- Cancer Center, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Background: Cellular immunotherapy has become a new and promising treatment for patients with liver tumor. However, as most immune cells are delivered by intravenous injection, the effect is limited and is likely to produce systemic toxicity. Here, the objective was to investigate the efficacy and safety of cellular immunotherapy by local infusion, which seems to be a promising approach and has not been well-studied.
Methods: The PubMed, Web of Science, Embase, and Cochrane Library databases were searched to obtain literature. The overall response rate (ORR), overall survival (OS) rates, and adverse events were investigated to evaluate the effectiveness and safety of locoregional therapy. The methodological quality of the articles was assessed using the methodological index for non-randomized studies (MINORS) score. The meta-analysis was performed using Stata 15.0.
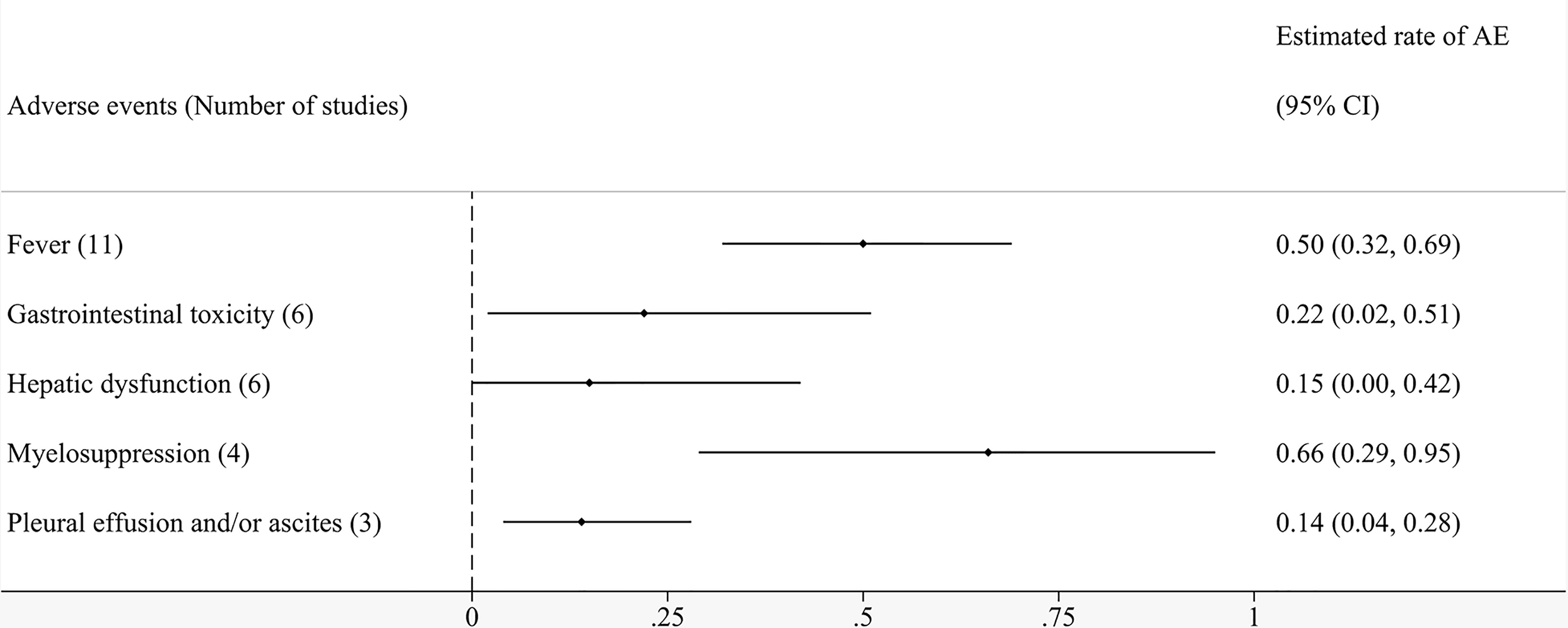
Results: The eligible 17 studies involved a total of 318 patients. The random-effects model demonstrated that the ORR of local cell infusion therapy was 48% (95% confidence interval [CI]: 26%–70%). The pooled OS rate was 94% (95% CI: 83%–100%) at 6 months, 87% (95% CI: 74%–96%) at 12 months, and 42% (95% CI: 16%–70%) at 24 months. Subgroup analyses suggested that minimally invasive treatment and absence of metastasis were significantly associated with better ORR. Fourteen studies reported a variety of adverse events related to cell therapy by local perfusion. The most common complications after regional infusion of immune cells were myelosuppression (66%), fever (50%), gastrointestinal toxicity (22%), hepatic dysfunction (15%), and pleural effusion and/or ascites (14%).
Conclusions: Immune cell therapy through local perfusion is effective for patients with liver cancer, with manageable toxicity. It demonstrates better prognosis when combined with minimally invasive therapy. Considering the potential limitations, more randomized controlled trials are needed to provide solid evidence for our findings.
Introduction
Liver cancer, including primary liver cancer and liver metastasis, ranks sixth among the most common cancers in the world, and is recognized as the fourth leading cause of cancer-related death (1). Progression and recurrence after treatment are the principal reasons for the poor prognosis of patients with hepatocellular carcinoma (HCC), which accounts for 75%–85% of primary liver cancer. Meanwhile, the liver has been identified as a common site of metastasis, and the presence of liver metastasis is usually associated with lower survival rates (2). As patients are often diagnosed with liver cancer in the advanced stages, only limited treatment options are available, and the outcome fails to live up to expectations in most cases (3). On one hand, surgery, ablation, and arterial embolization are therapies with a locoregional scope of targeting lesions, which probably leads to local changes in tumor biology and contributes to postoperative recurrence to some extent (4, 5). On the other hand, chemotherapy and radiotherapy easily bring about resistance through different mechanisms (6–9). In addition, the epidemiology of hepatocellular carcinoma is gradually changing over the past dozen years. For example, HCC patients are older and metabolic disorders have become a growing cause of liver cancer (10). Changes in etiology and clinical features complicates the management of liver cancer. Conventional treatments are not sufficient to satisfy the demands of individualized cancer therapy. Accordingly, there is an urgent need to develop new methods to aid liver cancer treatment and enhance the survival benefit for patients.
With the development of molecular biology and tumor immunology, cellular immunotherapy, a new and promising therapeutic strategy, has been promoted in recent years. Immune cells are isolated from blood and cultured in vitro. Based on the principles of immunology, these processed immune cells with antitumor properties can be injected into patients to kill tumor cells directly or indirectly. Previous studies have mainly involved lymphokine-activated killer (LAK) cells, cytokine-induced killer (CIK) cells, natural killer (NK) cells, dendritic cells (DC), tumor-infiltrating lymphocytes (TIL), chimeric antigen receptor (CAR)-engineered T (CAR-T) cells, CAR-engineered NK (CAR-NK) cells, and T cell receptor (TCR)-engineered T (TCR-T) cells. LAK cells and CIK cells are activated lymphocytes with direct killing effect. NK cells are major part of the innate immune response against viruses and tumors. By presenting antigens, DCs induce innate and adaptive immune responses. TILs are isolated and expanded ex vivo from lymphocytes that have infiltrated tumors. CAR and TCR gene engineered immune cells can specifically recognize tumor antigens by gene modifications. Cellular immunotherapy can strengthen the immune state of the body, alter the tumor immune microenvironment, and provide potential assistance in improving the therapeutic effect (11). Several studies and meta-analyses have shown that the combination of cellular immunotherapy and conventional therapy can have a longer-term and more stable anti-tumor effect (12, 13). However, most of the studies involve intravenous therapy, with limited efficacy and a tendency to generate systemic toxicity.
In this regard, some experts have proposed the idea of local infusion and have made relevant attempts. An animal experiment showed that a high concentration of LAK cells can be obtained in the first capillary bed 2 hours after local infusion, prompting the idea that local delivery of LAK cells may be more effective for tumor (14). This conclusion holds true for other cell therapy. The route of cell administration is an important factor for determining the biological distribution pattern of these cells, so it must be considered in the study of cellular immunotherapy. The typical methods for local infusion include transarterial therapies, such as transcatheter arterial embolization (TAE), hepatic arterial infusion chemotherapy (HAIC), transarterial chemoembolization (TACE), and transarterial radioembolization (TARE). As mature methods for locoregional treatment, they can selectively deliver drugs or radiation to the tumor site (15). The same should be true of immune cells. Theoretically, local infusion of immune cells can compensate for the disadvantage of the poor specificity of tumor antigens and avoid the systemic toxicity associated with intravenous infusion. However, there has been no systematic analysis of the efficacy and safety of cellular immunotherapy by local transfusion. The application value of immunocyte therapy with regional perfusion, such as via the hepatic artery or portal vein, remains controversial.
Therefore, we have summarized various cellular immunotherapy trials and have systematically evaluated the efficacy and safety of local perfusion with immune cells. We look forward to providing an objective foundation and meaningful reference for the clinical application of this treatment.
Methods
This systematic review and meta-analysis has been carried out according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16).
