- Department of Pharmacy, Daping Hospital, Army Medical University, Chongqing, China
Epidermal growth factor receptor (EGFR) inhibitors are widely used to treat various types of cancers such as non-small cell lung cancer, head and neck cancer, breast cancer, pancreatic cancer. Adverse reactions such as skin toxicity, interstitial lung disease, hepatotoxicity, ocular toxicity, hypomagnesemia, stomatitis, and diarrhea may occur during treatment. Because the EGFR signaling pathway is important for maintaining normal physiological skin function. Adverse skin reactions occurred in up to 90% of cancer patients treated with EGFR inhibitors, including common skin toxicities (such as papulopustular exanthemas, paronychia, hair changes) and rare fatal skin toxicities (e.g., Stevens–Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis). This has led to the dose reduction or discontinuation of EGFR inhibitors in the treatment of cancer. Recently, progress has been made about research on the skin toxicity of EGFR inhibitors. Here, we summarize the mechanism of skin toxicity caused by EGFR inhibitors, measures to prevent severe fatal skin toxicity, and provide reference for medical staff how to give care and treatment after adverse skin reactions.
Introduction
The epidermal growth factor receptor (EGFR, also named HER1) is a 170 kDa transmembrane glycoprotein receptor that is coded by the c-erbB1 proto-oncogene located on the human 7q22 chromosome (1). Asparagine-linked glycosylation is a post-translational modification necessary for its active function (2). EGFR is a member of the ErbB receptor family of tyrosine protein kinases, which also includes ErbB-2 (HER2), ErbB-3 (HER3), and ErbB-4 (HER4) (3). EGFR is highly expressed in lung cancer (4), breast cancer, human glioblastoma (5), gastric carcinoma (3), rectal cancer, and head and neck cancer (6) compared to healthy tissues. The EGFR signaling pathway is involved in normal biological processes of cells, and the destruction of the dynamic balance will lead to pathological changes in healthy tissues. Overexpression of EGFR promotes cell proliferation, adhesion, metastasis, and angiogenesis and inhibits apoptosis, all of which can induce tumorigenesis (7). Therefore, EGFR inhibitors have been utilized for cancer treatment.
EGFR inhibitors are divided into monoclonal antibodies (mAb) and small molecule intracellular tyrosine kinase inhibitors (TKIs). EGFR mAb competitively inhibit ligand binding to EGFR extracellular domain with higher affinity than ligand to reduce EGFR signaling pathway activity (8). The small molecule EGFR-TKIs are ATP analogs that competitively bind to the intracellular catalytic domain of EGFR, which blocks ATP-mediated phosphorylation (9). Although EGFR inhibitors have good efficacy for a variety of tumors, adverse reactions such as skin toxicity, interstitial lung disease, hepatotoxicity, ocular toxicity, hypomagnesemia, stomatitis, and diarrhea may occur during treatment (10). These adverse reactions lead to organ, tissue, and system damage, resulting in corresponding drug induced diseases. Finally reduce patient compliance and even lead to the withdrawal of antitumor drugs. Skin toxicities is one of the most common adverse reactions caused by EGFR inhibitors
These skin toxicities may result in fatal complications if they are ignored (11). Doctors, pharmacists, and nurses must consider how to avoid severe skin toxicity in their patients and determine which patients would be prone to fatal skin toxicity. Understanding the molecular and cellular mechanism of skin toxicity and the relationship between skin toxicity and drug efficacy is essential for safe, effective, and rational use of EGFR inhibitors. Here, we focus on the mechanism of skin toxicity and fatal skin toxicity caused by EGFR inhibitors and clinical countermeasures as a mean to alleviate adverse reactions and ultimately achieve the purpose of reducing adverse emotions of the patients during the treatment phase, improving medication compliance, and effectively treating related cancers.
Activation Mechanism of EGFR Signaling Pathway
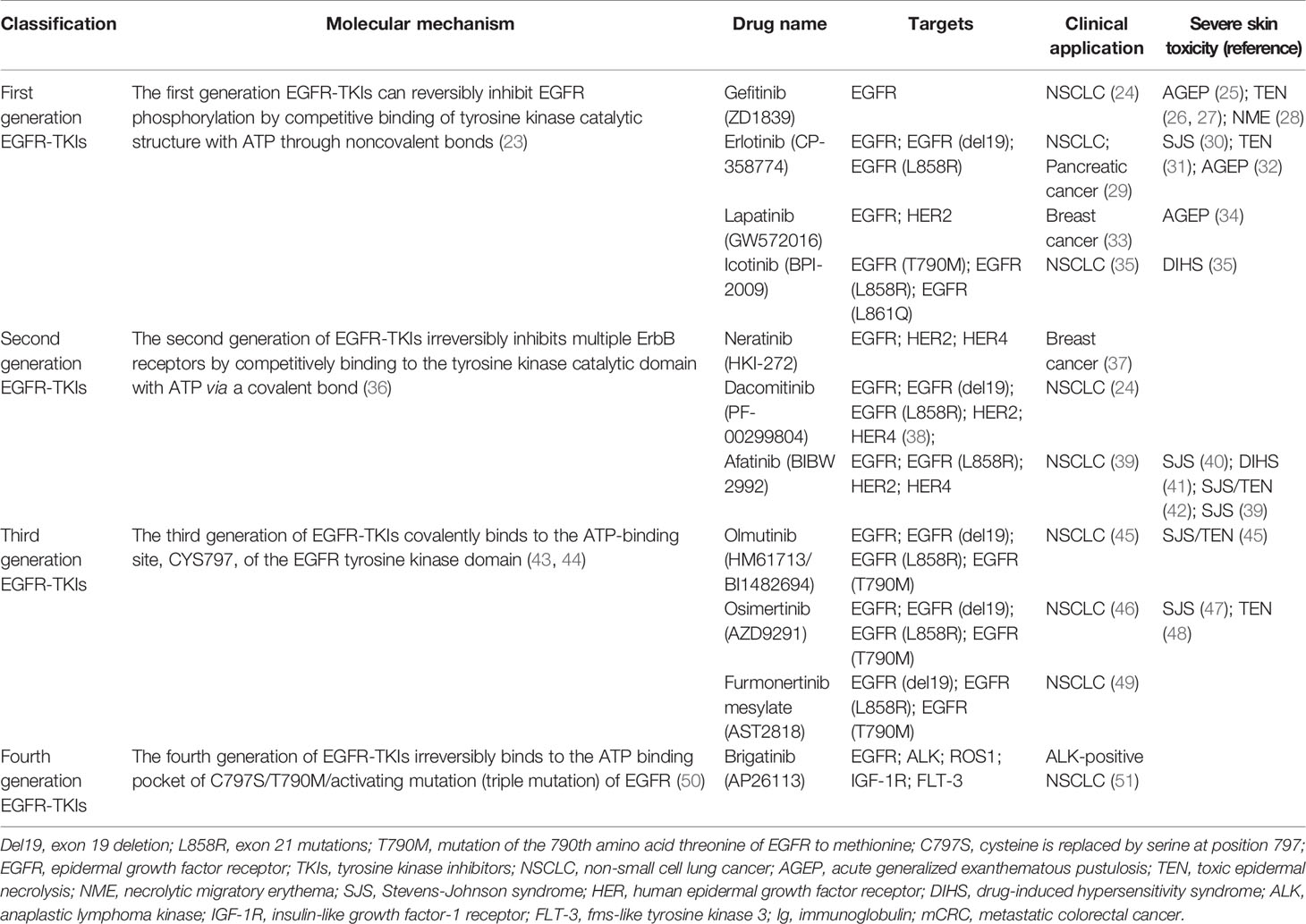
The four members of the human ErbB family have similar structures that are divided into the extracellular domain, transmembrane domain, cytoplasmic domain, and C-terminal tail domain (3). Mature EGFR consists of 1186 amino acid residues and is divided into three parts from N-terminal to C-terminal: extracellular domain (621 amino acids), hydrophobic lipophilic short transmembrane domain (23 amino acids), and cytoplasmic domain (542 amino acids) (Figure 1).
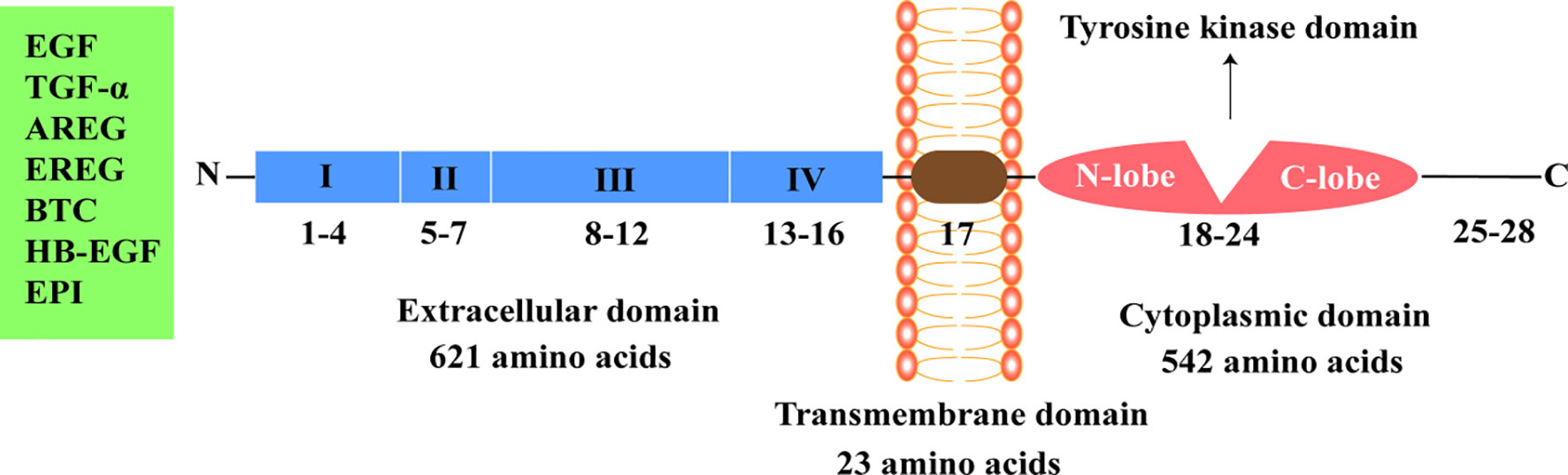
Figure 1 Epidermal growth factor receptor structure. EGF, epidermal growth factor; TGF-α, transforming growth factor-α; AREG, amphiregulin; EREG, epiregulin; BTC, betacellulin; HB-EGF, heparin binding epidermal growth factor-like growth factor; EPI, epiregulin.
The extracellular region of EGFR can be subdivided into four domains: I, II, III, and IV. Domains I (amino acids 1-133, exons 1-4) and III (amino acids 313-445, exons 8-12) are rich in leucine and are the main fragments involved in ligand binding in the extracellular domain (7). Domains II (amino acids 134-312, exons 5-7) and IV (amino acids 446-621, exons 13-16) contain 51 cysteine residues and are not involved in ligand binding. However, domain II is involved in the formation of homodimers and heterodimers with other members of the ErbB family (7, 12, 13).
The specific ligands of EGFR include epidermal growth factor (EGF), transforming growth factor-α, and amphiregulin, while non-specific ligands include epiregulin, betacellulin, heparin binding EGF-like growth factor, and epiregulin (14, 15). EGF, the ligand of EGFR, was first isolated from the mouse submandibular gland and is associated with epidermal proliferation and keratinization (16). Asparagine-linked glycosylation is a post-translational modification necessary for functional EGFR, and the extracellular domain of EGFR contains 12 sites for asparagine-linked glycosylation (2, 12). The transmembrane domain of EGFR (amino acids 622-644, exon 17) serves to link the two functional domains of the extracellular and cytoplasmic domains (13). The cytoplasmic domain of EGFR (amino acids 645-1186, exons 18-28) includes a tyrosine kinase domain (exons 18-24) and C-terminal tail (exons 25-28). The tyrosine kinase domain can be subdivided into the N-lobe and C-lobe. ATP binds to the gap formed by the two lobes. EGFR-TKIs inhibit the activation of tyrosine kinase and subsequent signaling pathways by competitively binding the ATP-binding site of the tyrosine kinase domain (17–19).
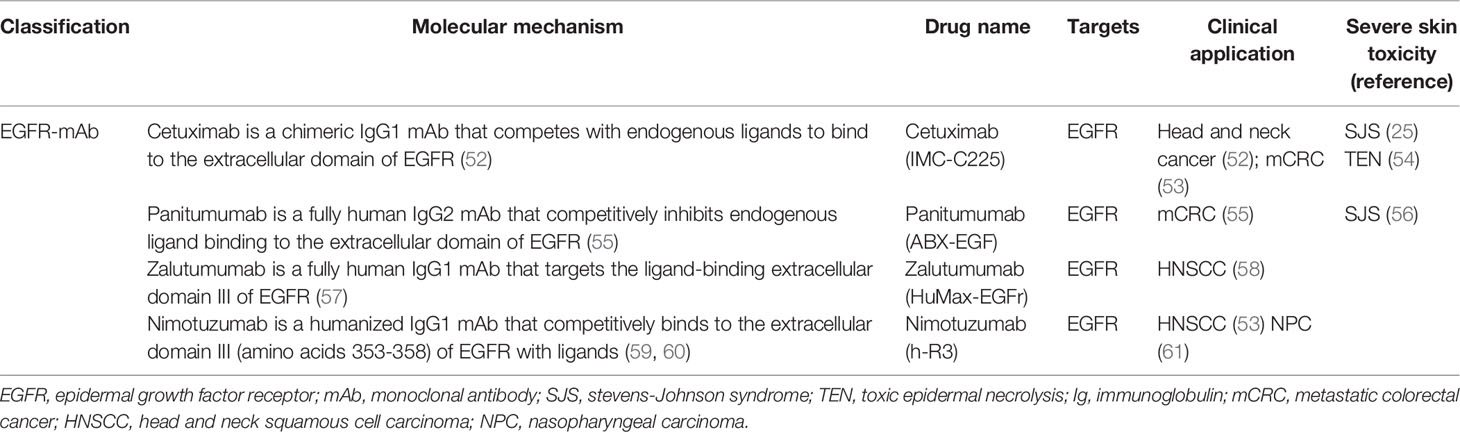
EGFR is activated in four phases (7, 20–22) (Figure 2): 1) The ligand binds to the extracellular domain of EGFR; 2) Homodimerization or heterodimerization with ErbB-2, ErbB-3, and ErbB-4 (also known as HER-2, HER-3, and HER-4, respectively) occurs. ErbB-2 is the most common heterodimerization partners of EGFR; 3) Autophosphorylation of tyrosine residues in the cytoplasmic domain occurs; 4) The activation of the intracellular signaling pathway occurs, which regulates cell proliferation, migration, differentiation, and apoptosis.
