- 1Division of Surgery and Interventional Science, University College London, London, United Kingdom
- 2Medical Sciences Division Brasenose College, University of Oxford, Oxford, United Kingdom
- 3Department of Biostatistics, University of Notre Dame, Fremantle, WA, Australia
- 4Department of Radiation Oncology, Sir Charles Gairdner Hospital, Perth, WA, Australia
- 5Department of Clinical Oncology, University College London Hospitals, London, United Kingdom
Micro abstract: Targeted intraoperative radiotherapy (TARGIT-IORT) is delivered immediately after lumpectomy for breast cancer. We estimated its impact. At least 44,752 patients with breast cancer were treated with TARGIT-IORT in 260 centres in 35 countries, saving >20 million miles of travel and preventing ~2,000 non–breast cancer deaths. The TARGIT-IORT website (https://targit.org.uk/travel) provides maps and tools to find the nearest centre offering TARGIT-IORT and travel savings.
Background: Targeted intraoperative radiotherapy (TARGIT-IORT) delivers radiotherapy targeted to the fresh tumour bed exposed immediately after lumpectomy for breast cancer. TARGIT-A trial found TARGIT-IORT to be as effective as whole-breast radiotherapy, with significantly fewer deaths from non–breast cancer causes. This paper documents its worldwide impact and provides interactive tools for clinicians and patients.
Method: Centres using TARGIT-IORT provided the date of the first case and the total number of patients. We plotted these data on a customised Google Map. An interactive web-based tool provided directions to the closest centre. Using the data from the TARGIT-A trial, we estimated the total savings in travel miles, carbon footprint, and the number of non–breast cancer deaths that might be prevented.
Results: Data from 242 (93%) of the 260 centres treating patients from 35 countries were available. From the first patient treated in 1998 to early 2020, at least 44,752 women with breast cancer have been treated with TARGIT-IORT. The TARGIT-IORT website (https://targit.org.uk/travel) displays the Google Map of centres with number of cases and an interactive tool for patients to find the nearest centre offering TARGIT-IORT and their travel savings. Scaling up to the already treated patients, >20 million miles of travel would have been saved and about 2,000 deaths prevented.
Conclusion: One can ascertain the number of patients treated with a novel treatment. These data show how widely TARGIT-IORT has now been adopted and gives an indication of its beneficial worldwide impact on a large number of women with breast cancer.
Clinical practice points
● What is already known about this subject? Targeted intraoperative radiotherapy (TARGIT-IORT) delivers radiotherapy targeted to the fresh tumour bed exposed immediately after lumpectomy for breast cancer. TARGIT-A trial found TARGIT-IORT to be as effective as whole-breast radiotherapy, with significantly fewer deaths from non–breast cancer causes. This paper documents its worldwide impact and provides interactive tools for clinicians and patients.
● What are the new findings? We ascertained that by early 2020, at least 44,752 women with breast cancer have been treated with TARGIT-IORT in 260 centres in 35 countries. We provide at the TARGIT-IORT website (https://targit.org.uk/travel) the Google Map of centres with number of cases and an interactive tool for patients to find the nearest centre offering TARGIT-IORT and their travel savings. We also estimated that, by now, >20 million miles of travel would have been saved and about 2,000 deaths prevented.
● How might it impact on clinical practice in the foreseeable future? In addition to hard randomised evidence proving survival and quality of life benefits clinical practice is often prompted by seeing what our peers are doing. Dissemination of these data showing widespread adoption of the technique would further increase awareness and utilisation of this patient-centred approach amongst patients, clinicians, and policymakers.
Introduction
A large proportion of patients with small breast cancers can be effectively treated by a lumpectomy and radiotherapy, rather than a mastectomy. Radiotherapy is traditionally given to the whole breast.
In the mid-1990s, targeted intraoperative radiotherapy (TARGIT-IORT) (1–3) was proposed as a radical new approach. This treatment delivers effective radiotherapy targeted to the fresh tumour bed exposed immediately after lumpectomy (4, 5) while sparing nearby tissues and nearby vital organs such as the heart and lung.
In pilot studies starting from 2 July 1998, the safety and feasibility of this novel approach combining surgery and radiotherapy were confirmed (1–3), and the TARGIT-A randomised trial was proposed in 1999 (6) comparing risk-adapted single-dose TARGIT-IORT during lumpectomy vs. conventional fractionated whole-breast external beam radiotherapy (EBRT) given daily for several weeks (6–8).
Long-term outcomes of the TARGIT-A trial found it to be as effective in terms of breast cancer outcomes and that it led to fewer deaths from other causes (9). Further pre-planned subgroup analysis found that these results are valid for all invasive ductal carcinoma tumour subtypes; there is an overall survival benefit of 4.4% at 12 years in those with grade 1 or 2 tumours (n = 1,797) and identical overall survival in grade 3 cancers (n = 443) (10). Unlike the poor prognosis faced by patients who have a local recurrence after EBRT, those who receive TARGIT-IORT maintain their excellent prognosis even after local recurrence (10). Other benefits included lower radiation related toxicity (11–18), reduced pain, and better quality of life (17, 19–23). When given a choice, TARGIT-IORT is preferred by patients over other methods of radiotherapy or “no-radiotherapy” (24–30). An online tool can guide clinicians in decisions about additional whole-breast radiotherapy after TARGIT-IORT (https://targit.org.uk/addrt) (10).
The adoption of TARGIT-IORT for standard clinical practice has grown considerably over the last 20 years. In this short paper, to assess the worldwide impact of TARGIT-IORT, we aimed to count the number of patients treated with TARGIT-IORT around the world, as well as to estimate the total benefits to the patient, in terms of the saving of travel distance, time, and reduction of transport-related carbon footprint and reduced deaths from other causes.
The TARGIT-A trial was initiated by an academic insight and collaboration with the industry was solely for the development of the device. The study was sponsored by University College London Hospitals (UCLH)/UCL Comprehensive Biomedical Research Centre. Funding was provided by UCLH Charities, National Institute for Health Research (NIHR) Health Technology Assessment programme (HTA 07/60/49), Ninewells Cancer Campaign, National Health and Medical Research Council, and German Federal Ministry of Education and Research (BMBF) FKZ 01ZP0508. The infrastructure of the trial operations office in London, UK, was supported by core funding from Cancer Research Campaign (now Cancer Research UK) when the trial was initiated. In the extended follow-up of the TARGIT-A trial (TARGIT-Ex; funded by the HTA programme of the National Institute for Health Research, Department of Health and Social Care in the UK, HTA 14/49/13), we are continuing the follow up of TARGIT-A trial patients in the UK by direct patient contact and via UK national databases.
