- 1Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
- 2Department of Pathology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
- 3Fujian Key Laboratory of Translational Cancer Medicine, Fuzhou, China
Aim: Tumor deposits (TDs) are an aggressive hallmark of rectal cancer, but their prognostic value has not been addressed in current staging systems. This study aimed to construct and validate a prognostic nomogram for rectal cancer patients with TDs.
Methods: A total of 1,388 stage III–IV rectal cancer patients who underwent radical surgical resection from the Surveillance, Epidemiology, and End Results (SEER) database were retrospectively analyzed to identify the clinical value of TDs. TD-positive rectal cancer patients in the SEER database were used as the training set to construct a prognostic model, which was validated by Fujian Cancer Hospital. Three models were constructed to predict the prognosis of rectal cancer patients with TDs, including the least absolute shrinkage and selection operator regression (LASSO, model 1), backward stepwise regression (BSR, model 2), and LASSO followed by BSR (model 3). A nomogram was established among the three models.
Results: In the entire cohort, TD was also identified as an independent risk factor for overall survival (OS), even after adjusting for baseline factors, stage, other risk factors, treatments, and all the included variables in this study (all P < 0.05). Among patients with TDs, model 3 exhibited a higher C-index and area under the curves (AUCs) at 3, 4, and 5 years compared with the American Joint Committee on Cancer staging system both in the training and validation sets (all P < 0.05). The nomogram obtained from model 3 showed good consistency based on the calibration curves and excellent clinical applicability by the decision curve analysis curves. In addition, patients were divided into two subgroups with apparently different OS according to the current nomogram (both P < 0.05), and only patients in the high-risk subgroup were found to benefit from postoperative radiotherapy (P < 0.05).
Conclusion: We identified a novel nomogram that could not only predict the prognosis of rectal cancer patients with TDs but also provide reliable evidence for clinical decision-making.
Introduction
Tumor deposits (TDs) were first reported in 1935 (1) and are associated with aggressive characteristics, advanced stage, and adverse prognosis of rectal cancers (2–4). The definition and origin of TD has always been disputed (3), although it was introduced in the fifth American Joint Committee on Cancer (AJCC) staging system for rectal cancer in 1997 (5). Until recently, TDs are defined as “irregular discrete tumor deposits in the perirectal fat that are away from the leading edge of the tumor and show no evidence of residual lymph node tissue, but that are within the lymphatic drainage of the primary tumor” in the latest edition of AJCC staging system (6). Considering that the detection rate of TDs is higher (4, 7), more concerns should be considered.
TDs are considered as an aggressive hallmark of rectal cancer not only in the absence of regional lymph node metastasis (LNM) (8, 9), but also in patients with LNM (10, 11). However, the clinical value of TDs is severely underestimated in the management of rectal cancer. TD-positive tumors are classified as N1c in the absence of LNM, while neither the presence nor the number of TDs is considered in the pN staging in cases of concomitant LNM (6). In addition, TD-positive patients have often been overlooked in the postoperative management of most of the current guidelines. As an efficient anti-recurrence prophylaxis and an alternative salvage strategy for recurrent tumors, postoperative radiotherapy (RT) is only recommended for N1c patients in the European Society for Medical Oncology (ESMO) clinical practice guideline for rectal cancer (12).
In the current study, we first identified the clinical significance of TDs in a population-based analysis of the Surveillance, Epidemiology, and End Results (SEER) database, and then constructed a nomogram to predict the prognosis of TD-positive rectal cancer patients, which was also validated by an external cohort from our center.
Materials and Methods
Ethics Statement
This study was conducted under the ethical guidelines of the Helsinki Declaration. We acquired approval from Fujian Cancer Hospital’s Ethics Committee (K2021-050-01), which waived back the individual informed consent owing to that the clinicopathological data were extracted retrospectively. On the other hand, we gained an official permit to access the research data from the SEER database.
Study Design
Figure 1 shows the flowchart of the current study. Stage III–IV rectal cancer patients who underwent radical surgical resection in the SEER database between January 2010 and December 2015 were studied, including age, sex, marital status, carcinoembryonic antigen (CEA) level, T stage, N stage, M stage, tumor size, tumor differentiation, perineural invasion (PNI) status, lymph node ratio (LNR), log odds of metastatic lymph nodes (LOODS), positive lymph node (PLNC), negative lymph node (NLNC), TD status, postoperative RT, postoperative chemotherapy, and follow-up. The exclusion criteria in this study were as follows: 1) underwent neoadjuvant therapy, 2) multiple primary cancers, and 3) T0/Tis.
