- 1Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China
- 2Institute of Cancer and Basic Medicine (IBMC), Chinese Academy of Sciences, Hangzhou, China
Background: Roughly one third of diffuse large B cell lymphoma (DLBCL) patients experience relapsed or refractory disease, and their prognosis is unsatisfactory. It is thus important to identify patients who respond poorly to first-line treatment. Some studies have evaluated the prognostic value of interim PET-CT (iPET-CT) or end-of-treatment PET-CT (ePET-CT) in lymphoma patients, but there have been few studies exploring the prognostic value of metabolic response rates in the evaluation of DLBCL patients.
Methods: Consecutive newly diagnosed DLBCL patients were screened from March 2013 to June 2020. Patients received at least four cycles of chemotherapy, and underwent baseline, iPET-CT and ePET-CT scanning. Kaplan-Meier survival curves with log-rank tests were employed to assess survival outcomes including overall survival (OS) and progression-free survival (PFS). Independent predictors of survival were identified through univariable and multivariable Cox regression analyses.
Results: 307 patients were evaluated. At the time of iPET-CT scanning, 250, 45, and 12 patients exhibited complete response (CR), partial response (PR), and stable disease (SD)/progressive disease (PD), respectively. The percentage of negative iPET-CT was 81.4% (250/307). Among 295 patients with ePET-CT, 262 (88.8%) achieved negativity and 33 (11.2%) exhibited positivity including 26 PR and 7 PD. The 2-year PFS and 2-year OS for patients with iPET-CT positivity were 50.7% and 76.5%, respectively, and were significantly shorter than those for patients with iPET-CT negativity (2-year PFS 82.7%, p<0.001; 2-year OS 94.2%, p<0.001). Patients with ePET-CT positivity had significant poorer 2-year PFS (48.1%) and 2-year OS (78.5%) compared with those ePET-CT negativity (2-year PFS 83.8%, p<0.001; 2-year OS 94.9%, p<0.001). The positivity rates on iPET-CT and ePET-CT evaluation were significantly higher in patients in the high/high-intermediate risk group compared with patients in the low/low-intermediate group. In a multivariable analysis, high/high-intermediate international prognostic index (IPI) and ePET-CT positivity were independently associated with poor PFS and OS.
Conclusions: Our results suggest that the speed of metabolic response to treatment is of limited prognostic value in newly diagnosed DLBCL patients. Patients exhibiting PR at iPET-CT evaluation should carefully consider whether to change chemotherapy regimen.
Introduction
Diffuse large B cell lymphoma (DLBCL) is the prevalent non-Hodgkin lymphoma subtype (1). Roughly 60% of patients with DLBCL can undergo successful curative first-line RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy (2). Unfortunately, one third of patients still experience relapsed or refractory disease (3). Just 30-35% of these relapsed/refractory patients will undergo successful rescue by high-dose chemotherapy following autologous stem-cell transplantation (ASCT) (4). Therefore, further work is needed to efficiently identify patients that respond poorly to first-line therapy so that their chances of cure can be increased by early intensification.
18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) is commonly employed in lymphoma patients for pretreatment staging, therapeutic efficacy evaluation, and transformation assessment (5). Positive end-of-treatment PET-CT (ePET-CT) scans are closely associated with residual/recurrent disease and with worse overall survival (OS) and progression-free survival (PFS) (6). However, the predictive role of the mid-treatment PET-CT remains controversial (7–9). As such, interim PET-CT-(iPET-CT)-guided therapy strategies in DLBCL patients have not been widely accepted to date.
In advanced mantle cell lymphoma (MCL), Jeon et al. suggested that the speed of metabolic response to treatment may be a powerful predictor of individual outcomes (10). It has been hypothesized that DLBCL patients who are rapid metabolic responders, as measured by reductions in the intensity of 18F-FDG uptake, are reflective of early tumor regression with a high likelihood of curative outcomes, whereas slow metabolic responders are more likely to relapse. To test this hypothesis, we conducted the present retrospective analysis to explore the prognostic value of metabolic response rate measured by iPET-CT and ePET-CT, indexed by the Deauville five-point scale, in a cohort of DLBCL patients undergoing treatment with a RCHOP-like regimen.
Materials and Methods
Patients and Study Design
Consecutive newly diagnosed DLBCL patients were screened from March 2013 to June 2020 at Zhejiang Cancer Hospital. The DLBCL diagnosis for these patients was confirmed via pathological review as performed by an independent experienced pathologist. Disease stage was judged according to the criteria of Lugano 2014 (11). First-line treatment consisted of at least four cycles of rituximab-containing anthracycline-based chemotherapy. Patients that completed fewer than four cycles were excluded. All patients underwent baseline whole-body PET-CT scans within four weeks before starting therapy, iPET-CT scans after four cycles of chemotherapy, and ePET-CT scans conducted within eight weeks after the completion of chemotherapy. Responses to chemotherapy were evaluated based upon the revised criteria published by Cheson et al. (12). The Deauville score (DS) was employed for measuring 18F-FDG-uptake in PET-CT (13). A DS 1 to 3 was defined as PET negativity. DS 4 or DS 5 were used to define PET positivity. After completion of first-line chemotherapy, all patients underwent regular follow-up CT scans every 3 months over the first two years, every 6 months for the next three years, and once a year from the sixth year onward. A retrospective analysis of data extracted from patient electronic medical records including demographic information, pathological features, treatment regimens, therapeutic responses to initial or salvage chemotherapy, and survival was performed. The Zhejiang Cancer Hospital ethics committee approved this study, which was consistent with the Declaration of Helsinki.