We are also currently inviting women who would fall outside the eligibility criteria of the TARGIT-A trial to participate in the TARGIT-B(oost) trial (funded by HTA 10/104/07), already opened in 38 centres internationally, which is comparing TARGIT-IORT as a tumour bed boost with EBRT boost in younger women or women who have higher risk disease to test for superiority in terms of local control and survival.
Method
Since the first case was performed in London in 1998, an international network has been developed between centres using TARGIT-IORT. Therefore, the contact details of a large proportion of the centres were available. Using Google forms and electronic communication, we requested the date when the first patient with breast cancer was treated with TARGIT-IORT at their centre and how many such patients were treated by their centre in total. We did not restrict this to those centres that had participated in the TARGIT trials. If, after repeated attempts, there was no response from a centre, then we included the name of the centre without the number of cases. We also queried the German National Database (https://www.destatis.de/) using the codes 8.52d, 8-523.6, and 8-521. Such databases were not available for other countries. We collected data until just before the COVID-19 pandemic started. Using Google My Maps, each hospital was displayed on an interactive map, showing the date of the first case and the total number of cases performed at the centre, along with directions to a chosen hospital.
In addition to avoiding the hospital visit required to plan radiotherapy, the large majority of patients (eight out of every 10) who received TARGIT-IORT would avoid 15 to 30 daily trips to the hospital where they would have undergone conventional whole-breast radiotherapy. Therefore, we made an estimate of the total savings by the patient—in terms of travel miles, travel time, and carbon footprint, using the methodology described previously (31). Our previous work (31) had found that patients in the TARGIT-A trial, mostly from urban areas in the UK, saved an average of 305 miles of travel, whereas those in semi-urban areas saved 753 miles. This calculation was based on the total number of hospital trips the patients saved when they were randomised to the TARGIT-IORT arm compared with the EBRT arm in the randomised TARGIT-A trial. The distance travelled for each trip was individually calculated by inputting in Google Maps API, the addresses of the patient, and the treating hospital where the EBRT was given. The total miles saved were used to calculate the amount of CO2 saved using standard emissions for a medium sized car. This estimate takes into account the additional travel required in the 20% of patients who are recommended whole-breast EBRT. It has been estimated that 55% of the world population lived in urban areas in 2018 (32). In this paper, we used the UK figures for travel savings and assumed a larger proportion of patients (66% rather than 55%) will be urban dwellers. We prepared an interactive web application to make individual estimates. These tools were tested by patients, and their feedback was used for making improvements.
Long-term results of the TARGIT-A trial (9) (Supplementary Figure 1) found no difference any breast cancer outcome or breast cancer–specific mortality but a significant reduction in non–breast cancer mortality (HR, 0.59; 95% CI, 0.40 to 0.86; P = 0.005) such that it was 5.41% for TARGIT-IORT and 9.85% for EBRT. The difference was 4.44% (95% CI of the difference being 2.5% to 6.4%). This estimate is consistent with that of overall survival improvement in patients with grade 1 and grade 2 cancers that formed a large subgroup of patients in the trial contributing 1,796 out of the total of 2,298. In a pre-specified subgroup analysis (with its usual caveats), overall survival was significantly better in this subgroup by 4.4% (HR, 0.72; p = 0.0361). We used this absolute difference in deaths, i.e., 4.4 fewer deaths over 12 years per 100 patients treated, to estimate the global impact of using TARGIT-IORT in terms of mortality reduction amongst patients already treated around the world.
We used STATA 16 for statistical analysis.
Results
Data from 242 (93%) of the 260 centres were available. Data from 31 of 64 centres (n = 8021) in Germany were available directly from investigators and the remaining 33 (n = 8044) from the German national database. Of these 260 centres, 33 had participated in the TARGIT-A trial.
The first patient with breast cancer was treated with TARGIT-IORT on 2 July 1998 at the Middlesex hospital (now part of University College London Hospitals), University College London. Since then, we found that TARGIT-IORT has been used in 35 countries, and at least 44,752 patients with breast cancer have been treated (Table 1). The total numbers of patients known to have been treated are approximately 30,000 in Europe, 9,000 in North America, 3,000 in Asia Pacific, 2,000 in South/Central America, 500 in the Middle East, and 200 in Africa. Table 2 has the list the collaborating centres.
