
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 18 May 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.776920
This article is part of the Research Topic Minimal Residual Disease (MRD) Assessment in Multiple Myeloma Patients View all 9 articles
Objective: To explore the prognostic significance of the stage at which a minimal residual disease (MRD)-negative status is achieved for patients with newly diagnosed multiple myeloma (NDMM) who received autologous hematopoietic stem cell transplantation (ASCT).
Cases and Methods: A retrospective analysis of 186 NDMM patients who received “induction therapy-ASCT-maintenance therapy” in our center and achieved an MRD-negative status was performed. Patients were divided into three groups, A (induction therapy), B (3 months after ASCT), and C (maintenance therapy), according to the stage at which an MRD-negative status was achieved.
Results: The median time to progression (TTP) of 186 patients was not reached; the median overall survival (OS) was 113.8 months. The median TTP of the patients in three groups was not reached (P=0.013), and the median OS of the patients in three groups was not reached, not reached, and 71.2 months, respectively (P=0.026). Among patients with standard-risk cytogenetics, the median TTP of those in all three groups was not reached (P=0.121), and the median OS of the patients in three groups was not reached, not reached, and 99.6 months, respectively (P=0.091). Among patients with high-risk cytogenetics, the median TTP of those in three groups was not reached, 53.9 months, and 35.8 months (P=0.060), and the median OS was not reached, 71.2 months, and 60.2 months, respectively (P=0.625). Among patients with R-ISS stage I-II, the median TTP of those in three groups was not reached (P=0.174), and the median OS of the patients in three groups was not reached, not reached, and 99.6 months, respectively (P=0.186). Among the 29 patients with R-ISS stage III, the median TTP of those in the 3 groups were unreached, unreached, and 35.1 months (P<0.001), and the median OS was unreached, unreached, and 48.5 months, respectively (P=0.020). In all enrolled patients, the stage of reaching MRD-negative was an independent prognostic factor for TTP, rather than a prognostic factor for OS. The stage of reaching MRD-negative in patients with R-ISS III was an independent prognostic factor for OS.
Conclusion: For the same patients who are MRD-negative, the prognoses of those who achieve an MRD-negative status at different groups are different. The stage at which an MRD-negative status is achieved can predict the prognosis of patients with R-ISS stage III.
Multiple myeloma (MM) is a hematologic malignancy characterized by the accumulation of malignant plasma cells, renal insufficiency, hypercalcemia, anemia, and bone destruction. In the past two decades, the availability of new drugs and autologous hematopoietic stem cell transplantation (ASCT) for MM has led to significantly improved long-term outcomes (1). “Induction therapy-autologous hematopoietic stem cell transplantation-maintenance therapy” has become the preferred treatment option for MM patients eligible for ASCT (1, 2).
Minimal residual disease (MRD) assessment by multiparameter flow cytometry (MFC) on bone marrow is a sensitive strategy to accurately measure the response (3–6). At the end of each treatment stage, traditional efficacy and MRD need to be evaluated (1). Patients who are MRD-negative have a better prognosis (2, 3, 7). Regardless of the stage at which an MRD-negative status is achieved, the lower the MRD level detected with a highly sensitive technique is, the longer the progression-free survival (PFS) and overall survival (OS) periods (4, 8). The pretransplantation MRD level was an independent prognostic factor for event-free survival (EFS) and OS (9). MRD status by MFC at day 100 or 365 after ASCT was the most important independent prognostic factor for PFS and OS (3, 10). These findings demonstrate the clinical importance of MRD evaluation, and the MRD-negative status has become the goal of current treatment, and it has also become the endpoint of many clinical trials (11, 12).
However, previous studies were mostly based on the prognostic significance of MRD results at a single time point. It is believed that regardless of which stage the MRD is negative, the prognosis is better than that of the positive ones (4, 8). A few studies combined multiple MRD results at different time points to predict the prognosis of MM, and as our previous study found, patients with a sustained negative MRD status lasting for at least 24 months had a better prognosis (13). For MRD-negative patients, it is not clear whether there is a difference in clinical features and prognosis after reaching an MRD-negative status at different stages, especially for high-risk cytogenetics patients. To clarify this issue, we conducted a retrospective clinical study.
Patients with newly diagnosed multiple myeloma (NDMM) who received “induction therapy-ASCT-maintenance therapy” at the First Affiliated Hospital of Sun Yat-sen University between January 2007 and January 2021 were evaluated for inclusion in our retrospective study. Patients were divided into three groups, A (induction therapy), B (3 months after ASCT), and C (maintenance therapy), according to the stage at which an MRD-negative status was achieved. The clinical characteristics and prognoses of patients in the three stages were compared. All patients met the International Myeloma Working Group (IMWG) diagnostic criteria, and a total of 186 patients were analyzed (2). The inclusion criteria were as follows: 1) NDMM with complete clinical data; 2) received induction therapy-ASCT maintenance treatment, and 3) curative effect was evaluated and MRD was negative. The exclusion criteria were as follows: 1) past tumor history or diagnosis of combined tumors; 2) severe complications that may affect treatment or survival; 3) received double ASCT; 4) all MRD detection results were positive, and 5) discontinued treatment at any stage. It should be pointed out that for patients with rapid progression after treatment, if they achieved MRD-negative before, they were included in the analysis, otherwise they were excluded. In this study, those with 17p-, t(4;14) or t(14;16) were allocated to the high-risk cytogenetics group, and those without the abovementioned cytogenetic changes were allocated to the standard-risk cytogenetics group (1).
In this study, the patients were divided into three groups. The prognosis of the three groups of patients in different stages was compared, and the observed events were progression and death. The Kaplan-Meier survival curve was used to analyze the prognostic characteristics and the Log-Rank test was used. The study design conformed to the 3-rank vs. outcome event chi-square test model. The Df is set to 2, 1-β is set to 0.8, α is set to 0.05, w is set to 0.25 (medium effect size), and the sample size n=155 is calculated. When the test reaches the expected statistical significance, the ideal statistical test power can be achieved when the number of valid study cases is more than 155 cases.
Induction therapy comprised a regimen containing bortezomib or lenalidomide. Some patients were treated with a regimen containing bortezomib and lenalidomide at the same time, with a median induction of 4 (1–12) courses. The mobilizing regimen was cyclophosphamide (CTX 3 g/m2, iv drip d1) + granulocyte colony-stimulating factor (G-CSF, 300 μg, ih QD from d2) or G-CSF alone (G-CSF, 300 μg, ih QD d1–5). The pretreatment regimen was high-dose melphalan (melphalan 200 mg/m2, reduction in renal insufficiency) or CVB (CTX 50 mg/kg, iv drip, QD -3 d~-2 d; etoposide 10 mg/kg, iv drip, QD, -5 d~-4 d; busulfan 0.8 mg/kg, iv, q6h, -8 d~-6 d). The following numbers of collected cells were required: MNCs>2.0×108/kg and CD34+ cells>2.0×106/kg. Maintenance treatment was started as soon as the blood cells recovered after transplantation. Proteasome inhibitors, immunomodulators, interferon, glucocorticoids, and Daratumumab were applied individually or in combination for maintenance treatment.
