- 1Division of Medical Oncology, Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples, Italy
- 2Medical Oncology Unit, Ospedale del Mare, Naples, Italy
- 3Medical Oncology Unit, Ospedale Ave Gratia Plena, San Felice a Cancello, Caserta, Italy
- 4Department of Breast and Thoracic Oncology, Istituto Nazionale Tumori Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) “Fondazione G. Pascale”, Naples, Italy
- 5Polytechnique Montréal, Montréal, QC, Canada
- 6Department of Clinical Medicine and Surgery, Oncology Division, University of Naples “Federico II”, Naples, Italy
Introduction: In luminal-like early breast cancer (BC), the lack of Progesterone Receptor (PR) expression generally correlates with more aggressive behavior but the clinical validity of low PR levels remains a debated issue.
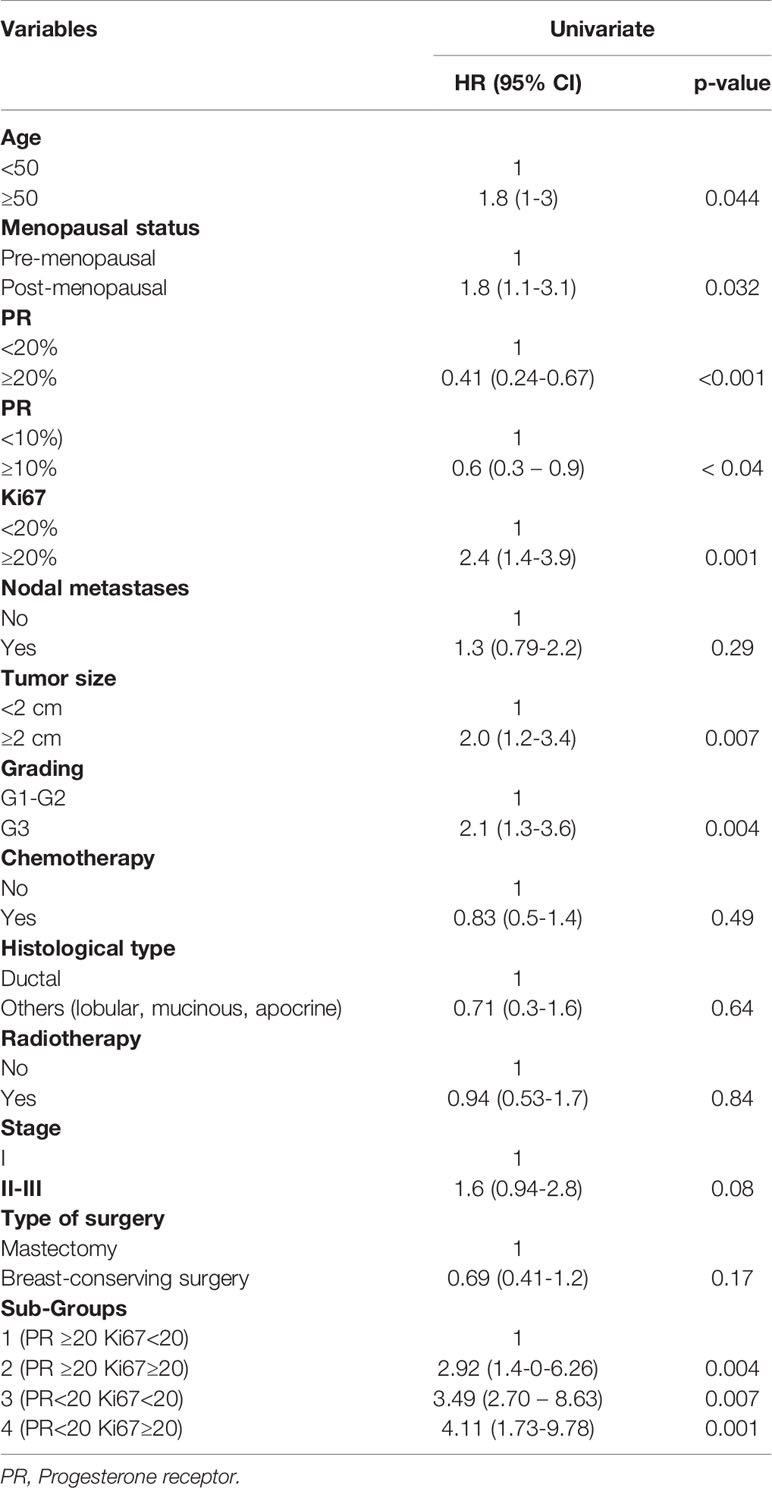
Methods: The main aim of this retrospective analysis was to assess the survival outcome (Breast cancer specific survival, BCSS) in a cohort of 687 luminal-like HER2 negative early BC patients treated at our Institutions from January 2000 to December 2018, using a sub-classification of tumors in subgroup 1 (PR high/Ki67 low), subgroup 2 (PR high/Ki67 high), subgroup 3 (PR low/Ki67 low), subgroup 4 (PR low/Ki67 high) according to PR and Ki67 values.