Search Strategy
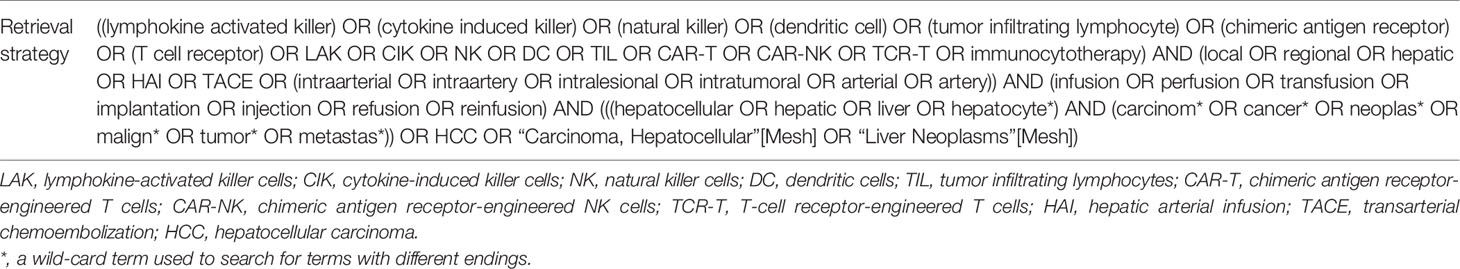
PubMed, Web of Science, Embase and the Cochrane Library were searched comprehensively to obtain the literatures related to cellular immunotherapy by local infusion for liver tumor. The retrieval was performed by two researchers respectively and disagreements were resolved by a third expert. Subject words and free terms were combined for searching. There was a subtle tweak on retrieval language towards different databases. The search period was set dating from January 1, 1990 to March 8, 2021. Taking PubMed as an example, the specific retrieval strategy has been shown in Table 1. The detailed search strategies for other databases are presented in Supplementary Methods. Only the published literatures were included. If necessary, authors would be contacted for additional information.
Inclusion and Exclusion Criteria
The studies included in the review should meet the following criteria (1): patients were diagnosed with liver tumor (including primary liver cancer and liver metastasis) (2); immune cells applied included lymphokine-activated killer (LAK) cells, cytokine-induced killer (CIK) cells, natural killer (NK) cells, dendritic cells (DC), tumor-infiltrating lymphocytes (TIL), chimeric antigen receptor (CAR)-engineered T (CAR-T) cells, CAR-engineered NK (CAR-NK) cells, T-cell receptor (TCR)-engineered T (TCR-T) cells, and so on (3); immunocytotherapy was conducted via local infusion (hepatic artery, portal vein, or intratumoral injection) (4); at least one of the following outcomes should be provided, including overall response rate (ORR), overall survival (OS) rates, and adverse events. Age and gender of the patients and concurrent combination therapy were not limited.
The exclusion criteria are as follows (1): duplicate articles or data (2); irrelevant studies, animal studies, case reports, reviews, and conference papers (3); immune cells were injected only through systemic intravenous or intradermal infusion (4); efficacy and safety were not mentioned (5); number of cases ≤ 8 (6); researches with incomplete or missing data (7); language barriers.
Data Extraction
Two authors independently performed data extraction. With title and abstract browsed, the literatures were preliminarily screened in compliance with the inclusion and exclusion criteria. Obviously unrelated articles would be excluded. Then the second screening was carried out by reading the full text to determine whether it was finally eligible. The extracted information included first author, published year, country, study design, number, age and gender of participants, neoplasm types, classes of cells injected, location of infusion, combination therapy, frequency of cell infusion, duration of treatment, follow up time, and outcomes. The primary outcome was effectiveness. Response rate and survival rate were used to evaluate the efficacy and overall response rate (ORR) was defined as sum of complete and partial response in this study. The secondary outcomes was safety, consisted of types of adverse events and their rates, as well as treatment-related mortality.
Quality Evaluation
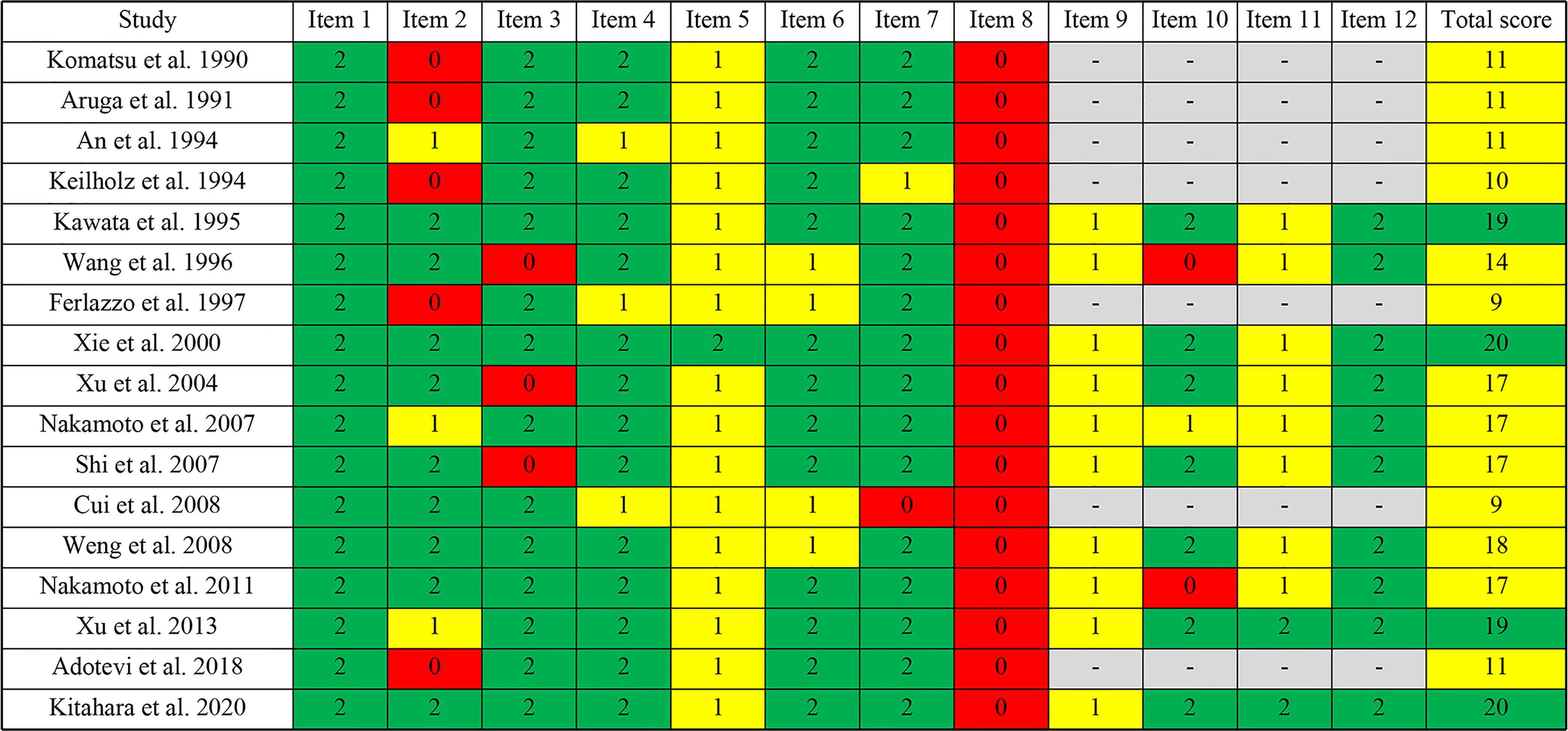
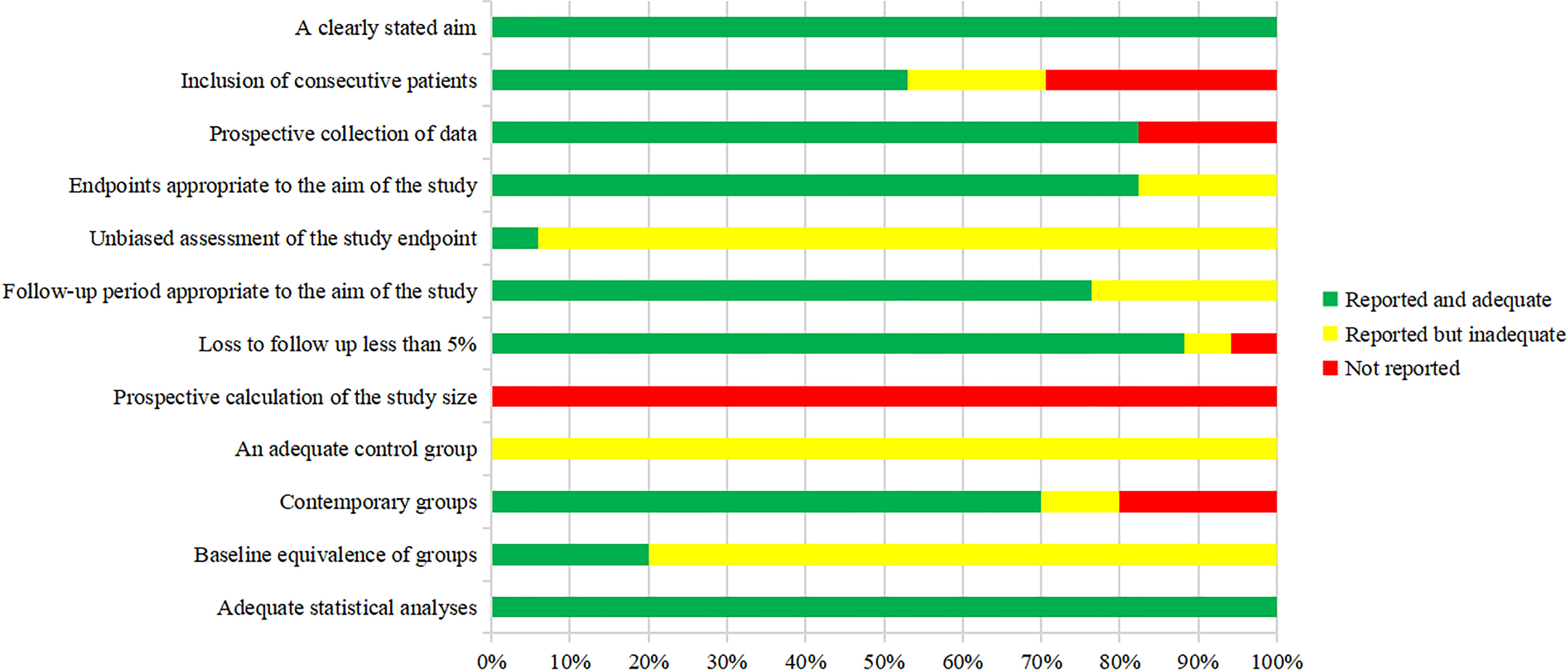
The methodological index for non-randomized studies (MINORS) score was used by two authors independently to evaluated the methodological quality of the articles (17). The MINORS tool is composed of eight items for non-comparative studies and four additional items for comparative studies. Items are scored as 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). The version with a total score of 16 was provided for non-comparative studies while 24 for comparative studies. In this review, ≤8 was considered poor quality, 9−12 moderate quality, and ≥13 good quality for non-comparative studies. For comparative studies, ≤12 was considered poor quality, 13−18 moderate quality, and ≥19 good quality. Items 1 to 12 are as follows (1): a clearly stated aim (2); inclusion of consecutive patients (3); prospective collection of data (4); endpoints appropriate to the aim of the study (5); unbiased assessment of the study endpoint (6); follow-up period appropriate to the aim of the study (7); loss to follow up less than 5% (8); prospective calculation of the study size (9); an adequate control group (10); contemporary groups (11); baseline equivalence of groups (12); adequate statistical analyses.