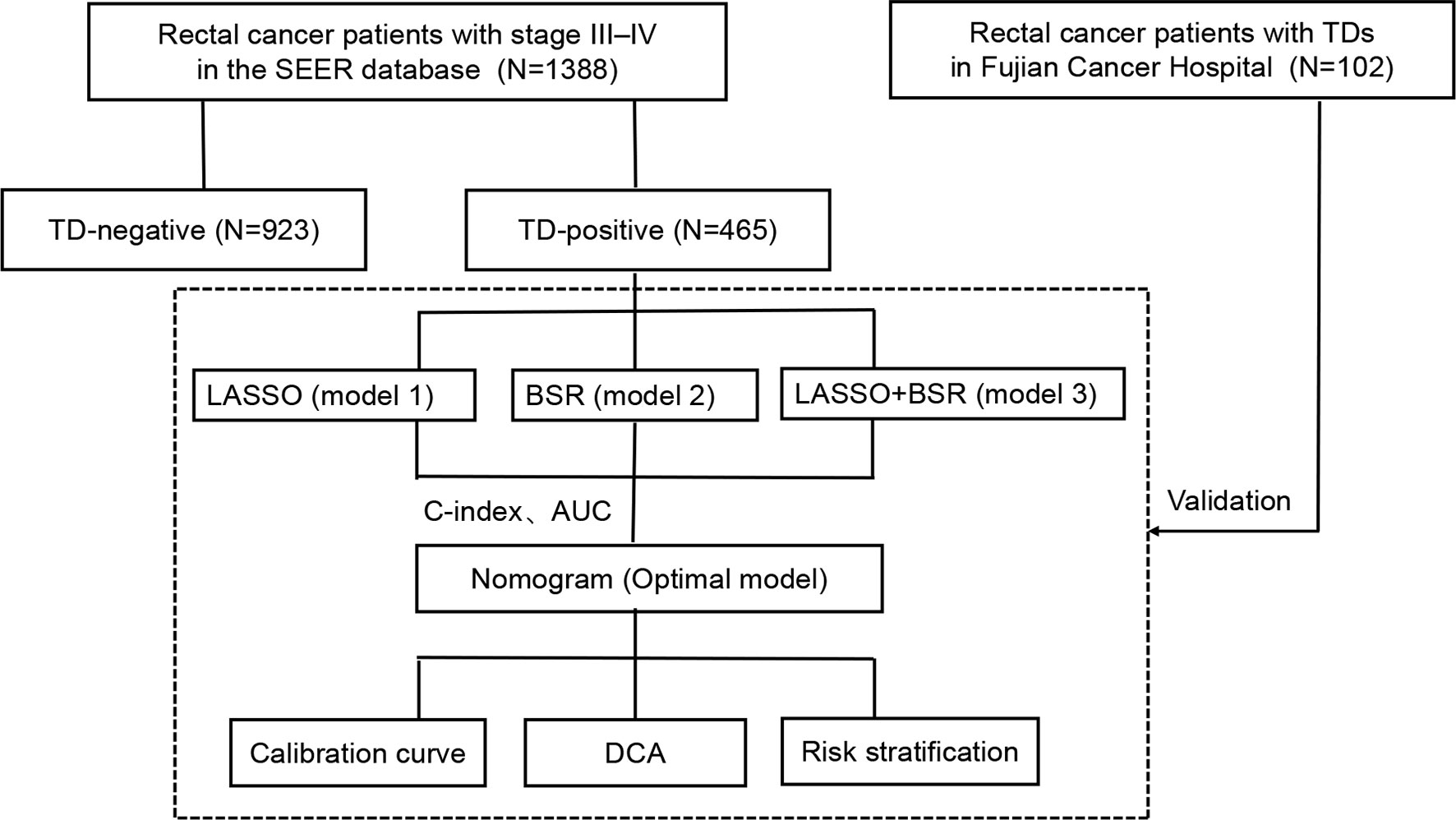
Figure 1 Flowchart of the study design. SEER, Surveillance, Epidemiology, and End Results; TD, tumor deposit; LASSO, least absolute shrinkage and selection operator regression; BSR, backward stepwise regression; C-index, concordance index; AUC, area under the curve; DCA, decision curve analysis.
First, we identified the clinical value of TDs in the entire cohort. TD-positive rectal cancer patients in the SEER database were used as the training set to construct a prognostic model. Data of rectal cancer patients with TDs from Fujian Cancer Hospital were used as an external cohort to verify the prognostic model.
Clinicopathological Variable Stratification
Using the “surv_cutpoint” function from the “survminer” R package, LNR, LOODS, and PLNC were categorized as tritaxic variables with optimal cutoff values of 0.038 and 0.600, −1.330 and 0.160, and 0 and 4, while NLNC was classified as a dichotomous variable with an optimal cutoff value of 7. Supplementary Figure 1 shows the excellent calibration of the current cutoff values of LNR, LOODS, PLNC, and NLNC in terms of overall survival (OS) (all P < 0.05).
Variables in this study were classified as follows: age at diagnosis (≤60 years, >60 years), sex (male or female), marital status (married, unmarried), CEA level (normal, elevated/borderline), stage (III, IV), T stage (T1–2, T3, T4), N stage (N1, N2), M stage (M0, M1), tumor size (≤5 cm, >5 cm), tumor differentiation (I/II and III/IV), PNI (absent, present), LNR (≤0.038, 0.038–0.600, >0.600), LOODS (≤−1.330, −1.330–0.160, >0.160), PLNC (0, 0–4, >4), NLNC (≤7, >7), postoperative RT (no, yes), postoperative chemotherapy (no, yes), and survival (months).
Outcome Definition
The primary outcome measure for this study was OS, which was defined from the data obtained from the date of diagnosis through the date of either death or the latest follow-up.
Variable Selection and Model Construction
To avoid underfitting and/or overfitting of the model, three advanced statistical methods, namely, the least absolute shrinkage and selection operator regression (LASSO, model 1) (13), backward stepwise regression (BSR, model 2) (14), and LASSO followed by BSR (model 3) (15), were adopted to screen the candidate variables in the training set. The optimal model was determined using Harrell’s concordance index (C-index) (16) and area under the curve (AUC) (17). All three models were compared with the eighth AJCC staging system.
Performance and Validation of the Nomogram
A nomogram was derived using the optimal model. The discrimination of the nomogram was evaluated by the C-index and AUC as described above, and the predictive accuracy was assessed by the calibration curve (18). Decision curve analysis (DCA) (19) was performed to assess the potential clinical applicability and benefits of the nomogram. Similar analyses were conducted in the validation set.
The patients were divided into low-risk and high-risk groups according to the optimal cutoff value of the prognostic model risk score, which was determined by the “surv cutpoint” function from the “survminer” R package in the training set. Finally, we evaluated the effects of postoperative RT in different groups to screen patients who benefited from postoperative RT.
Statistical Analyses
The Kaplan–Meier (K-M) method was used to compare OS among different groups using a log-rank test. LASSO and BSR were used to select variables. Multivariate Cox regression analysis was performed for model construction. Statistical tests were conducted using RStudio (version 1.3.1073), including xlsx, Table 1, survminer, survival, rms, nomogramFormula, timeROC, and stdca packages. All statistical tests were two-tailed, and statistical significance was set at P <0.05.
Results
Characteristics Comparison Between Patients With and Without TDs
A total of 1,338 patients were eligible for this study, including 465 (34.8%) patients with TDs. The clinicopathological characteristics of patients with and without TDs are depicted in Table 1. As expected, TD-positive patients typically present with aggressive characteristics, such as elevated CEA levels, advanced tumor-node-metastasis (TNM) stage, and PNI (all P < 0.05, Table 1). Notably, no significant differences were observed between patients with and without TDs in terms of receiving postoperative RT and postoperative chemotherapy (both P > 0.05, Table 1).
