- Department of Thoracic Surgery, Cancer Hospital of Shantou University Medical College, Shantou, China
Background: The goal of this study was to investigate the prognostic value of body mass index (BMI) in patients with esophageal squamous cell carcinoma (ESCC) when stratified by alcohol drinking status.
Methods: A total of 620 patients with ESCC who underwent esophagectomy were analyzed. A receiver operating characteristic curve was constructed to set the appropriate cutoff point for BMI. Alcohol drinking was divided into ever and never. Kaplan-Meier and multivariate Cox regression analyses were conducted to investigate the association between clinicopathological factors and survival.
Results: The cutoff point was 18.75 kg/m2 for BMI. Two hundred and twenty-nine patients were ever drinkers, while the other 391 patients were never drinkers. The ever drinker group was found to have more males, longer tumor lengths, advanced pT category disease, advanced pN category disease, and lower tumor locations. However, no significant difference in BMI was found between ever drinkers and never drinkers. For ever drinkers, low BMI was significantly correlated with worse overall survival (hazard ratio = 1.690; P=0.035) and cancer-specific survival (hazard ratio = 1.763; P=0.024) than high BMI after adjusting for other factors. However, BMI was not a prognostic factor in univariate and multivariate analyses for never drinkers.
Conclusions: BMI is a prognostic factor only in ever drinkers with ESCC but not in never drinkers. Further studies are needed to elucidate the mechanism underlying the effect of the interaction between BMI and alcohol consumption on the prognosis of patients with ESCC.
Introduction
Esophageal carcinoma is one of the most common digestive system malignancies, with esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) as the two main histological subtypes (1). Esophagectomy remains the most important tool for the treatment of resectable cases; however, the prognosis of patients with esophageal carcinoma is still poor.
Malnutrition is a frequent problem in cancer patients, and preoperative nutritional status has been found to be correlated with outcomes in patients with malignancies (2). Body mass index (BMI) is one of the preoperative nutritional parameters and has been found to be correlated with the survival of patients with various carcinomas (3–6). It has been reported that a lower BMI increases the risk of developing ESCC (7). However, the prognostic value of BMI in patients with ESCC after esophagectomy is still controversial (8). Some previous studies found that a lower BMI was associated with poorer survival in ESCC patients after esophagectomy (9–11); however, others found that a lower BMI had no impact on the prognosis of ESCC patients (12, 13) or even had a favorable impact (14, 15).
Alcohol abuse is a leading risk factor for death globally and has been found to be correlated with multiple carcinomas (16, 17). Globally, an estimated 741300 of all new cases of cancer in 2020 were attributable to alcohol consumption, while esophageal carcinoma was the most common alcohol-attributable cancer (18). Alcohol consumption has also been found to affect BMI in the general population (19–21). Although alcohol consumption is a well-established risk factor for multiple cancers, little is known about its influence on the prognosis of these cancers, and even less is known about the effect of the interaction between BMI and alcohol consumption on the prognosis of cancer patients. Some studies reported an adverse impact of alcohol consumption on the survival of cancer patients (22–25), whereas others could not obtain consistent results (26, 27).
Given that alcohol abuse affects BMI in the general population and that both BMI and alcohol abuse may affect the prognosis of ESCC patients, we hypothesize that the prognostic value of BMI in ESCC patients may be impacted by alcohol consumption status. However, to the best of our knowledge, no studies have investigated the effect of the interaction between BMI and alcohol consumption on the prognosis of ESCC patients after esophagectomy. Thus, in the current study, we aimed to investigate the value of BMI in patients with ESCC who underwent surgical resection when stratified by alcohol drinking status.
Patients and Methods
Patients
A total of 817 patients with esophageal cancer underwent esophagectomy at Shantou University Medical College Cancer Hospital between September 2014 and December 2017. Only patients with ESCC who chose surgery as their initial treatment were enrolled in this study. This study was approved by an independent ethics committee at our hospital. All patients provided informed consent.
Preoperative Examinations
For all patients, a chest radiograph, barium meal, Doppler ultrasound examination of the supraclavicular lymph nodes, and contrast enhanced computed tomography scan of the chest and abdomen were routinely administrated to evaluate the clinical stage of the tumor. Endoscopic ultrasonography (EUS) was also performed for most of these patients after the year 2010. Positron emission tomography (PET) was not routinely performed before surgery.
