- 1Novartis Pharmaceuticals Corporation, East Hanover, NJ, United States
- 2Genesis Research, Hoboken, NJ, United States
Background: MET exon 14 skipping mutation (METex14) is observed in ~3% of non-small cell lung cancer (NSCLC) cases and has been shown to be an independent poor prognostic factor associated with shorter overall disease-specific survival. Broad molecular testing can identify this biomarker in patients with advanced NSCLC (aNSCLC) and allow patients to be matched with the appropriate targeted therapy. This study examines biomarker testing patterns and clinical outcomes of chemotherapy and immuno-oncology (IO) monotherapy in aNSCLC patients with METex14.
Methods: A descriptive retrospective study was conducted using the Flatiron Health–Foundation Medicine Inc. (FMI) clinico-genomic database. Patients with METex14 aNSCLC treated with systemic therapies were included in the biomarker testing analysis. The duration from specimen collection to reported results was assessed for PD-L1– and METex14-tested patients. Clinical outcomes were assessed in patients treated with chemotherapy or IO monotherapy. First-line (1L) and second-line (2L) real-world progression-free survival (rw-PFS) were estimated using Kaplan-Meier analysis.
Results: Of 91 METex14 patients eligible for the biomarker testing analysis, 77% and 60% received PD-L1 and FMI next-generation sequencing (NGS) testing within 3 months post aNSCLC diagnosis. Of those assessed for both PD-L1 and METex14 (n=9), the median duration between specimen collection and reporting was 1 week shorter for PD-L1 than for FMI NGS. Median 1L rw-PFS was 5.7 months (95% CI, 4.6-7.1) and 2.4 months (95% CI, 1.4-3.2) in patients receiving 1L chemotherapy (n=59) and IO monotherapy (n=18), with 3-month 1L rw-PFS rates of 78% and 33%. Median 2L rw-PFS was 3.5 months (95% CI, 1.9-11.1) and 4.7 months (95% CI, 2.8-12.9) in patients receiving 2L chemotherapy (n=16) and IO monotherapy (n=23), with 3-month 2L rw-PFS rates of 54% and 67%.
Conclusions: The median time from biopsy to test results appears 1 week shorter for PD-L1 than for FMI NGS. Chemotherapy and IO monotherapy were the most common regimens utilized but with limited PFS.
Introduction
Non-small cell lung cancer (NSCLC), which accounts for ~85% of lung cancer diagnoses, is a heterogeneous disease consisting of multiple histologies and many known driver mutations (1–4). MET exon 14 skipping mutation (METex14) is observed in ~3% (95% CI, 1%-4%) of NSCLC cases; is more likely to occur in females, elderly patients (median age ranging from 65 to 76 years old), and nonsmokers (5); and typically occurs in the absence of other driver mutations (2, 3, 6). In retrospective studies of patients with resected NSCLC, METex14 has been shown to be an independent poor prognostic factor and is associated with shorter overall survival (7, 8), underscoring the need for identification of this biomarker in patients with NSCLC and for targeted therapies (9).
Capmatinib was approved by the US Food and Drug Administration (FDA) on May 6, 2020, for the treatment of adult patients with metastatic NSCLC whose tumors have a mutation that leads to METex14. Approval was based on results from the phase 2 GEOMETRY mono-1 trial (NCT02414139) (10, 11). In the trial, a blinded independent review committee confirmed an overall response rate (ORR) of 68% (95% CI, 48%-84%) and median duration of response (DOR) of 12.6 months (95% CI, 5.6 months-not estimable [NE]) among treatment-naive patients (N=28). An ORR of 41% (95% CI, 29%-53%) and median DOR of 9.7 months (95% CI, 5.6-13.0 months) were established among previously treated patients (N=69) (11).
Additionally, capmatinib has shown clinical evidence of intracranial activity. In the GEOMETRY mono-1 trial, among 13 patients who had data evaluable by an independent neuroradiologic review committee, 12 (92%) had intracranial disease control and 7 (54%) had an intracranial response. Of the patients with an intracranial response, 4 (57%) had a complete response (11).
Tepotinib, another MET inhibitor, was approved by the FDA on February 3, 2021, for the treatment of adult patients with metastatic NSCLC harboring METex14 mutations. Approval was based on results from the phase 2 VISION trial (NCT02864992) (12, 13). Among patients with METex14 NSCLC treated with tepotinib, the ORR was 46% (95% CI, 36%-57%) and the median DOR was 11.1 months (95% CI, 7.2 months-NE), according to independent review (12). In the 11 patients with brain metastases, the response rate by independent review was 55% (95% CI, 23%-83%), with a median DOR of 9.5 months (95% CI, 6.6 months-NE) (12).
Prior to the FDA approval of capmatinib, there were no US-approved therapies that specifically targeted METex14 in NSCLC (4, 10, 14), but several other targeted therapies were given off-label to patients with METex14 NSCLC (15). These included crizotinib, a tyrosine kinase inhibitor with activity against MET, ALK, and ROS1; and cabozantinib, a tyrosine kinase inhibitor with activity against a broad range of targets, including MET, VEGFR2, RET, FLT3, and KIT (16). Reports have shown promising clinical activity for these agents in patients with METex14 NSCLC (2, 16, 17).
For lung cancer patients with METex14, broad molecular profiling, such as next-generation sequencing (NGS) testing, can be successfully used to guide treatment decisions (2, 16, 17). Studies have shown that broad molecular profiling, which allows patients to match with an appropriate therapy, can result in improved outcomes (18–20). NGS testing also saves personnel working time and consumables related to biomarker tests and reduces the overall cost of testing per patient (21, 22).
A potential barrier to use of targeted agents may be the time required for NGS testing to identify METex14 as well as other actionable biomarkers in patients with NSCLC (23), which may result in some patients initiating treatment with systemic therapies prior to receiving broad molecular testing results. Over the years, testing companies have tried to expedite the processing time for NGS testing, but the time needed to receive testing results can still be multiple weeks (23). Although NGS panels have longer testing turnaround times compared with single tests, NGS can reliably cover a broader spectrum of alterations (24). Patients with METex14 NSCLC have commonly been treated with chemotherapy or immuno-oncology (IO) therapy (9). Programmed death-ligand 1 (PD-L1) expression ≥50% has been detected in nearly half of patients with METex14 (25); however, as seen in patients with many other targetable oncogenic drivers, patients with METex14 tend to have generally poor responses to immunotherapy (25, 26), and the evidence of the clinical effectiveness of these treatments, particularly IO therapy, on patients with METex14 has been mixed, potentially due to differences in study populations, designs, and outcomes assessments (9, 27–29). Insights into real-world testing patterns and clinical outcomes associated with current treatments in the METex14 advanced NSCLC (aNSCLC) population may help physicians to make appropriate treatment choices for these patients. This study used a nationwide US de-identified clinico-genomic database (CGDB) to describe real-world biomarker testing patterns and assess the clinical outcomes of the most frequently used first-line (1L) and second-line (2L) therapies (IO monotherapy or chemotherapy) in patients with METex14 aNSCLC.
Methods
Study Design and Objectives
This was a descriptive retrospective study using electronic health records (EHR) and genomic data from the Flatiron Health (FH)–Foundation Medicine Inc (FMI) NSCLC CGDB to examine real-world biomarker testing patterns and clinical outcomes of chemotherapy and IO in patients with METex14 aNSCLC for treatment in 1L and 2L. The start of 1L therapy after aNSCLC diagnosis served as the index date and the beginning of the post-index period (follow-up), which extended until the earlier of 2 events: death or the last activity date (the last observed encounter during the patient’s follow-up).
Data Source
Retrospective longitudinal clinical data were derived from EHR, comprising patient-level structured and unstructured data, curated via technology-enabled abstraction. During the study period, these data originated from approximately 280 cancer clinics (~800 sites of care) in the FH network, representing more than 2.4 million patients with cancer (30, 31). Genomic data were sourced from comprehensive genomic profiling (CGP) performed by FMI, with a genomic database of >400,000 sequenced tumor samples from patients in the US. The linking of patient data was performed by de-identified, deterministic matching using a third party in an institutional review board–approved, Health Insurance Portability and Accountability Act (HIPAA)-compliant fashion. Data collected from January 1, 2011, to December 31, 2019, were used in this study.
