
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 31 January 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.772076
This article is part of the Research Topic Beyond Chemotherapy and Immunotherapy in Thoracic Malignancies: Overcoming Resistance by Tackling New Molecular Pathways View all 9 articles
 Marc A. Schneider1,2
Marc A. Schneider1,2 Adriana Rozy3
Adriana Rozy3 Sabine Wrenger4,5
Sabine Wrenger4,5 Petros Christopoulos2,6
Petros Christopoulos2,6 Thomas Muley1,2
Thomas Muley1,2 Michael Thomas2,6
Michael Thomas2,6 Michael Meister1,2
Michael Meister1,2 Tobias Welte4,5
Tobias Welte4,5 Joanna Chorostowska-Wynimko3
Joanna Chorostowska-Wynimko3 Sabina Janciauskiene3,4,5*
Sabina Janciauskiene3,4,5*In the last decade, targeting the immune system became a promising therapy in advanced lung cancer stages. However, in a clinical follow-up, patient responses to immune checkpoint inhibitors widely differ. Peripheral blood is a minimally invasive source of potential biomarkers to explain these differences. We blindly analyzed serum samples from 139 patients with non-small cell lung cancer prior to anti-PD-1 or anti-PD-L1 therapies to assess whether baseline levels of albumin (ALB), alpha-1 acid glycoprotein (AGP), alpha1-antitrypsin (AAT), alpha2-macroglobulin (A2M), ceruloplasmin (CP), haptoglobin (HP), alpha1-antichymotrypsin (ACT), serum amyloid A (SAA), and high-sensitivity C-reactive protein (hs-CRP), have a predictive value for immunotherapy success. Disease progression-free survival (PFS) was calculated based on RECIST 1.1 criteria. A multivariate Cox regression analysis, including serum levels of acute-phase proteins and clinical parameters, revealed that higher pre-therapeutic levels of HP and CP are independent predictors of a worse PFS. Moreover, a combined panel of HP and CP stratified patients into subgroups. We propose to test this panel as a putative biomarker for assessing the success of immunotherapy in patients with NSCLC.
Programmed death ligand-1 (PD-L1) is a transmembrane protein induced by pro-inflammatory substances in many cell types, including cancer cells, and acts as a suppressor of immune response when bound to its complementary ligands. PD-L1 combines with programmed death (PD-1) receptor and inhibits immune cell activation (1). Checkpoint-inhibitor blockade is based on highly selective humanized monoclonal antibodies against PD-1 or PD-L1, helping the host immune system to identify and destroy tumour cells. Antibodies targeting the PD1-PDL1 axis are used both as a first- or second-line therapy for many malignancies, including lung cancers (2). The PD-1 inhibitors nivolumab and pembrolizumab, and PD-L1 inhibitors atezolizumab and durvalumab are currently considered as the breakthroughs and the most successful therapies for advanced non-small cell lung cancer (NSCLC). When assessed in different settings, these drugs seem to improve survival rates and show less severe toxicity than chemotherapy (3). Studies comparing survival benefits between standard platinum-based chemotherapy versus immunotherapy have shown significant benefit in the selected population of NSCLC patients both as the first (pembrolizumab, atezolizumab) or second-line therapies (4–6).
Despite certain advantages, immunotherapy does not show an expected benefit for all patients (7, 8). It seems that only about 20-25% of NSCLC patients positively respond to immunotherapy (9). Therefore, further studies are necessary to identify patients who may benefit from immune checkpoint blockade therapy (10) and to determine an appropriate sequence and/or combination of chemotherapy with immunotherapy. Concurrently, effective predictive biomarkers are necessary to enable personalized therapy and to guide designs of clinical trials.
In advanced NSCLC patients, evaluation of PD-L1 expression by immunohistochemistry is used as the primary biomarker for selecting patients as eligible for receiving anti-PD-L1 therapy. Yet, this assay is not able to identify conclusively non-benefiting patients (11). Likewise, other researched biomarker candidates have not been proven to be helpful in clinical settings. Only in a small subset of cancers mismatch repair /microsatellite instability and tumour mutations can serve as biomarkers for predicting immune checkpoint inhibitors efficacy (12).
The concentrations of plasma acute phase proteins (APPs) are changing during lung cancer development (13), and although these proteins are nonspecific inflammatory markers, they might be useful as biomarkers for disease management and prognosis. For example, elevated pre-operative levels of C-reactive protein (CRP) have been associated with inability to achieve complete resection in patients with NSCLC (14–16). Independently of tumour stage, patients with higher plasma CRP levels were found to show significantly lower overall survival than those with lower levels of CRP (15, 17). Higher serum levels of amyloid A (SAA) have also been found in patients with NSCLC as compared to healthy controls (18–22). Cho et al. reported that patients with a survival ≥ 5 years have significantly lower SAA than patients with a survival < 5 years (23). To date, various reports suggest that APPs have a profound impact on cancer development and the body’s innate immune system, however a putative prognostic value of combined serum APPs in NSCLC patients treated with immune therapy has not been explored.
We present data on serum levels of albumin (ALB), alpha-1 acid glycoprotein (AGP), alpha1-antitrypsin (AAT), alpha2-macroglobulin (A2M), ceruloplasmin (CP), haptoglobin (HP), alpha1-antichymotrypsin (ACT), serum amyloid A (SAA), and high-sensitivity C-reactive protein (hs-CRP), in NSCLC patients and discuss if the combinations of measured APPs could be exploited for assessing the success of immunotherapy with PD-1/PD-L1 checkpoint inhibitors.
Serum samples of patients with NSCLC were collected at the Thorax Clinic-Heidelberg and provided by Lung Biobank Heidelberg, a member of the accredited Tissue Bank of the National Center for Tumor Diseases (NCT) Heidelberg, the Biomaterial Bank Heidelberg, and the Biobank platform of the German Center for Lung Research (DZL). The use of biomaterial and data for this study was approved by the local ethics committee of the Medical Faculty Heidelberg (S-270/2001) and Hannover (9155_BO_K_2020). All patients included in the study signed informed consent and the study was performed according to the principles set out in the WMA Declaration of Helsinki. Investigated patient samples were part of a prospective cohort. The assembly of this cohort has started in 2018 and includes patients receiving checkpoint inhibitors at any therapy line. Up to now (May 2021), more than 400 patients with NSCLC have been included in the cohort, and these patients are currently followed up. At the time of analysis, 139 patients were available with a follow-up of at least 2 years. These patients were included in the study. Baseline blood sampling was performed up to 10 days prior to any immunotherapy. Blood was processed within 1 h after the blood draw. Serum aliquots were stored at −80°C until measurements. The patient cohort is described in Table 1 and Table S1. All following measurements were performed using the whole cohort.