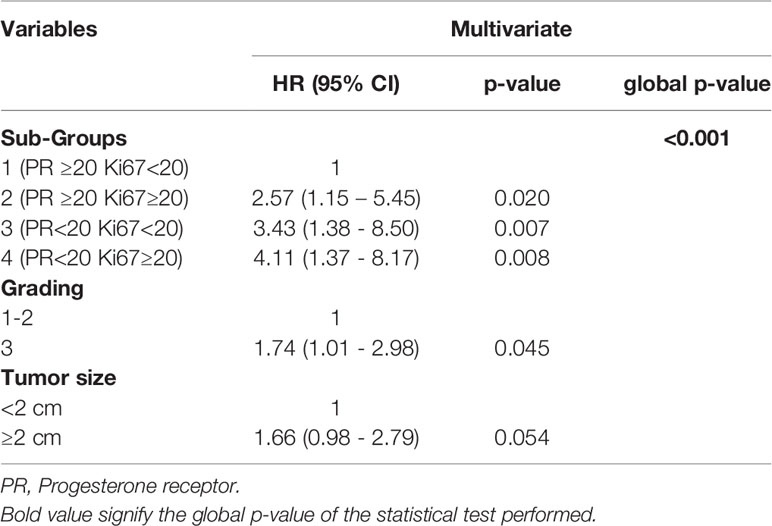
Results: At a median follow-up of 7 years, BCSS rates were 96.3%, 89%, 86.8% and 85% in the subgroup 1, 2, 3, 4 respectively. Overall, a statistically significant difference in BCSS rates was observed among the 4 subgroups (p=0.0036). On univariate analysis, post-menopause, older age (≥ 50 years), low PR and high Ki67 expression, poorly differentiated grade and size ≥ 2 cm as well as luminal B-like tumors (subgroups 2, 3, 4) were significantly associated with a worse BCSS. Multivariate analysis identified grade, size and subgroup classification of BC as independent prognostic markers of poorer outcome. In particular, subgroups 4, 3 and 2 displayed a significantly higher risk of BC-related death (HR=4.11; p=0.008; HR=3.43; p=0-007; HR=2.57; p=0.020, respectively) when compared to subgroup 1.
Conclusions: Our results support the usefulness of PR and Ki67 levels as prognostic markers, corroborating their crucial role in the decision-making process of patients with luminal-like HER2 negative early BC. Clinical application of these parameters should be assessed prospectively.
Introduction
Breast cancer (BC) is a heterogeneous disease. Based on gene expression analysis, it has been classified in five molecular or “intrinsic” subtypes linked to different prognosis and therapeutic responsiveness (1, 2). So far, high costs and technical issues have limited the use of genomic profile tools in routine clinical practice. Therefore, therapeutic decision-making process is still commonly based on clinical and immunohistochemical (IHC) characteristics, namely tumor size and grade, nodal status, hormone receptor (HR) expression, human epidermal growth factor receptor 2 (HER2) status and Ki67 values (3). According to IHC evaluation of HR, Ki67 expression levels and HER2 status, a “surrogate” classification of estrogen receptor (ER) positive (+)/HER2 negative BC in luminal A- and luminal B-like tumors has been established and widely used in clinical practice (4). A cut off value of 20% of progesterone receptor (PR) and Ki67 has been suggested to distinguish high versus low expression levels (5, 6) and is currently used to categorize luminal BC. In detail, Luminal A-like tumors, characterized by high PR (i.e. ≥ 20%) and low Ki67 (i.e. <20%) levels, have an excellent prognosis and endocrine sensitivity, while luminal B-like HER2 negative cancers, identified by low PR (i.e. < 20%) and/or high Ki67 (i.e. ≥ 20%) values, represent an extremely heterogeneous subgroup associated with a slightly unfavorable outcome (4). In this context, in the absence of available reimbursed genomic tests for widespread clinical use, the major challenge is to identify which type of luminal B-like patient could, actually, obtain additional benefit from adjuvant chemotherapy (CT) with respect to endocrine therapy (ET) alone.
Despite the well-known role of ER expression as a prognostic factor and predictor of ET sensitivity, the clinical utility of PR measurement for risk assessment and guidance for adjuvant treatment choice has long been debated and remains less clear (5). Several studies, however, correlated low or negative PR expression with a poorer prognosis (6–9) and, in the latest ASCO/CAP guidelines, the expert panel highlighted the relevance of PR levels as a prognostic marker in BC (5).
The aim of this retrospective analysis was to investigate the prognostic role of PR expression levels in a cohort of 687 luminal-like HER2 negative BC patients, using a sub-classification of luminal B-like BC according to PR and Ki67 expression.
Materials and Methods
Patients and Tumor Characteristics
Clinical records of 687 consecutive patients with primary resectable, N0-1 (up to 3 axillary lymph nodes involved), invasive, luminal-like HER2 negative BC referred to the Oncology Units at “Luigi Vanvitelli” and “Federico II” teaching hospitals in Naples, Italy, between January 1, 2000, and December 31, 2018, were retrieved. Follow-up was available until February 2021. Patients affected by in situ or de novo metastatic carcinoma at the time of diagnosis, as well as patients with 4 or more axillary lymph nodes involved (N2-3) were excluded. All women were treated with tamoxifen and/or aromatase inhibitors as adjuvant endocrine therapy for five years. Chemotherapy was administered to patients with high-risk features such as large tumor size, poorly differentiated grade, high Ki67, younger age and nodal involvement. Clinicopathological parameters including histological type, grade, ER, PR, Ki67 values were measured on surgical specimens by immunohistochemistry. BC was considered to be ER+ if at least 1% of invasive malignant cells exhibited nuclear staining or immunoreactivity, while a cut-off value of 20% was used to distinguish low versus high Ki67 and PR expression levels (4, 10).
All patients were categorized into four subgroups according to PR and Ki67 values, as follows: subgroup 1 or “Luminal-A like” (PR high/Ki67 low), subgroup 2 or “Luminal-B like with high Ki67” (PR high/Ki67 high), subgroup 3 or “Luminal-B like with low PR” (PR low/Ki67 low), subgroup 4 or “Luminal-B like with low PR and high Ki67” (PR low/Ki67 high).
Medical history, type of surgery, adjuvant treatments and clinicopathological characteristics of tumors were collected. All patients were treated in accordance with national and international guidelines.