Patient Selection
Participants first had to meet baseline criteria for the FH-FMI NSCLC CGDB, as follows:
● At least 2 documented clinical visits in the FH network on or after January 1, 2011, and on or before June 30, 2019
● International Classification of Diseases (ICD) code for lung cancer (ICD-9 162.x or ICD-10 C34x or C39.9); chart-confirmed diagnosis of NSCLC
● CGP test by FMI on or after the date of chart-confirmed diagnosis of NSCLC on a tumor sample (collected no earlier than 30 days before the FH diagnosis date) with pathologist-confirmed histology that is consistent with NSCLC
● Uniquely and deterministically matched demographic characteristics
Selected participants were eligible for inclusion if they were diagnosed with aNSCLC (initial diagnosis of stage IIIB, IIIC, IV, IVA, or IVB disease or diagnosis of early-stage NSCLC with subsequent development of recurrent or progressive disease) between January 1, 2011, and October 2, 2019 (patients diagnosed with aNSCLC with less than 90 days before data cutoff were not considered for analysis due to limited follow-up time); were treated with at least 1 line of therapy starting on or after January 1, 2011; were at least 18 years of age as of the index date; and had METex14 confirmed from a solid-tumor biopsy according to the FMI NGS report closest to the index date. Key exclusion criteria were a gap in structured activity after the advanced diagnosis date, defined as no records of vital information, medication administrations, non-canceled drug orders, or laboratory tests/results within 90 days after the advanced diagnosis date (as patients without observed activity during this timeframe may have been treated at a practice outside the FH network); evidence of treatment with a clinical trial drug in the 1L setting; and treatment with combination therapies of chemotherapy + IO or chemotherapy + MET inhibitor in the 1L setting.
Outcome Measurements
The duration between the specimen collection date and aNSCLC diagnosis was assessed for both PD-L1 and FMI NGS testing (specimen collection date could have occurred before or after aNSCLC diagnosis). Because the physician order date was incomplete for PD-L1 testing and not available for FMI NGS testing, the duration from the specimen collection date until date of availability of the results for PD-L1 and FMI NGS testing was explored. Only patients with the same specimen collection date for PD-L1 and FMI NGS testing in 2019 (the most recent year for which data were available) were included in this analysis to minimize potential overestimation due to tissue archiving and cross-institutional bias. Note that molecular biomarker testing methods such as CGP and immunohistochemistry are often performed on specimens that had originally been obtained for a different purpose (e.g., diagnostic biopsy). Therefore, the duration from specimen collection to result availability reflects a combination of physician decision-making and testing time. We advise interpreting this duration with this caveat in mind.
Real-world progression-free survival (rw-PFS) and overall survival (OS) by 1L and 2L therapy class were assessed only among those who initiated chemotherapy or IO monotherapy as 1L treatment, because a limited number of patients initiated other therapies during the study period. Progression data were curated by abstractors from the EHRs of patients in the FH-FMI CGDB (32). Progression events were defined as distinct episodes in which the treating clinician concluded that there had been growth or worsening in the disease of interest. Such episodes are abstracted using an abstraction approach that uses clinician assessment as the main source of evidence, with radiology, laboratory, and pathology results as confirmatory documentation. Overall survival events were based on documented death dates. All other patients were censored on their last activity date in the database.
Statistical Analysis
This study was descriptive in nature. 1L and 2L rw-PFS were estimated using a Kaplan-Meier analysis stratified by drug class (chemotherapy and IO monotherapy). An event for 1L or 2L rw-PFS was defined as the earlier of documented progression or death after the 1L or 2L treatment start date, respectively. Patients without an event were censored on their last clinic note date. Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc).
Ethics Approval and Consent to Participate
This study was designed, implemented, and reported in accordance with the Guidelines for Good Pharmacoepidemiology Practices (GPP) of the International Society for Pharmacoepidemiology (2016), the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, and the ethical principles laid down in the Declaration of Helsinki.
FH-FMI processed data were de-identified according to the Expert Determination method as outlined in HIPAA to prevent re-identification of patient-level data to protect patients’ confidentiality. The CGDB data source and research activities were reviewed and approved by WCG IRB, the independent central institutional review board (IRB) of record for FH and FMI. Based on minimal-risk research, a waiver of informed consent and HIPAA authorization was approved by WCG IRB. WCG IRB holds full accreditation of their human subjects research program by the Association for the Accreditation of Human Research Protection Programs, Inc. In addition, WCG IRB of record assures the ethical conduct of research and compliance with federal, state, and institutional guidelines.
This study fulfills the criteria of a European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) study and followed the ENCePP Code of Conduct (European Medicines Agency 2016).
Results
Patient Selection
Of the 9439 patients meeting baseline criteria for the NSCLC CGDB, 91 met all study-specific selection criteria and were included in the biomarker testing pattern analyses. Only patients who initiated chemotherapy or IO monotherapy as 1L or 2L were included in rw-PFS and OS analyses (Figure 1). Chemotherapy regimens consisted of carboplatin, cisplatin, docetaxel, etoposide, gemcitabine, paclitaxel, paclitaxel protein-bound, or pemetrexed, including combinations with afatinib, bevacizumab, and/or ramucirumab. IO therapies received were nivolumab, pembrolizumab, atezolizumab, and durvalumab. A total of 16 patients with confirmed METex14 were excluded due to initiation of combination therapies of chemotherapy and IO or chemotherapy and MET inhibitor in the 1L setting.
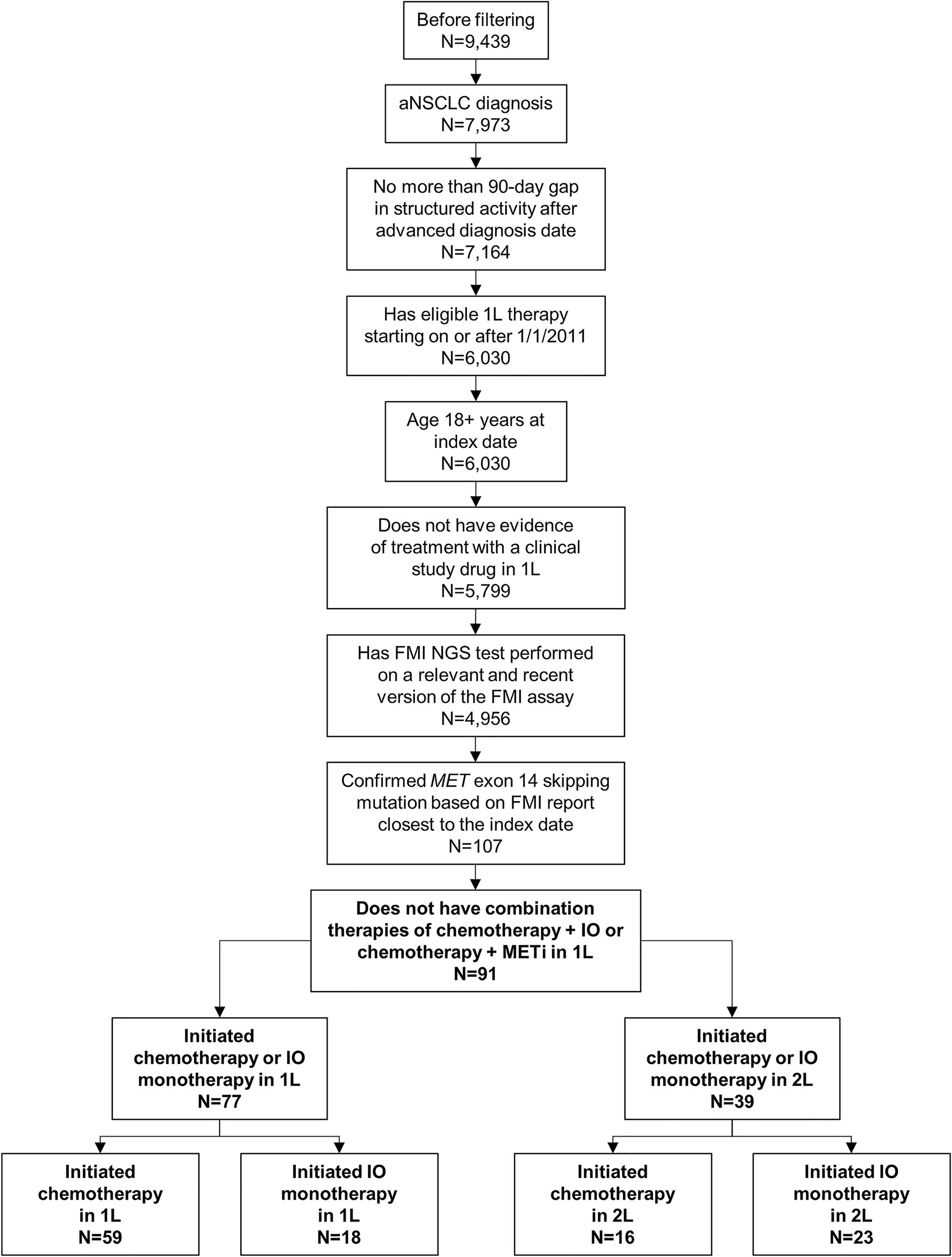
Figure 1 Patient Selection. Patients with non-small cell lung cancer (NSCLC) who were eligible for inclusion in the study were first required to meet baseline criteria for the Flatiron Health–Foundation Medicine Inc (FMI) NSCLC clinico-genomic database. Of those patients (N=9439), 91 also met the study-specific inclusion criteria and were included in the biomarker testing pattern analyses. Only patients who initiated chemotherapy or immuno-oncology (IO) monotherapy as first line (1L; n=77) or second line (2L; n=39) were included in clinical outcome analyses. aNSCLC, advanced non-small cell lung cancer; METi, MET inhibitor; NGS, next-generation sequencing.