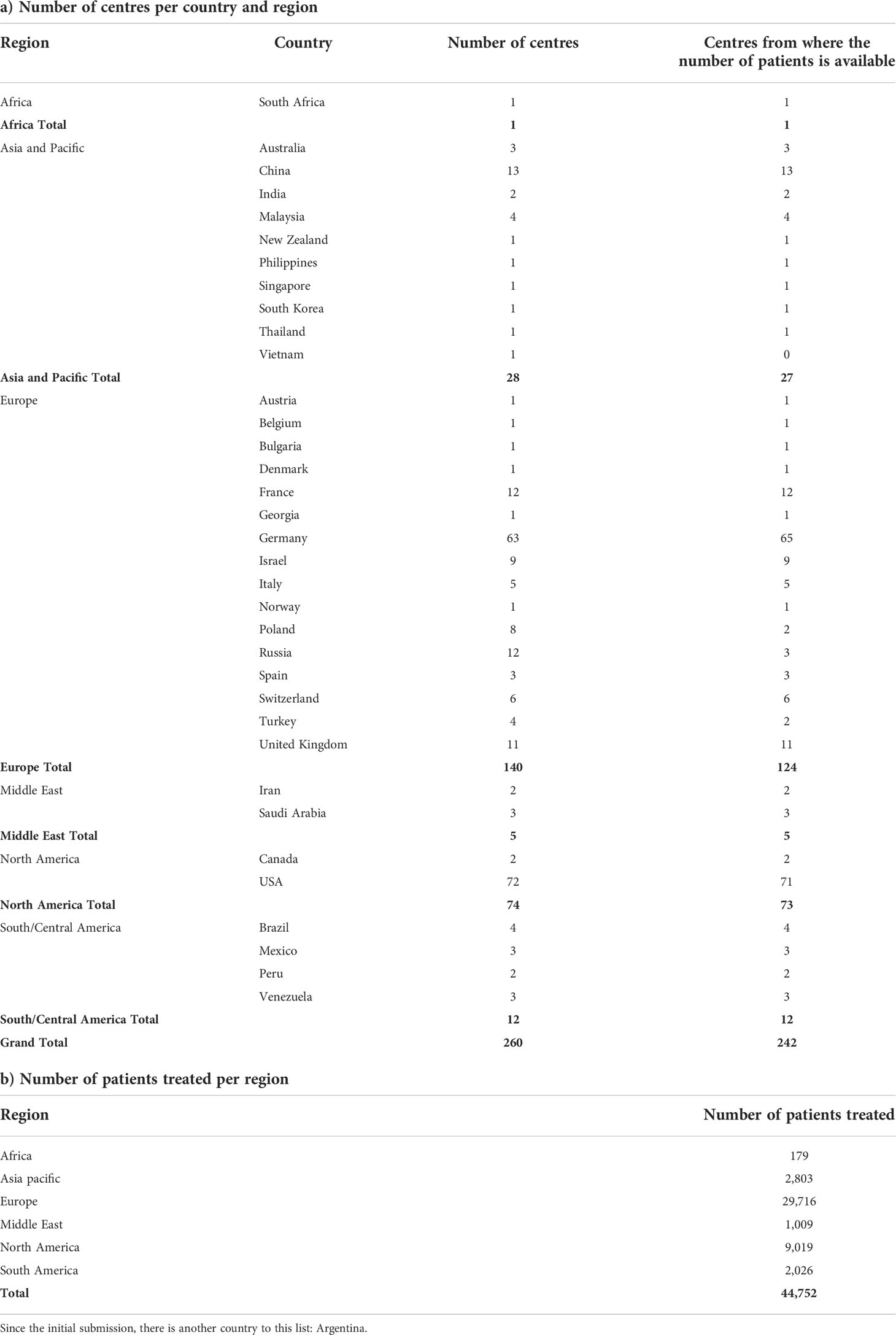
Table 1 a) Number of centres that have treated patients with breast cancer with TARGIT-IORT around the world and b) number of patients treated in each of the world regions.
Figure 1 is the screenshot of an interactive Google Map that shows the centres that have offered TARGIT-IORT for breast cancer, the year of their first case, and the number of cases performed as of August 2020. Once the reader clicks on a particular centre, they can get directions to the centre by clicking on the direction arrow on top left corner, next to the name of the centre. The interactive map in Supplementary Figure 2 shows the number of centres in each country. Supplementary Figure 3 shows how they have increased since 1998.

Figure 1 Screenshots of the map of the world with each dot representing a centre that has treated breast cancer with TARGIT-IORT. The name of the centre and the number of cases treated by the centre (if available) are seen in the left-hand pane when you click on the centre in 1b below (the map can be zoomed in). This map is interactive and available at https://targit.org.uk/travel.
Scaling up the journeys saved by avoiding EBRT, because of the use of TARGIT-IORT, to the 44,752 patients, we estimate that over 20 million (20,134,909) miles of travel have already been saved, representing a carbon footprint reduction of 5.6 million kg of CO2 emissions.
Figure 2 is the screenshot of the interactive tool with which one can find the centre offering TARGIT-IORT closest to one’s home. It will also estimate how much an individual patient would save by using TARGIT-IORT in terms of travel distance, time, and carbon footprint.
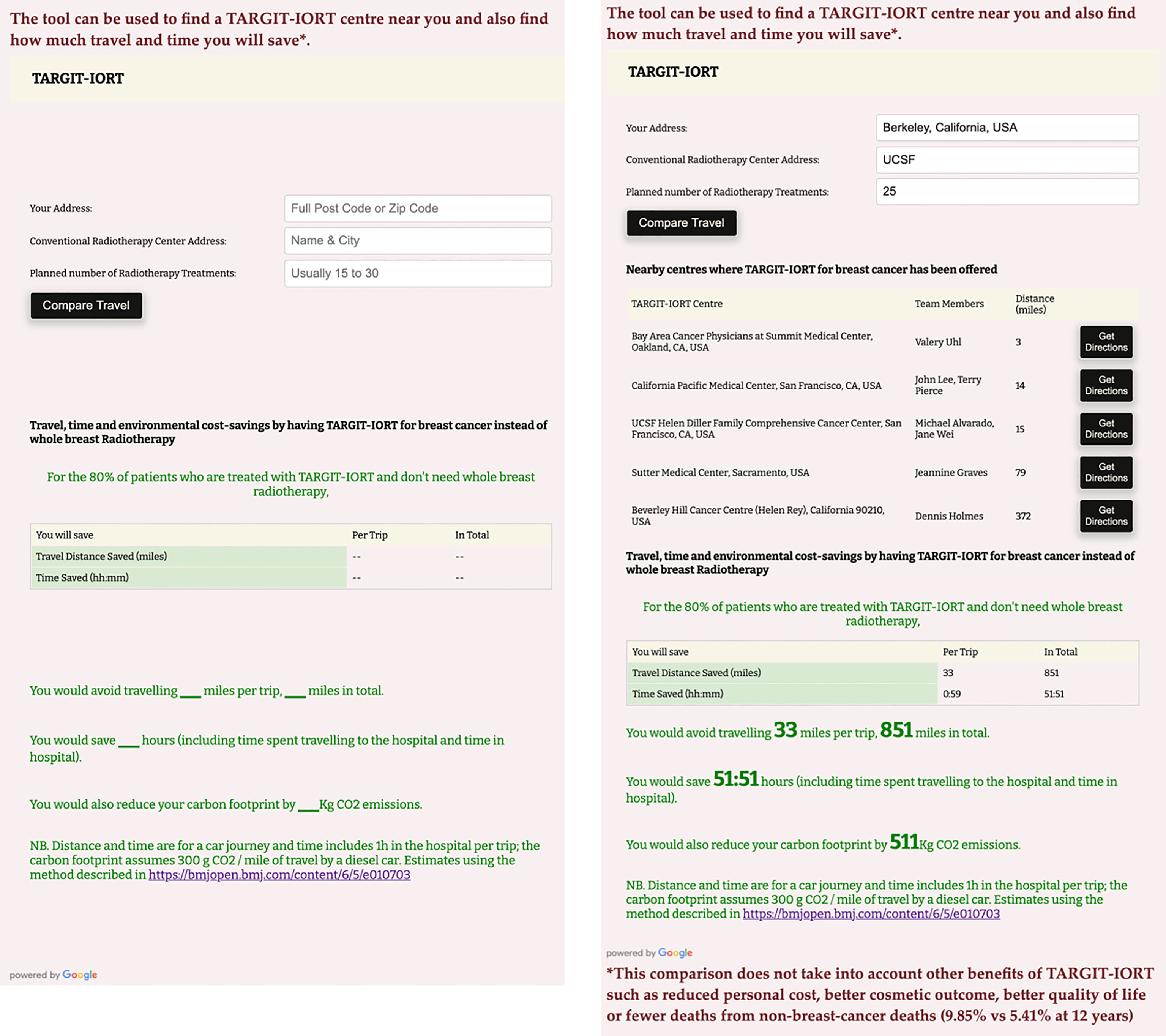
Figure 2 A screenshot of the interactive tool to assess how much an individual patient would save by using TARGIT-IORT in terms of travel distance, time, and carbon footprint. This example is for someone living in Berkeley, California, USA, for example, and going for radiotherapy at the University of Califoria San Francisco UCSF hospital, the closest radiotherapy centre from this house. This interative tool can be accessed at https://targit.org.uk/travel.
