
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 August 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.800813
This article is part of the Research Topic Locoregional Management of Breast Cancer: Multidisciplinary and Individualized Treatment View all 22 articles
 Min Xiao1,2
Min Xiao1,2 Pin Zhang1*
Pin Zhang1*Background: Conditional survival (CS) represents the probability of surviving for additional years after the patient has survived for several years, dynamically describing the survival rate of the patient with the varying time of survival. The aim of this study was to evaluate the conditional cause-specific survival (CCSS) after chemotherapy and local treatment for metastatic breast cancer, and to identify the prognostic factors affecting the CCSS.
Methods: Patients diagnosed with primary stage IV breast cancer in the Surveillance, Epidemiology, and End Results (SEER) database from 2010 to 2015 were included. CS is defined as the probability of additional survival for y years after the patient had survived x years with the calculation formula CCSS (x | y) = CSS (x + y)/CSS (x), where CSS(x) indicates the patient’s cause-specific survival rate at the time of x years. Cox proportional hazard models were used to evaluate predictors of CCSS.
Results: A total of 3,194 patients were included. The 5-year CSS was 39%, whereas the 5-year CCSS increased to 46%, 57%, 71%, and 85% after the diagnosis of 1, 2, 3, and 4 years. For patients with adverse clinical pathological features, CCSS had more pronounced increase with survival time and is more different from the CSS at diagnosis. No matter at the time of diagnosis or 1 year or 3 years after diagnosis, HER2 status, local treatment, and multisite metastasis were independent prognostic factors that affect the long-term survival of patients (all P < 0.05).
Conclusion: The 5-year CCSS of patients with stage IV breast cancer was extended as the survival years increased. HER2 status, multisite metastasis, and local treatment were independent prognostic factors even 3 years after diagnosis.
Breast cancer is the most common malignant tumor and is also the most frequent cause of death from cancer in women (1). Globally, over two million patients are diagnosed annually and over 600,000 die from the disease (2). About 5–10% patients at diagnosis have metastases (3). Despite the use of various traditional systemic treatments such as chemotherapy, endocrine treatment, and targeted therapy, the overall survival (OS) of patients with metastatic breast cancer is still not so satisfactory. Recently, some studies showed that chemotherapy combined with local treatment including primary tumor site surgery or radiotherapy or both may improve the prognosis of advanced breast cancer (4–6).
Most survival rates reported in the literature are static, being calculated from the day of diagnosis or surgery (7–10). This statistical method could only reflect the continuous hazard ratio and survival rate of patients from the beginning of follow-up. Since the survival rate, death risk, and risk ratio of patients will change with the extension of survival time, this approach has limitations, especially for long-term survival. Conditional survival (CS) represents the probability of surviving a certain number of years after diagnosis treatment based on the time the patient has already survived (11). Compared with the traditional survival evaluation, CS can provide more accurate information for long-term prognosis and is more meaningful in the process of follow-up. Thus, it has been used in many kinds of malignant tumors, such as gastrointestinal, liver, pancreatic, and urinary tract cancer (12–15).
As we know, there is no report on the conditional cause-specific survival (CCSS) in patients with metastatic breast cancer who underwent chemotherapy combined with local treatment. Our study aims to evaluate the dynamic cause-specific survival (CSS) of this type of population and prognostic factors that change with time.
A retrospective cohort study was performed with data extracted from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER program collects and publishes cancer incidence and survival data from population-based cancer registries covering approximately 34.6% of the U.S. population. The inclusion criteria were as follows: (1) patients histologically diagnosed as stage IV breast cancer according to the 7th edition of the American Joint Committee on Cancer (AJCC) TNM classification between 2010 and 2015, and (2) chemotherapy combined with local surgery and/or radiotherapy were performed. The exclusion criteria were as follows: (1) male, (2) more than 84 years old, (3) T0 local disease, (4) not the only primary tumor, (5) lack of information on distant metastatic lesion, (6) incomplete follow-up data, (7) 0 survival month, and (8) incomplete baseline data. A total of 3,194 cases entered the final analysis (Figure 1). All data obtained included age at diagnosis, race, tumor grade, human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), progesterone receptor (PR) status, AJCC TNM stage, metastatic organ, treatment, and follow-up information. The SEER program identifies only the first course of therapy, defined as those recorded in the treatment plan at diagnosis and administered before disease progression or recurrence. Surgery in the current research refers to the primary lesion (16). SEER data are publicly available, and a signed Research Data Agreement form was required to access the database. No institutional review board approval was required for this study.