Baseline Characteristics
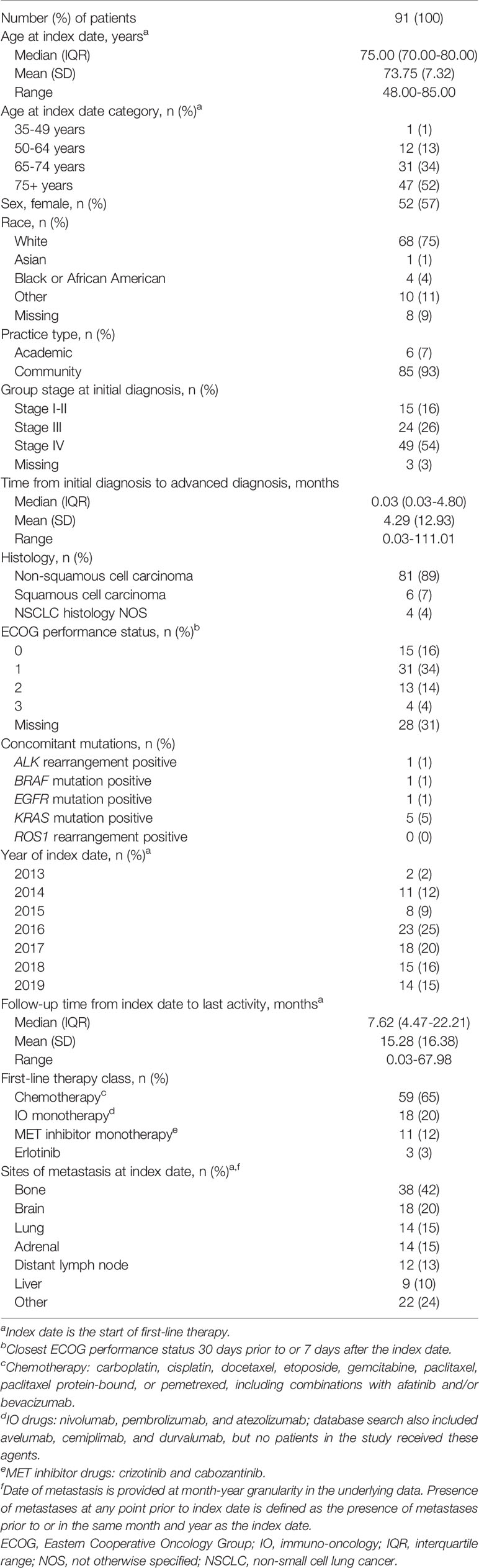
Patient characteristics at baseline are shown in Table 1. The median age of patients at the index date was 75 years, with a range from 48 to 85 years. A high proportion of patients (80%, n=73/91) were initially diagnosed with stage III or IV NSCLC, representing a primarily de novo aNSCLC population. A total of 89% of patients were diagnosed with non-squamous cell carcinoma. Bone (42%) and brain (20%) were the most common sites of metastases at the index date. Other oncogenic drivers (i.e., ALK rearrangement, BRAF mutation, EGFR mutation, KRAS mutation, ROS1 rearrangement) were rare in this patient population. Ninety-three percent of patients were managed in a community practice setting.
Testing Patterns
Out of 91 patients included in the study, 62% (n=56) had documentation of receiving PD-L1 testing. Among these patients, 39% (n=22/56) were tested through FMI, while 61% (n=34/56) were tested through external laboratories. These data may be underestimated, because patients could have received PD-L1 testing outside the FH network. PD-L1 status was ≥50% in 46% (n=26) of patients, 1% to 49% in 20% (n=11) of patients, negative or <1% in 29% (n=16) of patients, and missing/unknown in 5% (n=3) of patients.
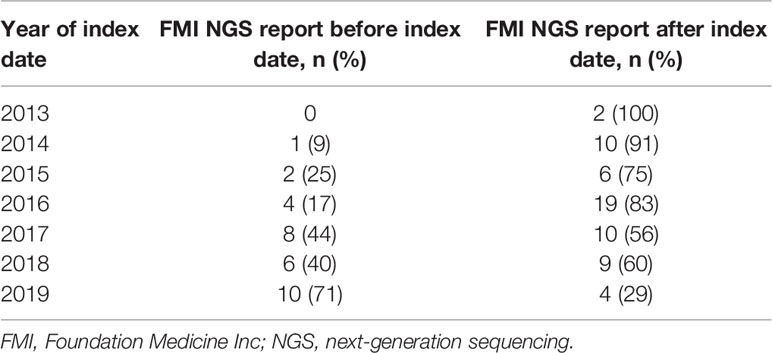
Overall, 34% (n=31/91) of patients received their FMI NGS testing reports before initiation of 1L therapy, whereas 66% (n=60/91) of patients received their reports after starting 1L therapy. Prior to 2019, most patients initiated 1L therapy before receiving their FMI reports (Table 2 and Figure 2). Generally, between 2013 and 2019, there was a trend toward an increasing proportion of patients initiating 1L treatment after FMI NGS reports were received (Figure 2). Of patients with de novo stage IV NSCLC, 24% (n=12/49) received FMI NGS testing results before initiation of 1L treatment and 76% (n=37/49) received FMI NGS testing results after initiation of 1L treatment.
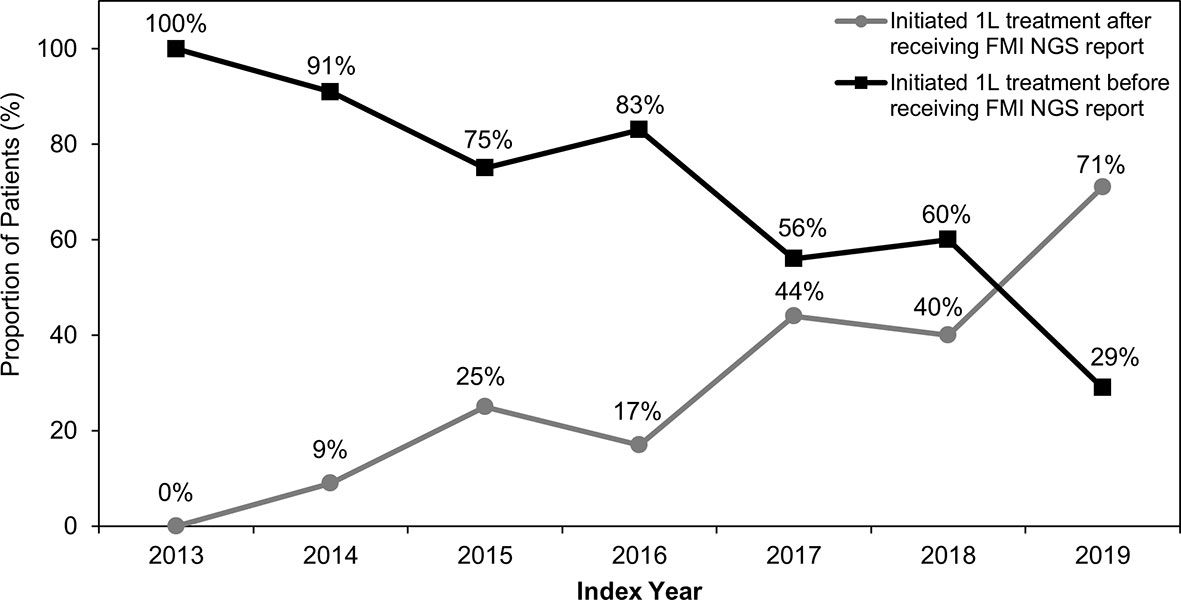
Figure 2 Treatment Initiation Relative to Receipt of FMI NGS Reports Between 2013 and 2019. Between 2013 and 2019, the percentage of patients who initiated first-line (1L) treatment before Foundation Medicine Inc (FMI) next-generation sequencing (NGS) testing reports were received generally decreased, while the percentage of patients initiating 1L treatment after receiving FMI NGS testing reports generally increased.