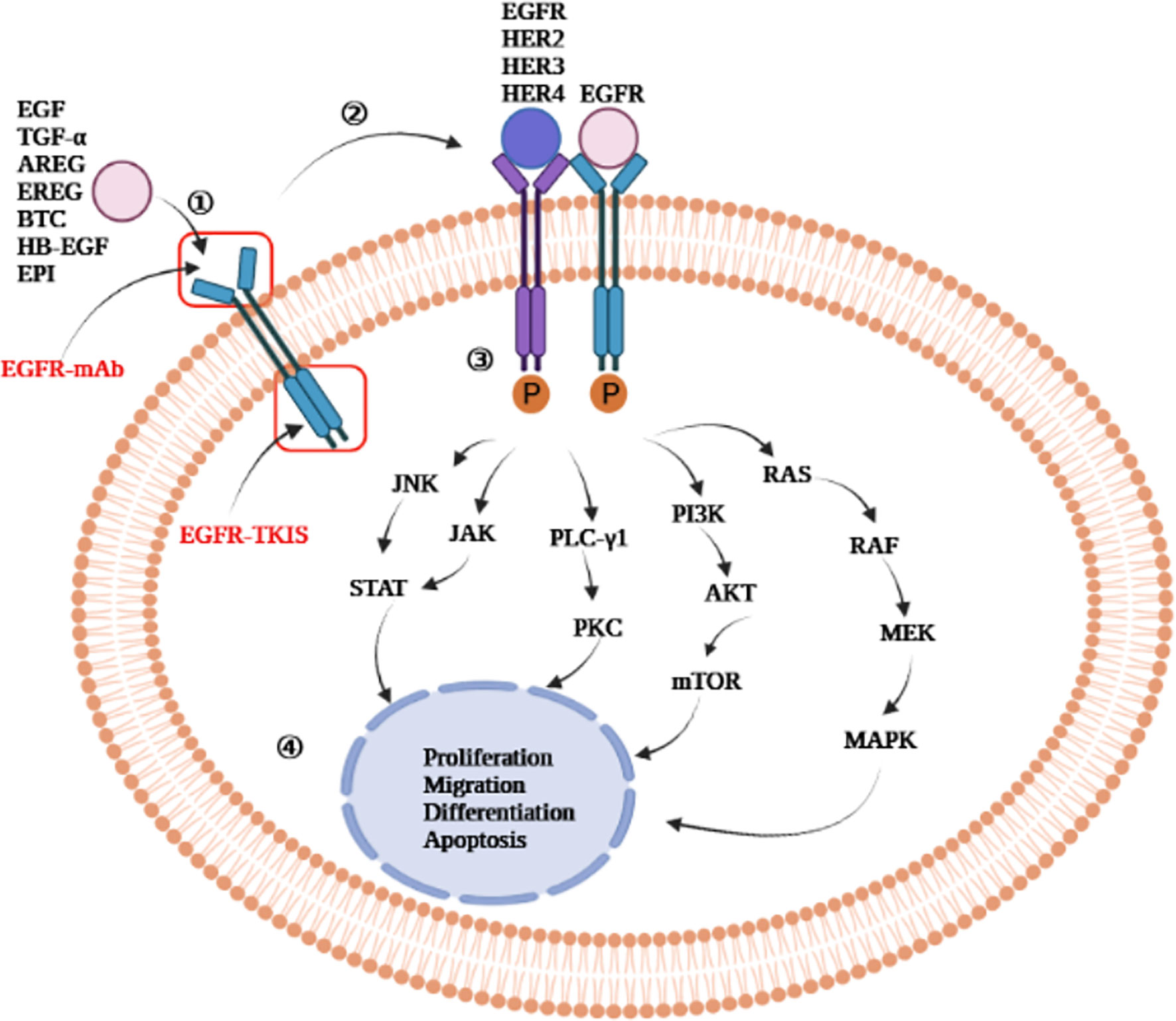
Figure 2 Epidermal growth factor receptor activation mechanism. EGFR-mAb: Cetuximab, panitumumab, zalutumumab, nimotuzumab; EGFR-TKIs: gefitinib, erlotinib, lapatinib, icotinib, neratinib, dacomitinib, afatinib, olmutinib, osimertinib, furmonertinib mesylate, brigatinib; JNK, jun amino-terminal kinase; JAK, janus activated kinase; STAT, signal transducer and activator of transcription; PLC-γ1, phospholipase C-γ1; PKC, protein kinase C; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol-3- kinase; mTOR, mammalian target of rapamycin; AKT, protein kinase B; RAS, rat sarcoma virus gene homolog; RAF, rapidly accelerated fibrosarcoma serine/threonine kinase; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; EGF, epidermal growth factor; TGF-α, transforming growth factor-α; AREG, amphiregulin; EREG, epiregulin; BTC, betacellulin; HB-EGF, heparin binding epidermal growth factor-like growth factor; EPI, epiregulin; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; mAb, monoclonal antibody; TKIs, tyrosine kinase inhibitors.
EGFR Inhibitors Used Clinically
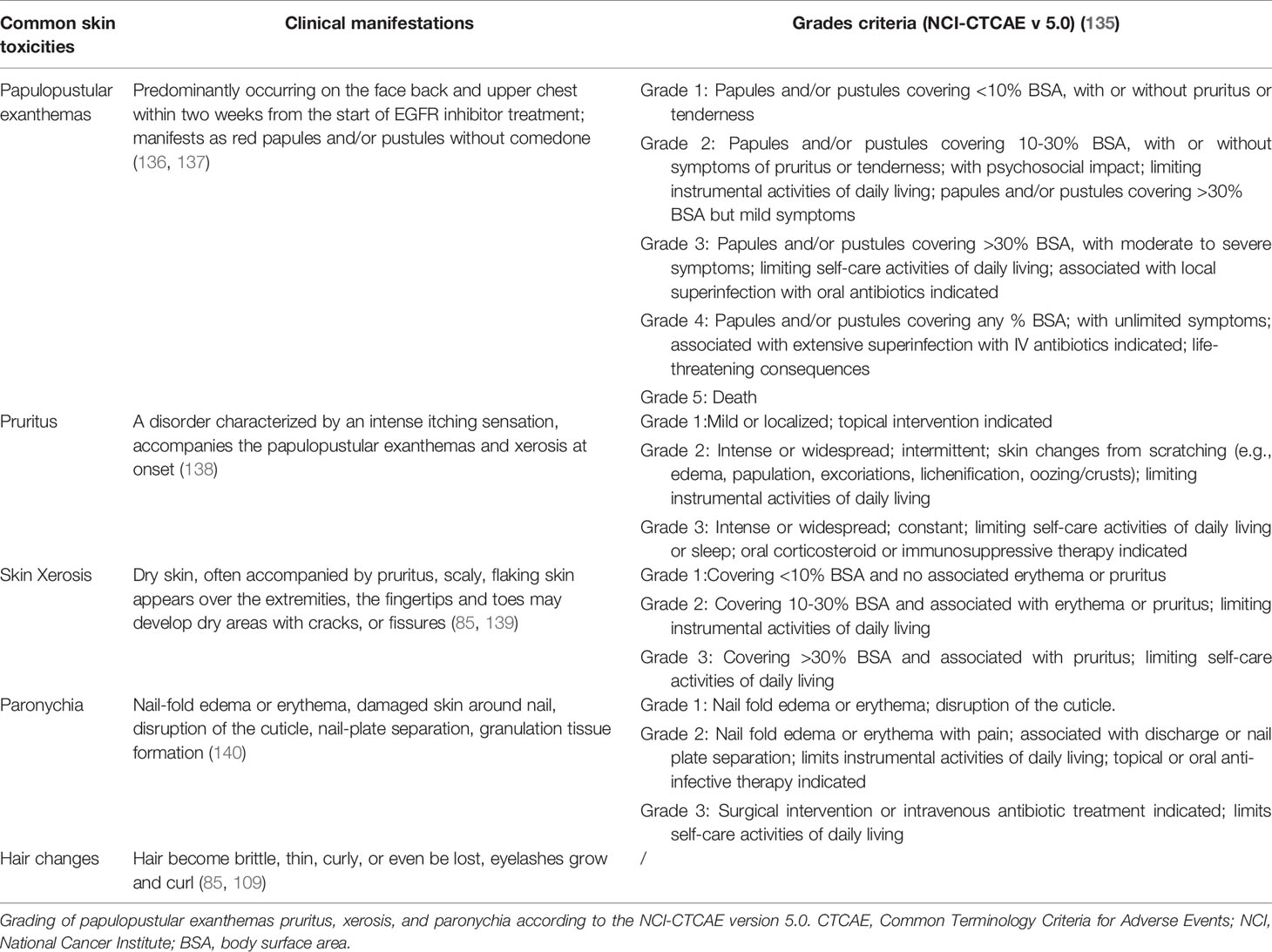
At present, there are four generations of EGFR-TKIs (Table 1) and multiple EGFR-mAbs (Table 2) that have been developed, such as cetuximab, panitumumab, zalutumumab, and nimotuzumab. The chemical formula of EGFR-TKIs for clinical use is shown in Figure 3.

Figure 3 Chemical formula of clinically used EGFR-TKIs. (A–D) are the first generation EGFR-TKIs; (E–G) are the second generation EGFR-TKIs; (H, I) are the third generation EGFR-TKIs; (J) is the fourth generation EGFR-TKIs.
The first-generation EGFR-TKIs includes gefitinib, erlotinib, lapatinib, and icotinib. Gefitinib was the first agent designed to receive approval from the United States Food and Drug Administration (FDA) for the treatment of lung cancer (62, 63). Gefitinib was considered to be safe and effective for adjuvant treatment of operable stage II-IIIA non-small cell lung cancer (NSCLC) in addition to the treatment of conventional mutated NSCLC (64, 65). Seong et al. (28) reported a rare case of necrolytic migratory erythema during the use of gefitinib. Gefitinib has also been reported to cause fatal skin toxicity such as toxic epidermal necrolysis (TEN) (26) and acute generalized exanthematous pustulosis (AGEP) (25). Although gefitinib shows excellent antitumor effects, it was eventually discontinued due to these severe skin toxicities. Erlotinib, a derivative of quinazoline was approved by the FDA on November 18, 2004 for use in NSCLC with exon 19 deletion (del19) or exon 21 point mutation (L858R) (66). Erlotinib often causes papulopustular exanthemas characterized by pruritus (67). Lapatinib (GW572016) is an ATP-competitive, reversible small-molecule inhibitor of ErbB-2 and EGFR tyrosine kinases that has been approved for the treatment of patients with metastatic breast cancer (68–71).
The mutation of amino acid 790 from threonine to methionine (T790M) increases the affinity of EGFR for ATP, which competitively reduces the efficacy of EGFR-TKIs. Therefore, EGFR (T790M) is one of the reasons for resistance to first-generation EGFR-TKIs (68, 72). In order to overcome drug resistance, the second-generation EGFR-TKIs, afatinib and dacomitinib, were developed (73). Dacomitinib was approved by the FDA on September 27, 2018 for the treatment of NSCLC patients with EGFR del19 or exon 21 L858R mutations (73). However, second-generation EGFR-TKIs were not able to be administered at full strength to inhibit T790M mutant lung cancer due to adverse side effects, such as rash caused by inhibition of normal cells (23). Ding et al. (74) concluded in a meta-analysis of clinical trials that afatinib resulted in a higher risk of rash than erlotinib or gefitinib.
In order to overcome the resistance of first and second-generation EGFR-TKIs, the third-generation EGFR-TKIs were developed. Osimertinib was approved by the FDA in 2015 to treat NSCLC patients with the EGFR T790M mutation (75, 76). In March 2021, furmonertinib mesylate was first approved in China for the treatment NSCLC patients with the EGFR T790M mutation (49). Osimertinib is no longer the only third generation EGFR-TKIs approved for the treatment of EGFR T790M mutant NSCLC. However, during the application of the third-generation EGFR-TKIs, a cysteine-to-serine mutation (C797S) occurred at C797 in the kinase binding site. The C797S mutation blocks the formation of a covalent bond at 797, which ultimately reduced the efficacy of the third-generation EGFR-TKIs (77, 78).
EGFR C797S is the most common tertiary mutation in patients with T790M-positive NSCLC treated with third-generation EGFR-TKI osimertinib. In order to overcome the EGFR C797S mutation, brigatinib was developed as a fourth-generation EGFR-TKI. It is effective against the EGFR C797S-T790M-del19 triple mutant (79). Brigatinib received approval for the treatment of anaplastic lymphoma kinase-positive metastatic NSCLC patients who had progressive disease while taking crizotinib or who were intolerant to crizotinib (80).