These interactive maps and tools can be accessed at https://targit.org.uk/travel.
Scaling up the 4.44% (95% CI, 2.5% to 6.4%) reduction in non–breast cancer mortality to the 44,752 patients treated to date (mid-2020), we estimate that 1,987 (95% CI, 1,129 to 2,845) non–breast cancer deaths from causes other than breast cancer such as cardiovascular and lung problems and other cancers would be prevented.
Discussion
This paper describes the worldwide adoption of TARGIT-IORT for treatment of early breast cancer over the past two decades. We could confirm that TARGIT-IORT has been used in 260 centres in 35 countries and about 45,000 patients in six continents have been treated. In the process, an estimated 20 million miles of journeys were avoided. Applying the reduction in non–breast cancer mortality, found in the TARGIT-A trial, to the patients already treated around the world suggests that the use of TARGIT-IORT would lead to 2,000 fewer deaths from causes other than breast cancer.
Over the last decade, there has been growing support for the use of partial breast irradiation (PBI) instead of whole-breast radiation therapy, and it is arguable that TARGIT-IORT is much better for patients than other methods of PBI (30, 33–35). The TARGIT-A trial cohort comprised a medium-risk population, with a substantial number of patients at a higher risk of relapse: 1,898 (83%) were younger than 70 years, 366 (16%) had tumours >2 cm in size, 443 (20%) patients had grade 3 cancers, 488 (22%) patients had involved nodes, and 426 (19%) had ER- or PgR-negative tumours. Therefore, its results would also be applicable to patients with breast cancer suitable for breast conserving surgery more widely than other methods of PBI (9, 30).
In many countries, patients live a considerable distance from the radiotherapy centre (31, 36, 37) and are more likely to receive a mastectomy than breast conservation (38). Even in the USA as recently as 2015, patients who lived farther away from the radiation facility (> 9.2 miles/19 min away by road) were 36%–44% more likely to receive a mastectomy than breast conservation (38). TARGIT-IORT is a much more convenient option (28, 39). We pointed out that wider availability and applicability of TARGIT-IORT should enable many more women to have the choice of having breast conservation when they would otherwise have a mastectomy because they are not able to have conventional radiotherapy (40–49). TARGIT-IORT also reduces the cost of providing treatment (50–55).
Importantly, TARGIT-IORT lowers the toxicity and reduces deaths from cardiovascular causes and other cancers by a substantial amount (4.4% by 12 years) (30), which has become increasingly important with the rising rates of survival with modern breast cancer treatment. This effect of improving survival appears to be a combination of avoiding the risks due to inadvertent scattered radiation from whole-breast radiotherapy and from a potential abscopal effect of delivery of intraoperative radiotherapy during the surgical excision of the cancer (10).
The strengths of this study are that the data were provided directly by the physicians and staff from each centre and that the response rate of 93% was excellent. In addition, we provide user-friendly interactive links (https://targit.org.uk/travel) for use by clinicians and patients. The obvious weakness is that this paper does not describe data about outcomes, but this is not the intention of this manuscript. Outcome data are best gained from comparative analysis within the prospective TARGIT-A randomised trial (9, 10), as well as data from several centres that have published their own experience of TARGIT-IORT and from prospective registry studies (https://targit.org.uk/publications) (18, 28, 39, 55–65). In addition, as the list of centres using TARGIT-IORT was compiled using personal contacts, we may have missed some centres, underestimating the number of cases. The network of centres using this approach is now been greatly strengthened and will in due course provide the foundation for a unified collection of outcome data.
TARGIT-IORT is now included in several national and international guidelines (66–79) (https://www.targit.org.uk/targit-iort-in-guidelines) for breast cancer treatment. Several of these guidelines specifically recommend using TARGIT-IORT during the COVID-19 pandemic caused by the SARS-CoV-2 virus to give the added advantage of reducing patient exposure to hospital environments and public places.
In this paper, we described the impact of a new treatment proven in a randomised clinical trial over the worldwide breast cancer community. It demonstrates how widely this evidence-based approach has now been adopted and how it has benefitted women with breast cancer around the world.
Author's note
A pre-print of this paper is available from UCL Discovery http://doi.org/10.14324/000.wp.10121050
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, and as permitted by individual clinical teams.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JV conceived the project and discussed it with UV, MBa, JST, and MBu and wrote the first draft; UV helped in making contacts, collecting data from centres and collating data, and programming for creating the figures and tables; JV, MBa, MBu, JT, DJ, and UV contributed to finalizing the draft. All other authors and contributors/collaborators contributed by treating patients and returning their own data for the compilation and approving the manuscript for submission.
