- 1Department of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pathology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background and Aims: Chronic atrophic gastritis (CAG) is closely related to the development of gastric cancer. However, the diagnostic accuracy of white light endoscopy (WLE) biopsy for CAG is poor. The diagnostic role and efficacy of confocal laser endomicroscopy (CLE) in CAG missed under WLE biopsy remain unclear.
Methods: This study is a single-center prospective study that included 21 patients from 1,349 patients who underwent WLE and biopsy and whose WLE results confirmed CAG, but pathological results did not. Then, all these patients received CLE examination and underwent targeted biopsies and five-point standard biopsies. The sensitivity, specificity, and accuracy of CLE diagnosis and targeted biopsy were analyzed.
Results: The pathological results of five-point standard biopsies in 21 patients confirmed CAG, and 17 patients (81.0%) were confirmed to have intestinal metaplasia (IM). According to the image diagnosis of CLE, there were 19 cases (90.5%) of CAG and 14 cases (66.7%) of IM among these 21 patients. According to the targeted biopsy of CLE, 17 cases (81.0%) of CAG and 14 cases (66.7%) of IM were diagnosed. There was no significant difference between CLE image diagnosis and five-point standard biopsies in terms of atrophy severity score (p = 0.927), IM severity score (p = 0.250), atrophy scope score (p = 0.781), and IM scope score (p = 0.195). For CAG, the sensitivity and accuracy of CLE image diagnosis were higher than those of CLE targeted biopsies (90.5% vs. 81.0%, p = 0.331), but for IM, the diagnosis was the same.
Conclusions: CLE can improve the diagnosis rate of CAG and can increase the comprehensive assessment of the scope and severity of CAG.
Introduction
As the fourth most common cancer worldwide, gastric cancer has great significance in prevention and treatment (1). Chronic atrophic gastritis (CAG) is a well-recognized precancerous lesion of gastric cancer and its diagnosis and follow-up are critical (2, 3). The most common diagnostic and follow-up method of CAG currently is white light endoscopy (WLE) and endoscopic biopsy.
However, WLE cannot accurately diagnose CAG, and the WLE diagnosis has two limitations. Firstly, WLE cannot accurately locate the lesion, resulting in targeted biopsy failure. Therefore, there may be inconsistencies between WLE diagnosis and pathological diagnosis (4). A national multicenter study in China found that the sensitivity of WLE to diagnose CAG was less than 50%, and most specificities and positive predictive values were less than 70% (5). Secondly, local biopsy under WLE not only cannot accurately reflect the severity of the lesion because it cannot achieve targeted biopsy but also cannot assess the extent of the lesion. However, the degree and scope of atrophy affect its prognosis (6, 7).
Confocal laser endomicroscopy (CLE) stands at the forefront of the novel endoscopic techniques. CLE permits direct in vivo identification and clear visualization of gastric patterns at the cellular level (8, 9), which makes it assess the severity and scope of atrophy effectively. Several studies reported that CLE is useful in the diagnosis of cancer and premalignant lesions (10–12). Furthermore, some studies showed that CLE had greater consistency with the pathological diagnosis result than WLE (12, 13).
Actually, a great number of patients had the CAG diagnosis confirmed with WLE and the chronic inflammation diagnosis confirmed with histopathology clinically (4), which brings difficulty in final diagnosis and treatment for clinicians. Therefore, in this study, we aim to use CLE to diagnose those patients who were diagnosed with CAG under WLE, and whose pathological results were chronic inflammation, to explore the diagnostic value of CLE for CAG patients.