Cellular and Molecular Mechanism of Skin Toxicity Caused by EGFR Inhibitors
Skin is the first line of defense against the invasion of external pathogens. Skin structure from the outside to the inside is the epidermis, dermis, and subcutaneous tissue. The skin contains accessory organs such as nails, sebaceous glands, sweat glands, hair follicles, cutaneous nerves, and subcutaneous blood vessels. EGFR is widely expressed in skin keratinocytes, dendritic cells, connective tissue cells, and skin appendage organelles (e.g. sebaceous glands, sweat glands, and hair follicles) and associated with proliferation, apoptosis, migration, and differentiation of normal cells (81–84). Normal activation of EGFR signaling promotes wound healing, inhibits inflammation, and stimulates capillary constriction (85).
EGFR is widely distributed in the skin, and skin toxicity is one of the most common adverse reactions for EGFR inhibitor treatment. EGFR-mAbs generally produce more severe skin toxicity than EGFR-TKIs (14). Rare purpuric drug eruptions have been reported when using EGFR-TKIs such as gefitinib, erlotinib and afatinib. The main clinical manifestations are purpuric macules, papules, and confluent plaques on the lower extremities. These adverse side effects occur because blocking EGFR leads to endothelial inflammation, decreased vascular tone, and ultimately increased vascular permeability (86). Besides the common rash, rare severe lethal skin toxicities from EGFR inhibitors, such as Stevens-Johnson syndrome (SJS), TEN, and AGEP, are often important causes of drug discontinuation. SJS (10% mortality) and TEN (50% mortality) are two related skin and mucosal diseases caused by delayed drug hypersensitivity. They are characterized by extensive epidermal necrosis and skin detachment (the range of detached surface area:SJS < 10%, TEN > 30%, and SJS/TEN = 10%-30%) (87, 88). AGEP is characterized by the formation of sterile non-follicular pustules on the base of the erythema, often accompanied by neutrophilia and fever, which can involve multiple organs in severe cases and may be life-threatening in approximately 4% of patients (25, 89).
Because EGFR homodimers are typically associated with normal skin tissue and primary keratinocytes (90), it is speculated that EGFR inhibitors block activation of EGFR due to the inability of EGFR to homodimerize. Therefore, skin toxicity in normal cells occurs. However, the pathophysiology and mechanisms of skin toxicity caused by EGFR inhibitors have not been fully elucidated. We explain the causes of skin toxicity caused by EGFR inhibitors from the following four aspects: destruction of the physical barrier of the skin by damage to the epidermal layer, damage of hair follicles, destruction of skin homeostasis, inflammation, and host immune activation, and radiotherapy.
Destruction of the Physical Barrier of the Skin by Damage to the Epidermal Layer
Keratinocytes stratify into enucleated flattened surface squames to form a skin barrier that moisturizes and isolates pathogens. The barrier is maintained by the precise proliferation and differentiation of keratinocytes (91, 92). EGF promotes keratinocyte proliferation by increasing Ki67 and filaggrin expression through the rapidly accelerated fibrosarcoma serine/threonine kinase/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling pathway (93). In addition, EGFR regulates the terminal differentiation of keratinocytes through the phospholipase C-γ1-protein kinase C pathway to maintain and continuously regenerate the epidermal barrier (94). EGFR inhibitors can lead to destruction of physical and immune balance barriers in the epidermis, which results in skin toxicity such as dryness and rashes (93). Claudins, as essential components for the formation of tight junctions, are critical for maintaining the normal skin barrier (95). Fang et al. (96) found that gefitinib may damage the skin barrier by reducing claudin-1 and claudin-4 and increasing claudin-2 expression in keratinocytes, resulting in skin toxicity.
The epidermis is composed of five parts: basal layer, spinous layer, granular layer, stratum lucidum, and stratum corneum. EGFR is abundant in keratinocytes in the basal layer of the epidermis (97). Upon separation of proliferating basal keratinocytes from the basement membrane, they cross the spinous and granular layers and enter the stratum corneum where they stop proliferating and terminally differentiate. Then, keratinization occurs (98). EGFR inhibitors reduce the expression of the proliferation marker Ki67 suggesting keratinocyte growth arrest and premature differentiation, which ultimately results in abnormal formation and thinning of the stratum corneum (the outermost layer of the epidermis) (99, 100). Moreover, when the EGFR signaling pathway is inhibited, patients become susceptible to pathogenic bacteria, such as Staphylococcus aureus. The aggravation of inflammation further inhibits epidermal differentiation and exacerbates keratinocyte damage, leading to the occurrence of eczema-like skin reactions (86). An EGFR knockout model demonstrated that the skin of the mouse became dry and fragile (101). Therefore, EGFR inhibitors damage the natural moisturizing function of the skin and destroy skin homeostasis by damaging the physical barrier of the stratum corneum, leading to dry skin, itching, and rashes.
Damage of Hair Follicles
EGFR is also expressed abundantly in undifferentiated keratinocytes proliferating in the external root sheath of hair follicles (102). Treatment with EGFR inhibitors induces secretion of pro-inflammatory factors and lymphocyte infiltration, which leads to folliculitis and hair follicle rupture as the disease progresses (85, 103). Folliculitis is also known as acneiform rash and papulopustular exanthema, and the primary lesions are inflammatory follicular papules and pustules. The histopathology of papulopustular exanthema demonstrates purulent folliculitis with ectatic follicular infundibula and rupture of the epithelial lining. Keratin plugs and microorganisms are seen in the dilated infundibulum (104, 105). EGFR plays an essential role during the hair growth cycle (106). In addition, in vitro studies have shown that the concentration of EGF regulates the conversion between hair follicle growth and inhibition (107). Some studies have also confirmed that EGFR inhibitors have different effects on hair on different parts of the body. Hair will become brittle, thin, curly, or even be lost, while eyelashes will grow and curl (105, 108, 109).
Destruction of Skin Homeostasis-Inflammation and Host Immune Activation
In human skin, keratinocytes differentiate to provide a physical barrier in the stratum corneum, but they will also secrete various cytokines, chemokines, and antimicrobial peptides to participate in the innate immune response to resist pathogen invasion (110). Park et al. (111) found that the expression of β-defensin, an antimicrobial peptide produced by human symbiotic bacteria, decreased after using EGFR inhibitors leading to bacterial susceptibility. This may be one of the reasons for skin toxicity.
EGFR inhibitors also activate nuclear factor-κB in both cancer and normal cells, leading to destruction of immune balance and an inflammatory microenvironment (21). When EGFR signaling was inhibited, CCL2, CCL5, and CXCL10 expression levels increased and CXCL8 expression level decreased, which increased leukocyte recruitment and inflammatory infiltration (112). Wan et al. (113) induced a skin rash in female Brown Norway rats with gefitinib and found that macrophages infiltrated to the skin and secreted large amounts of inflammatory cytokines such as TREM-1, CINC-2, and CINC-3.
Furthermore, when EGFR inhibitors are used, the expression level of proapoptotic genes (such as secreted frizzled related protein 1, the apoptosis inhibitor survivin, and BCL2 associated athanogene) are upregulated, and the expression level of antiapoptotic genes (such as death associated protein kinase-1 and apoptosis response zinc finger protein requiem) are downregulated (21). The combination of tumor-induced inflammation with iatrogenic apoptotic lysis may be an important factor of associated skin toxicity.
Severe disruption of skin homeostasis induced by microbial susceptibility, inflammatory activation, and increased apoptosis ultimately leads to the generation of cutaneous toxicity.
Radiotherapy
Radiation therapy is often combined with chemotherapy or targeted therapy during tumor therapy, and the duration, dose, and area of radiation have a significant impact on the severity of skin toxicity induced by EGFR inhibitors (114). EGFR inhibitors are associated with an increased risk of severe radiation dermatitis during the first few weeks of radiation therapy when radiation damages epidermal basal cells (115). Radiotherapy and chemotherapy cause the release of chemoradiation associated molecular patterns. They play an integral role in the generation of inflammation, which causes adverse skin reactions from EGFR inhibitors more severe and complex (116). In addition, skin xerosis caused by cetuximab may aggravate dermatitis caused by radiotherapy (117).
Factors Leading to Fatal Skin Toxicity
Severe and fatal skin toxicities occur in only a small number of patients treated with EGFR inhibitors, but the pathogenic mechanism remains unclear. Le-Rademacher et al. (118) found that androgens may mediate adverse skin reactions caused by EGFR inhibitors, and anti-androgen therapy may be a method to treat or alleviate skin toxicity. Another study showed that patients with high sebaceous gland activity and sebum secretion were more sensitive to EGFR inhibitors and developed acneiform rash more frequently (119). Takahashi et al. (120) also found that men and high-weight patients who used EGFR inhibitors were more susceptible to severe skin toxicity. High male hormones, high sebum secretion, and smoking (more prevalent in males than females) are risk factors causing male lung cancer patients to have more severe adverse skin reactions.
Other risk factors leading to skin toxicity after EGFR-TKI treatment require further investigation. For example, individuals with mutations in interleukin-36 receptor antagonist may be at an increased risk of AGEP development after drug treatment (89). Ethnicity may also be a risk factor. The frequency of EGFR-TKI-associated SJS/TEN is higher in Asian countries than in other regions (121). In addition, sulfonamides, anti-epileptic drugs (carbamazepine, phenytoin, phenobarbital, lamotrigine), nonsteroidal anti-inflammatory drugs of the oxicam type, and allopurinol have been shown to be high-risk drugs for inducing delayed type hypersensitivity SJS/TEN. Therefore, the possible risk of serious adverse effects should be considered when EGFR inhibitors are used in combination with these drugs (122). When more than one susceptibility factor for lethal skin toxicity exists, the treatment of related cancers with EGFR inhibitors should be evaluated early and continuously monitored. The treatment of skin toxicity should be started early to reduce the pain and death risk of patients.
The Relationship Between Skin Toxicity and Anticancer Efficacy
EGFR is essential for maintaining the development and normal physiological functions of the epidermis in the skin. The main cause of skin toxicity is the targeting effect of anti-tumor drugs on wild-type EGFR. It has been suggested that skin response can be used as a biomarker of EGFR efficacy (123). A review of 116 patients treated with cetuximab and panitumumab by Jaka et al. (124) also confirmed that more severe rashes were associated with better outcomes. Because of the observed positive correlation of rash with efficacy, studies have suggested a new administration in which the dose is increased until the rash is most tolerable to the patient. The severity of EGFR inhibitor-induced skin toxicity is positively correlated with the therapeutic effect, making related skin toxicity a potential marker for predicting drug efficacy.
Determining how to predict whether a patient will have skin toxicity is an important area of investigation In 2004, Amador et al. (125) found that the number of single sequence repeats in EGFR intron 1 was related to the skin toxicity and anti-tumor activity of EGFR inhibitors. Kimura et al. (126) found that compared with patients who did not show any skin toxicity, the plasma macrophage inflammatory protein level was significantly decreased in patients with skin toxicity, suggesting that macrophage inflammatory protein levels in plasma might be a predictor of dermal toxicity in patients treated with gefitinib. In 2012, Moreno Garcia et al. (127) observed that elevated plasma creatine kinase was associated with EGFR-TKI-induced rash, and in vitro experiments showed that the expression level of cytosolic isoforms of creatine kinase-brain increased after EGFR-TKIs stimulated human keratinocytes. Steffens et al. (128) found that patients treated with higher erlotinib/O-demethyl-erlotinib (O-demethyl-erlotinib is the main active metabolite of erlotinib) had longer progression free survival and overall survival. They were also more prone to adverse skin reactions. The occurrence of rash was positively correlated with progression free survival and overall survival. The identification of biomarkers for severe skin toxicity can help doctors to take preventive measures to prevent severe or even fatal skin toxicity in patients. These blood biomarkers can predict drug efficacy or serious skin toxicity earlier than the occurrence of a skin rash, and it is more appropriate to predict the effect of EGFR inhibitors for patients who are not prone to skin toxicity.
Whether the efficacy of all EGFR inhibitors can be measured by skin toxicity is debatable. When applying the less targetable first-generation EGFR-TKIs (e.g., erlotinib, gefitinib, afatinib), the targeted toxicity of the skin may serve as a biomarker to measure anticancer efficacy. However, skin toxicity as an indicator of efficacy is not applicable to all EGFR-TKIs. Osimertinib (third-generation EGFR-TKIs) is typically used for treatment of NSCLC patients with the T790M resistance mutation. It has significantly greater activity against tumor EGFR with mutations del19, L858R, and T790M than wild-type EGFR (129, 130). The incidence of adverse skin reactions is lower with osimertinib than with erlotinib, but it is an effective treatment for NSCLC with T790M mutations. Further investigations are needed because the use of skin toxicity as an indicator of EGFR inhibitor efficacy is incomplete (131).
Therapeutic Strategies for Skin Toxicity
Up to 90% of cancer patients treated with EGFR inhibitors have skin adverse reactions. Of these, 76% of patients reported that they interrupted the EGFR inhibitor therapy, 32% of patients completely discontinued the EGFR inhibitor therapy, and 60% of patients reduced the dose of the EGFR inhibitor (132, 133). The most common skin toxicity caused by EGFR inhibitors is follicular papulopustular exanthemas, also known as follicular rash. It usually occurs on the head, back, and upper chest in the first few weeks of treatment. The lesions disappear without sequelae upon withdrawal of the EGFR inhibitor (134). Sebostasis, epidermal atrophy, itchy eczema, skin xerosis, paronychia, and changes in hair (such as hair and eyelashes) often occur after 1 to 2 months of treatment (132). In addition, skin toxicity caused by EGFR inhibitors is often accompanied by severe pain and extreme itching causing patients to endure physical pain and psychological stress. The clinical manifestations and basic grades of common skin toxicities (e.g., papulopustular exanthemas, pruritus, xerosis, paronychia, hair changes) caused by EGFR inhibitors are shown in Table 3.