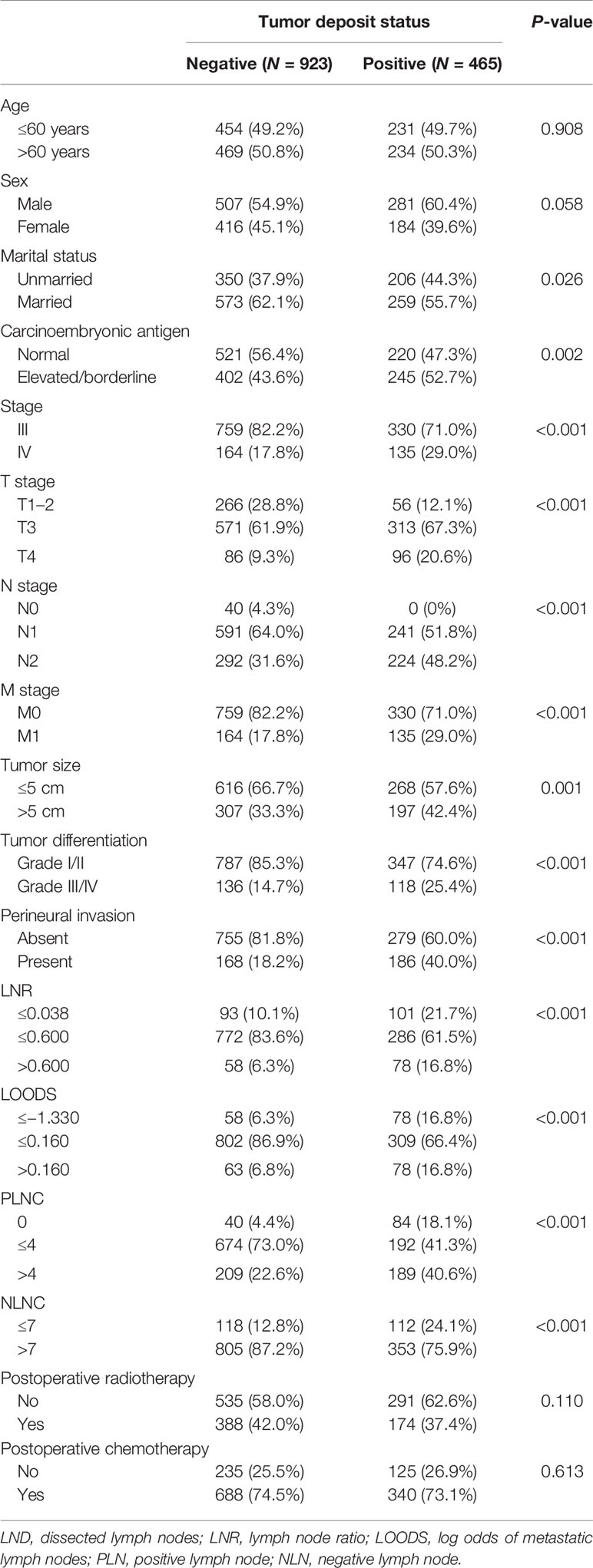
Table 1 Baseline clinicopathological characteristics and the status of tumor deposit in rectal cancer patients.
Significance of TDs in Patients With Rectal Cancer
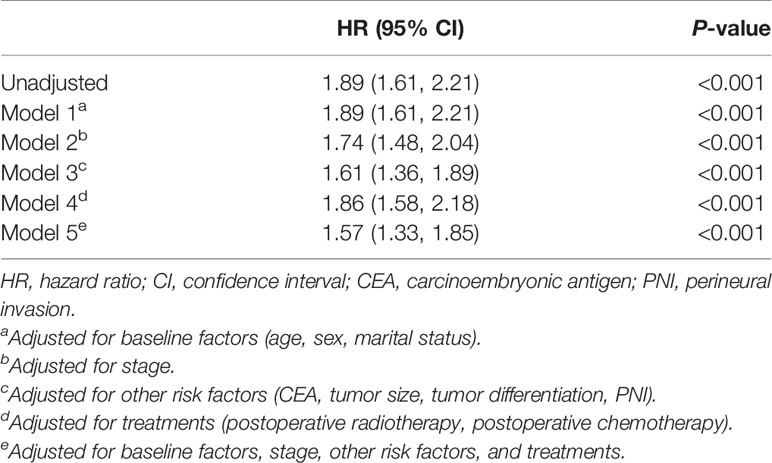
TDs were identified as a risk factor for OS using univariate Cox regression analysis (P < 0.001, Table 2). To decrease the potential confounding bias, adjusted hazard ratios (HRs) were adopted to determine the effect of TDs on the prognosis of rectal cancer. The results showed that TDs remained an independent risk factor for OS after adjusting for baseline factors (age, sex, marital status), stage, other risk factors (CEA, tumor size, tumor differentiation, PNI), treatments (postoperative RT, postoperative chemotherapy), and all the included variables (baseline, stage, treatment, and others), which are all shown in Table 2.
Clinicopathological Characteristics of Patients With TDs
Supplementary Table 1 shows the baseline clinical and pathological characteristics of the training and validation sets. Apparent differences were observed between the two cohorts. Briefly, in the training set, the proportions of stage IV, tumor size >5 cm, LNR ≤0.038, LOODS ≤−1.330, PLNC = 0, and receiving postoperative RT were 29.0%, 42.4%, 61.5%, 66.4%, 41.3%, and 37.4%, respectively; in the validation cohort, the corresponding proportions were 11.8%, 16.7%, 18.6%, 14.7%, 17.6%, and 9.8%, respectively.
Among the N stage, LNR, LOODS, PLNC, and NLNC, we identified the optimal lymph node staging system using the C-index and the Akaike information criterion (AIC). The LOODS exhibited the highest C-index (0.61) and minimum AIC (2,991), compared with LNR (C-index: 0.61, AIC: 2,995), PLNC (C-index: 0.59, AIC: 3,017), NLNC (C-index: 0.58, AIC: 3,019), and N stage (C-index: 0.61, AIC: 2,995). Therefore, LOODS was included in the further analysis.