Methods
Patients
We selected subjects from 1,349 patients with both biopsy and pathological diagnoses among 6,258 patients who underwent WLE in the Union Hospital of Tongji Medical College of Huazhong University of Science and Technology. Among them, 21 patients were included in this study according to the inclusion and exclusion criteria. Inclusion criteria were as follows: 1) 18–80 years old, can endure digestive endoscopy; 2) patients with WLE diagnosis of CAG, and pathological results were chronic inflammation in nearly 3 months; and 3) voluntarily joined the experiment and signed written informed consent. Exclusion criteria were as follows: 1) patients with gastrectomy history, acute gastrointestinal bleeding history, advanced gastric cancer, and other gastrointestinal malignancies; 2) patients suffering from disease who cannot tolerate gastroscopy, such as coagulation dysfunction, renal insufficiency, serious cardiopulmonary disease, and sodium fluorescein allergy; 3) pregnant and lactating women; and 4) no legal capacity or having medical and ethical reasons that affect the study. All patients included in this study signed an informed consent form.
Confocal Laser Endomicroscopy Procedure
All patients were given oral saline solution containing simethicone emulsion (Berlin-Chemie AG) to remove excess mucus from gastric mucosa before examination. And all patients underwent a skin test of fluorescein sodium (Baiyunshan Mingxing Pharmaceutical Co. Ltd., Guangzhou, China) before the examination to ensure that allergic reactions did not occur, which was used as a contrast agent intravenously before CLE.
First, the condition of the mucosa was observed through WLE, then eroded, elevated, hyperemic, and rough or granular mucosa and gray intestinal-type epithelium were selected as targets, then CLE was used for observation (14). CLE (YZB/FRA 5399-2012) was performed by an experienced endoscopist whose endoscopy experience was the same as that of WLE endoscopists. Five standardized intragastric sites were observed on CLE: lesser curvature of the antrum, greater curvature of the antrum, stomach angle, lesser curvature of the corpus, and greater curvature of the corpus. Meanwhile, 5–10 CLE images of different mucosal depths and CLE video were collected from each site, and the images and videos were stored for later assessment. The endoscopist performed real-time diagnosis, targeted biopsy, and five standard site biopsies under CLE. And another experienced endoscopist made diagnoses through images and videos and was blinded to the basic information and medical history of patients.