Treatment strategies for skin toxicity caused by EGFR inhibitors currently include empirical treatment and expert consensus in countries such as the United Kingdom (141), Germany (142), Taiwan (136), France (143, 144), Italy (145), and Spain (146). These consensuses general principles of treatment about skin toxicities are consistent but differ slightly. They mainly describe treatment strategies for common skin toxicities caused by EGFR inhibitors, but not for fatal skin toxicities. Moreover, most treatments are focused on alleviating symptoms without effective etiological treatment. There is no recognized authoritative guide for the treatment of EGFR inhibitors related skin toxicity. This is an urgent clinical problem that needs to be solved.
Symptomatic Treatment
Papulopustular Exanthemas
When grade 1 rash occurs, the patient can continue to use EGFR inhibitors and to use non-alcoholic emollients (141). Reduction or discontinuation of EGFR inhibitors should be considered when grade 2 rash duration is unmanageable or the patient is unable to tolerate it. EGFR inhibitor therapy should be temporarily discontinued when ≥ grade 3 rash appears (141). Therapeutic measures are shown in more detail in Table 4.
Pruritus Due to Papulopustular Exanthemas and Xerosis
Grade 1-2 can be treated with topical steroids (0.05% clobetasol), and oral antihistamines (cetirizine, loratadine, etc.) can be used for grade 3 pruritus. In addition to the drugs mentioned above, gamma-aminobutyric acid agonists, neurokinin-1 receptor antagonists, antidepressants, corticosteroids, and other drugs can be added for treatment (150, 151). However, caution should be taken to avoid systemic steroids as they can have acneiform rash-like side effects (152). However, when a rash of grade ≥ 3 occurs, systemic dexamethasone or prednisolone is usually used for treatment (83).
Once bacterial infection occurs, systemic antibiotics can be selected for treatment based on a drug sensitivity test (153). If local use of metronidazole is not enough to control symptoms of papulopustular lesions, then they can be treated by oral tetracycline (152, 154). For skin toxicity with pustules and a large amount of exudate (typically grade 3 or higher), the use of both tetracycline and saline compresses (15 minutes, 2 to 3 times a day) can effectively control inflammation (152, 155). Doxycycline is recommended for patients with renal insufficiency, and minocycline is recommended for patients living in areas with high ultraviolet exposure (156). At the same time, attention should be paid to the intestinal microflora disorder caused by long-term systemic antibiotics in the treatment of rash (157).
In addition to the conventional treatment mentioned above, Bavetta et al. (158) found a significant improvement in skin symptoms after 4 weeks of treatment with a cream containing 1.5% polydatin (a natural precursor of resveratrol), suggesting that it may be used as an adjunctive agent for prophylactic treatment of papulopustular exanthemas and as an alternative to corticosteroids. Lacouture et al. (159) showed that although BRAF inhibitors can activate mitogen-activated protein kinase downstream of EGFR, topical use of BRAF inhibitor LUT014 can improve the skin toxicity induced by EGFR inhibitors cetuximab or panitumumab. Additionally, topical use of recombinant human EGF may ameliorate the rash produced by EGFR inhibitors by regulating the normal proliferation and differentiation of keratinocytes and reducing the expression of inflammatory factors (93).
Skin Xerosis
Patients with dry skin should use moisturizing emollients several times a day, avoid bathing with soap and hot water, and use emollients to moisturize the skin after cleansing (147). Water-based creams aggravate dry skin and very greasy emollients increase the risk of folliculitis. Therefore, ointment is recommended for the care of dry skin. Specific emollients and soap substitutes are recommended by the United Kingdom EGFR-TKI expert consensus on adverse event management published in 2015 (141). In addition, skin dryness with eczematous lesions is treated with topical steroids (160).
Paronychia
Paronychia can be extremely painful to the patient, leading to difficulty in walking and limited mobility by affecting the nails of the fingers and toes (148). A retrospective study by Osio et al. (103) found that patients using EGFR inhibitors for more than 6 months had a > 50% chance of developing paronychia. Patients with paronychia can be treated with silver nitrate, preservatives, topical corticosteroids, and antibiotics. For grade 1 and 2 paronychia, topical betamethasone valerate (2-3 times, qd) is recommended; for grade 3 paronychia, local use of clobetasol cream (2-3 times, qd) is recommended. Patients with periungual granulomas can be treated with nitrate first. If there is no response, then curettage and cauterization can be utilized (133, 148, 161). Therapeutic measures are shown in more detail in Table 5.
Hair Changes
Topical minoxidil is recommended for non-cicatricial alopecia of the head caused by EGFR inhibitors. Topical steroids are recommended for inflammatory and cicatricial alopecia (160). Curled hypertrophic long eyelashes can be trimmed, and facial hirsutism can be treated with laser hair removal (160).
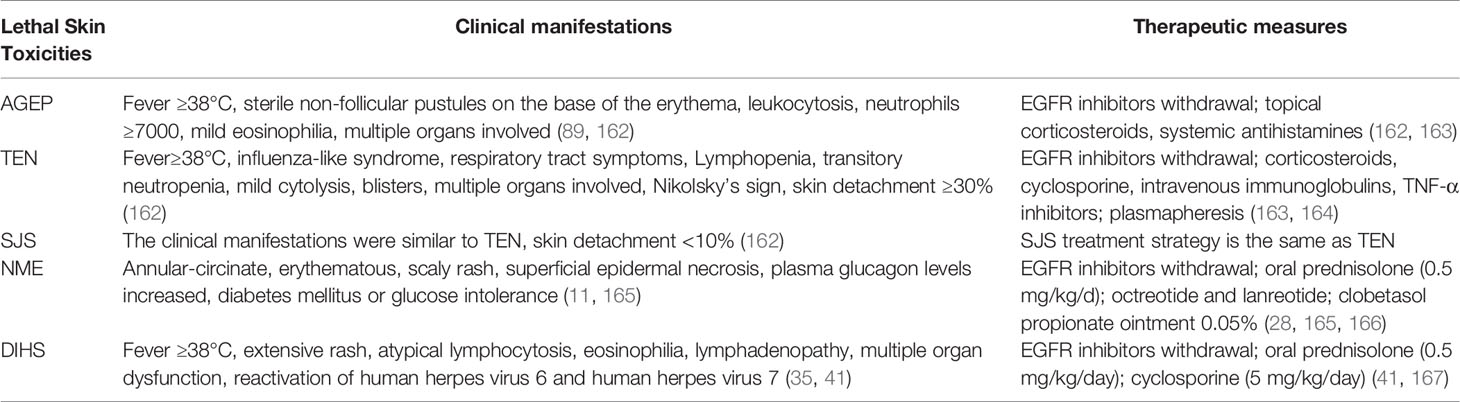
Management of Lethal Severe Cutaneous Adverse Reactions
Rare but fatal adverse skin reactions such as AGEP, SJS, TEN, and SJS/TEN may be caused when EGFR inhibitors are used. Their clinical manifestations and related therapeutic measures are shown in the Table 6. The general treatment principle is to stop the relevant EGFR inhibitors, reduce fluid loss, replenish body fluids, control pain, and provide adequate nutrition (88). AGEP symptoms usually resolve rapidly after discontinuing EGFR inhibitors. Topical corticosteroids and systemic antihistamines are recommended for symptom control (168).
For more severe skin toxicities such as SJS, TEN, SJS/TEN, conservative treatment (e.g., applying emollients) recommends maintaining skin integrity and preventing fluid loss. Surgical debridement is recommended only when infection occurs. Aggressive treatment recommends removing exfoliated epidermis that may be infected (88). In subsequent treatment, emollients and steroid creams can be used alternately for moisturizing and anti-inflammation. Gauze soaked with betadine can be used for bandaging (169).
A meta-analysis of the literature suggested that cyclosporine was effective in reducing mortality from SJS/TEN, and the combination of cyclosporine and systemic steroids may be an effective treatment for SJS/TEN (170–172). Mucosal damage caused by TEN often affects the eyes, gastrointestinal tract, and respiratory tract. TEN often causes eye keratitis and corneal erosion. It is recommended to consult an ophthalmologist, use antibiotic eye drops to prevent bacterial infection, and use eye lubricant combined with topical corticosteroids for the treatment of eye complications. Attention should be paid to secondary glaucoma caused by steroid treatment (173, 174). Oral ulcers are the most common in TEN and can be treated with topical lidocaine gel or cocaine mouthwash. In addition, the mucous membrane of the respiratory tract may fall off and cause respiratory distress that requires management by a specialized physician (169).
Preventive Measures
Due to the important role of EGFR in the normal physiological function of the skin, the incidence of adverse skin reactions caused by EGFR inhibitors is 60%-85%. These adverse reactions often lead to the reduction or even withdrawal of antitumor drugs (175). Therefore, preventing skin toxicity is increasingly gaining attention by investigators. Prophylactic use of emollients, sunscreen, mild body wash and facial cleansers, are ointment containing EGF are beneficial measures to prevent or reduce skin toxicity in patients treated with EGFR inhibitors (141, 142, 176).
A phase III clinical trial conducted in Canada in 2016 showed that the preventive use of minocycline (100 mg twice a day for 1 month) before erlotinib did not reduce the incidence of rashes but reduced the incidence of grade 3 skin toxicity while not affecting efficacy (177). Takahashi et al. (120) also showed that the grade of acneiform rash was lower after preventive use of minocycline. Meanwhile, Ichiki et al. (178) found that prophylactic use of minocycline (50 mg twice a day for 4 weeks) reduced rashes and paronychia induced by afatinib. In addition, preventive use of minocycline and topical corticosteroids may be effective for afatinib-induced paronychia, but elevated transaminase was found in patients during the use of minocycline. Therefore, long-term use of minocycline should be noted for possible liver damage (179).
Preventive use of doxycycline (100 mg twice a day for 4 weeks) can reduce the incidence of grade 2 or high adverse skin reactions caused by dacomitinib (180). In the treatment of refractory metastatic colorectal cancer with panitumumab, prophylactic doxycycline (100 mg twice a day for 6 weeks) and topical moisturizers, sunblock, and 1% hydrocortisone cream reduced the incidence of panitumumab-induced skin toxicity higher than grade 2 by 50% (181). Petrelli et al. (182) conducted a systematic review and meta-analysis of studies on the use of antibiotics to prevent skin rashes before 2016 and found that preventive use of minocycline or doxycycline reduced the absolute risk of all skin rashes (grade1-4) and severe skin rashes (grade 2-4) by 10% and 25%, respectively.
A randomized, open-label trial confirmed that tetracycline (250 mg twice a day for 4 weeks) was effective for afatinib-induced acneiform rash, and prophylactic use of tetracycline reduced the incidence and severity of rashes (183). However, Jatoi et al. (184) found that prophylactic use of tetracycline (500 mg orally twice a day for 28 days) did not reduce the incidence or severity of rashes induced by EGFR inhibitors. The different doses may be the reason for the inconsistent research results. Petrelli et al. (182) concluded that tetracycline could significantly reduce the incidence of severe rash induced by EGFR inhibitors after analyzing 13 clinical studies. Hofheinz et al. (185) recommended prophylactic use of antibiotics (such as tetracycline, doxycycline, and minocycline) on the first day of EGFR therapy to reduce the severity of adverse skin reactions and improve patient compliance. However, Italian experts do not recommend the preventive use of antibiotics as a treatment method to prevent serious skin toxicity of EGFR inhibitors (145). For skin toxicity caused by EGFR inhibitors, whether to use antibiotics prophylactically needs to be determined by comprehensively considering the situation of the patient.
In addition to the aforementioned studies on the preventive use of antibiotics, nonsteroidal anti-inflammatory drugs may also play a role in preventing EGFR inhibitor-related rashes (186). Local prophylactic use of 3% chloramphenicol + 0.5% prednisolone ointment significantly reduced the severity of facial papulopustular exanthemas induced by EGFR inhibitors (175). Although studies have shown that preventive use of vitamin K3 cream does not reduce the number of papulopustular exanthemas (187), preventive use of vitamin K1 cream can reduce the incidence of grade 2 or higher rashes (188). A randomized single-blind trial conducted by Chayahara et al. (189) showed that compared with the control group adapalene treatment did not prevent acneiform rash and may have harmful effects. Therefore, adapalene is not recommended to prevent acneiform rash caused by EGFR inhibitors.
Conclusion
To avoid the lethal skin toxicity caused by EGFR inhibitors, more targeted drugs need to be developed as well as conducting further investigations on efficacious preventive measures before cancer treatment and beneficial treatment measures after adverse skin reactions occur. In addition, there is no official or unified guidelines to deal with skin toxicities induced by EGFR inhibitors. Therefore, to create an authoritative guide would be beneficial to clinicians. The occurrence of adverse skin reactions and fatal skin toxicity are the most widespread reasons that limit anti-tumor treatments with EGFR inhibitors. Using the existing evidence for prevention and treatment should be an area of interest for medical staff and scientific researchers.
This paper summarized the cellular and molecular mechanisms of EGFR signaling and adverse skin reactions caused by EGFR inhibitors to provide ideas for the use of EGFR inhibitors and the prevention of related skin toxicity in cancer treatment. Effectively preventing and treating skin toxicity without damaging the anti-tumor efficacy of EGFR inhibitors is the ultimate goal we want to achieve. Treatment after the occurrence of skin toxicity is the key to effective anti-tumor treatment and a good prognosis of patients. This will require medical care providers to summarize and record more treatment details during their daily work, formulate a series of effective treatment schemes, and publish these results.
