- 1Division of Medical Oncology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 2Division of Medical Oncology, Department of Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 3Medical Oncology Unit, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 4Department of Clinical Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Background: Current guidelines recommend anti-epidermal growth factor receptor monoclonal antibodies (anti-EGFR Ab) as first-line treatment only in patients with left-sided RAS wild type (RASwt) metastatic colorectal cancer (mCRC). However, there are no guideline recommendations specific to tumor sidedness in subsequent-line treatment. This study aimed to investigate the effect of primary tumor location on second- or later-line treatment outcomes in patients with KRASwt mCRC.
Methods: Medical records of patients diagnosed with mCRC at 3 academic centers in Thailand (Siriraj, Chulalongkorn, and Ramathibodi hospital) between 2008 and 2019 were retrospectively reviewed. Patients with KRASwt mCRC who received anti-EGFR Ab in second- or later-line treatment were included. The impact of tumor sidedness on progression-free survival (PFS) was determined using Kaplan-Meier method, and those results were compared using log-rank test.
Results: Among the 2,102 patients who had KRAS analysis data, 1,130 (54%) patients had KRASwt. Of those, 413 patients received anti-EGFR Ab in second- or later-line treatment. One hundred and sixty-two of 413 (39%) patients had extended RAS analysis. Seventy (17%) patients had right-sided tumors. Two hundred and thirty-eight (58%) patients received anti-EGFR Ab in the third line, and 132 (32%) patients and 43 (10%) patients were treated in the second and more than third line, respectively. Single-agent irinotecan was the most commonly used backbone chemotherapy (303/413, 73%). Patients with right-sided tumors had non-significantly inferior PFS compared to patients with left-sided tumors (median PFS: 5.7 months (mo), 95% confidence interval [CI]: 3.9-7.5 vs. 7.5 mo, 95% CI 6.5-8.5; p=0.17). Subgroup analysis showed no difference in PFS when stratified by treatment lines. Patient with right-sided tumors had significantly inferior OS compared to patients with left-sided tumors (median OS: 23.3 mo vs. 29.9 mo; p=0.005).
Conclusions: To date, this is the largest real world data of the effect of primary tumor location on anti-EGFR Ab which demonstrated that tumor sidedness has no significant impact on treatment outcomes in KRASwt mCRC patients receiving second- or later-line therapy. Our findings do not support the utility of tumor sidedness for treatment selection in these settings. We confirmed that patients with right-sided tumors had significantly worse survival.
Introduction
Colorectal cancer (CRC) is the leading cause of cancer-related death worldwide with over 1.93 million new cases and 935,000 deaths in 2020 (1). However, due to recent advancements in targeted biological therapy, the median survival duration now exceeds 30 months in patients with metastatic CRC (mCRC) (2). The epidermal growth factor receptor (EGFR), which is a transmembrane receptor tyrosine kinase that is overexpressed in several human cancers, has become an important therapeutic target in mCRC. Anti-EGFR monoclonal antibodies (anti-EGFR Ab), including cetuximab and panitumumab, are widely used in mCRC treatment, and RAS mutations are a negative predictive marker of anti-EGFR Ab response (3).
Retrospective analyses of data from several randomized studies have assessed the clinical effect of anti-EGFR Ab in patients with mCRC according to the location of the primary tumor (2, 4–6). The results of those analyses revealed better survival outcomes after treatment with anti-EGFR Ab plus chemotherapy versus chemotherapy alone or combined with bevacizumab in patients with left-sided mCRC. In contrast, patients with right-sided tumors generally appeared to benefit more from chemotherapy combined with bevacizumab.
The current guideline of the European Society for Medical Oncology (ESMO) (7) recommends anti-EGFR Ab as a first-line treatment for patients with left-sided RASwt mCRC. However, there are no recommendations specific to tumor sidedness relative to subsequent-line treatments in RASwt mCRC patients. Accordingly, the aim of this study was to investigate the effect of primary tumor location on second- or later-line treatment outcomes in patients with KRASwt mCRC.
Materials and Methods
This multi-center, retrospective cohort study evaluated patients diagnosed with primary colorectal adenocarcinoma between 1 January 2008 and 31 December 2019 at 3 academic centers in Thailand (Faculty of Medicine Siriraj Hospital, Faculty of Medicine Chulalongkorn University hospital, and Faculty of Medicine Ramathibodi hospital, Bangkok, Thailand). We excluded patients with missing data, no histopathological data, no anti-EGFR treatment, and no treatment and follow-up at our centers. The study protocol was approved by the Siriraj Institutional Review Board (SIRB) EC3-356/2562, Med Chula IRB 664/62), MURA 2020/1287. This retrospective nature of this study ensures total anonymity of patient data, and patient health and wellbeing are in no way affected, so the requirement to obtain written informed consent from study participants was waived.
The primary objective of this study was to assess the effect of tumor location on progression-free survival (PFS) in KRASwt mCRC patients treated with anti-EGFR Ab as second- or later-line treatment. The secondary objective was to evaluate the impact of any influence of tumor sidedness on overall survival (OS) and objective response rate (ORR).
Clinical Characteristics
Demographic and clinical characteristics including age, gender, primary tumor site, line of treatment, RAS status, microsatellite instability (MSI) status, molecular analysis technique, chemotherapy regimen used, date of diagnosis of stage IV disease, date of disease recurrence, date of starting anti-EGFR Ab, date of stopping anti-EGFR Ab, reason for stopping anti-EGFR Ab, date of last follow-up, and date of death were collected from a review of patient electronic medical records. Disease staging was determined according to the American Joint Committee on Cancer (AJCC) TNM Staging Classification System for Colon Cancer 8th edition (8).
Assessment of Primary Tumor Location
All patients with KRASwt mCRC who received anti-EGFR Ab as second- or later-line treatment were classified into two groups according to primary tumor location. Primary tumors located in the cecum to the splenic flexure were classified as right-sided. Tumors located from the descending colon to the rectum were categorized as left-sided.
RAS Status Assessment
RAS analyses were performed at our centers or at local molecular analysis centers using Sanger sequencing, TheraScreen® (Qiagen, Hilden, Germany), real-time polymerase chain reaction (PCR) techniques, and next-generation sequencing (NGS). Extended panel RAS analysis included mutations in KRAS exons 2, 3, and 4, and in NRAS exons 2, 3, and 4.
Statistical Analysis
PFS was calculated from the date of starting anti-EGFR Ab treatment to the date of disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 or death (whichever occurred first). OS was calculated from the date of diagnosis of stage IV disease to the date of death from any cause.
Patient demographic and clinical characteristics were summarized descriptively. Median and range values were used for continuous variables, and frequency and percentage values were used for categorical variables. Pearson’s chi-square test or Fisher’s exact test was applied to evaluate associations between right-/left-sided tumor and clinicopathological variables. Patient survival outcome was analyzed using Kaplan-Meier method. Differences between curves were determined using log-rank test. Cox regression analysis was used to estimate the 95% confidence intervals (CIs) for PFS. A p-value ≤ 0.05 was the determiner of statistical significance. All analyses were performed using SPSS version 26.0 statistical software (SPSS, Inc.; IBM Corporation, Armonk, NY, USA).