Serum concentrations of APPs were measured blindly using the nephelometric method (IMMAGE 800 Protein Chemistry Analyzer, Beckman Coulter Inc., CA, USA) in the Department of Genetics and Clinical Immunology at the National Institute of Tuberculosis and Lung Diseases, Warsaw. Analysis sensitivity for measured APPs was: ALB (22.2 mg/dL), AAT (10 mg/dL), AGP (35 mg/dL) hs-CRP (0.02 mg/dL), AT3 (5 mg/dL), CP (2 mg/dL), HP (5.83mg/dL) and A2M (40 mg/dL). All serum samples were analyzed at the same time, to control for testing variability. For HP, one data point is missing due to too the low sample amount. Plasma levels of ACT and SAA were measured by ELISA sandwich kit from BT Lab Bioassay Technology Laboratory (Shanghai, China) at 450 nm in a spectrophotometric reader Infinite M200 (Tecan, Austria). Assay sensitivity for ACT was 5.17 µg/ml and for SAA was 0.024 µg/ml. All standards and samples were analyzed in duplicates.
Data of serum measurements were visualized and statistically analysed with GraphPad Prism 9 (GraphPad Software, San Diego, California, USA) and SPSS 26.0 (IBM, Ehningen, Germany). Data were statistically analysed under REMARK criteria (12). Progression-free survival (PFS) time was calculated from the date of medication start until the last date of contact or progression of the disease under RECIST 1.1 criteria (24). Patients were included in analyses if PFS was > 30 days after therapy initiation. The cut-offs used for survival analyses were selected using the software tool “cutoff-finder” (25). “Survival” method of the tool was used. This method fits Cox proportional hazard models to the dichotomized variable and the survival variable. The optimal cutoff is defined as the point with the most significant (log-rank test) split. Hazard ratios (HRs) including 95% confidence intervals are calculated. Uni and multivariate survival analyses were performed using the Cox proportional hazards model. Visualization of survival data was performed according to Kaplan and Meier. Correlation analyses were performed using the nonparametric Spearman’s rank test. A correlation with r > 0.5 was considered as a reliable correlation. P-values are interpreted in a descriptive manner, no formal sample size calculation was done in this exploratory study.
To investigate serum APPs as predictive biomarkers for the immunotherapy response, levels of nine APPs (ACT, SAA, AGP, HP, AAT, CRP, A2M, CP and ALB) were measured in 139 patients with NSCLC prior to anti-PD-1 or anti-PD-L1 therapy (Table 1 and Table S1). Serum concentrations of some investigated proteins, like AGP, HP, AAT, A2M, ALB and hs-CRP, varied strongly among patients (Figure 1A) while others, like ACT, CP, and SAA, were homogenous. To gain a better understanding of the relationships between analyzed APPs, we performed a correlation analysis (Figure 1B). Interestingly, we detected two sets of APPs, in which they correlated to each other. One set included AGP, HP, AAT, hs-CRP, CP, and ALB, with the highest correlations observed between hs-CRP and AGP (r = 0.85) and between hs-CRP and AAT (r = 0.79). Another set included ACT and SAA (r = 0.53) whereas A2M did not correlate with any of the measured APPs.