Author Contributions
LY conceived and supervised the project. LYP summed up the literature and drafted the manuscript. FRQ collected and organized the inhibitors and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Chongqing Clinical Pharmacy Key Specialties Construction Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sabbah DA, Hajjo R, Sweidan K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr Top Med Chem (2020) 20(10):815–34. doi: 10.2174/1568026620666200303123102
2. Lopez Sambrooks C, Baro M, Quijano A, Narayan A, Cui W, Greninger P, et al. Oligosaccharyltransferase Inhibition Overcomes Therapeutic Resistance to EGFR Tyrosine Kinase Inhibitors. Cancer Res (2018) 78(17):5094–106. doi: 10.1158/0008-5472.CAN-18-0505
3. Arienti C, Pignatta S, Tesei A. Epidermal Growth Factor Receptor Family and its Role in Gastric Cancer. Front Oncol (2019) 9:1308. doi: 10.3389/fonc.2019.01308
4. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR Mutations in Lung Cancer: Correlation With Clinical Response to Gefitinib Therapy. Science (2004) 304(5676):1497–500. doi: 10.1126/science.1099314
5. Sugawa N, Ekstrand AJ, James CD, Collins VP. Identical Splicing of Aberrant Epidermal Growth Factor Receptor Transcripts From Amplified Rearranged Genes in Human Glioblastomas. Proc Natl Acad Sci USA (1990) 87(21):8602–6. doi: 10.1073/pnas.87.21.8602
6. Arteaga CL, Engelman JA. ERBB Receptors: From Oncogene Discovery to Basic Science to Mechanism-Based Cancer Therapeutics. Cancer Cell (2014) 25(3):282–303. doi: 10.1016/j.ccr.2014.02.025
7. Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) (2017) 9(5):52. doi: 10.3390/cancers9050052
8. Reddavid R, Dagatti S, Franco C, Puca L, Tomatis M, Corso S, et al. Molecularly Targeted Therapies for Gastric Cancer. State Art Cancers (Basel) (2021) 13(16):4094. doi: 10.3390/cancers13164094
9. Ciardiello F, Tortora G. EGFR Antagonists in Cancer Treatment. N Engl J Med (2008) 358(11):1160–74. doi: 10.1056/NEJMra0707704
10. Shah RR, Shah DR. Safety and Tolerability of Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors in Oncology. Drug Saf (2019) 42(2):181–98. doi: 10.1007/s40264-018-0772-x
11. Abdelli W, Alaoui F, Souissi A, Sassi W, Chelly I, Haouet S, et al. Case of Delayed Diagnosis of Necrolytic Migratory Erythema. Clin Case Rep (2021) 9(12):e05179. doi: 10.1002/ccr3.5179
12. Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, et al. Human Epidermal Growth Factor Receptor Cdna Sequence and Aberrant Expression of the Amplified Gene in A431 Epidermoid Carcinoma Cells. Nature (1984) 309(5967):418–25. doi: 10.1038/309418a0
13. Roskoski R Jr. The Erbb/HER Family of Protein-Tyrosine Kinases and Cancer. Pharmacol Res (2014) 79:34–74. doi: 10.1016/j.phrs.2013.11.002
14. Li T, Perez-Soler R. Skin Toxicities Associated With Epidermal Growth Factor Receptor Inhibitors. Target Oncol (2009) 4(2):107–19. doi: 10.1007/s11523-009-0114-0
15. Singh B, Carpenter G, Coffey RJ. EGF Receptor Ligands: Recent Advances. F1000Res (2016) 5:1–11. doi: 10.12688/f1000research.9025.1
16. Cohen S, Elliott GA. The Stimulation of Epidermal Keratinization by a Protein Isolated From the Submaxillary Gland of the Mouse. J Invest Dermatol (1963) 40:1–5. doi: 10.1038/jid.1963.1
17. Walton GM, Chen WS, Rosenfeld MG, Gill GN. Analysis of Deletions of the Carboxyl Terminus of the Epidermal Growth Factor Receptor Reveals Self-Phosphorylation at Tyrosine 992 and Enhanced In Vivo Tyrosine Phosphorylation of Cell Substrates. J Biol Chem (1990) 265(3):1750–4. doi: 10.1016/S0021-9258(19)40080-X
18. Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the Epidermal Growth Factor Receptor Kinase Domain Alone and in Complex With a 4-Anilinoquinazoline Inhibitor. J Biol Chem (2002) 277(48):46265–72. doi: 10.1074/jbc.M207135200
19. Duggirala KB, Lee Y, Lee K. Chronicles of EGFR Tyrosine Kinase Inhibitors: Targeting EGFR C797S Containing Triple Mutations. Biomol Ther (Seoul) (2021) 30(1):19–27. doi: 10.4062/biomolther.2021.047
20. Graus-Porta D, Beerli RR, Daly JM, Hynes NE. Erbb-2, the Preferred Heterodimerization Partner of All Erbb Receptors, is a Mediator of Lateral Signaling. EMBO J (1997) 16(7):1647–55. doi: 10.1093/emboj/16.7.1647
21. Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M. Inhibition of the Epidermal Growth Factor Receptor Increases Expression of Genes That Stimulate Inflammation, Apoptosis, and Cell Attachment. Mol Cancer Ther (2005) 4(4):650–8. doi: 10.1158/1535-7163.MCT-04-0238
22. Lee CS, Milone M, Seetharamu N. Osimertinib in EGFR-Mutated Lung Cancer: A Review of the Existing and Emerging Clinical Data. Onco Targets Ther (2021) 14:4579–97. doi: 10.2147/OTT.S227032
23. Papini F, Sundaresan J, Leonetti A, Tiseo M, Rolfo C, Peters GJ, et al. Hype or Hope - can Combination Therapies With Third-Generation EGFR-Tkis Help Overcome Acquired Resistance and Improve Outcomes in EGFR-Mutant Advanced/Metastatic NSCLC? Crit Rev Oncol Hematol (2021) 166:103454. doi: 10.1016/j.critrevonc.2021.103454
24. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib Versus Gefitinib as First-Line Treatment for Patients With EGFR-Mutation-Positive Non-Small-Cell Lung Cancer (ARCHER 1050): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(11):1454–66. doi: 10.1016/S1470-2045(17)30608-3
25. Chen CB, Wu MY, Ng CY, Lu CW, Wu J, Kao PH, et al. Severe Cutaneous Adverse Reactions Induced by Targeted Anticancer Therapies and Immunotherapies. Cancer Manag Res (2018) 10:1259–73. doi: 10.2147/CMAR.S163391
26. Huang JJ, Ma SX, Hou X, Wang Z, Zeng YD, Qin T, et al. Toxic Epidermal Necrolysis Related to AP (Pemetrexed Plus Cisplatin) and Gefitinib Combination Therapy in a Patient With Metastatic Non-Small Cell Lung Cancer. Chin J Cancer (2015) 34(2):94–8. doi: 10.5732/cjc.014.10151
27. Jackman DM, Cioffredi LA, Jacobs L, Sharmeen F, Morse LK, Lucca J, et al. A Phase I Trial of High Dose Gefitinib for Patients With Leptomeningeal Metastases From Non-Small Cell Lung Cancer. Oncotarget (2015) 6(6):4527–36. doi: 10.18632/oncotarget.2886
28. Seong JY, Kong SH, Choi YS, Suh HS. Necrolytic Migratory Erythema-Like Skin Lesion During Gefitinib Treatment: A Rare Cutaneous Adverse Reaction. JAMA Dermatol (2016) 152(8):947–8. doi: 10.1001/jamadermatol.2016.1098
29. Modjtahedi H, Essapen S. Epidermal Growth Factor Receptor Inhibitors in Cancer Treatment: Advances, Challenges and Opportunities. Anticancer Drugs (2009) 20(10):851–5. doi: 10.1097/CAD.0b013e3283330590
30. Wnorowski AM, de Souza A, Chachoua A, Cohen DE. The Management of EGFR Inhibitor Adverse Events: A Case Series and Treatment Paradigm. Int J Dermatol (2012) 51(2):223–32. doi: 10.1111/j.1365-4632.2011.05082.x
31. Li X, Kamenecka TM, Cameron MD. Cytochrome P450-Mediated Bioactivation of the Epidermal Growth Factor Receptor Inhibitor Erlotinib to a Reactive Electrophile. Drug Metab Dispos (2010) 38(7):1238–45. doi: 10.1124/dmd.109.030361
32. Komiya N, Takahashi K, Kato G, Kubota M, Tashiro H, Nakashima C, et al. Acute Generalized Exanthematous Pustulosis Caused by Erlotinib in a Patient With Lung Cancer. Case Rep Oncol (2021) 14(1):599–603. doi: 10.1159/000514146
33. Ouyang DJ, Chen QT, Anwar M, Xie N, Ouyang QC, Fan PZ, et al. The Efficacy of Pyrotinib as a Third- or Higher-Line Treatment in HER2-Positive Metastatic Breast Cancer Patients Exposed to Lapatinib Compared to Lapatinib-Naive Patients: A Real-World Study. Front Pharmacol (2021) 12:682568. doi: 10.3389/fphar.2021.682568
34. Lakshmi C, Pillai S, Srinivas CR. Lapatinib-Induced Acute Generalized Exanthematous Pustulosis. Indian Dermatol Online J (2010) 1(1):14–7. doi: 10.4103/2229-5178.73251
35. Chen X, Wang S, Li L. A Case of Drug-Induced Hypersensitivity Syndrome Induced by Icotinib Managed by Intravenous Immunoglobulin and Systemic Corticosteroids. Indian J Dermatol Venereol Leprol (2018) 84(3):350–2. doi: 10.4103/ijdvl.IJDVL_490_17
36. Shah R, Lester JF. Tyrosine Kinase Inhibitors for the Treatment of EGFR Mutation-Positive Non-Small-Cell Lung Cancer: A Clash of the Generations. Clin Lung Cancer (2020) 21(3):e216–28. doi: 10.1016/j.cllc.2019.12.003
37. Le Du F, Dieras V, Curigliano G. The Role of Tyrosine Kinase Inhibitors in the Treatment of HER2+ Metastatic Breast Cancer. Eur J Cancer (2021) 154:175–89. doi: 10.1016/j.ejca.2021.06.026
38. Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O'Connell J, et al. Targeting HER2 Aberrations as Actionable Drivers in Lung Cancers: Phase II Trial of the Pan-HER Tyrosine Kinase Inhibitor Dacomitinib in Patients With HER2-Mutant or Amplified Tumors. Ann Oncol (2015) 26(7):1421–7. doi: 10.1093/annonc/mdv186
39. Doesch J, Debus D, Meyer C, Papadopoulos T, Schultz ES, Ficker JH, et al. Afatinib-Associated Stevens-Johnson Syndrome in an EGFR-Mutated Lung Cancer Patient. Lung Cancer (2016) 95:35–8. doi: 10.1016/j.lungcan.2016.02.015
40. Honda Y, Hattori Y, Katsura S, Terashima T, Manabe T, Otsuka A, et al. Stevens-Johnson Syndrome-Like Erosive Dermatitis Possibly Related to Afatinib. Eur J Dermatol (2016) 26(4):413–4. doi: 10.1684/ejd.2016.2807
41. Oyama B, Morikawa K, Sakaguchi T, Tsunoda A, Kida H, Inoue T, et al. Drug-Induced Hypersensitivity Syndrome by EGFR-TKI in a Patient With Lung Cancer. Intern Med (2021) 60(3):441–4. doi: 10.2169/internalmedicine.4237-19
42. Nuhnen VP, Schon MP, Mossner R. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Overlap in a NSCLC Patient Treated With Afatinib. J Dtsch Dermatol Ges (2018) 16(2):199–201. doi: 10.1111/ddg.13412
43. Wu SG, Shih JY. Management of Acquired Resistance to EGFR TKI-Targeted Therapy in Advanced Non-Small Cell Lung Cancer. Mol Cancer (2018) 17(1):38. doi: 10.1186/s12943-018-0777-1
44. Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel Mutant-Selective EGFR Kinase Inhibitors Against EGFR T790M. Nature (2009) 462(7276):1070–4. doi: 10.1038/nature08622
45. Nagasaka M, Zhu VW, Lim SM, Greco M, Wu F, Ou SI. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors for Advanced EGFR+ NSCLC. J Thorac Oncol (2021) 16(5):740–63. doi: 10.1016/j.jtho.2020.11.028
46. Hirashima T, Satouchi M, Hida T, Nishio M, Kato T, Sakai H, et al. Osimertinib for Japanese Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: A Pooled Subgroup Analysis. Cancer Sci (2019) 110(9):2884–93. doi: 10.1111/cas.14120
47. Lin YT, Chu CY. Osimertinib-Induced Stevens-Johnson Syndrome in a Patient With EGFR T790M Mutation-Positive Non-Small Cell Lung Cancer. Lung Cancer (2019) 129:110–1. doi: 10.1016/j.lungcan.2018.12.030
48. Wang J, Cheng X, Lu Y, Zhou B. A Case Report of Toxic Epidermal Necrolysis Associated With AZD-9291. Drug Des Devel Ther (2018) 12:2163–7. doi: 10.2147/DDDT.S168248
49. Deeks ED. Furmonertinib: First Approval. Drugs (2021) 81(15):1775–80. doi: 10.1007/s40265-021-01662-3
50. Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, et al. Brigatinib Combined With Anti-EGFR Antibody Overcomes Osimertinib Resistance in EGFR-Mutated Non-Small-Cell Lung Cancer. Nat Commun (2017) 8:14768. doi: 10.1038/ncomms14768
51. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib Versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med (2018) 379(21):2027–39. doi: 10.1056/NEJMoa1810171
52. Espinosa ML, Abad C, Kurtzman Y, Abdulla FR. Dermatologic Toxicities of Targeted Therapy and Immunotherapy in Head and Neck Cancers. Front Oncol (2021) 11:605941. doi: 10.3389/fonc.2021.605941
53. Kozakiewicz P, Grzybowska-Szatkowska L. Application of Molecular Targeted Therapies in the Treatment of Head and Neck Squamous Cell Carcinoma. Oncol Lett (2018) 15(5):7497–505. doi: 10.3892/ol.2018.8300
54. Lin WL, Lin WC, Yang JY, Chang YC, Ho HC, Yang LC, et al. Fatal Toxic Epidermal Necrolysis Associated With Cetuximab in a Patient With Colon Cancer. J Clin Oncol (2008) 26(16):2779–80. doi: 10.1200/JCO.2007.15.7883
55. McGregor M, Price TJ. Panitumumab in the Treatment of Metastatic Colorectal Cancer, Including Wild-Type RAS, KRAS and NRAS Mcrc. Future Oncol (2018) 14(24):2437–59. doi: 10.2217/fon-2017-0711
56. Fukata T, Ito Y, Miyagaki H, Nishida H, Toyoda Y, Shingai T, et al. [a Case of Stevens-Johnson Syndrome Induced by Chemotherapy for Metastatic Colon Cancer]. Gan To Kagaku Ryoho (2019) 46(4):748–50.
