
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 22 April 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.810096
 Xiaoyuan Qian1
Xiaoyuan Qian1 Jinzhou Xu1
Jinzhou Xu1 Chenqian Liu1
Chenqian Liu1 Mingliang Zhong1
Mingliang Zhong1 Senyuan Hong1
Senyuan Hong1 Can Qian2
Can Qian2 Jianning Zhu3
Jianning Zhu3 Jiaqiao Zhang1*†
Jiaqiao Zhang1*† Shaogang Wang1*†
Shaogang Wang1*†Objective: Renal collecting duct carcinoma (CDC) is an extremely rare disease with few studies, and the current understanding of its prognosis is limited. We used the Surveillance, Epidemiology, and End Results (SEER) registry data to explore the prognostic factors and effect of treatment modalities on the overall survival (OS) and cancer-specific survival (CSS) in patients with CDC.
Methods: Patients’ information of CDCs diagnosed by pathological examination between 2000 and 2018 was extracted from the SEER database. The Kaplan–Meier method was used to calculate OS and CSS and log-rank tests to evaluate the differences in OS and CSS. The associations between clinicopathological variables and survival outcomes were assessed with the Cox proportional hazard model. A directed acyclic graph (DAG) was drawn to recognize confounding factors and to obtain the multivariable regression model, and the impact of surgery, radiotherapy, and chemotherapy on OS and CSS was analyzed, respectively.
Results: A total of 242 patients with CDC were enrolled. The median OS and CSS time were 17 and 21 months, respectively. The OS rates at 1, 2, and 5 years were 56.9%, 41.9%, and 30.0%, respectively, while the CSS rates at 1, 2, and 5 years were 60.1%, 47.5%, and 34.8%, respectively. Patients who had a large tumor size, poor pathological grade, and advanced TNM classification exhibited worse survival outcomes. Univariable and multivariable Cox regression analyses revealed that surgery, chemotherapy, T stage, N stage, and M stage were independent prognostic factors for OS and CSS. The DAG-guided multivariate Cox regression model revealed that surgery and chemotherapy improved OS and CSS.
Conclusions: CDC is an exceedingly rare disease and has malignant behavior. Most patients have a high pathological grade and advanced TNM stage at diagnosis and exhibited poor survival. Resection of all visible tumors including metastatic lesions or chemotherapy can be beneficial to prognosis, while healthier benefits are less likely to receive radiotherapy. More relevant studies with larger samples are needed to verify the value of surgery and adjuvant therapy in the treatment of CDCs.
Collecting duct carcinoma (CDC) is a rare subtype of renal cell carcinoma (RCC), which is a malignant renal epithelial tumor, originating from the principal cells of the distal segment of the renal medullary collecting duct (1, 2). CDCs account for less than 2% of RCC and display aggressive features and poor prognosis (3–6). At the early stages of CDCs, the typical clinical symptoms and specific biomarkers are lacking and the majority of patients are found and diagnosed at the advanced TNM stage (6–8), which may result in a worse survival. Owing to the aggressive behavior of CDCs—a high incidence of distant metastasis and high pathological grade—early and accurate preoperative or postoperative diagnosis of CDCs is of importance for treatment strategies to improve the outcome of the patients with CDCs.
Robust studies about surgical treatment and prognostic factors of CDCs are lacking. Although some studies based on public databases have verified relevant prognostic factors affecting survival (4, 9, 10), the effect of different treatment modalities on outcomes of CDC was not clarified. Recently, Tang et al. reported the clinical characteristics and survival of patients with CDC. However, they failed to report oncologic outcomes for overall survival (OS) and explore the effect of different treatment methods on outcomes in detail. Therefore, we used data derived from the Surveillance, Epidemiology, and End Results (SEER) database to investigate the impact of surgery, radiotherapy, or chemotherapy, on OS and CSS respectively, using the DAG-guided multivariate Cox regression model. The clinical feature, survival outcomes, and independent prognostic factors of OS and CSS were also revealed.
Much information on cancer incidence and survival outcomes was collected in the SEER database, and about 35% of the US population was covered (11). By running the SEER*Stat 8.3.9.2 software (https://seer.cancer.gov/seerstat/), the “Incidence-SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018)” database was linked and the clinical and follow-up data of patients diagnosed with CDCs by postoperative pathology from 2000 to 2018 were retrieved and exported. According to the code of ICD-0-3: 8319/3: Collecting duct carcinoma, the patients were recruited into this study. The inclusion/exclusion criteria for patients were as follows: (1) survival data of patients were complete and available; (2) diagnosis was confirmed by histology; (3) the year of diagnosis was between 2000 and 2018; (4) laterality only contained left or right; (5) patient information came from the medical institution; (6) in terms of surgery, patients only receiving tumor destruction were not considered; and (7) cases with incomplete clinical information related to the study were excluded. Finally, a total of 242 patients with CDCs who met the criteria were recruited in this study.
Information of demographic characteristics including age, sex, and race was obtained. The data of tumor features containing tumor size, TNM stage, pathological grade, and laterality of tumor were also chosen. Next, the treatment strategies of CDCs which consisted of surgery, chemotherapy, or radiotherapy were extracted. Radiotherapy and chemotherapy were categorized into “no/unknown” or “yes.” Notably, the intent of radiotherapy for these cases is unclear and a significant proportion of patients were probably given radiotherapy for palliation. Additionally, OS was calculated from the date of diagnosis confirmed to any cause of death, regarded as the primary survival outcome. Additionally, CSS was defined as the time from the date of diagnosis to the date of death caused by CDCs.
All statistical analyses were performed, using R 3.6.3 software (The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables with a normal distribution were described as mean ± standard deviation (SD) while clinical characteristics conforming to the skewed distribution were exhibited by the median and interquartile range (IQR). Categorical variables were recorded in numbers and percentages during descriptive statistics. The Kaplan–Meier method was used to conduct survival curves, and log-rank tests were applied to compare differences between survival curves. The univariable and multivariable Cox proportional hazards regression model was used to analyze independent prognostic factors affecting OS and CSS. Moreover, under the guidance of DAG, three different multivariable regression models were constructed to explore the impact of surgery, chemotherapy, and radiotherapy on OS and CSS, respectively. p < 0.05 (two-sided) was considered to be statistically significant.
A total of 242 patients diagnosed with CDCs were enrolled in this study, and their baseline clinicopathological features are shown in Supplementary Table S1. Of the total patients, there were 71 (29.3%) females and 171 (70.7%) males. In terms of age, 161 (66.5%) of patients were less than 68 years old and 81 (33.5%) were more than 68 years old. The included population mainly consisted of 60 (24.8%) black, 166 (68.6%) white, and other races accounting for only 16 (6.6%).