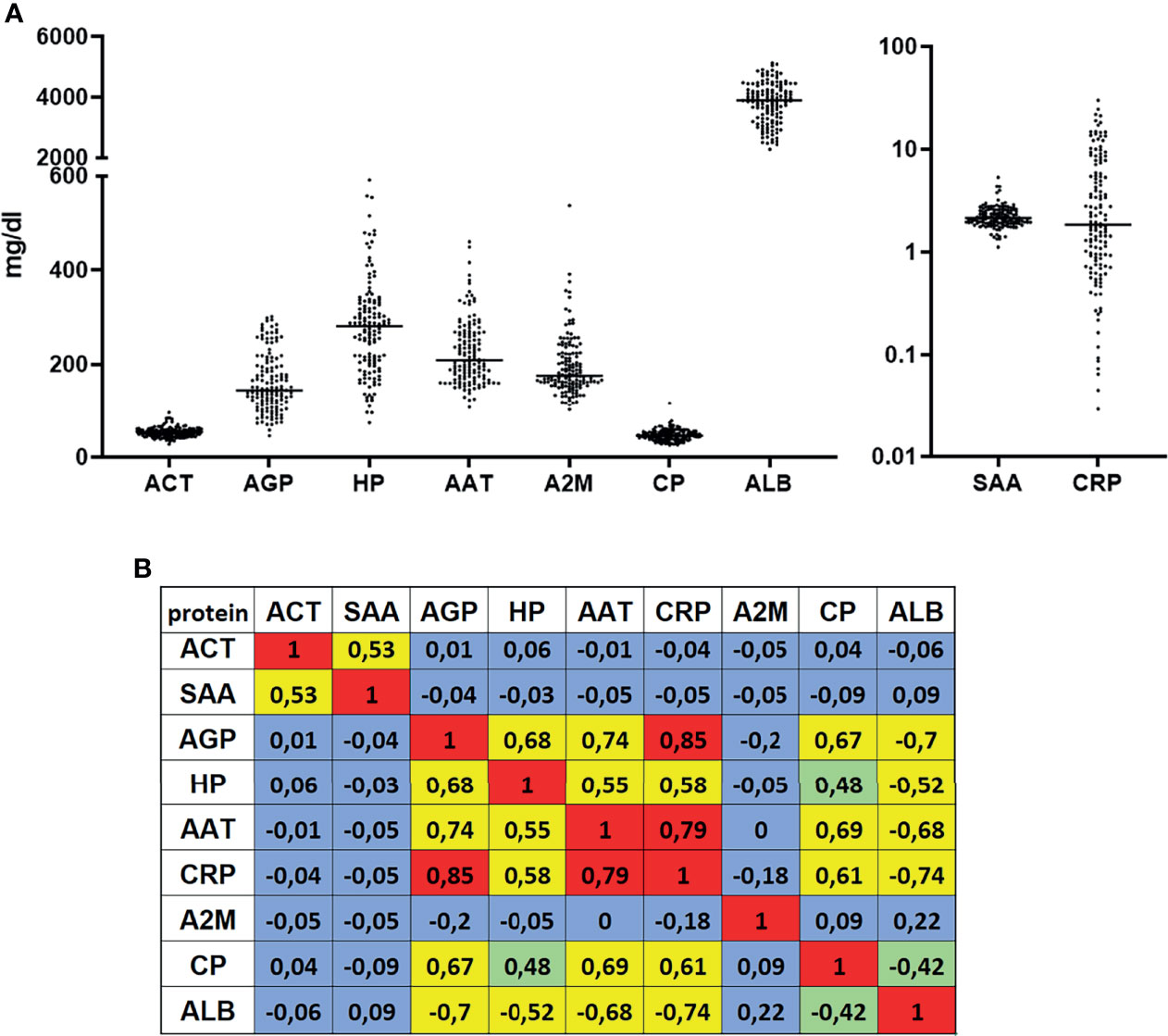
Figure 1 Serum concentrations and correlations of measured acute phase proteins. (A) Nine acute phase proteins were measured in serum of patients with NSCLC (n = 139) prior to a PD-1 or PD-L1 immunotherapy. (B) Spearman ranked correlation analyses of measured serum values. Values with r > 0.5 or r < -0.5 were considered as correlation. Colours indicate- red-strong correlation; yellow- reliable correlation; green-weak correlation; blue-no correlation.
Most of the patients were diagnosed with an adenocarcinoma of the lung (66 %), at clinical stage IV (70 %). The patients initially received a variety of treatments while the major therapy was immuno-chemotherapy for 31 % of patients. The levels of APPs were analyzed at the start of immunotherapy in 124 patients at clinical stage IV and in 15 patients at stage III. As illustrated in Figure S1, APP levels did not differ significantly within the two stages. Regarding the immunotherapy drugs, 41 % of the patients received pembrolizumab + chemotherapy followed by pembrolizumab alone (25 %). We included patients from 1st to 4th line immunotherapy whereas approximately half of the patients (52 %) received the immunotherapy as 1st line treatment. The median progression-free survival of the cohort was 204 days (33 - 750 days).
To get an idea of whether any of the measured proteins might be predictive for patient`s benefit receiving anti-PD-1 or anti-PD-L1 antibody immunotherapy, we performed a univariate Cox-regression analysis (Table S2). By using the software tool “cut-off finder”, optimal values of APPs were determined for the categorization of the patients (Table S3). Based on these cut-offs, two APPs (HP and CP) were highly predictive for the efficiency of immunotherapy when considered as single factors (Table S2). AGP, AAT, hsCRP and ALB failed to be highly robust markers after Bonferroni correction for multiple testing. Three other proteins, namely ACT, SAA and A2M, showed no significant predictive value.
Besides HP and CP, AGP, AAT, hs-CRP and ALB were also included for visualization by Kaplan Meier plots. The Cox regression model showed that these latter might be good predictors as well. In our cohort we observed that lower pre-therapy levels of AGP, HP, AAT, hs-CRP, and CP were predictive for significantly longer PFS (Figures 2A–E). By contrast, patients with higher ALB levels showed a longer PFS (Figure 2F). The predictive value of ACT and SAA was not significant while no optimal cut-off was discovered for A2M (Figure S3).