Statistical Analysis
This study was conducted to assess the survival outcome of a cohort of non-metastatic luminal-like HER2 negative BC patients. Breast cancer specific survival (BCSS) was calculated from the date of surgery to the date of cancer-related death or last follow up. Follow-up for patients who were alive at the time of database lock was censored at the date of the last follow up. Continuous variables (e.g., ER, PR and Ki67), discrete variables (e.g., age) and categorical variables (e.g., grading, histological type) were included in the analysis. Variables were dichotomized as follows: age (<50 vs ≥50 years), menopausal status (premenopausal vs postmenopausal), Ki67 (<20% vs ≥20%), PR (<20% vs ≥20%), grade (G1-G2 vs G3), histological type (ductal vs other), lymph nodal metastases (N0 vs N1), tumor size (<2 cm vs ≥2 cm), type of surgery (mastectomy vs conservative surgery), adjuvant chemotherapy (yes vs no).
The χ2 test was used to assess the differences in the distribution of clinicopathological variables among the subgroups. Whenever the number of expected observations were lower than five (namely for histological subtype), we also applied the Fisher exact test. The survival analysis was carried out using the Kaplan-Meier (KM) method and the log-rank test was performed to estimate the differences among the curves, while survminer R package was mainly used for curve visualization. Cox proportional hazard regressions were applied to univariate and multivariate analyses to identify independent factors affecting BCSS. Multivariate analysis includes only those variables resulted statistically significant in univariate analysis. All statistical analyses have been performed using the open source environment R, release 4.0 (see: https://cran.r-project.org/) on a MacBook Pro. In all analyses, significance was established at a p value < 0.05.
Results
Patients’ Characteristics
Patients’ characteristics are summarized in Table 1. The study enrolled 687 women stratified as follows: 267 (39%) patients fell into subgroup 1, 264 (38%) into subgroup 2, 76 (11%) into subgroup 3 and 80 (12%) into subgroup 4. Median age was 53 years (range: 25-83 years), with 60% of patients aged 50 or over. Younger age (<50 years) and premenopausal status were more frequently recorded in groups 2 and 4. About 60-70% of the tumors were smaller than 2 cm, well or moderately differentiated (G1-2) and node negative. A significant different distribution of patients by ER status in the four subgroups was found, with a higher prevalence (86.6%) of tumors with high ER positivity (≥50%).
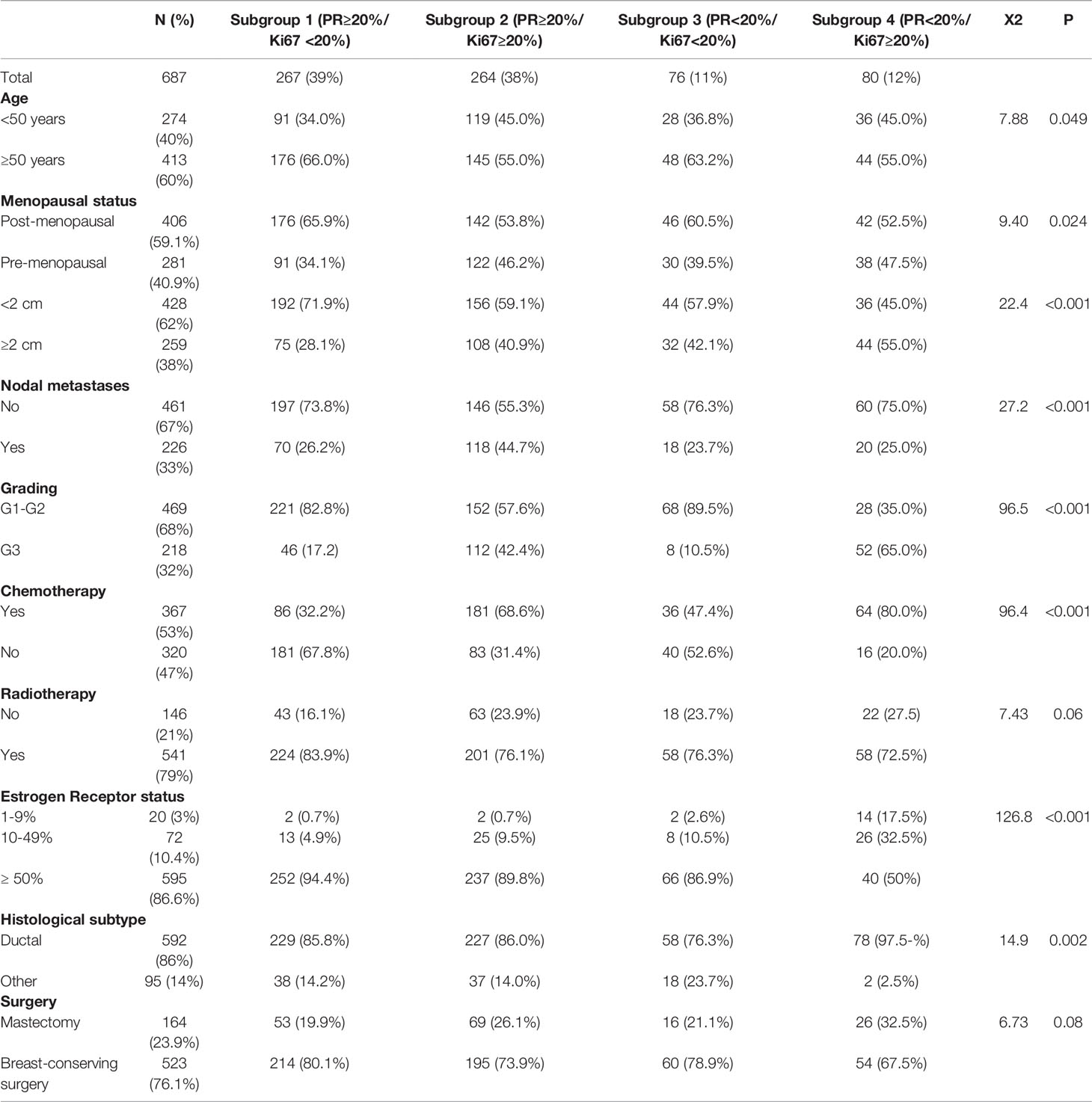
Table 1 Clinicopathological characteristics of 687 patients with luminal-like HER2-negative. BC according to different PR and Ki67 expression levels (subgroups).