The maximum tumor diameter ranged from 4.1 to 8.5 cm, and the median tumor size was 6.3 cm. According to the TNM stage system, 77 (31.8%) cases were in T1, 16 (6.6%) in T2, 121 (50.0%) in T3, and 21 (8.7%) in T4. Respectively, 94 (38.9%) and 86 (35.5%) patients had clinical lymph node metastasis and/or distant metastases at presentation. Based on the AJCC 6th edition staging system for renal carcinoma, there were 57 (23.6%) stage I, 11 (4.6%) stage II, 57 (23.6%) stage III, and 111 (45.9%) stage IV tumors, while pathological grade revealed that 3.72%, 10.7%, 38.0%, and 26.4% of the patients were in stages I, II, III, and IV disease, respectively. During the treatment, most patients (85.1%) with CDCs underwent surgical resection and the remaining patients (14.9%) received conservative treatment. However, only a few patients received radiotherapy or chemotherapy, accounting for 25 (10.3%) and 64 (26.4%), respectively.
To investigate the impact of clinical factors and treatment methods on OS and CSS, the Kaplan–Meier method and log-rank test were used. The median follow-up time of all patients was 17.0 months (IQR: 5.0–68.0 months). The overall 1-, 2-, and 5-year survival rates were 56.9%, 41.9%, and 30.0%, respectively, and for CSS, its 1-, 2-, and 5-year survival rates were 60.1%, 47.5%, and 34.8%, respectively. Patients with larger tumor sizes had shorter survival (median OS: 11 vs. 25 months, p < 0.001, and median CSS: 11 vs. 32 months, p < 0.001) than those with smaller tumor sizes (Supplementary Figures S1A and S2A). The survival rates were also affected by pathological grade. The median survival times of grades I, II, III, and IV for OS were 134, 97, 13.5, and 17.5 months, respectively (the total p < 0.001; I vs. IV, p = 0.017; II vs. III, p = 0.016; II vs. IV, p < 0.001) (Supplementary Figure S1B), for CSS not reached, not reached, 15 months, and 18 months (the total p < 0.001; I vs. III, p = 0.013; I vs. IV, p = 0.002; II vs. III, p = 0.002; II vs. IV, p < 0.001) (Supplementary Figure S2B). Similarly, tumors with a high AJCC stage were not good for the survival time of CDCs. The findings demonstrated that the median survival times of stages I, II, III, and IV for OS were 115, 91, 22, and 7 months, respectively (the total p < 0.001; I vs. III, p < 0.001; I vs. IV, p < 0.001; II vs. IV, p < 0.001; III vs. IV, p < 0.001) (Supplementary Figure S1C), for CSS not reached, not reached, 26 months, and 7 months (the total p < 0.001; I vs. III, p < 0.001; I vs. IV, p < 0.001; II vs. III, p = 0.041; II vs. IV, p < 0.001; III vs. IV, p < 0.001) (Supplementary Figure S2C). Besides, patients with the late T stage had a lower survival rate than those with the early T stage. For OS, 81, 56, 11, and 10 months were respectively matched to the median survival times of T1, T2, T3, and T4 (the total p < 0.001; T1 vs. T3, p < 0.001; T1 vs. T4, p < 0.001; T2 vs. T3, p = 0.030; T2 vs. T4, p = 0.007) (Supplementary Figure S1D). For CSS, the median survival times of T1, T2, T3, and T4 were 115, 91, 15, and 10 months, respectively (the total p < 0.001; T1 vs. T3, p < 0.001; T1 vs. T4, p < 0.001; T2 vs. T3, p = 0.013; T2 vs. T4, p = 0.003) (Supplementary Figure S2D).
Lymph node metastasis can result in worse survival. The median survival times of N0, N1, and N2 for OS were 42, 7, and 9 months, respectively (the total p < 0.001; N0 vs. N1 p < 0.001; N0 vs. N2, p < 0.001) (Supplementary Figure S1E), and 95, 9, and 9 months corresponded to the median survival times of N0, N1, and N2 for CSS (the total p < 0.001; N0 vs. N1 p < 0.001; N0 vs. N2, p = 0.001) (Supplementary Figure S2E). Compared with tumors with distant metastasis, patients without distant metastasis displayed better OS (median OS:41 vs. 5 months, p < 0.001) (Supplementary Figure S1F) and CSS (median CSS: 95 vs. 6 months, p < 0.001) (Supplementary Figure S2F). Patients who underwent surgery had longer survival than those who did not (median OS: 22 vs. 4 months, p < 0.001, and median CSS: 27 vs. 4 months, p < 0.001) (Supplementary Figures S1G and S2G). Interestingly, patients who underwent radiotherapy had a worse survival than those who did not (median OS: 7 vs. 20 months, p < 0.001, and median CSS: 10 vs. 25 months, p = 0.001) (Supplementary Figures S1H and S2H). Furthermore, patients who received chemotherapy had shorter survival than those who did not (median OS: 12.5 vs. 21 months, p < 0.001, and median CSS: 14 vs. 32 months, p < 0.001) (Supplementary Figures S1I and S2I).
Since most patients receiving chemotherapy and radiotherapy were in stage IV, we included these patients for subgroup analysis. Patients who underwent surgery had better survival than those who did not (median OS: 9 vs. 4 months, p < 0.001, and median CSS: 10 vs. 4 months, p < 0.001) (Figures 1A, D). However, compared with a patient who did not receive chemotherapy, patients receiving chemotherapy had a longer survival (median OS: 9 vs. 5 months, p = 0.008, and median CSS: 11 vs. 5 months, p = 0.02) (Figures 1B, E). Meanwhile, patients who underwent radiotherapy had the same survival as those who did not undergo radiotherapy (median OS: 7 vs. 7 months, p = 0.95, and median CSS: 10 vs. 7 months, p = 0.51) (Figures 1C, F).
