
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 07 April 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.812358
This article is part of the Research Topic Challenges and their Implications for the Clinical Practice of Head and Neck Cancer View all 23 articles
Objective: To develop and validate a bone metastasis prediction model based on skull base invasion (SBI) in patients with locally advanced nasopharyngeal carcinoma (LA-NPC).
Methods: This retrospective cohort study enrolled 290 patients with LA-NPC who received intensity-modulated radiation therapy in two hospitals from 2010 to 2020. Patient characteristics were grouped by SBI and hospital. Both unadjusted and multivariate-adjusted models were used to determine bone metastasis risk based on SBI status. Subgroup analysis was performed to investigate heterogeneity using a forest graph. Cox proportional hazard regression analysis was used to screen for risk factors of bone metastasis-free survival (BMFS). A nomogram of BMFS based on SBI was developed and validated using C-index, receiver operating characteristic curve, calibration curves, and decision curve analysis after Cox proportional hazard regression analysis.
Results: The incidence of bone metastasis was 14.83% (43/290), 20.69% (24/116), and 10.92% (19/174) in the overall population, SBI-positive group, and SBI-negative group, respectively. In the unadjusted model, SBI was associated with reduced BMFS [HR 2.43 (1.32–4.47), P = 0.004], and the results remained stable after three continuous adjustments (P <0.05). No significant interaction was found in the subgroup analyses (P for interaction >0.05). According to Cox proportional hazard regression analysis and clinical value results, potential risk factors included SBI, Karnofsky performance status, TNM stage, induction chemotherapy, concurrent chemoradiotherapy, and adjuvant chemotherapy. Using a training C-index of 0.80 and a validation C-index of 0.79, the nomogram predicted BMFS and demonstrated satisfactory prognostic capability in 2, 3, and 5 years (area under curve: 83.7% vs. 79.6%, 81.7% vs. 88.2%, and 79.0% vs. 93.8%, respectively).
Conclusion: Skull base invasion is a risk factor for bone metastasis in patients with LA-NPC. The SBI-based nomogram model can be used to predict bone metastasis and may assist in identifying LA-NPC patients at the highest risk of bone metastasis.
Nasopharyngeal carcinoma (NPC), a squamous cell carcinoma that develops on the nasopharyngeal epithelium, is one of the most common malignant tumors in South China, with more than 70% of patients diagnosed with locally advanced NPC (LA-NPC) (1–3). Although treatments like intensity-modulated radiation therapy (IMRT) can improve local control rate, the incidence of distant metastasis ranges from 11.00 to 27.08% and remains a significant concern (4–7). Multiple studies have correlated distant metastasis with poor prognosis (8, 9). NPC is associated with pulmonary, liver, and bone metastasis, with bone being the most common, occurring concurrent with or before other distant metastases (10, 11). Thus, it is critical to identify risk factors that may influence bone metastasis in LA-NPC patients.
The skull base is a common site of tumor invasion in LA-NPC patients (12). Zou et al. (13) studied 518 LA-NPC patients and found that those with skull base invasion (SBI) had a higher risk of bone metastasis than those without (16.4% vs. 6.6%, respectively; P < 0.05). Other studies have shown that SBI detected by computed tomography (CT) was predictive of bone metastasis in patients with early N-stage NPC [2.478 (1.146–5.358), P = 0.021] (14). However, more research is needed to determine the independent prognostic value of SBI to the risk of bone metastasis. Furthermore, there is no international consensus on the best model to predict bone metastasis in LA-NPC patients based on SBI (15).
A nomogram is a visual depiction of a complicated mathematical formula that offers the overall likelihood of a specific outcome (16). Nomograms generated by regression analysis are widely used in regimen selection, tumor recurrence/metastasis prediction, and prognostic evaluation (17, 18). In addition, the prediction model can be integrated into TNM staging (19). This retrospective study was designed to assess the relationship between SBI and bone metastasis and to develop a bone metastasis risk model based on SBI.
A retrospective study was conducted by consecutively enrolling LA-NPC patients seen at Taizhou Central Hospital (Taizhou University Hospital) and Taizhou Hospital from 2010 to 2020 (Figure 1). The local Institutional Review Boards approved the study (No. 2019-SC-019, Date: 2019/06/09). Because the study was retrospective, the requirement for informed consent was waived.
Inclusion criteria included (1) a pathological diagnosis of NPC, (2) complete imaging results confirming LA-NPC (stage III or IVa, AJCC 8th edition), (3) CT or magnetic resonance imaging (MRI) diagnosis of SBI, and (4) receipt of IMRT alone or in combination with induction chemotherapy (IC), concurrent chemoradiotherapy (CCRT), or adjuvant chemotherapy (AC). Exclusion criteria included (1) stage I, II, and IVb (n = 67), (2) presence of other primary malignant tumors (n = 10), (3) incomplete clinical data (n = 26), and (4) loss to follow-up (n = 27). Based on these criteria, 290 LA-NPC patients were included in the study.
The times from inclusion in the study to bone metastasis, distant metastasis, or death were defined as bone metastasis-free survival (BMFS), distant metastasis-free survival (DMFS), or overall survival (OS), respectively. Follow-up was conducted during outpatient visits or by phone every 3 months for the first 2 years and every 6 months for the next 3–5 years. The end of follow-up was defined as the date of death or June 2021.
Potential predictor variables were collected before and during treatment. Patient information, including demographics, clinical features, imaging findings, and treatment, was obtained from the hospital information systems. SBI was separately assessed for each patient by two radiologists using contrast-enhanced CT and/or MRI (14, 20). Any disagreements were reviewed until a consensus was reached.