Data Analysis
PFS was calculated from the start of first-line chemotherapy to the first recording of disease progression or disease relapse or death. OS was defined as the period from the start of first-line chemotherapy to the date of death from any cause or the last follow-up. Categorical variables are given as proportions and were analyzed with chi-squared tests and Fisher’s exact test. Continuous variables are given as medians and ranges. PFS and OS were calculated using the Kaplan–Meier survival method and log-rank tests. Univariable and multivariable Cox regression analyses were performed to determine the independent factors affecting PFS or OS. P <0.05 was the threshold of significance. To further explore exact survival differences, survival time distributions in four groups were compared pairwise. A Bonferroni corrected p-value was applied to the multifactorial logistic regression p–values to account for the multiple testing of six different comparisons (corrected α = 0.05/6 = 0.00833). Statistical analyses were performed with Statistical Package for Social Sciences (SPSS), version 24. Survival curves were drawn with GraphPad Prism 8.
Results
Clinical Characteristics
Initially, 505 total patients diagnosed with DLBCL were identified, of whom 198 were excluded due to ambiguous diagnoses (n=4), fewer than 4 chemotherapy cycles (n=12), or a lack of available iPET-CT or ePET-CT data (n=182). Therefore, 307 patients were analyzed in this study (Figure 1). Patient baseline clinical characteristics are shown in Table 1.
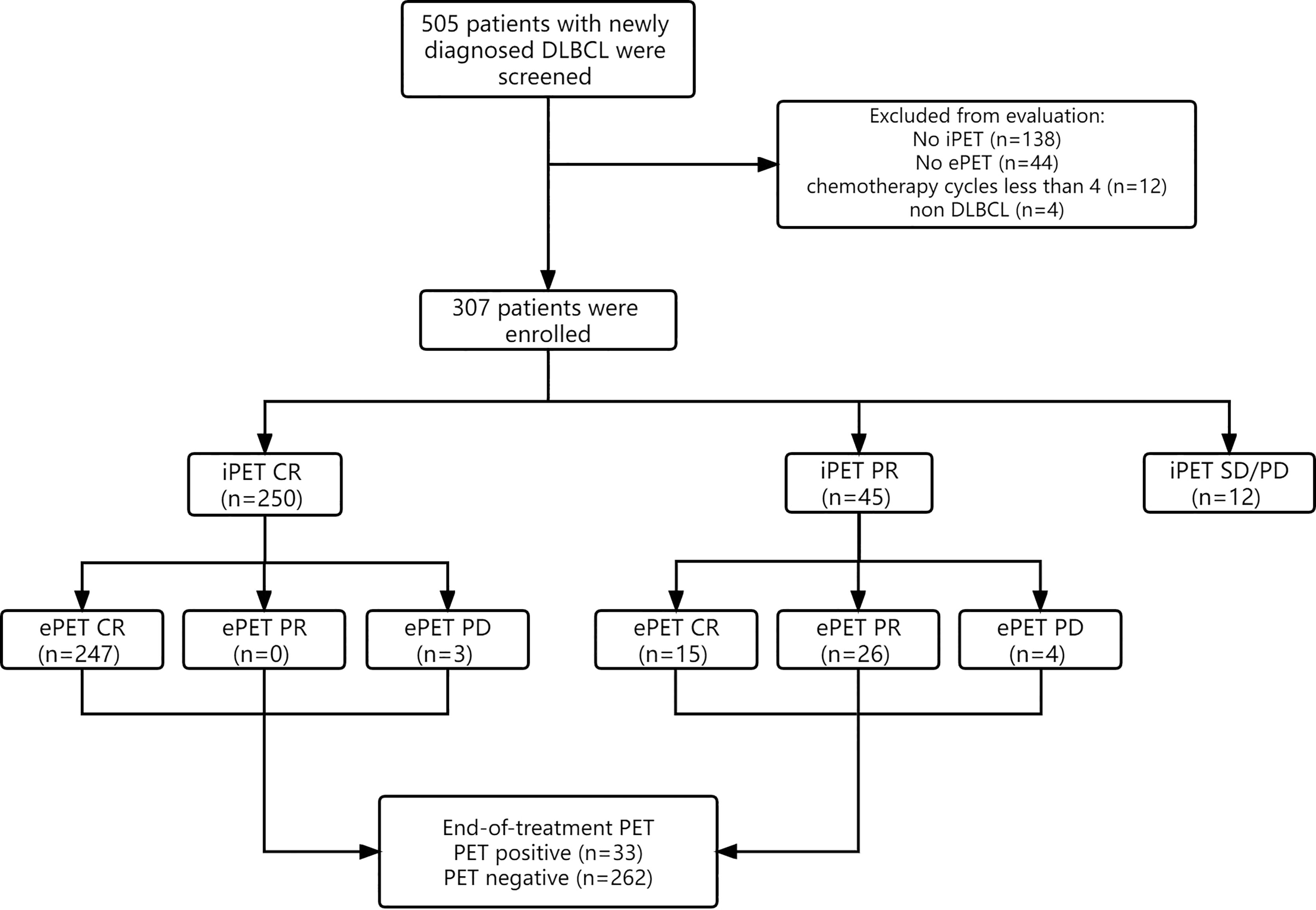
Figure 1 Flow diagram of 505 patients with newly diagnosed DLBCL. 198 patients were excluded from the study, resulting in 307 patients being analyzed. Response evaluation of iPET-CT and ePET-CT were showed.