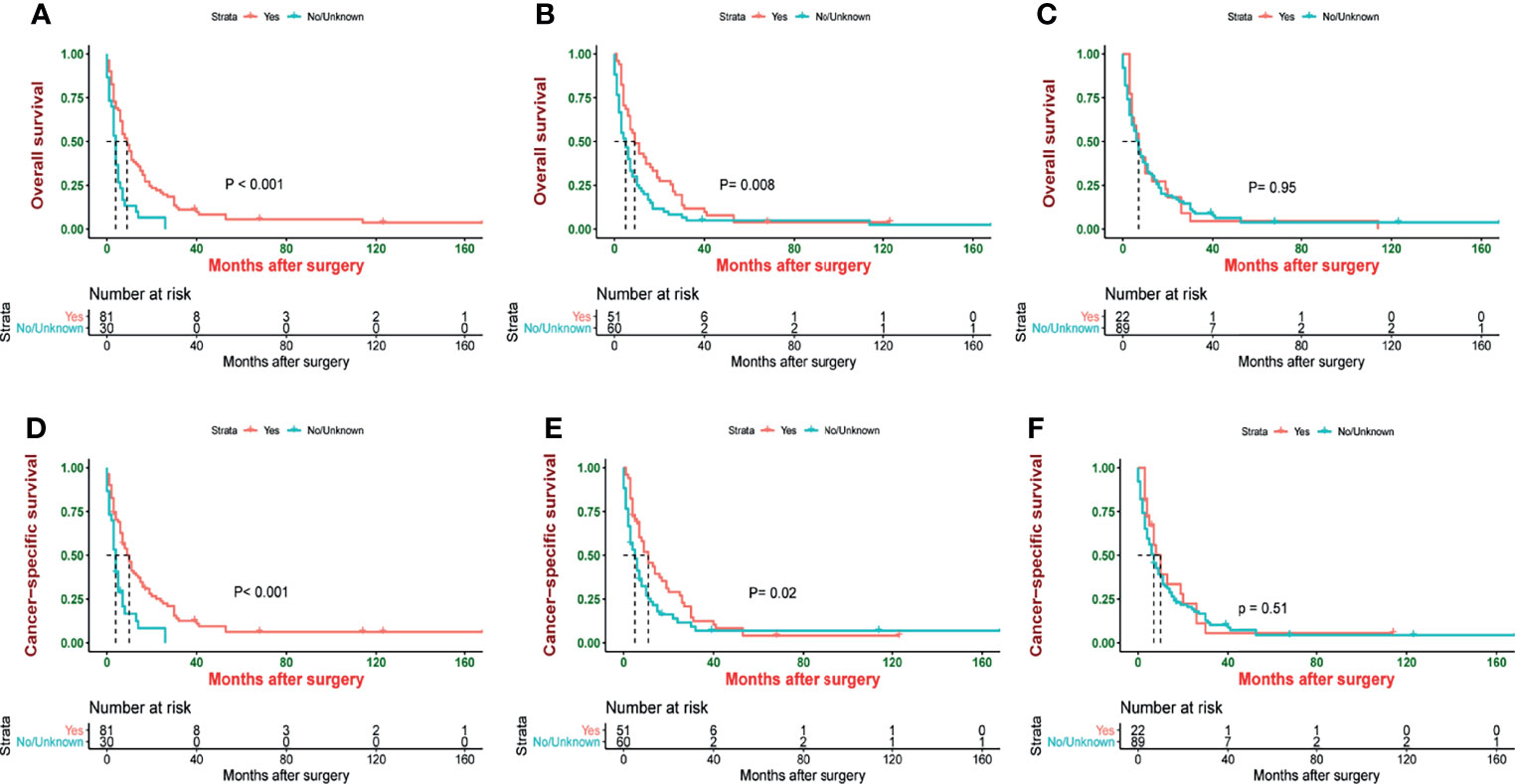
Figure 1 Kaplan–Meier estimate of overall survival (OS) and cancer-specific survival (CSS) stage IV patients by (A) surgery, (B) chemotherapy, (C) radiotherapy, (D) surgery, (E) chemotherapy, and (F) radiotherapy.
The Cox regression models were used to recognize the independent predictors of OS and CSS. By univariate analysis, the result showed that tumor size, pathological grade, AJCC stage, T stage, N stage, M stage, surgery, radiotherapy, and chemotherapy were closely related to OS and CSS (p < 0.05). All factors with statistical significance (p < 0.05) in univariate analysis were selected and submitted to multivariable Cox regression model analysis. Next, we detected multicollinearity between these variables and found that the variance inflation factors of the AJCC stage which was included in the model were more than 5. Therefore, the factor of the AJCC stage was not enrolled in the multivariable Cox regression model. In multivariate analysis, advanced T stage (T3 vs. T1 OS: HR: 1.896, 95% CI: 1.128–3.178; p = 0.016; CSS: HR: 2.105, 95% CI: 1.181–3.752; p = 0.012), N stage (N1 vs. N0: OS: HR: 2.105, 95% CI: 1.279–3.464; p = 0.003; CSS: HR: 2.093, 95% CI: 1.233–3.551; p = 0.006), M stage (OS: HR: 4.726, 95% CI: 2.843–7.856; p < 0.001; CSS: HR: 4.864, 95% CI: 2.850–8.299; p < 0.001), surgery (OS: HR: 4.526, 95% CI: 2.070–9.897; p < 0.001; CSS: HR: 4.844, 95% CI: 2.113–11.104; p < 0.001), chemotherapy (OS: HR: 3.154, 95% CI: 1.896–5.248; p < 0.001; CSS: HR: 2.871, 95% CI: 1.682–4.899; p < 0.001) remained significant prognostic factors for OS and CSS. The above findings of the Cox regression analysis of prognostic factors for OS and CSS are presented in Tables 1, 2, respectively.
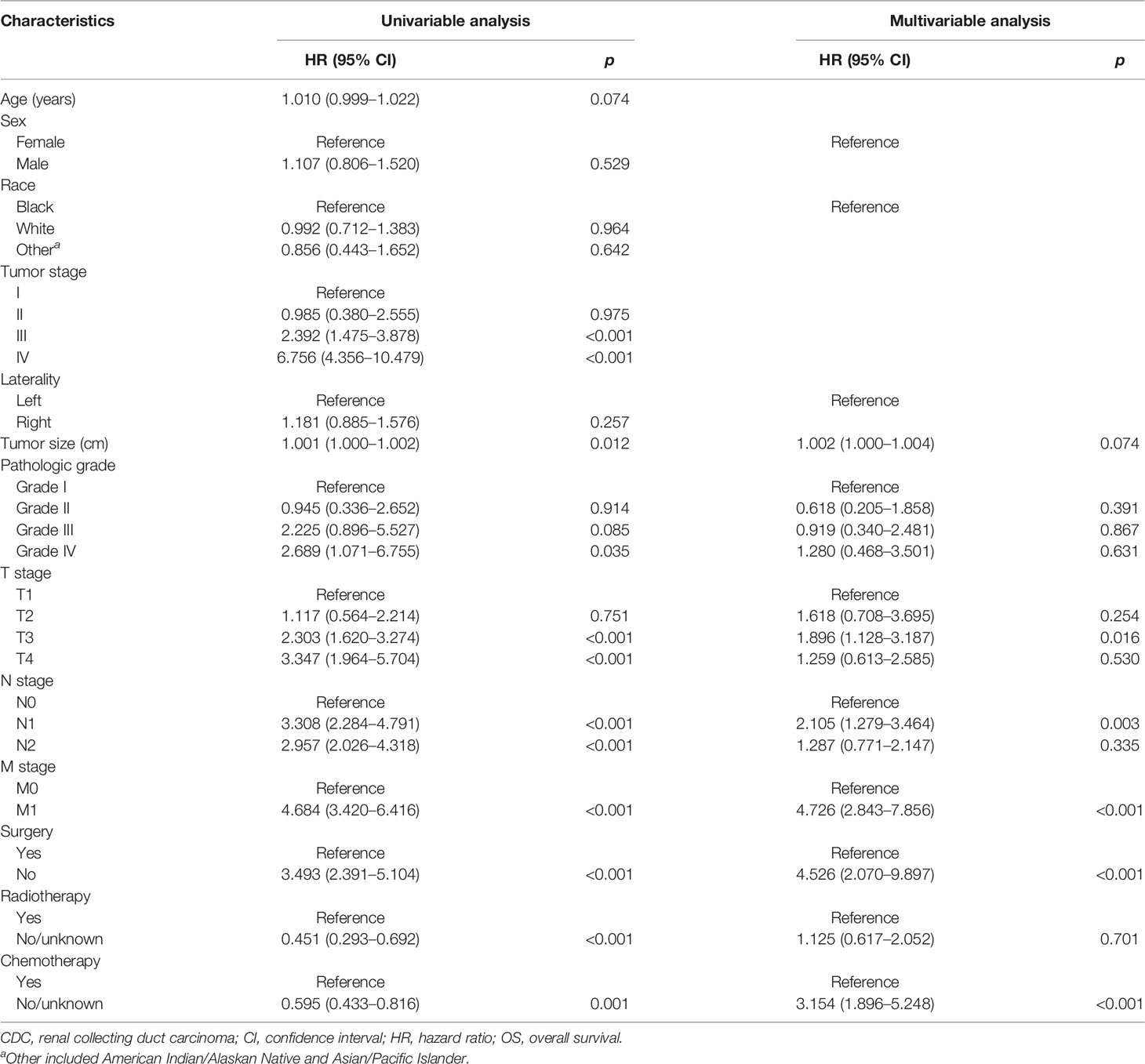
Table 1 Univariate and multivariate Cox regression analysis of the associations between clinicopathological features and OS in patients with CDCs.