Specimen Collection and Testing Results
For 84% (n=47/56) and 78% (n=71/91) of patients, a specimen was collected for PD-L1 and FMI NGS testing, respectively, on or after their advanced diagnosis date. Specimen collection occurred within 3 months post aNSCLC diagnosis for most patients (PD-L1: 43/56, 77%; FMI NGS: 55/91, 60%; Figure 3). For patients with the same PD-L1 and FMI NGS specimen collection date in 2019 (n=9), the median time from specimen collection to reported results for PD-L1 and FMI NGS testing was 20 days (interquartile range [IQR], 17-42 days) and 27 days (IQR, 27-54 days), respectively—a difference of 1 week (Table 3).
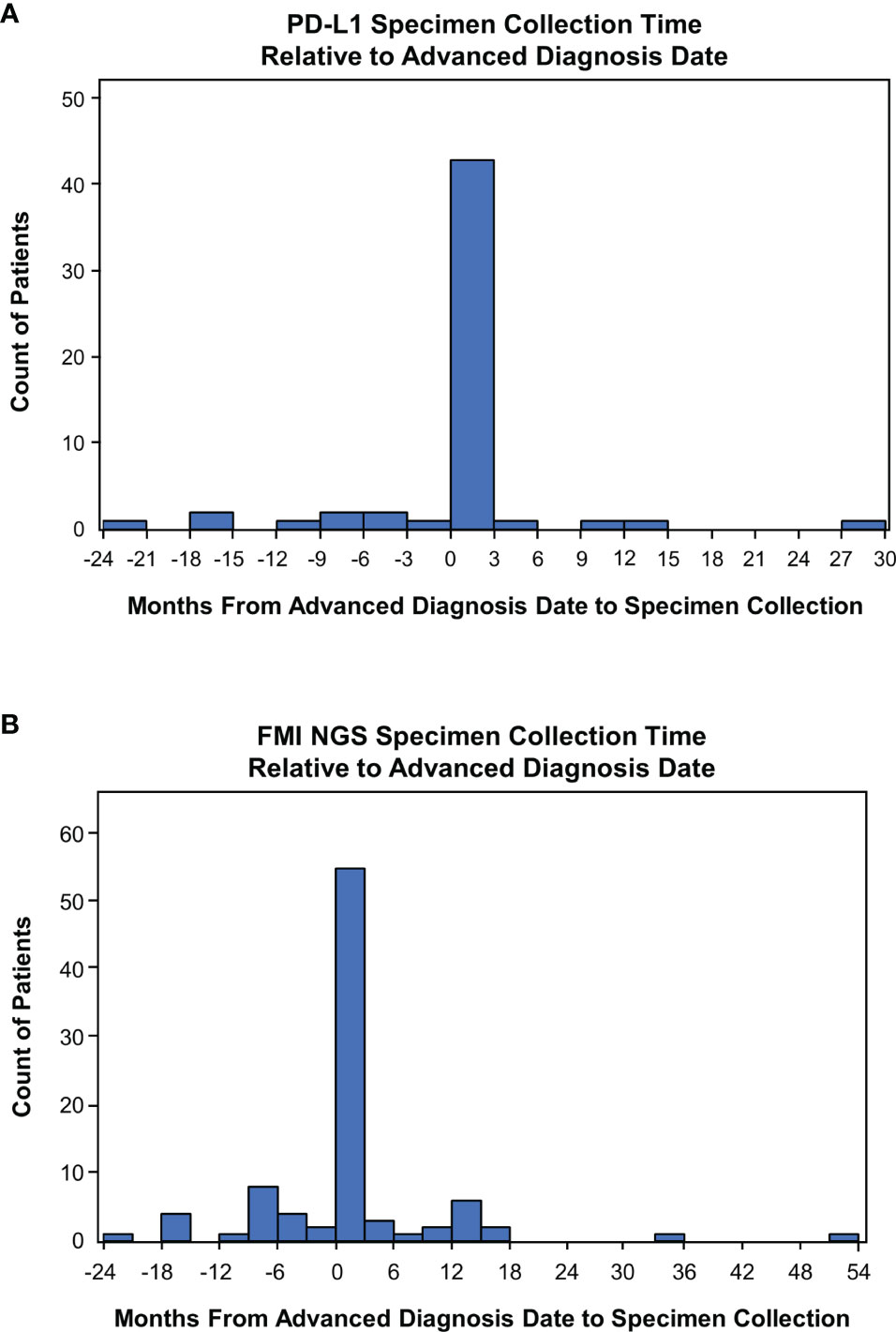
Figure 3 Time of Specimen Collection Relative to Advanced Non-Small Cell Lung Cancer Diagnosis. (A) Frequency histogram of PD-L1 testing times. (B) Frequency histogram of Foundation Medicine Inc (FMI) next-generation sequencing (NGS) testing times. Specimen collection times were defined as the duration from the date of advanced diagnosis to specimen collection.
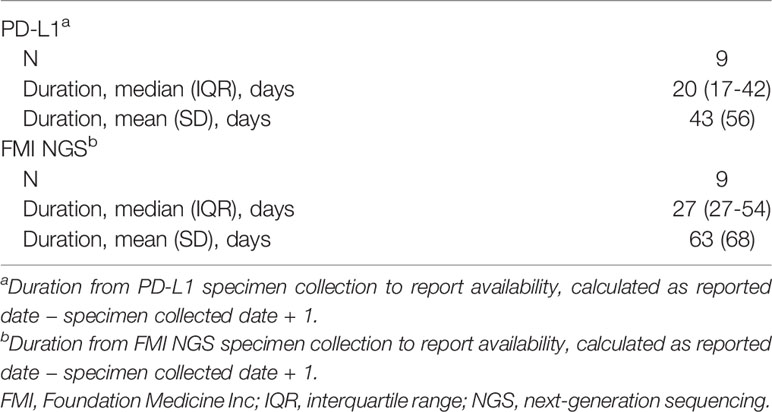
Table 3 Duration from specimen collection to reported results: same-day PD-L1 and FMI NGS specimen collection.
For 73% (n=41/56) of patients tested for PD-L1, specimens were collected for both FMI NGS and PD-L1 tests on the same date (Supplementary Table 1). For 18% (n=10/56) of patients tested for PD-L1, samples were collected for PD-L1 testing prior to the collection of samples for FMI NGS testing.
rw-PFS and OS
1L rw-PFS and OS were assessed in patients who initiated chemotherapy (n=59/77, 77%) or IO monotherapy (n=18/77, 23%) as 1L therapy and in patients who initiated chemotherapy (n=16/39, 41%) or IO monotherapy (n=23/39, 59%) as 2L therapy. 1L rw-PFS was not assessed for other therapy groups, because the sample sizes were inadequate for analyses (1L: MET inhibitor n=11, erlotinib n=3; 2L: MET inhibitor n=7, clinical study drug n=6, afatinib n=2, ceritinib n=1, crizotinib + pembrolizumab n=1). The most common 1L and 2L chemotherapy regimens were platinum doublets (platinum-based chemotherapy plus another chemotherapy agent), constituting 71% (n=42/59) and 50% (n=8/16) of patients, respectively. The most common 1L IO monotherapy regimen was pembrolizumab, constituting 83% (n=15/18) of patients. In the 2L setting, the most common IO monotherapy regimens were nivolumab and durvalumab, constituting 43% (n=10/23) and 30% (n=7/23) of patients, respectively.
In the 1L setting, median 1L rw-PFS was 5.7 months (95% CI, 4.6-7.1 months) and 2.4 months (95% CI, 1.4-3.2 months) in patients receiving chemotherapy and IO monotherapy, respectively (Figure 4A). The 3-, 6-, and 12-month rw-PFS rates for patients initiating chemotherapy in 1L were 78%, 46%, and 20%, respectively. For patients initiating IO in the 1L, the 3-, 6-, and 12-month 1L rw-PFS rates were 33%, 17%, and 17%, respectively. The median OS in the 1L setting was 20.0 months (95% CI, 10.9-24.6 months) and not reached (95% CI, 3.1 months-not reached) in patients receiving chemotherapy and IO monotherapy, respectively. The 3-, 6-, and 12-month OS rates for patients initiating chemotherapy in 1L were 95%, 82%, and 60%, respectively. For patients initiating IO in 1L, the 3-, 6-, and 12-month OS rates were 78%, 61%, and 52%, respectively.
