–20. doi: 10.3760/cma.j. issn.0253-3766.2016.04.013.
3. Cipriano CA, Jang E, Tyler W. Sarcoma Surveillance: A Review of Current Evidence and Guidelines. J Am Acad Orthop Surg (2020) 28:145–56. doi: 10.5435/JAAOS-D-19-00002
4. Osasan S, Zhang M, Shen F, Paul PJ, Persad S, Sergi C. Osteogenic Sarcoma: A 21st Century Review. Anticancer Res (2016) 36:4391–8. doi: 10.21873/anticanres.10982
6. Bosma SE, Ayu O, Fiocco M, Gelderblom H, Dijkstra PDS. Prognostic Factors for Survival in Ewing Sarcoma: A Systematic Review. Surg Oncol (2018) 27:603–10. doi: 10.1016/j.suronc.2018.07.016
7. Singhi EK, Moore DC, Muslimani A. Metastatic Soft Tissue Sarcomas: A Review Of Treatment and New Pharmacotherapies. P t (2018) 43:410–29.
8. Nagar SP, Mytelka DS, Candrilli SD, D’Yachkova Y, Lorenzo M, Kasper B, et al. Treatment Patterns and Survival Among Adult Patients With Advanced Soft Tissue Sarcoma: A Retrospective Medical Record Review in the United Kingdom, Spain, Germany, and France. Sarcoma (2018) 2018:5467057. doi: 10.1155/2018/5467057
9. Villalobos VM, Byfield SD, Ghate SR, Adejoro O. A Retrospective Cohort Study of Treatment Patterns Among Patients With Metastatic Soft Tissue Sarcoma in the US. Clin Sarcoma Res (2017) 7:18. doi: 10.1186/s13569-017-0084-4
10. Maki. Gemcitabine RG. And Docetaxel in Metastatic Sarcoma: Past, Present, and Future. Oncologist (2007) 12:999–1006. doi: 10.1634/theoncologist.12-8-999
11. Penel N, Lansiaux A, Adenis A. Angiosarcomas and Taxanes. Curr Treat Options Oncol (2007) 8:428–34. doi: 10.1007/s11864-007-0042-0
12. Kim JH, Park HS, Heo SJ, Kim SK, Han JW, Shin KH, et al. Differences in the Efficacies of Pazopanib and Gemcitabine/Docetaxel as Second-Line Treatments for Metastatic Soft Tissue Sarcoma. Oncology (2019) 96:59–69. doi: 10.1159/000492597
13. Ojima I, Lichtenthal B, Lee S, Wang C, Wang X. Taxane Anticancer Agents: A Patent Perspective. Expert Opin Ther Pat (2016) 26:1–20. doi: 10.1517/13543776.2016.1111872
14. Yared JA, Tkaczuk KH. Update on Taxane Development: New Analogs and New Formulations. Drug Des Devel Ther (2012) 6:371–84. doi: 10.2147/DDDT.S28997
15. Weaver BA. How Taxol/paclitaxel Kills Cancer Cells. Mol Biol Cell (2014) 25:2677–81. doi: 10.1091/mbc.E14-04-0916
16. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant Antitumor Agentsand Structure of Taxol, a Novel Antileukemic and Antitumor Agent From Taxus Brevifolia. J Am Chem Soc (1971) 93:2325–7. doi: 10.1021/ja00738a045
17. Verweij J. Docetaxel: An Interesting New Drug for the Treatment of Head and Neck Cancers and Soft Tissue Sarcomas. Anticancer Drugs (1995) 6:19–24. doi: 10.1097/00001813-199507004-00004
18. Zhao P, Astruc D. Docetaxel Nanotechnology in Anticancer Therapy. Chem Med Chem (2012) 7:952–72. doi: 10.1002/cmdc.201200052
19. de Weger VA, Beijnen JH, Schellens JH. Cellular and Clinical Pharmacology of the Taxanes Docetaxel and Paclitaxel–A Review. Anticancer Drugs (2014) 25:488–94. doi: 10.1097/CAD.0000000000000093
20. Andriguetti NB, Raymundo S, Antunes MV, Perassolo MS, Verza SG, Suyenaga ES, et al. Pharmacogenetic and Pharmacokinetic Dose Individualization of the Taxane Chemotherapeutic Drugs Paclitaxel and Docetaxel. Curr Med Chem (2017) 24:3559–82. doi: 10.2174/0929867324666170623093445
21. Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for Treatment of Solid Tumours: A Systematic Review of Clinical Data. Lancet Oncol (2005) 6:229–39. doi: 10.1016/s1470-2045(05)70094-2
22. Lee H, Park S, Kang JE, Lee HM, Kim SA, Rhie SJ. Efficacy and Safety of Nanoparticle-Albumin-Bound Paclitaxel Compared With Solvent-Based Taxanes for Metastatic Breast Cancer: A Meta-Analysis. Sci Rep (2020) 10:530. doi: 10.1038/s41598-019-57380-0
23. Louage B, De Wever O, Hennink WE, De Geest BG. Developments and Future Clinical Outlook of Taxane Nanomedicines. J Contr Rel (2017) 253:137–52. doi: 10.1016/j.jconrel.2017.03.027
24. Paal K, Muller J, Hegedus L. High Affinity Binding of Paclitaxel to Human Serum Albumin. Eur J Biochem (2001) 268:2187–91. doi: 10.1046/j.1432-1327.2001.02107.x