18F-FDG PET-CT Treatment and Efficacy Evaluation
All 307 patients underwent initial pretreatment PET-CT and iPET-CT scanning (Figure 1). At iPET-CT evaluation, 250 patients achieved complete response (CR) and the proportion of patients with negative metabolic uptake was 81.4% (250/307). Moreover, 45 patients achieved partial response (PR), all of whom continued to complete prior chemotherapy regimens for at least 2 cycles, and 15 of them (33.3%) achieved CR at ePET-CT. Twenty-six patients maintained PR, while 4 patients ultimately exhibited progressive disease (PD). Additionally, 12 patients exhibited SD/PD at iPET-CT, of whom just 3 underwent biopsy and 2 were confirmed to have progressive disease. Of these 12 patients, 10 underwent second-line treatment, while one underwent palliative radiotherapy. The remaining patient did not receive any treatment, and died 5 months later.
At time of ePET-CT evaluation (n=295), 262 patients (88.8%) achieved CR and were considered as negative ePET-CT, whereas 33 patients (11.2%) exhibited ePET-CT positivity, including 26 patients with PR and 7 patients with PD. Among the 26 patients with PR at time of ePET-CT, 10 received second-line chemotherapy and 2 of them underwent subsequent autologous stem-cell transplantation (ASCT) with no evidence of disease. Eight patients received palliative radiotherapy for residual lesions without chemotherapy. Another 8 patients did not receive any treatment, and 7 of them were still alive. All 7 patients with PD at ePET-CT received salvage chemotherapy, but only 3 patients remained alive at last follow-up.
PET-CT-Based Survival Outcomes
After a median follow-up of 45.1 months (range: 5.1 - 100 months), 81 patients (26.4%) experienced disease progression or relapse, and 36 patients (11.7%) were censored due to death. The 2-year PFS rate and 2-year OS rate for the whole cohort (n=307) were 76.6% (95% confidence interval (CI), 71.8 to 81.4%) and 91.0% (95% CI, 87.7 to 94.2%), respectively.
The iPET-CT and ePET-CT results for these patients were both significantly associated with survival outcomes (Figure 2). The 2-year PFS and 2-year OS for patients with iPET-CT positivity were 50.7% (95%CI, 37.6 to 63.8%) and 76.5% (95%CI, 65.3 to 87.7%), respectively, and were significantly shorter than those for patients with iPET-CT negativity (2-year PFS: 82.7% (95% CI, 78 to 87.4%), p<0.001; 2-year OS: 94.2% (95% CI, 91.3 to 97.1%), p<0.001). The survival outcomes for patients with SD/PD at iPET-CT were extremely poor, with median PFS and OS were only 3.2 months and 11.0 months, respectively. Similarly, patients with ePET-CT positivity had a significantly poorer 2-year PFS (48.1%, 95% CI, 30.9 to 65.3%) and 2-year OS (78.5%, 95% CI, 64.4 to 92.6%) rates compared with those of patients with ePET-CT negativity (2-year PFS: 83.8% (95% CI, 79.3 to 88.3%), p<0.001; 2-year OS: 94.9% (95% CI, 92.2 to 97.6%), p<0.001).