Most of larger (≥2 cm) and poorly differentiated (G3) tumors were in subgroup 4 (55% and 65%, respectively). Nodal involvement was found in 33% (n=226) of the total population, with about half of the cases (n=118) belonging to group 2. Ductal carcinoma accounted for 86% of all cases (n=592), while the remaining 24% of tumors (n=95) were found to be lobular in 88, mucinous in 5, and apocrine in 2 patients, respectively. The entire cohort received ET while CT was administered to 53% of patients (n=367), distributed as follow: 32%, 69%, 47% and 80% in subgroup 1, 2, 3, 4, respectively. In particular, in the 72% (n=264) of cases a sequential anthracycline and taxane-based regimen was prescribed.
Survival Analysis
After a median follow-up of 7 years, 61 patients (9%) died of breast cancer. The median survival of all patients since diagnosis of BC was 82 months, ranging from 56 to 103 months. The resulting BCSS rates were 96.3%, 89%, 86.8% and 85% in subgroups 1, 2, 3, 4 respectively (Table 2). Overall, significant differences in BCSS were registered among the four subgroups (p=0.0036) (Figure 1).
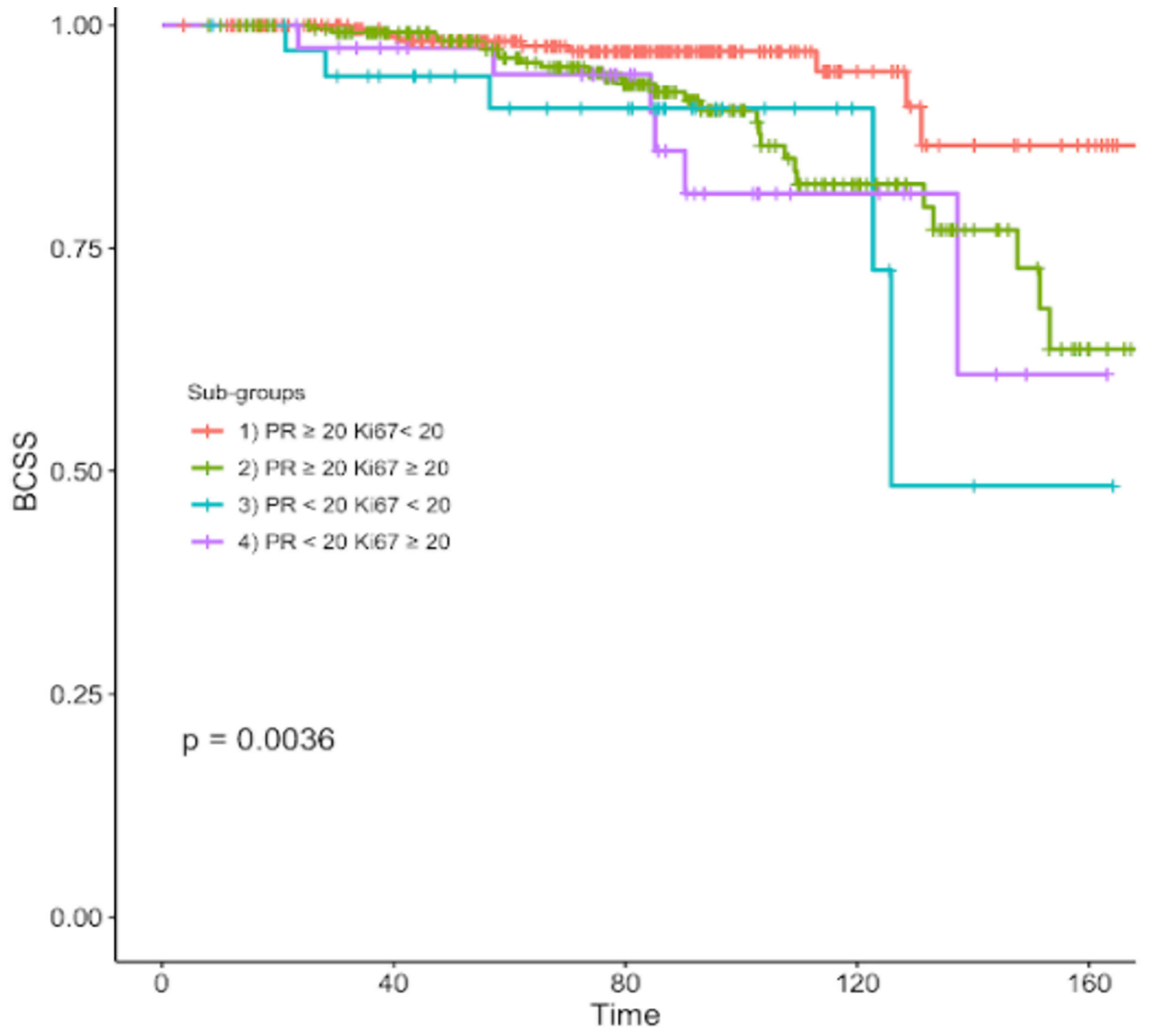
Figure 1 Kaplan-Meier curve of breast cancer specific survival of different subgroups according to different PR and Ki67 expression levels.
On univariate analysis, post-menopause, older age (≥ 50 years), low PR (i.e. < 10% and < 20%) and high Ki67 expression, poorly differentiated grade and size ≥ 2cm as well as the sub-classification of luminal B-like BC significantly correlated with a worse BCSS (Table 3).