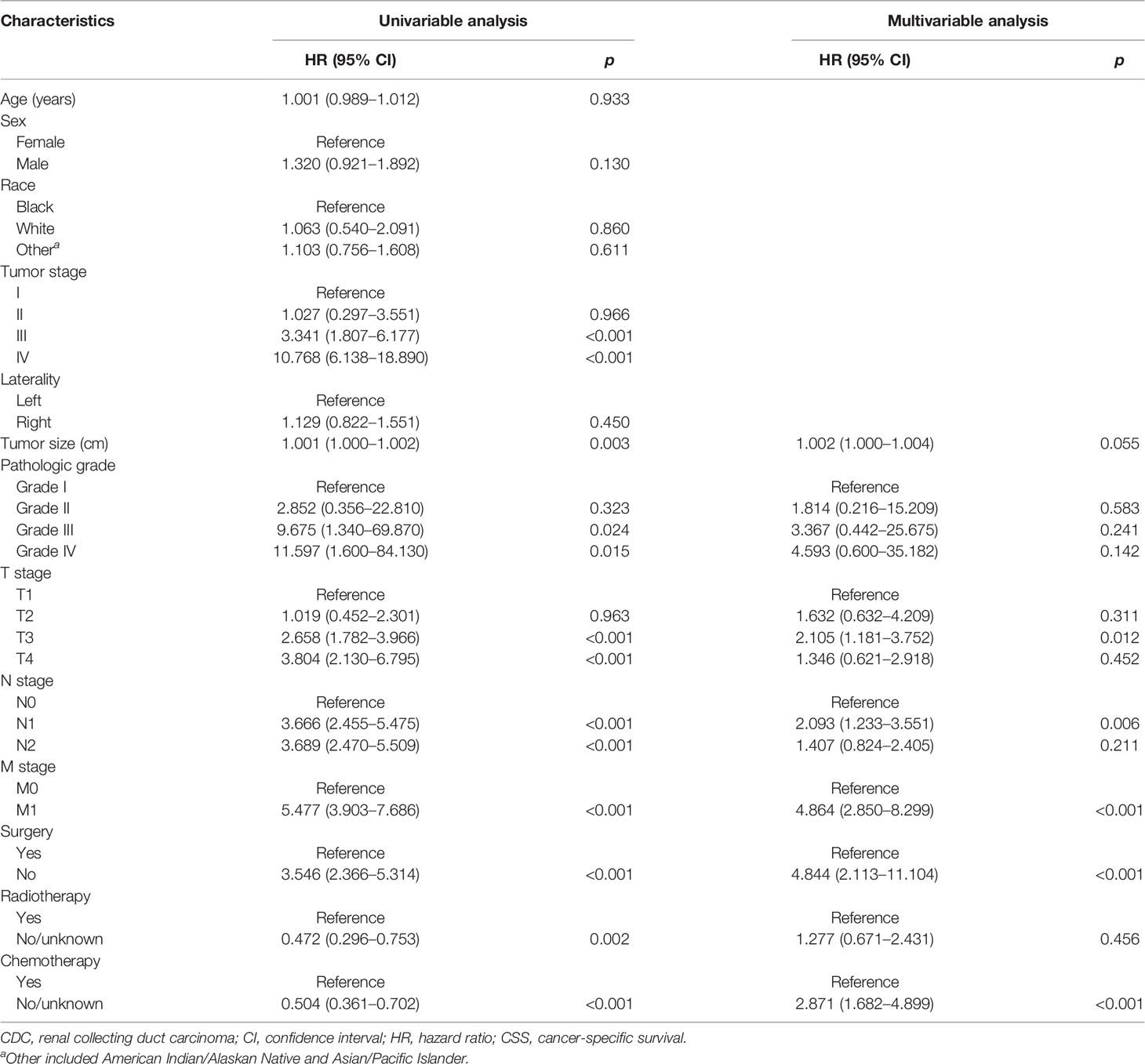
Table 2 Univariate and multivariate Cox regression analysis of the associations between clinicopathological features and CSS in patients with CDCs.
In an attempt to explore the influence of surgery, radiotherapy, and chemotherapy on OS and CSS, DAG was drawn to elucidate that the structure of the causal relation between surgery and survival outcomes (OS and CSS), chemotherapy and survival outcomes, or radiotherapy and survival outcomes, respectively, and the confounding factor in the Cox regression model were identified correctly. In the light of the relationship between each exposure factor and outcomes, a total of three DAGs were produced to direct the multivariable regression analysis model. When surgery was deemed as the main exposure factor, chemotherapy and radiotherapy as intermediate variables should be excluded from the Cox regression model (Supplementary Figure S3). The result of the multivariable analysis directed by DAGs showed that surgery was associated with OS (HR: 3.300, 95% CI: 1.568–6.946; p = 0.004) and CSS (HR: 3.398, 95% CI: 1.555–7.425; p = 0.002) (Table 3). However, chemotherapy as a confounding factor was chosen as an exposure factor, surgery was not excluded, and radiotherapy as an ancestor of outcome was excluded (Supplementary Figure S4). The DAGs which guided the multivariate regression model demonstrated that chemotherapy was associated with OS (HR: 2.918, 95% CI: 1.750–4.868; p < 0.001) and CSS (HR: 2.747: 95% CI: 1.604–4.703; p < 0.001) (Table 4). Additionally, if the exposure factor was radiotherapy, surgery seen as a confounding variable factor was also not excluded and chemotherapy as an ancestor of outcome was not included (Supplementary Figure S5). Finally, the findings presented that radiotherapy was not associated with OS (HR: 1.139, 95% CI: 0.613–12.115; p = 0.680) and CSS (HR: 1.292, 95% CI: 0.667–2.501; p = 0.448) (Table 5).