The IMWG 2016 efficacy standard was used to evaluate efficacy (2). The abovementioned efficacy evaluation was performed after induction chemotherapy, every 3 months in the first year after ASCT, and every six months after 1 year. At the same time, flow cytometry was used for MRD detection in the bone marrow. The antibody marker of flow cytometry MRD was CD38/CD45/CD19/CD20/CD56/CD54/CD138/cκ/cλ. The number of cells detected was 1×106, the detection of ≥20 abnormal phenotype plasma cells was considered positive, and the limit of quantification (LOD) was 2×10-5 to 10-5.
Follow-up was conducted by telephone. All patients were followed up until January 31, 2021.
Sample size was estimated by G*Power version 3.0 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, German). Statistical analyses were carried out using SPSS 26.0 software (SPSS, Chicago, IL, US) and GraphPad Prism (version 9.0; GraphPad Software, La Jolla, California). Baseline characteristics were evaluated using descriptive statistical analysis: frequency distributions (n, %) are presented for categorical variables and compared using the chi-squared test. The median (range) is presented for continuous variables and compared using a nonparametric test, only age was a continuous variable in our study. Time to progression (TTP) was calculated from the start of treatment to disease progression or the last follow-up, and overall survival (OS) was calculated from the start of treatment until death or the last follow-up. The Kaplan-Meier method was used to analyze the survival of patients who achieved an MRD-negative status at different stages. P<0.05 indicated that the difference was statistically significant.
This study reviewed 293 patients, 45 of whom were excluded due to a lack of MRD monitoring, 1 of whom was excluded due to having combined malignant tumors, 15 of whom received double ASCT, and 46 of whom had persistent MRD-positive results. Ultimately, 186 patients achieved an MRD-negative status, as shown in Figure 1.
The basic data of the enrolled patients are shown in Table 1. A comparison of the baseline information of patients in the three groups (A-C) is shown in Table 2. There was no significant difference in basic data between the groups (P>0.05).
A total of 178 patients received a bortezomib-containing regimen for induction therapy, 19 patients were treated with a lenalidomide-containing regimen for induction therapy, and 11 patients were treated with a bortezomib- and lenalidomide-containing regimen for induction therapy. Eighty patients (43.0%) achieved complete response (CR) after induction therapy, 81 (43.5%) achieved very good partial response (VGPR), 21 (11.3%) achieved partial response (PR), and 4 (2.2%) did not achieve PR. Seventy-three patients (39.2%) achieved an MRD-negative status after induction therapy, and another 113 patients (60.8%) remained MRD positive at this stage. A total of 180 patients received high-dose CTX+G-CSF for peripheral blood stem cell mobilization, and 15 patients failed after the first mobilization; of these patients, peripheral blood stem cells were successfully collected in 5 after receiving the same protocol again, peripheral blood + bone marrow stem cells were collected in 4 after receiving the same protocol again, and peripheral blood + bone marrow stem cells were collected after mobilization with G-CSF monotherapy in 6. Another 6 patients were mobilized with G-CSF (300 µg/d, d1–5) to collect bone marrow stem cells. 102 patients received CVB pretreatment; 69 patients received high-dose melphalan pretreatment, and 15 patients received a reduced-dose Mel regimen due to renal insufficiency[100mg/m2 (n=3), 120mg/m2 (n=1), 140mg/m2 (n=10), 150mg/m2 (n=1)]. The median count of CD34+ cells was 3.06 (0.13–17.8)×106/kg, the median reconstruction time of granulocytes was 10 d, and the median reconstruction time of megakaryocytes was 11 d. Maintenance therapy with bortezomib + thalidomide + dexamethasone after ASCT (n=1); Bortezomib+Dexamethasone (n=11); Daratumumab (n=1); Thalidomide (n=121); Interferon (n=31); lenalidomide (n=17); Ixazomib (n=4). Forty-two patients (22.6%) achieved an MRD-negative status after transplantation. The best curative effect after maintenance treatment was CR in 163 patients (87.6%), VGPR in 22 patients (11.8%), and PR in 1 patient (0.5%). Seventy-one patients (38.2%) achieved an MRD-negative status after maintenance treatment.
The median follow-up time was 67.6 (9.3–154.9) months. The median TTP of 186 patients was not reached, and the median OS was 113.0 months. Of the 73 patients who achieved an MRD-negative status after induction, 8 progressed during the follow-up period, 11 died, and neither TTP nor OS was reached; of the 42 patients who achieved an MRD-negative status after ASCT, 9 progressed and 13 died. The TTP and OS were not reached. Among the 71 patients who achieved an MRD-negative status after maintenance, 25 progressed, and 28 died. The TTP was not reached, and OS was 71.2 months. As shown in Figure 2, there were differences in TTP and OS between the groups (P values were 0.013 and 0.026, respectively).
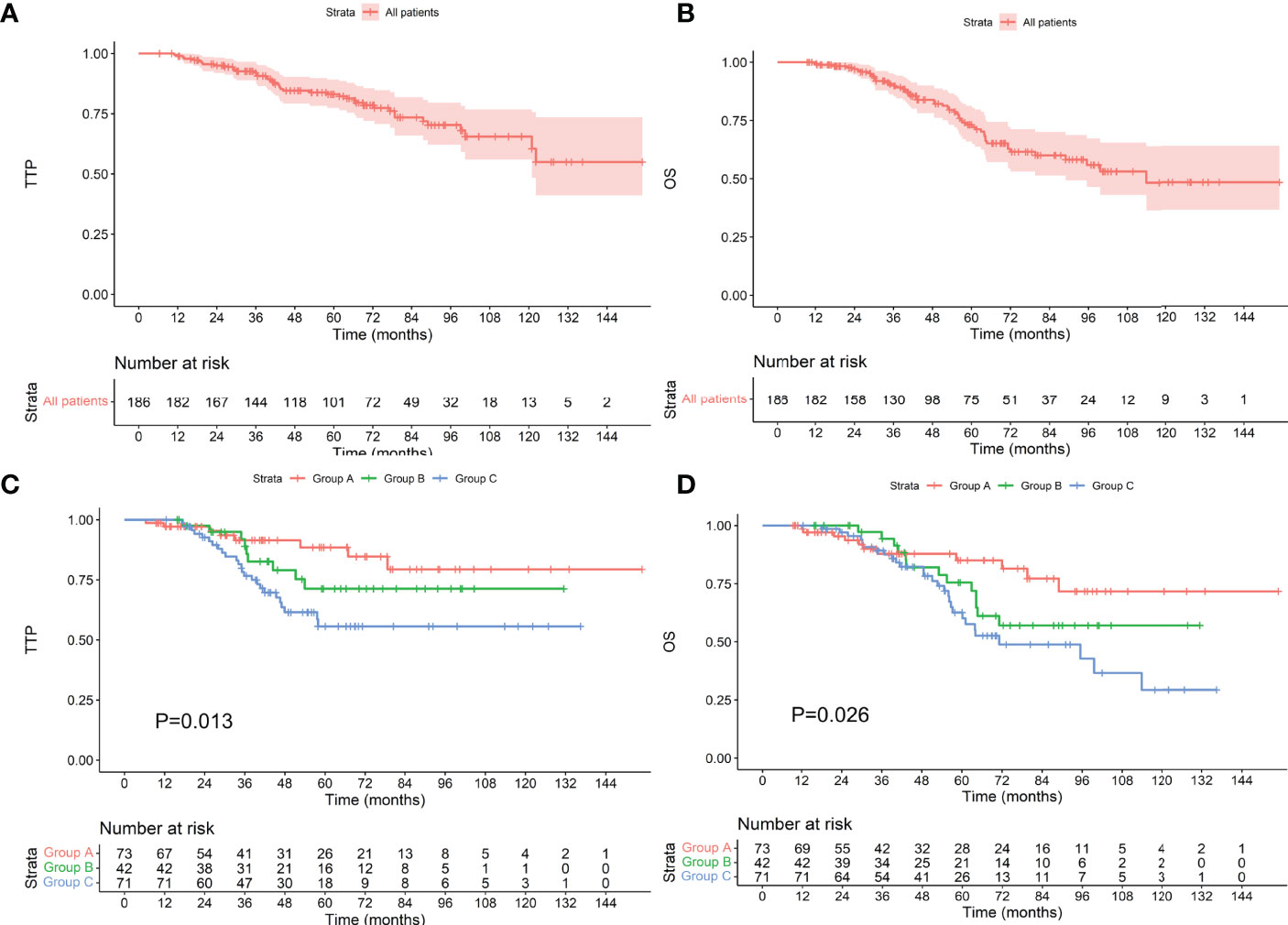
Figure 2 TTP (A) and OS (B) for all patients included. TTP (C) and OS (D) for patients who achieved an MRD-negative status at different stages. The Group A, Group B, and Group C represent achieving an MRD-negative status after induction therapy, ASCT, and maintenance therapy, respectively (the same below).