57. Cohen RB. Current Challenges and Clinical Investigations of Epidermal Growth Factor Receptor (EGFR)- and Erbb Family-Targeted Agents in the Treatment of Head and Neck Squamous Cell Carcinoma (HNSCC). Cancer Treat Rev (2014) 40(4):567–77. doi: 10.1016/j.ctrv.2013.10.002
58. Tang L, Liu T, Chen J, Dang J, Li G. Immune-Checkpoint Inhibitors Versus Other Systemic Therapies in Advanced Head and Neck Cancer: A Network Meta-Analysis. Immunotherapy (2021) 13(6):541–55. doi: 10.2217/imt-2020-0070
59. Koramati SL, Sarathy V, Varayathu H, Thomas BE, Naik R. Addition of Nimotuzumab to Standard TPF Regimen in Locally Advanced Head and Neck Cancer: A Single Institutional Study. J Oncol (2021) 2021:6641963. doi: 10.1155/2021/6641963
60. Cai WQ, Zeng LS, Wang LF, Wang YY, Cheng JT, Zhang Y, et al. The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells. Front Oncol (2020) 10:1249. doi: 10.3389/fonc.2020.01249
61. Liang R, Yang L, Zhu X. Nimotuzumab, an Anti-EGFR Monoclonal Antibody, in the Treatment of Nasopharyngeal Carcinoma. Cancer Control (2021) 28:1073274821989301. doi: 10.1177/1073274821989301
62. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med (2004) 350(21):2129–39. doi: 10.1056/NEJMoa040938
63. Sanford M, Scott LJ. Gefitinib: A Review of its Use in the Treatment of Locally Advanced/Metastatic Non-Small Cell Lung Cancer. Drugs (2009) 69(16):2303–28. doi: 10.2165/10489100-000000000-00000
64. Zhang Y, Fu F, Hu H, Wang S, Li Y, Hu H, et al. Gefitinib as Neoadjuvant Therapy for Resectable Stage II-IIIA Non-Small Cell Lung Cancer: A Phase II Study. J Thorac Cardiovasc Surg (2021) 161(2):434–42 e2. doi: 10.1016/j.jtcvs.2020.02.131
65. Xie H, Wang H, Xu L, Li M, Peng Y, Cai X, et al. Gefitinib Versus Adjuvant Chemotherapy in Patients With Stage II-IIIA Non-Small-Cell Lung Cancer Harboring Positive EGFR Mutations: A Single-Center Retrospective Study. Clin Lung Cancer (2018) 19(6):484–92. doi: 10.1016/j.cllc.2018.05.007
66. Shalata W, Jacob BM, Agbarya A. Adjuvant Treatment With Tyrosine Kinase Inhibitors in Epidermal Growth Factor Receptor Mutated Non-Small-Cell Lung Carcinoma Patients, Past, Present and Future. Cancers (Basel) (2021) 13(16):4119–33. doi: 10.3390/cancers13164119
67. Melosky B, Hirsh V. Management of Common Toxicities in Metastatic NSCLC Related to Anti-Lung Cancer Therapies With EGFR-Tkis. Front Oncol (2014) 4:238. doi: 10.3389/fonc.2014.00238
68. Bello M. Binding Mechanism of Kinase Inhibitors to EGFR and T790M, L858R and L858R/T790M Mutants Through Structural and Energetic Analysis. Int J Biol Macromol (2018) 118(Pt B):1948–62. doi: 10.1016/j.ijbiomac.2018.07.042
69. Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, et al. Activity of the Dual Kinase Inhibitor Lapatinib (GW572016) Against HER-2-Overexpressing and Trastuzumab-Treated Breast Cancer Cells. Cancer Res (2006) 66(3):1630–9. doi: 10.1158/0008-5472.CAN-05-1182
70. Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The Effects of the Novel, Reversible Epidermal Growth Factor Receptor/Erbb-2 Tyrosine Kinase Inhibitor, GW2016, on the Growth of Human Normal and Tumor-Derived Cell Lines In Vitro and In Vivo. Mol Cancer Ther (2001) 1(2):85–94. doi: 10.1097/00008390-200112000-00011
71. Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, et al. A Unique Structure for Epidermal Growth Factor Receptor Bound to GW572016 (Lapatinib): Relationships Among Protein Conformation, Inhibitor Off-Rate, and Receptor Activity in Tumor Cells. Cancer Res (2004) 64(18):6652–9. doi: 10.1158/0008-5472.CAN-04-1168
72. Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR Mutation and Resistance of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med (2005) 352(8):786–92. doi: 10.1056/NEJMoa044238
73. Lavacchi D, Mazzoni F, Giaccone G. Clinical Evaluation of Dacomitinib for the Treatment of Metastatic Non-Small Cell Lung Cancer (NSCLC): Current Perspectives. Drug Des Devel Ther (2019) 13:3187–98. doi: 10.2147/DDDT.S194231
74. Ding PN, Lord SJ, Gebski V, Links M, Bray V, Gralla RJ, et al. Risk of Treatment-Related Toxicities From EGFR Tyrosine Kinase Inhibitors: A Meta-Analysis of Clinical Trials of Gefitinib, Erlotinib, and Afatinib in Advanced EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol (2017) 12(4):633–43. doi: 10.1016/j.jtho.2016.11.2236
75. Zhang T, Qu R, Chan S, Lai M, Tong L, Feng F, et al. Discovery of a Novel Third-Generation EGFR Inhibitor and Identification of a Potential Combination Strategy to Overcome Resistance. Mol Cancer (2020) 19(1):90. doi: 10.1186/s12943-020-01202-9
76. Choi G, Kim D, Oh J. AI-Based Drug Discovery of Tkis Targeting L858R/T790M/C797S-Mutant EGFR in Non-Small Cell Lung Cancer. Front Pharmacol (2021) 12:660313. doi: 10.3389/fphar.2021.660313
77. Yu HA, Tian SK, Drilon AE, Borsu L, Riely GJ, Arcila ME, et al. Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol (2015) 1(7):982–4. doi: 10.1001/jamaoncol.2015.1066
78. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance Mechanisms to Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer. Br J Cancer (2019) 121(9):725–37. doi: 10.1038/s41416-019-0573-8
79. To C, Jang J, Chen T, Park E, Mushajiang M, De Clercq DJH, et al. Single and Dual Targeting of Mutant EGFR With an Allosteric Inhibitor. Cancer Discov (2019) 9(7):926–43. doi: 10.1158/2159-8290.CD-18-0903
80. Umbela S, Ghacha S, Matuknauth R, Gause S, Joshee S, Deshmukh RR. Brigatinib: New-Generation ALK Inhibitor for Nonsmall Cell Lung Cancer. Curr Probl Cancer (2019) 43(6):100477. doi: 10.1016/j.currproblcancer.2019.03.005
81. Joly-Tonetti N, Ondet T, Monshouwer M, Stamatas GN. EGFR Inhibitors Switch Keratinocytes From a Proliferative to a Differentiative Phenotype Affecting Epidermal Development and Barrier Function. BMC Cancer (2021) 21(1):5. doi: 10.1186/s12885-020-07685-5
82. Annunziata MC, De Stefano A, Fabbrocini G, Leo S, Marchetti P, Romano MC, et al. Current Recommendations and Novel Strategies for the Management of Skin Toxicities Related to Anti-EGFR Therapies in Patients With Metastatic Colorectal Cancer. Clin Drug Investig (2019) 39(9):825–34. doi: 10.1007/s40261-019-00811-7
83. Kozuki T. Skin Problems and EGFR-Tyrosine Kinase Inhibitor. Jpn J Clin Oncol (2016) 46(4):291–8. doi: 10.1093/jjco/hyv207
84. Yano S, Kondo K, Yamaguchi M, Richmond G, Hutchison M, Wakeling A, et al. Distribution and Function of EGFR in Human Tissue and the Effect of EGFR Tyrosine Kinase Inhibition. Anticancer Res (2003) 23(5A):3639–50. doi: 10.1097/01.cad.0000089693.26177.3c
85. Mitchell EP, Perez-Soler R, Van Cutsem E, Lacouture ME. Clinical Presentation and Pathophysiology of EGFRI Dermatologic Toxicities. Oncol (Williston Park) (2007) 21(11 Suppl 5):4–9.
86. Cho YT, Chen KL, Sheen YS, Yang CW, Liau JY, Cheng YP, et al. Purpuric Drug Eruptions Caused by Epidermal Growth Factor Receptor Inhibitors for Non-Small Cell Lung Cancer: A Clinicopathologic Study of 32 Cases. JAMA Dermatol (2017) 153(9):906–10. doi: 10.1001/jamadermatol.2017.0903
87. Roujeau JC, Stern RS. Severe Adverse Cutaneous Reactions to Drugs. N Engl J Med (1994) 331(19):1272–85. doi: 10.1056/NEJM199411103311906
88. Mustafa SS, Ostrov D, Yerly D. Severe Cutaneous Adverse Drug Reactions: Presentation, Risk Factors, and Management. Curr Allergy Asthma Rep (2018) 18(4):26. doi: 10.1007/s11882-018-0778-6
89. Hadavand MA, Kaffenberger B, Cartron AM, Trinidad JC. Clinical Presentation and Management of Atypical and Recalcitrant Acute Generalized Exanthematous Pustulosis (AGEP). J Am Acad Dermatol (2020) S0190-9622(20):32609–17. doi: 10.1016/j.jaad.2020.09.024
90. Laux I, Jain A, Singh S, Agus DB. Epidermal Growth Factor Receptor Dimerization Status Determines Skin Toxicity to HER-Kinase Targeted Therapies. Br J Cancer (2006) 94(1):85–92. doi: 10.1038/sj.bjc.6602875
91. Quiroz FG, Fiore VF, Levorse J, Polak L, Wong E, Pasolli HA, et al. Liquid-Liquid Phase Separation Drives Skin Barrier Formation. Science (2020) 367(6483):1–34. doi: 10.1126/science.aax9554
92. Madison KC. Barrier Function of the Skin: "La Raison D'etre" of the Epidermis. J Invest Dermatol (2003) 121(2):231–41. doi: 10.1046/j.1523-1747.2003.12359.x
93. Kim JM, Ji JH, Kim YS, Lee S, Oh SY, Huh SJ, et al. Rhegf Treatment Improves EGFR Inhibitor-Induced Skin Barrier and Immune Defects. Cancers (Basel) (2020) 12(11):3120–37. doi: 10.3390/cancers12113120
94. Wolf C, Qian Y, Brooke MA, Kelsell DP, Franzke CW. ADAM17/EGFR Axis Promotes Transglutaminase-Dependent Skin Barrier Formation Through Phospholipase C Gamma1 and Protein Kinase C Pathways. Sci Rep (2016) 6:39780. doi: 10.1038/srep39780
95. Lynn KS, Peterson RJ, Koval M. Ruffles and Spikes: Control of Tight Junction Morphology and Permeability by Claudins. Biochim Biophys Acta Biomembr (2020) 1862(9):183339. doi: 10.1016/j.bbamem.2020.183339
96. Fang H, Wang Y, Xu L, Zhou S, Bai J, Wu Y, et al. EGFR Inhibitor Gefitinib Regulates Barrier Function in Human Epidermal Keratinocytes via the Modulation of the Expression of Claudins. Int J Mol Med (2019) 43(3):1522–30. doi: 10.3892/ijmm.2018.4046
97. Nanney LB, Magid M, Stoscheck CM, King LE Jr. Comparison of Epidermal Growth Factor Binding and Receptor Distribution in Normal Human Epidermis and Epidermal Appendages. J Invest Dermatol (1984) 83(5):385–93. doi: 10.1111/1523-1747.ep12264708
98. Denning MF. Epidermal Keratinocytes: Regulation of Multiple Cell Phenotypes by Multiple Protein Kinase C Isoforms. Int J Biochem Cell Biol (2004) 36(7):1141–6. doi: 10.1016/j.biocel.2003.12.004
99. Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, et al. Phase I Safety, Pharmacokinetic, and Pharmacodynamic Trial of ZD1839, a Selective Oral Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor, in Patients With Five Selected Solid Tumor Types. J Clin Oncol (2002) 20(21):4292–302. doi: 10.1200/JCO.2002.03.100
100. Tan AR, Yang X, Hewitt SM, Berman A, Lepper ER, Sparreboom A, et al. Evaluation of Biologic End Points and Pharmacokinetics in Patients With Metastatic Breast Cancer After Treatment With Erlotinib, an Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. J Clin Oncol (2004) 22(15):3080–90. doi: 10.1200/JCO.2004.08.189
101. Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, et al. Targeted Disruption of Mouse EGF Receptor: Effect of Genetic Background on Mutant Phenotype. Science (1995) 269(5221):230–4. doi: 10.1126/science.7618084
102. Eames T, Kroth J, Flaig MJ, Ruzicka T, Wollenberg A. Perifollicular Xanthomas Associated With Epidermal Growth Factor Receptor Inhibitor Therapy. Acta Derm Venereol (2010) 90(2):202–3. doi: 10.2340/00015555-0792
103. Osio A, Mateus C, Soria JC, Massard C, Malka D, Boige V, et al. Cutaneous Side-Effects in Patients on Long-Term Treatment With Epidermal Growth Factor Receptor Inhibitors. Br J Dermatol (2009) 161(3):515–21. doi: 10.1111/j.1365-2133.2009.09214.x
104. Brodell LA, Hepper D, Lind A, Gru AA, Anadkat MJ. Histopathology of Acneiform Eruptions in Patients Treated With Epidermal Growth Factor Receptor Inhibitors. J Cutan Pathol (2013) 40(10):865–70. doi: 10.1111/cup.12202
105. Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, et al. Cutaneous Side-Effects of Kinase Inhibitors and Blocking Antibodies. Lancet Oncol (2005) 6(7):491–500. doi: 10.1016/S1470-2045(05)70243-6
106. Mak KK, Chan SY. Epidermal Growth Factor as a Biologic Switch in Hair Growth Cycle. J Biol Chem (2003) 278(28):26120–6. doi: 10.1074/jbc.M212082200
107. Zhang H, Nan W, Wang S, Zhang T, Si H, Wang D, et al. Epidermal Growth Factor Promotes Proliferation of Dermal Papilla Cells via Notch Signaling Pathway. Biochimie (2016) 127:10–8. doi: 10.1016/j.biochi.2016.04.015