Results
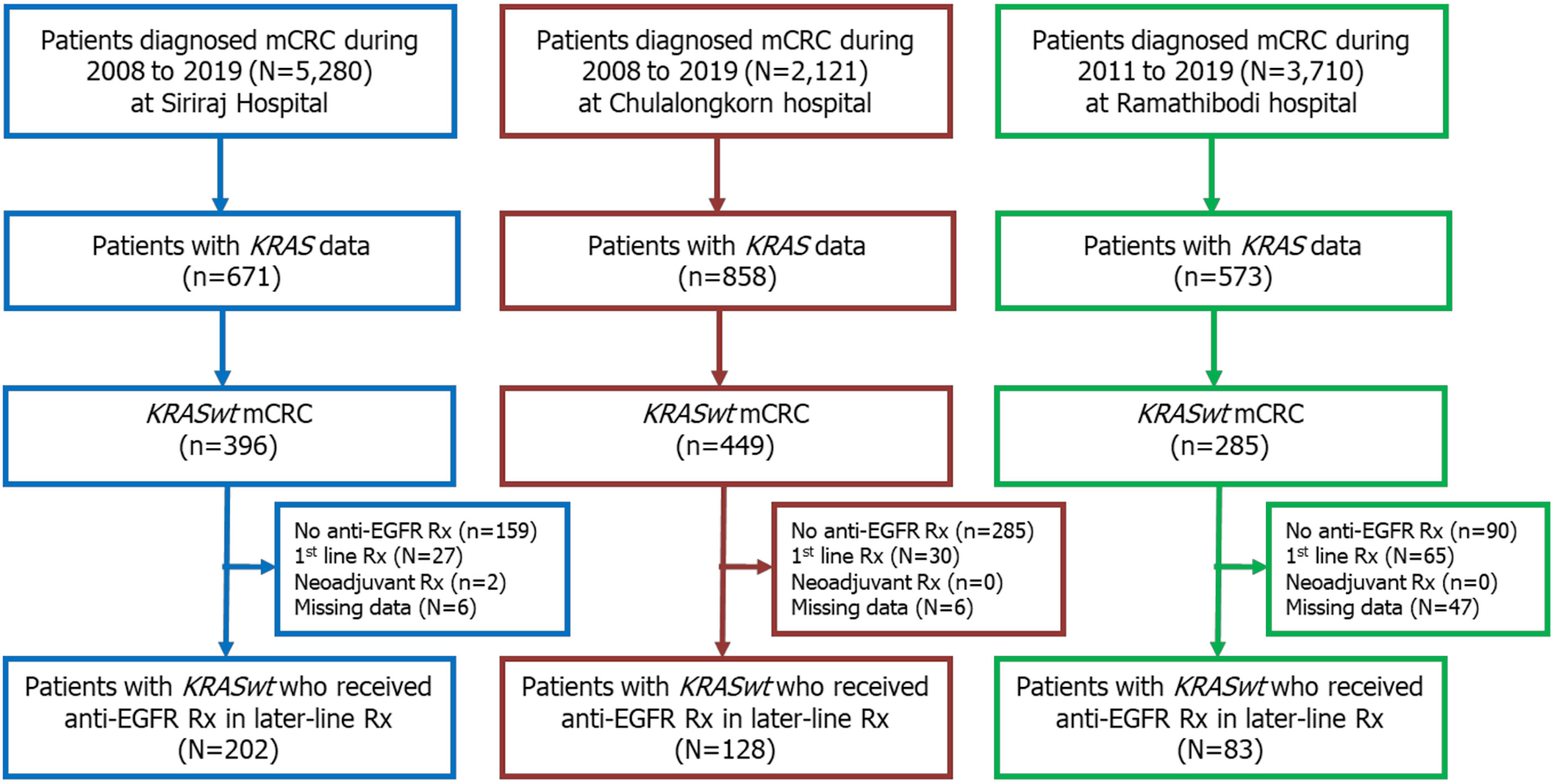
A total of 11,111 patients were diagnosed with CRC at our centers during the 12-year study period. Among those, 2,102 patients were classified as mCRC with known KRAS status. Of those, there were 1,130 patients (54%) with KRASwt mCRC. Among those with KRASwt data, 413 received anti-EGFR Ab as second- or later-line treatment, and those patients were included in this study. A Consolidated Standards of Reporting Trials (CONSORT) diagram of the patient enrollment process is shown in Figure 1.
Patient Characteristics
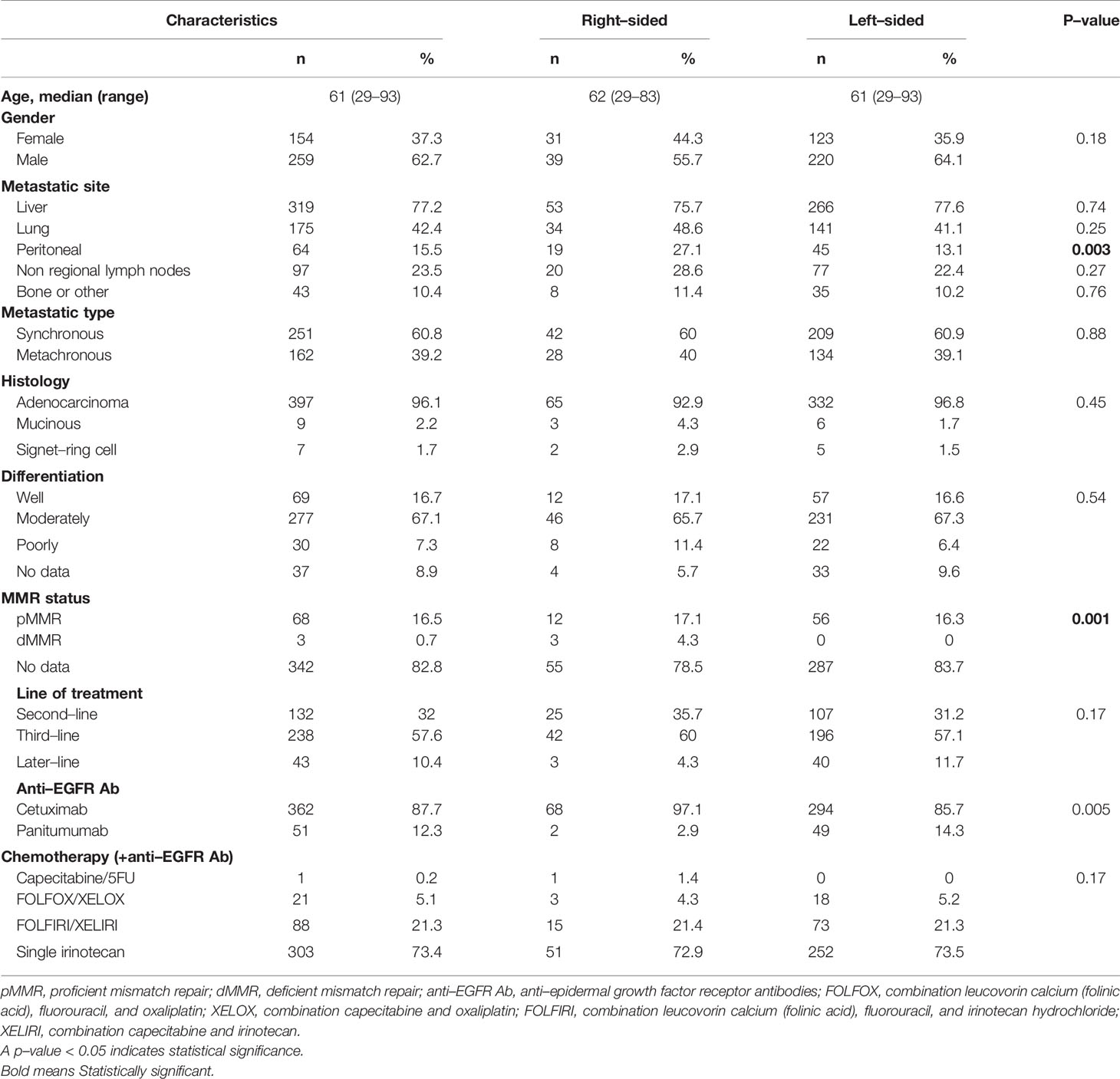
The median age of included patients was 61 years (range: 29-93), and the ratio of males to females was 1.7:1. Seventy (17%) patients had right-sided tumors, and 343 (83%) patients had left-sided tumors. The liver and lung were the most frequent sites of metastasis. Two hundred and thirty-eight (58%) patients received anti-EGFR Ab in the third-line, and 132 (32%) patients and 43 (10%) patients were treated in the second- and the later-line, respectively. Single-agent irinotecan therapy was the most frequently used chemotherapy (73%) in combination with anti-EGFR Ab therapy. Patient and tumor characteristics and details of treatments according to tumor sidedness are shown in Table 1.