All enrolled LA-NPC patients were treated with IMRT as described previously (21, 22). In brief, the prescription doses of 70-76Gy, 66-70Gy, 60-66Gy, or 56-60Gy were administered to the primary tumor volume of the gross tumor (GTVnx) and the involved lymph nodes (GTVnd), with the clinical target volume including high- and low-risk regions (CTV1/2). IC, CCRT, or AC was usually recommended for these patients in the form of single-agent cisplatin or platinum-based regimen. The combination chemotherapy regimens included platinum/fluorouracil, gemcitabine/platinum, docetaxel/platinum, and docetaxel/platinum/fluorouracil (Supplementary S1).
Unadjusted and multivariable-adjusted models were used to determine the relationship between SBI and LA-NPC outcomes. Covariables were added to a Cox regression model and dropped one by one. The coefficients of the corresponding regression were compared. Effect modification based on TNM stage, IC, CCRT, and AC treatment was investigated by including an interaction term with SBI in the Cox regression model for bone metastasis. The findings were presented as a hazard ratio (HR) with 95% confidence intervals (CI).
Univariate and multivariate Cox regression analyses were performed to identify clinically important variables related to bone metastasis risk (P < 0.1). A nomogram predicting bone metastasis was then established to visualize model efficiency using a training dataset from Taizhou Central Hospital (Taizhou University Hospital). The results were validated with a validation dataset from Taizhou Hospital. The area under the curve (AUC) of the receiver operating characteristic curve (ROC) was used to evaluate the accuracy of the nomogram model. The concordance index (C-index) was calculated to assess the model’s discrimination ability and a calibration curve was plotted to calibrate the model (23). The clinical usefulness of the nomogram was estimated using decision curve analysis (24).
Descriptive analysis was used to characterize the study participants. Categorical variables were expressed as proportions (%), and continuous variables were expressed as the mean plus standard deviation. The correlation between clinical covariables and SBI or hospital was analyzed using χ2 and t tests. P <0.05 denoted a statistically significant difference (two-tailed test). Pearson’s coefficients of association were calculated to assess the collinearity between SBI and the covariables. The threshold was set at r <0.6 with P <0.05. All data were processed using Free Statistics software version 1.3 and SPSS software version 25.0.
In total, 290 cases were included in the study with a median 49.5-month follow-up (range: 6–60 months). Of these, 198 cases were from Taizhou central hospital (Taizhou university hospital) and 92 cases were from Taizhou Hospital. Baseline characteristics of the patients, grouped by the presence or absence of SBI, are shown in Table 1. The patients were an average of 54.9 ± 11.6 years of age and 74.5% (216/290) were male. Most participants (71.3%, 207/290) had TNM stage III, while the remaining 83 had TNM stage IVa. Forty percent (116/290) of the patients had SBI. There were significant differences in T category, N category, TNM stage, and IC between the SBI-positive and SBI-negative groups (P <0.05). However, no statistically significant differences in hospital, age, sex, Karnofsky performance status (KPS), smoking index, histological type, CCRT, and AC were observed between the groups (P >0.05). Table S1 summarizes the baseline characteristics by hospital. While there was a significant difference in CCRT (77.3% vs. 65.2%, P = 0.043), no statistically significant differences were reported in SBI, bone metastasis, age, sex, KPS, smoking index, histological type, T category, N category, TNM stage, IC, and AC (P >0.05).
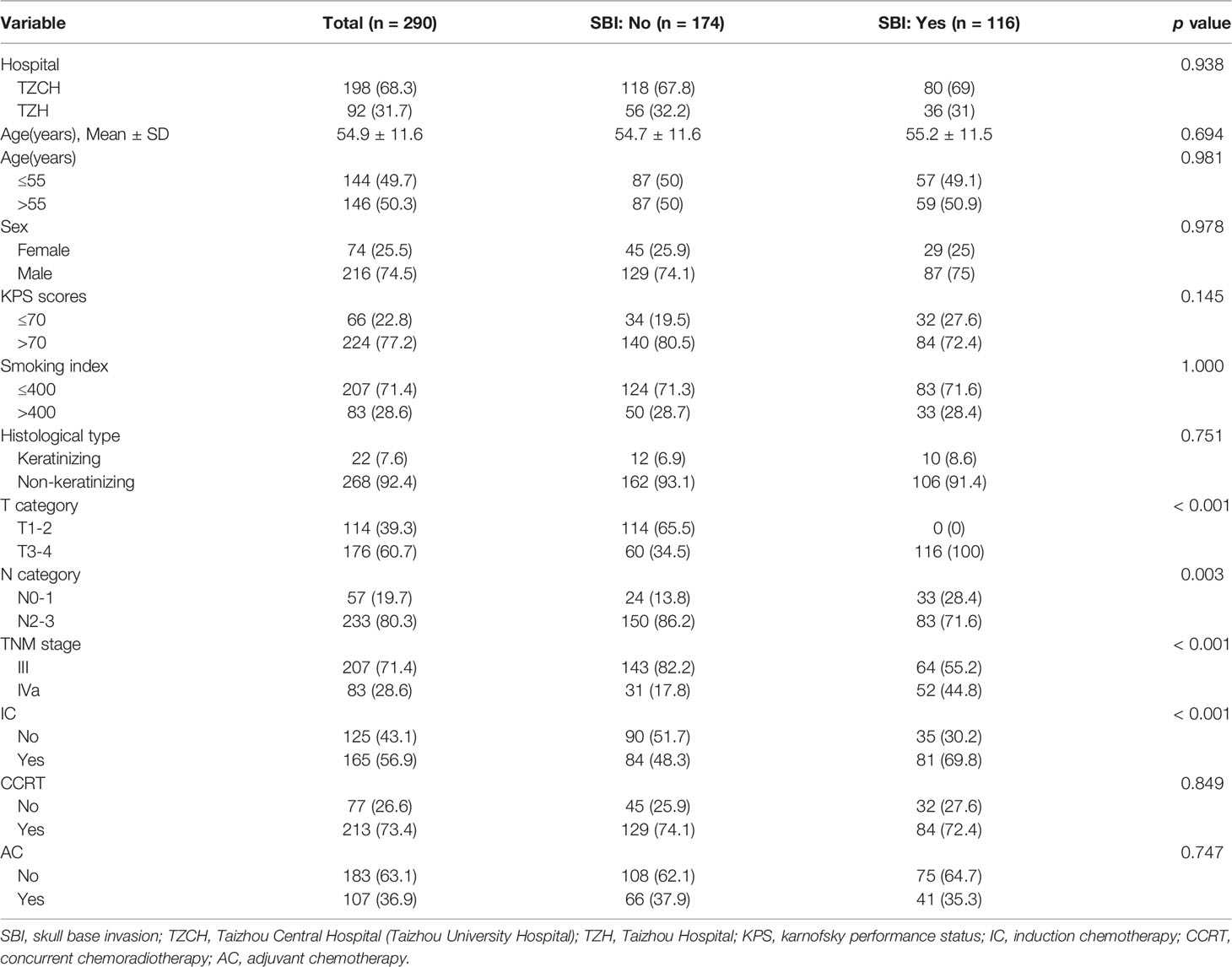
Table 1 Baseline characteristics of 290 locally advanced nasopharyngeal carcinoma patients grouped by presence of skull base invasion.