The standard criteria for endoscopists to diagnose CAG with CLE are that stomach pits are sparse and there are interstitial widening, irregular arrangement, severely reduced number of gastric pits, dilated openings, and a decreased number of subepithelial capillaries (15). The criteria for diagnosing IM with CLE are goblet cells, villous‐like pits, absorptive cells, and brush borders (16) (Figure 1).
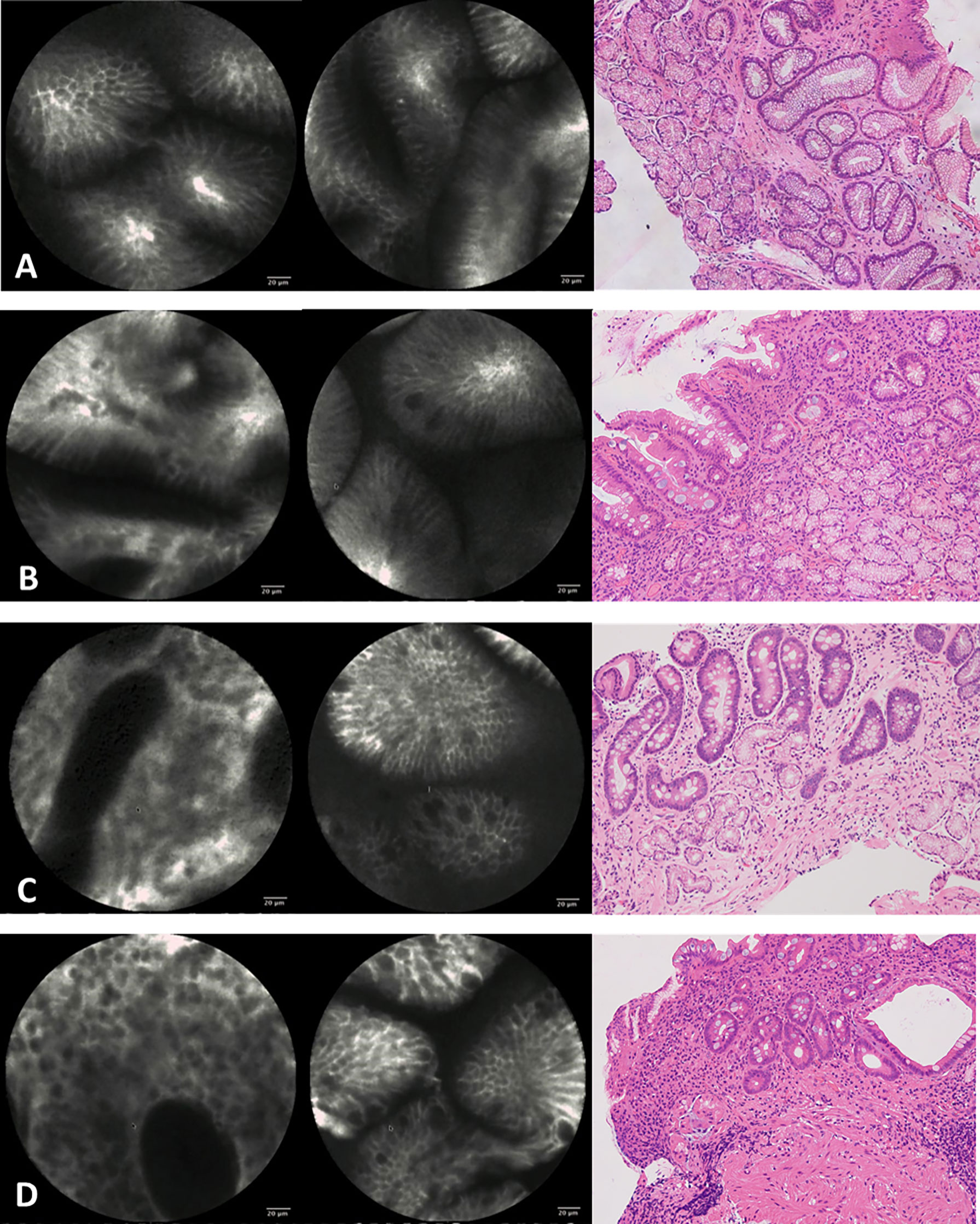
Figure 1 Confocal imaging and histopathology of gastric epithelium. (A) Normal gastric epithelium. (B) Mild atrophic gastritis and intestinal metaplasia. (C) Moderate atrophic gastritis and intestinal metaplasia. (D) Severe atrophic gastritis and intestinal metaplasia.
Histopathology
Mucosal biopsy specimens were placed in 4% “methanal” or “formaldehyde” sectioned into 4-μm thickness, and stained with hematoxylin and eosin (HE). Two experienced pathologists reviewed all the biopsy specimens of targeted biopsy under WLE and CLE. Meanwhile, they were blinded to both endoscopy information and results and the medical history of patients, and they were unaware of each other’s diagnoses. CAG was recognized morphologically by mild epithelial abnormalities, including a decrease in the number of cytoplasmic mucins, an increase in the nucleus and nucleolus, and an increase in mitotic figures in the pits of the stomach, when inflammation was extensive and accompanied by glandular atrophy (17). And the CAG was further divided into mild, moderate, and severe by a rough assessment of the relationship between the thickness of the gland and the thickness of the entire mucosa. IM was recognized morphologically by goblet cells and absorptive cells and was further divided into mild, moderate, and severe according to the number of abnormal cells (18) (Figure 1).
Study Definitions
Atrophy range score was a diagnosis of CLE and five standard site biopsy pathologies for the scope of atrophy. The diagnosis results of different parts of the stomach with or without atrophy were recorded as 1 point and 0 points, respectively. Atrophy severe score was a diagnosis to evaluate the severity of atrophy by CLE and five standard site biopsy pathologies. Diagnosis of different parts of the stomach as mild to moderate atrophy was recorded as 1 point, 2 points, and 3 points. IM range score and IM severe score were defined in the same way.