Statistical Analysis
Stata 15.0 was used for meta-analysis of single group rate. The pooled effect size (ES) and 95% confidence interval (CI) were constructed using fixed-effect or random-effects meta-analysis. After data processing, the heterogeneity test was carried out by Q test and I2 statistic. If I2 < 25%, it was judged that the heterogeneity was small; if 25% < I2 < 50%, it was judged moderate heterogeneity; if I2 > 50%, then substantial heterogeneity. When there was statistical homogeneity among the studies (P > 0.10, I2 < 25%), the fixed-effect model was selected for analysis; when there was statistical heterogeneity among the studies (P < 0.10, I2 > 25%), the random-effects model was used for analysis. If sufficient data were available, we performed subgroup analyses. Sensitivity analysis was performed to evaluate the influences of individual studies on the pooled effect sizes. Funnel plot and Egger’s test were used to examine publication bias. P < 0.05 indicated that the results were of statistical significance.
Results
Study Selection and Search Results
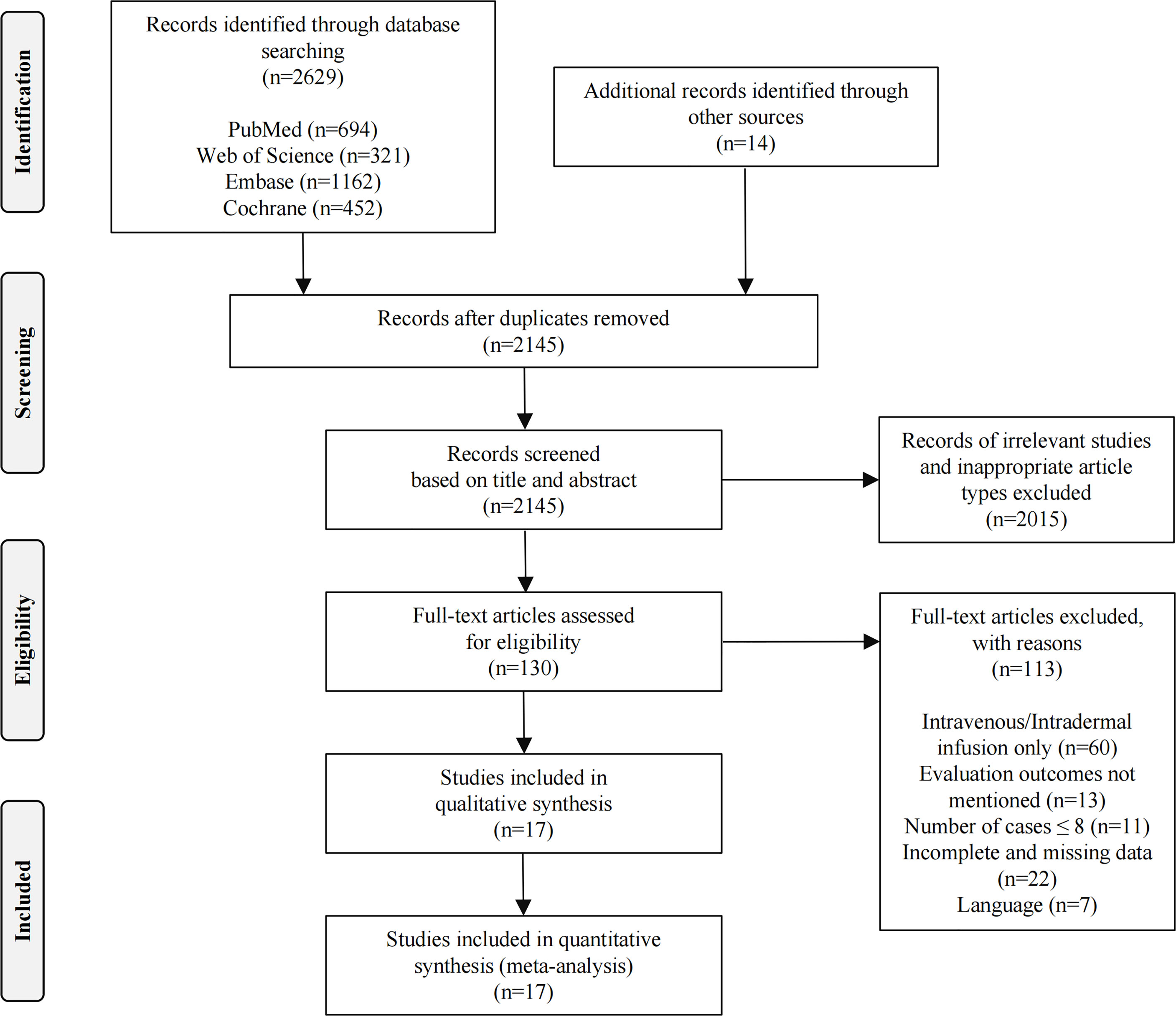
Initially, 2643 related studies were retrieved (694 articles in PubMed, 321 articles in Web of Science, 1162 articles in Embase, 452 articles in the Cochrane Library, and 14 articles from other sources). After eliminating 498 duplicate studies, a total of 2145 articles remained. Title and abstract screening resulted in the exclusion of 1880 unrelated studies, 93 animal studies, 11 case reports, 22 reviews, and nine conference papers, leaving 130 articles for full-text analysis. Through full-text review, we removed 113 studies: 60 were not in accordance with the infusion method studied, 13 had no evaluation endpoints, 11 had ≤8 patients, 22 had incomplete or missing data, and 7 had language barriers. Ultimately, 17 articles were considered eligible for further study (18−34). Figure 1 presents the flow diagram of the search results according to PRISMA guidelines.