108. Celik T, Kosker M. Ocular Side Effects and Trichomegaly of Eyelashes Induced by Erlotinib: A Case Report and Review of the Literature. Cont Lens Anterior Eye (2015) 38(1):59–60. doi: 10.1016/j.clae.2014.08.005
109. Koksal UI, Pilanci KN, Ordu C, Okutur K, Saglam S, Demir G. Trichomegaly Induced by Cetuximab: Case Series and Review the Literature. Am J Ther (2016) 23(5):e1226–9. doi: 10.1097/MJT.0000000000000189
110. Chieosilapatham P, Kiatsurayanon C, Umehara Y, Trujillo-Paez JV, Peng G, Yue H, et al. Keratinocytes: Innate Immune Cells in Atopic Dermatitis. Clin Exp Immunol (2021) 204(3):296–309. doi: 10.1111/cei.13575
111. Park K, Ommori R, Imoto K, Asada H. Epidermal Growth Factor Receptor Inhibitors Selectively Inhibit the Expressions of Human Beta-Defensins Induced by Staphylococcus Epidermidis. J Dermatol Sci (2014) 75(2):94–9. doi: 10.1016/j.jdermsci.2014.04.011
112. Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF Receptor Induces a Deranged Chemokine Expression in Keratinocytes Leading to Enhanced Skin Inflammation. Am J Pathol (2003) 163(1):303–12. doi: 10.1016/S0002-9440(10)63654-1
113. Wan L, Wang Y, Tang Y, Tan Y, He F, Zhang Y, et al. Gefitinib-Induced Cutaneous Toxicities in Brown Norway Rats are Associated With Macrophage Infiltration. Inflammation (2020) 43(6):2137–46. doi: 10.1007/s10753-020-01281-2
114. Russi EG, Moretto F, Rampino M, Benasso M, Bacigalupo A, De Sanctis V, et al. Acute Skin Toxicity Management in Head and Neck Cancer Patients Treated With Radiotherapy and Chemotherapy or EGFR Inhibitors: Literature Review and Consensus. Crit Rev Oncol Hematol (2015) 96(1):167–82. doi: 10.1016/j.critrevonc.2015.06.001
115. Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, et al. Clinical Practice Guidelines for the Prevention and Treatment of EGFR Inhibitor-Associated Dermatologic Toxicities. Support Care Cancer (2011) 19(8):1079–95. doi: 10.1007/s00520-011-1197-6
116. Russi EG, Raber-Durlacher JE, Sonis ST. Local and Systemic Pathogenesis and Consequences of Regimen-Induced Inflammatory Responses in Patients With Head and Neck Cancer Receiving Chemoradiation. Mediators Inflammation (2014) 2014:518261. doi: 10.1155/2014/518261
117. Russi EG, Numico G, Merlano MC, Pinto C. Cetuximab-Related Radiation Dermatitis in Head-and-Neck Cancer Patients: In Regard to Studer Et al. (Int J Radiat Oncol Biol Phys in Press). Int J Radiat Oncol Biol Phys (2011) 79(4):1278. author reply -9. doi: 10.1016/j.ijrobp.2010.10.047
118. Le-Rademacher JG, Rowland K, Atherton PJ, Dakhil C, Sun Z, Tan A, et al. Androgen Mediation-and Antiandrogens Mitigation-of the Epidermal Growth Factor Receptor (EGFR) Inhibitor-Induced Rash: Results From a Pilot Randomized Trial and Small Translational Case Series. Am J Hosp Palliat Care (2019) 36(6):519–25. doi: 10.1177/1049909118819820
119. Nakahara T, Moroi Y, Takayama K, Itoh E, Kido-Nakahara M, Nakanishi Y, et al. Changes in Sebum Levels and the Development of Acneiform Rash in Patients With Non-Small Cell Lung Cancer After Treatment With EGFR Inhibitors. Oncol Targets Ther (2015) 8:259–63. doi: 10.2147/OTT.S76860
120. Takahashi H, Asaka J, Tairabune T, Ujiie H, Matsuura Y, Nihei S, et al. Analysis of Risk Factors for Skin Disorders Caused by Anti-Epidermal Growth Factor Receptor Antibody Drugs and Examination of Methods for Their Avoidance. J Clin Pharm Ther (2021) 46(5):1404–11. doi: 10.1111/jcpt.13475
121. Sato I, Mizuno H, Kataoka N, Kunimatsu Y, Tachibana Y, Sugimoto T, et al. Osimertinib-Associated Toxic Epidermal Necrolysis in a Lung Cancer Patient Harboring an EGFR Mutation-a Case Report and a Review of the Literature. Medicina (Kaunas) (2020) 56(8):403–9. doi: 10.3390/medicina56080403
122. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clin Rev Allergy Immunol (2018) 54(1):147–76. doi: 10.1007/s12016-017-8654-z
123. Shah DR, Shah RR, Morganroth J. Tyrosine Kinase Inhibitors: Their on-Target Toxicities as Potential Indicators of Efficacy. Drug Saf (2013) 36(6):413–26. doi: 10.1007/s40264-013-0050-x
124. Jaka A, Gutierrez-Rivera A, Lopez-Pestana A, del Alcazar E, Zubizarreta J, Vildosola S, et al. Predictors of Tumor Response to Cetuximab and Panitumumab in 116 Patients and a Review of Approaches to Managing Skin Toxicity. Actas Dermosifiliogr (2015) 106(6):483–92. doi: 10.1016/j.ad.2015.01.006
125. Amador ML, Oppenheimer D, Perea S, Maitra A, Cusatis G, Iacobuzio-Donahue C, et al. An Epidermal Growth Factor Receptor Intron 1 Polymorphism Mediates Response to Epidermal Growth Factor Receptor Inhibitors. Cancer Res (2004) 64(24):9139–43. doi: 10.1158/0008-5472.CAN-04-1036
126. Kimura H, Kasahara K, Sekijima M, Tamura T, Nishio K. Plasma MIP-1beta Levels and Skin Toxicity in Japanese Non-Small Cell Lung Cancer Patients Treated With the EGFR-Targeted Tyrosine Kinase Inhibitor, Gefitinib. Lung Cancer (2005) 50(3):393–9. doi: 10.1016/j.lungcan.2005.07.012
127. Moreno Garcia V, Thavasu P, Blanco Codesido M, Molife LR, Vitfell Pedersen J, Puglisi M, et al. Association of Creatine Kinase and Skin Toxicity in Phase I Trials of Anticancer Agents. Br J Cancer (2012) 107(11):1797–800. doi: 10.1038/bjc.2012.482
128. Steffens M, Paul T, Hichert V, Scholl C, von Mallek D, Stelzer C, et al. Dosing to Rash?–the Role of Erlotinib Metabolic Ratio From Patient Serum in the Search of Predictive Biomarkers for EGFR Inhibitor-Mediated Skin Rash. Eur J Cancer (2016) 55:131–9. doi: 10.1016/j.ejca.2015.11.022
129. Chen P, Chen F, Lei J, Zhou B. Curative Effectiveness and Safety of Osimertinib in the Treatment for Non-Small-Cell Lung Cancer: A Meta-Analysis of the Experimental Evidence. Onco Targets Ther (2018) 11:9033–47. doi: 10.2147/OTT.S182077
130. Bollinger MK, Agnew AS, Mascara GP. Osimertinib: A Third-Generation Tyrosine Kinase Inhibitor for Treatment of Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer With the Acquired Thr790Met Mutation. J Oncol Pharm Pract (2018) 24(5):379–88. doi: 10.1177/1078155217712401
131. Liao BC, Lin CC, Lee JH, Yang JC. Update on Recent Preclinical and Clinical Studies of T790M Mutant-Specific Irreversible Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. J BioMed Sci (2016) 23(1):86. doi: 10.1186/s12929-016-0305-9
132. Gutzmer R, Wollenberg A, Ugurel S, Homey B, Ganser A, Kapp A. Cutaneous Side Effects of New Antitumor Drugs: Clinical Features and Management. Dtsch Arztebl Int (2012) 109(8):133–40. doi: 10.3238/arztebl.2012.0133
133. Aw DC, Tan EH, Chin TM, Lim HL, Lee HY, Soo RA. Management of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-Related Cutaneous and Gastrointestinal Toxicities. Asia Pac J Clin Oncol (2018) 14(1):23–31. doi: 10.1111/ajco.12687
134. Gutzmer R, Becker JC, Enk A, Garbe C, Hauschild A, Leverkus M, et al. Management of Cutaneous Side Effects of EGFR Inhibitors: Recommendations From a German Expert Panel for the Primary Treating Physician. J Dtsch Dermatol Ges (2011) 9(3):195–203. doi: 10.1111/j.1610-0387.2010.07561.x
135. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Washington: U.S. Department of Health and Human Services (2017).
136. Chu CY, Chen KY, Wen-Cheng Chang J, Wei YF, Lee CH, Wang WM. Taiwanese Dermatological Association Consensus for the Prevention and Management of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-Related Skin Toxicities. J Formos Med Assoc (2017) 116(6):413–23. doi: 10.1016/j.jfma.2017.03.001
137. Tohyama M, Hamada M, Harada D, Kozuki T, Nogami N, Monden N, et al. Clinical Features and Treatment of Epidermal Growth Factor Inhibitor-Related Late-Phase Papulopustular Rash. J Dermatol (2020) 47(2):121–7. doi: 10.1111/1346-8138.15170
138. Fischer A, Rosen AC, Ensslin CJ, Wu S, Lacouture ME. Pruritus to Anticancer Agents Targeting the EGFR, BRAF, and CTLA-4. Dermatol Ther (2013) 26(2):135–48. doi: 10.1111/dth.12027
139. Clabbers JMK, Boers-Doets CB, Gelderblom H, Stijnen T, Lacouture ME, van der Hoeven KJM, et al. Xerosis and Pruritus as Major EGFRI-Associated Adverse Events. Support Care Cancer (2016) 24(2):513–21. doi: 10.1007/s00520-015-2781-y
140. Beech J, Germetaki T, Judge M, Paton N, Collins J, Garbutt A, et al. Management and Grading of EGFR Inhibitor-Induced Cutaneous Toxicity. Future Oncol (2018) 14(24):2531–41. doi: 10.2217/fon-2018-0187
141. Califano R, Tariq N, Compton S, Fitzgerald DA, Harwood CA, Lal R, et al. Expert Consensus on the Management of Adverse Events From EGFR Tyrosine Kinase Inhibitors in the UK. Drugs (2015) 75(12):1335–48. doi: 10.1007/s40265-015-0434-6
142. Potthoff K, Hofheinz R, Hassel JC, Volkenandt M, Lordick F, Hartmann JT, et al. Interdisciplinary Management of EGFR-Inhibitor-Induced Skin Reactions: A German Expert Opinion. Ann Oncol (2011) 22(3):524–35. doi: 10.1093/annonc/mdq387
143. Bachmeyer C, Reguiai Z, Peuvrel L, Bachet JB, Bensadoun RJ, Ychou M, et al. [Cutaneous Adverse Reactions of EGFR (Epidermal Growth Factor Receptor)-Inhibitors: Therapeutic Algorithm of the French PROCUR Group]. Bull Cancer (2013) 100(5):417–26. doi: 10.1684/bdc.2013.1735
144. Peuvrel L, Bachmeyer C, Reguiai Z, Bachet JB, Andre T, Bensadoun RJ, et al. Survey on the Management of Skin Toxicity Associated With EGFR Inhibitors Amongst French Physicians. J Eur Acad Dermatol Venereol (2013) 27(4):419–29. doi: 10.1111/j.1468-3083.2011.04421.x
145. Pinto C, Barone CA, Girolomoni G, Russi EG, Merlano MC, Ferrari D, et al. Management of Skin Reactions During Cetuximab Treatment in Association With Chemotherapy or Radiotherapy: Update of the Italian Expert Recommendations. Am J Clin Oncol (2016) 39(4):407–15. doi: 10.1097/COC.0000000000000291
146. Gravalos C, Sanmartin O, Gurpide A, Espana A, Majem M, Suh Oh HJ, et al. Clinical Management of Cutaneous Adverse Events in Patients on Targeted Anticancer Therapies and Immunotherapies: A National Consensus Statement by the Spanish Academy of Dermatology and Venereology and the Spanish Society of Medical Oncology. Clin Transl Oncol (2019) 21(5):556–71. doi: 10.1007/s12094-018-1953-x
147. Hofheinz RD, Segaert S, Safont MJ, Demonty G, Prenen H. Management of Adverse Events During Treatment of Gastrointestinal Cancers With Epidermal Growth Factor Inhibitors. Crit Rev Oncol Hematol (2017) 114:102–13. doi: 10.1016/j.critrevonc.2017.03.032
148. Melosky B, Leighl NB, Rothenstein J, Sangha R, Stewart D, Papp K. Management of Egfr Tki-Induced Dermatologic Adverse Events. Curr Oncol (2015) 22(2):123–32. doi: 10.3747/co.22.2430
149. Lacouture ME, Sibaud V, Gerber PA, van den Hurk C, Fernandez-Penas P, Santini D, et al. Prevention and Management of Dermatological Toxicities Related to Anticancer Agents: ESMO Clinical Practice Guidelines(). Ann Oncol (2021) 32(2):157–70. doi: 10.1016/j.annonc.2020.11.005
150. Wu J, Lacouture ME. Pruritus Associated With Targeted Anticancer Therapies and Their Management. Dermatol Clin (2018) 36(3):315–24. doi: 10.1016/j.det.2018.02.010
151. Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous Adverse Effects of Targeted Therapies: Part I: Inhibitors of the Cellular Membrane. J Am Acad Dermatol (2015) 72(2):203–18. quiz 19-20. doi: 10.1016/j.jaad.2014.07.032
152. Segaert S, Van Cutsem E. Clinical Signs, Pathophysiology and Management of Skin Toxicity During Therapy With Epidermal Growth Factor Receptor Inhibitors. Ann Oncol (2005) 16(9):1425–33. doi: 10.1093/annonc/mdi279
153. Farahnik B, Kwong B, Murase J. General Management Strategy for Epidermal Growth Factor Receptor Inhibitor-Associated Papulopustular Eruption. J Am Acad Dermatol (2016) 75(5):e191. doi: 10.1016/j.jaad.2016.07.036
154. Segaert S, Van Cutsem E. Clinical Management of EGFRI Dermatologic Toxicities: The European Perspective. Oncol (Williston Park) (2007) 21(11 Suppl 5):22–6.