Multivariate analysis identified grade, size and subgroup classification of tumors as variables associated with a poorer outcome (Table 4). In detail, subgroups 4 (PR low/Ki67 high), 3 (PR low/Ki67low) and 2 (PR high/Ki67 high) all displayed a significantly increased risk of BC-related death (HR=4.11; p=0.008; HR=3.43; p=0-007; HR=2.57; p=0.020, respectively) when compared to subgroup 1 (PR low/Ki67 low) (Table 4). An additional multivariate analysis keeping Ki67 as covariate confirmed the role of PR status (<20% or ≥20%) as an independent predictor of BC survival (p=0.015). (Table S1).
Discussion
The risk stratification process remains a critical issue in selecting the adjuvant treatment for early BC. Gene expression analysis has categorized BC into distinct molecular subtypes associated with different outcomes and treatment sensitivity (2). ER+/HER2 negative BC, representing 75-80% of all cases, include “Luminal-A like” tumors associated with good prognosis as well as ET responsiveness and “Luminal-B like” cancers, representing a heterogeneous disease with a more aggressive behavior often requiring CT (11).
The role of gene expression profiling (GEP) assays in risk stratification and treatment decisions for early BC patients is undisputed, but, unfortunately, these tests are not fully integrated in daily clinical practice due to high costs and other logistic issues. Therefore, researchers have recently re-focused on the value of traditional clinicopathological features for prognosis estimation and treatment planning, in order to assess whether IHC-based biomarkers could substitute or integrate information obtained from GEP (12, 13). ER expression represents the most important prognostic biomarker in luminal-like HER2 negative BC and the main predictor of ET responsiveness (7). Recent analyses showed that the risk of relapse and death persists despite the completion of 5 years of adjuvant ET in ER+/HER2 negative BC patients (14–16), suggesting that additional parameters, beyond ER status, should be considered to identify patients who may obtain additional benefit from CT and/or extended ET. Although 1% is the recommended cut-off to define ER positivity (5), recent evidences revealed that tumors with low ER levels (1-9%) display a clinical behavior more similar to ER-negative BC, both in terms of response to neoadjuvant chemotherapy and prognosis thus suggesting that threshold of 10% should be used in clinical practice for therapeutic decisions (17).
In this scenario, PR and Ki67 evaluation deserve a special attention. Despite the optimal threshold has not been defined yet, high Ki67 levels demonstrated to be associated with an increased risk of relapse and a worse survival (18). Furthermore, recent studies indicate that changes in Ki67 expression after neo-adjuvant ET may predict long-term outcomes, supporting its prognostic value (19). Conversely, the clinical utility of semi-quantitative assessment of PR levels is still debated and not fully elucidated. Previous exploratory analyses of multiple independent datasets have demonstrated that quantitative scoring of PR positive tumor cells (but not ER positive tumor cells) might predict BC outcome when an empiric cut-off of more than 20% for PR percentage to discriminate luminal A and luminal B-like was chosen (20). Additional data confirmed that tumors with low (i.e. <20%) or negative PR expression display a more aggressive phenotype, although its prognostic value seems to decrease after long-term follow-up (21–24).
Results from gene expression studies revealed a specific molecular profile of single HR+ BC associated with poor prognostic factors and response to endocrine therapy as well as less favorable clinical behaviour compared to double HR+ cancers (25). In particular, PAM 50 analysis showed that ER-/PR+ tumors, accounting less than 1% of all BC, are mostly basal like (50-60%), a molecular subtype generally observed in TNBC which can be easily detectable through immunohistochemistry-based method (TFF1, CK5, and EGFR positivity) (26).
Moreover, at immunohistochemical level too, ER-/PR+ BC compared to ER+/PR+ cases are more likely associated to biomarkers predicting worse prognosis such as p53 and basal cytokeratin expression, high Ki67 and MKI67 mRNA levels, as well as low E-cadherin and absence of androgen receptor (27).
Otherwise, PR represents a molecular rheostat controlling ERα transcriptional activity and regulates chromatin binding events, resulting in a unique gene expression signature associated with good prognosis (28). Therefore, the absence of PR leads to the activation of genes related to aggressive features, including myc, cyclin D1, and insulin-like growth factor receptor 1 (29, 30). Unfortunately, in our series, the number of patients with PR negative breast tumors is too small (7% of all population) to provide meaningful results.
With regard to the predictive value of PR levels, clinical studies reported controversial results (31, 32). Some authors concluded that patients affected by PR negative tumors, unlikely to obtain benefit from adjuvant ET, could gain a survival advantage from adjuvant CT (21, 23). Moreover, a retrospective analysis from three adjuvant clinical trials supported this hypothesis showing that low PR expression could be predictive of additional benefit from CT compared to ET alone, regardless of ER positivity (33). A large metanalysis including 20 trials and more than 20,000 patients with ER+ early BC revealed that tamoxifen improves relapse-free survival regardless of PR status, age, nodal status, or use of adjuvant CT (7). Similarly, a retrospective analysis from the ATAC and BIG 1-98 trials showed that PR expression did not affect the survival advantage obtained from tamoxifen or an aromatase inhibitor, but confirmed a significant role for outcome prediction in both treatment arms (33, 34). Overall, these trials suggest that PR expression has an intrinsic prognostic effect, although its predictive relevance remains controversial (35).
Additionally, a large amount of data reported that IHC PR expression levels correlate with the Oncotype DX recurrence score (RS) and support the combined use of PR and mitotic rate as a surrogate marker for Oncotype RS (36–38).
Based on these observations, expert panels agreed that low PR expression can be used as a prognostic determinant for Luminal-like tumors (4), recommending its use in combination with others pathological factors such as Ki67, histological grade and tumor stage (4, 6).