Characteristics of the Included Studies
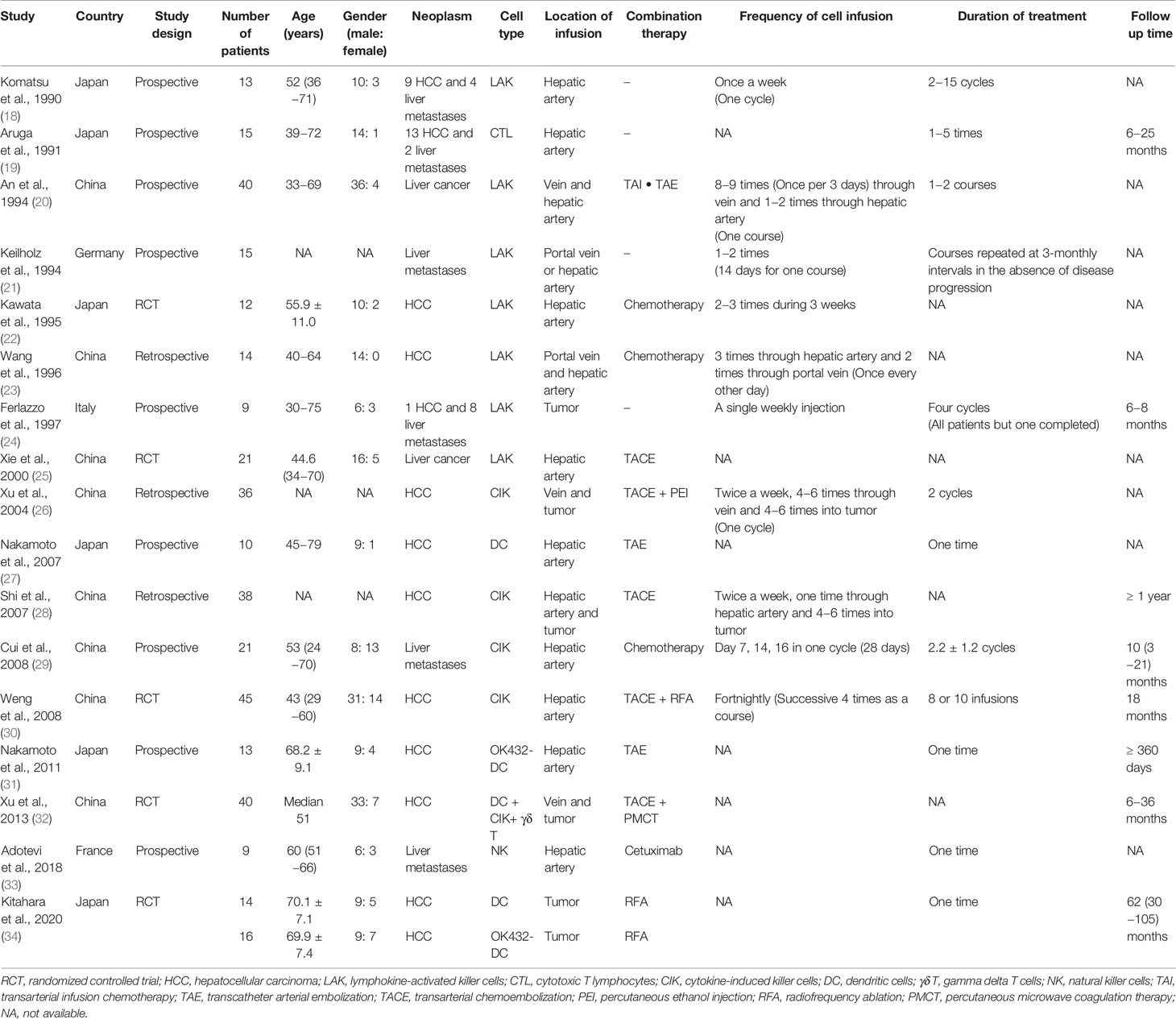
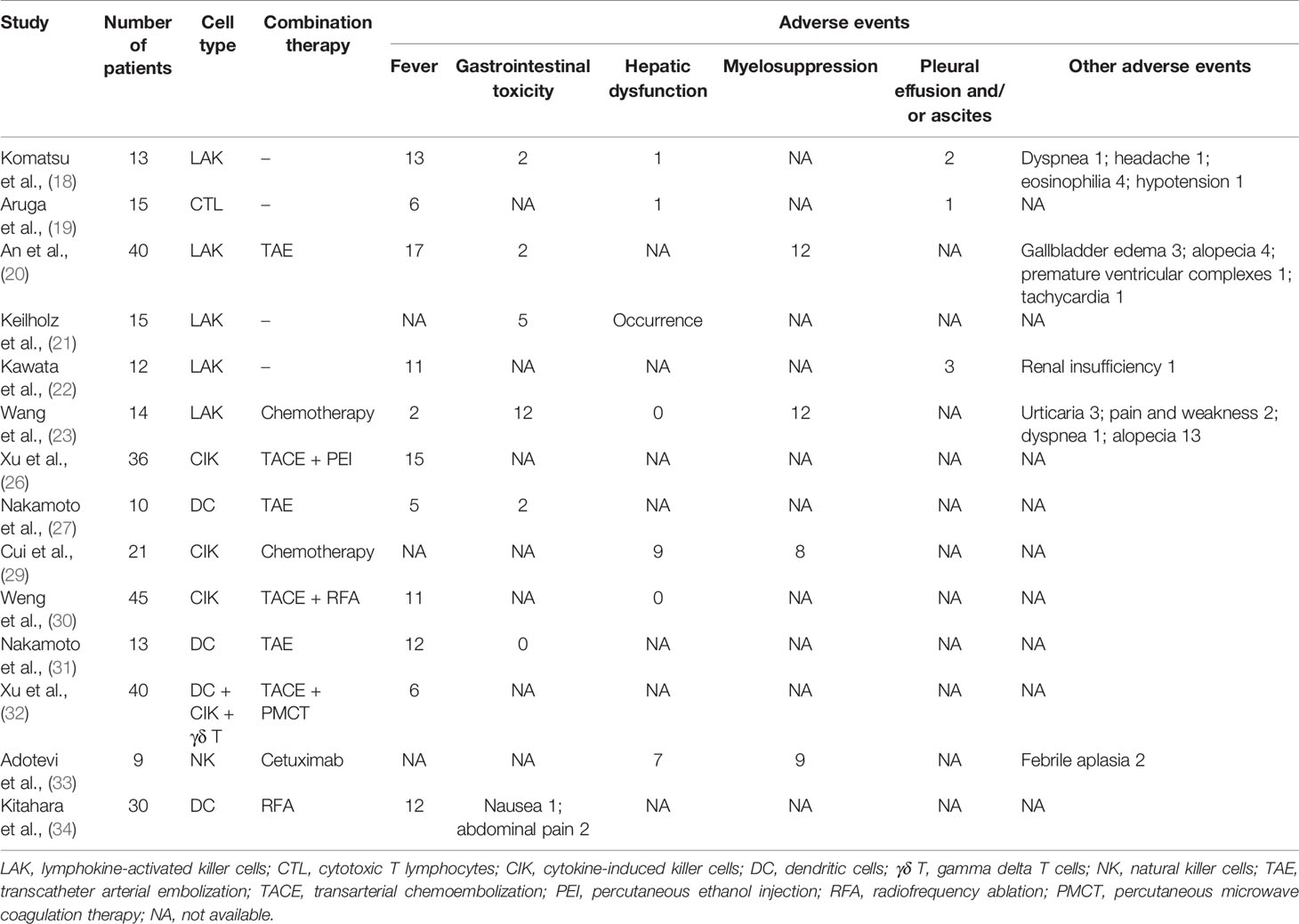
The 17 studies included involved a total of 381 participants: 261 patients in 12 articles were definitely diagnosed with HCC and 59 patients in six articles were diagnosed with liver metastasis. There were five randomized controlled trials (RCTs), nine prospective studies, and three retrospective studies. Eight studies had been performed in China, six in Japan, and one each in Germany, Italy, and France. Seven studies used LAK cells, four used CIK cells, three used DC, one combined DC with CIK and γδ T cells, and one each utilized cytotoxic T lymphocytes (CTL) and NK cells. Further, immune cell treatment in nine studies had been combined with minimally invasive treatments such as TAE or radiofrequency ablation (RFA). The different geographic locations and combined therapies were possible sources of heterogeneity. Table 2 shows the relevant information on the characteristics of the included studies.
Quality Assessment
The methodological quality of the included studies was assessed by the MINORS tool. The scores are shown in Figures 2 and 3. Seven reports were non-comparative studies evaluated by eight items; the remaining 10 were comparative studies with an additional four criteria. Notably, no study had a prospective calculation of the study size. Based on separate grading standards, four studies had low risk of bias, with a total score indicating good quality; 13 studies were identified as moderate quality.