Data Collection
All clinicopathological data and laboratory data were obtained from the patients’ medical records. Tumors were staged according to the 8th edition American Joint Committee on Cancer TNM staging system for ESCC. Weight and height were collected within 1 week before surgery. Alcohol drinking was divided into ever and never. An ever drinker was defined as a person who drank ≥1 time per week. BMI was calculated as follows: (weight, kg)/(height2, m2).
Surgery
Most of the patients underwent esophagectomy through a right thoracotomy, while other patients underwent a left thoracotomy. For lymphadenectomy, the regional lymph nodes in the middle mediastinal, lower mediastinal, and upper abdominal regions were routinely dissected for all patients. For patients who underwent esophagectomy through a right thoracotomy, the lymph nodes around the left and right recurrent laryngeal nerves were also dissected.
Statistical Analysis
Categorical variables were compared by the χ2 test or Fisher’s exact test. Overall survival (OS) and cancer-specific survival (CSS) were estimated using the Kaplan-Meier method, and the survival differences were assessed by the log-rank test. Multivariate Cox regression analysis was applied to identify independent prognostic factors, and all clinicopathological factors were included in the analysis due to the highly stable results. A receiver operating characteristic (ROC) curve was constructed to evaluate the sensitivity and specificity for 5-year OS, and the highest Youden’s index was used to identify the appropriate cutoff point for BMI. P< 0.05 was set to indicate significance. All statistical analyses were conducted in SPSS 20.0 software (IBM, Armonk, New York, USA).
Results
Patient Characteristics
Of the 817 patients with esophageal carcinoma who underwent esophagectomy between September 2014 and December 2017, 761 patients were diagnosed with ESCC. We excluded 116 patients who received neoadjuvant therapy (including 94 patients who received neoadjuvant chemoradiotherapy, 13 patients who received neoadjuvant radiotherapy, and 9 patients who received neoadjuvant chemotherapy) and 25 patients lacking any follow-up data, leaving 620 patients for analysis in this study. The study cohort included 229 ever drinkers and 391 never drinkers. Most patients were males (76.9%), and the median age was 61 years (range, 38 to 84 years). The mean number of lymph nodes dissected was 26.8 ± 11.0, with a median number of 26 (range, 6–74). Based on the 8th edition TNM staging system, 283 patients (45.6%) had pN0 disease, 207 patients (33.4%) had pN1 disease, 102 patients (16.5%) had pN2 disease, and 28 patients (4.5%) had pN3 disease. Radical resection was performed in 594 patients (95.8%), while palliative resection was performed in 26 patients (4.2%). The hospital mortality rate was 0.5% (3/620).
Twenty-two patients in this study had multiple primary malignancies (including 5 patients with synchronous malignancy and 17 patients with metachronous malignancy). The most common sites for multiple primary malignancies were head and neck in 10 cases, the esophagogastric junction in 5 cases, the lung in 3 cases, the stomach in 2 cases, the breast in 1 case, and the colon in 1 case.
Cutoff Point for BMI
The median preoperative BMI was 20.90 kg/m2 (range, 13.30–32.70). One hundred seventeen patients were underweight (BMI <18.5 kg/m2), 448 patients were normal weight (BMI ≥ 18.5 to 24.9 kg/m2), and the other 55 patients were overweight or obese (BMI ≥ 25.0 kg/m2). To maximize the predictive value for BMI, we used the ROC curve to determine the appropriate cutoff point, which was 18.75 kg/m2 in this study. We further subdivided all the patients into two groups for analysis: the low BMI group (≤18.75 kg/m2) and the high BMI group (>18.75 kg/m2).