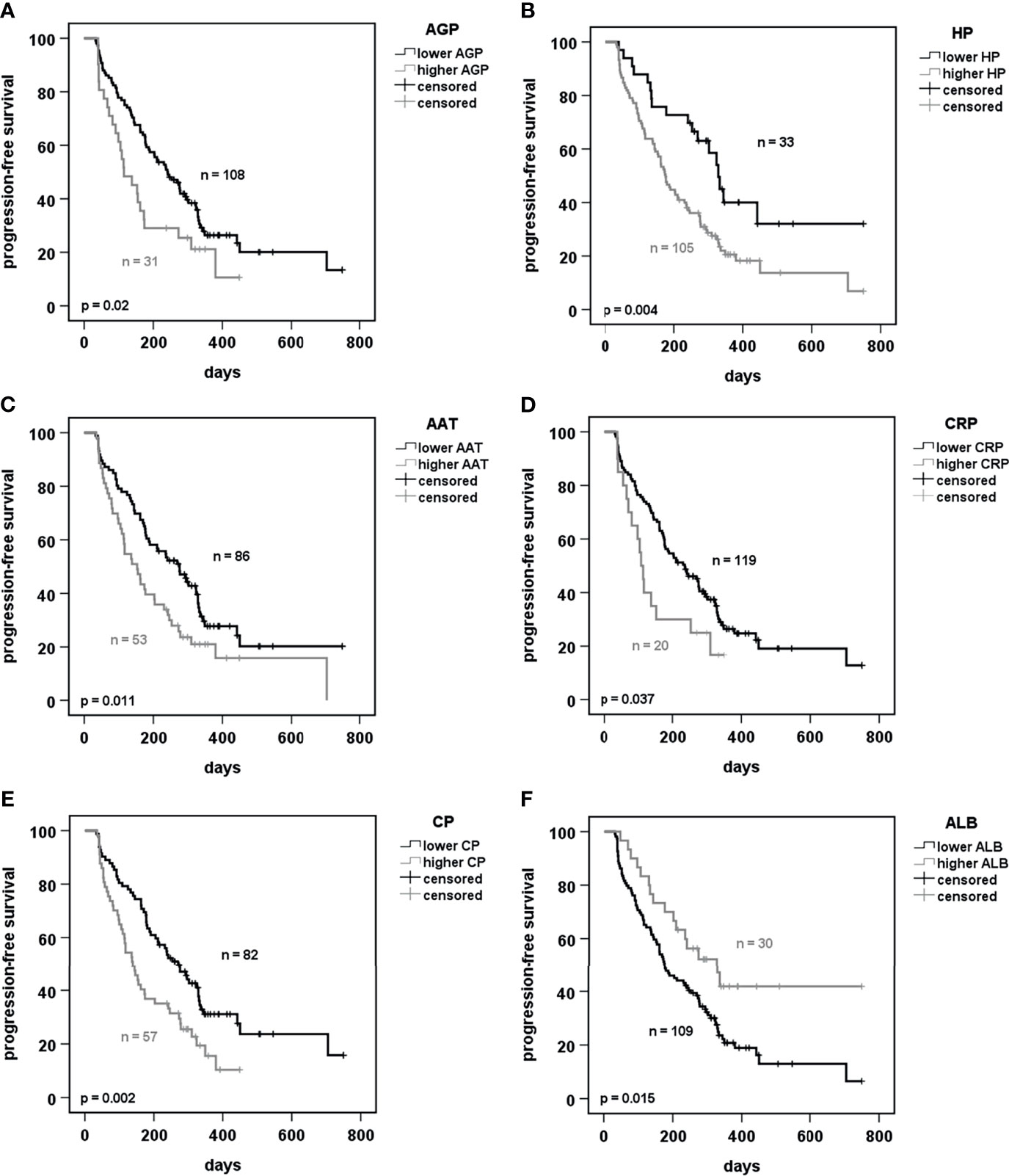
Figure 2 Prediction of progression-free survival of patients receiving PD-1 or PD-L1 immunotherapy dependent on acute phase proteins. (A–F) Kaplan-Meier curves of progression-free survival under immunotherapy in prediction to baseline serum concentrations of the indicated acute phase proteins. Cut-offs were calculated using the software tool “cutoff-finder” (25). p < 0.05 was considered as significant. AGP, alpha-1 acid glycoprotein; HP, haptoglobin; AAT, alpha1-antitrypsin; CRP, C-reactive protein; CP, ceruloplasmin; ALB, albumin.
We also checked for any putative effect of anti-PD‐1 and PD‐L1 antibodies alone or when used with chemotherapy on the patient’s PFS (Figure S2). While a specific targeted therapy showed no influence (Figure S2A), line of therapy showed a borderline significance with the tendency for a benefit if immunotherapy was given as a 1st-line treatment (Figure S2B).
To further strengthen the power of APPs as biomarkers, we combined the cut-offs of HP and CP, since both proteins did not correlate (Figure 1B). We performed multivariate Cox regression analysis including the clinical parameters such as age, sex, cancer histology and clinical stage, line of treatment and target of immunotherapy (Table S4) and investigated every single factor for its robustness. We observed that HP and CP, when combined with clinical parameters, remained as significantly predictive markers. In fact, in all performed multivariate analyses, the serum concentration of the APPs was the factor with the highest predictive value for PFS. Interestingly, further multivariate analysis including HP and CP as well as the clinical parameters from Table S4 showed that HP is the strongest independent marker (p = 0.007) (Table S5).
We next hypothetically divided all patients into 3 subgroups as having different risks for disease progression: i) a low risk (serum values of HP and CP below cut-offs from Table S3), ii) a high-risk (serum values above cut-offs from Table S3) and iii) an intermediate risk (serum values between) (Figure 3). The results demonstrate that patients from the low-risk group showed a highly increased PFS (upper curve), whereas high-risk patient group displayed a dramatic drop-down of progression-free survival. These results support a panel of two APPs as highly predictive for the success of immunotherapy in patients with NSCLC.
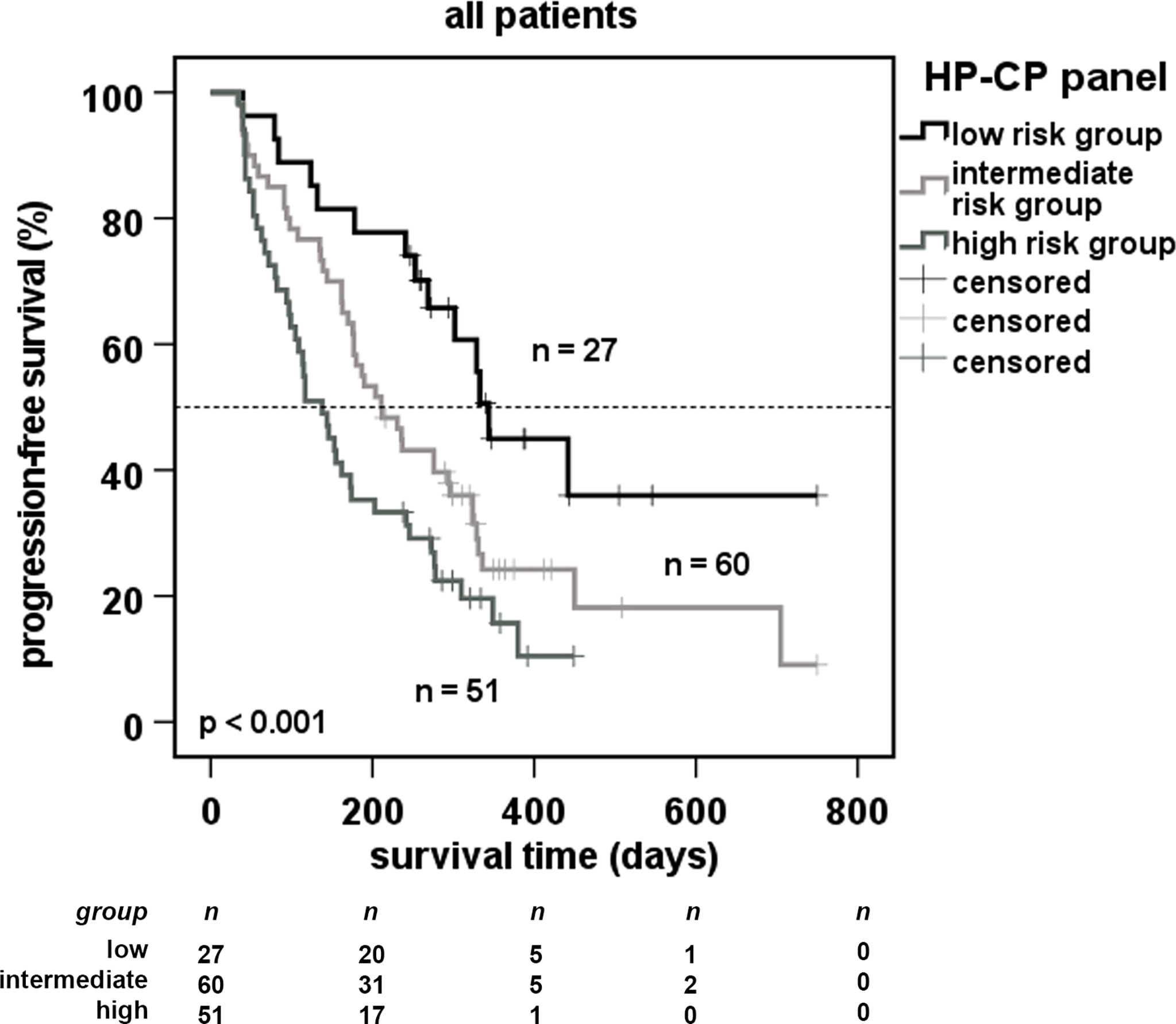
Figure 3 Predictive progression-free survival of patients using a 2-marker-panel of acute phase proteins. Cut-off values used in Figure 2 were combined for robust markers of multivariate analysis (Table S4). Patients were separated in a low-risk group (serum values below cut-offs), a high-risk group (serum values above cut-offs) and an intermediate risk group (all other patients). Dotted line indicates the median survival of the cohort.