Conflict of interest
JV has received a research grant from Photoelectron Corp (1996–99) and from Carl Zeiss for supporting data management at the University of Dundee (Dundee, UK, 2004–2008) and has received honorariums. JV and JT received funding from HTA, NIHR, Department of Health and Social Care for some activities related to the TARGIT trials. MBa was briefly on the scientific advisory board of Carl Zeiss and was paid consultancy fees before 2010. Carl Zeiss sponsors some of the travel and accommodation for meetings of the international steering committee and data monitoring committee and when necessary for conferences where a presentation about targeted intraoperative radiotherapy is being made for all authors apart from UV, who has declared no conflict of interest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.786515/full#supplementary-material
Supplementary Figure 1 | Kaplan-Meier curves showing breast cancer mortality (top left) and non–breast cancer mortality (top right), overall mortality for grade 1 or 2 cancers (bottom left), and grade 3 cancers (bottom left) for TARGIT-IORT v EBRT in the TARGIT-A trial. Figures under titles are hazard ratios (95% confidence intervals) and log rank test P values. EBRT=external beam radiotherapy; TARGIT = targeted intraoperative radiotherapy = TARGIT-IORT (taken from BMJ 2020;370:m2836 https://www.bmj.com/content/370/bmj.m2836.full.pdf and BJC 2021 125, pages380–389 (2021) https://www.nature.com/articles/s41416-021-01440-8.pdf.
Supplementary Figure 2 | World map showing countries in which TARGIT-IORT is offered for breast cancer. The shading correlates with the number of centres in each country. For an interactive map see https://targit.org.uk/travel.
Supplementary Figure 3 | The number of centres offering TARGIT-IORT increased worldwide from 1998 onward. The graph below includes only those centres from which the date of first case was returned to us.
References
1. Vaidya JS, Baum M, Tobias JS, et al. Targeted intra-operative radiotherapy (TARGIT): an innovative method of treatment for early breast cancer. Ann Oncol Off J Eur Soc Med Oncol / ESMO (2001) 12(8):1075–80.
2. Vaidya JS, Baum M, Tobias JS, Morgan S S, D'Souza D. The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2002) 28(4):447–54. doi: S0748798302912758
3. Vaidya JS. A novel approach for local treatment of early breast cancer. PhD thesis, university college London. University of London (2002). Available at: http://www.ucl.ac.uk/~rmhkjsv/papers/thesis.htm.
4. Vaidya JS, Vyas JJ, Chinoy RF, Merchant N, Sharma OP, Mittra I, et al. Multicentricity of breast cancer: whole-organ analysis and clinical implications. Br J Cancer (1996) 74(5):820–4.
5. Baum M, Vaidya JS, Mittra I. Multicentricity and recurrence of breast cancer [letter; comment]. Lancet (1997) 349(9046):208–08.
6. Vaidya JS, Baum M, Tobias JS, Houghton J. Targeted intraoperative radiothearpy (TARGIT)- trial protocol. Lancet (1999).
7. Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-a trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet (2010) 376(9735):91–102. doi: 10.1016/S0140-6736(10)60837-9
8. Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-a randomised trial. Lancet (2014) 383(9917):603–13. doi: 10.1016/S0140-6736(13)61950-9
9. Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-a randomised clinical trial. BMJ (2020) 370:m2836. doi: 10.1136/bmj.m2836
10. Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. New clinical and biological insights from the international TARGIT-a randomised trial of targeted intraoperative radiotherapy during lumpectomy for breast cancer. Br J Cancer (2021) 125(3):380–89. doi: 10.1038/s41416-021-01440-8
11. Kraus-Tiefenbacher U, Bauer L, Kehrer T, Hermann B, Melchert F, Wenz F, et al. Intraoperative radiotherapy (IORT) as a boost in patients with early-stage breast cancer – acute toxicity. Onkologie (2006) 29(3):77–82.
12. Kraus-Tiefenbacher U, Bauer L, Scheda A, Fleckenstein K, Keller A, Herskind C, et al. Long-term toxicity of an intraoperative radiotherapy boost using low energy X-rays during breast-conserving surgery. Int J Radiat Oncol Biol Phys (2006) 66(2):377–81.
13. Wenz F, Welzel G, Keller A, Blank E, Vorodi F, Herskind C, et al. Early initiation of external beam radiotherapy (EBRT) may increase the risk of long-term toxicity in patients undergoing intraoperative radiotherapy (IORT) as a boost for breast cancer. Breast (2008) 17(6):617–22. doi: 10.1016/j.breast.2008.05.009. doi: S0960-9776(08)00148-3.
14. Kraus-Tiefenbacher U, Welzel G, Brade J, Hermann B, Siebenlist K, Wasser KS, et al. Postoperative seroma formation after intraoperative radiotherapy using low-kilovoltage X-rays given during breast-conserving surgery. Int J Radiat Oncol Biol Phys (2010) 77(4):1140–5. doi: 10.1016/j.ijrobp.2009.06.008
15. Aziz MH, Schneider F, Clausen S, Blank E, Herskind C, Afzal M, et al. Can the risk of secondary cancer induction after breast conserving therapy be reduced using intraoperative radiotherapy (IORT) with low-energy x-rays? Radiat Oncol (2011) 6:174. doi: 10.1186/1748-717X-6-174
16. Sperk E, Welzel G, Keller A, Kraus-Tiefenbacher U, Gerhardt A, Sutterlin M, et al. Late radiation toxicity after intraoperative radiotherapy (IORT) for breast cancer: results from the randomized phase III trial TARGIT a. Breast Cancer Res Treat (2012) 135(1):253–60. doi: 10.1007/s10549-012-2168-4
17. Welzel G, Boch A, Sperk E, Hofmann F, Kraus-Tiefenbacher U, Gerhardt A, et al. Radiation-related quality of life parameters after targeted intraoperative radiotherapy versus whole breast radiotherapy in patients with breast cancer: results from the randomized phase III trial TARGIT-a. Radiat Oncol (2013) 8(1):9. doi: 10.1186/1748-717X-8-9
18. Celejewsak A, Wydmansky J, Majewski W, Majewski W, Wozniak G, Kaniewska-Dorsz Z, et al. The evaluation of tolerance and efficacy of intraoperative radiation therapy (IORT) combined with external beam radiation therapy (EBRT) in patients with breast cancer, after breast-conserving surgery (BCT). Int J Radiat Oncol Biol Phys (2016) 96(2 Suppl):D.