RAS Testing Methods
Sixty-one percent (251/413) of patients had only KRAS exon 2 data, whereas 39% (162/413) of patients had extended RAS analysis data.
Pattern of Response to Anti-EGFR Ab
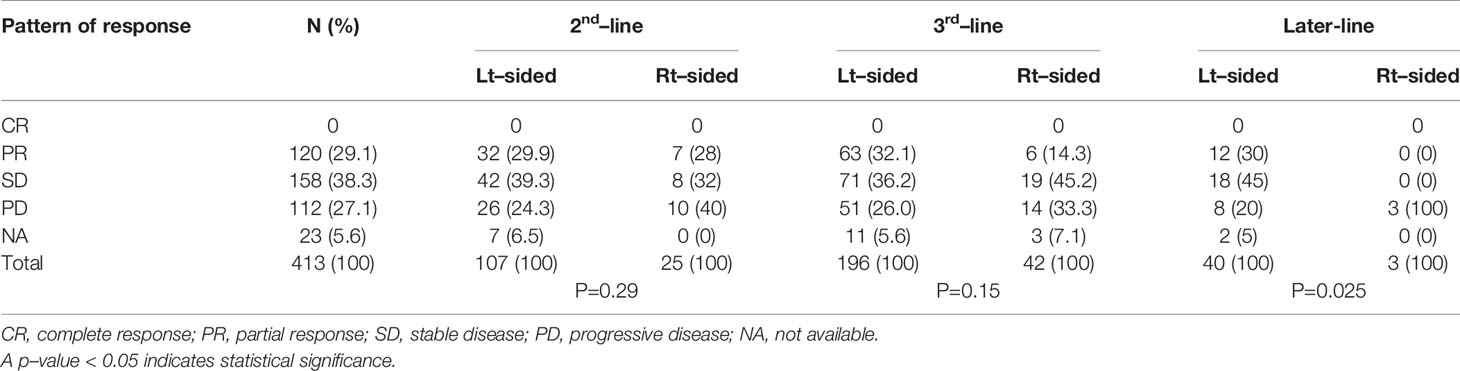
An overall response rate (complete or partial response) was observed in 31.2% of the patients with left-sided tumor as compared with 18.6% in patients with right-sided tumor. There was no complete response. The percentage of patients with progressive disease was higher in the right-sided tumors group than in the left-sided tumors group (38.6% vs. 24.8%). Tumor response assessment was not available in 23 patients. Details of treatment response according to tumor sidedness are shown in Table 2.
Reasons for Termination of Anti-EGFR Treatment
Disease progression was the most common cause of anti-EGFR therapy discontinuation followed by 6-month treatment completion of anti-EGFR therapy (51% and 36%, respectively). Eight percent of patients discontinued anti-EGFR Ab due to toxicities. Details of reason for termination of anti-EGFR treatment are shown in Table 3.
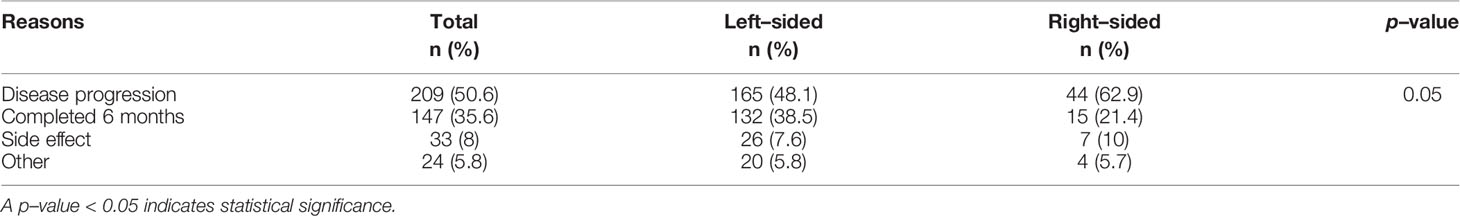
Table 3 Reasons for termination of anti–epidermal growth factor receptor (anti–EGFR) treatment (N=413).
Survival Analysis
The median follow-up time was 28.7 months (mo). At the last follow-up visit (1 September 2021), there were 23 patients (5.6%) alive, and the remaining 390 patients (94.4%) had succumbed to their disease.