CSS was measured by the time between diagnosis and breast cancer–related death. Survival curves were constructed according to the Kaplan–Meier (K–M) method, and difference curves were analyzed using the log-rank test.
CS is defined as the possibility of surviving an additional number of y years given that a patient has already survived for x years. The CCSS formula is CCSS (x | y) = CSS (x + y)/CSS (x), where CSS (x) represents the cause-specific survival at x year calculated by the K–M curve. For example, CS for surviving another year among patients who had already survived 4 years, CCSS (1|4), was calculated by dividing the 5-year K–M survival estimate CSS (5) by the 4-year survival estimate CSS (4).
Multivariate Cox proportional-hazards regression was performed to evaluate the hazard of CSS at the time of diagnosis and CCSS for multiple survival periods (1 and 3 years after diagnosis). For instance, to compute the CCSS at 1 year after diagnosis, 1-year survivors were selected. After subtraction of 12 months from their survival time, a multivariate analysis was performed. Only the variables that were prognostic with P-value less than 0.1 in the analysis of the previous period were selected and incorporated in the next period’s multivariate analysis sequentially. Differences were statistically significant when P < 0.05. Statistical analyses were performed using the SPSS 22.0 statistical software.
This study included 3,194 breast cancer patients who met the criteria in the SEER database (Table 1). Most of the patients were younger than 65 years old (78.4%). Majority of the patients were White (71.1%) followed by Black (19.6%). The most frequent histopathological grade was poorly