19. Andersen KG, Gartner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: A randomized trial. Breast (2012) 21(1):46–9. doi: 10.1016/j.breast.2011.07.011
20. Keshtgar MR, Williams NR, Bulsara M, Saunders C, Flyger H, Cardoso JS, et al. Objective assessment of cosmetic outcome after targeted intraoperative radiotherapy in breast cancer: results from a randomised controlled trial. Breast Cancer Res Treat (2013) 140(3):519–25. doi: 10.1007/s10549-013-2641-8
21. Corica T, Nowak AK, Saunders CM, Bulsara M, Taylor M, Vaidya JS, et al. Cosmesis and breast-related quality of life outcomes after intraoperative radiation therapy for early breast cancer: A substudy of the TARGIT-a trial. Int J Radiat Oncol Biol Phys (2016) 96(1):55–64. doi: 10.1016/j.ijrobp.2016.04.024
22. Corica T, Nowak AK, Saunders CM, Bulsara MK, Taylor M, Williams NR, et al. Cosmetic outcome as rated by patients, doctors, nurses and BCCT.core software assessed over 5 years in a subset of patients in the TARGIT-a trial. Radiat Oncol (2018) 13(1):68. doi: 10.1186/s13014-018-0998-x
23. Sosin M, Gupta SS, Wang JS, Costellic CD, Gulla A, Bartholomew AJ, et al. A prospective analysis of quality of life and toxicity outcomes in treating early breast cancer with breast conservation therapy and intraoperative radiation therapy. Front Oncol (2018) 8:545. doi: 10.3389/fonc.2018.00545
24. Corica T, Nowak A, Saunders C, Bulsara M, Joseph D, et al. Patient preferences for adjuvant radiotherapy in early breast cancer – an Australian Sub-study of the international TARGIT trial. Eur J Cancer (2012) 48(Suppl 1):S187: Abstract 482.
25. Alvarado MD, Conolly J, Park C, Sakata T, Mohan AJ, Harrison BL, et al. Patient preferences regarding intraoperative versus external beam radiotherapy following breast-conserving surgery. Breast Cancer Res Treat (2014) 143(1):135–40. doi: 10.1007/s10549-013-2782-9
26. Corica T, Joseph D, Saunders C, Saunders C, Bulsara M, Nowak AK, et al. Intraoperative radiotherapy for early breast cancer: do health professionals choose convenience or risk? Radiat Oncol (2014) 9:33. doi: 10.1186/1748-717X-9-33
27. Spaich S, Krickeberg S, Hetjens S, Wenz F, Gerhardt A, Sutterlin M, et al. Patient preferences regarding intraoperative versus external beam radiotherapy for early breast cancer and the impact of socio-demographic factors. Arch Gynecol Obstet (2019) 299(4):1121–30. doi: 10.1007/s00404-018-5025-9
28. Ramdas Y, Benn C-A, Heerden MV. First intraoperative radiation therapy center in Africa: First 2 years in operation, including COVID-19 experiences. JCO Global Oncol 2020(6):1696–703. doi: 10.1200/go.20.00258
29. Tang A, Cohan CM, Beattie G, Cureton EL, Svahn JD, Lyon LL, et al. Patients older 65 years with early breast cancer prefer intraoperative radiation as a locoregional treatment choice. Ann Surg Oncol (2021). doi: 10.1245/s10434-021-09618-3
30. Vaidya JS, Bulsara M, Baum M, Tobias JS. Single-dose intraoperative radiotherapy during lumpectomy for breast cancer: an innovative patient-centred treatment. Br J Cancer (2021) 124(9):1469–74. doi: 10.1038/s41416-020-01233-5
31. Coombs NJ, Coombs JM, Vaidya UJ, Singer J, Bulsara M, Tobias JS, et al. Environmental and social benefits of the targeted intraoperative radiotherapy for breast cancer: data from UK TARGIT-a trial centres and two UK NHS hospitals offering TARGIT IORT. BMJ Open (2016) 6(5):e010703. doi: 10.1136/bmjopen-2015-010703
32. Ritchie H. How urban is the world? (2018). University of Oxford. Available at: https://ourworldindata.org/how-urban-is-the-world#un-estimates-55-of-people-live-in-urban-areas (Accessed 1 Feb 2021 2018).
33. Douek M, De Silva-Minor S, Davies L, Jones B. Breast cancer radiation therapy. Lancet (2020) 396(10262):1558–59. doi: 10.1016/S0140-6736(20)32323-0
34. Vaidya JS, Bulsara M, Sperk E, Massarut S, Douek M, Alvarado M, et al. TARGIT-IORT during lumpectomy for breast cancer - better for patients than other PBI approaches. Int J Radiat Oncol Biol Phys (2021). doi: 10.1016/j.ijrobp.2021.01.059
35. Vaidya JS, Bulsara M, Baum M, Alvarado M, Bernstein M, Massarut S, et al. Intraoperative radiotherapy for breast cancer: powerful evidence to change practice. Nat Rev Clin Oncol (2021) 18(3):187–88. doi: 10.1038/s41571-021-00471-7
36. Bargallo-Rocha JE, Soto-Perez-de-Celis E, Pico-Guzman FJ, Quintero-Rodriguez CE, Almog D, Santiago-Concha G, et al. The impact of the use of intraoperative radiotherapy on costs, travel time and distance for women with breast cancer in the Mexico city metropolitan area. J Surg Oncol (2017) 116(6):683–89. doi: 10.1002/jso.24712
37. Larson KE, Valente SA, Shah C, Tendulkar RD, Cherian S, Yanda C, et al. Are patients traveling for intraoperative radiation therapy? Int J Breast Cancer (2017) 2017:6395712. doi: 10.1155/2017/6395712
38. Goyal S, Chandwani S, Haffty BG, Demissie K. Effect of travel distance and time to radiotherapy on likelihood of receiving mastectomy. Ann Surg Oncol (2015) 22(4):1095–101. doi: 10.1245/s10434-014-4093-8
39. Lorenzen AW, Kiriazov B, De Andrade JP, Lizarraga IM, Scott-Conner CE, Sugg SL, et al. Intraoperative radiotherapy for breast cancer treatment in a rural community. Ann Surg Oncol (2018) 25(10):3004–10. doi: 10.1245/s10434-018-6574-7
40. Athas WF, Adams-Cameron M, Hunt WC, Hunt WC, Amir-Fazli A, Key C. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. JNCI J Natl Cancer Inst (2000) 92(3):269–71.