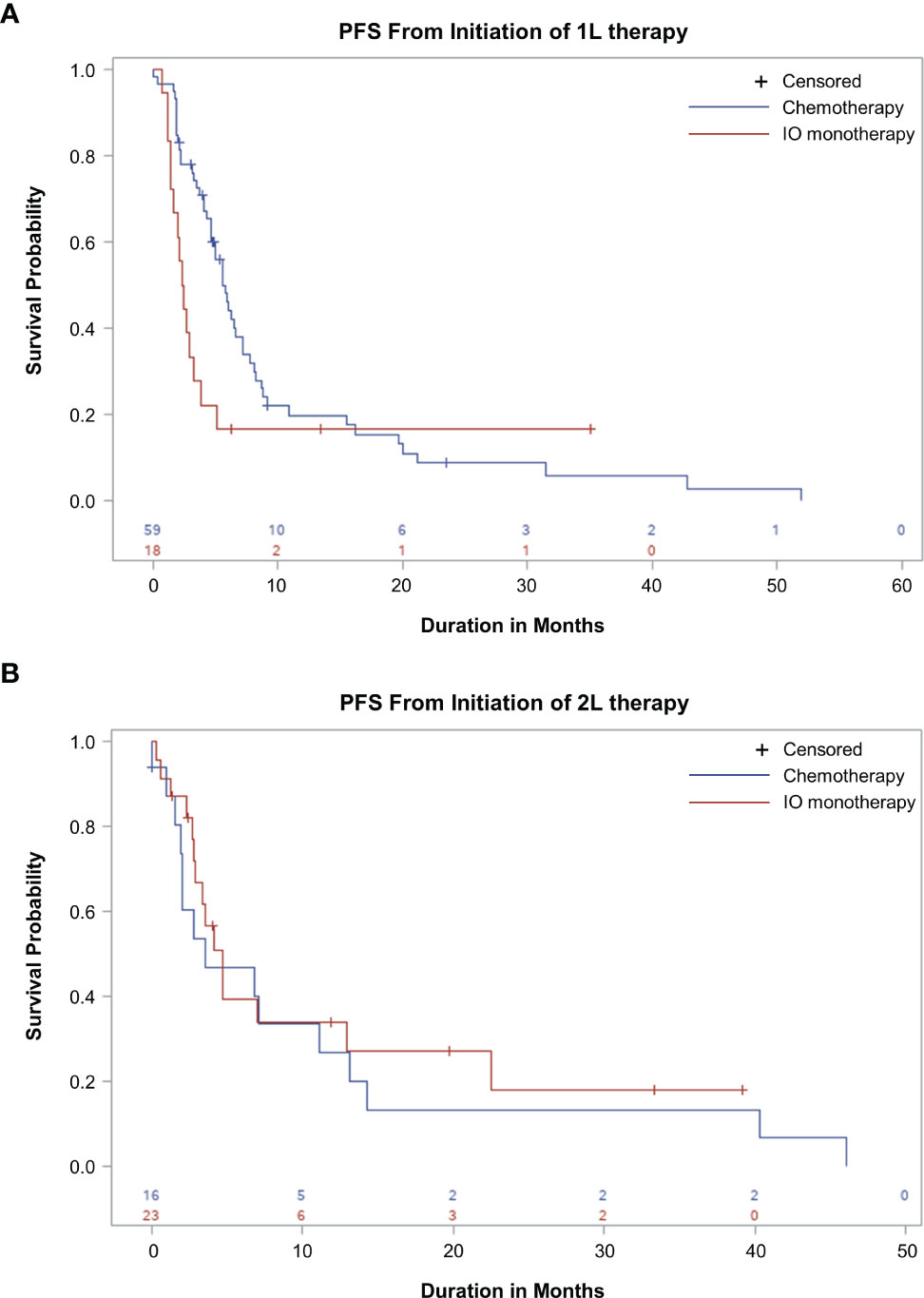
Figure 4 Real-World Progression-Free Survival (rw-PFS) With First-line (1L) and Second-line (2L) Therapy. Kaplan-Meier curves showing rw-PFS for patients treated with chemotherapy or immuno-oncology (IO) in 1L (A) or 2L setting (B). The start of the analysis is the start of 1L therapy (A) and the start of 2L therapy (B). This study is descriptive in nature, and comparisons between treatment groups should not be made.
In the 2L setting, median 2L rw-PFS was 3.5 months (95% CI, 1.9-11.1 months) and 4.7 months (95% CI, 2.8-12.9 months) in patients receiving chemotherapy and IO monotherapy, respectively (Figure 4B). The 3-, 6-, and 12-month 2L rw-PFS rates for patients initiating chemotherapy in 2L were 54%, 47%, and 27%, respectively. For patients initiating IO in 2L, the 3-, 6-, and 12-month 2L rw-PFS rates were 67%, 40%, and 34%, respectively. The median OS in the 2L setting was 15.3 months (95% CI, 4.0-41.1 months) and 19.3 (95% CI, 11.6 months-not reached) in patients receiving chemotherapy and IO monotherapy, respectively. The 3-, 6-, and 12-month OS rates for patients initiating chemotherapy in 2L were 87%, 73%, and 51%, respectively. For patients initiating IO in 2L, the 3-, 6-, and 12-month OS rates were 76%, 76%, and 70%, respectively.
For the limited number of patients initiating MET inhibitor monotherapy (n=11) or erlotinib (n=3) in 1L, the median 1L rw-PFS was 4.5 months (95% CI, 2.2 months-not reached) and 4.1 (95% CI, 0 months-not reached), respectively. Median OS for these patients was 10.0 months (95% CI, 4.9 months-not reached) and 24.7 months (95% CI, 0 months-not reached), respectively. However, no FDA-approved MET inhibitors were included in this study and these findings are based on a small number of patients; caution should be taken in interpreting these results.
Discussion
The time associated with biomarker testing in aNSCLC is known as a critical operational issue impacting treatment decision-making (26); however, few studies have provided up-to-date assessments on this topic. We found only two studies in this domain (33, 34), neither of which looked at testing time for NGS in recent years. Our study utilized the most recent data available to provide contemporary assessment on the timing associated with biomarker testing (both PD-L1 and NGS) from collection of tumor specimen to receipt of test results among patients with METex14 aNSCLC. For patients with advanced cancer, the time needed for receiving NGS testing results may affect real-world treatment decisions regarding the adoption of targeted therapy. Importantly, NGS testing requires about 2 weeks for results and costs more than individual single-gene tests; however, the overall cost is lower and the time to all results is shorter compared with multiple sequential single-gene tests for all potentially actionable oncogenic drivers, which makes NGS testing a preferred method for comprehensive molecular profiling (22). It should be noted that the assessment of time from biopsy to receiving testing results, in relation to the subsequent clinical benefit of patients receiving various therapies, has not been well studied. This study examined real-world testing patterns for PD-L1 and FMI NGS testing and assessed the clinical outcomes with chemotherapy and IO monotherapy in patients with METex14 aNSCLC prior to capmatinib US approval.
Our results show that the time from specimen collection to reported results was shorter for PD-L1 testing than for FMI NGS testing in 2019, with a median difference of 1 week. Whether this arose from differences in clinical decision-making, characteristics of the 2 forms of testing, or some combination of these factors could not be ascertained with the data available. Additionally, the duration between specimen collection and report date in this study should not be interpreted as turnaround time, which is defined as the time between test order and report date; note that specimen collection could have occurred before or after the test order in real-world practice. In this study, based on a primarily community oncology setting in the US, we found that twice as many patients with METex14 initiated 1L therapy prior to receiving their FMI NGS reports as initiated 1L therapy after receiving their reports. Although multiple lung cancer guidelines recommend early comprehensive genomic profiling prior to systemic treatment initiation (4, 35, 36), our study showed that testing was often postponed until after the treatment decisions were made, and therefore the optimal clinical outcomes were not achieved. Our study sample was restricted to those who received NGS testing; if otherwise assessed in a broader sample of patients with NSCLC, it is expected that comprehensive biomarker testing may be further delayed or not completed. One of the primary reasons for this delay or lack of NGS testing, excluding cost, is the consideration around the additional waiting or turnaround time to obtain testing results. Because NGS testing turnaround time may be shortened due to advancement of testing technology in recent years, we attempted to further explore the most recent data, which showed a difference of only about 1 week between NGS testing and PD-L1 testing. In our analysis, we saw a trend toward more patients receiving an NGS report prior to treatment initiation in recent years, and fewer patients having the opposite order (Table 2 and Figure 2). The shortened turnaround time and increased awareness of the importance of early NGS testing could be reasons leading to the positive shift with regard to testing pattern shown in Table 2 and Figure 2. The data we presented here will further help physicians to rationalize the best treatment options for patients, as well as communicate to patients the importance of waiting for NGS testing results to achieve the best clinical outcomes. Furthermore, a few studies also support the idea of waiting in order to ensure that the most appropriate treatment is initiated first (19, 37, 38). Importantly, earlier treatment initiation for advanced NSCLC may not be associated with longer survival (37, 38), and a large retrospective study has shown that in patients with actionable oncogenic drivers, receiving a targeted therapy is associated with longer survival (19).