differentiated (60.7%). Bone metastasis (35.6%) was the most common site of metastasis, followed by lung metastasis (10.9%) and brain metastasis (1.6%). In terms of treatment, more than 70% of the patients received chemotherapy combined with surgery, of which 1,222 (38.3%) patients received chemotherapy combined with surgery and radiotherapy.
With a median follow-up time of 26 (1–83) months until 2018, the CSS of patients at 1, 3, and 5 years was 84%, 55%, and 39%, respectively. The CCSS related to the number of years are shown in Table 2, and the K–M survival curves are shown in Figure 2. The 5-year CCSS increased from 39% directly after diagnosis to 46% (Δ 7%), 57% (Δ 18%), 71% (Δ 32%), and 85% (Δ 46%), given 1, 2, 3, and 4 years already survived, respectively. The longer the patients have survived, the more likely they are to survive for additional years. This growth leveled off after many years.
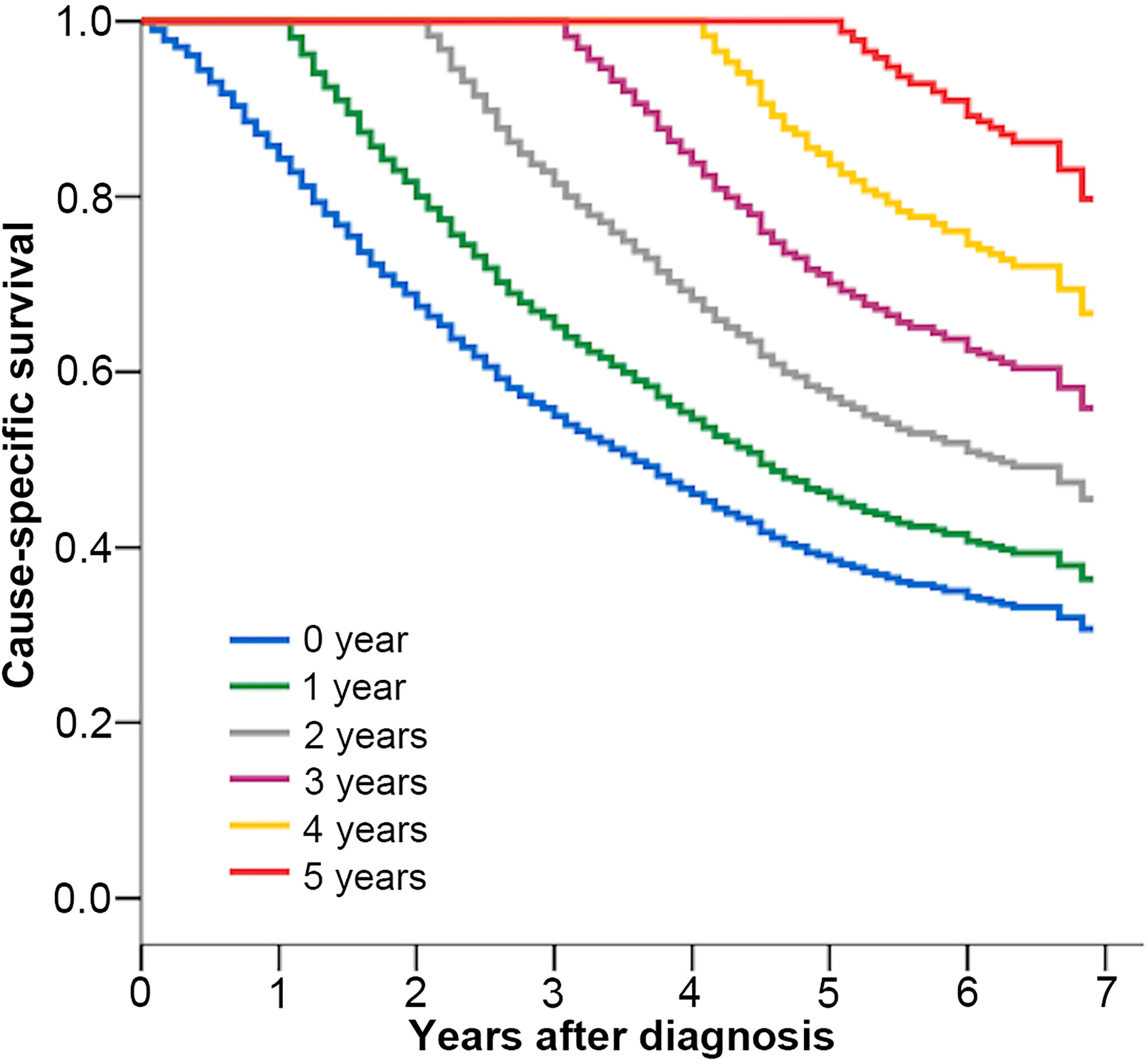
Figure 2 Kaplan–Meier estimates of cancer-specific survival after diagnosis (0 year) and conditional cancer-specific survival, according to years already survived after diagnosis (1–5 years).
Multivariate analysis showed that age, race, AJCC T, N categories, tumor grade, HER2, ER, PR status, metastatic organ, and treatment were independent prognostic factors for CSS of metastatic breast cancer (all P < 0.05, Table 3) at diagnosis. For patients surviving for 1 year after diagnosis, multivariate analysis identified that T4, poorly grade, HER2 positive, brain, and multisite metastasis were independent risk factors (all P < 0.05), whereas ER positive, PR positive, and surgery or surgery combined with radiotherapy were independent protective factors (all P < 0.05). After 3 years of diagnosis, only HER2 positive (HR = 0.598, P < 0.001), multisite metastasis (HR = 1.621, P = 0.002), and surgery (HR = 0.507, P < 0.001) or surgery combined with radiotherapy (HR = 0.521, P < 0.001) were still independent prognostic factors.
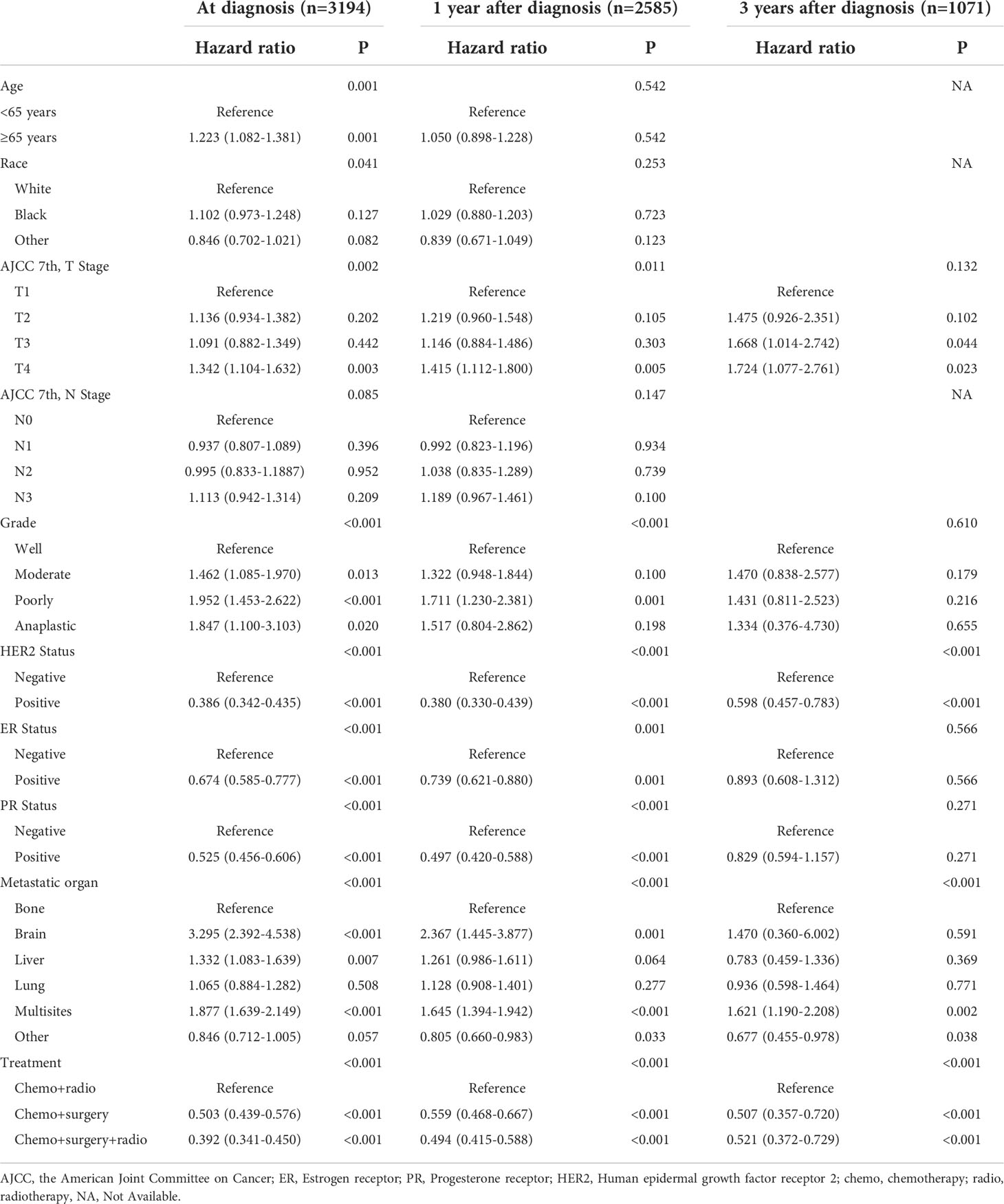
Table 3 Multivariable Cox proportional hazards analysis of risk factors associated with cause-specific survival.
All patients were divided into subgroups according to the independent prognostic factors to evaluate their effects on CSS and CCSS. Figure 3 shows that the 5-year CSS of HER2 positive patients was significantly better than that of HER2 negative patients (52% vs. 31%, P < 0.001, Figure 3A). In the subgroup analysis according to the metastatic site, the 5-year CSS of patients with brain metastasis (9%) was significantly worse than that of patients with bone (47%), liver (44%), and other sites (49%) (P < 0.001, Figure 3C). The 5-year CCSS of patients with bone, liver, and other site metastasis who have survived 4 years after diagnosis increased to 84%, 88%, and 92%, respectively, whereas the 5-year CCSS of patients with brain metastasis was only 53% (Figure 3D), which indicated that patients with brain metastasis disease at diagnosis still experience disease progression despite surviving 4 years. The subgroup analysis according to the treatment methods showed that the prognosis of patients who underwent surgery with or without radiotherapy was significantly better than that of patients who only underwent radiotherapy (5-year CSS of radiotherapy = 16%, 5-year CSS of surgery = 43%, 5-year CSS of surgery combined with radiotherapy = 47%, P < 0.001) (Figure 3E).