Statistical Analysis
Data were entered into Excel data sheets and analyzed with SPSS software v25.0 (IBM, USA) or SAS software, version 9.4. Clinical characteristics were expressed as median and range, absolute value or fractions. Patients characteristics were compared using χ2 or Fisher exact test for categorical variables. A Mann–Whitney U-test was used to compare the median duration between groups. p < 0.05 was considered statistically significant.
Results
Patient Characteristics
We included 21 patients with WLE diagnosis of CAG and chronic inflammation on pathological examination for nearly 3 months in this prospective study. The overall median age was 54 years (range: 27–66 years), with a women-to-men ratio of 1.33. Fourteen (66.7%) patients had a history of proton pump inhibitor (PPI) medication. Eight (38.1%) patients had a history of smoking and drinking. And 8 (38.1%) patients were anxious, and 3 (14.3%) patients were depressed. The demographic characteristics of patients identified in this study are summarized in Table 1.
Confocal Laser Endomicroscopy Results and Pathological Diagnosis Results
Among the 21 patients, pathological results of five standard site biopsies all confirmed CAG, and 17 (81.0%) of them had IM. The real-time diagnosis results through CLE and the post-diagnosis results through images and videos were consistent. And the diagnoses of the two pathologists were also consistent. Among the 21 patients, 19 (90.5%) patients were diagnosed with CAG by CLE, and 14 (66.7%) patients were diagnosed with IM. In the case of targeted biopsy, 17 (81.0%) patients were diagnosed with CAG, and 14 (66.7%) patients had IM.
Most patients had CAG and IM in multiple sites. Compared with targeted biopsy, CLE image diagnosis was more consistent with five-point standard biopsies in the diagnosis of CAG and IM. There was no significant difference between the CLE diagnosis and the pathological diagnosis of five standard site biopsies for the atrophy range score and atrophy severe score (p = 0.781; p = 0.927) and also for the IM range score and IM severe score (p = 0.195; p = 0.250). The results of confocal diagnosis, targeted biopsy, and five standard site biopsies are shown in Table 2.