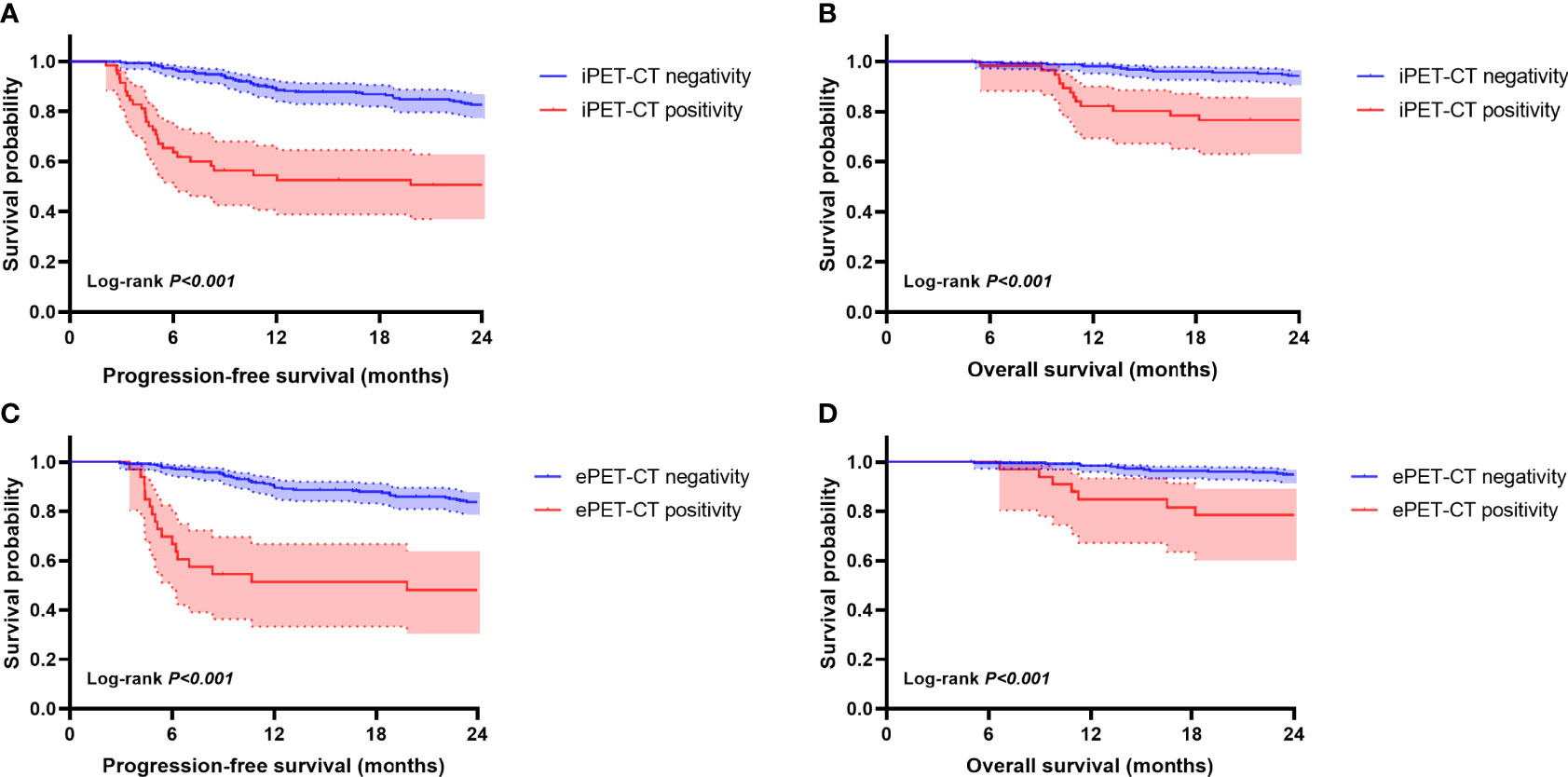
Figure 2 Kaplan-Meier survival curves according to interim PET-CT (iPET-CT) and end-of-treatment (ePET-CT). Progression-free survival (PFS) (A) and overall survival (OS) (B) according to iPET-CT evaluation. PFS (C) and OS (D) according to ePET-CT.
These results suggest that there are significant relationships between PET avidity at different follow-up time points and DLBCL patient survival. In light of these results, we conducted a further examination of the prognostic value of the speed of metabolic response. As patients with SD/PD at iPET-CT began undergoing second-line chemotherapy and lacked available ePET-CT scans, so they were excluded in this section. The remaining 295 patients were divided into the following 4 groups: EMR (early metabolic responders, iPET-CR+ePET-CR, n=247), DMR (delayed metabolic responders, iPET-PR+ePET-CR, n=15), IMR (incomplete metabolic responders, iPET-PR+ePET-PR, n=26), and MP (metabolic progressors, iPET-CR/PR+ePET-PD, n=7). The 2-year PFS rates were significantly different in these four groups (83.7%, 86.2%, 61.1%, and 0%, respectively; p<0.001). The 2-year OS rates were also significantly different in these four groups (94.6%, 100%, 84.3%, and 57.1%, respectively; p<0.001). The survival distribution of the four groups was compared in a pairwise manner. For 2-year PFS rate, there was a significant difference between MP and EMR (p<0.001), DMR (p<0.001), and IMR (p<0.001). There was also a difference between EMR and IMR (p=0.002). Between the other groups, no significant difference was found (p>0.0083). For 2-year OS, there was a difference between MP and EMR (p<0.001), and DMR (p=0.006). No significant difference was found between the other groups (p>0.0083). After Bonferroni correction, results showed a significant prognostic difference between MP and EMR/DMR. In Figure 3, a Kaplan-Meier plot for PFS and OS of the different groups of patients is shown.