Among the 124 patients with standard-risk cytogenetics, the median TTP was not reached, and the median OS was 113.8 months. Among the 54 patients who achieved an MRD-negative status after induction, 7 progressed during the follow-up period, and 7 died. The median TTP and median OS were not reached. Among the 24 patients who achieved an MRD-negative status after ASCT, 3 progressed, and 8 died. The median TTP and median OS were not reached. Among the 46 patients who achieved an MRD-negative status after maintenance, 13 progressed, and 13 died. The median TTP was not reached, and the median OS was 99.6 months. There was no difference in TTP or OS between the groups (as shown in Figure 3, P values were 0.121 and 0.091, respectively). Among the 38 patients with high-risk cytogenetics, among the 16 who achieved an MRD-negative statusafter induction, 1 progressed during the follow-up period, and 3 died. The median TTP and median OS were not reached. Among the 10 patients who achieved an MRD-negative status after ASCT, 3 progressed, and 3 died. The median TTP was 53.9 months, and the median OS was 71.2 months. Among the 12 patients who achieved an MRD-negative status after maintenance, 7 progressed, and 7 died. The median TTP was 35.8 months, and the median OS was 60.2 months. There was no difference in TTP or OS between the groups (as shown in Figure 4, P values were 0.060 and 0.624, respectively).
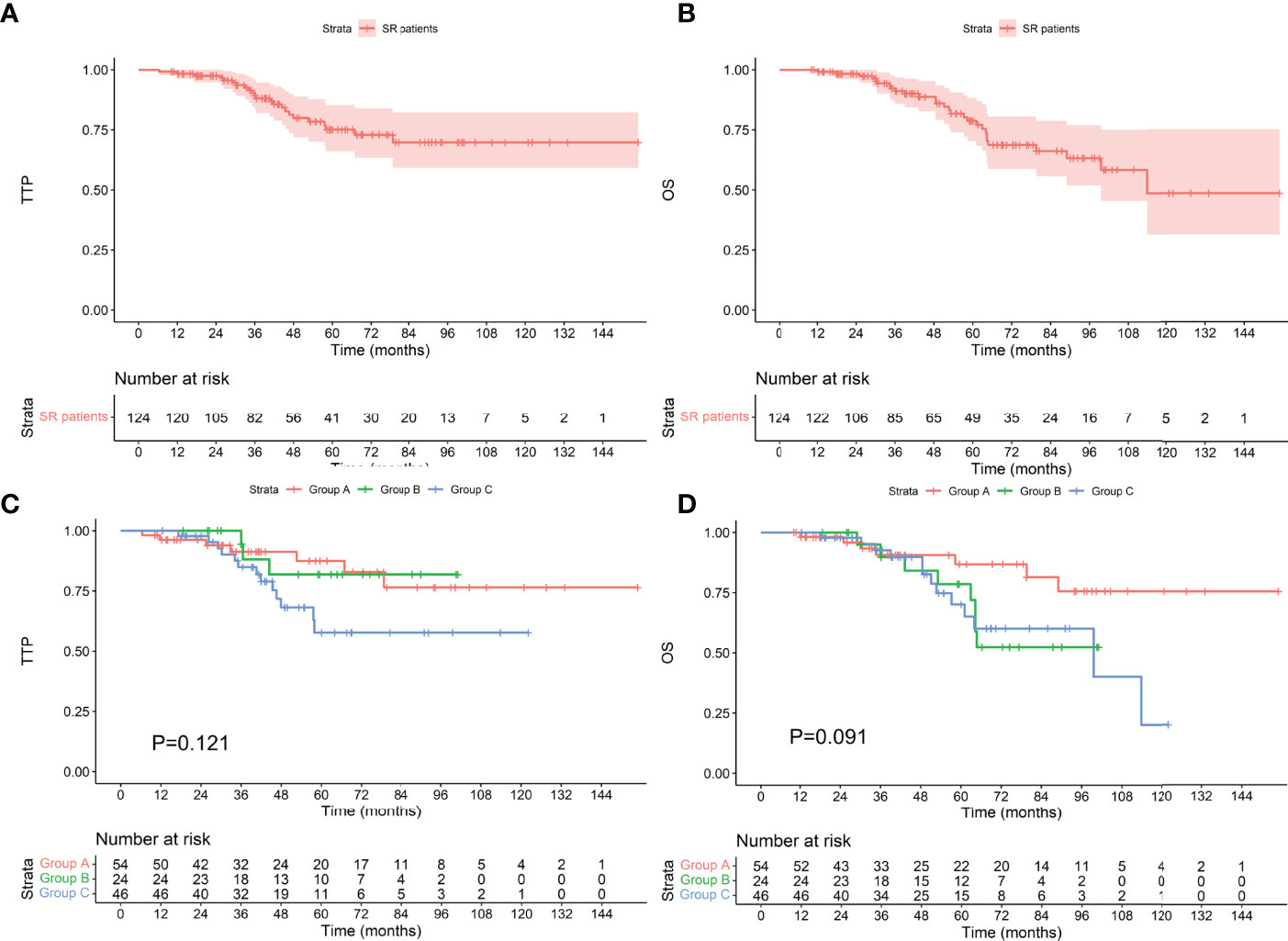
Figure 3 TTP (A) and OS (B) for patients with standard-risk cytogenetics and TTP (C) and OS (D) for patients who achieved an MRD-negative status at different stages. SR, standard-risk cytogenetics.