Variable Selection
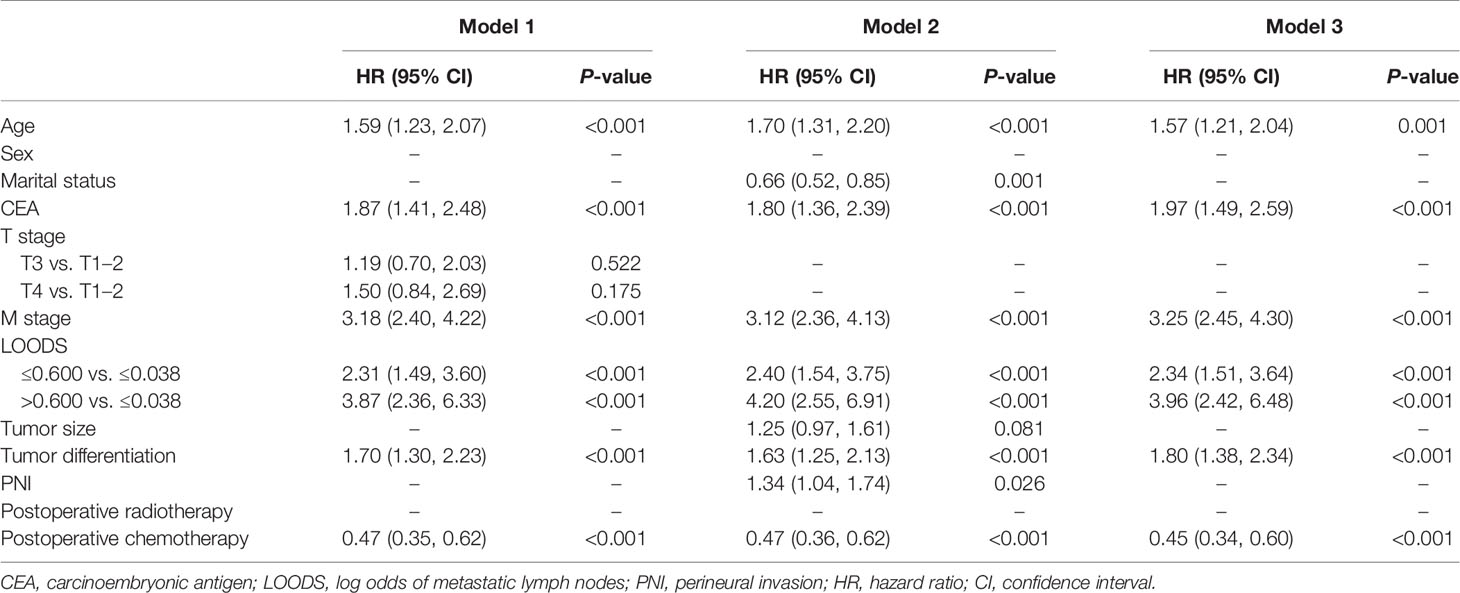
Model 1 was constructed using the variables identified from the LASSO. As shown in Supplementary Figures 2A, B, a coefficient profile figure was produced against the ln (λ) sequence. With a lambda of 0.144, the LASSO regression analysis identified the seven non-zero coefficients: age, CEA, T stage, M stage, LOODS, tumor differentiation, and postoperative chemotherapy (Table 3).
Model 2 was constructed using potential factors via the BSR. With a minimum AIC of 2,815, nine potential factors, namely, age, marital status, CEA, M stage, LOODS, tumor size, tumor differentiation, PNI, and postoperative chemotherapy, were selected and incorporated into model 2 (Table 3).
Considering that we aimed to establish an accurate and convenient model for predicting OS of patients with TDs, the seven factors identified from LASSO were used in the BSR analysis (model 3). Finally, the LASSO-BSR identified the following six most powerful factors: age, CEA, M stage, LOODS, tumor differentiation, and postoperative chemotherapy. All selected variables showed significant statistical differences (all P < 0.05, Table 3), and model 3 was constructed.
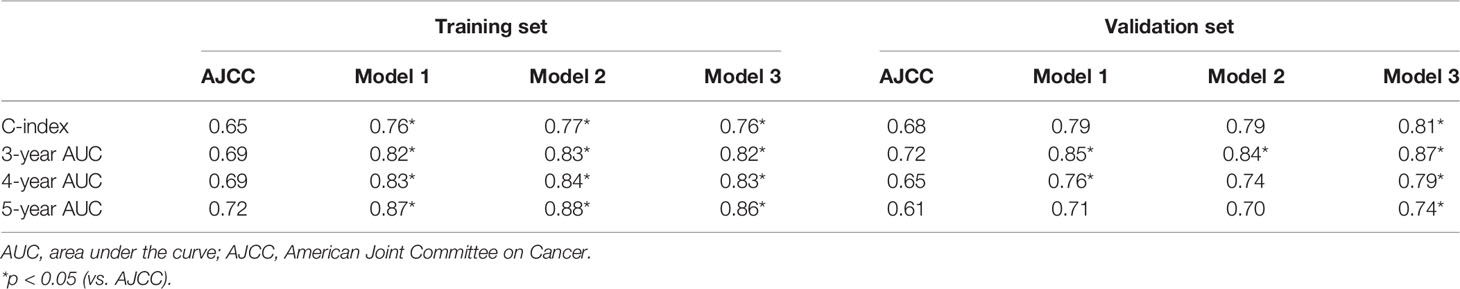
The final prediction model was determined by C-index and AUC at 3, 4, and 5 years (Table 4). In the training set, all three models exhibited higher C-indexes and AUCs at 3, 4, and 5 years than AJCC (all P < 0.05), but there were no significant differences among the three models. In the validation set, only model 3 exhibited better performance than AJCC in terms of C-index and AUCs at 3, 4, and 5 years (all P < 0.05). Therefore, model 3 was chosen as the final prognostic model.
Construction and Validation of the Nomogram
A nomogram was established based on model 3 (Figure 2). The C-indexes of the nomogram in the training and validation sets were 0.76 [95% confidence intervals (CI) = 0.73–0.79] and 0.81 (95% CI = 0.73–0.88), respectively. Calibration plots showed better consistency between the predicted outcomes of the nomogram and the actual outcomes in terms of 3- and 5-year OS in the training set (Supplementary Figures 3A, B). Similar results were observed in the validation set (Supplementary Figures 3C, D).