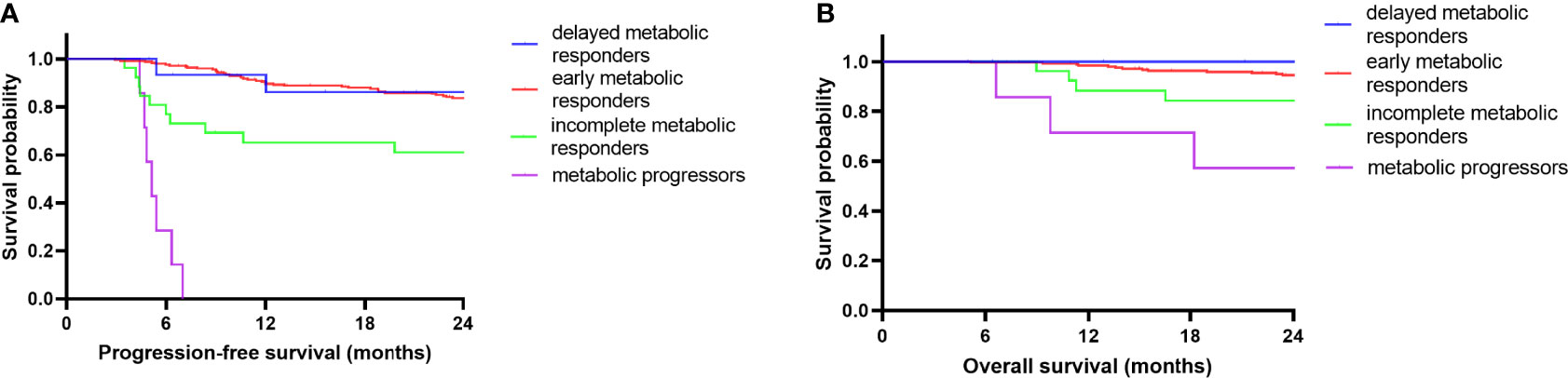
Figure 3 Kaplan-Meier survival curves according to serial changes in PET-CT response. PFS (A) and OS (B) according to early metabolic responders (n=247), delayed metabolic responders (n=15), incomplete metabolic responders (n=26) and metabolic progressors (n=7) during frontline RCHOP.
The iPET-CT and ePET-CT positivity rates in different international prognostic index (IPI) risk groups were significantly different. Overall, 13.9% (29/209) patients with low/low-intermediate risk exhibited iPET-CT positivity, while 29.6% (29/98) patients with high/high-intermediate risk exhibited iPET-CT positivity (p=0.001). Moreover, 8.8% (18/205) patients with low/low-intermediate risk exhibited ePET-CT positivity, while 16.7% (15/90) patients with high/high-intermediate risk exhibited ePET-CT positivity (p=0.048). Additionally, bulky nodes (> 5 cm) and elevated serum C reactive protein (CRP) were more common in patients with positive iPET-CT (28.2% vs 15.7%, p=0.015; 26.1% vs 14.6%, p=0.013).
Analysis of Prognostic Factors Associated With Patient Survival Outcomes
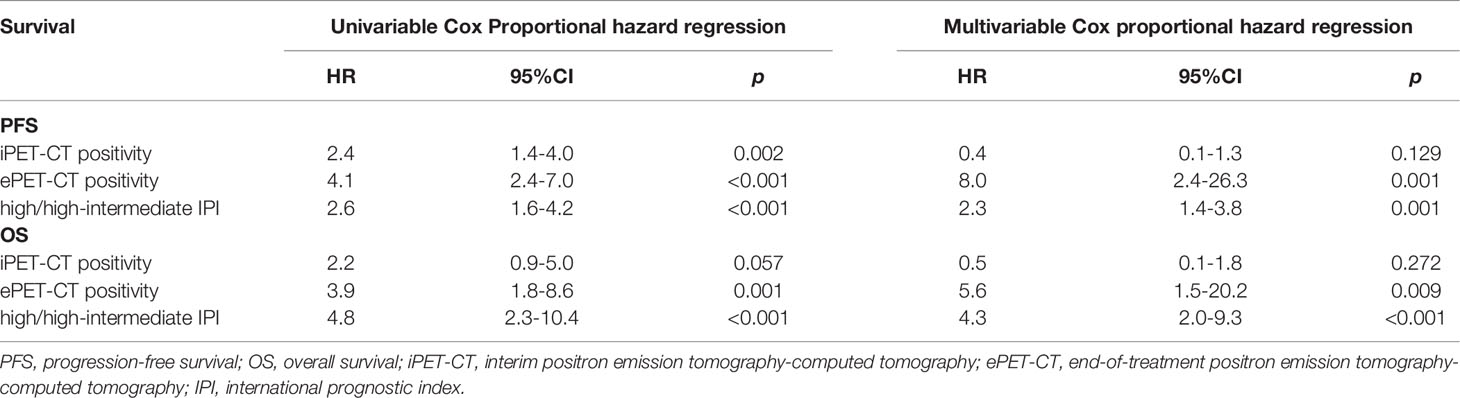
Factors including iPET-CT (positivity vs negativity), ePET-CT (positivity vs negativity) and IPI (high/high-intermediate vs low/low-intermediate) were analyzed in univariable and multivariable analysis for potential significance in terms of PFS and OS. In univariable analysis, positive iPET-CT, positive ePET-CT and high/high-intermediate IPI were all associated with inferior PFS and latter two factors were also associated with inferior OS. In multivariable analysis, positive ePET-CT and high/high-intermediate IPI were independent prognostic factors for poor PFS and OS (Table 2).