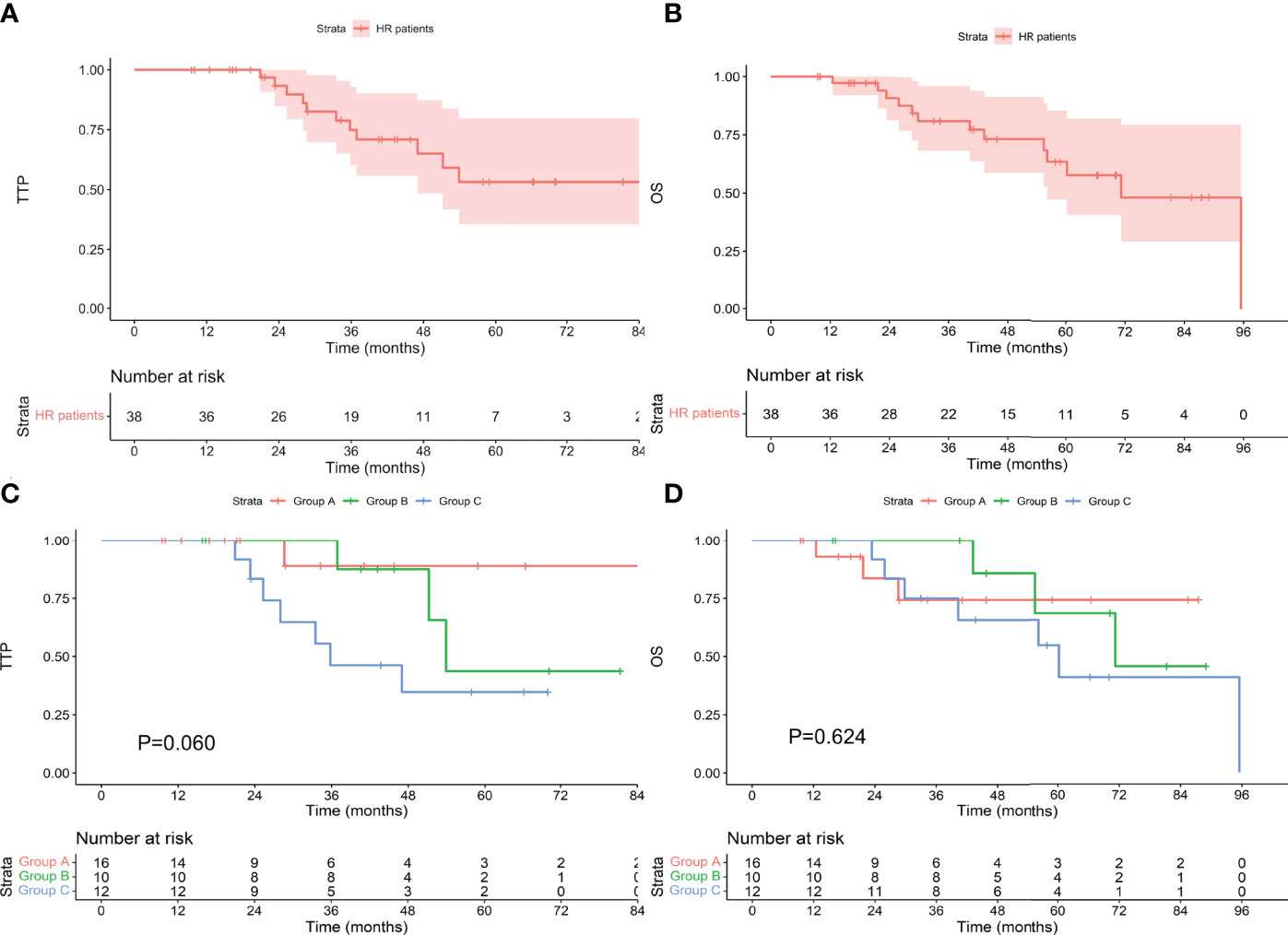
Figure 4 TTP (A) and OS (B) for patients with high-risk cytogenetics and TTP (C) and OS (D) for patients who achieved an MRD-negative status at different stages. HR, high-risk cytogenetics.
There were 26 patients with t(4;14). Among the 10 patients who achieved an MRD-negative status after induction, 1 progressed during the follow-up period, and 2 died. The median TTP and OS were not reached. Among the 5 patients who achieved an MRD-negative status after ASCT, 2 progressed, and 1 died. The median TTP was 52.6 months, and the median OS was 80.1 months. Among the 11 patients who achieved an MRD-negative status after maintenance, 6 progressed, and 6 died. The median TTP was 35.8 months, and the median OS was 56.2 months. There was no difference in TTP or OS between the groups (P values were 0.324 and 0.345, respectively). There were 6 patients with t(14;16), and none of them progressed during the follow-up period. One patient died, and he achieved an MRD-negative status after ASCT. There was no difference in TTP (P>0.999) or OS (P=0.607) between the groups. There were 12 patients with 17p-; among the 5 patients who achieved an MRD-negative status after induction, none progressed, and one died. The median TTP and OS were not reached. Among the 4 patients who achieved an MRD-negative status after ASCT, 1 progressed, and 1 died. The median TTP was not reached, and the median OS was 62.9 months. Of the 3 patients who achieved an MRD-negative status after maintenance, 2 progressed, and 2 died. The median TTP was 47.0 months, and the median OS was 95.5 months. There was no significant difference in TTP or OS between the groups (P values were 0.337 and 0.997, respectively). There was no difference in TTP or OS among those who achieved an MRD-negative status at different stages among the three types of patients with high-risk cytogenetics, as shown in Figure 5.