Study Results
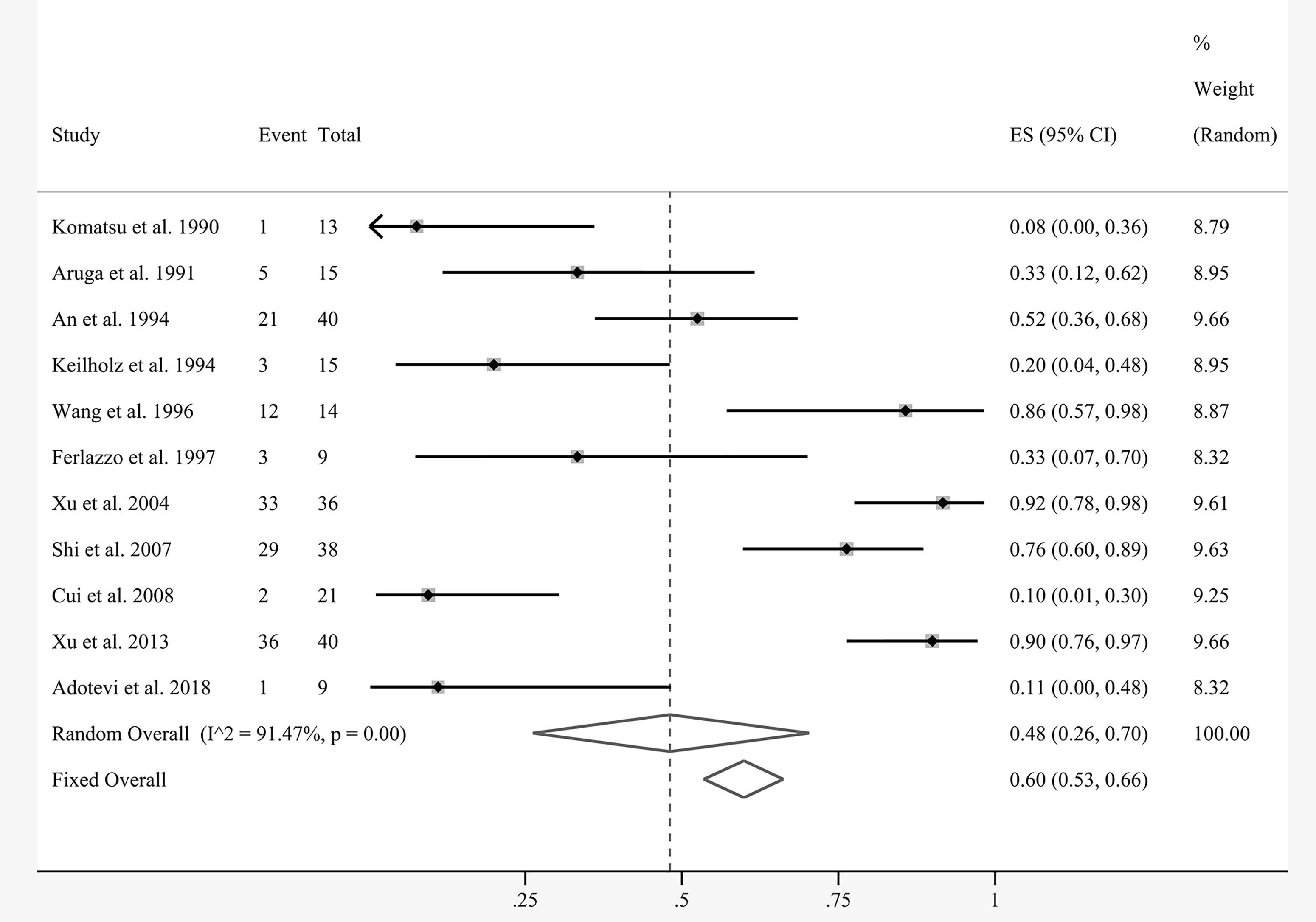
The efficacy results of the meta-analysis are shown in Figures 4 and 5. Due to the nature of the single-arm rate study, the corresponding outcome indices were pooled directly. Eleven articles were included for pooled analysis of the overall response rate (ORR), which equaled the complete response (CR) plus partial response (PR). There was substantial statistical heterogeneity between the involved studies (I2 = 91.47%, P = 0.00). The random-effects model demonstrated that the pooled ORR of local cell infusion therapy was 48% (95% confidence interval [CI]: 26%–70%) (Figure 4).
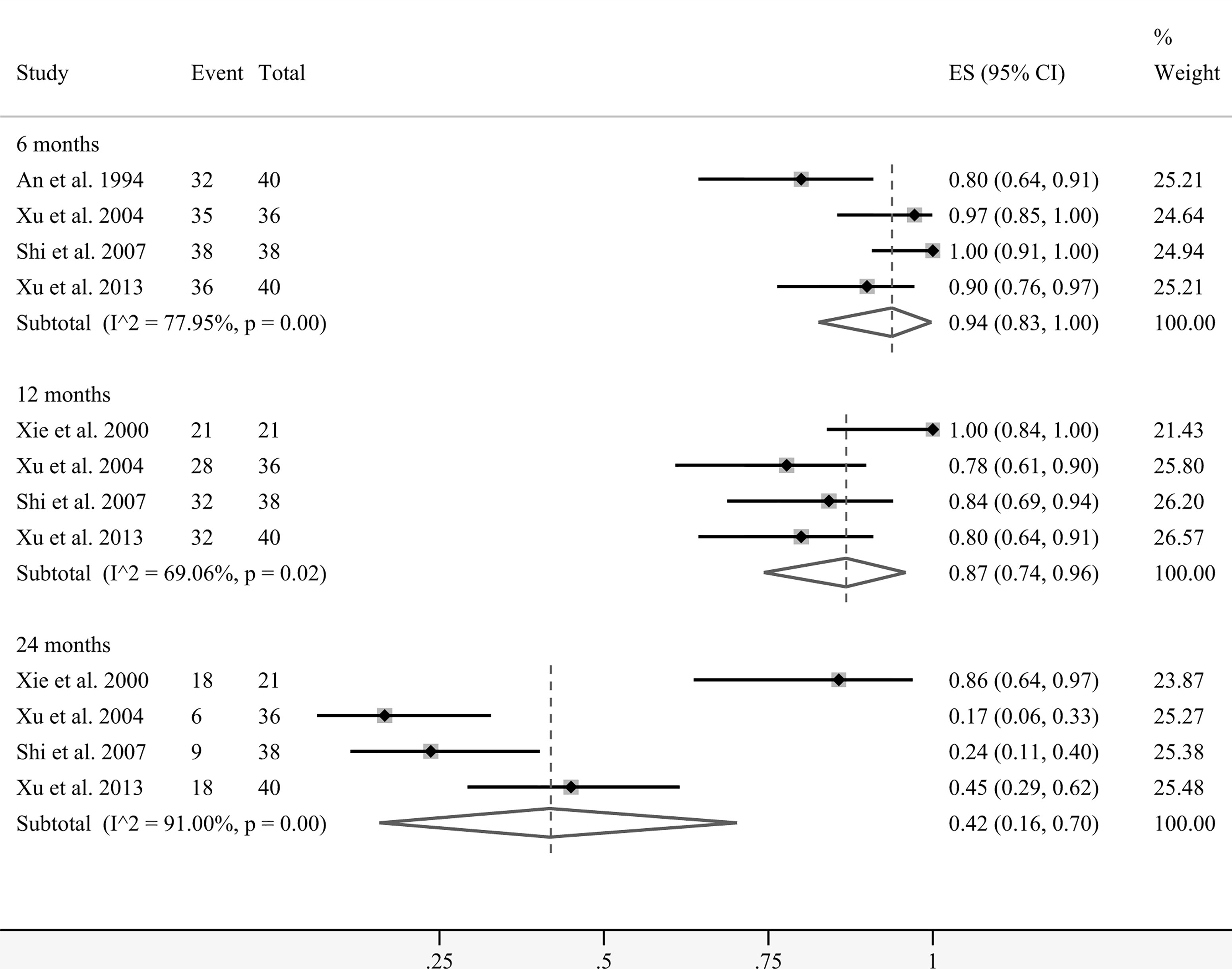
A total of five studies presented data on the overall survival (OS) rates at several time points. Coincidentally, the patients in these groups all received interventional treatment as combined therapy. Figure 5 demonstrates that the pooled survival rates of patients undergoing regional cellular immunotherapy in combination with transarterial therapy at 6, 12, and 24 months were 94% (95% CI: 83%–100%), 87% (95% CI: 74%–96%), and 42% (95% CI: 16%–70%), respectively.
Subgroup analyses were performed based on cell type, combination treatment, region, and primary or secondary cancer status. Subgroups with <3 studies after classification were not included in the analyses. Table 3 shows that combination therapy with minimally invasive intervention seemed to be significantly associated with higher ORR compared with cell therapy alone (0.79 vs. 0.22, P = 0.014). Furthermore, the HCC group had a far greater ORR than the liver metastases group (0.87 vs. 0.13, P = 0.008). There was no statistical difference between the other two pairs (cell type, P = 0.442; region, P = 0.152). Supplementary Figures 1-8 present the forest plots of each group. The meta-regression analysis suggested that interventional management and metastasis status might influence the effectiveness of local cellular immunotherapy.
Fourteen studies reported adverse events related to local perfusion cell therapy. Table 4 shows that the complications patients were likely to experience after regional infusion of immune cells primarily included fever, gastrointestinal toxicity, hepatic dysfunction, myelosuppression, and pleural effusion and/or ascites. Figure 6 shows the pooled analyses on the proportion adverse reactions in the patients. The estimated rates of fever, gastrointestinal toxicity, hepatic dysfunction, myelosuppression, and pleural effusion and/or ascites were 50% (95% CI: 32%–69%), 22% (95% CI: 2%–51%), 15% (95% CI: 0%–42%), 66% (95% CI: 29%–95%), and 14% (95% CI: 4%–28%), respectively (Supplementary Figures 9-13). Although reported in most of the studies, fever did not have the highest incidence. Myelosuppression was the most common adverse event because of its uppermost estimated rate. Symptomatic treatment relieved nearly all complications.
One study reported five deaths from hepatic failure, two from HCC rupture, two from varix rupture, one from subarachnoid hemorrhage, and one from respiratory failure. Another study reported one death from upper gastrointestinal bleeding. The other studies did not specify treatment-associated death.
Sensitivity Analysis and Publication Bias
This study conducted sensitivity analyses on the pooled effect sizes with articles >10. Results of sensitivity analysis were shown in Supplementary Figure 14 (for pooled ORR) and 15 (for pooled fever rate). Changes in the two pooled effect sizes were not large, indicating that our results were stable. The publication bias was objectively evaluated with funnel plots and Egger’s test. The relative asymmetry of the funnel plots indicated the presence of publication bias, with P < 0.05 in Egger’s test (P = 0.025 for ORR pooled analysis; P = 0.035 for fever rate pooled analysis) (Figure 7). If there are <10 studies, the power of Egger’s test can be too low to distinguish chance from real asymmetry, so other pooled analyses were not considered.