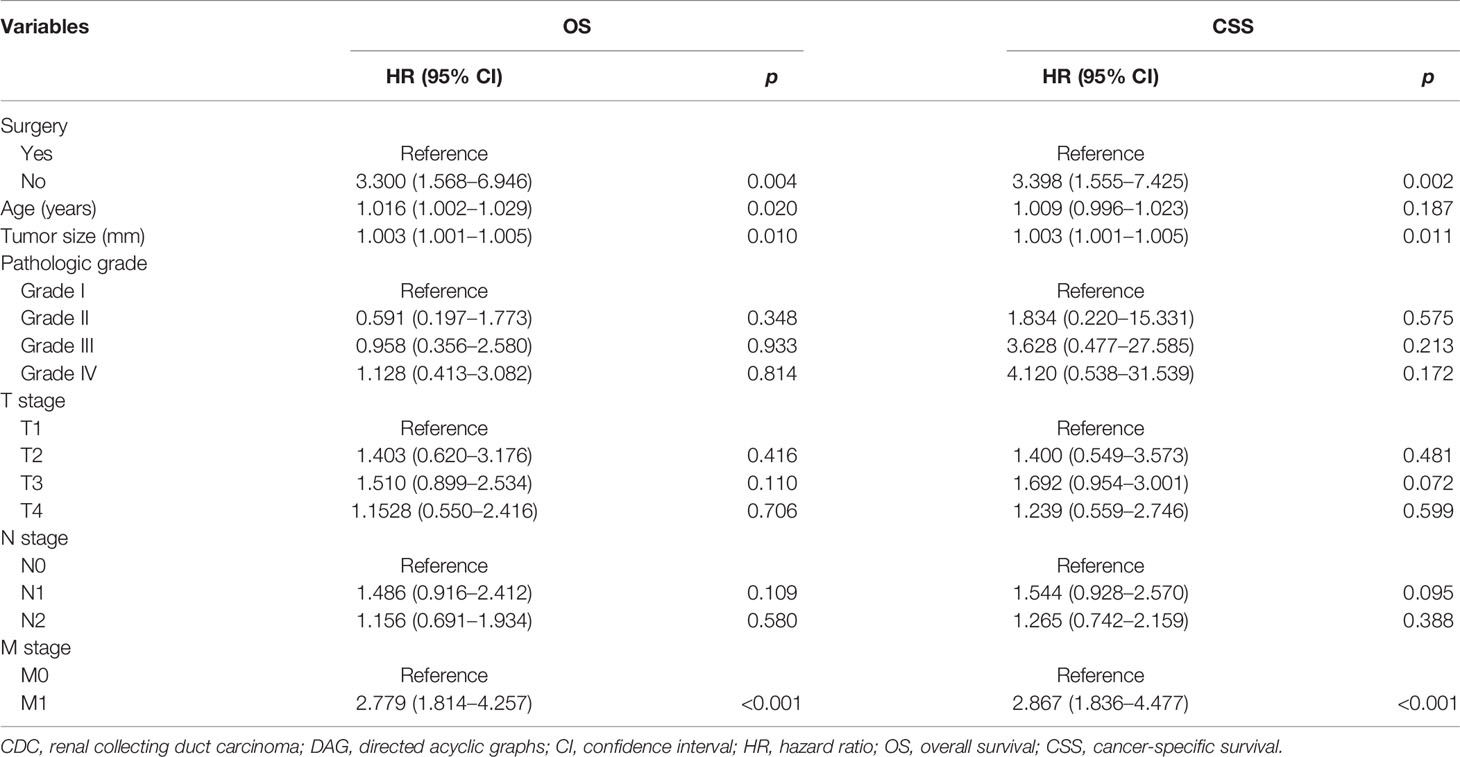
Table 3 DAG-guided multivariable Cox regression model analysis of causal effect of surgery on OS and CSS.
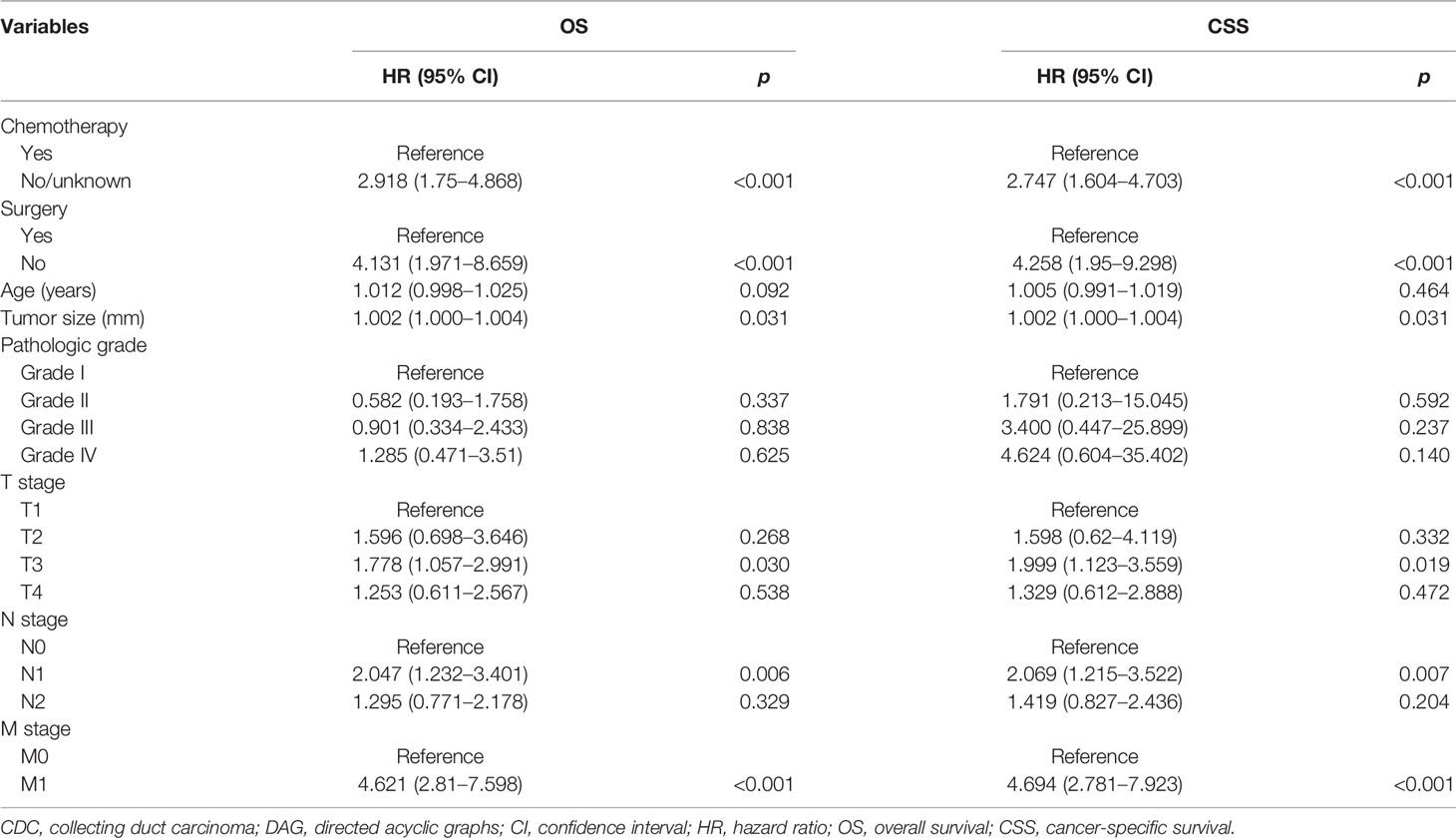
Table 4 DAG-guided multivariable Cox regression model analysis of causal effect of chemotherapy on OS and CSS.