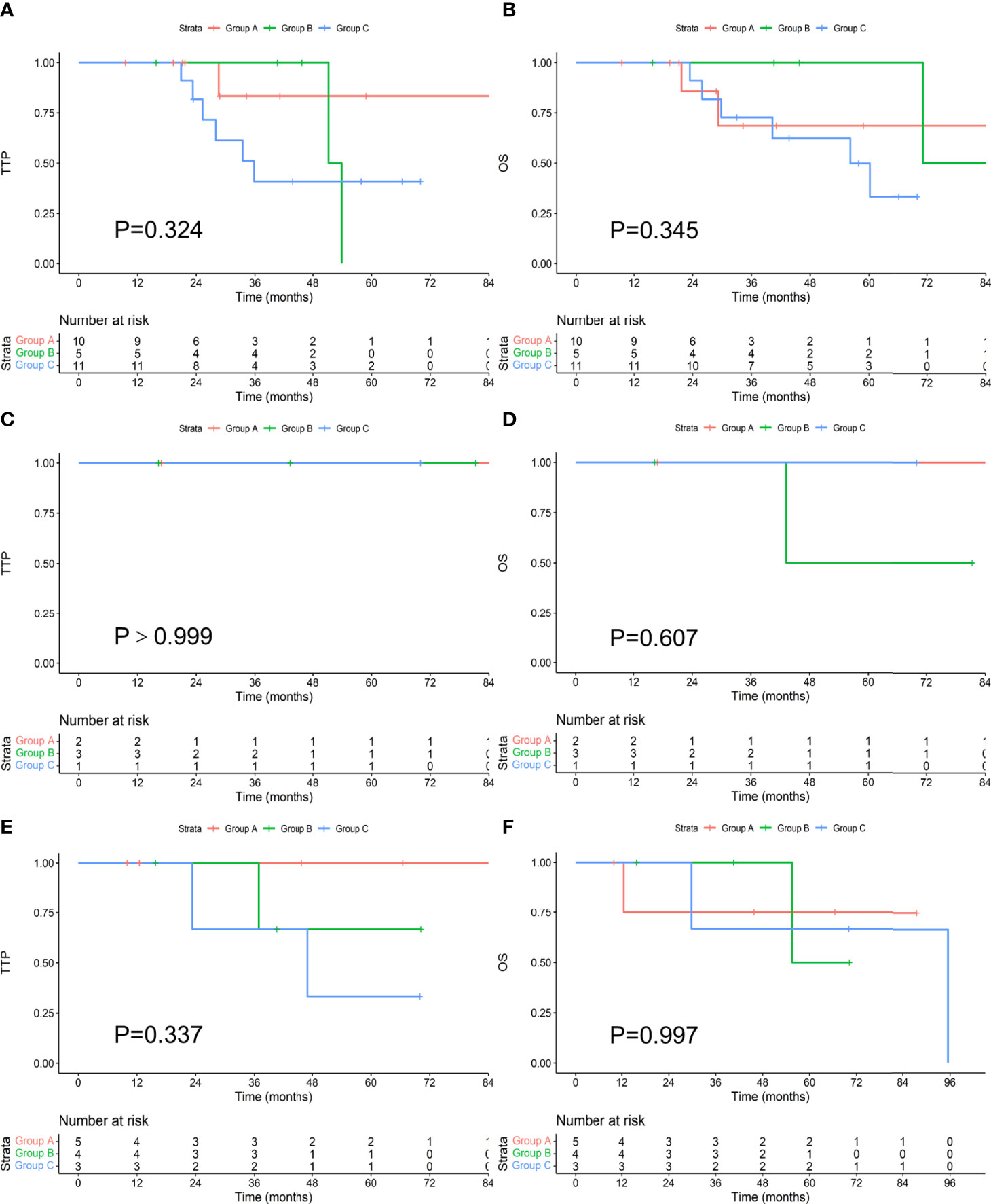
Figure 5 Comparison of TTP (A, C, E) and OS (B, D, F) in patients with t(4;14), t(14;16), and 17p- who achieved an MRD-negative status at different stages.
Among the 157 patients with R-ISS stage I-II, the median TTP and OS were not reached. Among the 61 patients who achieved an MRD-negative status after induction, 7 progressed during the follow-up period, and 9 died. The median TTP and OS were not reached. Among the 35 patients who achieved an MRD-negative status after ASCT, 9 progressed, and 11 died. The median TTP and OS were not reached. Among the 61 patients who achieved an MRD-negative status after maintenance, 17 progressed, and 19 died. The median TTP was not reached, and the median OS was 99.6 months. There was no difference in TTP or OS between the groups (as shown in Figure 6, P values were 0.174 and 0.186, respectively). Among the 29 patients with R-ISS stage III, the median TTP was not reached, and the median OS was 61.1 months. Among the 12 patients who achieved an MRD-negative status after induction, 1 progressed during the follow-up period, and 2 died. The median TTP and OS were not reached. Among the 7 patients who achieved an MRD-negative status after ASCT, none progressed, and 2 died. The median TTP and OS were not reached. Among the 10 patients who achieved an MRD-negative status after maintenance, 8 progressed, and 9 died. The median TTP was 35.1 months, and the median OS was 48.5 months. As shown in Figure 7, there was a significant difference in TTP (Chi-square=15.691, P<0.001) and OS (Chi-square=7.776, P=0.020) between the groups.
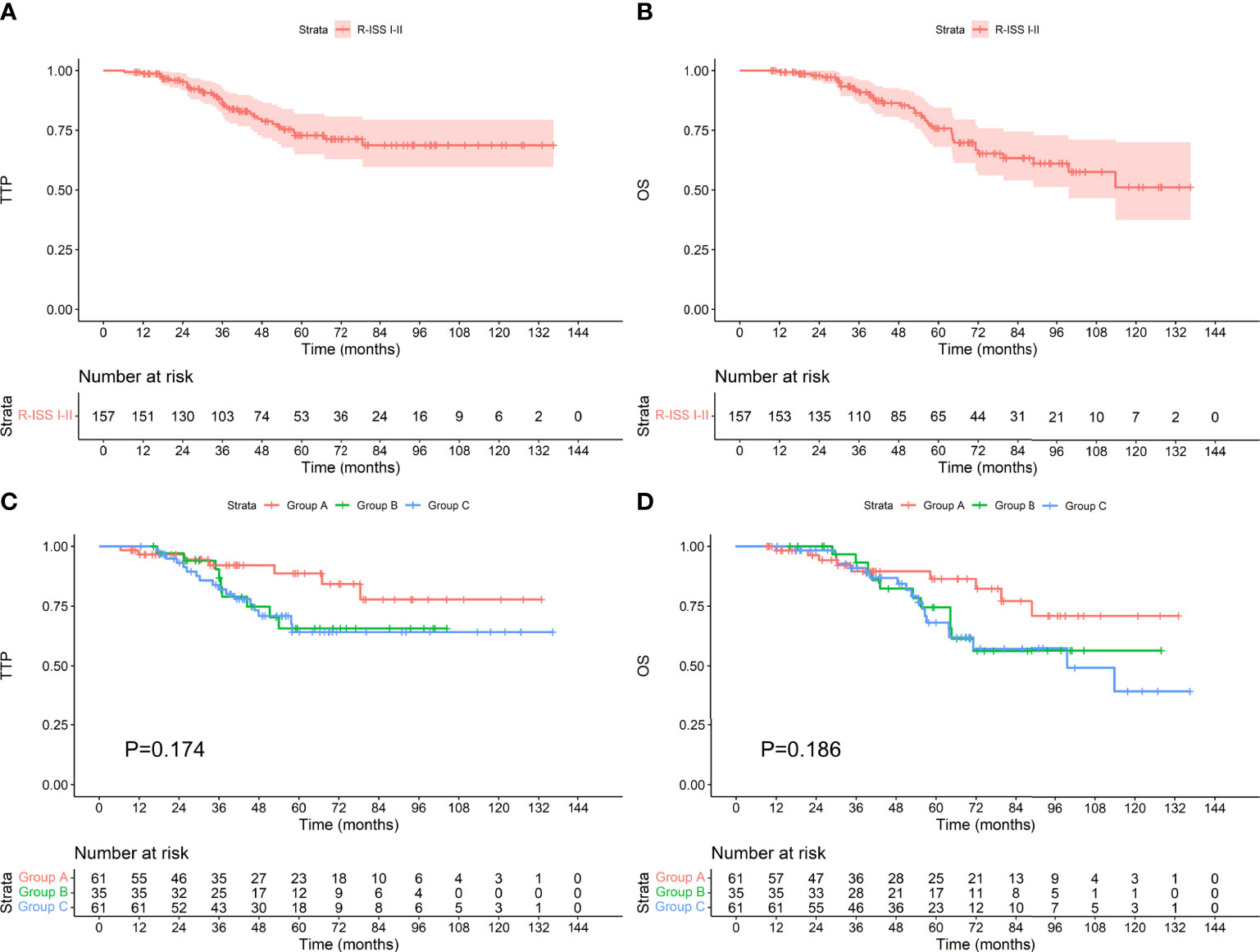
Figure 6 TTP (A) and OS (B) for patients with R-ISS stage I-II and TTP (C) and OS (D) for patients who achieved an MRD-negative status at different stages.