Currently approved criteria for patient selection to receive ICI monotherapy or ICI and chemotherapy combination do not reliably exclude non-responders or patients who develop treatment resistance over time (5). Therefore, individually adjusted treatment, based on predictive biomarkers of PFS, would be extremely helpful for enhancing therapeutic benefits and avoiding unnecessary costs of ICI treatment.
PD-L1 expression is typically used as a biomarker for PD-1/PD-L1 therapy since PD-1/PD-L1 signal pathway is a key target of ICIs. However, the predictive value of PD-L1 in NSCLC has been confirmed for the 1st but not for the 2nd line of treatment (26, 27). Besides PD-L1, another approved biomarker is microsatellite instability-high resulting from an impaired DNA mismatch repair. However, this is a rare condition and reflects an exceedingly small subpopulation of NSCLC patients, about 0,53% in lung adenocarcinoma, although different trials came with opposite conclusions (28).
Other biomarkers with a putative predictive value regarding the benefits of ICI therapy have also been examined. Tumour mutation burden (TMB), defined as the frequency of certain mutations within a tumour’s genes, has been considered as a very promising biomarker for dual ICI with significantly positive predictive value for better PFS and overall survival including patients negative for PD-L1 (29). Yet, its clinical application has been crashed by the inconsistency in the detection methods, and the lack of a standardized cut-off to define high TMB status. Likewise, the significance of tumour-infiltrating lymphocytes is difficult to implement as a biomarker for ICI response due to the technical constraints of assessment methods and biopsy material representativeness. The transcriptional signatures of immune responsiveness, like a ratio of CD8+ T versus T helper 1 cell cytokine mRNA, red blood cell distribution width (30), baseline serum sodium concentration (31), blood levels of prolactin (32) and positron emission tomography (33) were used to predict the PFS and overall survival in response to ICI therapy. Despite advances in new methodologies, routine measurement of specific tumour markers remains challenging because some of them are rapidly degraded, difficult to assay and/or masked by highly abundant blood proteins like ALB, AAT, or HP.
Different reports support an important role of inflammation in cancer cell proliferation, angiogenesis, and migration (34, 35). On the other hand, cancer cells themselves mediate systemic inflammation, which is coordinated by immune cells, cytokines/chemokines, and APPs, among others (36), and it is thought to contribute to cancer progression and cancer-related complications. Various APPs, specifically CRP, may reflect cancer-induced inflammatory processes (37). CRP has been shown to correlate with low levels of CD4+ T-cells, which play a key role in ICI-mediated antitumor immune response (38). Recent studies have indicated that pre-ICI therapy levels of CRP may represent a valuable prognostic marker in NSCLC (39–41).
In general, single APPs have been reported to have a potential value in guiding decision-making for patients undergoing chemotherapy and targeted ICI therapy. In the present study, we assessed whether a panel of APPs could have better prognostic and predictive value than single APPs and could be useful to predict a patient`s response to immunotherapy. Indeed, data from a retrospective cohort of patients harboring NSCLC treated with ICIs revealed that the pre-therapeutic levels of some APPs have good prognostic value. Lower pretherapeutic (baseline) concentrations of AGP, HP, AAT, hs-CRP, and CP but higher ALB were predictive for better PFS in our cohort. Finally, a panel of HP and CP preserved a powerful prognostic value even after multivariate Cox regression analysis combining APPs with clinical data. According to some reports, CRP/ALB ratio may have a prognostic value in cancer (42), unfortunately, we were not able to confirm this in our patient’s cohort. It is also important to notify that targeting therapy using anti-PD-1 or anti-PD-L1 alone or in combination with chemotherapy showed no influence on PFS time in our NSCLC cohort. However, a tendency towards a longer PFS was found if immunotherapy was given as a 1st-line treatment. The clinical decision of 1st line therapy is typically based on both, tumour and patients’, characteristics. Therefore, it remains incredibly important to decide when immunotherapy, using PD-1/PD-L1 immune checkpoint blockade as a 1st line therapy, is the right strategy for NSCLC patients (43).