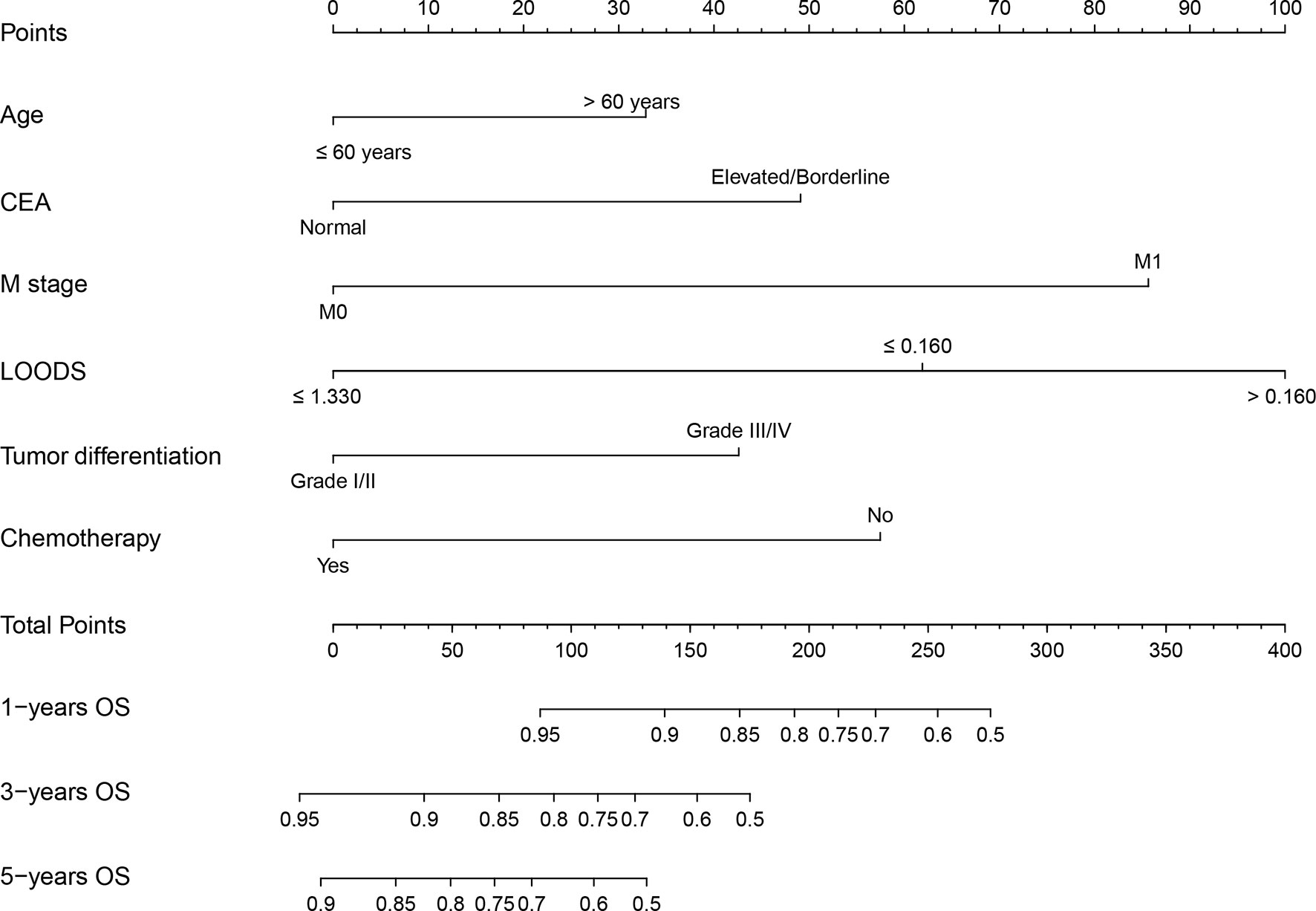
Figure 2 Nomogram for predicting overall survival (OS) of rectal cancer patients with tumor deposits. CEA, carcinoembryonic antigen; LOODS, log odds of metastatic lymph nodes.
In addition, each patient received a corresponding total point according to the nomogram. The median total points were 144 (0–342) in the training set and 105 (0–330) in the validation set. A cutoff value of 100 was used to categorize the patients into two risk subgroups (low-risk and high-risk groups). K-M curves showed good predictive performance of the nomogram both in the training and validation sets (both P < 0.001, Figures 3A, B).
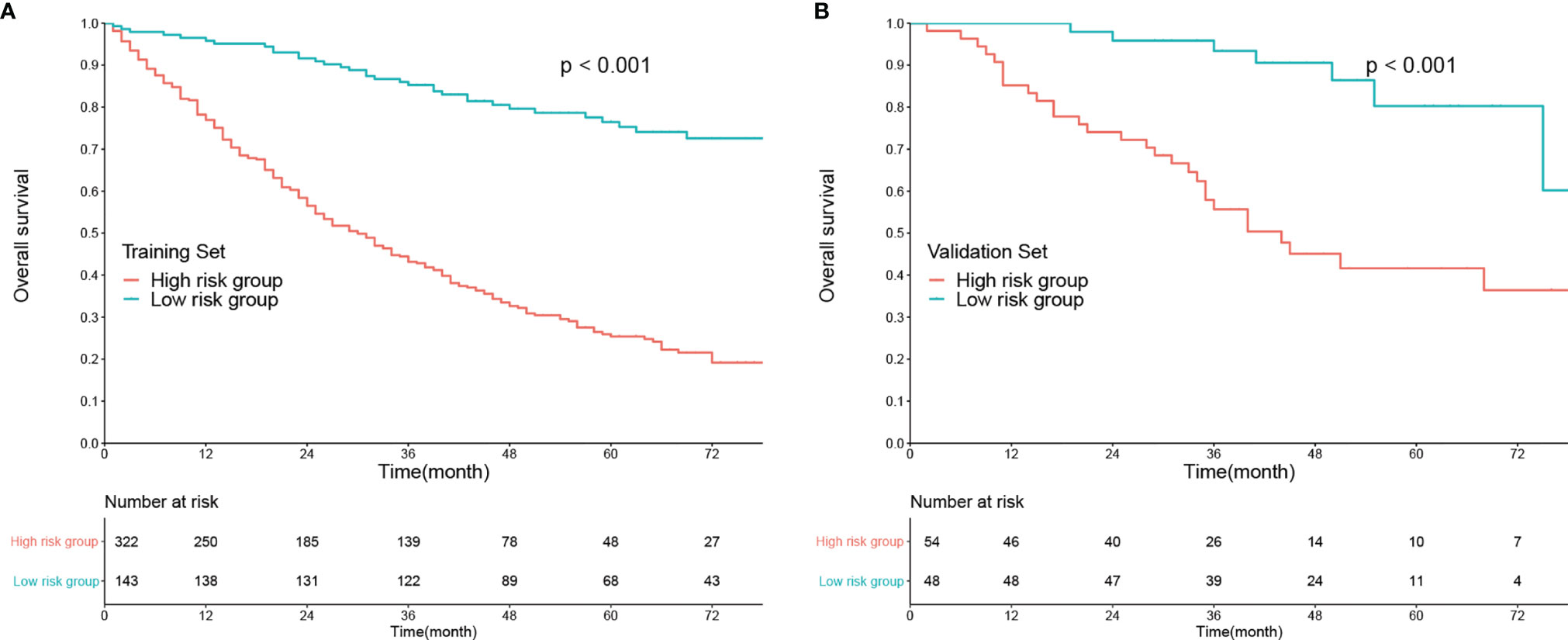
Figure 3 Kaplan–Meier analysis of overall survival according to risk stratification in the (A) training set and (B) validation set.