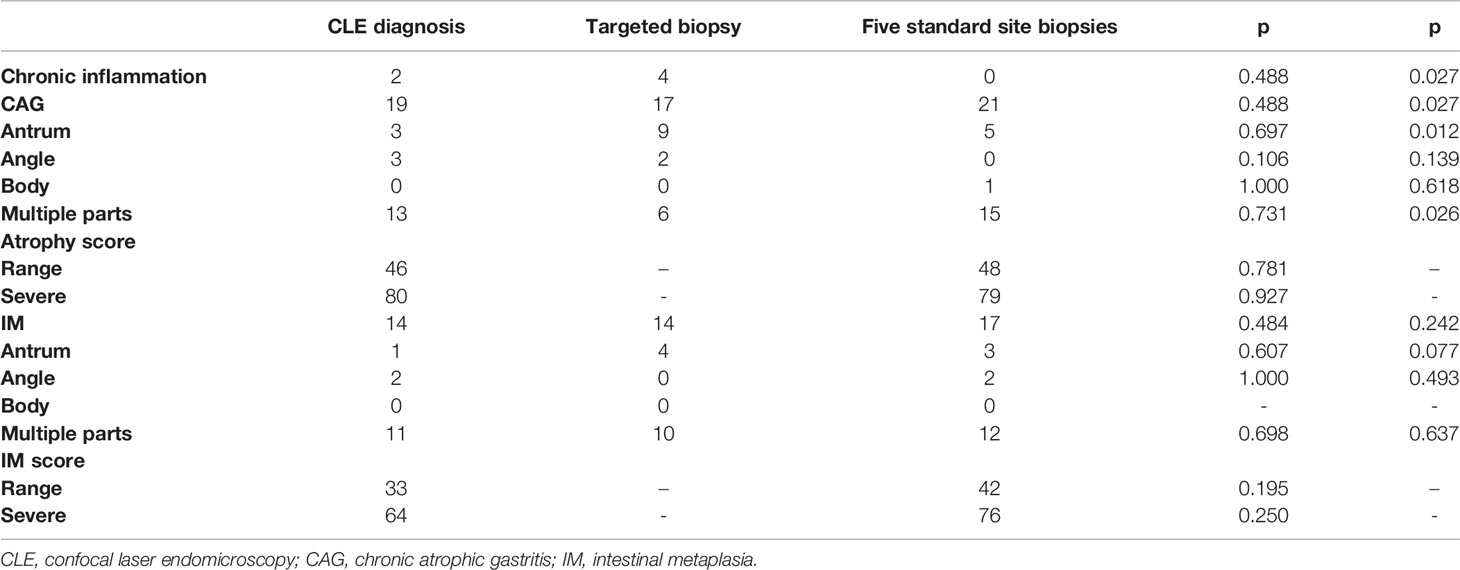
Table 2 The diagnosis results of CLE image diagnosis, CLE targeted biopsies, and five-point standard biopsies.
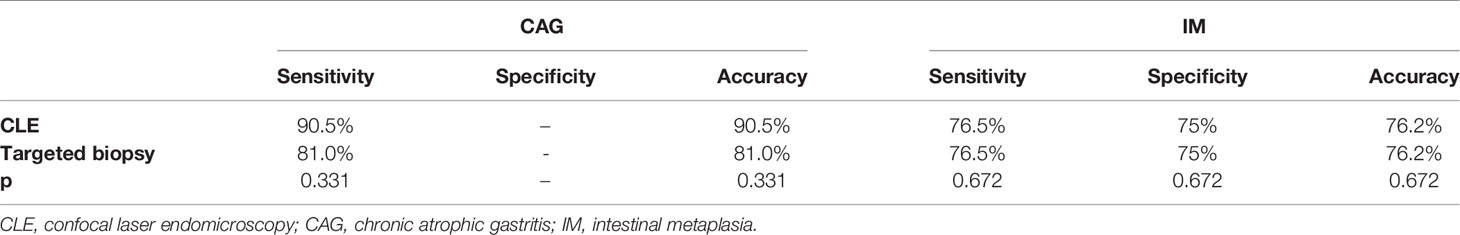
The sensitivity and accuracy of the CLE image in the diagnosis of CAG were both 90.5% and of CLE targeted biopsy were both 81.0%. For CAG, CLE image diagnosis had higher sensitivity and accuracy than CLE targeted biopsies but not statistically significant (p = 0.331), but for IM, they were the same. The sensitivities of the CLE image and CLE targeted biopsy in the diagnosis of IM were both 76.5%, the specificity was 75%, and the accuracy was 76.2%. The results of diagnostic efficiency of CLE and targeted biopsy on CAG and IM are shown in Table 3.
Discussion
Each year, there are more than 900,000 new diagnoses of gastric cancer worldwide, and gastric cancer is the second and fourth most common cause of cancer death in men and women, respectively (19). As a precancerous lesion of gastric cancer, CAG is of great significance in diagnosis. There was a great number of patients with inconsistent results of WLE and pathological diagnosis of CAG clinically (4), which made difficulties in clinical diagnosis and treatment. But there were few studies for the diagnosis of these contradictory patients. CLE can realize real-time diagnosis at the cellular level and a larger evaluated area in vivo identification compared to biopsies (8, 9). And many studies have shown that CLE has a high diagnostic value for precancerous lesions (20, 21), but few studies had used CLE to diagnose patients with inconsistent WLE diagnosis and pathology diagnosis. Therefore, this paper made a prospective study of these patients with conflicting diagnosis results and made diagnoses of CAG and IM by CLE, targeted biopsy, and five standard site biopsies that were regarded as the gold standard, so as to evaluate the diagnostic efficacy of CLE for CAG patients.