The incidence of bone metastasis was 14.83% (43/290), 20.69% (24/116), and 10.92% (19/174) in the study population, SBI-positive group, and SBI-negative group, respectively. Collinearity analysis revealed strong collinearity between SBI and T category (r = 0.657) (Table S2), while SBI and TNM stage (r = 0.293) did not show collinearity. Thus, TNM stage was chosen for subsequent analyses.
The HRs and 95% CIs for tumor progression and survival analyses determined by SBI are shown in Table 2. SBI-positive patients had a shorter BMFS in the unadjusted model [HR: 2.43, 95%CI (1.32–4.47)] (Table 2 and Figure 2A). After adjusting for hospital, age, sex, KPS, smoking index, histological type, TNM stage, IC, CCRT, and AC, the HRs were 2.52 (1.36–4.66), 2.28 (1.23–4.22), and 2.31 (1.17–4.54), respectively (P <0.05). A correlation was observed between SBI and bone metastasis in unadjusted and multivariable-adjusted models. While the Kaplan–Meier survival curve showed that SBI-positive patients had a lower DMFS and OS than SBI-negative ones, the HRs was 1.56 (0.92–2.65) and 1.56 (0.85–2.89) for DMFS and OS after adjusting for all covariables (Table 2 and Figures 2B, C).
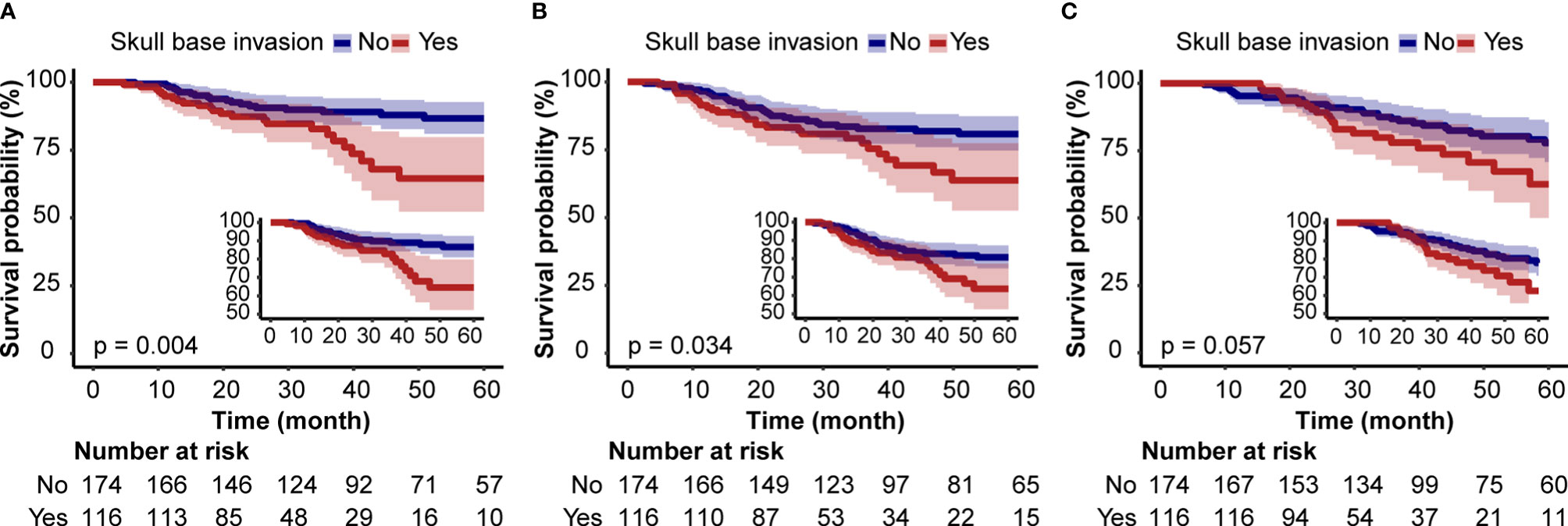
Figure 2 Kaplan–Meier Survival Curves for bone metastasis-free survival (A), distant metastasis-free survival, (B) and overall survival (C) of locally advanced nasopharyngeal carcinoma patients based on skull base invasion.
Stratified and interactive analyses were used to determine if the relationships between SBI and bone metastasis were stable in the TNM stage, IC, CCRT, and AC subgroups (Figure 3). However, no significant interaction effects were found in the four subgroups (P for interaction > 0.05).