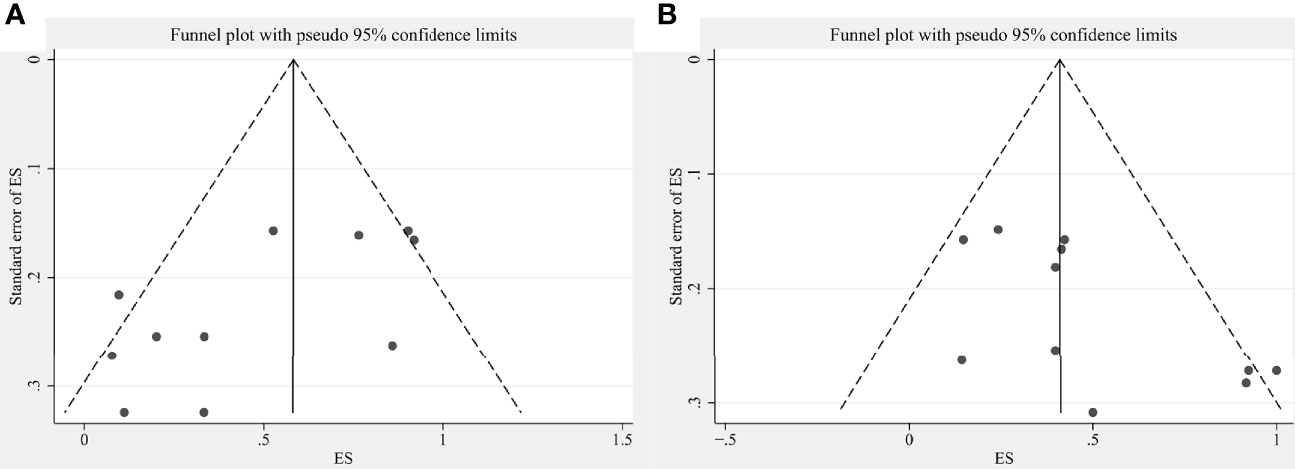
Figure 7 Funnel plots showing publication bias for (A) ORR pooled analysis and (B) fever rate pooled analysis.
Discussion
Lacking typical symptoms in the early stage, liver cancer is one of the most common malignancies, with complicated therapeutic strategies. Transplantation, hepatectomy, ablation, transarterial embolization, chemotherapy, radiotherapy, and drug therapy can prolong survival to some extent. However, the curative effect remains poor, mainly owing to tumor recurrence and drug resistance of the liver cancer cells (35). By directly recognizing and killing tumor cells or enhancing the anti-tumor immune response, cellular immunotherapy has become a new method for improving the survival and curative effect in patients with liver tumor in recent years. Initial immunotherapy involves LAK cells, CIK cells, and other non-specific cell therapy, but the specificity is poor. In recent years, this problem has been solved with the development of specific cell therapy such as that involving CAR-T, CAR-NK, and TCR-T cells (11). However, most studies on cellular immunotherapy are still in phase I/II clinical trials. A meta-analysis showed that the administration of immune cells induced in vitro can decrease the early recurrence and mortality of postoperative HCC (36).
Cell-based immunotherapy has a strong anti-tumor effect on cancer cells. One of the treatments with the most potential in recent years, it has an extraordinary effect on blood tumors (37–39), but challenges remain, i.e., liver cancer, one of the solid tumors (40, 41). As most of the existing therapies use systemic infusion (i.e., intravenous infusion), these cells first reach the lung after entering the body, peak in 2–4 hours, and then are distributed to the liver, kidney, spleen, and other parts, tending to stabilize in 24 hours (42). Similar to the first-pass effect of drugs, the organ at which the infused immune cells first arrive is intercepted to the maximum extent. Therefore, for liver cancer, the cells cannot completely enter the tumor tissue. In contrast, direct regional infusion of immune cells can transport the cells effectively and avoid the physical barrier in systemic circulation (43). In theory, locoregional therapy can achieve the liver aggregation of immune cells to bring them into full effect. Different means of cell perfusion can influence the outcome of cancer therapy, and local injection improves the effectiveness and prolongs the response time (44, 45). Hence, there is more expectation that local infusion is used instead of systemic infusion alone. In recent reports on cell therapy, Schmidt et al. described the methods of combined antigen targeting, regional delivery, and improving the persistence of CAR-T cells in a malignant tumor microenvironment (40). Li et al. proved that CIK cell infusion therapy had a synergistic effect on the long-term survival of patients with liver cancer after minimally invasive treatment (12). However, no meta-analysis has focused on local perfusion in cell therapy. In the present review, we selected studies that transfused immunocytes via the hepatic artery, portal vein, or intratumoral injection instead of systemic infusion. It is worth noting that the sample size of each research was small and that the results varied greatly. There remains a lack of high-level evidence-based validation on the efficacy and safety of cellular immunotherapy via local perfusion.
Here, we comprehensively analyzed the results of 17 individual articles from four databases to investigate the efficacy and safety of local cell immunotherapy in the prognosis of patients with liver tumor. Meta-analysis demonstrated that the total response rate was 48% (95% CI: 26%–70%). The OS rates of patients treated with regional cellular immunotherapy in combination with interventional therapy at 6, 12, and 24 months were 94% (95% CI: 83%–100%), 87% (95% CI: 74%–96%), and 42% (95% CI: 16%–70%), respectively. The subgroup analysis and meta-regression results suggested that combination therapy and metastasis status might affect the outcome of immunocyte therapy. In particular, the ORR of local cell perfusion combined with interventional therapy was significantly higher than that of cell therapy alone. HCC had a superior response rate compared to liver metastasis, indicating that local cell immunotherapy has better application prospects in HCC. As the number of eligible studies in some subgroups was too small (n < 3) after classification, the pooled results and meta-regression analysis may be subject to bias. Therefore, we excluded groups with <3 studies from the subgroup analysis, such as the CTL group (n = 1), DC group (n = 1) and NK group (n = 1) in cell classification, and the chemotherapy group (n = 2) and cetuximab group (n = 1) in classification of combination therapy. We also excluded four studies due to their indeterminate cancer metastasis status. With respect to safety, one advantage of local cell infusion is that it has few special adverse effects. The most common adverse reactions were myelosuppression and fever, followed by gastrointestinal toxicity, hepatic dysfunction, and pleural effusion and/or ascites. These complications also frequently occur in routine treatment of liver cancer and can be well controlled by symptomatic treatment. No deaths directly related to local perfusion of cell therapy have been reported.
Immune cells are an important component of the tumor microenvironment (TME) and play critical roles in tumorigenesis (46). Tumor-antagonizing immune cells and tumor-promoting immune cells participate in tumor immunity regulation through producing various cytokines. Because of the multiple immunosuppressive mechanisms in the tumor microenvironment, immune cells in liver cancer often fail to work (47). It has been reported that CD4+CD25+Foxp3+ regulatory T cells (Tregs) play a immunosuppressive role in TME which contributes to HCC initiation and progression (48). CD3+, CD8+, activated NKs, and Foxp3+ T cells were also found to have a suppression function in HCC (49). Several included literatures demonstrated altered T cell subpopulations in patients after CIK cell infusions (26, 28, 30). In another included article, the level of CD4+CD25+FOXP3+/CD4+ cells in patients treated with cellular immunotherapy was significantly lower than before, suggesting that the immunosuppression status improved (32). We inferred that improving the composition of immune-cell subsets might be a crucial step for promoting tumor immunity in liver cancer. Cell therapy varies in function and efficacy, depending in part on the type of immune cells infused. It was a shame that only the LAK group and the CIK group were compared in the subgroup analysis, given the potential bias. The CIK group seemed to have a higher ORR than the LAK group, but the statistical difference was not significant (0.61 vs. 0.40, P = 0.442). CIK cells are a heterogeneous cell population comprised of CD3+CD56+ cells, CD3−CD56+ NK cells, and CD3+CD56− T cells, while LAK cells predominantly contain CD3−CD56+ NK cells and CD3+ T cells (50, 51). Some studies argued that CIK cells were better, as they showed easier availability and more effective cytotoxicity (52–54). In recent years, CAR and TCR gene engineered immune cells have gained increased attention due to their ability of specifically recognition. More large-scale studies are expected to optimize the strategy of cell therapy.
In view of the occult occurrence of liver neoplasms, patients are often in the middle and late stage when diagnosed, missing the chance for surgery. TACE is generally considered the first choice for these unresectable liver tumors. However, for most patients treated with TACE alone, even with repeated treatment, active cancer cells remain in the tumor lesions. It is difficult for patients with liver cancer to maintain long-term survival because the tumor cannot be completely eliminated by TACE. Applying local cell therapy as an auxiliary means to interventional therapy such as TACE to control localized advanced diseases is a novel and exciting prospect. Cell therapy after minimally invasive treatment in patients with liver cancer is helpful for removing tumor microlesions, preventing recurrence or metastasis, and ultimately improving the patient’s quality of life and prognosis (12, 55, 56). Immune cells injected into the tumor can significantly increase the number of working cells in the tumor area, and further kill the residual cancer cells after the interventional therapy. We have verified that our results are consistent with this viewpoint. The ORR and survival of combination groups were all elevated as compared with their respective control groups treated with interventional therapy alone (if any).