Our analysis of measures of effectiveness for chemotherapy or IO monotherapy in patients with METex14 demonstrated a median 1L rw-PFS of 5.7 months and 2.4 months in patients receiving 1L chemotherapy and 1L IO monotherapy, respectively, and a median 2L rw-PFS of 3.5 months and 4.7 months in patients receiving 2L chemotherapy and 2L IO monotherapy, respectively. Patients who received IO monotherapy in 1L showed 3- and 6-month 1L rw-PFS rates of 33% and 17%, respectively, indicating that most patients experienced rapid disease progression even with these treatments. Our analysis also found a median OS of 20.0 months in patients receiving 1L chemotherapy but not reached in patients receiving 1L IO monotherapy, respectively, and a median OS of 15.3 months and 19.3 months in patients receiving 2L chemotherapy and 2L IO monotherapy, respectively. These results are consistent with previous reports showing lower overall survival in patients with METex14 NSCLC receiving nontargeted therapies (chemotherapy and IO) relative to those receiving targeted therapies (9, 39). In a retrospective chart review study, patients who received treatment with a MET inhibitor had a median overall survival of 25.3 months, whereas patients who did not receive a MET inhibitor had a median overall survival of 10.9 months (39). In another retrospective review of stage IV NSCLC patients with METex14 comparing those who received a MET tyrosine kinase inhibitor in 1 or more lines of therapy (n=27) with those who did not (n=34), the median overall survival was 24.6 months and 8.1 months, respectively. Among patients who received crizotinib as their first MET inhibitor, the median PFS was 7.4 months (9). Patients in these studies could have received a MET inhibitor in any line of treatment, not just in 1L. A report from the Sarah Cannon Research Institute showed that patients with METex14 NSCLC were responsive to MET inhibitor therapy after receiving standard-of-care therapy in 1L (40).
A phase 2 study of capmatinib in patients with aNSCLC and confirmed METex14 (GEOMETRY mono-1) demonstrated substantial antitumor activity regardless of the line of therapy in which capmatinib was used. The median progression-free survival (PFS) was 12.4 months (95% CI, 8.2 months-NE) and 5.4 months (95% CI, 4.2-7.0 months) among treatment-naive patients in the 1L setting and previously treated patients in the 2L or third-line (3L) setting, respectively (11). A post hoc analysis of patients treated with capmatinib in the 2L or 3L setting in the GEOMETRY mono-1 trial reviewed responses in patients who did or did not receive IO in the 1L or 2L setting. Among patients who received prior IO, the median PFS was 8.3 months (95% CI, 4.2-12.6 months; data not mature). Among patients without prior IO, the median PFS was 5.4 months (95% CI, 4.2-6.9 months) (41). Furthermore, a recent analysis reviewed treatment-naive patients with METex14 aNSCLC treated with 1L capmatinib in the GEOMETRY mono-1 trial compared with a matched real-world cohort treated with 1L chemotherapy and/or immunotherapy. The median PFS was 12.0 months (95% CI, 5.5-20.7 months) and 6.2 months (95% CI, 3.4-9.1 months) for patients treated with 1L capmatinib or 1L chemotherapy and/or immunotherapy, respectively. Additionally, after left truncation was accounted for in the real-world dataset, the median overall survival was 20.8 months (95% CI, 12.6 months-not reached) and 14.8 months (95% CI, 9.0 months-not reached) for patients treated with 1L capmatinib or 1L chemotherapy and/or immunotherapy, respectively (31). Taken together, both real-world and clinical trial evidence highlights the need to test all patients with NSCLC for oncogenic driver mutations, including METex14, and the importance of early testing.
A retrospective review of 24 patients with METex14 NSCLC receiving single-agent (anti–PD-L1) or combination (anti–PD-1 and anti–CTLA-4) IO reported an ORR of 17%, with short DOR and PFS (28). In the 21 assessable patients, the median PFS was 1.9 months (95% CI, 1.7-2.7 months) (28). Furthermore, in the retrospective IMMUNOTARGET registry study, 23 patients with advanced METex14 treated with immunotherapy had a median PFS of 4.7 months (95% CI, 1.8-7.8 months) (26). These results are consistent with case reports showing limited efficacy of IO in patients with METex14 NSCLC (29, 42). In each of these 4 studies or reports, responses were limited even in tumors with high PD-L1 expression (26, 28, 29, 42). A recent study with a small sample size (N=13) by Mayenga et al, however, showed prolonged responses to 2L IO treatment in 6 patients with METex14 NSCLC previously treated with chemotherapy (N=13), with 5 patients showing a partial or complete response within the first 4 months (27). This study finding contradicts the results of all other studies with larger sample sizes, although it indicates that the response to IO in patients with METex14 is still not fully understood; results based on only 13 patients pose the risk of overinterpretation of the finding (27, 28). The importance of broad molecular testing for driver mutations is further underscored by evidence showing that patients harboring driver mutations may not respond well to IO, in the case of genes such as EGFR and ALK (26, 43–45), or may have only modest responses, in the case of BRAF (26, 46). Accordingly, the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for NSCLC now considers the presence of oncogenes that predict lack of benefit from single-agent IO or combination IO-chemotherapy a contraindication to the use of these agents (4). In particular, the guidelines note that patients with METex14 and high PD-L1 expression do not generally respond to IO (4).
Our study found that pembrolizumab and other PD-L1 inhibitors and chemotherapies were used frequently as 1L treatments in this patient population during the study time frame (January 1, 2011, to December 31, 2019). Although newly approved MET inhibitors will likely change the treatment landscape, the clinical outcomes associated with IO or chemotherapy in this study are still very much relevant and reflective of the current outcomes associated with these regimens. In our study, a small number of patients received IO plus chemotherapy or MET inhibitors as 1L treatments during the study period; however, due to the limited number of patients receiving these treatments, we are unable to provide data on the clinical outcomes with these treatment regimens.
Limitations
This was an observational study using retrospective data. As such, differences in endpoints with small sample sizes should be interpreted with caution, and this study was not designed to provide comparisons between treatments. Data for this study were obtained from the EHRs of a network of oncology practices; any treatments or outcomes that occur outside of these practices may not be captured in the EHR system and thus are unaccounted for in this study.
Analyses for this study were based upon data available within the FH-FMI NSCLC CGDB. Given this constraint, testing times in our analysis reflected time from specimen collection to the reported test results, which does not reflect true testing turnaround time from the physician order date of the test to the reporting of results. This difference would necessarily be increased in patients whose samples may be sequentially tested and patients whose specimen was collected prior to aNSCLC diagnosis. In addition, cross-institutional bias may be introduced for testing not performed by FMI, such as PD-L1 immunohistochemistry testing routinely performed by institutions.
Furthermore, these analyses did not examine the effect of PD-L1 levels or tumor mutational burden (TMB) on rw-PFS. These biomarkers have been shown to be predictive of responses to IO monotherapy in non–oncogene-driven NSCLC (47, 48); however, patients with METex14 tend to have low TMB. Furthermore, in a retrospective analysis of patients with METex14 NSCLC, responses to IO monotherapy were modest compared with an unselected patient population, and responses were not enriched in the subset of patients with either high PD-L1 expression or high TMB (28).
Ultimately, the quality of information extracted from real-world data depends on the quality of information available in the data source. Although the dataset has the potential for missing, inaccurate, or incomplete data, having access to source genomic data and technology-enabled abstraction by specially trained human abstractors using documented policies and procedures and defined quality assurance and control activities aims to reduce data issues and increase completeness.