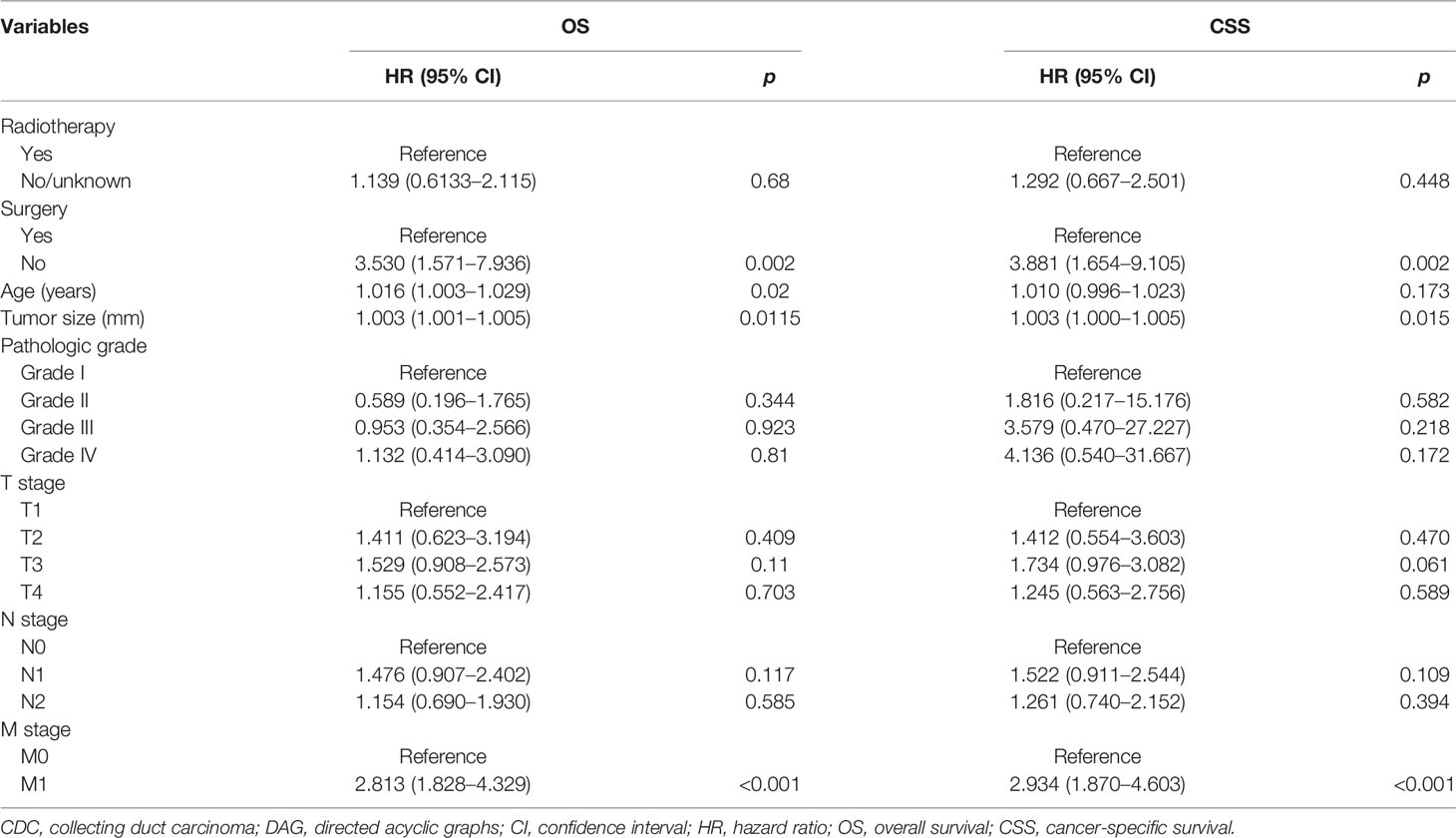
Table 5 DAG-guided multivariable Cox regression model analysis of causal effect of radiotherapy on OS and CSS.
CDC, also called Bellini duct carcinoma, is a very rare subtype of renal malignancies and presents an aggressive clinical course, with low incidence and worse outcomes (6, 10). Currently, most of the works mainly focus on a case report and case series reports and systematic studies with large sample cases are lacking. How to recognize CDC early, make the correct diagnosis, and make appropriate treatment strategies is extremely important to improve the prognosis of CDCs. Additionally, standard treatment strategies are still not available. Most patients with CDC received adjuvant radiotherapy, chemotherapy, and immunotherapy after surgery including partial nephrectomy (PN), radical nephrectomy (RN), and cytoreductive nephrectomy (CNx). There exist several questions that need to be addressed during the treatment. As we know, patients with cT1−2/N0M0 clear cell RCC receiving PN or RN can obtain equivalent oncologic outcomes (12). Doubtfully, whether patients with T1−2/N0M0 CDC receiving surgery can get similar oncologic outcomes needs to be resolved. For metastatic CDC, whether CNx benefits the patient is urgent to be illuminated. Moreover, whether all patients with CDC should receive adjuvant therapy is questioned. Here, we obtained the data from the SEER database to describe the clinical characteristics of the CDC and demonstrated its independent risk factors. Moreover, we verified the impact of different treatment methods on the survival of patients with CDC. Our findings suggested that CDCs have malignant behavior and that resection of all visible tumors or chemotherapy is significantly associated with outcomes. For patients with advanced CDC, no correlation between radiotherapy and outcomes is seen.