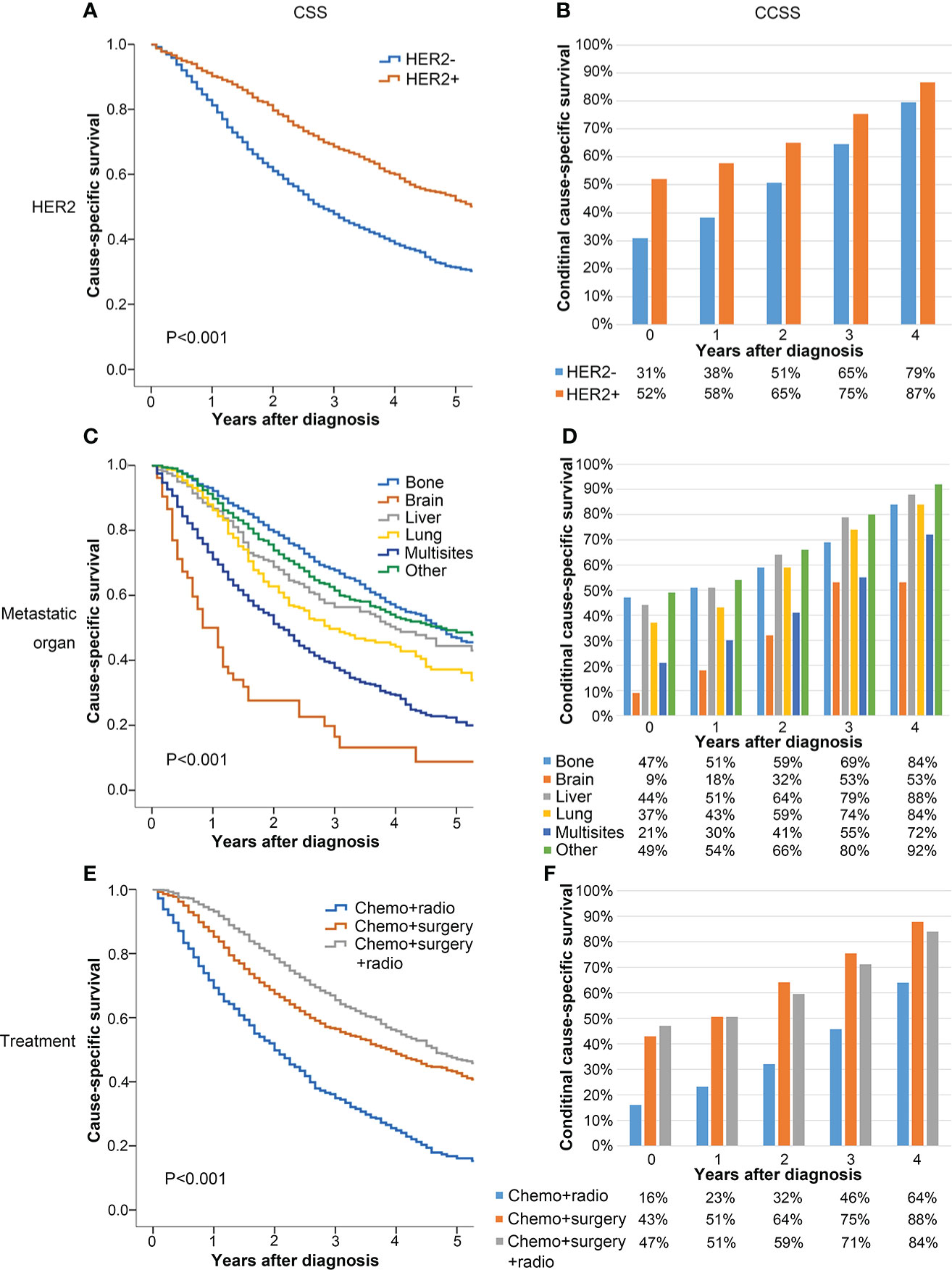
Figure 3 Comparison between CSS (A, C, E) and CCSS (B, D, F) according to HER2 (A, B), metastatic organ (C, D), and treatment (E, F).
In each subgroup, CSS showed a downward trend, whereas the 5-year CCSS gradually went up with the passage of survival time. In each subgroup, the 5-year CCSS was better than the 5-year CSS. Moreover, the difference between the CSS and the 5-year CCSS was more significant in patients with poor clinicopathological factors at baseline. In contrast, this difference was relatively small in patients with good initial clinicopathological factors at baseline. For example, the 5-year CSS (baseline) of patients with HER2 positive was 52%, whereas the 5-year CCSS of 4 years after diagnosis was 87% (Δ 35%). For patients with HER2 negative, the 5-year CCSS was 31% at diagnosis and the 5-year CCSS increased to 79% (Δ 47%) at 4 years after diagnosis (Figure 3B).
To the best of our knowledge, this is the first study evaluating the CS of metastatic breast cancer. More than 3,000 cases of metastatic breast cancer with chemotherapy and local treatment in the SEER database were included in this study. It has been found that although the population has poor prognosis with the 5-year CSS only 39%, the 5-year CCSS increased with the extension of survival time. For patients who have survived for 4 years, the 5-year CCSS is as high as 85%, especially for patients with adverse prognostic factors. Furthermore, HER2 status, multisite metastasis, and treatment were independent prognostic factors at the time of diagnosis, and their prognostic effects persisted until 3 years after diagnosis.