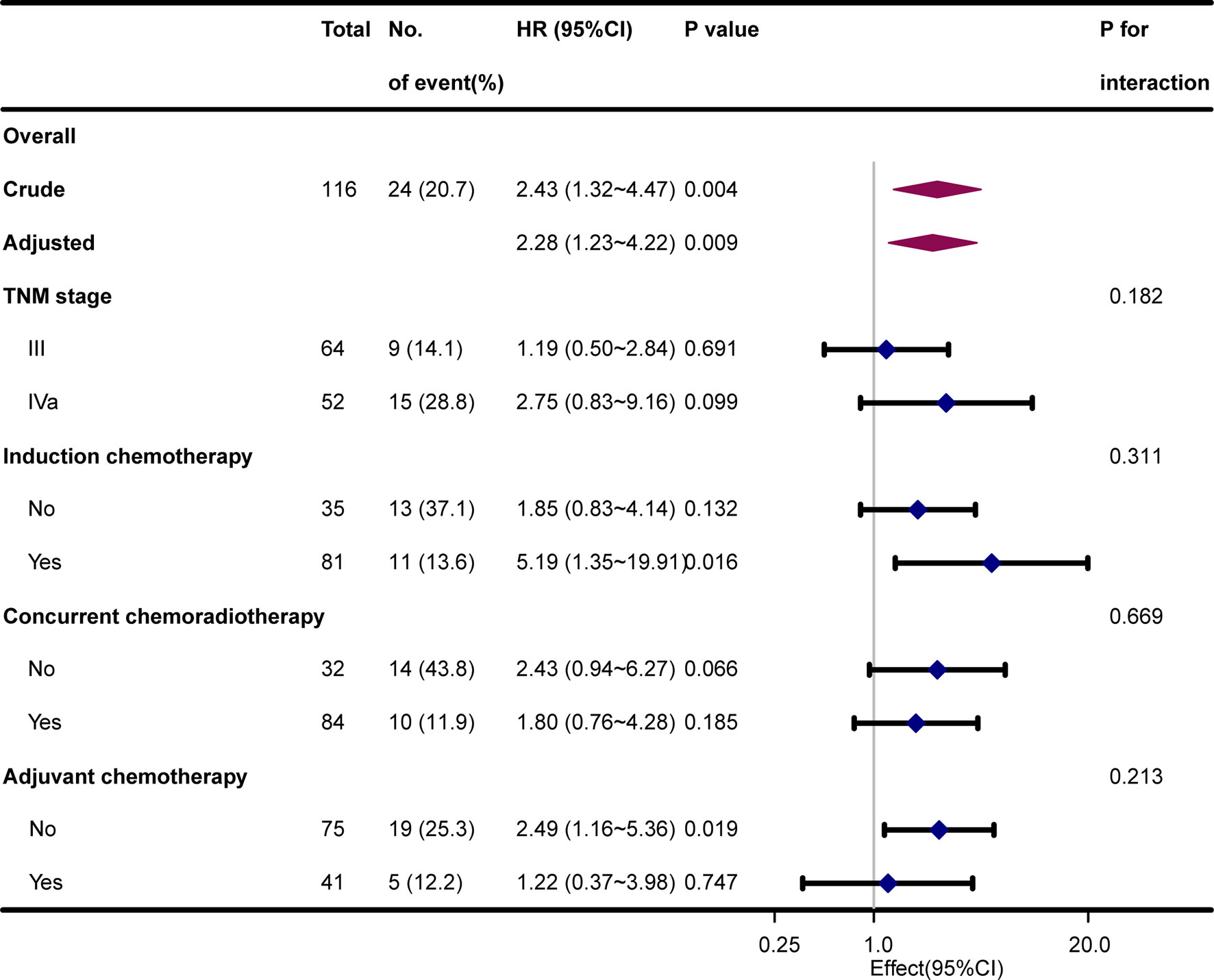
Figure 3 Hazard risk of bone metastasis in subgroup analyses after adjustment for hospital, age, sex, Karnofsky performance status, smoking index, and histological type.
Cox proportional hazard regression revealed that SBI, KPS, TNM stage, IC, and CCRT were independent risk factors for BMFS (Table 3). AC was also selected due to its clinical value for tumor prognosis. A nomogram with the six factors is shown in Figure 4. The C-index for BMFS prediction in the training and validation datasets was 0.80 (95% CI 0.694–0.905) and 0.79 (95% CI 0.621–0.963), respectively. According to ROC analyses on both the training and validation datasets, the AUCs were 83.7% vs. 79.6%, 81.7% vs. 88.2%, and 79.0% vs. 93.8% for predicting 2-, 3-, and 5-year BMFS, respectively (Figures 5A, B). In addition, the calibration plot of the nomogram for the probability of BMFS at 2, 3, and 5 years showed strong agreement (Figures 6A–F), and the decision curve results indicated that the nomogram was clinically applicable (Figures 7A–F).