Our study has some limitations. There are differences in the patients’ disease severity, age, sex, and other basic characteristics, so there was large clinical heterogeneity between the studies. Only studies with commonly used immunocyte therapy were included, because normative nomenclature and classification of the therapy had not been provided. In addition, the study designs were not uniform. The inherent limitations of non-randomized studies in meta-analysis may have influenced our results. More prospective-design RCTs are needed. As the immune cell types used, combined therapies, patient location, and tumor metastasis status are quite different, especially in the different countries, the evaluation criteria of outcomes can be diverse. These problems are solved with the random-effects method and subgroup analysis. The meta-analysis is also limited by the literature quality. Several studies had inconsistent endpoints and the data were insufficient for conducting further exploration. Potential publication bias cannot be ignored, as most of the studies had been performed in Asia. Due to the relatively new development period, there are no eligible articles with published results related to specific cells such as CAR-T, CAR-NK, and TCR-T cells. Considering the above limitations, our meta-analysis results should be interpreted with caution.
Conclusions
As a new approach to tumor treatment, cellular immunotherapy has the advantages of good safety, high efficiency, and wide indications. Although widely used in a variety of tumors, its curative effect on liver cancer remains limited. Local infusion is an improved mode for enhancing antineoplastic efficacy and reducing systemic toxicity. We demonstrate that cell therapy through local perfusion is safe and effective for patients with liver cancer. It is more efficacious when combined with minimally invasive interventional therapy. Our findings may help promote the clinical application of cellular immunotherapy in cancer treatment. Given the limitations on the quantity and quality of the included studies, more high-quality prospective clinical trials are warranted to confirm our conclusion. Moreover, how the specific recognition ability of immune cells for liver tumor can be improved and further increase therapeutic efficacy, is also the focus of cellular immunotherapy in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
SC and WL conceived and designed the study. SC and HC performed the literature search and data abstraction. SC and YZ were involved in the data analysis and interpretation. SC wrote the initial draft. WL reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Capital’s Funds for Health Improvement and Research (CFH 2020-2-2175) and Beijing Talents Project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the authors of the included studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.772509/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Horn SR, Stoltzfus KC, Lehrer EJ, Dawson LA, Tchelebi L, Gusani NJ, et al. Epidemiology of Liver Metastases. Cancer Epidemiol (2020) 67:101760. doi: 10.1016/j.canep.2020.101760
3. Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in Liver Cancer and Possible Treatment Approaches. Biochim Biophys Acta Rev Cancer (2020) 1873(1):188314. doi: 10.1016/j.bbcan.2019.188314
4. Govaert KM, Emmink BL, Nijkamp MW, Cheung ZJ, Steller EJ, Fatrai S, et al. Hypoxia After Liver Surgery Imposes an Aggressive Cancer Stem Cell Phenotype on Residual Tumor Cells. Ann Surg (2014) 259(4):750–9. doi: 10.1097/SLA.0b013e318295c160
5. Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current State of Liver-Directed Therapies and Combinatory Approaches With Systemic Therapy in Hepatocellular Carcinoma (HCC). Cancers (Basel) (2019) 11(8):1085. doi: 10.3390/cancers11081085
6. Marin JJG, Macias RIR, Monte MJ, Romero MR, Asensio M, Sanchez-Martin A, et al. Molecular Bases of Drug Resistance in Hepatocellular Carcinoma. Cancers (Basel) (2020) 12(6):1663. doi: 10.3390/cancers12061663
7. Cheng Z, Wei-Qi J, Jin D. New Insights on Sorafenib Resistance in Liver Cancer With Correlation of Individualized Therapy. Biochim Biophys Acta Rev Cancer (2020) 1874(1):188382. doi: 10.1016/j.bbcan.2020.188382
8. Wang J, Zhao H, Yu J, Xu X, Liu W, Jing H, et al. MiR-92b Targets P57kip2 to Modulate the Resistance of Hepatocellular Carcinoma (HCC) to Ionizing Radiation (IR) -Based Radiotherapy. BioMed Pharmacother (2019) 110:646–55. doi: 10.1016/j.biopha.2018.11.080
9. Zhang Y, Zheng L, Ding Y, Li Q, Wang R, Liu T, et al. MiR-20a Induces Cell Radioresistance by Activating the PTEN/PI3K/Akt Signaling Pathway in Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys (2015) 92(5):1132–40. doi: 10.1016/j.ijrobp.2015.04.007
10. Bucci L, Garuti F, Lenzi B, Pecorelli A, Farinati F, Giannini EG, et al. The Evolutionary Scenario of Hepatocellular Carcinoma in Italy: An Update. Liver Int (2017) 37(2):259–70. doi: 10.1111/liv.13204
11. Mizukoshi E, Kaneko S. Immune Cell Therapy for Hepatocellular Carcinoma. J Hematol Oncol (2019) 12(1):52. doi: 10.1186/s13045-019-0742-5
12. Li X, Dai D, Song X, Liu J, Zhu L, Xu W. A Meta-Analysis of Cytokine-Induced Killer Cells Therapy in Combination With Minimally Invasive Treatment for Hepatocellular Carcinoma. Clin Res Hepatol Gastroenterol (2014) 38(5):583–91. doi: 10.1016/j.clinre.2014.04.010
13. Li YC, Zhao L, Wu JP, Qu CX, Song QK, Wang RB. Cytokine-Induced Killer Cell Infusion Combined With Conventional Treatments Produced Better Prognosis for Hepatocellular Carcinoma Patients With Barcelona Clinic Liver Cancer B or Earlier Stage: A Systematic Review and Meta-Analysis. Cytotherapy (2016) 18(12):1525–31. doi: 10.1016/j.jcyt.2016.09.002
14. Kuppen PJ, Marinelli A, Camps JA, Pauwels EK, van de Velde CJ, Fleuren GJ, et al. Biodistribution of Lymphokine-Activated Killer (LAK) Cells in Wag Rats After Hepatic-Artery or Jugular-Vein Infusion. Int J Cancer (1992) 52(2):266–70. doi: 10.1002/ijc.2910520219
15. Chapiro J, Tacher V, Geschwind JF. Intraarterial Therapies for Primary Liver Cancer: State of the Art. Expert Rev Anticancer Ther (2013) 13(10):1157–67. doi: 10.1586/14737140.2013.845528
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
17. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
18. Komatsu T, Yamauchi K, Furukawa T, Obata H. Transcatheter Arterial Injection of Autologous Lymphokine-Activated Killer (LAK) Cells Into Patients With Liver Cancers. J Clin Immunol (1990) 10(3):167–74. doi: 10.1007/BF00917917
19. Aruga A, Yamauchi K, Takasaki K, Furukawa T, Hanyu F. Induction of Autologous Tumor-Specific Cytotoxic T Cells in Patients With Liver Cancer. Characterizations and Clinical Utilization. Int J Cancer (1991) 49(1):19–24. doi: 10.1002/ijc.2910490105
20. An YM. The Treatment of Liver Cancer With Transcatheter Artery Embolization (TAE) in Combination With LAK/IL-2. Chin J Clin Oncol (1994) 21(11):828–30.
21. Keilholz U, Scheibenbogen C, Brado M, Georgi P, Maclachlan D, Brado B, et al. Regional Adoptive Immunotherapy With Interleukin-2 and Lymphokine-Activated Killer (LAK) Cells for Liver Metastases. Eur J Cancer (1994) 30A(1):103–5. doi: 10.1016/s0959-8049(05)80028-0
22. Kawata A, Une Y, Hosokawa M, Wakizaka Y, Namieno T, Uchino J, et al. Adjuvant Chemoimmunotherapy for Hepatocellular Carcinoma Patients. Adriamycin, Interleukin-2, and Lymphokine-Activated Killer Cells Versus Adriamycin Alone. Am J Clin Oncol (1995) 18(3):257–62. doi: 10.1097/00000421-199506000-00014
23. Wang ZG, Zhang AL, Ding FQ, Liu GZ. Local Transfusion of LAK Cell With Chemotherapy in the Treatment of Patients and Advanced Liver Cancer With IDDS. Chin J N Gastroenterol (1996) 4(8):448–9.
24. Ferlazzo G, Scisca C, Iemmo R, Cavaliere R, Quartarone G, Adamo V, et al. Intralesional Sonographically Guided Injections of Lymphokine-Activated Killer Cells and Recombinant Interleukin-2 for the Treatment of Liver Tumors: A Pilot Study. J Immunother (1997) 20(2):158–63. doi: 10.1097/00002371-199703000-00008
25. Xie L, Pang R, Jin Y, Xiang S, Li H. Effects of Hepatic Artery Chemotherapeutic Embolization Combined With Perfusing LAK Cells Into Hepatic Artery After Radical Operation of Liver Cancer. Zhonghua Gan Zang Bing Za Zhi [Chin J Hepatol] (2000) 8(3):142–3.
26. Xu YM, Zhang NZ, Zhang GL, Ma WQ, Gao CJ, Xu WD, et al. Transcatheter Hepatic Arterial Chemoembolization and Percutaneous Ethanol Injection Combined With Cytokine-Induced Killer Cells in Treatment of Advanced Hepatocellular Carcinoma. World Chin J Digestol (2004) 12(6):1288–91. doi: 10.11569/wcjd.v12.i6.1288
27. Nakamoto Y, Mizukoshi E, Tsuji H, Sakai Y, Kitahara M, Arai K, et al. Combined Therapy of Transcatheter Hepatic Arterial Embolization With Intratumoral Dendritic Cell Infusion for Hepatocellular Carcinoma: Clinical Safety. Clin Exp Immunol (2007) 147(2):296–305. doi: 10.1111/j.1365-2249.2006.03290.x
28. Shi Y, Gao CJ, Dong SL, Chen FX, Xu YM. Cytokine-Induced Killer Cell for Interventional Chemotherapy of Hepatocellular Carcinoma. J Intervent Radiol (2007) 16(4):235–9.