In the present study, we reconfirmed that CDCs showed some aggressive behavioral characteristics, and patients with CDCs had a poor prognosis. Many studies have reported that the majority of patients had high pathological grade, advanced T stage, positive lymph node, and distant metastasis at diagnosis and these factors were the independent predictor of CDCs. In our previous study (6), most patients had obvious invasive pathologic features and half of the patients had distant metastasis. They all had short survival times. Similar results can also be seen in previous studies. In a study published by Karakiewicz and his colleagues, T3 or higher accounted for 80.5%, positive node for 48.8%, and metastatic disease for 19.5% at diagnosis. In 78.0% of patients, their Fuhrman grades were grade III or higher, and the 5-year CSS for CDCs was 48% (3). Another large multi-institutional cohort from Japan revealed that more than 50% of patients had a late T stage and 97.8% of patients had a poor Fuhrman grade and that disease-specific survival was 34.3% (8). Similarly, the OS rate reduced to 26.8% in the recent work based on the SEER database (9). The authors discovered that CDC presented more often with T3 (52.8%), node-positive (40.6%), and metastatic (42.0%) diseases. An early study from 16 European and American institutions also reported that the 5-year CSS rate for CDCs was 40.3% (13). Compared to these works, our findings demonstrate the same incidence of late T stage, lymph node positive, or metastatic disease. Furthermore, the OS and CSS at 5 years were 30.0% and 34.8%, respectively, which were inferior to the survival time of RCC (14). Moreover, more than 50% of the patients with CDC died within 5 years after diagnosis and treatment and a poor prognosis was discovered. Additionally, older age, larger tumor size, late T stage, positive node, distant metastasis, poorly Fuhrman grade, and lymphovascular invasion were closely associated with worse survival outcomes (4, 10, 13), which was similar to our results. In our study, larger tumor size, advanced TNM stage, late AJCC stage, and poor pathological grade exhibited an extremely detrimental prognosis. Finally, the above factors remained independent prognosis factors for OS and CSS in the multivariable regression analysis model.
For the treatment of CDC, patients with CDC could benefit from surgical treatment and chemotherapy. When we included patients in all stages for the survival analysis, patients who received chemotherapy and radiotherapy displayed shorter survival than those who did not. However, in the next subgroup analysis, we found that patients with advanced CDC who underwent chemotherapy had longer survival than those who did not, while patients with advanced CDC who received radiotherapy had the same survival time as those who did not. The reason for this phenomenon is that for most patients receiving chemotherapy or radiotherapy, their intent of chemotherapy or radiotherapy was likely palliative and these patients themselves had a poorer prognosis than those in the earlier stages. Although no explicit treatment strategy is established, there is no doubt that surgery is still the primary treatment. Generally, patients with CDCs after surgery can obtain a longer survival (4, 10, 15). Consistent results were presented in our study. Additionally, our study revealed that advanced CDC patients benefited from surgery. In terms of surgical methods, at present, RN is suggested if tumors are suspected to be CDC before surgery for their malignant biological behavior. A minority of patients with low-stage and low-grade receiving RN did not show signs of tumor progression, suggesting that RN during the treatment of CDCs can be effective and curative (6, 16). Certainly, a few patients with early TNM stage achieved better outcomes after nephron-sparing surgery (17). In clinical practice, the surgical strategies often depend upon complicating factors such as preoperative patient status, surgical risks, survival outcomes, and distant metastasis (18). Thus, for the patient with node or distant metastasis or unable to receive CNx, adjuvant therapy may play an important role in improving the survival of CDCs. Wilson and colleagues reported that CNx combined with chemo/radiotherapy or chemo/radiotherapy alone was associated with a survival benefit over a single CNx in patients with CDCs, indicating the potential benefit for combination treatment (4). A partial response or complete remission acquired in patients receiving the therapy of chemotherapeutic agents (gemcitabine and either cisplatin or carboplatin) was discovered in previous studies (8, 18–21). Nevertheless, the only slight improvement in OS has been revealed, and progression of tumor and failure of first-line therapy was often observed in CDCs (22). Different from chemotherapy, although radiotherapy was found to play a certain role in delaying tumor progression reported in Wilson and colleagues’ study (4), the benefit from radiotherapy in the treatment of CDCs has not been unfolded in other relevant works (8, 10). In this study, most of the patients in stage IV received radiotherapy and the effect of radiotherapy on the survival of patients was not seen. Furthermore, we used DAG that can explicitly exclude irrelevant variables to enroll the real confounding factors into the Cox regression model to analyze the impact of surgery, chemotherapy, and radiotherapy, respectively, on the survival of CDCs. Finally, we found that surgery and chemotherapy were beneficial to prognosis, while healthier benefits are less likely to receive radiotherapy.
Undoubtedly, there exist several limitations in the present study. First, despite the data we used in this study from the SEER database, the number of cases included is still small. Second, this study belongs to a retrospective study and potential selection bias is inevitable. Third, chemotherapy schedule and administration time are unclear and the effect of specific chemotherapeutic drugs on the survival of CDCs remains to be studied. Fourth, the type of surgery is unknown and the role of NSS and RN in the treatment of patients with early CDCs is also folded. Equally importantly, the intent of radiotherapy—palliative or curative—is not known and the value of radiotherapy is not clear. Additionally, because pathological sections are not centrally re-confirmed by professional pathologists, CDC is easily misdiagnosed as others, including medullary carcinoma and FH-deficient RCC. These defects are unavoidable. However, this study is of tremendous value to help clinicians comprehend the prognosis of CDCs and make the right treatment strategies for this tumor.
CDC is an extremely rare renal carcinoma, with an invasive biological behavior. Most patients have a high pathological grade and advanced TNM stage at diagnosis and exhibited poor survival. Larger tumor size, advanced TNM stage, later AJCC stage, and higher pathological grade may indicate an extremely detrimental prognosis. Healthier benefits are more likely to undergo surgery or chemotherapy than more comorbid ones. Certainly, to make systemic therapeutic options for this tumor, long-term large-scale prospective trials are necessary.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
XYQ, JQZ and SGW conceived and designed the study. JZX, and CQL collected and assembled the data. XYQ, MLZ, and SYH, contributed to data processing, interpretation of results, and drafting. XYQ, JNZ, and CQ critically revised the manuscript. All authors read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Surveillance, Epidemiology, and End Results (SEER) Program for providing the data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.810096/full#supplementary-material
CDC, renal collecting duct carcinoma; SEER, Surveillance, Epidemiology, and End Results; OS, overall survival; CSS, cancer-specific survival; DAG, directed acyclic graphs; IQR, interquartile range; HR, hazard ratio; CI, confidence interval; Cox, proportional hazards regression model; TNM, tumor node metastasis; RN, radical nephrectomy; CNx, cytoreductive nephrectomy.