25. Bashour SI, Ibrahim NK. CCR 20th Anniversary Commentary: Setting the Stage for Nanoparticle Albumin-Bound Paclitaxel-How Far Science Has Come. Clin Cancer Res (2015) 21:1975–7. doi: 10.1158/1078-0432.CCR-14-2554
26. Crombag MBS, Dorlo TPC, van der Pan E, van Straten A, Bergman AM, van Erp NP, et al. Exposure to Docetaxel in the Elderly Patient Population: A Population Pharmacokinetic Study. Pharm Res (2019) 36:181. doi: 10.1007/s11095-019-2706-4
27. Hurria A, Blanchard MS, Synold TW, Mortimer J, Chung CT, Luu T, et al. Age-Related Changes in Nanoparticle Albumin-Bound Paclitaxel Pharmacokinetics and Pharmacodynamics: Influence of Chronological Versus Functional Age. Oncologist (2015) 20:37–44. doi: 10.1634/theoncologist.2014-0202
28. Crombag MBS, de Vries Schultink AHM, Koolen SLW, Wijngaard S, Joerger M, Schellens JHM, et al. Impact of Older Age on the Exposure of Paclitaxel: A Population Pharmacokinetic Study. Pharm Res (2019) 36:33. doi: 10.1007/s11095-018-2563-6
29. Zhang E, Xing R, Liu S, Li P. Current Advances in Development of New Docetaxel Formulations. Expert Opin Drug Deliv (2019) 16:301–12. doi: 10.1080/17425247.2019.1583644
30. Li Y, Chen N, Palmisano M, Zhou S. Pharmacologic Sensitivity of Paclitaxel to its Delivery Vehicles Drives Distinct Clinical Outcomes of Paclitaxel Formulations. Mol Pharm (2015) 12:1308–17. doi: 10.1021/acs.molpharmaceut.5b00026
31. Chen N, Brachmann C, Liu X, Pierce DW, Dey J, Kerwin WS, et al. Albumin-Bound Nanoparticle (Nab) Paclitaxel Exhibits Enhanced Paclitaxel Tissue Distribution and Tumor Penetration. Cancer Chemother Pharmacol (2015) 76:699–712. doi: 10.1007/s00280-015-2833-5
32. Lazzaro C, Bordonaro R, Cognetti F, Fabi A, De Placido S, Arpino G, et al. An Italian Cost-Effectiveness Analysis of Paclitaxel Albumin (Nab-Paclitaxel) Versus Conventional Paclitaxel for Metastatic Breast Cancer Patients: The COSTANza Study. Clinicoecon Outcomes Res (2013) 5:125–35. doi: 10.2147/CEOR.S41850. (2013).
33. Dranitsaris G, Cottrell W, Spirovski B, Hopkins S. Economic Analysis of Albumin-Bound Paclitaxel for the Treatment of Metastatic Breast Cancer. J Oncol Pharm Pract (2009) 15:67–78. doi: 10.1177/1078155208098584
34. Dranitsaris G, Yu B, King J, Kaura and A Zhang. Nab-paclitaxel S. Docetaxel, or Solvent-Based Paclitaxel in Metastatic Breast Cancer: A Cost-Utility Analysis From a Chinese Health Care Perspective. Clinicoecon Outcomes Res (2015) 7:249–56. doi: 10.2147/CEOR.S82194
35. Alqahtani FY, Aleanizy FS, EEl Tahir HM. Alkahtani and BT AlQuadeib. Paclitaxel. Profiles Drug Subst Excip Relat Methodol (2019) 44:205–38. doi: 10.1016/bs.podrm.2018.11.001
36. Mekhail TM, Markman M. Paclitaxel in Cancer Therapy. Expert Opin Pharmacother (2002) 3:755–66. doi: 10.1517/14656566.3.6.755
37. Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, et al. Phase II Trial of Weekly Paclitaxel for Unresectable Angiosarcoma: The ANGIOTAX Study. J Clin Oncol (2008) 26:5269–74. doi: 10.1200/JCO.2008.17.3146
38. Engels FK, Mathot RA, Verweij J. Alternative Drug Formulations of Docetaxel: A Review. Anticancer Drugs (2007) 18:95–103. doi: 10.1097/CAD.0b013e3280113338
39. Strauss SJ, Seddon B, Whelan J, Leahy M, Woll PJ, Cowie F, et al. Gemcitabine and Docetaxel Versus Doxorubicin as First-Line Treatment in Previously Untreated Advanced Unresectable or Metastatic Soft-Tissue Sarcomas (GeDDiS): A Randomised Controlled Phase 3 Trial. Lancet Oncol (2017) 18:1397–410. doi: 10.1016/S1470-2045(17)30622-8
40. Xu J, Guo W, Xie L. Combination of Gemcitabine and Docetaxel: A Regimen Overestimated in Refractory Metastatic Osteosarcoma? BMC Cancer (2018) 18:987. doi: 10.1186/s12885-018-4872-x
41. Sofias AM, Dunne M, Storm G, Allen C. The Battle of “Nano” Paclitaxel. Adv Drug Deliv Rev (2017) 122:20–30. doi: 10.1016/j.addr.2017.02.003
42. Kudlowitz D, Muggia F. Clinical Features of Taxane Neuropathy. Anticancer Drugs (2014) 25:495–501. doi: 10.1097/CAD.0000000000000051
43. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly Nab-Paclitaxel in Combination With Carboplatin Versus Solvent-Based Paclitaxel Plus Carboplatin as First-Line Therapy in Patients With Advanced Non-Small-Cell Lung Cancer: Final Results of a Phase III Trial. J Clin Oncol (2012) 30:2055–62. doi: 10.1200/JCO.2011.39.5848
44. Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased Antitumor Activity, Intratumor Paclitaxel Concentrations, and Endothelial Cell Transport of Cremophor-Free, Albumin-Bound Paclitaxel, ABI-007, Compared With Cremophor-Based Paclitaxel. Clin Cancer Res (2006) 12:1317–24. doi: 10.1158/1078-0432.CCR-05-1634
45. Viudez A, Ramirez N, Hernandez-Garcia I, Carvalho FL, Vera R, Hidalgo M. Nab-Paclitaxel: A Flattering Facelift. Crit Rev Oncol Hematol (2014) 92:166–80. doi: 10.1016/j.critrevonc.2014.06.001
46. Kudlowitz D, Muggia F. Nanoparticle Albumin-Bound Paclitaxel (Nab-Paclitaxel): Extending Its Indications. Expert Opin Drug Saf (2014) 13:681–5. doi: 10.1517/14740338.2014.910193
47. Kundranda MN, Niu J. Albumin-Bound Paclitaxel in Solid Tumors: Clinical Development and Future Directions. Drug Des Devel Ther (2015) 9:3767–77. doi: 10.2147/DDDT.S88023
48. Parisi A, Palluzzi E, Cortellini A, Sidoni T, Cocciolone V, Lanfiuti Baldi P, et al. First-Line Carboplatin/Nab-Paclitaxel in Advanced Ovarian Cancer Patients, After Hypersensitivity Reaction to Solvent-Based Taxanes: A Single-Institution Experience. Clin Transl Oncol (2020) 22:158–62. doi: 10.1007/s12094-019-02122-x
49. Takashima A, Shitara K, Fujitani K, Koeda K, Hara H, Nakayama N, et al. Peritoneal Metastasis as a Predictive Factor for Nab-Paclitaxel in Patients With Pretreated Advanced Gastric Cancer: An Exploratory Analysis of the Phase III ABSOLUTE Trial. Gastric Cancer (2019) 22:155–63. doi: 10.1007/s10120-018-0838-6
50. Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, et al. Nab-Paclitaxel and Gemcitabine as First-Line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol (2018) 4:1707–12. doi: 10.1001/jamaoncol.2018.3277
51. Wang HY, Yao ZH, Tang H, Zhao Y, Zhang XS, Yao SN, et al. Weekly Nanoparticle Albumin-Bound Paclitaxel in Combination With Cisplatin Versus Weekly Solvent-Based Paclitaxel Plus Cisplatin as First-Line Therapy in Chinese Patients With Advanced Esophageal Squamous Cell Carcinoma. Onco Targets Ther (2016) 9:5663–9. doi: 10.2147/OTT.S108580
52. Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, et al. Nab-Paclitaxel Versus Solvent-Based Paclitaxel in Patients With Previously Treated Advanced Gastric Cancer (ABSOLUTE): An Open-Label, Randomised, non-Inferiority, Phase 3 Trial. Lancet Gastroenterol Hepatol (2017) 2:277–87. doi: 10.1016/s2468-1253(16)30219-9
53. Ley J, Wildes TM, Daly K, Oppelt P, Adkins D. Clinical Benefit of Nanoparticle Albumin-Bound-Paclitaxel in Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma Resistant to Cremophor-Based Paclitaxel or Docetaxel. Med Oncol (2017) 34:28. doi: 10.1007/s12032-017-0884-7
54. Moreno L, Casanova M, Chisholm JC, Berlanga P, Chastagner PB, Baruchel S, et al. Phase I Results of a Phase I/II Study of Weekly Nab-Paclitaxel in Paediatric Patients With Recurrent/Refractory Solid Tumours: A Collaboration With Innovative Therapies for Children With Cancer. Eur J Cancer (2018) 100:27–34. doi: 10.1016/j.ejca.2018.05.002
55. Houghton PJ, Kurmasheva RT, Kolb EA, Gorlick R, Maris JM, Wu J, et al. Initial Testing (Stage 1) of the Tubulin Binding Agent Nanoparticle Albumin-Bound (Nab) Paclitaxel (Abraxane((R))) by the Pediatric Preclinical Testing Program (PPTP). Pediatr Blood Cancer (2015) 62:1214–21. doi: 10.1002/pbc.25474
56. Metts JL, Alazraki AL, Clark D, Amankwah EK, Wasilewski-Masker KJ, George BA, et al. Gemcitabine/nab-Paclitaxel for Pediatric Relapsed/Refractory Sarcomas. Pediatr Blood Cancer (2018) 65:e27246. doi: 10.1002/pbc.27246
57. Higuchi T, Kawaguchi K, Miyake K, Oshiro H, Zhang Z, Razmjooei S, et al. The Combination of Gemcitabine and Nab-Paclitaxel as a Novel Effective Treatment Strategy for Undifferentiated Soft-Tissue Sarcoma in a Patient-Derived Orthotopic Xenograft (PDOX) Nude-Mouse Model. BioMed Pharmacother (2019) 111:835–40. doi: 10.1016/j.biopha.2018.12.110
58. Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women With Breast Cancer. J Clin Oncol (2005) 23:7794–803. doi: 10.1200/jco.2005.04.937
59. Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Phase II Trial of Nab-Paclitaxel Compared With Docetaxel as First-Line Chemotherapy in Patients With Metastatic Breast Cancer: Final Analysis of Overall Survival. Clin Breast Cancer (2012) 12:313–21. doi: 10.1016/j.clbc.2012.05.001
60. Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, et al. Randomized Phase III Trial of Paclitaxel Once Per Week Compared With Nanoparticle Albumin-Bound Nab-Paclitaxel Once Per Week or Ixabepilone With Bevacizumab As First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol (2015) 33:2361–9. doi: 10.1200/JCO.2014.59.5298
61. Brufsky A. Nab-Paclitaxel for the Treatment of Breast Cancer: An Update Across Treatment Settings. Exp Hematol Oncol (2017) 6:7. doi: 10.1186/s40164-017-0066-5
62. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379:2108–21. doi: 10.1056/NEJMoa1809615
63. Liu M, Liu S, Yang L, Wang S. Comparison Between Nab-Paclitaxel and Solvent-Based Taxanes as Neoadjuvant Therapy in Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer (2021) 21:118. doi: 10.1186/s12885-021-07831-7
64. Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary Results From IMpassion131, a Double-Blind, Placebo-Controlled, Randomised Phase III Trial of First-Line Paclitaxel With or Without Atezolizumab for Unresectable Locally Advanced/Metastatic Triple-Negative Breast Cancer. Ann Oncol (2021) 32:994–1004. doi: 10.1016/j.annonc.2021.05.801
65. Sharma P, Abramson VG, O’Dea A, Nye L, Mayer I, Pathak HB, et al. Clinical and Biomarker Results From Phase I/II Study of PI3K Inhibitor Alpelisib Plus Nab-Paclitaxel in HER2-Negative Metastatic Breast Cancer. Clin Cancer Res (2021) 27:3896–904. doi: 10.1158/1078-0432.CCR-20-4879
66. Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, et al. Quality of Life in a Randomized Phase II Study to Determine the Optimal Dose of 3-Week Cycle Nab-Paclitaxel in Patients With Metastatic Breast Cancer. Breast Cancer (2021) 29:131–43. doi: 10.1007/s12282-021-01290-5
67. Tsurutani J, Hara F, Kitada M, Takahashi M, Kikawa Y, Kato H, et al. Randomized Phase II Study to Determine the Optimal Dose of 3-Week Cycle Nab-Paclitaxel in Patients With Metastatic Breast Cancer. Breast (2021) 55:63–8. doi: 10.1016/j.breast.2020.12.002
68. Yuan Y, Lee JS, Yost SE, Li SM, Frankel PH, Ruel C, et al. Phase II Trial of Neoadjuvant Carboplatin and Nab-Paclitaxel in Patients With Triple-Negative Breast Cancer. Oncologist (2021) 26:e382–93. doi: 10.1002/onco.13574
69. Socinski MA, Langer CJ, Okamoto I, Hon JK, Hirsh V, Dakhil SR, et al. Safety and Efficacy of Weekly Nab(R)-Paclitaxel in Combination With Carboplatin as First-Line Therapy in Elderly Patients With Advanced non-Small-Cell Lung Cancer. Ann Oncol (2013) 24:314–21. doi: 10.1093/annonc/mds461
70. Miyauchi E, Inoue A, Usui K, Sugawara S, Maemondo M, Saito H, et al. Phase II Study of Modified Carboplatin Plus Weekly Nab-Paclitaxel in Elderly Patients With Non-Small Cell Lung Cancer: North Japan Lung Cancer Study Group Trial 1301. Oncologist (2017) 22:640–e59. doi: 10.1634/theoncologist.2017-0059
71. Gajra A, Karim NA, Mulford DA, Villaruz LC, Matrana MR, Ali HY, et al. Nab-Paclitaxel-Based Therapy in Underserved Patient Populations: The ABOUND.PS2 Study in Patients With NSCLC and a Performance Status of 2. Front Oncol (2018) 8:253. doi: 10.3389/fonc.2018.00253
72. Asahina H, Oizumi S, Takamura K, Harada T, Harada M, Yokouchi H, et al. A Prospective Phase II Study of Carboplatin and Nab-Paclitaxel in Patients With Advanced Non-Small Cell Lung Cancer and Concomitant Interstitial Lung Disease (HOT1302). Lung Cancer (2019) 138:65–71. doi: 10.1016/j.lungcan.2019.09.020