Results from our exploratory cohort of 139 patients show that five APPs - AGP, HP, AAT, CP, and ALB - are promising biomarkers to predict the beneficial response of NSCLC patients to ICI therapy. Specifically, we show that a combination of HP and CP can highly improve our findings. HP, a marker of red blood cell destruction and a main hemoglobin-binding protein, which increases in many inflammatory diseases, including lung cancer. HP seems to be involved in the pathogenesis of tumors through innate/acquired immunity, effects on cell migration and angiogenesis, and glycolytic activity (44–46). Different authors found that circulating levels of HP increase with cancer stage and that HP is potentially useful in the clinical biomarker of lung cancer (47–49). CP is a well-known copper-binding protein, which has been associated with cancer development (50) and suggested as a useful biomarker for lung adenocarcinoma (51). Although CP is associated with tumor growth, invasiveness and prognosis in lung cancer patients, its biological role in tumorigenesis remains not fully understood. Other APPs, which are included in our analysis, have also been related to lung cancer and discussed as putative biomarkers. For example, in lung cancer patients’ serum level alterations were documented for AGP (52, 53), AAT (13), and ALB (54). A retrospective study evaluated the association of baseline serum ALB with the clinical outcome in a cohort of 457 patients with advanced-stage NSCLC treated with erlotinib, targeting the epidermal growth factor receptor. Remarkably, before the treatment initiation, low albumin was associated with poor outcomes of patients (55). Our findings are in line, as in our panel higher levels of ALB combined with lower levels of AGP, HP, AAT, and CP, are associated with better PFS of patients with NSCLC.
The changes in serum/plasma concentrations of APPs correlate with their increased hepatic synthesis (56) in response to tissue injury, inflammation, infection, or various malignancies. Although APPs are mainly produced in the liver, other organs/tissues may contribute like skin, lungs, kidneys, adipose tissue (57). In general, it has been thought that APPs are not tumour-derived and represent cancer epiphenomena rather than direct tumour-derived proteins. Recent proteomics studies profiling serum proteins of cancer and non-cancer individuals indicated that the altered levels of specific APPs can be observed in distinct types, subtypes, and stages of cancer (58). Moreover, others and we previously reported that cancer cells express and release APPs such as AAT or SAA (13).
The reproducibly of prognostic values of our suggested panel of APPs needs to be confirmed in an independent and larger sample set by us and other investigators. Since we are still increasing the cohort of NSCLC patients with immunotherapy, we will validate our findings in a larger cohort. If confirmed, this panel can be a valuable a predictive marker for NSCLC patient response to ICI therapy. In general, the analysis of APPs is a non-invasive and reliable method, available in all clinical chemistry laboratories, suggesting its high potential.
A panel of two serum APPs, namely HP and CP, provides a clinically relevant pretherapeutic tool to predict the efficiency of PD-1/PD-L1 checkpoint inhibitor therapy as displayed in the progression-free survival of 139 NSCLC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the local ethics committees of the Medical Faculty of the University of Heidelberg (S-270/2001) and of the Hannover Medical School (9155_BO_K_2020). The patients/participants provided their written informed consent to participate in this study.
MS: data analysis and presentation. AR, acute phase protein assay. SW: manuscript drafting. PC, TM, MT, and MM: providing patient cohort and clinical data. TW and JC-W: manuscript drafting. SJ: concept, manuscript preparation. All authors read and added comments to the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by German Center for Lung Research (DZL), grant numbers 82DZL00402 and 82DZL002A1.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Ingrid Heinzmann-Groth, Saskia Oestringer and Karin Schnorr-Teichert for the collection of the blood samples.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.772076/full#supplementary-material
1. Han Y, Liu D, Li L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am J Cancer Res (2020) 10:727–42.
2. Koshkin VS, Barata PC, Zhang T, George DJ, Atkins MB, Kelly WJ, et al. Clinical Activity of Nivolumab in Patients With non-Clear Cell Renal Cell Carcinoma. J Immunother Cancer (2018) 6:9. doi: 10.1186/s40425-018-0319-9
3. Havel JJ, Chowell D, Chan TA. The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat Rev Cancer (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
4. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
5. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
6. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol (2019) 37:537–46. doi: 10.1200/JCO.18.00149
7. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med (2017) 376:2415–26. doi: 10.1200/JCO.18.00149
8. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
9. Horvath L, Thienpont B, Zhao L, Wolf D, Pircher A. Overcoming Immunotherapy Resistance in Non-Small Cell Lung Cancer (NSCLC) - Novel Approaches and Future Outlook. Mol Cancer (2020) 19:141. doi: 10.1186/s12943-020-01260-z
10. Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, et al. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. J Immunol Res (2018) 2018:6984948. doi: 10.1155/2018/6984948
11. Grigg C, Rizvi NA. PD-L1 Biomarker Testing for Non-Small Cell Lung Cancer: Truth or Fiction? J Immunother Cancer (2016) 4:48. doi: 10.1186/s40425-016-0153-x
12. Cabezon-Gutierrez L, Custodio-Cabello S, Palka-Kotlowska M, Alonso-Viteri S, Khosravi-Shahi P. Biomarkers of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Beyond PD-L1. Clin Lung Cancer (2021) 22:381–9. doi: 10.1016/j.cllc.2021.03.006
13. Janciauskiene S, Wrenger S, Gunzel S, Grunding AR, Golpon H, Welte T. Potential Roles of Acute Phase Proteins in Cancer: Why Do Cancer Cells Produce or Take Up Exogenous Acute Phase Protein Alpha1-Antitrypsin? Front Oncol (2021) 11:622076. doi: 10.3389/fonc.2021.622076
14. Jones JM, Mcgonigle NC, Mcanespie M, Cran GW, Graham AN. Plasma Fibrinogen and Serum C-Reactive Protein are Associated With Non-Small Cell Lung Cancer. Lung Cancer (2006) 53:97–101. doi: 10.1016/j.lungcan.2006.03.012
15. Hara M, Matsuzaki Y, Shimuzu T, Tomita M, Ayabe T, Enomoto Y, et al. Preoperative Serum C-Reactive Protein Level in Non-Small Cell Lung Cancer. Anticancer Res (2007) 27:3001–4.