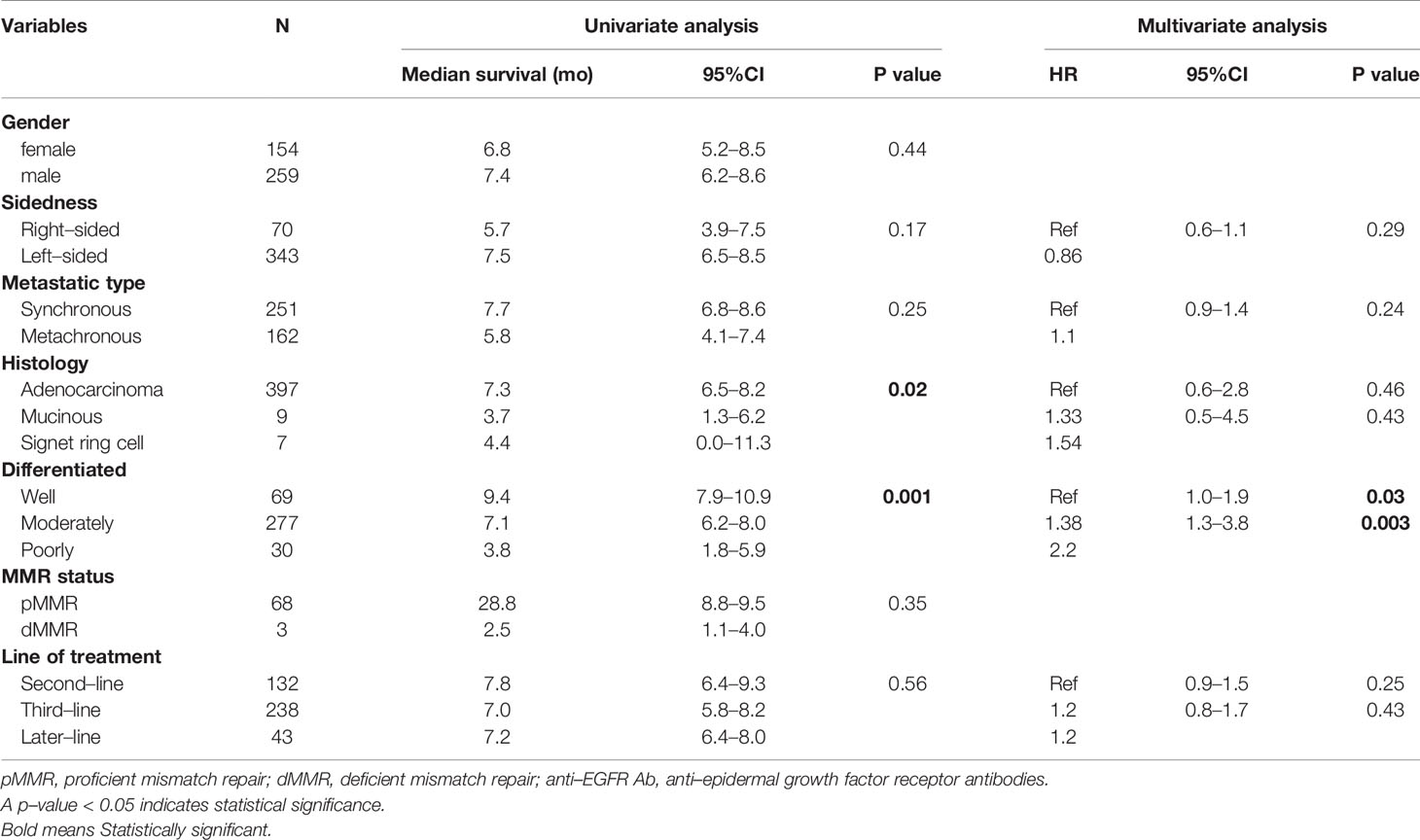
Univariate analysis of PFS was performed using previously established prognostic factors. Factors that were associated with statistically significantly worse PFS in this analysis included mucinous/signet ring cell histology (p=0.02) and poorly differentiation (p=0.001). Patients with right-sided tumors had a non-significantly inferior PFS compared to those with left-sided tumors (median PFS: 5.7 mo, 95% CI: 3.9-7.5 vs. 7.5 mo, 95% CI: 6.5-8.5, respectively; p=0.17) (Table 4 and Figure 2). Subgroup analysis of the impact of primary tumor location showed no significant difference in PFS between tumor locations when stratified by treatment lines except for later-line (Figure 3). Subgroup analysis of patients who had extended RAS analysis data showed no significant difference in PFS between the right-sided and left-sided groups (median PFS: 7.0 mo, 95% CI: 3.8-10.3 vs. 8.7 mo, 95% CI: 8.1-9.3, respectively; p=0.46) (Figure 4). Multivariate Cox proportional hazards regression analysis of PFS was performed using the factors mentioned above. In this analysis, moderately and poorly differentiated tumors were associated with worse PFS (HR 1.38, 95% CI 1.0-1.9; p=0.03 and HR 2.2, 95% CI 1.3-3.8; p=0.003, respectively) (Table 4). Among the entire cohort, patients with left-sided colon tumors had significantly better OS than those with right-sided tumors (median OS: 29.9 mo, 95% CI: 28.0-31.7 vs. 23.3 mo, 95% CI: 18.8-27.8, respectively; p=0.005) (Figure 5).
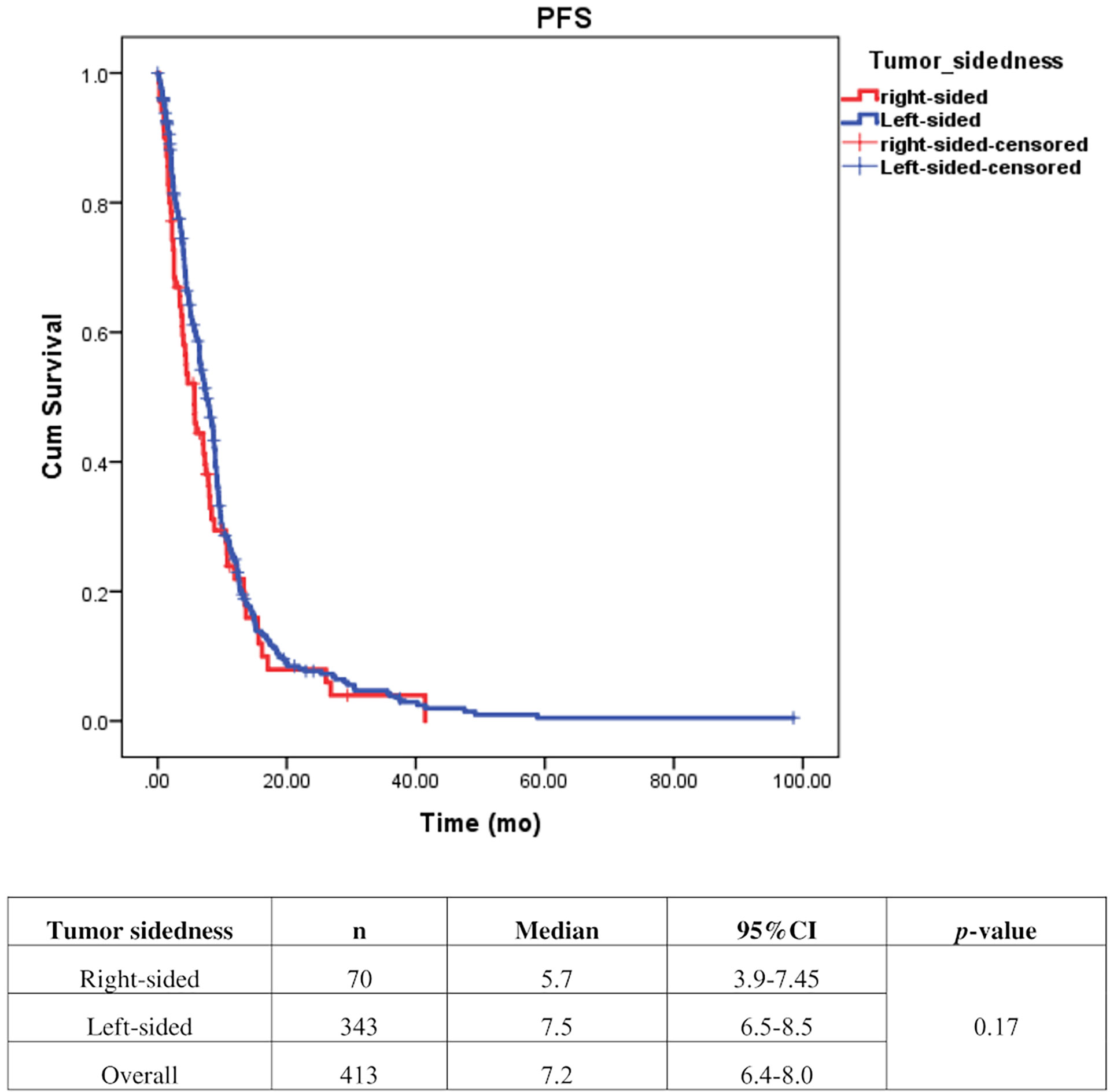
Figure 2 Effect of anti–EGFR treatment on PFS compared between right–sided tumor and left–sided tumor.