We studied 21 patients with conflicting diagnosis results, then all patients were diagnosed as CAG by the gold standard method, and 90.5% of patients were diagnosed with CAG through CLE. The sensitivity of CLE diagnosis of atrophy in the study was as high as in other studies (ranging from 85% to 90%) (12, 15, 22–29). Besides, targeted biopsy under CLE also diagnosed 81.0% of patients with CAG, which indicated that CLE can improve biopsy accuracy. As a result, CLE can improve the diagnosis rate of CAG. However, the accuracy of CAG diagnosis by targeted biopsy was lower than that of CLE. This may be because the biopsy under CLE can only be performed after the confocal probe was removed, and there may be displacement during this process. If there is a device that can simultaneously perform a biopsy in the CLE magnified field of view, this problem may be solved. Furthermore, CLE did not differ significantly from the gold standard method in assessing the scope and severity of atrophy and IM. This shows that CLE can achieve a comprehensive assessment of the scope and severity of CAG and IM compared with WLE.
In conclusion, experienced endoscopists can use WLE or CLE to diagnose CAG effectively, but the consistency of WLE diagnosis and pathological diagnosis of biopsy needs to be improved. This study shows that CLE can solve the contradiction between WLE and pathological diagnosis and increase the diagnostic rate of patients with different WLE and pathological diagnoses. CLE can achieve targeted biopsy, and as a real-time pathology, the diagnostic efficiency was even higher than that of targeted biopsy. The most important point was that CLE had a good advantage in the diagnosis, follow-up, and comprehensive evaluation of CAG, and it was significantly better than WLE and biopsy under WLE. CLE had good clinical significance for the diagnosis of CAG.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RL designed and supervised the study and data analysis. SP performed most of the investigation and data analysis and wrote the manuscript. HY and CJ contributed to interpretation of the data and analyses. QZ provided pathological diagnosis assistance. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (nos. 81770539, 81974068, and 81900580).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to the units and individuals who have given us support and help, especially the Digestive Endoscopy Center of Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology, which has provided great support for this study.
Abbreviations
CAG, chronic atrophic gastritis; WLE, white light endoscopy; CLE, confocal laser endomicroscopy; IM, intestinal metaplasia.
References
1. Kamangar F, Dores G, Anderson W. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J Clin Oncol Off J Am Soc Clin Oncol (2006) 24(14):2137–50. doi: 10.1200/JCO.2005.05.2308
2. Islami F, Sheikhattari P, Ren J, Kamangar F. Gastric Atrophy and Risk of Oesophageal Cancer and Gastric Cardia Adenocarcinoma–A Systematic Review and Meta-Analysis. Ann Oncol Off J Eur Soc Med Oncol (2011) 22(4):754–60. doi: 10.1093/annonc/mdq411
3. Weck MN, Brenner H. Prevalence of Chronic Atrophic Gastritis in Different Parts of the World. Cancer Epidemiol Biomark Prev (2006) 15(6):1083–94. doi: 10.1158/1055-9965.EPI-05-0931