CS represents the possibility that a patient can survive a certain number of years after diagnosis or treatment based on the time the patient has already survived. It can dynamically describe the survival rate of patients as time progresses (17). In this study, the 5-year CCSS of metastatic breast cancer increased year by year with the increase in survival years. For example, the probability of survival at 5 years after diagnosis went from 39% at 0 years to 71% at 3 years. In the subgroup analysis, this increasing trend was more obvious in patients with poor clinicopathological factors. The prognosis of surviving patients with high risk factors will be close to those of patients with some low risk factors as time goes on, which can reduce anxiety and improve the quality of life, especially for high-risk patients. For instance, the 5-year CSS of HER2 positive and HER2 negative patients at diagnosis were 52% and 31% (difference of 21%), and the 5-year CCSS of 4 years after diagnosis were 87% and 79% (difference of 8%). This may be due to the rapid death of high-risk patients after diagnosis. In the traditional survival analysis, patients with risk factors tend to have worse CSS. Therefore, cumulative survival analysis is somewhat crude for accurately assessing long-term survival, especially for patients who have survived for a period of time (18).
Currently, the treatment of metastatic breast cancer is still controversial. Three prospective randomized trials (the MF07-01, an Indian study, and the recent ECOG-ACRIN 2108 Trial) have shown different effects of the local treatments (19–22). The 3-year OS was similar between systemic therapy and primary surgery arms in all of them. However, the MF07-01 trial showed a better 5-year and 10-year OS in patients who underwent local treatment followed by system therapy compared with those who received only system therapy. There are some pitfalls in the above studies. The imbalance of baseline variables, insufficiency of system therapy, and high tumor burden are thought to lead to bias. Thus, it is very difficult to conduct a perfect random trial about the local therapy for primary stage IV breast cancer in a real world. Of particular note is oligometastatic disease, which can achieve long-term remission and even be cured through different treatment strategies (23). The BOMET MF14-01 study showed that bone metastasis only (especially oligometastatic bone and solitary bone) may take more advantage from local surgery (24). The subgroup analysis of the MF07-01 trial also favored the fact that the solitary bone metastasis was the proper candidate for local therapy (19, 20). So, it is very important to recognize those patients who can really benefit from the local treatment, and CS may be a better predictor of continued survival for people with long-term survival benefits.
Our study found that surgery combined with radiotherapy as the local treatment was more efficient compared with surgery or radiotherapy alone. The 5-year CSS increased from 16% to 43% (Δ 27%, P < 0.001), and it further increased to 47% (Δ 31%, P < 0.001) in patients accepting surgery combined with radiotherapy. Lian et al. collected data from SEER between 2004 and 2012 and also drew a similar conclusion (25). The 3-year CSS were 35.9%, 57.1%, and 63.9% in patients who underwent radiotherapy alone, surgery alone, and surgery combined with radiotherapy. Our study suggests that the local treatment can affect the prognosis for a long time. Due to the inability to obtain metastatic tumor load from the SEER database, we were unable to perform further analysis. In addition, the patients who have a good initial prognosis (low tumor burden, metastatic clearance with system therapy, fewer complications, and younger age), as evaluated subjectively by the physician, were more likely to opt for surgery, leading to bias.
Previous studies have shown that age, HER2 status, hormone receptor state, metastatic sites, and treatment were important factors affecting the prognosis of metastatic breast cancer (8, 26, 27), but there is no study on the prognostic factors for patients with metastatic breast cancer who have survived for several years. In this study, we found that age, race, grade, HER2, ER, PR status, metastatic organ, and local treatment were independent prognostic factors for CSS, which is consistent with the previous studies (8). However, at 1 year and 3 years after diagnosis, only HER2 status, metastatic organ, and local treatment continued to affect the prognosis. With HER2-targeted therapy, the prognosis of HER2 positive metastatic breast cancer has been improved (28). Our study also showed that the prognosis of HER2 positive patients was significantly better than HER2 negative, and this factor continued to influence the long-term survival during follow-up, which verified that the targeted treatment of HER2 had long-term survival benefits to the metastatic breast cancer. The common metastatic sites were bone, lung, brain, and liver, of which the prognosis of brain and multisite metastasis was the worst (29, 30). In this study, the 5-year CSS of brain metastasis and multisite metastasis patients were only 9% and 21%, and the latter remained an independent risk factor for prognosis as years of survival increased. Obviously, the more the metastases, the higher the tumor burden. As a result, these patients have a poor prognosis.