29. Cui CL, Chi ZH, Yuan XQ, Lian HY, Si L, Guo J. Hepatic Intra-Arterial Bio-Chemotherapy for the Treatment of Melanoma Patients With Liver Metastasis: A Phase II Clinical Study. Ai Zheng [Chin J Cancer] (2008) 27(8):845–50.
30. Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, et al. Minimally Invasive Treatment Combined With Cytokine-Induced Killer Cells Therapy Lower the Short-Term Recurrence Rates of Hepatocellular Carcinomas. J Immunother (2008) 31(1):63–71. doi: 10.1097/CJI.0b013e31815a121b
31. Nakamoto Y, Mizukoshi E, Kitahara M, Arihara F, Sakai Y, Kakinoki K, et al. Prolonged Recurrence-Free Survival Following OK432-Stimulated Dendritic Cell Transfer Into Hepatocellular Carcinoma During Transarterial Embolization. Clin Exp Immunol (2011) 163(2):165–77. doi: 10.1111/j.1365-2249.2010.04246.x
32. Xu YM, Xu DY, Zhang NZ, Shi Y, Luan ZY, Liu JQ. TACE Combined PMCT Sequential Own Cancer Vaccine and Immunoeffector Cells to Treat Large Hepatocellular Carcinoma Clinical Observation. Chin J Cancer Prev Treat (2013) 20(24):1928–32.
33. Adotevi O, Godet Y, Galaine J, Lakkis Z, Idirene I, Certoux JM, et al. In Situ Delivery of Allogeneic Natural Killer Cell (NK) Combined With Cetuximab in Liver Metastases of Gastrointestinal Carcinoma: A Phase I Clinical Trial. Oncoimmunology (2018) 7(5):e1424673. doi: 10.1080/2162402X.2018.1424673
34. Kitahara M, Mizukoshi E, Terashima T, Nakagawa H, Horii R, Iida N, et al. Safety and Long-Term Outcome of Intratumoral Injection of OK432-Stimulated Dendritic Cells for Hepatocellular Carcinomas After Radiofrequency Ablation. Transl Oncol (2020) 13(7):100777. doi: 10.1016/j.tranon.2020.100777
35. Yoo SY, Badrinath N, Woo HY, Heo J. Oncolytic Virus-Based Immunotherapies for Hepatocellular Carcinoma. Mediators Inflammation (2017) 2017:5198798. doi: 10.1155/2017/5198798
36. Zhao H, Zheng M, Wang K, Wang L, He H, Wang M, et al. A Meta-Analysis of Adoptive Immunotherapy in Postoperative Hepatocellular Carcinoma. J Cancer Res Ther (2018) 14(4):807–14. doi: 10.4103/jcrt.JCRT_858_17
37. Biernacki MA, Brault M, Bleakley M. T-Cell Receptor-Based Immunotherapy for Hematologic Malignancies. Cancer J (2019) 25(3):179–90. doi: 10.1097/PPO.0000000000000378
38. Tanaka J, Miller JS. Recent Progress in and Challenges in Cellular Therapy Using NK Cells for Hematological Malignancies. Blood Rev (2020) 44:100678. doi: 10.1016/j.blre.2020.100678
39. Cirillo M, Tan P, Sturm M, Cole C. Cellular Immunotherapy for Hematologic Malignancies: Beyond Bone Marrow Transplantation. Biol Blood Marrow Transplant (2018) 24(3):433–42. doi: 10.1016/j.bbmt.2017.10.035
40. Schmidts A, Maus MV. Making CAR T Cells a Solid Option for Solid Tumors. Front Immunol (2018) 9:2593. doi: 10.3389/fimmu.2018.02593
41. Zhang L, Ding J, Li HY, Wang ZH, Wu J. Immunotherapy for Advanced Hepatocellular Carcinoma, Where Are We? Biochim Biophys Acta Rev Cancer (2020) 1874(2):188441. doi: 10.1016/j.bbcan.2020.188441
42. Ai J, Bai YM, Li QX, Zhang C, Shan BE. Anti-Tumor Activity of CIK Cells on Cervical Cancer HeLa Cells In Vitro and In Vivo and Their Distribution Characteristics in Tumor-Bearing Mice. Chin J Cancer Biother (2012) 19(4):374–80.
43. Sridhar P, Petrocca F. Regional Delivery of Chimeric Antigen Receptor (CAR) T-Cells for Cancer Therapy. Cancers (Basel) (2017) 9(7):92. doi: 10.3390/cancers9070092
44. Keilholz U, Schlag P, Tilgen W, Brado B, Galm F, Görich J, et al. Regional Administration of Lymphokine-Activated Killer Cells Can Be Superior to Intravenous Application. Cancer (1992) 69(8):2172–5. doi: 10.1002/1097-0142(19920415)69:8<2172::aid-cncr2820690826>3.0.co;2-m
45. Hagenaars M, Ensink NG, Koelemij R, Basse PH, Eggermont AM, van de Velde CJ, et al. Regional Administration of Natural Killer Cells in a Rat Hepatic Metastasis Model Results in Better Tumor Infiltration and Anti-Tumor Response Than Systemic Administration. Int J Cancer (1998) 75(2):233–8. doi: 10.1002/(sici)1097-0215(19980119)75:2<233::aid-ijc11>3.0.co;2-e
46. Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune Cells Within the Tumor Microenvironment: Biological Functions and Roles in Cancer Immunotherapy. Cancer Lett (2020) 470:126–33. doi: 10.1016/j.canlet.2019.11.009
47. Fu Y, Liu S, Zeng S, Shen H. From Bench to Bed: The Tumor Immune Microenvironment and Current Immunotherapeutic Strategies for Hepatocellular Carcinoma. J Exp Clin Cancer Res (2019) 38(1):396. doi: 10.1186/s13046-019-1396-4
48. Granito A, Muratori L, Lalanne C, Quarneti C, Ferri S, Guidi M, et al. Hepatocellular Carcinoma in Viral and Autoimmune Liver Diseases: Role of CD4+ CD25+ Foxp3+ Regulatory T Cells in the Immune Microenvironment. World J Gastroenterol (2021) 27(22):2994–3009. doi: 10.3748/wjg.v27.i22.2994
49. Zheng X, Jin W, Wang S, Ding H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Front Immunol (2021) 12:729705. doi: 10.3389/fimmu.2021.729705
50. Wang X, Yu W, Li H, Yu J, Zhang X, Ren X, et al. Can the Dual-Functional Capability of CIK Cells Be Used to Improve Antitumor Effects? Cell Immunol (2014) 287(1):18–22. doi: 10.1016/j.cellimm.2013.11.009
51. Phillips JH, Lanier LL. Dissection of the Lymphokine-Activated Killer Phenomenon. Relative Contribution of Peripheral Blood Natural Killer Cells and T Lymphocytes to Cytolysis. J Exp Med (1986) 164(3):814–25. doi: 10.1084/jem.164.3.814
52. Scheffold C, Brandt K, Johnston V, Lefterova P, Degen B, Schöntube M, et al. Potential of Autologous Immunologic Effector Cells for Bone Marrow Purging in Patients With Chronic Myeloid Leukemia. Bone Marrow Transplant (1995) 15(1):33–9.
53. Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, et al. Sensitivity of Multidrug-Resistant Tumor Cell Lines to Immunologic Effector Cells. Cell Immunol (1996) 169(1):85–90. doi: 10.1006/cimm.1996.0094
54. Ren X, Yu J, Liu H, Zhang P, An X, Zhang N, et al. Th1 Bias in PBMC Induced by Multicycles of Auto-CIKs Infusion in Malignant Solid Tumor Patients. Cancer Biother Radiopharm (2006) 21(1):22–33. doi: 10.1089/cbr.2006.21.22
55. Ding M, Wang Y, Chi J, Wang T, Tang X, Cui D, et al. Is Adjuvant Cellular Immunotherapy Essential After TACE-Predominant Minimally-Invasive Treatment for Hepatocellular Carcinoma? A Systematic Meta-Analysis of Studies Including 1774 Patients. PloS One (2016) 11(12):e0168798. doi: 10.1371/journal.pone.0168798
Keywords: liver neoplasms, cellular immunotherapy, local infusion, efficacy, safety, systematic review
Citation: Chen S, Chen H, Zhang Y and Li W (2022) Efficacy and Safety of Cellular Immunotherapy by Local Infusion for Liver Tumor: A Systematic Review and Meta-Analysis. Front. Oncol. 12:772509. doi: 10.3389/fonc.2022.772509
Received: 08 September 2021; Accepted: 01 February 2022;
Published: 28 February 2022.
Edited by:
Khurum Hayat Khan, University College London, United KingdomReviewed by:
Alessandro Granito, University of Bologna, ItalyHan Wu, Eastern Hepatobiliary Surgery Hospital, China
Copyright © 2022 Chen, Chen, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, d2VpbGk4OTg5QGNjbXUuZWR1LmNu
 Shanshan Chen
Shanshan Chen Hualei Chen
Hualei Chen Wei Li
Wei Li