Discussion
In this cohort of 307 newly diagnosed DLBCL patients undergoing first-line rituximab-containing anthracycline-based chemotherapy treatment, the 2-year PFS and OS were 76.6% and 91.0%, respectively, in line with previous reports (14, 15).
Our study had several important findings. First, 81.4% (250/307) of patients achieved negative iPET-CT, of whom 98.8% (247/250) maintained CR after the completion of chemotherapy. These early metabolic responders had excellent survival outcomes, with a 2-year PFS of 83.7% and a 2-year OS of 94.6%. Second, only approximately 3.9% (12/307) of patients exhibited rapid disease progression and were considered as SD/PD at iPET-CT. The survival outcomes for these patients were poor, with median PFS and OS of just 3.2 months and 11.0 months, respectively. Third, although patients achieved negative iPET-CT findings, about 1.2% (3/250) of them still exhibited new metabolic lesions at ePET-CT. The survival outcomes of these 3 patients were poor. Intriguingly, among patients with PR at iPET-CT, 33.3% (15/45) of patients achieved CR at the end of chemotherapy. These delayed metabolic responders exhibited durable remission outcomes similar to those of early metabolic responders. Multivariable analyses further confirmed that ePET-CT positivity, but not iPET-CT positivity, was independently associated with patient prognosis. In summary, our study failed to confirm the hypothesis that there is a survival difference between early metabolic responders and delayed metabolic responders when evaluating DLBCL patients. These findings also indicate that the intensification of treatment regimens based upon iPET-CT positivity would likely expose many patients to the risk of unnecessary treatment.
A delayed metabolic response group has been noted in a few previous studies (10, 16, 17). A large, multinational, prospective study analyzed survival of patients with different metabolic response rates and found that 192 of 312 (62%) patients had negative iPET-CT and ePET-CT findings consistent with a rapid response, with a 2-year EFS of 97% and a 2-year OS of 97%. Moreover, 58 of 107 (54%) patients with positive iPET-CT findings achieved CR at ePET-CT, with an EFS of 86% and OS of 92%. The remaining 49 (16%) cases with positive iPET-CT and ePET-CT findings had a 2-year EFS of 35% and continuing relapses beyond 2 years. The delayed metabolic responders had approximately double the risk of 2-year relapse compared with early metabolic responders (18). Therefore, serial PET scans are important tools for the evaluation of lymphoma patients.
One possible explanation for delayed metabolic response is false-positive PET-CT results. Persistent 18F-FDG uptake can be indicative not only of residual lymphoma lesions but also of inflammatory reactions within necrotic tumor tissue (19). Such false positivity is more common in areas exposed to rituximab treatment (20). According to previous reports, the positive predictive value of iPET-CT ranged from 18% to 74% (16, 17, 21–23). This indicates that a single iPET-CT scan offers limited value as a means of identifying patients with poor outcomes. In addition, in patients exhibiting persistent FDG uptake in only one locus or the appearance of FDG uptake in a previously non-avid site, unrelated secondary neoplasms should be excluded (20). Particularly in cases of highly metabolically active PET-CT lesions within 1.5 cm in diameter, contrast-enhanced CT scans are important to exclude lymphoma lesions. Unfortunately, in this study, only a small number of patients with positive iPET-CT/ePET-CT findings underwent biopsy to confirm the presence of lymphoma and rule out potential secondary neoplasms.
Different criteria for the interpretation of PET results have certain limitations. The Deauville criteria, which is a visual assessment method, has been recommended by international guidelines and adopted for current clinical practice throughout the globe. In the present study, Deauville scores of 1-3 were considered as CR and PET negativity. But some patients with high Deauville scores could still achieve long survival time. As such, other semi-quantitative response assessment methods, including International Harmonization Project (24), Gallamini criteria (25), △SUVmax (26) and SUVmax-liver-based interpretation (27) can be used for response evaluation in patients with DLBCL.
There are certain limitations to this analysis that warrant consideration when interpreting these results. For one, this was a retrospective, single-center study without any prospective surveillance, and so these results may have been influenced by biases and other confounding variables. Secondly, this study excluded patients that only underwent CT scanning in order to focus on patients that had undergone iPET-CT and ePET-CT, thereby introducing selection bias. For survival analysis, we excluded patients with SD/PD at iPET-CT. The selection bias might influence the final survival outcome. Lastly, in most cases, disease progression was diagnosed in these patients based on imaging findings rather than biopsy results.