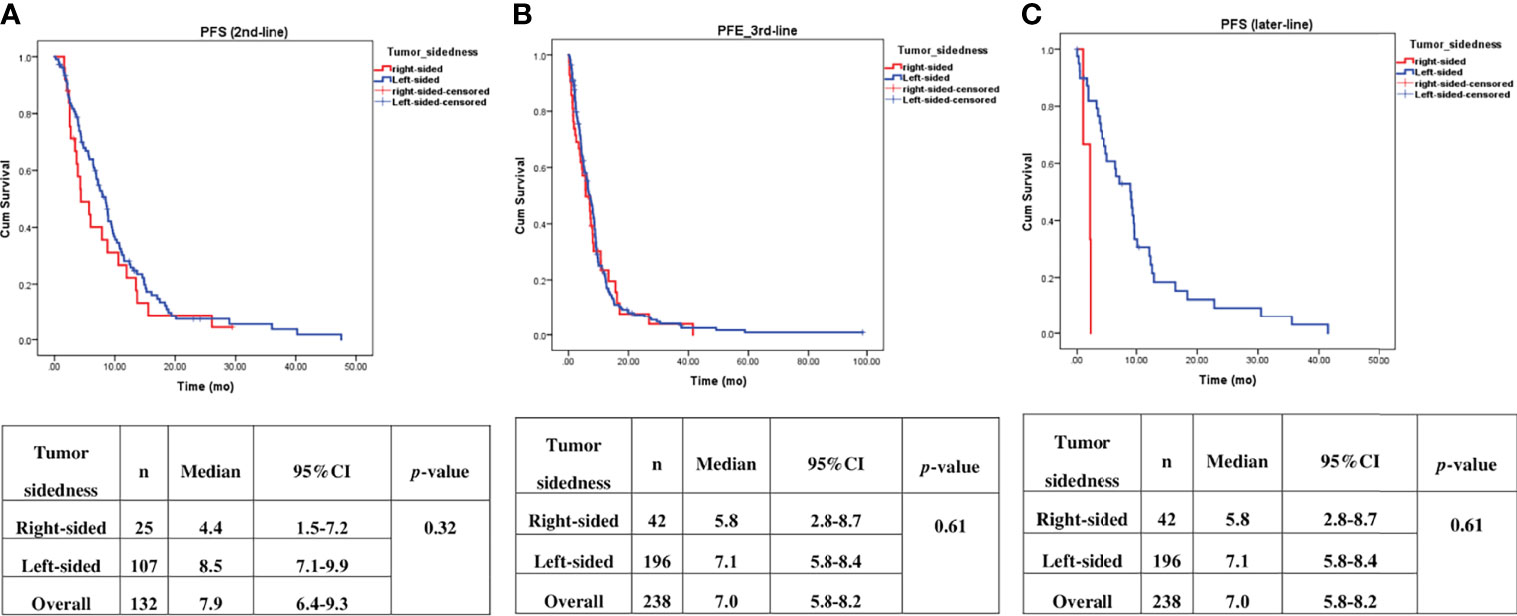
Figure 3 Effect of anti–EGFR treatment on PFS compared between right–sided tumor and left–sided tumor and stratified by treatment lines, (A) 2nd–line, (B) 3rd–line, (C) later–line.
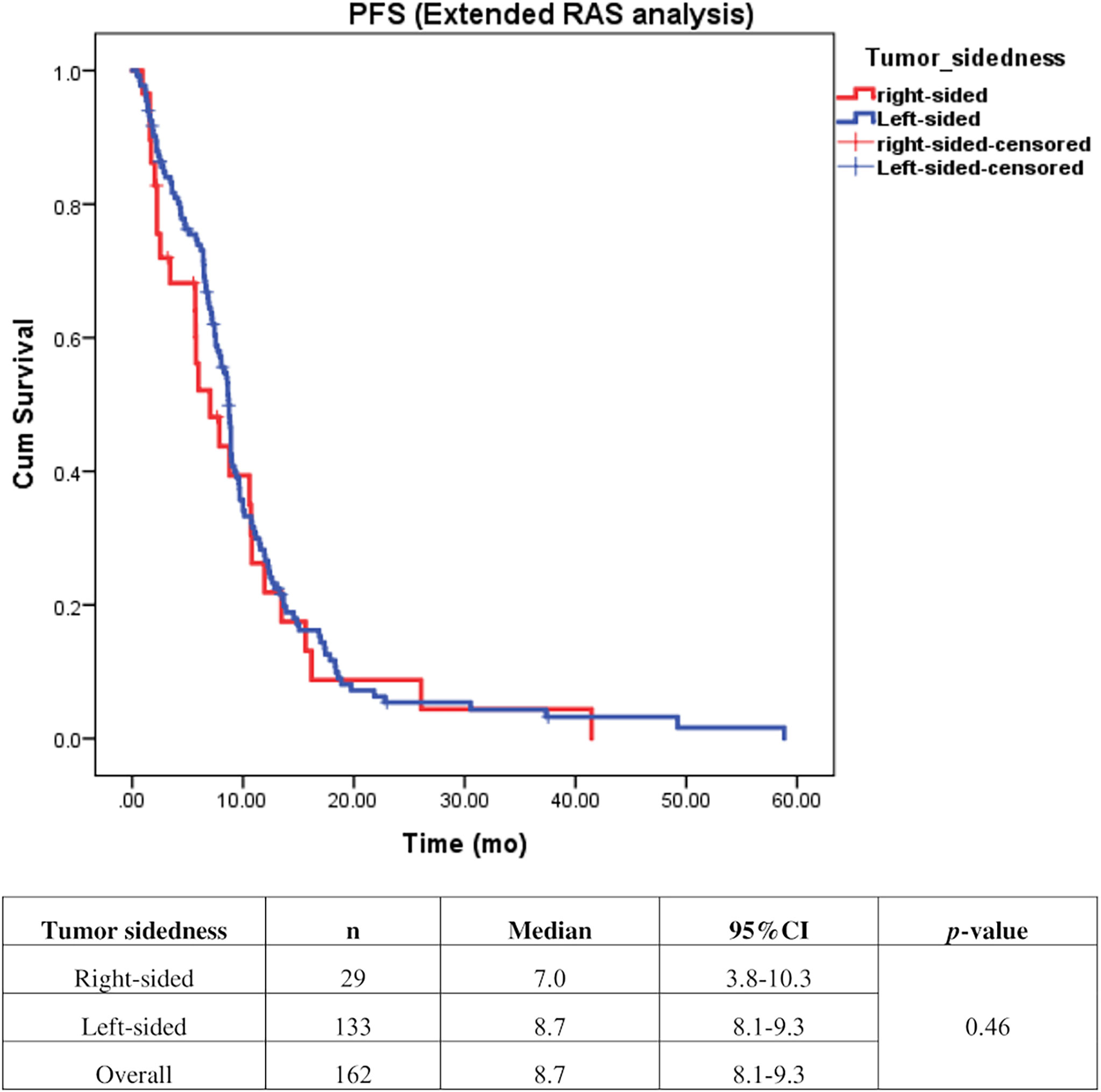
Figure 4 Effect of anti–EGFR treatment on PFS compared between right–sided tumor and left–sided tumor among patients who underwent extended RAS analysis (n=162).