155. Higgins PJ, Draper M, Nelson M. Anti-Inflammatory Activity of Tetracyclines: Applications to Human Disease. Antiinflamm Antiallergy Agents Med Chem (2011) 10(2):132–52. doi: 10.2174/1871523011109020132
156. Stulhofer Buzina D, Martinac I, Ledic Drvar D, Ceovic R, Bilic I, Marinovic B. The Most Common Cutaneous Side Effects of Epidermal Growth Factor Receptor Inhibitors and Their Management. Acta Dermatovenerol Croat (2015) 23(4):282–8.
157. Mihai MM, Ion A, Giurcaneanu C, Nitipir C, Popa AM, Chifiriuc MC, et al. The Impact of Long-Term Antibiotic Therapy of Cutaneous Adverse Reactions to EGFR Inhibitors in Colorectal Cancer Patients. J Clin Med (2021) 10(15):3219–38. doi: 10.3390/jcm10153219
158. Bavetta M, Silvaggio D, Campione E, Sollena P, Formica V, Coletta D, et al. The Effects of Association of Topical Polydatin Improves the Preemptive Systemic Treatment on EGFR Inhibitors Cutaneous Adverse Reactions. J Clin Med (2021) 10(3):466–74. doi: 10.3390/jcm10030466
159. Lacouture ME, Wainberg ZA, Patel AB, Anadkat MJ, Stemmer SM, Shacham-Shmueli E, et al. Reducing Skin Toxicities From EGFR Inhibitors With Topical BRAF Inhibitor Therapy. Cancer Discov (2021) 11(9):2158–67. doi: 10.1158/2159-8290.CD-20-1847
160. Cury-Martins J, Eris APM, Abdalla CMZ, Silva GB, Moura VPT, Sanches JA. Management of Dermatologic Adverse Events From Cancer Therapies: Recommendations of an Expert Panel. Bras Dermatol (2020) 95(2):221–37. doi: 10.1016/j.abd.2020.01.001
161. Gisondi P, Geat D, Mattiucci A, Lombardo F, Santo A, Girolomoni G. Incidence of Adverse Cutaneous Reactions to Epidermal Growth Factor Receptor Inhibitors in Patients With Non-Small-Cell Lung Cancer. Dermatology (2021) 237(6):929–33. doi: 10.1159/000513233
162. Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe Cutaneous Adverse Reactions to Drugs. Lancet (2017) 390(10106):1996–2011. doi: 10.1016/S0140-6736(16)30378-6
163. Chen CB, Abe R, Pan RY, Wang CW, Hung SI, Tsai YG, et al. An Updated Review of the Molecular Mechanisms in Drug Hypersensitivity. J Immunol Res (2018) 2018:6431694. doi: 10.1155/2018/6431694
164. Hasegawa A, Abe R. Recent Advances in Managing and Understanding Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. F1000Res (2020) 9:1–12. doi: 10.12688/f1000research.24748.1
165. Tolliver S, Graham J, Kaffenberger BH. A Review of Cutaneous Manifestations Within Glucagonoma Syndrome: Necrolytic Migratory Erythema. Int J Dermatol (2018) 57(6):642–5. doi: 10.1111/ijd.13947
166. Foss MG, Ferrer-Bruker SJ. Necrolytic Migratory Erythema. Treasure Island (FL: StatPearls (2021).
167. Kuschel SL, Reedy MS. Cyclosporine Treatment of Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS) Syndrome: A Case Report and Brief Review of the Literature. Pract Dermatol (2018) 2018:41–3.
168. Ingen-Housz-Oro S, Hotz C, Valeyrie-Allanore L, Sbidian E, Hemery F, Chosidow O, et al. Acute Generalized Exanthematous Pustulosis: A Retrospective Audit of Practice Between 1994 and 2011 at a Single Centre. Br J Dermatol (2015) 172(5):1455–7. doi: 10.1111/bjd.13540
169. Abela C, Hartmann CE, De Leo A, de Sica Chapman A, Shah H, Jawad M, et al. Toxic Epidermal Necrolysis (TEN): The Chelsea and Westminster Hospital Wound Management Algorithm. J Plast Reconstr Aesthet Surg (2014) 67(8):1026–32. doi: 10.1016/j.bjps.2014.04.003
170. Gonzalez-Herrada C, Rodriguez-Martin S, Cachafeiro L, Lerma V, Gonzalez O, Lorente JA, et al. Cyclosporine Use in Epidermal Necrolysis is Associated With an Important Mortality Reduction: Evidence From Three Different Approaches. J Invest Dermatol (2017) 137(10):2092–100. doi: 10.1016/j.jid.2017.05.022
171. Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-Analysis. JAMA Dermatol (2017) 153(6):514–22. doi: 10.1001/jamadermatol.2016.5668
172. Roujeau JC, Mockenhaupt M, Guillaume JC, Revuz J. New Evidence Supporting Cyclosporine Efficacy in Epidermal Necrolysis. J Invest Dermatol (2017) 137(10):2047–9. doi: 10.1016/j.jid.2017.07.828
173. Ueta M. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis With Severe Ocular Complications. Expert Rev Clin Immunol (2020) 16(3):285–91. doi: 10.1080/1744666X.2020.1729128
174. Sotozono C, Ueta M, Koizumi N, Inatomi T, Shirakata Y, Ikezawa Z, et al. Diagnosis and Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis With Ocular Complications. Ophthalmology (2009) 116(4):685–90. doi: 10.1016/j.ophtha.2008.12.048
175. Amitay-Laish I, Prag-Naveh H, Ollech A, Davidovici B, Leshem YA, Snast I, et al. Prophylactic Topical Treatment for EGFR Inhibitor-Induced Papulopustular Rash: A Randomized Clinical Trial. Dermatology (2020) 237(6):988–94. doi: 10.1159/000511869
176. Papoui E, Papastavrou E, Merkouris A, Charalambous A. The Extent to Which the Last Decade has Yielded Additional Treatment Options for EGFR-Associated Rash Besides Classic Treatment With Antibiotics and Corticosteroids - a Systematic Review. Eur J Oncol Nurs (2021) 50:101896. doi: 10.1016/j.ejon.2021.101896
177. Melosky B, Anderson H, Burkes RL, Chu Q, Hao D, Ho V, et al. Pan Canadian Rash Trial: A Randomized Phase III Trial Evaluating the Impact of a Prophylactic Skin Treatment Regimen on Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor-Induced Skin Toxicities in Patients With Metastatic Lung Cancer. J Clin Oncol (2016) 34(8):810–5. doi: 10.1200/JCO.2015.62.3918
178. Ichiki M, Wataya H, Yamada K, Tsuruta N, Takeoka H, Okayama Y, et al. Preventive Effect of Kampo Medicine (Hangeshashin-to, TJ-14) Plus Minocycline Against Afatinib-Induced Diarrhea and Skin Rash in Patients With Non-Small Cell Lung Cancer. Onco Targets Ther (2017) 10:5107–13. doi: 10.2147/OTT.S145613
179. Okajima M, Miura S, Watanabe S, Tanaka H, Ito K, Ishida T, et al. A Prospective Phase II Study of Multimodal Prophylactic Treatment for Afatinib-Induced Adverse Events in Advanced Non-Small Cell Lung Cancer (Niigata Lung Cancer Treatment Group 1401). Transl Lung Cancer Res (2021) 10(1):252–60. doi: 10.21037/tlcr-20-649
180. Lacouture ME, Keefe DM, Sonis S, Jatoi A, Gernhardt D, Wang T, et al. A Phase II Study (ARCHER 1042) to Evaluate Prophylactic Treatment of Dacomitinib-Induced Dermatologic and Gastrointestinal Adverse Events in Advanced Non-Small-Cell Lung Cancer. Ann Oncol (2016) 27(9):1712–8. doi: 10.1093/annonc/mdw227
181. Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, et al. Skin Toxicity Evaluation Protocol With Panitumumab (STEPP), a Phase II, Open-Label, Randomized Trial Evaluating the Impact of a Pre-Emptive Skin Treatment Regimen on Skin Toxicities and Quality of Life in Patients With Metastatic Colorectal Cancer. J Clin Oncol (2010) 28(8):1351–7. doi: 10.1200/JCO.2008.21.7828
182. Petrelli F, Borgonovo K, Barni S. Preventing or Treating Anti-EGFR Related Skin Rash With Antibiotics? Ann Transl Med (2016) 4(16):312. doi: 10.21037/atm.2016.07.01
183. Arrieta O, Vega-Gonzalez MT, Lopez-Macias D, Martinez-Hernandez JN, Bacon-Fonseca L, Macedo-Perez EO, et al. Randomized, Open-Label Trial Evaluating the Preventive Effect of Tetracycline on Afatinib Induced-Skin Toxicities in Non-Small Cell Lung Cancer Patients. Lung Cancer (2015) 88(3):282–8. doi: 10.1016/j.lungcan.2015.03.019
184. Jatoi A, Dakhil SR, Sloan JA, Kugler JW, Rowland KM Jr, Schaefer PL, et al. Prophylactic Tetracycline Does Not Diminish the Severity of Epidermal Growth Factor Receptor (EGFR) Inhibitor-Induced Rash: Results From the North Central Cancer Treatment Group (Supplementary N03CB). Support Care Cancer (2011) 19(10):1601–7. doi: 10.1007/s00520-010-0988-5
185. Hofheinz RD, Deplanque G, Komatsu Y, Kobayashi Y, Ocvirk J, Racca P, et al. Recommendations for the Prophylactic Management of Skin Reactions Induced by Epidermal Growth Factor Receptor Inhibitors in Patients With Solid Tumors. Oncologist (2016) 21(12):1483–91. doi: 10.1634/theoncologist.2016-0051
186. Iimura Y, Shimomura H, Yasu T, Imanaka K, Ogawa R, Ito A, et al. Nsaids may Prevent EGFR-TKI-Related Skin Rash in Non-Small Cell Lung Cancer Patients. Int J Clin Pharmacol Ther (2018) 56(11):551–4. doi: 10.5414/CP203323
187. Eriksen JG, Kaalund I, Clemmensen O, Overgaard J, Pfeiffer P. Placebo-Controlled Phase II Study of Vitamin K3 Cream for the Treatment of Cetuximab-Induced Rash. Support Care Cancer (2017) 25(7):2179–85. doi: 10.1007/s00520-017-3623-x
188. Hofheinz RD, Lorenzen S, Trojan J, Ocvirk J, Ettrich TJ, Al-Batran SE, et al. EVITA-a Double-Blind, Vehicle-Controlled, Randomized Phase II Trial of Vitamin K1 Cream as Prophylaxis for Cetuximab-Induced Skin Toxicity. Ann Oncol (2018) 29(4):1010–5. doi: 10.1093/annonc/mdy015
189. Chayahara N, Mukohara T, Tachihara M, Fujishima Y, Fukunaga A, Washio K, et al. Adapalene Gel 0.1% Versus Placebo as Prophylaxis for Anti-Epidermal Growth Factor Receptor-Induced Acne-Like Rash: A Randomized Left-Right Comparative Evaluation (APPEARANCE). Oncologist (2019) 24(7):885–e413. doi: 10.1634/theoncologist.2019-0156
Keywords: EGFR inhibitors, lethal skin toxicities, drug induced disease, treatment strategies, preventive measures
Citation: Li Y, Fu R, Jiang T, Duan D, Wu Y, Li C, Li Z, Ni R, Li L and Liu Y (2022) Mechanism of Lethal Skin Toxicities Induced by Epidermal Growth Factor Receptor Inhibitors and Related Treatment Strategies. Front. Oncol. 12:804212. doi: 10.3389/fonc.2022.804212
Received: 29 October 2021; Accepted: 17 January 2022;
Published: 10 February 2022.
Edited by:
Husain Yar Khan, Wayne State University, United StatesReviewed by:
Valentina Audrito, University of Turin, ItalyMarcelo Sobral-Leite, The Netherlands Cancer Institute (NKI), Netherlands
Copyright © 2022 Li, Fu, Jiang, Duan, Wu, Li, Li, Ni, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Liu, c3dobGl1eWFvQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yanping Li
Yanping Li Ruoqiu Fu†
Ruoqiu Fu† Yuanlin Wu
Yuanlin Wu Yao Liu
Yao Liu