Conclusions
Our results suggest that the speed of metabolic response to treatment offers limited prognostic value in newly diagnosed DLBCL patients. Patients exhibiting PR at iPET-CT evaluation should carefully consider whether to change chemotherapy regimen.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The Zhejiang Cancer Hospital ethics committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HYY, CL, and HFY participated in study design, evaluated the results, wrote the first and revised manuscript. CL performed the statistical analyses. SYH supervised patient care and collected data. CL, TL, HFY, XC, SP, and SH contributed to patient care and collected clinical information. All authors critically revised the manuscript and have approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All colleagues contributing to the manuscript have been listed as authors.
References
1. Vaidya R, Witzig TE. Prognostic Factors for Diffuse Large B-Cell Lymphoma in the R(X)CHOP Era. Ann Oncol Off J Eur Soc Med Oncol (2014) 25:2124–33. doi: 10.1093/annonc/mdu109
2. Flowers CR, Sinha R, Vose JM. Improving Outcomes for Patients With Diffuse Large B-Cell Lymphoma. CA Cancer J Clin (2010) 60:393–408. doi: 10.3322/caac.20087
3. Friedberg JW. Relapsed/refractory Diffuse Large B-Cell Lymphoma. Hematol Am Soc Hematol Educ Progr (2011) 2011:498–505. doi: 10.1182/asheducation-2011.1.498
4. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage Regimens With Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28:4184–90. doi: 10.1200/JCO.2010.28.1618
5. Barrington SF, Johnson PWM. (18)F-FDG PET/CT in Lymphoma: Has Imaging-Directed Personalized Medicine Become a Reality? J Nucl Med (2017) 58:1539–44. doi: 10.2967/jnumed.116.181347
6. El-Galaly TC, Villa D, Gormsen LC, Baech J, Lo A, Cheah CY. FDG-PET/CT in the Management of Lymphomas: Current Status and Future Directions. J Intern Med (2018) 284:358–76. doi: 10.1111/joim.12813
7. Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M, et al. Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography for Interim Response Assessment of Advanced-Stage Hodgkin’s Lymphoma and Diffuse Large B-Cell Lymphoma: A Systematic Review. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27:1906–14. doi: 10.1200/JCO.2008.16.0861
8. Horning SJ, Juweid ME, Schöder H, Wiseman G, McMillan A, Swinnen LJ, et al. Interim Positron Emission Tomography Scans in Diffuse Large B-Cell Lymphoma: An Independent Expert Nuclear Medicine Evaluation of the Eastern Cooperative Oncology Group E3404 Study. Blood (2010) 115:775–7; quiz 918. doi: 10.1182/blood-2009-08-234351
9. Haioun C, Itti E, Rahmouni A, Brice P, Rain J-D, Belhadj K, et al. [18F]Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography (FDG-PET) in Aggressive Lymphoma: An Early Prognostic Tool for Predicting Patient Outcome. Blood (2005) 106:1376–81. doi: 10.1182/blood-2005-01-0272
10. Jeon Y-W OJ-H, Park K-S, Min GJ, Park S-S, Yoon J-H, Eom K-S, et al. Prognostic Impact of Interim Positron Emission Tomography in Mantle Cell Lymphoma Patients Treated With Frontline R-CHOP. Br J Haematol (2020) 188:860–71. doi: 10.1111/bjh.16257
11. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol Off J Am Soc Clin Oncol (2014) 32:3059–68. doi: 10.1200/JCO.2013.54.8800
12. Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano Classification Lymphoma Response Criteria in the Era of Immunomodulatory Therapy. Blood (2016) 128:2489–96. doi: 10.1182/blood-2016-05-718528
13. Meignan M, Gallamini A, Itti E, Barrington S, Haioun C, Polliack A. Report on the Third International Workshop on Interim Positron Emission Tomography in Lymphoma Held in Menton, France, 26-27 September 2011 and Menton 2011 Consensus. Leuk Lymphoma (2012) 53:1876–81. doi: 10.3109/10428194.2012.677535
14. Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone in Patients With Newly Diagnosed Diffuse Large B-Cell non-Hodgkin Lymphoma: A Phase 3 Comparison of Dose Intensification With 14-Day Versus 21-Day Cycles. Lancet (London England) (2013) 381:1817–26. doi: 10.1016/S0140-6736(13)60313-X
15. Ennishi D, Asai H, Maeda Y, Shinagawa K, Ikeda K, Yokoyama M, et al. Statin-Independent Prognosis of Patients With Diffuse Large B-Cell Lymphoma Receiving Rituximab Plus CHOP Therapy. Ann Oncol Off J Eur Soc Med Oncol (2010) 21:1217–21. doi: 10.1093/annonc/mdp490