73. Grilley-Olson JE, Keedy VL, Sandler A, Moore DT, Socinski MA, Stinchcombe TE. A Randomized Phase II Study of Carboplatin With Weekly or Every-3-Week Nanoparticle Albumin-Bound Paclitaxel (Abraxane) in Patients With Extensive-Stage Small Cell Lung Cancer. Oncologist (2015) 20:105–6. doi: 10.1634/theoncologist.2014-0327
74. Gelsomino F, Tiseo M, Barbieri F, Riccardi F, Cavanna L, Frassoldati A, et al. Phase 2 Study of NAB-Paclitaxel in SensiTivE and Refractory Relapsed Small Cell Lung Cancer (SCLC) (NABSTER TRIAL). Br J Cancer (2020) 123:26–32. doi: 10.1038/s41416-020-0845-3
75. Liu Z, Wei Z, Hu Y, Gao F, Hao L, Fang P, et al. A Phase II Open-Label Clinical Study of Comparing Nab-Paclitaxel With Pemetrexed as Second-Line Chemotherapy for Patients With Stage IIIB/IV non-Small-Cell Lung Cancer. Med Oncol (2015) 32:216. doi: 10.1007/s12032-015-0660-5
76. Wang Z, Huang C, Yang JJ, Song Y, Cheng Y, Chen GY, et al. A Randomised Phase II Clinical Trial of Nab-Paclitaxel and Carboplatin Compared With Gemcitabine and Carboplatin as First-Line Therapy in Advanced Squamous Cell Lung Carcinoma (C-Tong1002). Eur J Cancer (2019) 109:183–91. doi: 10.1016/j.ejca.2019.01.007
77. Kawashima Y, Harada T, Fujita Y, Nakagawa T, Watanabe K, Morikawa N, et al. Randomized Phase II Trial of Carboplatin + Nab-Paclitaxel Versus Cisplatin + Gemcitabine for Chemotherapy-Naive Squamous Cell Carcinoma: North Japan Lung Cancer Study Group 1302. Int J Clin Oncol (2021) 26:515–22. doi: 10.1007/s10147-020-01828-1
78. Yoneshima Y, Morita S, Ando M, Nakamura A, Iwasawa S, Yoshioka H, et al. Phase 3 Trial Comparing Nanoparticle Albumin-Bound Paclitaxel With Docetaxel for Previously Treated Advanced NSCLC. J Thorac Oncol (2021) 16:1523–32. doi: 10.1016/j.jtho.2021.03.027
79. Tanaka H, Hasegawa Y, Makiguchi T, Okumura F, Tabe C, Shiratori T, et al. A Phase I/II Study of Biweekly Carboplatin and Nab-Paclitaxel With Concurrent Radiotherapy for Patients With Locally Advanced Unresectable Stage III Non-Small-Cell Lung Cancer. Clin Lung Cancer (2021) 22:42–8. doi: 10.1016/j.cllc.2020.09.016
80. Tsuchiya-Kawano Y, Sasaki T, Yamaguchi H, Hirano K, Horiike A, Satouchi M, et al. Updated Survival Data for a Phase I/II Study of Carboplatin Plus Nab-Paclitaxel and Concurrent Radiotherapy in Patients With Locally Advanced Non-Small Cell Lung Cancer. Oncologist (2020) 25:475–e891. doi: 10.1634/theoncologist.2019-0746
81. Wu K, Zhu L, Wang J, Pan K, Wang B, Li X, et al. A Phase II Study of Concurrent Nab-Paclitaxel/Carboplatin Combined With Thoracic Radiotherapy in Locally Advanced Squamous Cell Lung Cancer. J Thorac Dis (2019) 11:4529–37. doi: 10.21037/jtd.2019.10.81
82. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant Atezolizumab and Chemotherapy in Patients With Resectable Non-Small-Cell Lung Cancer: An Open-Label, Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol (2020) 21:786–95. doi: 10.1016/s1470-2045(20)30140-6
83. Shen D, Wang J, Wu J, Chen S, Li J, Liu J, et al. Neoadjuvant Pembrolizumab With Chemotherapy for the Treatment of Stage IIB-IIIB Resectable Lung Squamous Cell Carcinoma. J Thorac Dis (2021) 13:1760–8. doi: 10.21037/jtd-21-103
84. Wang J, Li J, Cai L, Chen S, Jiang Y. The Safety and Efficacy of Neoadjuvant Programmed Death 1 Inhibitor Therapy With Surgical Resection in Stage IIIA Non-Small Cell Lung Cancer. Ann Transl Med (2021) 9(6):486. doi: 10.21037/atm-21-670
85. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-Line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
86. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased Survival in Pancreatic Cancer With Nab-Paclitaxel Plus Gemcitabine. N Engl J Med (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
87. Prager GW, Oehler L, Gerger A, Mlineritsch B, Andel J, Petzer A, et al. Comparison of Nab-Paclitaxel Plus Gemcitabine in Elderly Versus Younger Patients With Metastatic Pancreatic Cancer: Analysis of a Multicentre, Prospective, non-Interventional Study. Eur J Cancer (2021) 143:101–12. doi: 10.1016/j.ejca.2020.11.003
88. Macarulla T, Pazo-Cid R, Guillén-Ponce C, López R, Vera R, Reboredo M, et al. Phase I/II Trial to Evaluate the Efficacy and Safety of Nanoparticle Albumin-Bound Paclitaxel in Combination With Gemcitabine in Patients With Pancreatic Cancer and an ECOG Performance Status of 2. J Clin Oncol (2019) 37:230–8. doi: 10.1200/JCO.18.00089