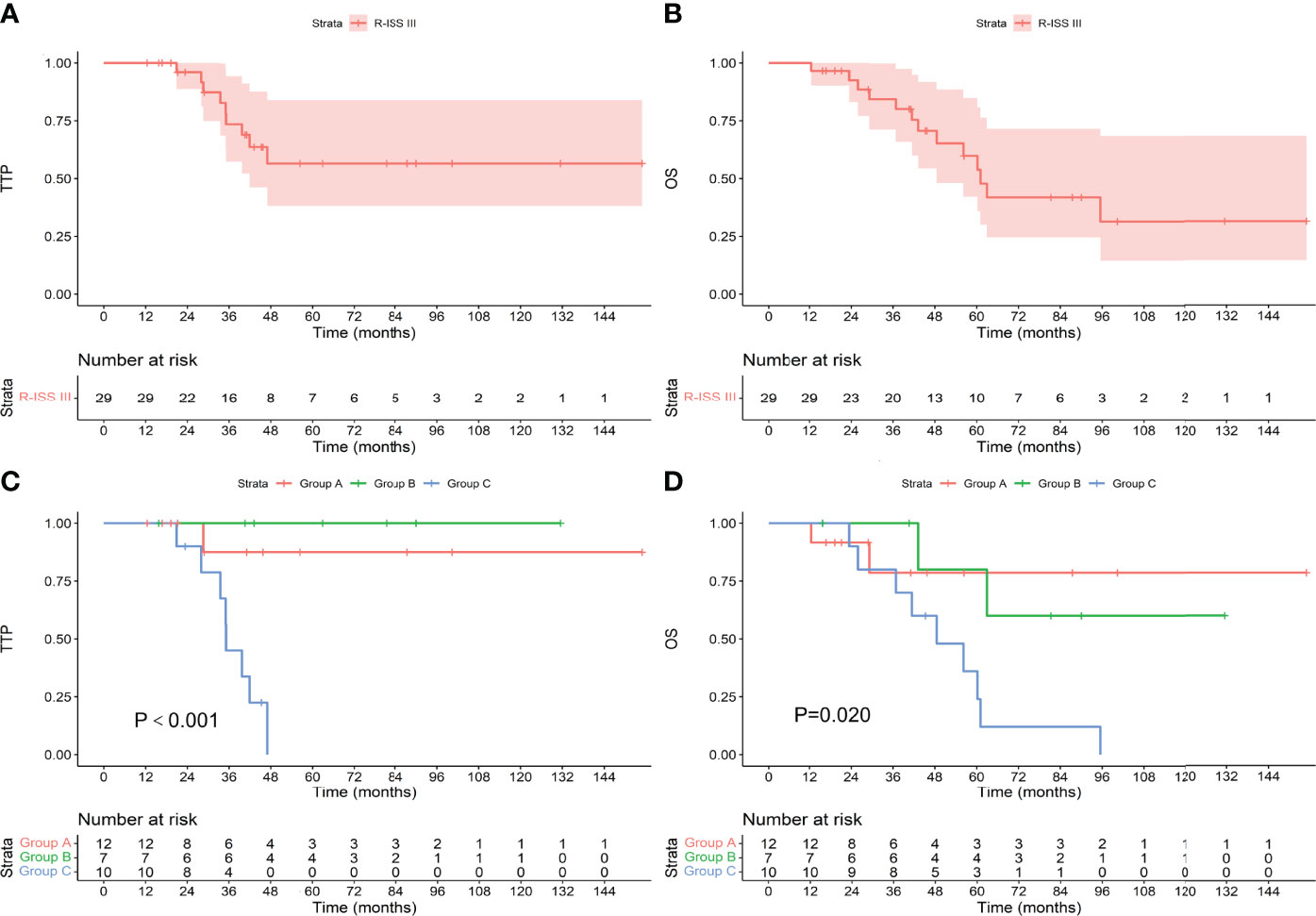
Figure 7 TTP (A) and OS (B) for patients with R-ISS stage III and TTP (C) and OS (D) for patients who achieved an MRD-negative status at different stages.
COX regression analysis showed that in 186 patients enrolled, high-risk cytogenetics and the stage of reaching MRD-negative were independent prognostic factors for TTP. For patients who achieved MRD-negative after maintenance therapy, compared with those who achieved MRD-negative after induction therapy patients had a shorter TTP. Only age was an independent prognostic factor for OS, but the stage of reaching MRD-negative was not. However, patients who achieved MRD-negative after maintenance therapy had worse OS than those who achieved MRD-negative after induction therapy (Table 3, HR=2.409, P=0.025). For patients with R-ISS I-II, there was no prognosis factor for TTP. Age, creatinine, and HBsAg-positive were independent prognostic factors for OS, and the stage of reaching MRD-negative was not a prognostic factor for patients with R-ISS I-II. However, patients who achieved MRD-negative after maintenance therapy had worse OS than those who achieved MRD-negative after induction therapy (Table 4, HR=2.289, P=0.043). Univariate analysis showed that the stage of reaching MRD-negative was a risk factor for TTP. There was no prognosis factor for OS. Multivariate analysis showed that the stage of reaching MRD-negative was an independent prognostic factor for OS (P=0.039). Patients who achieved MRD-negative after maintenance therapy had worse OS than those who achieved MRD-negative after induction therapy (Table 5, HR=4.922, P=0.044).
The detection of MRD has emerged as a significant tool in the management of MM since it has become viewed as highly important for evaluating the response and is strongly associated with PFS and OS (2, 6). However, it is not clear whether there are differences in clinical features and prognosis after reaching an MRD-negative status at different stages.