This study has some limitations. First of all, this is a retrospective study and inevitably leads to selection bias. Second, information such as the treatment of targeted and endocrine, the sequence of chemotherapy and surgery, and the therapeutic effect evaluation cannot be obtained from the SEER database. However, this is the first study to assess the 5-year CCSS of metastatic breast cancer and to analyze the potential factors that continue to influence the prognosis. The results of this study can be used as an important basis for improving treatment options as well as the prognosis of patients with metastatic breast cancer in the future.
CCSS of metastatic breast cancer was dynamic and increases with each additional year survived. Compared with CSS, CCSS provided a more individualized prognosis. Furthermore, HER2 status, multisite metastasis, and local treatment were independent prognostic factors that continued to influence the survival of metastatic breast cancer. These patients seemed to benefit more from surgery combined with radiotherapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
PZ and MX designed and performed the research. MX performed the statistical analyses, interpreted the data, and wrote the manuscript. Both authors critically reviewed and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer (2018) 144(8):1941–53. doi: 10.1002/ijc.31937
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Heller DR, Chiu AS, Farrell K, Killelea BK, Lannin DR. Why has breast cancer screening failed to decrease the incidence of de novo stage IV disease? Cancers (2019) 11(4):500. doi: 10.3390/cancers11040500
4. Lane WO, Thomas SM, Blitzblau RC, Plichta JK, Rosenberger LH, Fayanju OM, et al. Surgical resection of the primary tumor in women with de novo stage IV breast cancer. Ann Surg (2019) 269(3):537–44. doi: 10.1097/SLA.0000000000002621
5. Kim Y-J, Jung S-Y, Kim K. Survival benefit of radiotherapy after surgery in de novo stage IV breast cancer: A population-based propensity-score matched analysis. Sci Rep (2019) 9(1):8527. doi: 10.1038/s41598-019-45016-2
6. Nguyen DHA, Truong PT, Alexander C, Walter CV, Hayashi E, Christie J, et al. Can locoregional treatment of the primary tumor improve outcomes for women with stage IV breast cancer at diagnosis? Int J Radiat Oncol BiologyPhysics (2012) 84(1):39–45. doi: 10.1016/j.ijrobp.2011.11.046
7. Chia SK, Speers CH, D'Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer (2007) 110(5):973–9. doi: 10.1002/cncr.22867
8. Kim BH, Kim S, Kim YI, Chang JH, Hwang K-T, Kim S, et al. Development of an individualized prediction calculator for the benefit of postoperative radiotherapy in patients with surgically resected de novo stage IV breast cancer. Cancers (2020) 12(8):2103. doi: 10.3390/cancers12082103
9. Short NJ, Jabbour E, Sasaki K, Patel K, O'Brien SM, Cortes JE, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood (2016) 128(4):504–7. doi: 10.1182/blood-2016-03-707562
10. Lin JP, Zhao YJ, He QL, Hao HK, Tian YT, Zou BB, et al. Adjuvant chemotherapy for patients with gastric neuroendocrine carcinomas or mixed adenoneuroendocrine carcinomas. Br J Surg (2020) 107(9):1163–70. doi: 10.1002/bjs.11608
11. Bischof DA, Kim Y, Dodson R, Jimenez MC, Behman R, Cocieru A, et al. Conditional disease-free survival after surgical resection of gastrointestinal stromal tumors. JAMA Surg (2015) 150(4):299. doi: 10.1001/jamasurg.2014.2881
12. Wang P, Sun Z, Wang W, Deng J, Wang Z, Liang H, et al. Conditional survival of patients with gastric cancer who undergo curative resection: A multi-institutional analysis in China. Cancer (2018) 124(5):916–24. doi: 10.1002/cncr.31160
13. Shah MM, Meyer BI, Rhee K, NeMoyer RE, Lin Y, Tzeng CWD, et al. Conditional survival analysis of hepatocellular carcinoma. J Surg Oncol (2020) 122(4):684–90. doi: 10.1002/jso.26049
14. Rosiello G, Palumbo C, Knipper S, Pecoraro A, Luzzago S, Deuker M, et al. Contemporary conditional cancer-specific survival after radical nephroureterectomy in patients with nonmetastatic urothelial carcinoma of upper urinary tract. J Surg Oncol (2020) 121(7):1154–61. doi: 10.1002/jso.25877