89. Hasegawa R, Okuwaki K, Kida M, Yamauchi H, Kawaguchi Y, Matsumoto T, et al. A Clinical Trial to Assess the Feasibility and Efficacy of Nab-Paclitaxel Plus Gemcitabine for Elderly Patients With Unresectable Advanced Pancreatic Cancer. Int J Clin Oncol (2019) 24:1574–81. doi: 10.1007/s10147-019-01511-0
90. Perri G, Prakash L, Qiao W, Varadhachary GR, Wolff R, Fogelman D, et al. Response and Survival Associated With First-Line FOLFIRINOX vs Gemcitabine and Nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg (2020) 155:832–9. doi: 10.1001/jamasurg.2020.2286
91. Chun JW, Lee SH, Kim JS, Park N, Huh G, Cho IR, et al. Comparison Between FOLFIRINOX and Gemcitabine Plus Nab-Paclitaxel Including Sequential Treatment for Metastatic Pancreatic Cancer: A Propensity Score Matching Approach. BMC Cancer (2021) 21:537. doi: 10.1186/s12885-021-08277-7
92. Kunzmann V, Siveke JT, Algül H, Goekkurt E, Siegler G, Martens U, et al. Nab-Paclitaxel Plus Gemcitabine Versus Nab-Paclitaxel Plus Gemcitabine Followed by FOLFIRINOX Induction Chemotherapy in Locally Advanced Pancreatic Cancer (NEOLAP-AIO-PAK-0113): A Multicentre, Randomised, Phase 2 Trial. Lancet Gastroenterol Hepatol (2021) 6:128–38. doi: 10.1016/s2468-1253(20)30330-7
93. Riedl JM, Posch F, Horvath L, Gantschnigg A, Renneberg F, Schwarzenbacher E, et al. Gemcitabine/nab-Paclitaxel Versus FOLFIRINOX for Palliative First-Line Treatment of Advanced Pancreatic Cancer: A Propensity Score Analysis. Eur J Cancer (2021) 151:3–13. doi: 10.1016/j.ejca.2021.03.040
94. Reni M, Zanon S, Balzano G, Passoni P, Pircher C, Chiaravalli M, et al. A Randomised Phase 2 Trial of Nab-Paclitaxel Plus Gemcitabine With or Without Capecitabine and Cisplatin in Locally Advanced or Borderline Resectable Pancreatic Adenocarcinoma. Eur J Cancer (2018) 102:95–102. doi: 10.1016/j.ejca.2018.07.007
95. Sohal DPS, Duong M, Ahmad SA, Gandhi NS, Beg MS, Wang-Gillam A, et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol (2021) 7:421–7. doi: 10.1001/jamaoncol.2020.7328
96. Raufi AG, Breakstone R, Leonard K, Charpentier K, Beard R, Renaud J, et al. Adjuvant FOLFOX+Nab-Paclitaxel (FOLFOX-A) for Pancreatic Cancer: A Brown University Oncology Research Group Phase II Study (Bruog295). Am J Clin Oncol (2020) 43:857–60. doi: 10.1097/COC.0000000000000762
97. Reni M, Zanon S, Peretti U, Chiaravalli M, Barone D, Pircher C, et al. Nab-Paclitaxel Plus Gemcitabine With or Without Capecitabine and Cisplatin in Metastatic Pancreatic Adenocarcinoma (PACT-19): A Randomised Phase 2 Trial. Lancet Gastroenterol Hepatol (2018) 3:691–7. doi: 10.1016/s2468-1253(18)30196-1
98. Ahmad SA, Duong M, Sohal DPS, Gandhi NS, Beg MS, Wang-Gillam A, et al. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg (2020) 272:481–6. doi: 10.1097/SLA.0000000000004155
99. Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL, et al. Nab-Paclitaxel Plus Gemcitabine in Patients With Locally Advanced Pancreatic Cancer (LAPACT): A Multicentre, Open-Label Phase 2 Study. Lancet Gastroenterol Hepatol (2020) 5:285–94. doi: 10.1016/s2468-1253(19)30327-9
100. Zeh HJ, Bahary N, Boone BA, Singhi AD, Miller-Ocuin JL, Normolle DP, et al. A Randomized Phase II Preoperative Study of Autophagy Inhibition With High-Dose Hydroxychloroquine and Gemcitabine/Nab-Paclitaxel in Pancreatic Cancer Patients. Clin Cancer Res (2020) 26:3126–34. doi: 10.1158/1078-0432.CCR-19-4042
101. Hersh EM, Del Vecchio M, Brown MP, Kefford R, Loquai C, Testori A, et al. A Randomized, Controlled Phase III Trial of Nab-Paclitaxel Versus Dacarbazine in Chemotherapy-Naive Patients With Metastatic Melanoma. Ann Oncol (2015) 26:2267–74. doi: 10.1093/annonc/mdv324
102. Teneriello MG, Tseng PC, Crozier M, Encarnacion C, Hancock K, Messing MJ, et al. Phase II Evaluation of Nanoparticle Albumin-Bound Paclitaxel in Platinum-Sensitive Patients With Recurrent Ovarian, Peritoneal, or Fallopian Tube Cancer. J Clin Oncol (2009) 27:1426–31. doi: 10.1200/JCO.2008.18.9548