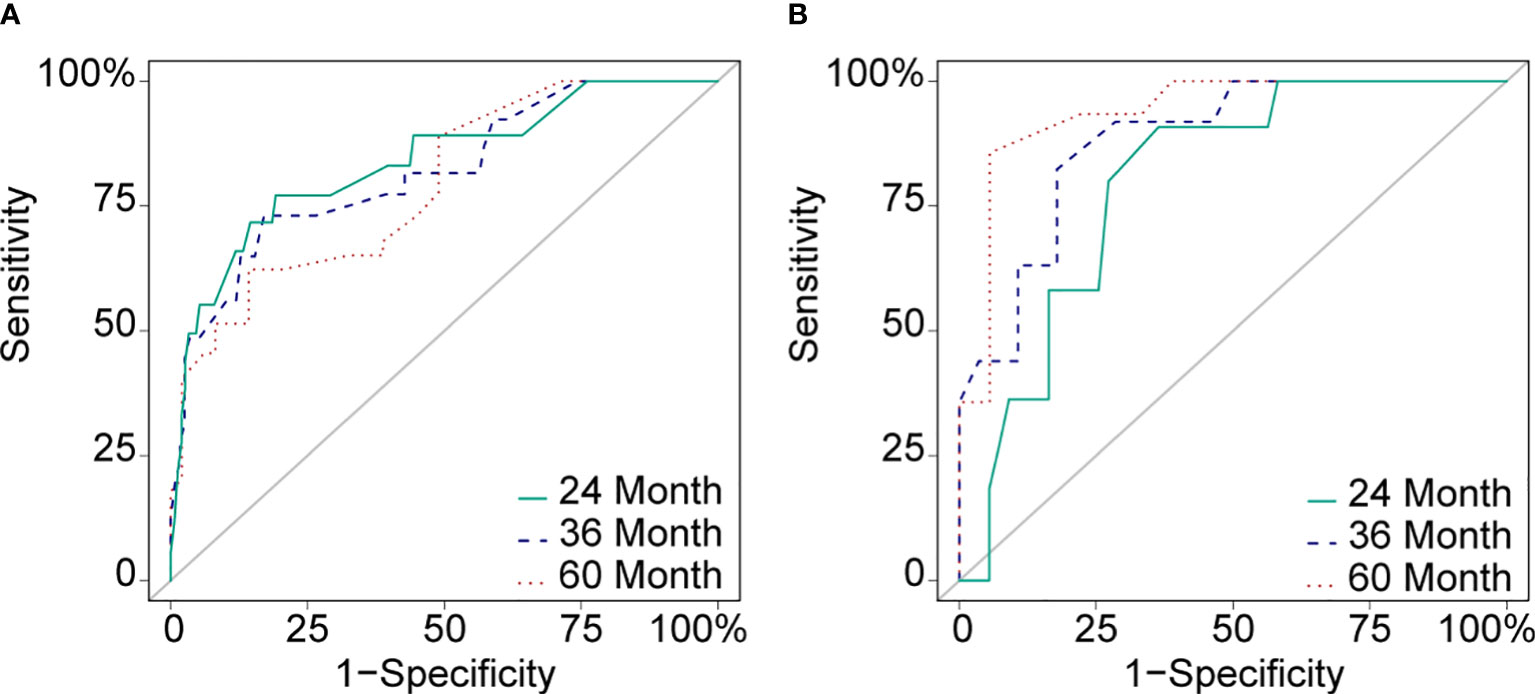
Figure 5 ROC curves of the training dataset (A) and the validation dataset (B) in 24 months (AUC: 83.7% vs. 79.6%), 36 months (AUC: 81.7% vs. 88.2%) and 60 months (AUC: 79.0% vs. 93.8%).

Figure 6 Calibration curves of the training dataset (A–C) and the validation dataset (D–F) in 24, 36, and 60 months.
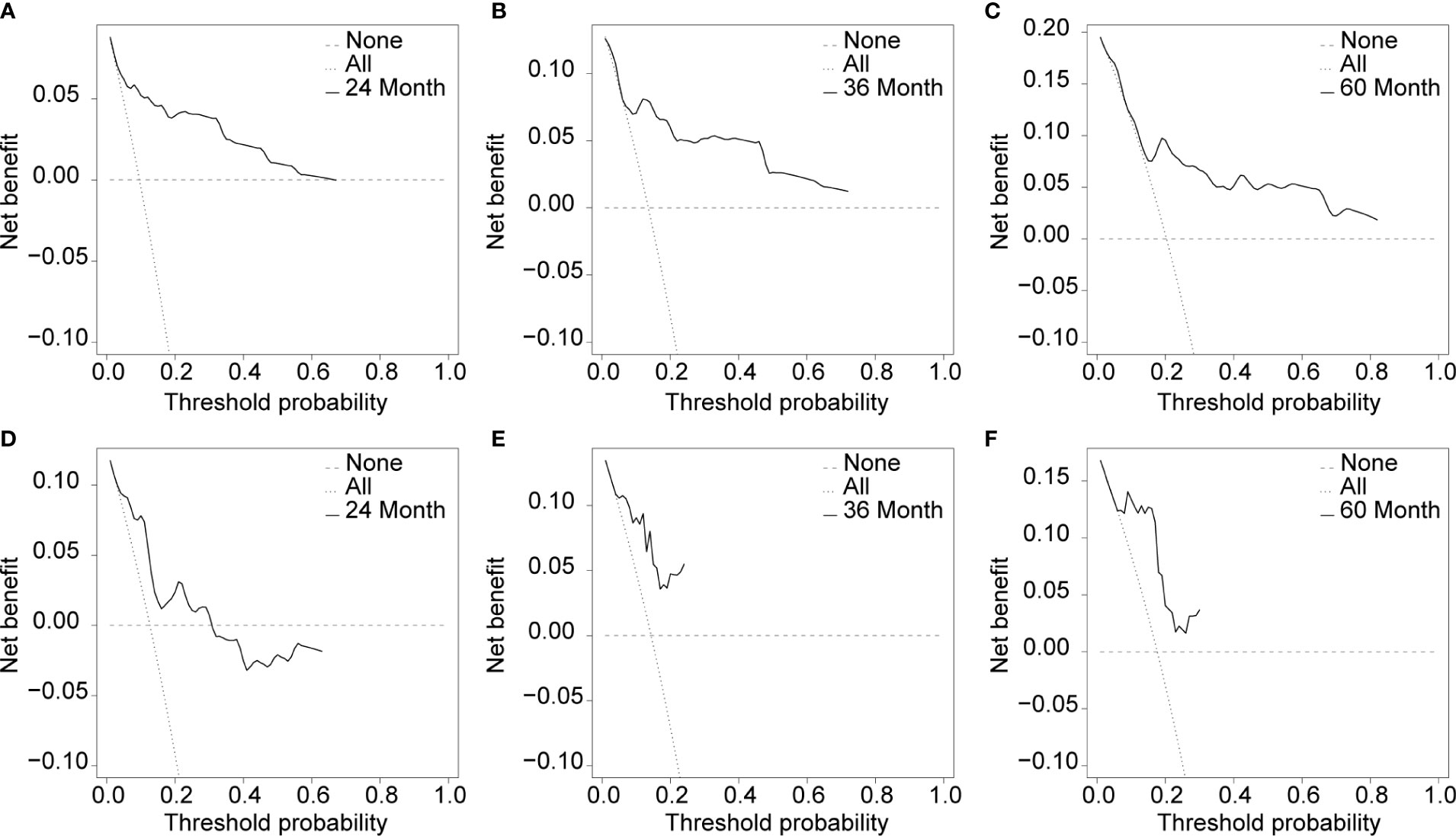
Figure 7 Usefulness evaluation of the training dataset (A–C) and the validation dataset (D–F) in 24, 36, and 60 months.