1. Seo AN, Yoon G, Ro JY. Clinicopathologic and Molecular Pathology of Collecting Duct Carcinoma and Related Renal Cell Carcinomas. Adv Anat Pathol (2017) 24(2):65–77. doi: 10.1097/PAP.0000000000000138
2. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol (2016) 70(1):93–105. doi: 10.1016/j.eururo.2016.02.029
3. Karakiewicz PI, Trinh QD, Rioux-Leclercq N, de la Taille A, Novara G, Tostain J, et al. Collecting Duct Renal Cell Carcinoma: A Matched Analysis of 41 Cases. Eur Urol (2007) 52(4):1140–5. doi: 10.1016/j.eururo.2007.01.070
4. Sui W, Matulay JT, Robins DJ, James MB, Onyeji IC, RoyChoudhury A, et al. Collecting Duct Carcinoma of the Kidney: Disease Characteristics and Treatment Outcomes From the National Cancer Database. Urol Oncol (2017) 35(9):540 e13– e18. doi: 10.1016/j.urolonc.2017.04.010
5. Saad AM, Gad MM, Al-Husseini MJ, Ruhban IA, Sonbol MB, Ho TH. Trends in Renal-Cell Carcinoma Incidence and Mortality in the United States in the Last 2 Decades: A SEER-Based Study. Clin Genitourinary Cancer (2019) 17(1):46–57 e5. doi: 10.1016/j.clgc.2018.10.002
6. Qian X, Wang Z, Zhang J, Wang Q, Zhou P, Wang S, et al. Clinical Features and Prognostic Outcome of Renal Collecting Duct Carcinoma: 12 Cases From a Single Institution. Cancer Manag Res (2020) 12:3589–95. doi: 10.2147/CMAR.S244094
7. Gupta R, Billis A, Shah RB, Moch H, Osunkoya AO, Jochum W, et al. Carcinoma of the Collecting Ducts of Bellini and Renal Medullary Carcinoma: Clinicopathologic Analysis of 52 Cases of Rare Aggressive Subtypes of Renal Cell Carcinoma With a Focus on Their Interrelationship. Am J Surg Pathol (2012) 36(9):1265–78. doi: 10.1097/PAS.0b013e3182635954
8. Tokuda N, Naito S, Matsuzaki O, Nagashima Y, Ozono S, Igarashi T. Collecting Duct (Bellini Duct) Renal Cell Carcinoma: A Nationwide Survey in Japan. J Urol (2006) 176(1):40–3. doi: 10.1016/s0022-5347(06)00502-7
9. Pepek JM, Johnstone PA, Jani AB. Influence of Demographic Factors on Outcome of Collecting Duct Carcinoma: A Surveillance, Epidemiology, and End Results (SEER) Database Analysis. Clin Genitourinary Cancer (2009) 7(2):E24–7. doi: 10.3816/CGC.2009.n.017
10. Tang C, Zhou Y, Ge S, Yi X, Lv H, Zhou W. Incidence, Clinical Characteristics, and Survival of Collecting Duct Carcinoma of the Kidney: A Population-Based Study. Front Oncol (2021) 11:727222. doi: 10.3389/fonc.2021.727222
11. Shi S, Xie H, Yin W, Zhang Y, Peng X, Yu F, et al. The Prognostic Significance of the 8th Edition AJCC TNM Staging System for non-Small-Cell Lung Cancer is Not Applicable to Lung Cancer as a Second Primary Malignancy. J Surg Oncol (2020) 121(8):1233–40. doi: 10.1002/jso.25903
12. Simone G, Tuderti G, Anceschi U, Papalia R, Ferriero M, Misuraca L, et al. Oncological Outcomes of Minimally Invasive Partial Versus Minimally Invasive Radical Nephrectomy for Ct1-2/N0/M0 Clear Cell Renal Cell Carcinoma: A Propensity Score-Matched Analysis. World J Urol (2017) 35(5):789–94. doi: 10.1007/s00345-016-1923-2
13. May M, Ficarra V, Shariat SF, Zigeuner R, Chromecki T, Cindolo L, et al. Impact of Clinical and Histopathological Parameters on Disease Specific Survival in Patients With Collecting Duct Renal Cell Carcinoma: Development of a Disease Specific Risk Model. J Urol (2013) 190(2):458–63. doi: 10.1016/j.juro.2013.02.035
14. Chen S, Zhang N, Jiang L, Gao F, Shao J, Wang T, et al. Clinical Use of a Machine Learning Histopathological Image Signature in Diagnosis and Survival Prediction of Clear Cell Renal Cell Carcinoma. Int J Cancer (2021) 148(3):780–90. doi: 10.1002/ijc.33288
15. Abern MR, Tsivian M, Polascik TJ, Coogan CL. Characteristics and Outcomes of Tumors Arising From the Distal Nephron. Urology (2012) 80(1):140–6. doi: 10.1016/j.urology.2012.03.034
16. Vazquez-Lavista LG, Uribe-Uribe N, Gabilondo-Navarro F. Collecting Duct Renal Cell Carcinoma: Two Different Clinical Stages, Two Different Clinical Outcomes. Urol Int (2008) 81(1):116–8. doi: 10.1159/000137652
17. Matsumoto H, Wada T, Aoki A, Hoshii Y, Takahashi M, Aizawa S, et al. Collecting Duct Carcinoma With Long Survival Treated by Partial Nephrectomy. Int J Urol (2001) 8(7):401–3. doi: 10.1046/j.1442-2042.2001.00321.xd
18. Cabanillas G, Montoya-Cerrillo D, Kryvenko ON, Pal SK, Arias-Stella JA 3rd. "Collecting Duct Carcinoma of the Kidney: Diagnosis and Implications for Management". Urol Oncol (2021) S1078-1439(21):00204-0. doi: 10.1016/j.urolonc.2021.04.041
19. Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment Outcome and Survival Associated With Metastatic Renal Cell Carcinoma of non-Clear-Cell Histology. J Clin Oncol Off J Am Soc Clin Oncol (2002) 20(9):2376–81. doi: 10.1200/JCO.2002.11.123
20. Oudard S, Banu E, Vieillefond A, Fournier L, Priou F, Medioni J, et al. Prospective Multicenter Phase II Study of Gemcitabine Plus Platinum Salt for Metastatic Collecting Duct Carcinoma: Results of a GETUG (Groupe d'Etudes Des Tumeurs Uro-Genitales) Study. J Urol (2007) 177(5):1698–702. doi: 10.1016/j.juro.2007.01.063
21. Pecuchet N, Bigot F, Gachet J, Massard C, Albiges L, Teghom C, et al. Triple Combination of Bevacizumab, Gemcitabine and Platinum Salt in Metastatic Collecting Duct Carcinoma. Ann Oncol Off J Eur Soc Med Oncol (2013) 24(12):2963–7. doi: 10.1093/annonc/mdt423
Keywords: collecting duct carcinoma, clinical characteristics, treatment methods, prognosis, directed acyclic graphs
Citation: Qian X, Xu J, Liu C, Zhong M, Hong S, Qian C, Zhu J, Zhang J and Wang S (2022) Impact of Treatment Modalities on Prognosis in Patients With Renal Collecting Duct Carcinoma: A Population-Based Study. Front. Oncol. 12:810096. doi: 10.3389/fonc.2022.810096
Received: 06 November 2021; Accepted: 17 March 2022;
Published: 22 April 2022.
Edited by:
Fabio Grizzi, Humanitas Research Hospital, ItalyReviewed by:
Giuseppe Simone, Hospital Physiotherapy Institutes (IRCCS), ItalyCopyright © 2022 Qian, Xu, Liu, Zhong, Hong, Qian, Zhu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaqiao Zhang, bWVkempxQDE2My5jb20=; Shaogang Wang, c2d3YW5ndGptQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.