41. Malter W, Kirn V, Richters L, Fridrich C, Markiefka B, Bongartz R, et al. Intraoperative boost radiotherapy during targeted oncoplastic breast surgery: Overview and single center experiences. Int J Breast Cancer (2014) 2014:637898. doi: 10.1155/2014/637898
42. Banks A, Coronado G, Zimmerman R, Iyengar G, Holmes DR. Breast conserving surgery with targeted intraoperative radiotherapy for the management of ductal carcinoma in situ. J Surg Oncol (2019) 119(4):409–20. doi: 10.1002/jso.25347 [published Online First: 2018/12/28
43. Chin C, Hirji S, Onishi M, Ha R, Taback B, Horowitz DP, et al. A single-institution experience in the preoperative selection of DCIS patients for IORT using the ASTRO consensus guidelines. Adv Radiat Oncol (2019) 4(2):253–60. doi: 10.1016/j.adro.2018.11.004
44. Kraus-Tiefenbacher U, Bauer L, Scheda A, Schoeber C, Schaefer J, Steil V, et al. Intraoperative radiotherapy (IORT) is an option for patients with localized breast recurrences after previous external-beam radiotherapy. BMC Cancer (2007) 7:178. doi: 10.1186/1471-2407-7-178
45. Keshtgar MR, Vaidya JS, Tobias JS, Wenz F, Joseph D, Stacey C, et al. Targeted intraoperative radiotherapy for breast cancer in patients in whom external beam radiation is not possible. Int J Radiat Oncol Biol Phys (2011) 80(1):31–8. doi: 10.1016/j.ijrobp.2010.01.045
46. Kraus-Tiefenbacher U, Blank E, Wenz F. Intraoperative radiotherapy during a second breast-conserving procedure for relapsed breast cancer after previous external beam radiotherapy. Int J Radiat Oncol Biol Phys (2011) 80(4):1279–80. doi: 10.1016/j.ijrobp.2011.02.038
47. Thangarajah F, Heilmann J, Malter W, Kunze S, Marnitz S, Mallmann P, et al. Breast conserving surgery in combination with intraoperative radiotherapy after previous external beam therapy: an option to avoid mastectomy. Breast Cancer Res Treat (2018) 168(3):739–44. doi: 10.1007/s10549-017-4657-y
48. Keshtgar MR, Eaton DJ, Reynolds C, Pigott K, Davidson T, Gauter-Fleckenstein B, et al. Pacemaker and radiotherapy in breast cancer: is targeted intraoperative radiotherapy the answer in this setting? Radiat Oncol (2012) 7(1):128. doi: 10.1186/1748-717X-7-128
49. Kolberg HC, Uhl V, Massarut S, Holmes D, Kolberg-Liedtke C, Kelly EW, et al. Targeted intraoperative radiotherapy during breast-conserving surgery for breast cancer in patients after implant augmentation. Anticancer Res (2019) 39(8):4215–18. doi: 10.21873/anticanres.13582
50. Alvarado M, Ozanne E, Mohan A, Esserman L. Cost-effectiveness of intraoperative radiation therapy for breast conservation. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29(Suppl):abstr 6081.
51. Alvarado MD, Mohan AJ, Esserman LJ, Park CC, Harrison BL, Howe RJ, et al. Cost-effectiveness analysis of intraoperative radiation therapy for early-stage breast cancer. Ann Surg Oncol (2013) 20(9):2873–80. doi: 10.1245/s10434-013-2997-3
52. Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph D, Saunders C, et al. An international randomised controlled trial to compare targeted intra-operative radiotherapy (TARGIT) with conventional post-operative radiotherapy after conservative breast surgery for women with early stage breast cancer (The TARGIT-a trial). Health Technol Assess (2016) 20(73):1–188. doi: 10.3310/hta20730
53. Patel R, Ivanov O, Voigt J. Lifetime cost-effectiveness analysis of intraoperative radiation therapy versus external beam radiation therapy for early stage breast cancer. Cost Eff Resour Alloc (2017) 15:22. doi: 10.1186/s12962-017-0084-5
54. Vaidya A, Vaidya P, Both B, et al. Health economics of targeted intraoperative radiotherapy (TARGIT- IORT) for early breast cancer: a cost- effectiveness analysis in the united kingdom. BMJ Open (2017) 7:e014944. doi: 10.1136/bmjopen-2016-014944
55. Grobmyer SR, Lightsey JL, Bryant CM, et al. Low-kilovoltage, single-dose intraoperative radiation therapy for breast cancer: results and impact on a multidisciplinary breast cancer program. J Am Coll Surg (2013) 216(4):617–23. doi: 10.1016/j.jamcollsurg.2012.12.038
56. Zioueche-Mottet A, Houvenaeghel G, Classe JM, et al. Eligibility criteria for intraoperative radiotherapy for breast cancer: study employing 12,025 patients treated in two cohorts. BMC Cancer (2014) 14:868. doi: 10.1186/1471-2407-14-868
57. Muñoz GH, Hany RP, Cosson A, et al. Intraoperative radiation therapy (INTRABEAM) experience at the mastology unit leopoldo aguerrevere clinic. J Cancer Ther (2015) 06(10):932–42. doi: 10.4236/jct.2015.610101
58. Abbott AM, Dossett LA, Loftus L, et al. Intraoperative radiotherapy for early breast cancer and age: clinical characteristics and outcomes. Am J Surg (2015) 210(4):624–8. doi: 10.1016/j.amjsurg.2015.05.012
59. Valente SA, Tendulkar RD, Cherian S, et al. TARGIT-r (Retrospective): North American experience with intraoperative radiation using low-kilovoltage X-rays for breast cancer. Ann Surg Oncol (2016) 23(9):2809–15. doi: 10.1245/s10434-016-5240-1
60. Thomas TO, Small W Jr.. Editorial: Intraoperative radiotherapy (IORT)-a new frontier for personalized medicine as adjuvant treatment and treatment of locally recurrent advanced malignancy. Front Oncol (2018) 8:234. doi: 10.3389/fonc.2018.00234