Novel treatments like IMRT have steadily reduced the rate of local/regional recurrence during LA-NPC, but distant metastasis still results in treatment failures (2). According to the “seed and soil” theory, bone metastasis most often results from nutrient-rich bone tissue, chemokine and cytokine mediation, and the unique ecological niche of the bone metastasis (5, 25). The present study developed a risk prediction model by investigating the relationship between SBI and bone metastasis. SBI was significantly correlated with a higher incidence of bone metastasis and shorter BMFS. Even in multivariable-adjusted models, the results remained robust and stable. A nomogram of BMFS was developed and validated based on SBI and found to perform well in terms of calibration and discrimination.
Prior studies have assessed the risk factors for bone metastasis in NPC patients. Zhao et al. (26) suggested that bone metastasis is related to N but not T classification. Another study yielded comparable results (27). These studies did not specifically investigate the risk of bone metastasis caused by SBI, however. In the current study, 14.83% (43/290) patients had bone metastasis and SBI was significantly associated with increased risk of bone metastasis (20.69% vs. 10.92% for SBI-positive vs. SBI-negative patients, respectively) and shorter BMFS [HR 2.43 (1.32–4.47), P < 0.05]. A Cox proportional hazard model with major covariable adjustment was used to examine the effect of SBI on bone metastasis. The results remained robust and stable even after three adjustments (P < 0.05). Yi et al. (14) demonstrated the predictive value of SBI for bone metastasis, particularly in patients with early N-staging NPC. While SBI was associated with poor DMFS and OS in this study, however, the covariable adjusted model showed that SBI may not be an independent factor. Feng et al. has demonstrated that extensive SBI is not an independent prognostic factor for DMFS and OS (28). In a separate study of 181 N0 NPC patients, the high-risk advanced T category, which included SBI, was an independent prognostic factor for PFS, OS, and locoregional relapse-free survival (29). Subgroup analysis assessed the relationship between SBI and bone metastasis based on TNM stage, IC, CCRT, and AC. Of note, no significant interaction effects were found in the four subgroups (P for interaction >0.05). There is a strong link between SBI and bone metastasis in different subgroups, which is consistent with prior studies (30–32). Collectively, these data confirm a correlation between SBI and a greater risk of bone metastasis.
This study suggests that the development of a prediction model of bone metastasis based on SBI is both feasible and meaningful. Collinearity, which exists in variables that are similar or have a strong association, should be checked before modeling, and variables with significant collinearity should not be included (33–35). Given that SBI and T category had strong collinearity (r = 0.657) in this study, while TNM stage did not (r = 0.293), TNM stage was selected for subsequent analyses. Chen et al. (36) developed a prognostic score for NPC patients with bone metastasis based on clinical routine factors. Another study (15) developed a nomogram using data from the Surveillance, Epidemiology, and End Results database to predict the prognosis of distant metastases. Yao et al. (37) used a nomogram to assess the benefits of adding IC to CCRT for N2-3 NPC patients and found that those in the high-risk group benefited more from combination therapy (DMFS: 69.5% vs. 56.7%, P = 0.004). There is no prediction model for bone metastasis risk in the Chinese population, however. Thus, a BMFS predictive model was developed based on SBI and visualized using a nomogram. The model included the following six components: SBI, KPS, TNM stage, IC, CCRT, and AC, and the nomogram performed well in both the training and validation datasets (C-index 0.80 vs. 0.79), which was consistent with the AUC at 2, 3, and 5 years. Calibration curves and DCA demonstrated the effectiveness of the nomogram. As a result, a nomogram based on SBI may provide an individual assessment of bone metastasis risk in LA-NPC patients.
The present study has several limitations. First, because it is a retrospective study, there is the possibility of both selection bias and confounding bias. Second, although the established nomogram model was trained and validated using data from two different hospitals, the sample size was small and external validation was not performed. Third, several variables including Epstein Barr Virus were not included in the analysis. Fourth, this study was conducted in an endemic area. So, extrapolation of the current results should be done with caution. Future studies should consider establishing an updated model with a large sample size and detailed data that is subject to external validation.
In conclusion, both unadjusted and adjusted analyses showed that SBI is strongly associated with the risk of bone metastasis. The established SBI-based nomogram can be used to assess the risk of bone metastasis in individual LA-NPC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Institutional Review Boards of Taizhou Central Hospital (Taizhou University Hospital). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BW and YT conceived the presented idea. YG and Q-GG collected the data. YG and HY analyzed the data, BW drafted the manuscript. YT improved the standard of English. All authors reviewed the manuscript and approved the submitted version.
This study was funded by the Medical and Health Science and Technology Program of Zhejiang Province (2020RC040).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Team of Clinical Scientists for their helpful review and comments regarding the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.812358/full#supplementary-material
Supplementary Table 1 | Baseline characteristics of 290 locally advanced nasopharyngeal carcinoma patients grouped by hospital.
Supplementary Table 2 | Collinearity analysis of skull base invasion and covariables.