This study showed that for MM patients who received ASCT, the prognoses of those who achieved an MRD-negative status at different stages were different, and those who achieved an MRD-negative status after induction therapy had a better prognosis than those who achieved MRD-negative after maintenance. In most previous studies, MRD was detected 3 months after ASCT, and the test results were used as the basis for the efficacy evaluation and prognosis prediction (4, 7, 14). However, our research shows that there are differences in the prognoses of patients who achieve an MRD-negative status at different stages. This may be because patients who achieve an MRD-negative status in the initial stage are more sensitive to chemotherapeutics, and subsequent long-term treatment can fully consolidate the therapeutic effect (15). Multivariate analysis showed that in all enrolled patients, the stage of reaching MRD-negative was an independent prognostic factor for TTP, rather than a prognostic factor for OS. In R-ISS I-II patients, the stage of reaching MRD-negative does not predict prognosis. The stage of reaching MRD-negative in patients with R-ISS III was an independent prognostic factor for OS. Although this is not an independent prognostic factor for TTP but the patients who achieved MRD-negative after maintenance therapy had worse TTP. We found that, as in the previous Kaplan-Meier group comparison, patients who achieved MRD-negative after maintenance therapy appeared to have a worse prognosis than those who had MRD-negative after induction therapy.
In patients with high-risk cytogenetics, the stage at which an MRD-negative status is achieved does not affect the prognosis, and there is no difference in patients with standard-risk cytogenetics. In patients with t(4;14), t(14;16), and 17p-, there was no difference in the prognosis of those who achieved an MRD-negative status at different stages. We believe that under the conditions of this study, for patients with high-risk cytogenetics, regardless of the stage at which an MRD-negative is achieved, there is no longer a significant difference in OS. However, it seems that for patients with high-risk cytogenetics, there are certain differences in TTP among those who achieve an MRD-negative status in different stages (P=0.060). This may be because an MRD-negative status is a prognostic factor independent of cytogenetics (14). At present, studies have reported that an MRD-negative status can overcome the prognostic significance of poor cytogenetics, but some studies have reported that an MRD-negative status cannot overcome the prognostic impact of poor cytogenetics (7, 16–19). However, there is currently no research discussing whether the stage at which an MRD-negative status is achieved can predict the prognosis of patients with high-risk cytogenetics; therefore, we believe that our conclusions are worthy of further verification with larger-scale data.
In patients with R-ISS I-II, the stage at which an MRD-negative status is achieved cannot predict the prognosis, but in patients with R-ISS stage III, the stage at which an MRD-negative status is achieved can predict the prognosis. Indeed, achieving undetectable MRD may also abrogate some adverse risk factors, such as R-ISS III (20). Patients who achieve an MRD-negative status after induction therapy have a better prognosis, especially compared with patients who achieve an MRD-negative status after maintenance treatment (21). We found that in high-risk cytogenetics patients, reaching an MRD-negative status cannot predict the prognosis, but it can be in R-ISS III patients. This may be because the R-ISS staging system is a new risk stratification algorithm with improved prognostic power compared with the individual chromosomal abnormality parameters (22). Achievement of an MRD-negative status is affected by both tumor burden and cytogenetics (11, 12). We speculate that for patients with R-ISS III, it is possible to improve long-term survival after ASCT by increasing the MRD negative rate before ASCT by adding induction chemotherapy and other methods (15, 23). We are convinced that the time has come to use it to adapt the treatment strategy to a dynamic risk (10, 20). The current guidelines recommend that patients with NDMM eligible for transplantation require 4–6 courses of induction chemotherapy (1). For patients with R-ISS stage III, it is possible to consider increasing the course of chemotherapy to achieve an MRD-negative status before ASCT to obtain a better prognosis. Although the baseline characteristics and treatment regimens of the patients enrolled in this study were inconsistent, multivariate analysis showed that induction therapy and maintenance therapy were never prognostic factors, so we believe that the conclusions of this study are still of some significance.
In previous studies, MRD results at a single time point were mainly used to predict the prognosis of patients, but an increasing number of studies have found that a single-time MRD test result does not necessarily well predict the prognosis of patients (4, 24). The prediction of prognosis requires multiple cycles and long-term monitoring of MRD. Therefore, at present, some studies are devoted to analyzing the prognostic significance of persistent MRD-negative results, and some studies are focusing on the clinical characteristics of MM patients with persistent MRD-positive results but can survive for a long time (13). Recent research has focused on new methods of MRD detection, such as next-generation flow cytometry, NGS, and liquid biopsy, which have attracted much attention (25–28). These new studies mainly improve the detection depth of MRD by upgrading the detection methods, thereby increasing its clinical value (26). We tested MRD with a popular 10-color flow cytometer and found that even if the stage at which an MRD-negative status was achieved was the same, patients who achieved an MRD-negative status at different stages had different prognostic characteristics. We can predict the prognosis of patients early according to the stage at which an MRD-negative status is achieved.
It should be noted that there were only 29 patients with R-ISS III in this study, which is lower than the ratio of R-ISS III in the population of MM patients because only patients with MRD-negative can be included in this study. After induction therapy-ASCT-maintenance therapy, it is more difficult for patients with R-ISS III to achieve MRD-negative. However, there were statistical differences in the TTP and OS of these 29 patients, the Chi-square was 15.691 and 7.776, respectively, and the 1-β values were both 100%, so the conclusion of the prognostic analysis in R-ISS III patients was established. However, this study was a retrospective clinical study. The clinical characteristics and the process of treatment in included patients were not very consistent, and the number of cases was limited. The conclusions drawn need to be verified in a larger prospective study.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Clinical Research and Animal Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
QS collected the data, analyzed the data, and drafted the manuscript. XL guided the writing and participated in the revision of the manuscript. JG directed the statistical analysis. BH reviews the original data. JRL and MC review and modify the manuscript. JL designed the study, reviewed the research results, and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (grant number 82070220), Sun Yat-sen University Medical Clinical Trial “5010 Plan” (grant number 2017005), and General Project of the Natural Science Foundation of Guangdong Province (grant number 2021A1515011715).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the staff involved in this research.