In order to get a deeper insight into the prognostic significance of PR, we retrospectively analyzed a cohort of 687 non-metastatic N0-1 luminal-like HER2 negative BC patients stratified into four subgroups based on PR and Ki67 expression levels as measured by IHC, with a cut-off of 20% according with the 2013 Saint Gallen International Breast Cancer Conference (4). The survival analysis showed a statistically significant difference in terms of BCSS among the four subgroups. As expected, the luminal-B like subtype displayed a more aggressive clinical behavior and an unfavorable prognosis when compared to luminal-A like cancers. Moreover, when looking at the survival curves, each subclass of luminal-B like BC patients presented a significantly different risk of death. Of note patients belonging to subgroup 4 (PR-low/Ki67-high) were more commonly younger and premenopausal women affected by tumor with more aggressive features (large size, poorly differentiated grade). All these unfavorable characteristics could have contributed to the worst prognosis of these patients, for whom a 4-fold increased risk of cancer-related death was reported compared with patients with Luminal-A like BC, followed by subgroup 3 (PR<20% and Ki67<20%), and 2 (PR≥20% and Ki67≥20%). Looking at the hazard ratio of each subgroup of luminal B-like tumors, patients with low PR (subgroup 3) had a higher risk of BC mortality than those with high Ki67 (subgroup 2) (HR= 3.43 and HR= 2.57, respectively). In addition, to further explore the prognostic role of PR levels, we performed an additional multivariate analysis keeping Ki67 as covariate which confirmed PR status as a powerful and independent predictor of BC survival.
Our study and findings have strengths and limitations. Our cohort of patients is fully characterized with regard to clinical and tumor features and was evaluated and treated in two teaching hospitals, which ensure high quality pathological evaluation and medical treatments in line with the best international standards. Furthermore, in the present study, the sub-classification of ER+/HER2 negative BC according to PR and Ki67 levels proved its prognostic significance despite the majority of cases exhibited strong ER positivity (only 3% of patients with ER< 10%). Our results underline that additional factors, beyond the ER status, should be collectively considered to provide a reliable prediction of survival outcomes assisting physicians’ treatment decisions process.
Our study has several limitations. First, the possibility of bias with respect to different chemotherapy regimens adopted over the course of 18 years cannot be completely ruled out due to the retrospective nature of our study. However, we performed an additional exploratory analysis showing no statically significant differences in patients survival outcome according to the various adjuvant chemotherapy regimens used (data not shown).
In addition, it should be noted that during this observation period, several methods for hormone receptor testing have been developed and applied, while specific guidance on the best antibody, assay, and scoring system to improve reproducibility and reduce interobserver variation is still lacking. In our study, although a comprehensive immunohistochemical re-evaluation of PR expression was not performed, the Ventana 1E2 clone PR IHC assay was found to be widely used. However, a potential limitation of the present analysis could be represented by the variability in the preanalytical procedures and immunohistochemical evaluation of PR status over the years and between the two different academic laboratories.
Our findings, according to previous retrospective analyses and metanalyses (28, 33, 39, 40, 41), suggest a prognostic role for PR expression levels in luminal-like HER2 negative BC that should be confirmed prospectively.
Conclusions
In conclusion, despite the limitations due to the retrospective nature of the study, our findings support the importance of IHC-based evaluation of PR expression levels combined with Ki67 status to sub-classify, among patients with Luminal-B like BC, groups with different prognosis, which could be useful in modulating and personalizing BC adjuvant treatments. Therefore, semi-quantitative measurement of PR and Ki67 expression levels maintains clinical relevance for risk estimation and treatment guidance even in the era of genomic profiling.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of the University of Campania “Luigi Vanvitelli” (Naples, Italy). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: AD, FCa, and MO. Methodology: GAn, VF, GB, and AD. Resources: CDA, SC, and AP. Data curation: GAn, VF, AD, FDV, FCa. Writing—original draft preparation: AD and FCa. Writing—review and editing: MO, AD, FCa and GB. Supervision: CDA, GrA, BD. Validation: CDA, FDV, and FCi, BD, GAr, and MO. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.813462/full#supplementary-material
Supplementary Table 1 | Prognostic variables for breast cancer specific-survival in multivariate analysis keeping Ki67 as covariate.