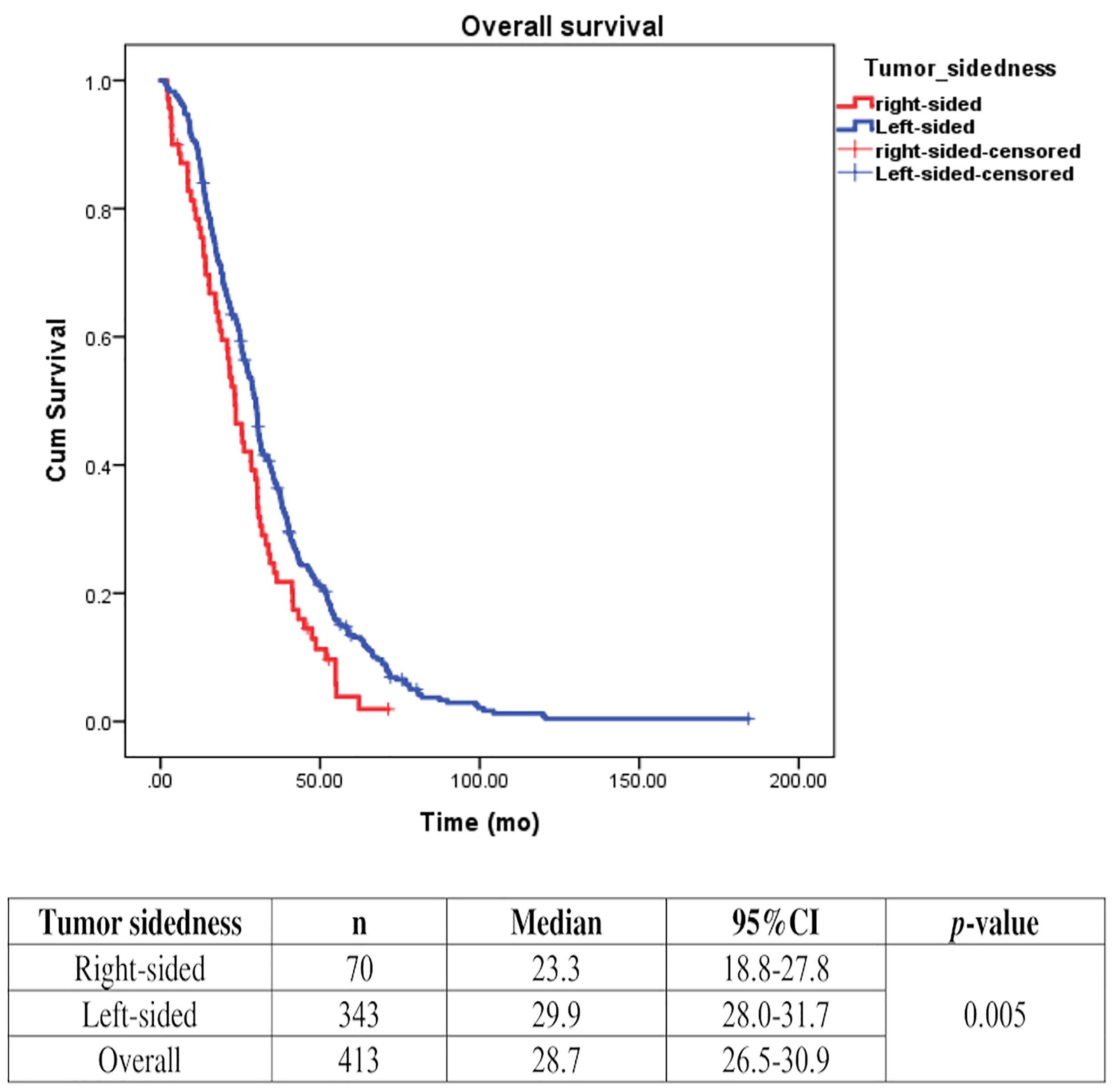
Figure 5 Effect of anti-EGFR treatment on OS compared between rightsided tumor and left-sided tumor.
Discussion
The results of this study demonstrated that tumor sidedness has no significant impact on treatment outcomes in KRASwt mCRC patients treated with anti-EGFR Ab in second- or later-line treatment. We also confirmed that patients with right sided tumors had worse outcome.
Metastatic CRC is a genetically heterogeneous disease with tumors arising from different sides of the colon (left versus right), and having different clinical and molecular characteristics (9). The incidence rates of left- and right-sided CRC also differ markedly with approximately two-thirds of CRC developing on the left side, and the remaining one-third developing on the right side (10).
Primary tumor location plays a significant role in estimating prognosis in mCRC. A retrospective analysis of CALGB/SWOG80405 (11) revealed that patients with KRASwt (codons 12 and 13) mCRC had significantly prolonged median OS in patients with left-sided tumors when compared to those with right-sided tumors irrespective of allocation to the cetuximab or bevacizumab groups. A recent retrospective analysis of six randomized trials that included 2,159 unresectable RASwt mCRC patients also found a worse prognosis for OS, PFS, and ORR in patients with right-sided tumors compared with those with left-sided tumors (12). These findings were consistent with the results from two recent meta-analyses (13, 14). Our study also demonstrated that patients with right-sided KRASwt tumors had significantly worse outcome (median OS: 23.3 mo and 29.9 mo for right-sided tumors and left-sided tumors, respectively (p=0.005). Therefore, our data confirmed that tumor sidedness does have prognostic impact on survival outcome in mCRC.
Regarding the predictive value of primary tumor location, CALGB/SWOG80405 (11) reported the median OS with cetuximab-based therapy to be 37.5 mo in left sided tumors as compared to 32.1 mo with bevacizumab-based therapy (HR: 0.77, p<0.05). For the right sided tumors, the bevacizumab arm had an OS of 24.5 mo compared to 16.4 mo in the cetuximab arm. Arnold, et al. (12) published the results of a retrospective pooled analysis of six randomized studies of tumor sidedness and anti-EGFR therapy in patients with RASwt (KRAS/NRASwt) mCRC. Of those six trials, five trials were first-line therapy [CRYSTAL (15), FIRE-3 (5), PRIME (16), PEAK (17), and CALGB/SWOG 80405 (2)]. In patients with left-sided tumors, doublet chemotherapy (either FOLFOX or FOLFIRI) plus anti-EGFR Ab (either cetuximab or panitumumab) was found to be associated with improved survival compared with doublet chemotherapy with or without bevacizumab (HR for OS: 0.75 and HR for PFS: 0.78). However, no benefit of anti-EGFR therapy was observed in patients with right-sided mCRC (HR for OS: 1.12 and HR for PFS: 1.12). Holch, et al. (14) performed a meta-analysis of 13 first-line randomized controlled trials and one pharmacogenetic study. Pooled data from all first-line anti-EGFR vs. anti-VEGF studies in RASwt mCRC patients (CALGB/SWOG 80405, FIRE-3, PEAK) showed significant OS benefit of anti-EGFR therapy in left-sided tumors (HR: 0.71, p=0.0003). Non-significant OS benefit favoring anti-VEGF (HR: 1.3, p=0.081) in patients with right-sided tumors was observed.