Conclusions
This study attempted to explore real-world biomarker testing patterns of PD-L1 and FMI NGS among patients with aNSCLC with METex14. We observed a difference in median duration between specimen collection and reporting of PD-L1 and FMI NGS results of 1 week from a small sample of the original cohort. Nontargeted therapies (chemotherapy and IO) were the most frequently used 1L and 2L therapies, although they demonstrated limited clinical benefit in patients with METex14 aNSCLC. The findings of our study may prompt physicians to order NGS testing earlier to initiate appropriate treatment and achieve optimal clinical outcomes when treating aNSCLC with METex14. Future studies are needed to assess the testing turnaround time of NGS testing in the real-world setting and evaluate the effectiveness of targeted therapies, such as MET inhibitors, in the real-world clinical setting.
Data Availability Statement
The data for this study are available from Flatiron, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Flatiron.
Ethics Statement
The studies involving human participants were reviewed and approved by WCG IRB. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
FAZVK was involved in the conceptualization, investigation, formal analysis, and supervision. BC was involved in the conceptualization, investigation, formal analysis, and supervision. HK was involved in the investigation and formal analysis. JK was involved in the investigation and formal analysis. VD was involved in the conceptualization and formal analysis. SZ was involved in the formal analysis. MS was involved in the conceptualization and formal analysis. All authors were involved in the writing, review, and editing of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
FAZVK is an employee of Novartis Pharma AG. BC, VD, SZ, and MS are employees of Novartis Pharmaceuticals Corporation. HK and JK are employees of Genesis Research.
The authors declare that this study received funding from Pharmaceuticals Corporation, East Hanover, NJ, USA. The funder had the following involvement with the study: Employees of the company were involved in medical accuracy review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Lindsay Tannenholz, PhD (Chameleon Communications International with funding from Novartis Pharmaceuticals Corporation) for editorial assistance in the preparation of this report, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.786124/full#supplementary-material
Abbreviations
1L, first-line; 2L, second-line; aNSCLC, advanced non-small cell lung cancer; CGDB, clinico-genomic database; CGP, comprehensive genomic profiling; DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; EHR, electronic health record; FDA, US Food and Drug Administration; FMI, Foundation Medicine Inc; HIPAA, Health Insurance Portability and Accountability Act; ICD, International Classification of Diseases; IO, immuno-oncology; IQR, interquartile range; METex14, MET exon 14 skipping mutation; NE, not estimable; NGS, next-generation sequencing; NOS, not otherwise specified; NSCLC, non-small cell lung cancer; OS, overall survival; rw-PFS, real-world progression-free survival.
References
1. American Cancer Society. About Lung Cancer. Available at: https://www.cancer.org/cancer/lung-cancer/about.html (Accessed 23 July 2020).
2. Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM, et al. Activation of MET via Diverse Exon 14 Splicing Alterations Occurs in Multiple Tumor Types and Confers Clinical Sensitivity to MET Inhibitors. Cancer Discovery (2015) 5(8):850–9. doi: 10.1158/1538-7445.AM2015-1118
3. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung Cancer: Current Therapies and New Targeted Treatments. Lancet (2017) 389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8
4. National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 4.2021). Available at: https://www.nccn.org/professionals/physician_gls/default.aspx (Accessed 5 March 2021).
5. Vuong HG, Ho ATN, Altibi AMA, Nakazawa T, Katoh R, Kondo T. Clinicopathological Implications of MET Exon 14 Mutations in Non-Small Cell Lung Cancer – A Systematic Review and Meta-Analysis. Lung Cancer (2018) 123:76–82. doi: 10.1016/j.lungcan.2018.07.006
6. Awad MM, Lee JK, Madison R, Classon A, Kmak J, Frampton GM, et al. Characterization of 1,387 NSCLCs With MET Exon 14 (METex14) Skipping Alterations (SA) and Potential Acquired Resistance (AR) Mechanisms. J Clin Oncol (2020) 38(15 suppl):9511. doi: 10.1200/JCO.2020.38.15_suppl.9511
7. Tong JH, Yeung SF, Chan AW, Chung LY, Chau SL, Lung RW, et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of non-Small Cell Lung Carcinoma With Poor Prognosis. Clin Cancer Res (2016) 22(12):3048–56. doi: 10.1158/1078-0432.CCR-15-2061
8. Yeung SF, Tong JHM, Law PPW, Chung LY, Lung RWM, Tong CYK, et al. Profiling of Oncogenic Driver Events in Lung Adenocarcinoma Revealed MET Mutation as Independent Prognostic Factor. J Thorac Oncol (2015) 10(9):1292–300. doi: 10.1097/JTO.0000000000000620
9. Awad MM, Leonardi GC, Kravets S, Dahlberg SE, Drilon A, Noonan SA, et al. Impact of MET Inhibitors on Survival Among Patients With Non-Small Cell Lung Cancer Harboring MET Exon 14 Mutations: A Retrospective Analysis. Lung Cancer (2019) 133:96–102. doi: 10.1016/j.lungcan.2019.05.011
11. Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med (2020) 383(10):944–57. doi: 10.1056/NEJMoa2002787
12. Paik PK, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in Non–Small-Cell Lung Cancer With MET Exon 14 Skipping Mutations. N Engl J Med (2020) 383(10):931–43. doi: 10.1056/NEJMoa2004407
14. US Food and Drug Administration. FDA Approves First Targeted Therapy to Treat Aggressive Form of Lung Cancer. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-treat-aggressive-form-lung-cancer (Accessed 13 August 2020).
15. Pilotto S, Gkountakos A, Carbognin L, Scarpa A, Tortora G, Bria E. MET Exon 14 Juxtamembrane Splicing Mutations: Clinical and Therapeutical Perspectives for Cancer Therapy. Ann Transl Med (2017) 5(1):2. doi: 10.21037/atm.2016.12.33
16. Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS, et al. Response to MET Inhibitors in Patients With Stage IV Lung Adenocarcinomas Harboring MET Mutations Causing Exon 14 Skipping. Cancer Discov (2015) 5(8):842–9. doi: 10.1158/2159-8290.CD-14-1467
17. Heist RS, Shim HS, Gingipally S, Mino-Kenudson M, Le L, Gainor JF, et al. MET Exon 14 Skipping in non-Small Cell Lung Cancer. Oncologist (2016) 21(4):481–6. doi: 10.1634/theoncologist.2015-0510
18. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med (2020) 383(7):640–9. doi: 10.1056/NEJMoa1916623
19. Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non-Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA (2019) 321(14):1391–9. doi: 10.1001/jama.2019.3241
20. Chawla A, Janku F, Wheler JJ, Miller VA, Ryan J, Anhorn R, et al. Estimated Cost of Anticancer Therapy Directed by Comprehensive Genomic Profiling in a Single-Center Study. JCO Precis Oncol (2018) 2:1–11. doi: 10.1200/PO.18.00074
21. Pisapia P, Pepe F, Baggi A, Barberis M, Galvano A, Gristina V, et al. Next Generation Diagnostic Algorithm in Non-Small Cell Lung Cancer Predictive Molecular Pathology: The KWAY Italian Multicenter Cost Evaluation Study. Crit Rev Oncol Hematol (2021) 169:103525. doi: 10.1016/j.critrevonc.2021.103525
22. Pennell NA, Mutebi A, Zhou Z-Y, Ricculli ML, Tang W, Wang H, et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precis Oncol (2019) 3:1–9. doi: 10.1200/PO.18.00356
23. Pennell NA, Arcila ME, Gandara DR, West H. Biomarker Testing for Patients With Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am Soc Clin Oncol Educ Book (2019) 39:531–42. doi: 10.1200/EDBK_237863
24. Russo A, Incorvaia L, Del Re M, Malapelle U, Capoluongo E, Gristina V, et al. The Molecular Profiling of Solid Tumors by Liquid Biopsy: A Position Paper of the AIOM-SIAPEC-IAP-SIBioC-SIC-SIF Italian Scientific Societies. ESMO Open (2021) 6(3):100164. doi: 10.1016/j.esmoop.2021.100164
25. Listì A, Barraco N, Bono M, Insalaco L, Castellana L, Cutaia S, et al. Immuno-Targeted Combinations in Oncogene-Addicted Non-Small Cell Lung Cancer. Trans Cancer Res (2019) 8(Suppl 1):S55–63. doi: 10.21037/tcr.2018.10.04
26. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune Checkpoint Inhibitors for Patients With Advanced Lung Cancer and Oncogenic Driver Alterations: Results From the IMMUNOTARGET Registry. Ann Oncol (2019) 30(8):1321–8. doi: 10.1093/annonc/mdz167
27. Mayenga M, Assié JB, Monnet I, Massiani MA, Tabeze L, Friard S, et al. Durable Responses to Immunotherapy of Non-Small Cell Lung Cancers Harboring MET Exon-14-Skipping Mutation: A Series of 6 Cases. Lung Cancer (2020) 150:21–5. doi: 10.1016/j.lungcan.2020.09.008
28. Sabari JK, Leonardi GC, Shu CA, Umeton R, Montecalvo J, Ni A, et al. PD-L1 Expression, Tumor Mutational Burden, and Response to Immunotherapy in Patients With MET Exon 14 Altered Lung Cancers. Ann Oncol (2018) 29(10):2085–91. doi: 10.1093/annonc/mdy334
29. Baba K, Tanaka H, Sakamoto H, Shiratori T, Tsuchiya J, Ishioka Y, et al. Efficacy of Pembrolizumab for Patients With Both High PD-L1 Expression and an MET Exon 14 Skipping Mutation: A Case Report. Thorac Cancer (2019) 10(2):369–72. doi: 10.1111/1759-7714.12939
30. Birnbaum B, Nussbaum N, Seidl-Rathkopf K, Agrawal M, Estevez M, Estola E, et al. Model-Assisted Cohort Selection With Bias Analysis for Generating Large-Scale Cohorts From the EHR for Oncology Research. arXiv (2020) arXiv:2001.09765.