Clinical Applicability of the Nomogram
DCA was used to evaluate the clinical applicability of the nomogram. Compared with the eighth AJCC staging system, the DCA showed that the current nomogram had a better overall net benefit across a wide range of reasonable threshold probabilities in both the training set (Supplementary Figure 4A) and the validation set (Supplementary Figure 4B).
The current nomogram was also taken as an index to guide the management of postoperative RT. As shown in Figure 4A, there was no significant difference in terms of OS between subgroups receiving postoperative RT or not among low-risk patients (1-year OS: 96.38% vs. 93.33%; 3-year OS: 84.34% vs. 86.63%; 5-year OS: 74.33% vs. 79.48%; P > 0.05). On the contrary, among high-risk patients, survival benefit was observed between subgroups receiving postoperative RT or not (1-year OS: 90.11% vs. 71.75%; 3-year OS: 59.98% vs. 36.53%; 5-year OS: 36.57% vs. 21.07%; P < 0.01; Figure 4B).

Figure 4 The effect of postoperative radiotherapy (RT) on overall survival of rectal cancer patients with tumor deposits. Kaplan–Meier curves in the high-risk group (A) and low-risk group (B). S, surgery.
Discussion
Growing concerns have been raised regarding TDs with increasing detection rates (4, 7), but the clinical value of TDs has been far from being explored. To the best of our knowledge, this is the first model to predict the prognosis of rectal cancer patients with TDs. We first identified the clinical significance of TDs in a population-based analysis, and then constructed a nomogram to predict the prognosis of TD-positive rectal cancer patients, which exhibited better performance and applicability than the AJCC staging system. Furthermore, the nomogram was validated in an external cohort at our center.
There has been controversy since the introduction of TD into the AJCC staging system (5). The majority considered TDs to come from lymph nodes (20), but some regarded TDs as destructive venous invasions (2, 21) or remnants of neoadjuvant treatment (4). Hence, in this study, we excluded all patients who had received neoadjuvant treatment. Nonetheless, TDs are quite an aggressive hallmark of rectal cancer (2–4). In the present study, TDs were significantly associated with adverse prognosis in patients with rectal cancer. Compared with TD-negative rectal cancer patients, TD-positive patients typically present with adverse features, such as elevated CEA levels, advanced TNM staging, and PNI (all P < 0.05), as previously reported (2, 10, 22, 23). In addition, TDs were still identified as an independent risk factor for OS, even after adjusting for baseline factors, stage, other risk factors, treatments, and all the included variables in this study (all P < 0.05).
However, the positioning of TDs in the TNM system has always been underestimated. TDs are only embodied in N1c in the eighth AJCC staging system (6). However, the presence of TDs in LNM patients also indicates a poor prognosis (10, 11), and Mayo et al. (9) showed that TDs were associated with worse 3-year OS in patients with any known and unknown N categories, both of which indicated that the current staging system might not be enough to predict the prognosis of TD-positive patients. In this study, we established a novel prognostic nomogram for rectal cancer patients with TDs, which exhibited better predictive performance than the eighth AJCC staging system in both the training and validation sets (C-index: 0.76 vs. 0.65, P < 0.05, in the training set; 0.81 vs. 0.68, P < 0.05, in the validation set, respectively). DCA curves showed that the nomogram had better net benefits than the eighth AJCC staging system. Similar findings were confirmed in the external validation set, indicating the robustness of the nomogram.
Considering the ignorance of dissected lymph node (LND) numbers in the current N staging system, we introduced the variables of LNR, LOODS, PLN, and NLN to avoid the phenomenon of “stage migration” in case of unsatisfactory LND (24). As previously reported (25, 26), all nodal staging systems exhibited good calibration among patients with TDs (all P < 0.05), and LOODS were chosen as the optimal nodal staging in the current study, with the highest C-index and the minimum AIC. The reasons why LOODS were superior to others, in our opinion, might be as follows:1) LOODS took full account of PLN and NLN numbers to minimize the possibility of “staging migration” due to poor lymph node dissection; 2) LOODS could be further stratified in patients without lymph node metastasis, which would decrease with the increasing PLN number; and 3) LNR would lose the value in cases where all lymph nodes are positive, but LOODS would not (25).
To avoid overfitting or underfitting of the model, we adopted three statistical methods to select the candidate variables. As is known to all, the big disadvantage of the Cox regression analysis is its unmanageable confounders (15), which will result in overfitting of the model. LASSO regression (13, 27) can process all independent variables simultaneously and introduce the variable λ (lambda). With the increase in λ, the regression coefficient β of each variable decreases, and some of them turn to 0, indicating that the variable makes little contribution to the model at this time and can be eliminated. In addition, BSR (14, 28) can eliminate factors that have an impact on outcome but not that important to make the model much more practical. The combination of these two methods, LASSO followed by BSR (29), solves the collinearity between independent variables but does not weaken the predictive efficiency. Hence, in this study, we adopted LASSO followed by BSR to establish model 3, which exhibited non-inferiority in discrimination and calibration compared with models 1 and 2 based on LASSO and BSR alone, but comprised the minimum variables with wider external application, which was subsequently validated in an external set. Of note, there were apparent differences in baseline characteristics between sets of training and validation, which indicated that the current model might have universal applicability.