References
1. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses With Clinical Implications. Proc Natl Acad Sci (2001) 98(19):10869–74. doi: 10.1073/pnas.191367098
2. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular Portraits of Human Breast Tumours. Nature (2000) 406(6797):747–52. doi: 10.1038/35021093
3. Walsh EM, Smith KL, Stearns V. Management of Hormone Receptor-Positive, HER2-Negative Early Breast Cancer. Semin Oncol (2020) 47(4):187–200. doi: 10.1053/j.seminoncol.2020.05.010
4. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the Treatment of Women With Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol (2013) 24(19):2206–23. doi: 10.1093/annonc/mdt303
5. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. JCO (2020) 38(12):1346–66. doi: 10.1200/JCO.19.02309
6. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2019) 30(8):1194–220. doi: 10.1093/annonc/mdz173
7. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of Breast Cancer Hormone Receptors and Other Factors to the Efficacy of Adjuvant Tamoxifen: Patient-Level Meta-Analysis of Randomised Trials. Lancet (2011) 378(9793):771–84. doi: 10.1016/S0140-6736(11)60993-8
8. Albert JM, Gonzalez-Angulo AM, Guray M, Sahin A, Tereffe W, Woodward WA, et al. Patients With Only 1 Positive Hormone Receptor Have Increased Locoregional Recurrence Compared With Patients With Estrogen Receptor-Positive Progesterone Receptor-Positive Disease in Very Early Stage Breast Cancer. Cancer (2011) 117(8):1595–601. doi: 10.1002/cncr.25694
9. Ahn SG, Yoon CI, Lee JH, Lee HS, Park SE, Cha YJ, et al. Low PR in ER (+)/HER2 (–) Breast Cancer: High Rates of TP53 Mutation and High SUV. Endocrine-Rel Cancer (2019) 26(2):177–85. doi: 10.1530/ERC-18-0281
10. Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. JCO (2010) 28(16):2784–95. doi: 10.1200/JCO.2009.25.6529
11. Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical Implications of the Intrinsic Molecular Subtypes of Breast Cancer. Breast (2015) 24:S26–35. doi: 10.1016/j.breast.2015.07.008
12. Dunnwald LK, Rossing MA, Li CI. Hormone Receptor Status, Tumor Characteristics, and Prognosis: A Prospective Cohort of Breast Cancer Patients. Breast Cancer Res (2007) 9(1):R6. doi: 10.1186/bcr1639
13. Hanna MG, Bleiweiss IJ, Nayak A, Jaffer S. Correlation of Oncotype DX Recurrence Score With Histomorphology and Immunohistochemistry in Over 500 Patients. Int J Breast Cancer (2017) 2017:1–6. doi: 10.1155/2017/1257078
14. Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, et al. Assessment of Letrozole and Tamoxifen Alone and in Sequence for Postmenopausal Women With Steroid Hormone Receptor-Positive Breast Cancer: The BIG 1-98 Randomised Clinical Trial at 8.1 Years Median Follow-Up. Lancet Oncol (2011) 12(12):1101–8. doi: 10.1016/S1470-2045(11)70270-4
15. Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thürlimann B, et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results From the International Breast Cancer Study Group Trials I to V. JCO (2016) 34(9):927–35. doi: 10.1200/JCO.2015.62.3504
16. Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year Risks of Breast-Cancer Recurrence After Stopping Endocrine Therapy at 5 Years. N Engl J Med (2017) 377(19):1836–46. doi: 10.1056/NEJMoa1701830
17. Dieci MV, Griguolo G, Bottosso M, Tsvetkova V, Giorgi CA, Vernaci G, et al. Impact of Estrogen Receptor Levels on Outcome in Non-Metastatic Triple Negative Breast Cancer Patients Treated With Neoadjuvant/Adjuvant Chemotherapy. NPJ Breast Cancer (2021) 7(1):101. doi: 10.1038/s41523-021-00308-7
18. de Azambuja E, Cardoso F, de Castro G, Colozza M, Mano MS, Durbecq V, et al. Ki-67 as Prognostic Marker in Early Breast Cancer: A Meta-Analysis of Published Studies Involving 12155 Patients. Br J Cancer (2007) 96(10):1504–13. doi: 10.1038/sj.bjc.6603756
19. Smith I, Robertson J, Kilburn L, Wilcox M, Evans A, Holcombe C, et al. Long-Term Outcome and Prognostic Value of Ki67 After Perioperative Endocrine Therapy in Postmenopausal Women With Hormone-Sensitive Early Breast Cancer (POETIC): An Open-Label, Multicentre, Parallel-Group, Randomised, Phase 3 Trial. Lancet Oncol (2020) 21(11):1443–54. doi: 10.1016/S1470-2045(20)30458-7
20. Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, Caballero R, et al. Prognostic Significance of Progesterone Receptor-Positive Tumor Cells Within Immunohistochemically Defined Luminal A Breast Cancer. J Clin Oncol (2013) 31(2):203–9. doi: 10.1200/JCO.2012.43.4134
21. Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone Receptor Status Significantly Improves Outcome Prediction Over Estrogen Receptor Status Alone for Adjuvant Endocrine Therapy in Two Large Breast Cancer Databases. JCO (2003) 21(10):1973–9. doi: 10.1200/JCO.2003.09.099
22. Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW, et al. Comparison of PAM50 Risk of Recurrence Score With Onco Type DX and IHC4 for Predicting Risk of Distant Recurrence After Endocrine Therapy. JCO (2013) 31(22):2783–90. doi: 10.1200/JCO.2012.46.1558
23. Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Progesterone Receptor Loss Identifies Luminal B Breast Cancer Subgroups at Higher Risk of Relapse. Ann Oncol (2013) 24(3):661–8. doi: 10.1093/annonc/mds430
24. Zong Y, Zhu L, Wu J, Chen X, Huang O, Fei X, et al. Progesterone Receptor Status and Ki-67 Index May Predict Early Relapse in Luminal B/HER2 Negative Breast Cancer Patients: A Retrospective Study. PloS One (2014) 9(8):e95629. doi: 10.1371/journal.pone.0095629
25. Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, et al. Biologic and Clinical Characteristics of Breast Cancer With Single Hormone Receptor Positive Phenotype. J Clin Oncol (2007) 25(30):4772–8. doi: 10.1200/JCO.2007.12.2747
26. Yu KD, Jiang YZ, Hao S, Shao ZM. Molecular Essence and Endocrine Responsiveness of Estrogen Receptor-Negative, Progesterone Receptor-Positive, and HER2-Negative Breast Cancer. BMC Med (2015) 13:254. doi: 10.1186/s12916-015-0496-z
27. Kunc M, Biernat W, Senkus-Konefka E. Estrogen Receptor-Negative Progesterone Receptor-Positive Breast Cancer - “Nobody’s Land” or Just an Artifact? Cancer Treat Rev (2018) 67:78–87. doi: 10.1016/j.ctrv.2018.05.005
28. Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, et al. Progesterone Receptor Modulates Erα Action in Breast Cancer. Nature (2015) 523(7560):313–7. doi: 10.1038/nature14583
29. Nishimukai A, Yagi T, Yanai A, Miyagawa Y, Enomoto Y, Murase K, et al. High Ki-67 Expression and Low Progesterone Receptor Expression Could Independently Lead to a Worse Prognosis for Postmenopausal Patients With Estrogen Receptor-Positive and HER2-Negative Breast Cancer. Clin Breast Cancer (2015) 15(3):204–11. doi: 10.1016/j.clbc.2014.12.007
30. Thakkar JP, Mehta DG. A Review of an Unfavorable Subset of Breast Cancer: Estrogen Receptor Positive Progesterone Receptor Negative. Oncol (2011) 16(3):276–85. doi: 10.1634/theoncologist.2010-0302
31. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and Predictive Factors in Breast Cancer by Immunohistochemical Analysis. Modern Pathol (1998) 11(2):155–68.