From these data, the ESMO consensus guidelines (7) recommend adding anti-EGFR Ab as a first-line treatment only in patients with left-sided RASwt mCRC. These findings are now incorporated into the latest clinical practice guidelines in European countries. However, the National Comprehensive Cancer Network (NCCN) still has no clear preference or recommendation relative to the addition of a biological agent in patients with left-sided tumors (18).
Although there are published recommendations specific to first-line treatment in mCRC, there are no recommendations relative to tumor sidedness in second- and subsequent-line treatment. The NCCN panel stated that there is not enough evidence to include tumor sidedness in treatment selection in these settings (18). To date, only few data have been reported specific to the effect of primary tumor location on the outcomes for RASwt patients receiving second- or later-line anti-EGFR treatment.
A retrospective analysis by Boeckx, et al. (19) evaluating RASwt data from 2 randomized studies [study 20050181 (20), and 20020408 (21)] revealed that RASwt left-sided tumors had clinical benefit when panitumumab was added in the second- or later-line treatment. In study 20050181, the addition of panitumumab to FOLFIRI resulted in a numerically improved median OS (20.1 mo vs. 16.6 mo; HR: 0.96; p=0.74) and PFS (8.0 mo vs. 5.8 mo; HR: 0.88; p=0.31) when compared with FOLFIRI alone in patients with RASwt left-sided primary tumors. In right-sided tumors, the HR for PFS favored panitumumab (4.8 mo vs. 2.4 mo; HR: 0.75; p=0.29), but the HR for OS favored FOLFIRI alone (10.3 mo vs. 8.1 mo; HR: 1.14; p=0.62). In study 20020408, a significant PFS benefit (5.5 mo vs. 1.6 mo; HR: 0.31; p<0.0001) was observed when panitumumab was added to best supportive care (BSC) to treat RASwt left-sided mCRC patients. No significant difference in PFS was observed in patients with right-sided tumors (1.7 mo vs. 1.5 mo; HR: 0.50; p=0.10). The OS results in that study were difficult to interpret because most patients in the BSC arm crossed over to panitumumab at progression.
Our finding demonstrated that patients with right-sided tumors had a non-significantly inferior PFS compared to those with left-sided tumors (median PFS: 5.7 mo, vs. 7.5 mo, p=0.17). Subgroup analysis also showed no significant difference in PFS between left-or right-sided when stratified by treatment lines (p=0.32 and 0.61 in second-and third-line, respectively). Due to small number of patients in later-line having right-sided tumors, no conclusion can be drawn from our cohort. A previous retrospective study reported that a KRAS exon 2 mutation alone cannot fully explain the heterogeneity of treatment responses to anti-EGFR therapy in mCRC (3). Strong evidence suggests that extended RAS analysis is needed to facilitate the identification of patients who most likely to benefit from anti-EGFR therapy. We then analyzed the patients who had extended RAS analysis data, and we found no significant difference in PFS between left- and right-sided tumors (median PFS: 7 mo for right-sided tumors, and 8.7 mo for left-sided tumors; p=0.46), which confirmed that sidedness had no significant impact on subsequent lines of treatment. Therefore, there was insufficient evidence to support the use of tumor sidedness in treatment selection in second- or later-line.
In this study, we did not directly compare the outcome in patients receiving chemotherapy with or without anti-EGFR Ab, since the majority of our patients were treated in the third-line setting. It might not be justified to compare with regorafenib or TAS102 which is considered to be the standard third-line therapy. Therefore, the predictive impact of sidedness for benefit of adding anti-EGFR Ab to chemotherapy in later-line treatment cannot be elucidated.
In our study, the ORR was approximately 30% in patients with left-sided tumors across treatment lines. For right-sided tumor, the ORR was 28% and 14% in second- and third-line respectively. Since the ORR was only 1-3% in patients who were treated with regorafenib (22, 23) or TAS102 (24, 25) in third- or later-line setting, our data supported the use of anti-EGFR Ab plus irinotecan in third-line treatment in patients with no prior anti-EGFR Ab before regorafenib or TAS102 regardless of tumor sidedness.
Study Strengths and Limitations
The strength of this study is a multi-center study which reduces the risk of bias. We also included patients with extended RAS analysis, which reflected the current guideline for using anti-EGFR Ab. However, owing to retrospective nature of the study, there are some inherent limitations. Firstly, some patients had missing or incomplete data. Secondly, due to relatively small number of right-sided tumors, the statistical power of the subgroup analysis might be insufficient for identifying all significant differences and should be interpreted with caution. Lastly, considering that the health benefit scheme in Thailand limits for only 6-month coverage for anti-EGFR Ab treatment, one-third of patients had to stop anti-EGFR Ab after 6-month treatment, even in cases that were responding to treatment. This factor could confound the duration of PFS. Nevertheless, our study is one of the few real-world studies that specifically addressed the effect of primary tumor location on the clinical outcomes of patients with KRASwt mCRC that were treated with anti-EGFR Ab therapy as second- or later-line treatment.
Conclusion
To date, this is the largest real world data of the effect of primary tumor location on anti-EGFR Ab which demonstrated that tumor sidedness has no significant impact on treatment outcomes in KRASwt mCRC patients receiving second- or later-line therapy. Our findings do not support the utility of tumor sidedness for treatment selection in these settings. We confirmed that patients with right-sided tumors had significantly worse survival.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
This retrospective nature of this study ensures total anonymity of patient data, and patient health and wellbeing are in no way affected, so the requirement to obtain written informed consent from study participants was waived.
Author Contributions
Conception and design: KK. Administrative support: KK. Provision of study materials or patients: AA, NT, TS, NP, ST, ES, CA, and KK. Collection and assembly of data: KK, AA, NT, and TS. Data analysis and interpretation: KK. Manuscript writing: all authors. Final approval of manuscript: all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge Dr. Sarppasit Ariyapanya for his help in preparing the figures.
References
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today (2020). Lyon: International Agency for Research on Cancer. Available at: https://gco.iarc.fr/today (Accessed February 2021).
2. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of First–Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild–Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA (2017) 317(23):2392–401. doi: 10.1001/jama.2017.7105
3. Hecht JR, Douillard JY, Schwartzberg L, Grothey A, Kopetz S, Rong A, et al. Extended RAS Analysis for Anti–Epidermal Growth Factor Therapy in Patients With Metastatic Colorectal Cancer. Cancer Treat Rev (2015) 41(8):653–9. doi: 10.1016/j.ctrv.2015.05.008
4. Rivera F, Karthaus M, Hecht JR, Sevilla I, Forget F, Fasola G, et al. Final Analysis of the Randomised PEAK Trial: Overall Survival and Tumour Responses During First–Line Treatment With Mfolfox6 Plus Either Panitumumab or Bevacizumab in Patients With Metastatic Colorectal Carcinoma. Int J Colorectal Dis (2017) 32(8):1179–90. doi: 10.1007/s00384-017-2800-1
5. Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling–Kaiser U, Al–Batran SE, et al. FOLFIRI Plus Cetuximab Versus FOLFIRI Plus Bevacizumab as First–Line Treatment for Patients With Metastatic Colorectal Cancer (FIRE–3): A Randomised, Open–Label, Phase 3 Trial. Lancet Oncol (2014) 15(10):1065–75. doi: 10.1016/S1470-2045(14)70330-4
6. Boeckx N, Koukakis R, Op de Beeck K, Rolfo C, Van Camp G, Siena S, et al. Primary Tumor Sidedness Has an Impact on Prognosis and Treatment Outcome in Metastatic Colorectal Cancer: Results From Two Randomized First–Line Panitumumab Studies. Ann Oncol (2017) 28(8):1862–8. doi: 10.1093/annonc/mdx119
7. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO Consensus Guidelines for the Management of Patients With Metastatic Colorectal Cancer. Ann Oncol (2016) 27(8):1386–422. doi: 10.1093/annonc/mdw235
8. Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol (2018) 25(6):1454–5. doi: 10.1245/s10434-018-6462-1
9. Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and Proximal Colon Cancers Differ in Terms of Molecular, Pathological, and Clinical Features. Ann Oncol (2014) 25(10):1995–2001. doi: 10.1093/annonc/mdu275
10. Meza R, Jeon J, Renehan AG, Luebeck EG. Colorectal Cancer Incidence Trends in the United States and United Kingdom: Evidence of Right– to Left–Sided Biological Gradients With Implications for Screening. Cancer Res (2010) 70(13):5419–29. doi: 10.1158/0008-5472.CAN-09-4417
11. Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O'Neil BH, et al. Impact of Primary Tumor Location on Overall Suvival (OS) and Progression–Free Survival (PFS) in Patients (Pts) With Metastatic Colorectal Cancer (Mcrc): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol (2016) 34( Suppl ):Abstract 3504. doi: 10.1200/JCO.2016.34.15_suppl.3504
12. Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and Predictive Value of Primary Tumour Side in Patients With RAS Wild–Type Metastatic Colorectal Cancer Treated With Chemotherapy and EGFR Directed Antibodies in Six Randomized Trials. Ann Oncol (2017) 28(8):1713–29. doi: 10.1093/annonc/mdx175
13. Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic Survival Associated With Left–Sided vs Right–Sided Colon Cancer: A Systematic Review and Meta–Analysis. JAMA Oncol (2017) 3(2):211–9. doi: 10.1001/jamaoncol.2016.4227
14. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The Relevance of Primary Tumour Location in Patients With Metastatic Colorectal Cancer: A Meta–Analysis of First–Line Clinical Trials. Eur J Cancer (2017) 70:87–98. doi: 10.1016/j.ejca.2016.10.007
15. Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N Engl J Med (2009) 360(14):1408–17. doi: 10.1056/NEJMoa0805019
16. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, Phase III Trial of Panitumumab With Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone as First–Line Treatment in Patients With Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J Clin Oncol (2010) 28(31):4697–705. doi: 10.1200/JCO.2009.27.4860
17. Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (Mfolfox6) or Bevacizumab Plus Mfolfox6 in Patients With Previously Untreated, Unresectable, Wild–Type KRAS Exon 2 Metastatic Colorectal Cancer. J Clin Oncol (2014) 32(21):2240–7. doi: 10.1200/JCO.2013.53.2473
18. National Comprehensive Cancer Network. Colon Cancer (Version 1.2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (Accessed April 28, 2021).
19. Boeckx N, Koukakis R, Op de Beeck K, Rolfo C, Van Camp G, Siena S, et al. Effect of Primary Tumor Location on Second– or Later–Line Treatment Outcomes in Patients With RAS Wild–Type Metastatic Colorectal Cancer and All Treatment Lines in Patients With RAS Mutations in Four Randomized Panitumumab Studies. Clin Colorectal Cancer (2018) 17(3):170–8.e3. doi: 10.1016/j.clcc.2018.03.005
20. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final Results From a Randomized Phase 3 Study of FOLFIRI {+/–} Panitumumab for Second–Line Treatment of Metastatic Colorectal Cancer. Ann Oncol (2014) 25(1):107–16. doi: 10.1093/annonc/mdt523
21. Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, et al. A Phase 3 Trial Evaluating Panitumumab Plus Best Supportive Care vs Best Supportive Care in Chemorefractory Wild–Type KRAS or RAS Metastatic Colorectal Cancer. Br J Cancer (2016) 115(10):1206–14. doi: 10.1038/bjc.2016.309
22. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo–Controlled, Phase 3 Trial. Lancet (2013) 381(9863):303–12. doi: 10.1016/S0140-6736(12)61900-X
23. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib Plus Best Supportive Care Versus Placebo Plus Best Supportive Care in Asian Patients With Previously Treated Metastatic Colorectal Cancer (CONCUR): A Randomised, Double–Blind, Placebo–Controlled, Phase 3 Trial. Lancet Oncol (2015) 16(6):619–29. doi: 10.1016/S1470-2045(15)70156-7
24. Xu J, Kim TW, Shen L, Sriuranpong V, Pan H, Xu R, et al. Results of a Randomized, Double–Blind, Placebo–Controlled, Phase III Trial of Trifluridine/Tipiracil (TAS–102) Monotherapy in Asian Patients With Previously Treated Metastatic Colorectal Cancer: The TERRA Study. J Clin Oncol (2018) 36(4):350–8. doi: 10.1200/JCO.2017.74.3245
Keywords: primary tumor location, later-line treatment, anti-epidermal growth factor receptor (EGFR) antibodies, metastatic colorectal cancer, sidedness
Citation: Archwamety A, Teeyapun N, Siripoon T, Poungvarin N, Tanasanvimon S, Sirachainan E, Akewanlop C and Korphaisarn K (2022) Effect of Primary Tumor Location on Second- or Later-Line Treatment With Anti-Epidermal Growth Factor Receptor Antibodies in Patients With Metastatic Colorectal Cancer: A Retrospective Multi-Center Study. Front. Oncol. 12:813009. doi: 10.3389/fonc.2022.813009
Received: 17 November 2021; Accepted: 24 January 2022;
Published: 15 February 2022.
Edited by:
Dario Baratti, Fondazione Istituto Nazionale Tumori (IRCCS), ItalyReviewed by:
Lorena Martin Roman, Mater Misericordiae University Hospital, IrelandLuca Sorrentino, National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2022 Archwamety, Teeyapun, Siripoon, Poungvarin, Tanasanvimon, Sirachainan, Akewanlop and Korphaisarn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krittiya Korphaisarn, a3JpdHRpeWEua29yQG1haGlkb2wuYWMudGg=
†These authors have contributed equally to this work and share first authorship
 Anita Archwamety1†
Anita Archwamety1† Krittiya Korphaisarn
Krittiya Korphaisarn