16. Pregno P, Chiappella A, Bellò M, Botto B, Ferrero S, Franceschetti S, et al. Interim 18-FDG-PET/CT Failed to Predict the Outcome in Diffuse Large B-Cell Lymphoma Patients Treated at the Diagnosis With Rituximab-CHOP. Blood (2012) 119:2066–73. doi: 10.1182/blood-2011-06-359943
17. Yoo C, Lee DH, Kim JE, Jo J, Yoon DH, Sohn BS, et al. Limited Role of Interim PET/CT in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. Ann Hematol (2011) 90:797–802. doi: 10.1007/s00277-010-1135-6
18. Carr R, Fanti S, Paez D, Cerci J, Györke T, Redondo F, et al. Prospective International Cohort Study Demonstrates Inability of Interim PET to Predict Treatment Failure in Diffuse Large B-Cell Lymphoma. J Nucl Med (2014) 55:1936–44. doi: 10.2967/jnumed.114.145326
19. Spaepen K, Stroobants S, Dupont P, Bormans G, Balzarini J, Verhoef G, et al. [(18)F]FDG PET Monitoring of Tumour Response to Chemotherapy: Does [(18)F]FDG Uptake Correlate With the Viable Tumour Cell Fraction? Eur J Nucl Med Mol Imaging (2003) 30:682–8. doi: 10.1007/s00259-003-1120-6
20. Han HS, Escalón MP, Hsiao B, Serafini A, Lossos IS. High Incidence of False-Positive PET Scans in Patients With Aggressive non-Hodgkin’s Lymphoma Treated With Rituximab-Containing Regimens. Ann Oncol Off J Eur Soc Med Oncol (2009) 20:309–18. doi: 10.1093/annonc/mdn629
21. Zinzani PL, Gandolfi L, Broccoli A, Argnani L, Fanti S, Pellegrini C, et al. Midtreatment 18F-Fluorodeoxyglucose Positron-Emission Tomography in Aggressive non-Hodgkin Lymphoma. Cancer (2011) 117:1010–8. doi: 10.1002/cncr.25579
22. Safar V, Dupuis J, Itti E, Jardin F, Fruchart C, Bardet S, et al. Interim [18F]Fluorodeoxyglucose Positron Emission Tomography Scan in Diffuse Large B-Cell Lymphoma Treated With Anthracycline-Based Chemotherapy Plus Rituximab. J Clin Oncol Off J Am Soc Clin Oncol (2012) 30:184–90. doi: 10.1200/JCO.2011.38.2648
23. Yang D-H, Ahn J-S, Byun BH, Min JJ, Kweon S-S, Chae YS, et al. Interim PET/CT-Based Prognostic Model for the Treatment of Diffuse Large B Cell Lymphoma in the Post-Rituximab Era. Ann Hematol (2013) 92:471–9. doi: 10.1007/s00277-012-1640-x
24. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of Positron Emission Tomography for Response Assessment of Lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol Off J Am Soc Clin Oncol (2007) 25:571–8. doi: 10.1200/JCO.2006.08.2305
25. Manohar K, Mittal BR, Raja S, Bhattacharya A, Malhotra P, Varma S. Comparison of Various Criteria in Interpreting End of Therapy F-18 Labeled Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients With Aggressive non-Hodgkin Lymphoma. Leuk Lymphoma (2013) 54:714–9. doi: 10.3109/10428194.2012.717693
26. Yang D-H, Min J-J, Song H-C, Jeong YY, Chung W-K, Bae S-Y, et al. Prognostic Significance of Interim 18F-FDG PET/CT After Three or Four Cycles of R-CHOP Chemotherapy in the Treatment of Diffuse Large B-Cell Lymphoma. Eur J Cancer (2011) 47:1312–8. doi: 10.1016/j.ejca.2010.12.027
27. Fan Y, Zhang Y, Yang Z, Ying Z, Zhou N, Liu C, et al. Evaluating Early Interim Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography With the SUV(max-Liver)-Based Interpretation for Predicting the Outcome in Diffuse Large B-Cell Lymphoma. Leuk Lymphoma (2017) 58:1–9. doi: 10.1080/10428194.2016.1277384
Keywords: diffuse large B cell lymphoma (DLBCL), interim 18 F-FDG PET, prognosis, RCHOP, treatment response
Citation: Li C, Yu H, Chen X, Han S, Peng S, Lei T and Yang H (2022) The Prognostic Utility of 18F-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography-Based Analyses of Metabolic Response Rates in Newly Diagnosed Diffuse Large B Cell Lymphoma Patients. Front. Oncol. 12:772773. doi: 10.3389/fonc.2022.772773
Received: 08 September 2021; Accepted: 22 April 2022;
Published: 23 May 2022.
Edited by:
Claudia Vener, University of Milan, ItalyReviewed by:
Monica Balzarotti, Humanitas Research Hospital, ItalyYi Miao, Nanjing Medical University, China
Copyright © 2022 Li, Yu, Chen, Han, Peng, Lei and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Yang, eWFuZ2h5QHpqY2Mub3JnLmNu
 Cong Li
Cong Li Haifeng Yu1,2
Haifeng Yu1,2