4. Yu X, Chen J, Zheng L, Song J, Lin R, Hou X, et al. Quantitative Diagnosis of Atrophic Gastritis by Probe-Based Confocal Laser Endomicroscopy. BioMed Res Int (2020) 2020:9847591. doi: 10.1155/2020/9847591
5. Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, et al. Chronic Gastritis in China: A National Multi-Center Survey. BMC Gastroenterol (2014) 14:21. doi: 10.1186/1471-230X-14-21
6. den Hoed CM, Holster IL, Capelle LG, de Vries AC, den Hartog B, Ter Borg F, et al. Follow-Up of Premalignant Lesions in Patients at Risk for Progression to Gastric Cancer. Endoscopy (2013) 45(4):249–56. doi: 10.1055/s-0032-1326379
7. Correa P, Piazuelo MB. The Gastric Precancerous Cascade. J Dig Dis (2012) 13(1):2–9. doi: 10.1111/j.1751-2980.2011.00550.x
8. Dunbar KB. Endomicroscopy in Barrett's Esophagus. Gastrointest Endosc Clin N Am (2013) 23(3):565–79. doi: 10.1016/j.giec.2013.03.003
9. Wallace MB, Sharma P, Lightdale C, Wolfsen H, Coron E, Buchner A, et al. Preliminary Accuracy and Interobserver Agreement for the Detection of Intraepithelial Neoplasia in Barrett's Esophagus With Probe-Based Confocal Laser Endomicroscopy. Gastrointest Endosc (2010) 72(1):19–24. doi: 10.1016/j.gie.2010.01.053
10. Li WB, Zuo XL, Zuo F, Gu XM, Yu T, Zhao YA, et al. Characterization and Identification of Gastric Hyperplastic Polyps and Adenomas by Confocal Laser Endomicroscopy. Surg Endosc (2010) 24(3):517–24. doi: 10.1007/s00464-009-0608-y
11. Banno K, Niwa Y, Miyahara R, Nakamura M, Nagaya T, Nagasaka T, et al. Confocal Endomicroscopy for Phenotypic Diagnosis of Gastric Cancer. J Gastroenterol Hepatol (2010) 25(4):712–8. doi: 10.1111/j.1440-1746.2009.06169.x
12. Li WB, Zuo XL, Li CQ, Zuo F, Gu XM, Yu T, et al. Diagnostic Value of Confocal Laser Endomicroscopy for Gastric Superficial Cancerous Lesions. Gut (2011) 60(3):299–306. doi: 10.1136/gut.2010.223586
13. Hoffman A, Manner H, Rey JW, Kiesslich R. A Guide to Multimodal Endoscopy Imaging for Gastrointestinal Malignancy - an Early Indicator. Nat Rev Gastroenterol Hepatol (2017) 14(7):421–34. doi: 10.1038/nrgastro.2017.46
14. Dai YC, Tang ZP, Zhang YL. How to Assess the Severity of Atrophic Gastritis. World J Gastroenterol (2011) 17(13):1690–3. doi: 10.3748/wjg.v17.i13.1690
15. Liu T, Zheng H, Gong W, Chen C, Jiang B. The Accuracy of Confocal Laser Endomicroscopy, Narrow Band Imaging, and Chromoendoscopy for the Detection of Atrophic Gastritis. J Clin Gastroenterol (2015) 49(5):379–86. doi: 10.1097/MCG.0000000000000164
16. Bai T, Zhang L, Sharma S, Jiang YD, Xia J, Wang H, et al. Diagnostic Performance of Confocal Laser Endomicroscopy for Atrophy and Gastric Intestinal Metaplasia: A Meta-Analysis. J Dig Dis (2017) 18(5):273–82. doi: 10.1111/1751-2980.12470
17. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and Grading of Gastritis. The Updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol (1996) 20(10):1161–81. doi: 10.1097/00000478-199610000-00001
18. Ji R, Zuo XL, Yu T, Gu XM, Li Z, Zhou CJ, et al. Mucosal Barrier Defects in Gastric Intestinal Metaplasia: In Vivo Evaluation by Confocal Endomicroscopy. Gastrointest Endosc (2012) 75(5):980–7. doi: 10.1016/j.gie.2011.12.016
19. Forman D, Burley VJ. Gastric Cancer: Global Pattern of the Disease and an Overview of Environmental Risk Factors. Best Pract Res Clin Gastroenterol (2006) 20(4):633–49. doi: 10.1016/j.bpg.2006.04.008