Postoperative management, regardless of prophylactic or salvage therapy, is also a concern in order to improve the long-term prognosis of rectal cancer. Substantial evidence has shown that TDs are correlated with increased local recurrence and distant metastasis and impaired DFS and OS (10, 11, 30). Delattre et al. (2) found that postoperative chemotherapy would benefit patients with TDs, which was also validated by Shi et al. (31). As one of the most common modalities, postoperative RT also plays an important role in the postoperative management of rectal cancer, especially for those who do not receive preoperative RT. In our previous study (32), we identified the survival benefit of postoperative RT for patients with pT3N0 disease in the high-risk subgroup. Nonetheless, it remains controversial whether all TD-positive patients should receive postoperative RT (33). In the current study, we also found that postoperative RT could only prolong the median OS of patients in the high-risk subgroup, but not in the low-risk subgroup. In summary, the current nomogram could also be used for decision-making in the management of postoperative RT.
However, this study has several limitations. First, both the training and validation sets were retrospective; therefore, the current model needs to be further validated by a prospective cohort. Second, considering that the etiology and management of rectal cancer are slightly different between the United States and China, as depicted in Supplementary Table 1, international multicenter cohorts are warranted to verify the performance of the current nomogram. Third, considering the uncertainty of TD origin (2, 4, 20, 21), we only included patients who did not receive neoadjuvant treatment in the present study; hence, the nomogram might not be appropriate for those receiving neoadjuvant treatment. Finally, considering that most of the TD numbers were unattainable in the SEER database and it was insufficient to regard the number of TDs as a prognostic parameter (2, 10), the variable of TD number was not considered in the present study.
Conclusion
In conclusion, we identified a novel nomogram for predicting the prognosis of rectal cancer patients with TD. The current model could provide reliable evidence for clinical decision-making, although it still deserves further validation.
Data Availability Statement
The dataset analyzed in this study from SEER can be obtained from: https://seer.cancer.gov/data/. Other data supporting the conclusions of this article are available from the corresponding author upon reasonable request.
Ethics Statement
This study was conducted under the ethical guidelines of the Helsinki Declaration. We acquired approval from Fujian Cancer Hospital’s Ethics Committee (K2021-050-01), which waived back the individual informed consent owing to that the clinicopathological data were extracted retrospectively. On the other hand, we gained an official permit to access the research data from the SEER database.
Author Contributions
XHZ, LW, LS, XQZ, LH, GC, JW contributed to conception and design. XHZ, LW conducted data collection and analyzed the data. XHZ, LW, LS, XQZ, LH, GC, JW interpreted the data. XHZ, LW, LS drafted the manuscript. XQZ, LH, GC, JW contributed critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the Science and Technology Program of Fujian Province, China (Nos. 2019L3018 and 2019YZ016006); the Fujian Province Finance Department Project (No. (2019)827); the Fujian Province Natural Science Foundation (2021J01438); the Fujian Provincial Clinical Research Center for Cancer Radiotherapy and Immunotherapy (2020Y2012); the National Clinical Key Specialty Construction Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the SEER database for providing valuable and public datasets.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.808557/full#supplementary-material
References
1. Gabriel WB, Dukes C, Bussey H. Lymphatic Spread in Cancer of the Rectum. Br J Surg (2010) 23(90):395–413. doi: 10.1002/bjs.1800239017
2. Delattre JF, Cohen R, Henriques J, Falcoz A, Emile JF, Fratte S, et al. Prognostic Value of Tumor Deposits for Disease-Free Survival in Patients With Stage III Colon Cancer: A Post Hoc Analysis of the IDEA France Phase III Trial (PRODIGE-GERCOR). J Clin Oncol (2020) 38(15):1702–10. doi: 10.1200/JCO.19.01960
3. Gallo G. The Emerging Role of Tumor Deposits as an Independent Prognostic Factor in Advanced Colorectal Cancer. J Invest Surg (2021) 16:1–2. doi: 10.1080/08941939.2021.1936304
4. Lord AC, Graham Martínez C, D'Souza N, Pucher PH, Brown G, Nagtegaal ID. The Significance of Tumour Deposits in Rectal Cancer After Neoadjuvant Therapy: A Systematic Review and Meta-Analysis. Eur J Cancer (2019) 122:1–8. doi: 10.1016/j.ejca.2019.08.020
5. Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, Fifth Edition (1997). Union Internationale Contre Le Cancer and the American Joint Committee on Cancer. Cancer (1997) 80(9):1803–4. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9
6. Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Rectal Cancer, Version2.2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/rec%20tal.pdf.
7. Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, et al. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol (2017) 35(10):1119–27. doi: 10.1200/JCO.2016.68.9091
8. Basnet S, Lou QF, Liu N, Rana R, Shah A, Khadka M, et al. Tumor Deposit is an Independent Prognostic Indicator in Patients Who Underwent Radical Resection for Colorectal Cancer. J Cancer (2018) 9(21):3979–85. doi: 10.7150/jca.27475
9. Mayo E, Llanos AA, Yi X, Duan SZ, Zhang L. Prognostic Value of Tumour Deposit and Perineural Invasion Status in Colorectal Cancer Patients: A SEER-Based Population Study. Histopathology (2016) 69(2):230–8. doi: 10.1111/his.12936
10. Cohen R, Shi Q, Meyers J, Jin Z, Svrcek M, Fuchs C, et al. Combining Tumor Deposits With the Number of Lymph Node Metastases to Improve the Prognostic Accuracy in Stage III Colon Cancer: A Post Hoc Analysis of the CALGB/SWOG 80702 Phase III Study (Alliance). Ann Oncol (2021) 32(10):1267–75. doi: 10.1016/j.annonc.2021.07.009
11. Zheng H, Zhang J, Liu Y, Wang X. Prognostic Value of Tumor Deposits in Locally Advanced Rectal Cancer: A Retrospective Study With Propensity Score Matching. Int J Clin Oncol (2021) 26(6):1109–19. doi: 10.1007/s10147-021-01885-0
12. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28(suppl_4):iv22–40. doi: 10.1093/annonc/mdx224
13. Tibshirani R. The Lasso Method for Variable Selection in the Cox Model. Stat Med (1997) 16(4):385–95. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3
14. Harrell FE Jr, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med (1996) 15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO
15. Walter S, Tiemeier H. Variable Selection: Current Practice in Epidemiological Studies. Eur J Epidemiol (2009) 24(12):733–6. doi: 10.1007/s10654-009-9411-2
16. Brentnall AR, Cuzick J. Use of the Concordance Index for Predictors of Censored Survival Data. Stat Methods Med Res (2018) 27(8):2359–73. doi: 10.1177/0962280216680245
17. Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-Dependent ROC Curve Analysis in Medical Research: Current Methods and Applications. BMC Med Res Methodol (2017) 17(1):53. doi: 10.1186/s12874-017-0332-6
18. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and Calibration of Clinical Prediction Models: Users' Guides to the Medical Literature. JAMA (2017) 318(14):1377–84. doi: 10.1001/jama.2017.12126
19. Fitzgerald M, Saville BR, Lewis RJ. Decision Curve Analysis. JAMA (2015) 313(4):409–10. doi: 10.1001/jama.2015.37
20. Nagtegaal ID, Quirke P. Colorectal Tumour Deposits in the Mesorectum and Pericolon; a Critical Review. Histopathology (2007) 51(2):141–9. doi: 10.1111/j.1365-2559.2007.02720.x
21. Maguire A, Sheahan K. Controversies in the Pathological Assessment of Colorectal Cancer. World J Gastroenterol (2014) 20(29):9850–61. doi: 10.3748/wjg.v20.i29.9850
22. Song JS, Chang HJ, Kim DY, Kim SY, Baek JY, Park JW, et al. Is the N1c Category of the New American Joint Committee on Cancer Staging System Applicable to Patients With Rectal Cancer Who Receive Preoperative Chemoradiotherapy? Cancer (2011) 117(17):3917–24. doi: 10.1002/cncr.25968
23. Yu L, Xu T, Zhang L, Zhu Y, Fang H, Zhang H. Tumor Deposits Should Not Be Ignored in the AJCC TNM Staging System for Ypn(+) Stage Rectal Cancer With Neoadjuvant Chemoradiotherapy. J Gastrointest Surg (2020) 24(10):2298–301. doi: 10.1007/s11605-020-04675-7
24. Ueno H, Hase K, Hashiguchi Y, Shinto E, Shimazaki H, Yamamoto J, et al. Potential Causes of Stage Migration and Their Prognostic Implications in Colon Cancer: A Nationwide Survey of Specialist Institutions in Japan. Jpn J Clin Oncol (2014) 44(6):547–55. doi: 10.1093/jjco/hyu043
25. Han L, Mo S, Xiang W, Li Q, Wang R, Xu Y, et al. Comparison of Four Lymph Node Staging Systems for Predicting Prognosis for Stage IV Rectum Cancer. Ann Transl Med (2020) 8(4):111. doi: 10.21037/atm.2019.12.90
26. Petrucciani N, Carra MC, Martínez-Pérez A, Vitali GC, Landi F, Genova P, et al. Comparison of Different Nodal Staging in Patients With Locally Advanced Mid-Low Rectal Cancer After Long-Term Neoadjuvant Chemoradiation Therapy. Anticancer Res (2019) 39(4):2113–20. doi: 10.21873/anticanres.13324
27. Van HJ. Shrinkage and Penalized Likelihood as Methods to Improve Predictive 337 Accuracy. Statistica Neerlandica (2001) 55(1):17–34. doi: 10.1111/1467-9574.00154
28. Zhang Z. Variable Selection With Stepwise and Best Subset Approaches. Ann Transl Med (2016) 4(7):136. doi: 10.21037/atm.2016.03.35
29. Li H, Zhou F, Cao Z, Tang Y, Huang Y, Li Y, et al. Development and Validation of a Nomogram Based on Nutritional Indicators and Tumor Markers for Prognosis Prediction of Pancreatic Ductal Adenocarcinoma. Front Oncol (2021) 11:682969. doi: 10.3389/fonc.2021.682969
30. Zheng K, Zheng N, Xin C, Zhou L, Sun G, Wen R, et al. The Prognostic Significance of Tumor Deposit Count for Colorectal Cancer Patients After Radical Surgery. Gastroenterol Res Pract (2020) 2020:2052561. doi: 10.1155/2020/2052561
31. Shi M, Zhang H, Yao G, Wu J, Zhu C, Zhang X, et al. The Role of Tumor Deposits in Predicting the Efficacy of Chemotherapy in Stage III Colon Cancer. Front Oncol (2020) 10:586603:586603. doi: 10.3389/fonc.2020.58660
32. Huang YX, Lin YZ, Li JL, Zhang XQ, Tang LR, Zhuang QY, et al. Role of Postoperative Radiotherapy in Pt3n0 Rectal Cancer: A Risk-Stratification System Based on Population Analyses. Cancer Med (2019) 8(3):1024–33. doi: 10.1002/cam4.1991
Keywords: rectal cancer, tumor deposits, nomogram, OS, SEER
Citation: Zhong X, Wang L, Shao L, Zhang X, Hong L, Chen G and Wu J (2022) Prognostic Nomogram for Rectal Cancer Patients With Tumor Deposits. Front. Oncol. 12:808557. doi: 10.3389/fonc.2022.808557
Received: 03 November 2021; Accepted: 12 January 2022;
Published: 02 February 2022.
Edited by:
Georg F. Weber, University of Erlangen Nuremberg, GermanyReviewed by:
Maximilian Brunner, University Hospital Erlangen, GermanyMelanie Langheinrich, Universitätsmedizin Greifswald, Germany
Copyright © 2022 Zhong, Wang, Shao, Zhang, Hong, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junxin Wu, anVueGlud3VmakBhbGl5dW4uY29t; Gang Chen, bmFpY2hlbmdhbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Xiaohong Zhong1†
Xiaohong Zhong1† Lei Wang
Lei Wang Lingdong Shao
Lingdong Shao Junxin Wu
Junxin Wu