1. Chen Y, Chan A, Le Q, Blanchard P, Sun Y, Ma J. Nasopharyngeal Carcinoma. Lancet (London England) (2019) 394(10192):64–80. doi: 10.1016/s0140-6736(19)30956-0
2. Ji MF, Sheng W, Cheng WM, Ng MH, Wu BH, Yu X, et al. Incidence and Mortality of Nasopharyngeal Carcinoma: Interim Analysis of a Cluster Randomized Controlled Screening Trial (PRO-NPC-001) in Southern China. Ann Oncol: Off J Eur Soc Med Oncol (2019) 30(10):1630–7. doi: 10.1093/annonc/mdz231
3. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. New Engl J Med (2019) 381(12):1124–35. doi: 10.1056/NEJMoa1905287
4. Liao S, Xie Y, Feng Y, Zhou Y, Pan Y, Fan J, et al. Superiority of Intensity-Modulated Radiation Therapy in Nasopharyngeal Carcinoma With Skull-Base Invasion. J Cancer Res Clin Oncol (2020) 146(2):429–39. doi: 10.1007/s00432-019-03067-y
5. Caglar M, Ceylan E, Ozyar E. Frequency of Skeletal Metastases in Nasopharyngeal Carcinoma After Initiation of Therapy: Should Bone Scans be Used for Follow-Up? Nucl Med Commun (2003) 24(12):1231–6. doi: 10.1097/00006231-200312000-00005
6. Liu FY, Chang JT, Wang HM, Liao CT, Kang CJ, Ng SH, et al. [18F]Fluorodeoxyglucose Positron Emission Tomography is More Sensitive Than Skeletal Scintigraphy for Detecting Bone Metastasis in Endemic Nasopharyngeal Carcinoma at Initial Staging. J Clin Oncol: Off J Am Soc Clin Oncol (2006) 24(4):599–604. doi: 10.1200/jco.2005.03.8760
7. Al Tamimi AS, Zaheer S, Ng DC, Osmany S. (18)F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Imaging of Metastatic Nasopharyngeal Cancer With Emphasis on the Distribution of Bone Metastases. World J Nucl Med (2017) 16(3):192–6. doi: 10.4103/1450-1147.207273
8. Lu T, Guo Q, Cui X, Chen Z, Lin S, Xu L, et al. Prognostic Evaluation of Nasopharyngeal Carcinoma With Bone-Only Metastasis After Therapy. Yonsei Med J (2016) 57(4):840–5. doi: 10.3349/ymj.2016.57.4.840
9. Yang H, Liu C, Luo S, Ma R, Zhou Y, Yin Y, et al. Prognostic Analysis of 152 Patients With Distant Metastasis After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. Ann Palliat Med (2021) 10(6):6824–32. doi: 10.21037/apm-21-1279
10. Yang X, Ren H, Yu W, Li H, Yang X, Fu J. Bone Metastases Pattern in Newly Diagnosed Metastatic Nasopharyngeal Carcinoma: A Real-World Analysis in the SEER Database. BioMed Res Int (2020) 2020:2098325. doi: 10.1155/2020/2098325
11. Li A-C, Xiao W-W, Shen G-Z, Wang L, Xu A-A, Cao Y-Q, et al. Distant Metastasis Risk and Patterns of Nasopharyngeal Carcinoma in the Era of IMRT: Long-Term Results and Benefits of Chemotherapy. Oncotarget (2015) 6(27):24511–21. doi: 10.18632/oncotarget.4312
12. Li YZ, Cai PQ, Xie CM, Huang ZL, Zhang GY, Wu YP, et al. Nasopharyngeal Cancer: Impact of Skull Base Invasion on Patients Prognosis and its Potential Implications on TNM Staging. Eur J Radiol (2013) 82(3):e107–11. doi: 10.1016/j.ejrad.2012.10.016
13. Zou GR, Li YH, Zeng WH, He Y, Gong Z, Cao XL. Correlation Between Skull Base Invasion and Bone Metastases in Locally Advanced Nasopharyngeal Carcinoma Patients. Cancer Res Prev Treat (2016) 43(10):4. doi: 10.3971/j.issn.1000-8578.2016.10.006
14. Yi W, Liu ZG, Li X, Tang J, Jiang CB, Hu JY, et al. CT-Diagnosed Severe Skull Base Bone Destruction Predicts Distant Bone Metastasis in Early N-Stage Nasopharyngeal Carcinoma. OncoTarg Ther (2016) 9:7011–7. doi: 10.2147/ott.S99717
15. Qu W, Li S, Zhang M, Qiao Q. Pattern and Prognosis of Distant Metastases in Nasopharyngeal Carcinoma: A Large-Population Retrospective Analysis. Cancer Med (2020) 9(17):6147–58. doi: 10.1002/cam4.3301
16. Iasonos A, Schrag D, Raj GV, Panageas KS. How to Build and Interpret a Nomogram for Cancer Prognosis. J Clin Oncol: Off J Am Soc Clin Oncol (2008) 26(8):1364–70. doi: 10.1200/jco.2007.12.9791
17. Peng H, Chen L, Mao Y, Tian L, Liu LJO. Nomogram-Aided Individual Induction Chemotherapy Regimen Selection in Advanced Nasopharyngeal Carcinoma. Oral Oncol (2021) 122:105555. doi: 10.1016/j.oraloncology.2021.105555
18. Zhang LL, Xu F, Song D, Huang MY, Huang YS, Deng QL, et al. Development of a Nomogram Model for Treatment of Nonmetastatic Nasopharyngeal Carcinoma. JAMA Netw Open (2020) 3(12):e2029882. doi: 10.1001/jamanetworkopen.2020.29882
19. Yang K, Zhang Q, Zhang M, Xie W, Li M, Zeng L, et al. A Nomogram for the Determination of the Necessity of Concurrent Chemotherapy in Patients With Stage II-IVa Nasopharyngeal Carcinoma. Front Oncol (2021) 11:640077. doi: 10.3389/fonc.2021.640077
20. Cheng YK, Liu LZ, Jiang N, Yue D, Tang LL, Zhang F, et al. MRI-Detected Skull-Base Invasion: Prognostic Value and Therapeutic Implication in Intensity-Modulated Radiotherapy Treatment for Nasopharyngeal Carcinoma. Strahlentherapie Und Onkol: Organ Der Deutschen Rontgengesellschaft (2014) 190(10):905–11. doi: 10.1007/s00066-014-0656-7
21. Hu W, Wang W, Yang P, Zhou C, Yang W, Wu B, et al. Phase I Study of Icotinib, an EGFR Tyrosine Kinase Inhibitor Combined With IMRT in Nasopharyngeal Carcinoma. Int J Clin Exp Med (2015) 8(9):15675–83.