20. Kollar M, Spicak J, Honsova E, Krajciova J, Vackova Z, Martinek J, et al. Role of Confocal Laser Endomicroscopy in Patients With Early Esophageal Neoplasia. Minerva Chir (2018) 73(4):417–27. doi: 10.23736/S0026-4733.18.07795-7
21. Zhang HP, Yang S, Chen WH, Hu TT, Lin J. The Diagnostic Value of Confocal Laser Endomicroscopy for Gastric Cancer and Precancerous Lesions Among Asian Population: A System Review and Meta-Analysis. Scand J Gastroenterol (2017) 52(4):382–8. doi: 10.1080/00365521.2016.1275770
22. Zhang JN, Li YQ, Zhao YA, Yu T, Zhang JP, Guo YT, et al. Classification of Gastric Pit Patterns by Confocal Endomicroscopy. Gastrointest Endosc (2008) 67(6):843–53. doi: 10.1016/j.gie.2008.01.036
23. Wang P, Ji R, Yu T, Zuo XL, Zhou CJ, Li CQ, et al. Classification of Histological Severity of Helicobacter Pylori-Associated Gastritis by Confocal Laser Endomicroscopy. World J Gastroenterol (2010) 16(41):5203–10. doi: 10.3748/wjg.v16.i41.5203
24. Li Z, Zuo XL, Yu T, Gu XM, Zhou CJ, Li CQ, et al. Confocal Laser Endomicroscopy for In Vivo Detection of Gastric Intestinal Metaplasia: A Randomized Controlled Trial. Endoscopy (2014) 46(4):282–90. doi: 10.1055/s-0033-1359215
25. Lim LG, Yeoh KG, Salto-Tellez M, Khor CJ, Teh M, Chan YH, et al. Experienced Versus Inexperienced Confocal Endoscopists in the Diagnosis of Gastric Adenocarcinoma and Intestinal Metaplasia on Confocal Images. Gastrointest Endosc (2011) 73(6):1141–7. doi: 10.1016/j.gie.2011.01.068
26. Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, et al. Diagnosis of Gastric Intestinal Metaplasia With Confocal Laser Endomicroscopy In Vivo: A Prospective Study. Endoscopy (2008) 40(7):547–53. doi: 10.1055/s-2007-995633
27. Lim LG, Yeoh KG, Srivastava S, Chan YH, Teh M, Ho KY, et al. Comparison of Probe-Based Confocal Endomicroscopy With Virtual Chromoendoscopy and White-Light Endoscopy for Diagnosis of Gastric Intestinal Metaplasia. Surg Endosc (2013) 27(12):4649–55. doi: 10.1007/s00464-013-3098-x
28. Pittayanon R, Rerknimitr R, Wisedopas N, Ridtitid W, Kongkam P, Treeprasertsuk S, et al. Flexible Spectral Imaging Color Enhancement Plus Probe-Based Confocal Laser Endomicroscopy for Gastric Intestinal Metaplasia Detection. J Gastroenterol Hepatol (2013) 28(6):1004–9. doi: 10.1111/jgh.12185
Keywords: chronic atrophic gastritis, gastric cancer, white light endoscopy, confocal laser endomicroscopy, pathology
Citation: Pang S, Yao H, Jiang C, Zhang Q and Lin R (2022) Confocal Laser Endomicroscopy Can Improve the Diagnosis Rate and Range Assessment of Patients With Conflicting Chronic Atrophic Gastritis Results of White Light Endoscopic and Pathological Diagnosis. Front. Oncol. 12:809822. doi: 10.3389/fonc.2022.809822
Received: 05 November 2021; Accepted: 28 February 2022;
Published: 24 March 2022.
Edited by:
Jingnan Li, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Yingxuan Chen, Shanghai JiaoTong University, ChinaStefan Urbanski, University of Calgary, Canada
Yongquan Shi, Fourth Military Medical University, China
Copyright © 2022 Pang, Yao, Jiang, Zhang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Lin, c2VsaW5hbGluMzVAc2luYS5jb20=
 Suya Pang
Suya Pang Hailing Yao
Hailing Yao Chen Jiang1
Chen Jiang1