61. Obi E, Tom MC, Manyam BV, et al. Outcomes with intraoperative radiation therapy for early-stage breast cancer. Breast J (2020) 26(3):454–57. doi: 10.1111/tbj.13574
62. Moini N, Akbari ME, Mirzaei H, et al. Intraoperative boost radiotherapy with 50 kV X-rays versus external radiotherapy in breast cancer: Single-center experiences. Int J Cancer Manag (2020) 13(3):e98561. doi: 10.5812/ijcm.98561
63. Tallet A, Racadot S, Boher JM, et al. The actual benefit of intraoperative radiation therapy using 50 kV x-rays in early breast cancer: A retrospective study of 676 patients. Breast J (2020) 26(11):2145–50. doi: 10.1111/tbj.13827
64. Lemanski C, Bourgier C, Draghici R, et al. Intraoperative partial irradiation for highly selected patients with breast cancer: Results of the INTRAOBS prospective study. Cancer Radiother J Soc Fr Radiother Oncol (2020) 24(2):114–19. doi: 10.1016/j.canrad.2020.01.007
65. Mi Y, Lv P, Wang F, Li L, Zhu M, Wang Y, et al. Targeted intraoperative radiotherapy is non-inferior to conventional external beam radiotherapy in Chinese patients with breast cancer: A propensity score matching study. Front Oncol (2020) 10:550327. doi: 10.3389/fonc.2020.550327
66. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol: Off J Eur Soc Med Oncol / ESMO (2011) 22(8):1736–47. doi: 10.1093/annonc/mdr304
67. Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the international society of geriatric oncology (SIOG) and European society of breast cancer specialists (EUSOMA). Lancet Oncol (2012) 13(4):e148–60. doi: 10.1016/S1470-2045(11)70383-7
68. Marmot M, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. Independent UK panel on breast cancer screening replies to Michael baum. BMJ (2013) 346:f873.
69. Mooney H. NICE gives go ahead to intrabeam radiotherapy for breast cancer. BMJ (2014) 349:g4863. doi: 10.1136/bmj.g4863
70. Vaidya JS, Bulsara M, Wenz F, Coombs N, Singer J, Ebbs S, et al. Reduced mortality with partial-breast irradiation for early breast cancer: A meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys (2016) 96(2):259–65. doi: 10.1016/j.ijrobp.2016.05.008
71. Medical Services Advisory Committee A. 1189 - targeted intraoperative radiotherapy (IORT) for early breast cancer 2016 (2020). Available at: http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1189-public (Accessed 23 Mar 2020).
72. Wise J. NICE recommends controlled intrabeam use for breast cancer after three year delay. BMJ (2017) 356:j725. doi: 10.1136/bmj.j72510.1136/bmj.h2874
73. (NICE) NIfHaCE. Intrabeam radiotherapy system for adjuvant treatment of early breast cancer: Technology appraisal guidance [TA501] (2018). Available at: https://www.nice.org.uk/guidance/ta501 (Accessed 23 Mar 2020).
74. Surgeons ASoB. Consensus guideline on accelerated partial breast irradiation 2018. Available at: https://www.breastsurgeons.org/docs/statements/Consensus-Statement-for-Accelerated-Partial-Breast-Irradiation.pdf.
75. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol Off J Eur Soc Med Oncol / ESMO (2019) 30(8):1194–220. doi: 10.1093/annonc/mdz173
76. Simcock R, Thomas TV, Estes C, Filippi AR, Katz MS, Pereira IJ, et al. COVID-19: Global radiation oncology’s targeted response for pandemic preparedness. Clin Trans Radiat Oncol (2020) 22:55–68. doi: 10.1016/j.ctro.2020.03.009
77. Chan JJ, Sim Y, Ow SGW, Lim JSJ, Kusumawidjaja G, Zhuang Q, et al. The impact of COVID-19 on and recommendations for breast cancer care: the Singapore experience. Endocr Relat Cancer (2020) 27(9):R307–R27. doi: 10.1530/ERC-20-0157
78. Battisti NML, Mislang AR, Cooper L, O'Donovan A, Audisio RA, Cheung KL, et al. Adapting care for older cancer patients during the COVID-19 pandemic: Recommendations from the international society of geriatric oncology (SIOG) COVID-19 working group. J Geriatr Oncol (2020) 11(8):1190–98. doi: 10.1016/j.jgo.2020.07.008
Keywords: TARGIT IORT, breast cancer, radiation therapy, lumpectomy (breast conserving surgery), TARGIT, IORT, radiotherapy, partial breast irradiation
Citation: Vaidya JS, Vaidya UJ, Baum M, Bulsara MK, Joseph D and Tobias JS (2022) Global adoption of single-shot targeted intraoperative radiotherapy (TARGIT-IORT) for breast cancer—better for patients, better for healthcare systems. Front. Oncol. 12:786515. doi: 10.3389/fonc.2022.786515
Received: 30 September 2021; Accepted: 28 June 2022;
Published: 11 August 2022.
Edited by:
William Small Jr., Loyola University Chicago, United StatesReviewed by:
Anil Vaidya, Masimo Inc., United StatesEmanuela Esposito, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2022 Vaidya, Vaidya, Baum, Bulsara, Joseph and Tobias. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jayant Sharad Vaidya, amF5YW50LnZhaWR5YUB1Y2wuYWMudWs=; amF5YW50dmFpZHlhQGdtYWlsLmNvbQ==
†The full list of authors along with their affiliations is given at the end of this document
 Jayant Sharad Vaidya
Jayant Sharad Vaidya Uma Jayant Vaidya2
Uma Jayant Vaidya2 Max Kishor Bulsara
Max Kishor Bulsara