22. Wang W, Yang H, Hu W, Shan G, Ding W, Yu C, et al. Clinical Study of the Necessity of Replanning Before the 25th Fraction During the Course of Intensity-Modulated Radiotherapy for Patients With Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys (2010) 77(2):617–21. doi: 10.1016/j.ijrobp.2009.08.036
23. Alba A, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux P, et al. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA (2017) 318(14):1377–84. doi: 10.1001/jama.2017.12126
24. Van Calster B, Wynants L, Verbeek J, Verbakel J, Christodoulou E, Vickers A, et al. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Europ Urol (2018) 74(6):796–804. doi: 10.1016/j.eururo.2018.08.038
25. Paget S. The Distribution of Secondary Growths in Cancer of the Breast. 1889. Cancer Metastasis Rev (1989) 8(2):98–101.
26. Zhao CL, Qian GQ, Chen XY, Chen C. Retrograde Analysis of Clinical Characteristics of Bone Metastasis in 1,031 Cases of Preliminarily Diagnosed Nasopharyngeal Carcinoma. Asian Pacific J Cancer Prevention: APJCP (2014) 15(8):3785–8. doi: 10.7314/apjcp.2014.15.8.3785
27. Guo Q, Lu T, Chen Y, Su Y, Zheng Y, Chen Z, et al. Genetic Variations in the PI3K-PTEN-AKT-mTOR Pathway are Associated With Distant Metastasis in Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiation Therapy. Sci Rep (2016) 6:37576. doi: 10.1038/srep37576
28. Feng Y, Cao C, Hu Q, Chen X. Grading of MRI-Detected Skull-Base Invasion in Nasopharyngeal Carcinoma With Skull-Base Invasion After Intensity-Modulated Radiotherapy. Radiat Oncol (London England) (2019) 14(1):10. doi: 10.1186/s13014-019-1214-3
29. Chen YP, Tang LL, Zhang WN, Mao YP, Chen L, Sun Y, et al. Prognostic Value and Grading of MRI-Based T Category in Patients With Nasopharyngeal Carcinoma Without Lymph Node Metastasis Undergoing Intensity-Modulated Radiation Therapy. Medicine (2015) 94(43):e1624. doi: 10.1097/MD.0000000000001624
30. Wong KCW, Hui EP, Lo KW, Lam WKJ, Johnson D, Li L, et al. Nasopharyngeal Carcinoma: An Evolving Paradigm. Nat Rev Clin Oncol (2021) 18(11):1–17. doi: 10.1038/s41571-021-00524-x
31. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and Radiotherapy in Nasopharyngeal Carcinoma: An Update of the MAC-NPC Meta-Analysis. Lancet Oncol (2015) 16(6):645–55. doi: 10.1016/s1470-2045(15)70126-9
32. Chen YP, Tang LL, Yang Q, Poh SS, Hui EP, Chan ATC, et al. Induction Chemotherapy Plus Concurrent Chemoradiotherapy in Endemic Nasopharyngeal Carcinoma: Individual Patient Data Pooled Analysis of Four Randomized Trials. Clin Cancer Res (2018) 24(8):1824–33. doi: 10.1158/1078-0432.CCR-17-2656
33. Wu M, Li X, Zhang T, Liu Z, Zhao Y. Identification of a Nine-Gene Signature and Establishment of a Prognostic Nomogram Predicting Overall Survival of Pancreatic Cancer. Front Oncol (2019) 9:996. doi: 10.3389/fonc.2019.00996
34. Xie C, Li H, Yan Y, Liang S, Li Y, Liu L, et al. A Nomogram for Predicting Distant Metastasis Using Nodal-Related Features Among Patients With Nasopharyngeal Carcinoma. Front Oncol (2020) 10:616. doi: 10.3389/fonc.2020.00616
35. Chen S, Qiu A, Tao Z, Zhang H. Clinical Impact of Cardiovascular Disease on Patients With Bronchiectasis. BMC Pulm Med (2020) 20(1):101. doi: 10.1186/s12890-020-1137-7
36. Chen C, Wu JB, Jiang H, Gao J, Chen JX, Pan CC, et al. A Prognostic Score for Nasopharyngeal Carcinoma With Bone Metastasis: Development and Validation From Multicenter. J Cancer (2018) 9(5):797–806. doi: 10.7150/jca.22663
Keywords: nasopharyngeal carcinoma, skull base invasion, bone metastasis, bone metastasis-free survival, prediction model, nomogram, intensity modulated radiation therapy
Citation: Wu B, Guo Y, Yang H-h, Gao Q-g and Tian Y (2022) Predicting Bone Metastasis Risk Based on Skull Base Invasion in Locally Advanced Nasopharyngeal Carcinoma. Front. Oncol. 12:812358. doi: 10.3389/fonc.2022.812358
Received: 10 November 2021; Accepted: 16 March 2022;
Published: 07 April 2022.
Edited by:
Steffi Ulrike Pigorsch, Technical University of Munich, GermanyReviewed by:
Victor Lewitzki, University Hospital Würzburg, GermanyCopyright © 2022 Wu, Guo, Yang, Gao and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Tian, ZHJ5ZXRpYW5AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.