1. Kumar SK, Callander NS, Adekola K, Anderson L, Baljevic M, Campagnaro E, et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2020) 18:1685–717. doi: 10.6004/jnccn.2020.0057
2. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol (2016) 17:e328–46. doi: 10.1016/S1470-2045(16)30206-6
3. Paiva B, Vidriales MB, Cervero J, Mateo G, Perez JJ, Montalban MA, et al. Multiparameter Flow Cytometric Remission Is the Most Relevant Prognostic Factor for Multiple Myeloma Patients Who Undergo Autologous Stem Cell Transplantation. Blood (2008) 112:4017–23. doi: 10.1182/blood-2008-05-159624
4. Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE, et al. Minimal Residual Disease Assessed by Multiparameter Flow Cytometry in Multiple Myeloma: Impact on Outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol (2013) 31:2540–7. doi: 10.1200/JCO.2012.46.2119
5. Landgren O, Devlin S, Boulad M, Mailankody S. Role of MRD Status in Relation to Clinical Outcomes in Newly Diagnosed Multiple Myeloma Patients: A Meta-Analysis. Bone Marrow Transplant (2016) 51:1565–8. doi: 10.1038/bmt.2016.222
6. Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-Analysis. JAMA Oncol (2017) 3:28–35. doi: 10.1001/jamaoncol.2016.3160
7. Gupta R, Kumar L, Dahiya M, Mathur N, Harish P, Sharma A, et al. Minimal Residual Disease Evaluation in Autologous Stem Cell Transplantation Recipients With Multiple Myeloma. Leuk Lymphoma (2017) 58:1234–7. doi: 10.1080/10428194.2016.1228930
8. Ferrero S, Ladetto M, Drandi D, Cavallo F, Genuardi E, Urbano M, et al. Long-Term Results of the GIMEMA VEL-03-096 Trial in MM Patients Receiving VTD Consolidation After ASCT: MRD Kinetics' Impact on Survival. Leukemia (2015) 29:689–95. doi: 10.1038/leu.2014.219
9. Korthals M, Sehnke N, Kronenwett R, Bruns I, Mau J, Zohren F, et al. The Level of Minimal Residual Disease in the Bone Marrow of Patients With Multiple Myeloma Before High-Dose Therapy and Autologous Blood Stem Cell Transplantation is an Independent Predictive Parameter. Biol Blood Marrow Transplant (2012) 18:423–31.e3. doi: 10.1016/j.bbmt.2011.07.002
10. Patel DA, Gopalakrishnan R, Engelhardt BG, McArthur E, Sengsayadeth S, Culos KA, et al. Minimal Residual Disease Negativity and Lenalidomide Maintenance Therapy are Associated With Superior Survival Outcomes in Multiple Myeloma. Bone Marrow Transplant (2020) 55:1137–46. doi: 10.1038/s41409-020-0791-y
11. Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, Lenalidomide, Bortezomib, and Dexamethasone for Transplant-Eligible Newly Diagnosed Multiple Myeloma: The GRIFFIN Trial. Blood (2020) 136:936–45. doi: 10.1182/blood.2020005288
12. Goicoechea I, Puig N, Cedena MT, Burgos L, Cordon L, Vidriales MB, et al. Deep MRD Profiling Defines Outcome and Unveils Different Modes of Treatment Resistance in Standard- and High-Risk Myeloma. Blood (2021) 137:49–60. doi: 10.1182/blood.2020006731
13. Gu J, Liu J, Chen M, Huang B, Li J. Longitudinal Flow Cytometry Identified "Minimal Residual Disease" (MRD) Evolution Patterns for Predicting the Prognosis of Patients With Transplant-Eligible Multiple Myeloma. Biol Blood Marrow Transplant (2018) 24:2568–74. doi: 10.1016/j.bbmt.2018.07.040
14. Paiva B, Gutierrez NC, Rosinol L, Vidriales MB, Montalban MA, Martinez-Lopez J, et al. High-Risk Cytogenetics and Persistent Minimal Residual Disease by Multiparameter Flow Cytometry Predict Unsustained Complete Response After Autologous Stem Cell Transplantation in Multiple Myeloma. Blood (2012) 119:687–91. doi: 10.1182/blood-2011-07-370460
15. Xu XS, Moreau P, Usmani SZ, Lonial S, Jakubowiak A, Oriol A, et al. Split First Dose Administration of Intravenous Daratumumab for the Treatment of Multiple Myeloma (MM): Clinical and Population Pharmacokinetic Analyses. Adv Ther (2020) 37:1464–78. doi: 10.1007/s12325-020-01247-8
16. van Rhee F, Giralt S, Barlogie B. The Future of Autologous Stem Cell Transplantation in Myeloma. Blood (2014) 124:328–33. doi: 10.1182/blood-2014-03-561985
17. Li H, Li F, Zhou X, Mei J, Song P, An Z, et al. Achieving Minimal Residual Disease-Negative by Multiparameter Flow Cytometry May Ameliorate a Poor Prognosis in MM Patients With High-Risk Cytogenetics: A Retrospective Single-Center Analysis. Ann Hematol (2019) 98:1185–95. doi: 10.1007/s00277-019-03609-x
18. Kunacheewa C, Lee HC, Patel K, Thomas S, Amini B, Srour S, et al. Minimal Residual Disease Negativity Does Not Overcome Poor Prognosis in High-Risk Multiple Myeloma: A Single-Center Retrospective Study. Clin Lymphoma Myeloma Leuk (2020) 20:e221–38. doi: 10.1016/j.clml.2020.01.001
19. Paiva B, Puig N, Cedena MT, Rosinol L, Cordon L, Vidriales MB, et al. Measurable Residual Disease by Next-Generation Flow Cytometry in Multiple Myeloma. J Clin Oncol (2020) 38:784–92. doi: 10.1200/JCO.19.01231
20. Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal Residual Disease Negativity Using Deep Sequencing Is a Major Prognostic Factor in Multiple Myeloma. Blood (2018) 132:2456–64. doi: 10.1182/blood-2018-06-858613
21. Hu B, Thall P, Milton DR, Sasaki K, Bashir Q, Shah N, et al. High-Risk Myeloma and Minimal Residual Disease Postautologous-HSCT Predict Worse Outcomes. Leuk Lymphoma (2019) 60:442–52. doi: 10.1080/10428194.2018.1485908
22. Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol (2015) 33:2863–9. doi: 10.1200/JCO.2015.61.2267
23. Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab Plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med (2019) 380:2104–15. doi: 10.1056/NEJMoa1817249
24. Paiva B, Puig N, Garcia-Sanz R, San Miguel JF. Is This the Time to Introduce Minimal Residual Disease in Multiple Myeloma Clinical Practice? Clin Cancer Res (2015) 21:2001–8. doi: 10.1158/1078-0432.CCR-14-2841
25. Yao Q, Bai Y, Kumar S, Au E, Orfao A, Chim CS. Minimal Residual Disease Detection by Next-Generation Sequencing in Multiple Myeloma: A Comparison With Real-Time Quantitative PCR. Front Oncol (2020) 10:611021. doi: 10.3389/fonc.2020.611021
26. Medina A, Puig N, Flores-Montero J, Jimenez C, Sarasquete ME, Garcia-Alvarez M, et al. Comparison of Next-Generation Sequencing (NGS) and Next-Generation Flow (NGF) for Minimal Residual Disease (MRD) Assessment in Multiple Myeloma. Blood Cancer J (2020) 10:108. doi: 10.1038/s41408-020-00377-0
27. Vrabel D, Sedlarikova L, Besse L, Rihova L, Bezdekova R, Almasi M, et al. Dynamics of Tumor-Specific cfDNA in Response to Therapy in Multiple Myeloma Patients. Eur J Haematol (2020) 104:190–7. doi: 10.1111/ejh.13358
Keywords: autologous stem cell transplantation, minimal residual disease, multiple myeloma, overall survival, time to progression
Citation: Sun Q, Li X, Gu J, Huang B, Liu J, Chen M and Li J (2022) Prognostic Significance of the Stage at Which an MRD-Negative Status Is Achieved for Patients With Multiple Myeloma Who Received ASCT. Front. Oncol. 12:776920. doi: 10.3389/fonc.2022.776920
Received: 14 September 2021; Accepted: 12 April 2022;
Published: 18 May 2022.
Edited by:
Alberto Orfao, University of Salamanca, SpainCopyright © 2022 Sun, Li, Gu, Huang, Liu, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, MTM3MTkyMDkyNDBAMTYzLmNvbQ==; bGp1YW5AIG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.