15. Latenstein AEJ, van Roessel S, van der Geest LGM, Bonsing BA, Dejong CHC, Groot Koerkamp B, et al. Conditional survival after resection for pancreatic cancer: A population-based study and prediction model. Ann Surg Oncol (2020) 27(7):2516–24. doi: 10.1245/s10434-020-08235-w
16. Wang Z, Cheng Y, Chen S, Shao H, Chen X, Wang Z, et al. Novel prognostic nomograms for female patients with breast cancer and bone metastasis at presentation. Ann Trans Med (2020) 8(5):197–7. doi: 10.21037/atm.2020.01.37
17. Moriwaki Y, Kunisaki C, Kobayashi S, Harada H, Imai S, Kido Y, et al. Progressive improvement of prognosis for patients with gastric cancer (dynamic stage grouping) with increasing survival interval from initial staging: How much longer can a given survivor expect to live? Surgery (2003) 133(2):135–40. doi: 10.1067/msy.2003.95
18. Ai B, Wang X, Kong X, Wang Z, Fang Y, Wang J. Conditional survival of female patients with operable invasive breast cancer in US: A population-based study. J Cancer (2020) 11(19):5782–91. doi: 10.7150/jca.46183
19. Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: Protocol MF07-01. Ann Surg Oncol (2018) 25(11):3141–9. doi: 10.1245/s10434-018-6494-6
20. Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Primary surgery with systemic therapy in patients with de Novo stage IV breast cancer: 10-year follow-up; protocol MF07-01 randomized clinical trial. J Am Coll Surg (2021) 233(6):742–51.e5. doi: 10.1016/j.jamcollsurg.2021.08.686
21. Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol (2015) 16(13):1380–8. doi: 10.1016/S1470-2045(15)00135-7
22. Khan SA, Zhao F, Goldstein LJ, Cella D, Basik M, Golshan M, et al. Early local therapy for the primary site in de novo stage IV breast cancer: Results of a randomized clinical trial (EA2108). J Clin Oncol (2022) 40(9):978–87. doi: 10.1200/JCO.21.02006
23. Barberi V, Pietragalla A, Franceschini G, Marazzi F, Paris I, Cognetti F, et al. Oligometastatic breast cancer: How to manage it? J Pers Med (2021) 11(6):532. doi: 10.3390/jpm11060532
24. Soran A, Dogan L, Isik A, Ozbas S, Trabulus DC, Demirci U, et al. The effect of primary surgery in patients with de novo stage IV breast cancer with bone metastasis only (Protocol BOMET MF 14-01): A multi-center, prospective registry study. Ann Surg Oncol (2021) 28(9):5048–57. doi: 10.1245/s10434-021-09621-8
25. Lian C-L, Guo L-Y, Zhang L, Wang J, Lei J, Hua L, et al. Aggressive local treatment improves survival in stage IV breast cancer with synchronous metastasis. Front Oncol (2020) 10:522580. doi: 10.3389/fonc.2020.522580
26. Li S, Zhao J, Zhu L, Su F, Chen K. Development and validation of a nomogram predicting the overall survival of stage IV breast cancer patients. Cancer Med (2017) 6(11):2586–94. doi: 10.1002/cam4.1224
27. Leone BA, Vallejo CT, Romero AO, Machiavelli MR, Pérez JE, Leone J, et al. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res Treat (2016) 161(3):537–48. doi: 10.1007/s10549-016-4066-7
28. Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer (2020) 126(19):4278–88. doi: 10.1002/cncr.33102
29. Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER-2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol (2017) 18(6):732–42. doi: 10.1016/S1470-2045(17)30312-1
Keywords: Breast cancer, conditional survival, prognosis, therapy, SEER program
Citation: Xiao M and Zhang P (2022) Conditional cause-specific survival after chemotherapy and local treatment for primary stage IV breast cancer: A population-based study. Front. Oncol. 12:800813. doi: 10.3389/fonc.2022.800813
Received: 24 October 2021; Accepted: 07 July 2022;
Published: 05 August 2022.
Edited by:
Xiaosong Chen, Shanghai Jiao Tong University, ChinaReviewed by:
Serdar Ozbas, Private Practitioner, Ankara, TurkeyCopyright © 2022 Xiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pin Zhang, enBwdW1jQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.