103. Ko YJ, Canil CM, Mukherjee SD, Winquist E, Elser C, Eisen A, et al. Nanoparticle Albumin-Bound Paclitaxel for Second-Line Treatment of Metastatic Urothelial Carcinoma: A Single Group, Multicentre, Phase 2 Study. Lancet Oncol (2013) 14:769–76. doi: 10.1016/s1470-2045(13)70162-1
104. Oppelt P, Ley J, Daly M, Rich J, Paniello R, Jackson RS, et al. Nab-Paclitaxel and Cisplatin Followed by Cisplatin and Radiation (Arm 1) and Nab-Paclitaxel Followed by Cetuximab and Radiation (Arm 2) for Locally Advanced Head and Neck Squamous-Cell Carcinoma: A Multicenter, non-Randomized Phase 2 Trial. Med Oncol (2021) 38:35. doi: 10.1007/s12032-021-01479-w
105. Watson S, de la Fouchardiere C, Kim S, Cohen R, Bachet JB, Tournigand C, et al. Oxaliplatin, 5-Fluorouracil and Nab-Paclitaxel as Perioperative Regimen in Patients With Resectable Gastric Adenocarcinoma: A GERCOR Phase II Study (FOXAGAST). Eur J Cancer (2019) 107:46–52. doi: 10.1016/j.ejca.2018.11.006
106. Spitler LE, Boasberg P, O’Day S, Hamid O, Cruickshank S, Mesko S, et al. Phase II Study of Nab-Paclitaxel and Bevacizumab as First-Line Therapy for Patients With Unresectable Stage III and IV Melanoma. Am J Clin Oncol (2015) 38:61–7. doi: 10.1097/COC.0b013e318287bbae
107. Giannatempo P, Raggi D, Marandino L, Bandini M, Fare E, Calareso G, et al. Pembrolizumab and Nab-Paclitaxel as Salvage Therapy for Platinum-Treated, Locally Advanced or Metastatic Urothelial Carcinoma: Interim Results of the Open-Label, Single-Arm, Phase II PEANUT Study. Ann Oncol (2020) 31:1764–72. doi: 10.1016/j.annonc.2020.09.012
108. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, Cisplatin, and Nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol (2019) 5:824–30. doi: 10.1001/jamaoncol.2019.0270
109. von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J Natl Compr Canc Netw (2020) 18:1604–12. doi: 10.6004/jnccn.2020.0058
110. Ni. Update M. And Interpretation of 2021 National Comprehensive Cancer Network (NCCN) “Clinical Practice Gu Idelines for Bone Tumors”. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi (2021) 35:1186–91. doi: 10.7507/1002-1892.202103073
111. Casper ES, Waltzman RJ, Schwartz GK, Sugarman A, Pfister D, Ilson D, et al. Phase II Trial of Paclitaxel in Patients With Soft-Tissue Sarcoma. Cancer Invest (1998) 16:442–6. doi: 10.3109/07357909809011697
112. Frezza AM, Ravi V, Lo Vullo S, Vincenzi B, Tolomeo F, Chen TW, et al. Systemic Therapies in Advanced Epithelioid Haemangioendothelioma: A Retrospective International Case Series From the World Sarcoma Network and a Review of Literature. Cancer Med (2021) 10:2645–59. doi: 10.1002/cam4.3807
113. Chen TW, Pang A, Puhaindran ME, Maw MM, Loong HH, Sriuranpong V, et al. The Treatment Landscape of Advanced Angiosarcoma in Asia-A Multi-National Collaboration From the Asian Sarcoma Consortium. Cancer Sci (2021) 112:1095–104. doi: 10.1111/cas.14793
114. Pink D, Andreou D, Bauer S, Brodowicz T, Kasper B, Reichardt P, et al. Treatment of Angiosarcoma With Pazopanib and Paclitaxel: Results of the EVA (Evaluation of Votrient((R)) in Angiosarcoma) Phase II Trial of the German Interdisciplinary Sarcoma Group (GISG-06). Cancers (Basel) (2021) 13. doi: 10.3390/cancers13061223
115. Verweij J, Lee SM, Ruka W, Buesa J, Coleman R, van Hoessel R, et al. Randomized Phase II Study of Docetaxel Versus Doxorubicin in First- and Second-Line Chemotherapy for Locally Advanced or Metastatic Soft Tissue Sarcomas in Adults: A Study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol (2000) 18:2081–6. doi: 10.1200/JCO.2000.18.10.2081
116. Leu KM, Ostruszka LJ, Shewach D, Zalupski M, Sondak V, Biermann JS, et al. Laboratory and Clinical Evidence of Synergistic Cytotoxicity of Sequential Treatment With Gemcitabine Followed by Docetaxel in the Treatment of Sarcoma. J Clin Oncol (2004) 22:1706–12. doi: 10.1200/JCO.2004.08.043
117. Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized Phase II Study of Gemcitabine and Docetaxel Compared With Gemcitabine Alone in Patients With Metastatic Soft Tissue Sarcomas: Results of Sarcoma Alliance for Research Through Collaboration Study 002. J Clin Oncol (2007) 25:2755–63. doi: 10.1200/JCO.2006.10.4117
118. Munhoz RR, D’Angelo SP, Gounder MM, Keohan ML, Chi P, Carvajal RD, et al. A Phase Ib/II Study of Gemcitabine and Docetaxel in Combination With Pazopanib for the Neoadjuvant Treatment of Soft Tissue Sarcomas. Oncologist (2015) 20:1245–6. doi: 10.1634/theoncologist.2015-0245
119. Zhang L, Marrano P, Kumar S, Leadley M, Elias E, Thorner P, et al. Nab-Paclitaxel is an Active Drug in Preclinical Model of Pediatric Solid Tumors. Clin Cancer Res (2013) 19:5972–83. doi: 10.1158/1078-0432.CCR-13-1485
120. Pascual-Pasto G, Castillo-Ecija H, Unceta N, Aschero R, Resa-Pares C, Gómez-Caballero A, et al. SPARC-Mediated Long-Term Retention of Nab-Paclitaxel in Pediatric Sarcomas. J Cont Rel (2021) 342:81–92. doi: 10.1016/j.jconrel.2021.12.035
121. Wagner LM, Yin H, Eaves D, Currier M, Cripe TP. Preclinical Evaluation of Nanoparticle Albumin-Bound Paclitaxel for Treatment of Pediatric Bone Sarcoma. Pediatr Blood Cancer (2014) 61:2096–8. doi: 10.1002/pbc.25062
122. Hara N, Fujimoto N, Miyamoto Y, Yamagishi T, Asano M, Fuchimoto Y, et al. Angiosarcoma of the Thoracic Wall Responded Well to Nanoparticle Albumin-Bound Paclitaxel: A Case Report. Drug Discovery Ther (2016) 10:114–6. doi: 10.5582/ddt.2016.01005
123. Honda K, Ando M, Sugiyama K, Mitani S, Masuishi T, Narita Y, et al. Successful Treatment of Cardiac Angiosarcoma Associated With Disseminated Intravascular Coagulation With Nab-Paclitaxel: A Case Report and Review of the Literature. Case Rep Oncol (2017) 10:863–70. doi: 10.1159/000481194
124. Amoroso L, Castel V, Bisogno G, Casanova M, Marquez-Vega C, Chisholm JC, et al. Phase II Results From a Phase I/II Study to Assess the Safety and Efficacy of Weekly Nab-Paclitaxel in Paediatric Patients With Recurrent or Refractory Solid Tumours: A Collaboration With the European Innovative Therapies for Children With Cancer Network. Eur J Cancer (2020) 135:89–97. doi: 10.1016/j.ejca.2020.04.031
125. Oesterheld JE, Reed DR, Setty BA, Isakoff MS, Thompson P, Yin H, et al. Phase II Trial of Gemcitabine and Nab-Paclitaxel in Patients With Recurrent Ewing Sarcoma: A Report From the National Pediatric Cancer Foundation. Pediatr Blood Cancer (2020) 67:e28370. doi: 10.1002/pbc.28370
126. Tian Z, Zhang F, Li P, Wang J, Yang J, Zhang P, et al. Albumin-Bound Paclitaxel and Gemcitabine Combination Therapy in Soft Tissue Sarcoma. BMC Cancer (2020) 20:698. doi: 10.1186/s12885-020-07199-0
127. Kikuchi Y, Nishikawa Y, Amanuma M, Kishimoto Y, Takuma K, Wakayama M, et al. Successful Treatment of Advanced Pancreatic Leiomyosarcoma Treated With Gemcitabine Plus Nab-Paclitaxel: A Case Report and Literature Review. Int Cancer Conf J (2021) 10:63–7. doi: 10.1007/s13691-020-00452-0
128. Pham LM, Poudel K, Ou W, Phung CD, Nguyen HT, Nguyen BL, et al. Combination Chemotherapeutic and Immune-Therapeutic Anticancer Approach via Anti-PD-L1 Antibody Conjugated Albumin Nanoparticles. Int J Pharm (2021) 605:120816. doi: 10.1016/j.ijpharm.2021.120816
129. Yang M, Li J, Gu P, Fan X. The Application of Nanoparticles in Cancer Immunotherapy: Targeting Tumor Microenvironment. Bioact Mater (2021) 6:1973–87. doi: 10.1016/j.bioactmat.2020.12.010
Keywords: albumin-bound paclitaxel, taxanes, sarcoma, breast cancer, lung cancer, pancreatic cancer
Citation: Tian Z and Yao W (2022) Albumin-Bound Paclitaxel: Worthy of Further Study in Sarcomas. Front. Oncol. 12:815900. doi: 10.3389/fonc.2022.815900
Received: 16 November 2021; Accepted: 20 January 2022;
Published: 10 February 2022.
Edited by:
Maria Felice Brizzi, University of Turin, ItalyReviewed by:
Vuong Trieu, University of Valencia, SpainAlessandro De Vita, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Copyright © 2022 Tian and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weitao Yao, eXd0MDAwMDFAMTYzLmNvbQ==
 Zhichao Tian
Zhichao Tian Weitao Yao
Weitao Yao