31. Wolf J, Neal J, Mansfield A, Doban V, Kanakamedala H, Wu W, et al. Comparison of Clinical Outcomes of Patients With MetΔex14 NSCLC Treated With First-Line Capmatinib in the GEOMETRY Mono-1 Study With Those of a Cohort of Real-World Patients. Ann Oncol (2020) 31(Suppl 4):S863. doi: 10.1016/j.annonc.2020.08.1660
32. Griffith SD, Tucker M, Bowser B, Calkins G, Chang CJ, Guardino E, et al. Generating Real-World Tumor Burden Endpoints From Electronic Health Record Data: Comparison of RECIST, Radiology-Anchored, and Clinician-Anchored Approaches for Abstracting Real-World Progression in Non-Small Cell Lung Cancer. Adv Ther (2019) 36(8):2122–36. doi: 10.1007/s12325-019-00970-1
33. Lim C, Tsao MS, Le LW, Shepherd FA, Feld R, Burkes RL, et al. Biomarker Testing and Time to Treatment Decision in Patients With Advanced Nonsmall-Cell Lung Cancer. Ann Oncol (2015) 26(7):1415–21. doi: 10.1093/annonc/mdv208
34. Krigsfeld GS, Prince EA, Pratt J, Chizhevsky V, William Ragheb J, Novotny J, et al. Analysis of Real-World PD-L1 IHC 28-8 and 22C3 pharmDx Assay Utilisation, Turnaround Times and Analytical Concordance Across Multiple Tumour Types. J Clin Pathol (2020) 73(10):656–64. doi: 10.1136/jclinpath-2020-206466
35. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn (2018) 20(2):129–59. doi: 10.1016/j.jmoldx.2017.11.004
36. Hanna N, Robinson A, Temin S, Baker S, Brahmer J, Ellis P, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol (2021) 39(9):1040–91. doi: 10.1200/JCO.20.03570
37. Bullard J, Eberth J, Arrington A, Adams S, Cheng X, Salloum R. Timeliness of Treatment Initiation and Associated Survival Following Diagnosis of Non-Small-Cell Lung Cancer in South Carolina. South Med J (2017) 110(2):107–13. doi: 10.14423/SMJ.0000000000000601
38. Azzouqa A, Chen R, Lou Y, Ailawadhi S, Manochakian R. Impact of Time to Treatment Initiation (TTI) on Survival of Patients With Newly Diagnosed Non-Small Cell Lung Cancer (NSCLC). J Clin Oncol (2019) 37(15 suppl):9058. doi: 10.1200/JCO.2019.37.15_suppl.9058
39. Wolf J, Baik C, Heist RS, Neal JW, Mansfield AS, Buettner R, et al. Natural History, Treatment (Tx) Patterns, and Outcomes in MET Dysregulated Non-Small Cell Lung Cancer (NSCLC) Patients (Pts). Eur J Cancer (2018) 103(Suppl):E131. doi: 10.1016/S0959-8049(18)31491-6
40. McKenzie A, Fisher A, Correll M, Jones C, Correia J, Thurber J, et al. Clinical and Genomic Analysis of Non-Small Cell Lung Cancer (NSCLC) Patients With MET Exon14 Skipping (METex14) Mutations and Responses to Anti-MET Therapy. J Clin Oncol (2020) 38(15 suppl):9613. doi: 10.1200/JCO.2020.38.15_suppl.9613
41. Vansteenkiste JF, Smit EF, Groen HJM, Garon EB, Heist RS, Hida T, et al. Capmatinib in Patients With METex14-Mutated Advanced Non-Small Cell Lung Cancer Who Received Prior Immunotherapy: The Phase II GEOMETRY Mono-1 Study. Ann Oncol (2020) 31:S830. doi: 10.1016/j.annonc.2020.08.1599
42. Reis H, Metzenmacher M, Goetz M, Savvidou N, Darwiche K, Aigner C, et al. MET Expression in Advanced Non-Small-Cell Lung Cancer: Effect on Clinical Outcomes of Chemotherapy, Targeted Therapy, and Immunotherapy. Clin Lung Cancer (2018) 19(4):e441–e63. doi: 10.1016/j.cllc.2018.03.010
43. Cavanna L, Citterio C, Orlandi E. Immune Checkpoint Inhibitors in EGFR-Mutation Positive TKI-Treated Patients With Advanced Non-Small-Cell Lung Cancer Network Meta-Analysis. Oncotarget (2019) 10(2):209–15. doi: 10.18632/oncotarget.26541
44. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR Mutations and ALK Rearrangements Are Associated With Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res (2016) 22(18):4585–93. doi: 10.1158/1078-0432.CCR-15-3101
45. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J Thorac Oncol (2018) 13(8):1138–45. doi: 10.1016/j.jtho.2018.03.035
46. Dudnik E, Peled N, Nechushtan H, Wollner M, Onn A, Agbarya A, et al. BRAF Mutant Lung Cancer: Programmed Death Ligand 1 Expression, Tumor Mutational Burden, Microsatellite Instability Status, and Response to Immune Check-Point Inhibitors. J Thorac Oncol (2018) 13(8):1128–37. doi: 10.1016/j.jtho.2018.04.024
47. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 Expression and Tumor Mutational Burden Are Independent Biomarkers in Most Cancers. JCI Insight (2019) 4(6):e126908. doi: 10.1172/jci.insight.126908
48. Galvano A, Gristina V, Malapelle U, Pisapia P, Pepe F, Barraco N, et al. The Prognostic Impact of Tumor Mutational Burden (TMB) in the First-Line Management of Advanced Non-Oncogene Addicted non-Small-Cell Lung Cancer (NSCLC): A Systemic Review and Meta-Analysis of Randomized Controlled Trials. ESMO Open (2021) 6(3):100124. doi: 10.1016/j.esmoop.2021.100124
Keywords: next-generation sequencing, PD-L1, chemotherapy, immuno-oncology therapy, real world outcomes
Citation: Asad Zadeh Vosta Kolaei F, Cai B, Kanakamedala H, Kim J, Doban V, Zhang S and Shi M (2022) Biomarker Testing Patterns and Treatment Outcomes in Patients With Advanced Non-Small Cell Lung Cancer and MET Exon 14 Skipping Mutations: A Descriptive Analysis From the US. Front. Oncol. 12:786124. doi: 10.3389/fonc.2022.786124
Received: 29 September 2021; Accepted: 27 January 2022;
Published: 25 February 2022.
Edited by:
Umberto Malapelle, University of Naples Federico II, ItalyReviewed by:
Gang Zheng, Mayo Clinic, United StatesPasquale Pisapia, University of Naples Federico II, Italy
Valerio Gristina, University of Palermo, Italy
Copyright © 2022 Asad Zadeh Vosta Kolaei, Cai, Kanakamedala, Kim, Doban, Zhang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beilei Cai, YmVpbGVpLmNhaUBub3ZhcnRpcy5jb20=
 Fatemeh Asad Zadeh Vosta Kolaei1
Fatemeh Asad Zadeh Vosta Kolaei1 Beilei Cai
Beilei Cai Shiyu Zhang
Shiyu Zhang Michael Shi
Michael Shi