16. Lee JG, Cho BC, Bae MK, Lee CY, Park IK, Kim DJ, et al. Preoperative C-Reactive Protein Levels are Associated With Tumor Size and Lymphovascular Invasion in Resected Non-Small Cell Lung Cancer. Lung Cancer (2009) 63:106–10. doi: 10.1016/j.lungcan.2008.04.011
17. O'dowd C, Mcrae LA, Mcmillan DC, Kirk A, Milroy R. Elevated Preoperative C-Reactive Protein Predicts Poor Cancer Specific Survival in Patients Undergoing Resection for Non-Small Cell Lung Cancer. J Thorac Oncol (2010) 5:988–92. doi: 10.1097/JTO.0b013e3181da78f9
18. Benson MD, Eyanson S, Fineberg NS. Serum Amyloid A in Carcinoma of the Lung. Cancer (1986) 57:1783–7. doi: 10.1002/1097-0142(19860501)57:9<1783::AID-CNCR2820570912>3.0.CO;2-L
19. Howard BA, Wang MZ, Campa MJ, Corro C, Fitzgerald MC, Patz EF Jr. Identification and Validation of a Potential Lung Cancer Serum Biomarker Detected by Matrix-Assisted Laser Desorption/Ionization-Time of Flight Spectra Analysis. Proteomics (2003) 3:1720–4. doi: 10.1002/pmic.200300514
20. Gao WM, Kuick R, Orchekowski RP, Misek DE, Qiu J, Greenberg AK, et al. Distinctive Serum Protein Profiles Involving Abundant Proteins in Lung Cancer Patients Based Upon Antibody Microarray Analysis. BMC Cancer (2005) 5:110. doi: 10.1186/1471-2407-5-110
21. Dowling P, O'driscoll L, Meleady P, Henry M, Roy S, Ballot J, et al. 2-D Difference Gel Electrophoresis of the Lung Squamous Cell Carcinoma Versus Normal Sera Demonstrates Consistent Alterations in the Levels of Ten Specific Proteins. Electrophoresis (2007) 28:4302–10. doi: 10.1002/elps.200700246
22. Liu DH, Wang XM, Zhang LJ, Dai SW, Liu LY, Liu JF, et al. Serum Amyloid A Protein: A Potential Biomarker Correlated With Clinical Stage of Lung Cancer. BioMed Environ Sci (2007) 20:33–40.
23. Cho WC, Yip TT, Cheng WW, Au JS. Serum Amyloid A is Elevated in the Serum of Lung Cancer Patients With Poor Prognosis. Br J Cancer (2010) 102:1731–5. doi: 10.1038/sj.bjc.6605700
24. Schwartz LH, Litiere S, De Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and Clarification: From the RECIST Committee. Eur J Cancer (2016) 62:132–7. doi: 10.1016/j.ejca.2016.03.081
25. Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff Finder: A Comprehensive and Straightforward Web Application Enabling Rapid Biomarker Cutoff Optimization. PLoS One (2012) 7:e51862. doi: 10.1371/journal.pone.0051862
26. Gibney GT, Weiner LM, Atkins MB. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy. Lancet Oncol (2016) 17:e542–51. doi: 10.1016/S1470-2045(16)30406-5
27. Hersom M, Jorgensen JT. Companion and Complementary Diagnostics-Focus on PD-L1 Expression Assays for PD-1/PD-L1 Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Ther Drug Monit (2018) 40:9–16. doi: 10.1097/FTD.0000000000000460
28. Gregg JP, Li T, Yoneda KY. Molecular Testing Strategies in Non-Small Cell Lung Cancer: Optimizing the Diagnostic Journey. Transl Lung Cancer Res (2019) 8:286–301. doi: 10.21037/tlcr.2019.04.14
29. Strickler JH, Hanks BA, Khasraw M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin Cancer Res (2021) 27:1236–41. doi: 10.1158/1078-0432.CCR-20-3054
30. Kiriu T, Yamamoto M, Nagano T, Koyama K, Katsurada M, Tamura D, et al. Prognostic Value of Red Blood Cell Distribution Width in Non-Small Cell Lung Cancer Treated With Anti-Programmed Cell Death-1 Antibody. In Vivo (2019) 33:213–20. doi: 10.21873/invivo.11462
31. Fuca G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, et al. Low Baseline Serum Sodium Concentration Is Associated With Poor Clinical Outcomes in Metastatic Non-Small Cell Lung Cancer Patients Treated With Immunotherapy. Target Oncol (2018) 13:795–800. doi: 10.1007/s11523-018-0599-5
32. Caponnetto S, Iannantuono GM, Barchiesi G, Magri V, Gelibter A, Cortesi E. Prolactin as a Potential Early Predictive Factor in Metastatic Non-Small Cell Lung Cancer Patients Treated With Nivolumab. Oncology (2017) 93:62–6. doi: 10.1159/000464328
33. Wu Q, Liu J, Zhang Y, Wu S, Xie X. Predictive Value of Positron Emission Tomography for the Prognosis of Immune Checkpoint Inhibitors (ICIs) in Malignant Tumors. Cancer Immunol Immunother (2020) 69:927–36. doi: 10.1007/s00262-020-02515-w
34. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
35. Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
36. Diakos CI, Charles KA, Mcmillan DC, Clarke SJ. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
37. Riedl JM, Barth DA, Brueckl WM, Zeitler G, Foris V, Mollnar S, et al. C-Reactive Protein (CRP) Levels in Immune Checkpoint Inhibitor Response and Progression in Advanced Non-Small Cell Lung Cancer: A Bi-Center Study. Cancers (Basel) (2020) 12:2319. doi: 10.3390/cancers12082319
38. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature (2014) 515:568–71. doi: 10.1038/nature13954
39. Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C, Shimizu J, et al. Predictive Clinical Parameters for the Response of Nivolumab in Pretreated Advanced Non-Small-Cell Lung Cancer. Oncotarget (2017) 8:103117–28. doi: 10.18632/oncotarget.21602
40. Naqash AR, Stroud CRG, Butt MU, Dy GK, Hegde A, Muzaffar M, et al. Co-Relation of Overall Survival With Peripheral Blood-Based Inflammatory Biomarkers in Advanced Stage Non-Small Cell Lung Cancer Treated With Anti-Programmed Cell Death-1 Therapy: Results From a Single Institutional Database. Acta Oncol (2018) 57:867–72. doi: 10.1080/0284186X.2017.1415460
41. Iivanainen S, Ahvonen J, Knuuttila A, Tiainen S, Koivunen JP. Elevated CRP Levels Indicate Poor Progression-Free and Overall Survival on Cancer Patients Treated With PD-1 Inhibitors. ESMO Open (2019) 4:e000531. doi: 10.1136/esmoopen-2019-000531
42. Wu M, Guo J, Guo L, Zuo Q. The C-Reactive Protein/Albumin Ratio Predicts Overall Survival of Patients With Advanced Pancreatic Cancer. Tumour Biol (2016) 37:12525–33. doi: 10.1007/s13277-016-5122-y
43. Li L, Xu F, Chen Y, Ren X, Liu Y, Chen Y, et al. Indirect Comparison Between Immunotherapy Alone and Immunotherapy Plus Chemotherapy as First-Line Treatment for Advanced Non-Small Cell Lung Cancer: A Systematic Review. BMJ Open (2020) 10:e034010. doi: 10.1136/bmjopen-2019-034010
44. De Kleijn DP, Smeets MB, Kemmeren PP, Lim SK, Van Middelaar BJ, Velema E, et al. Acute-Phase Protein Haptoglobin is a Cell Migration Factor Involved in Arterial Restructuring. FASEB J (2002) 16:1123–5. doi: 10.1096/fj.02-0019fje
45. Van Vlierberghe H, Langlois M, Delanghe J. Haptoglobin Polymorphisms and Iron Homeostasis in Health and in Disease. Clin Chim Acta (2004) 345:35–42. doi: 10.1016/j.cccn.2004.03.016
46. Chen J, Cheuk IW, Siu MT, Yang W, Cheng AS, Shin VY, et al. Human Haptoglobin Contributes to Breast Cancer Oncogenesis Through Glycolytic Activity Modulation. Am J Cancer Res (2020) 10:2865–77.