32. Osborne CK, Schiff R. Growth Factor Receptor Cross-Talk With Estrogen Receptor as a Mechanism for Tamoxifen Resistance in Breast Cancer. Breast (2003) 12(6):362–7. doi: 10.1016/S0960-9776(03)00137-1
33. Viale G, Regan MM, Maiorano E, Mastropasqua MG, Golouh R, Perin T, et al. Chemoendocrine Compared With Endocrine Adjuvant Therapies for Node-Negative Breast Cancer: Predictive Value of Centrally Reviewed Expression of Estrogen and Progesterone Receptors—International Breast Cancer Study Group. JCO (2008) 26(9):1404–10. doi: 10.1200/JCO.2007.10.6393
34. Viale G, Regan MM, Dell’Orto P, Mastropasqua MG, Maiorano E, Rasmussen BB, et al. Which Patients Benefit Most From Adjuvant Aromatase Inhibitors? Results Using a Composite Measure of Prognostic Risk in the BIG 1-98 Randomized Trial. Ann Oncol (2011) 22(10):2201–7. doi: 10.1093/annonc/mdq738
35. Olivotto IA, Truong PT, Speers CH, Bernstein V, Allan SJ, Kelly SJ, et al. Time to Stop Progesterone Receptor Testing in Breast Cancer Management. JCO (2004) 22(9):1769–70. doi: 10.1200/JCO.2004.99.251
36. Clark BZ, Dabbs DJ, Cooper KL, Bhargava R. Impact of Progesterone Receptor Semiquantitative Immunohistochemical Result on Oncotype DX Recurrence Score: A Quality Assurance Study of 1074 Cases. Appl Immunohistochem Mol Morphol (2013) 21(4):287–91. doi: 10.1097/PAI.0b013e31826f80c9
37. Tang P, Wang J, Hicks DG, Wang X, Schiffhauer L, McMahon L, et al. A Lower Allred Score for Progesterone Receptor Is Strongly Associated With a Higher Recurrence Score of 21-Gene Assay in Breast Cancer. Cancer Invest (2010) 28(9):978–82. doi: 10.3109/07357907.2010.496754
38. Thibodeau S, Voutsadakis IA. Prediction of Oncotype Dx Recurrence Score Using Clinical Parameters: A Comparison of Available Tools and a Simple Predictor Based on Grade and Progesterone Receptor. Hematology/Oncol Stem Cell Ther (2019) 12(2):89–96. doi: 10.1016/j.hemonc.2019.02.001
39. Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, et al. Prognostic Value of a Combined Estrogen Receptor, Progesterone Receptor, Ki-67, and Human Epidermal Growth Factor Receptor 2 Immunohistochemical Score and Comparison With the Genomic Health Recurrence Score in Early Breast Cancer. JCO (2011) 29(32):4273–8. doi: 10.1200/JCO.2010.31.2835
40. Ono M, Tsuda H, Yoshida M, Shimizu C, Kinoshita T, Tamura K. Prognostic Significance of Progesterone Receptor Expression in Estrogen-Receptor Positive, HER2-Negative, Node-Negative Invasive Breast Cancer With a Low Ki-67 Labeling Index. Clin Breast Cancer (2017) 17(1):41–7. doi: 10.1016/j.clbc.2016.06.012
41. Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, et al. Relationship Between Quantitative Estrogen and Progesterone Receptor Expression and Human Epidermal Growth Factor Receptor 2 (HER-2) Status With Recurrence in the Arimidex, Tamoxifen, Alone or in Combination Trial. JCO (2008) 29(32):1059–65. doi: 10.1200/JCO.2007.12.9437
Keywords: breast cancer, progesterone receptor, Ki67, luminal-like subtype, prognosis
Citation: Diana A, Carlino F, Buono G, Antoniol G, Famiglietti V, De Angelis C, Carrano S, Piccolo A, De Vita F, Ciardiello F, Daniele B, Arpino G and Orditura M (2022) Prognostic Relevance of Progesterone Receptor Levels in Early Luminal-Like HER2 Negative Breast Cancer Subtypes: A Retrospective Analysis. Front. Oncol. 12:813462. doi: 10.3389/fonc.2022.813462
Received: 11 November 2021; Accepted: 02 March 2022;
Published: 28 March 2022.
Edited by:
José Bines, National Cancer Institute (INCA), BrazilReviewed by:
Ossama Tawfik, Saint Luke’s Health System, United StatesNicoletta Staropoli, Magna Græcia University of Catanzaro, Italy
Copyright © 2022 Diana, Carlino, Buono, Antoniol, Famiglietti, De Angelis, Carrano, Piccolo, De Vita, Ciardiello, Daniele, Arpino and Orditura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Diana, YW5uYWRpYW5hODhAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Anna Diana
Anna Diana Francesca Carlino
Francesca Carlino Giuseppe Buono
Giuseppe Buono Giuliano Antoniol5
Giuliano Antoniol5 Antonio Piccolo
Antonio Piccolo Ferdinando De Vita
Ferdinando De Vita Fortunato Ciardiello
Fortunato Ciardiello Grazia Arpino
Grazia Arpino