47. Heo SH, Lee SJ, Ryoo HM, Park JY, Cho JY. Identification of Putative Serum Glycoprotein Biomarkers for Human Lung Adenocarcinoma by Multilectin Affinity Chromatography and LC-Ms/MS. Proteomics (2007) 7:4292–302. doi: 10.1002/pmic.200700433
48. Abdullah M, Schultz H, Kahler D, Branscheid D, Dalhoff K, Zabel P, et al. Expression of the Acute Phase Protein Haptoglobin in Human Lung Cancer and Tumor-Free Lung Tissues. Pathol Res Pract (2009) 205:639–47. doi: 10.1016/j.prp.2009.04.007
49. Chang YK, Lai YH, Chu Y, Lee MC, Huang CY, Wu S. Haptoglobin is a Serological Biomarker for Adenocarcinoma Lung Cancer by Using the ProteomeLab PF2D Combined With Mass Spectrometry. Am J Cancer Res (2016) 6:1828–36.
50. Knekt P, Aromaa A, Maatela J, Rissanen A, Hakama M, Aaran RK, et al. Serum Ceruloplasmin and the Risk of Cancer in Finland. Br J Cancer (1992) 65:292–6. doi: 10.1038/bjc.1992.58
51. Matsuoka R, Shiba-Ishii A, Nakano N, Togayachi A, Sakashita S, Sato Y, et al. Heterotopic Production of Ceruloplasmin by Lung Adenocarcinoma is Significantly Correlated With Prognosis. Lung Cancer (2018) 118:97–104. doi: 10.1016/j.lungcan.2018.01.012
52. Duche JC, Urien S, Simon N, Malaurie E, Monnet I, Barre J. Expression of the Genetic Variants of Human Alpha-1-Acid Glycoprotein in Cancer. Clin Biochem (2000) 33:197–202. doi: 10.1016/S0009-9120(00)00048-5
53. Ayyub A, Saleem M, Fatima I, Tariq A, Hashmi N, Musharraf SG. Glycosylated Alpha-1-Acid Glycoprotein 1 as a Potential Lung Cancer Serum Biomarker. Int J Biochem Cell Biol (2016) 70:68–75. doi: 10.1016/j.biocel.2015.11.006
54. Ikeda S, Yoshioka H, Ikeo S, Morita M, Sone N, Niwa T, et al. Serum Albumin Level as a Potential Marker for Deciding Chemotherapy or Best Supportive Care in Elderly, Advanced Non-Small Cell Lung Cancer Patients With Poor Performance Status. BMC Cancer (2017) 17:797. doi: 10.1186/s12885-017-3814-3
55. Fiala O, Pesek M, Finek J, Racek J, Minarik M, Benesova L, et al. Serum Albumin is a Strong Predictor of Survival in Patients With Advanced-Stage Non-Small Cell Lung Cancer Treated With Erlotinib. Neoplasma (2016) 63:471–6. doi: 10.4149/318_151001N512
56. Fournier T, Medjoubi NN, Porquet D. Alpha-1-Acid Glycoprotein. Biochim Biophys Acta (2000) 1482:157–71. doi: 10.1016/S0167-4838(00)00153-9
57. Jain S, Gautam V, Naseem S. Acute-Phase Proteins: As Diagnostic Tool. J Pharm Bioallied Sci (2011) 3:118–27. doi: 10.4103/0975-7406.76489
Keywords: NSCLC, checkpoint inhibitors, immunotherapy, acute phase proteins, progression-free survival
Citation: Schneider MA, Rozy A, Wrenger S, Christopoulos P, Muley T, Thomas M, Meister M, Welte T, Chorostowska-Wynimko J and Janciauskiene S (2022) Acute Phase Proteins as Early Predictors for Immunotherapy Response in Advanced NSCLC: An Explorative Study. Front. Oncol. 12:772076. doi: 10.3389/fonc.2022.772076
Received: 07 September 2021; Accepted: 10 January 2022;
Published: 31 January 2022.
Edited by:
Christian Rolfo, University of Maryland Medical System, United StatesReviewed by:
Vincenzo L’Imperio, University of Milano-Bicocca, ItalyCopyright © 2022 Schneider, Rozy, Wrenger, Christopoulos, Muley, Thomas, Meister, Welte, Chorostowska-Wynimko and Janciauskiene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabina Janciauskiene, amFuY2lhdXNraWVuZS5zYWJpbmFAbWgtaGFubm92ZXIuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.