Correlation Between Alcohol Consumption Status and Clinicopathological Factors
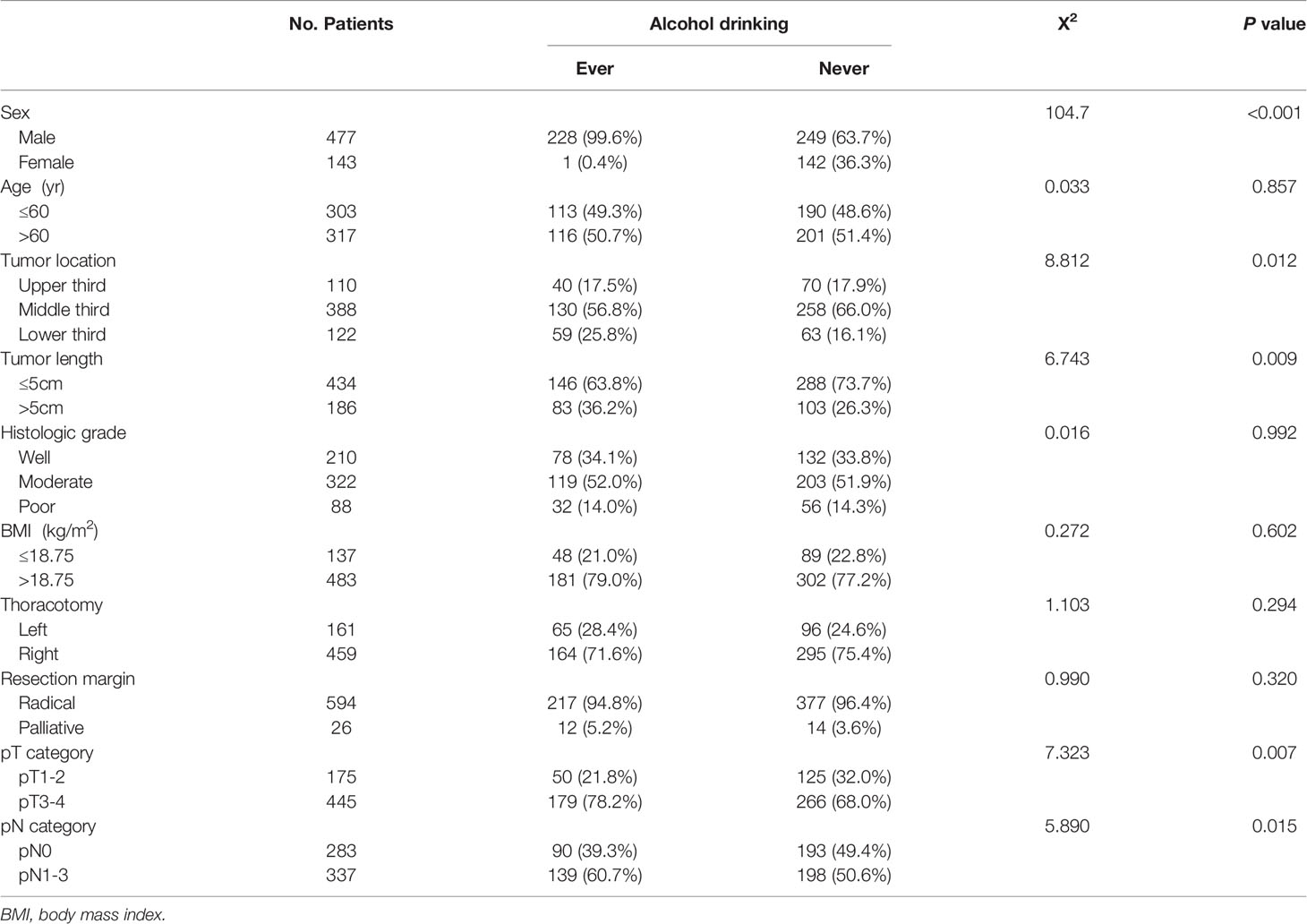
Table 1 shows the correlation between alcohol consumption status and patient clinicopathological factors. Of the 229 ever drinkers, there was only one female patient (0.4%), which was a significantly lower percentage than that of female never drinkers (36.3%) (P<0.001). Ever drinkers were also found to have longer tumor lengths, advanced pT category disease and advanced pN category disease (P<0.05). Moreover, the tumors of ever drinkers were more often located in the lower third of the esophagus (P=0.012). However, there was no significant difference in BMI between ever drinkers and never drinkers.
Survival Analysis for the Entire Patient Cohort
The last follow-up was conducted in December 2020, with a mean follow-up time of 34.7 months (range, 1–69 months). Two hundred and fifteen patients died, and 10 patients were lost to follow-up (1.6%).
The 1-, 3- and 5-year OS rates for the entire group were 88.5%, 66.0% and 61.3%, respectively, and the 1-, 3- and 5-year CSS rates were 88.7%, 66.3% and 62.2%. In univariate analysis for OS, tumor length, BMI, thoracotomy, resection margin, pT category, and pN category were significantly correlated with survival (Table 2). Patients with high BMI had a significantly better OS than those with low BMI (63.5% vs. 53.5%, P=0.045). Although the 5-year OS of 57.1% for ever drinkers was lower than that of 63.8% for never drinkers, the difference was not significant (P=0.077). In univariate analysis for CSS, only tumor length, thoracotomy, resection margin, pT category, and pN category were significantly correlated with survival. Although the 5-year CSS of 55.4% for patients with low BMI was lower than that of 64.1% for patients with high BMI, the difference was not significant (P=0.069).
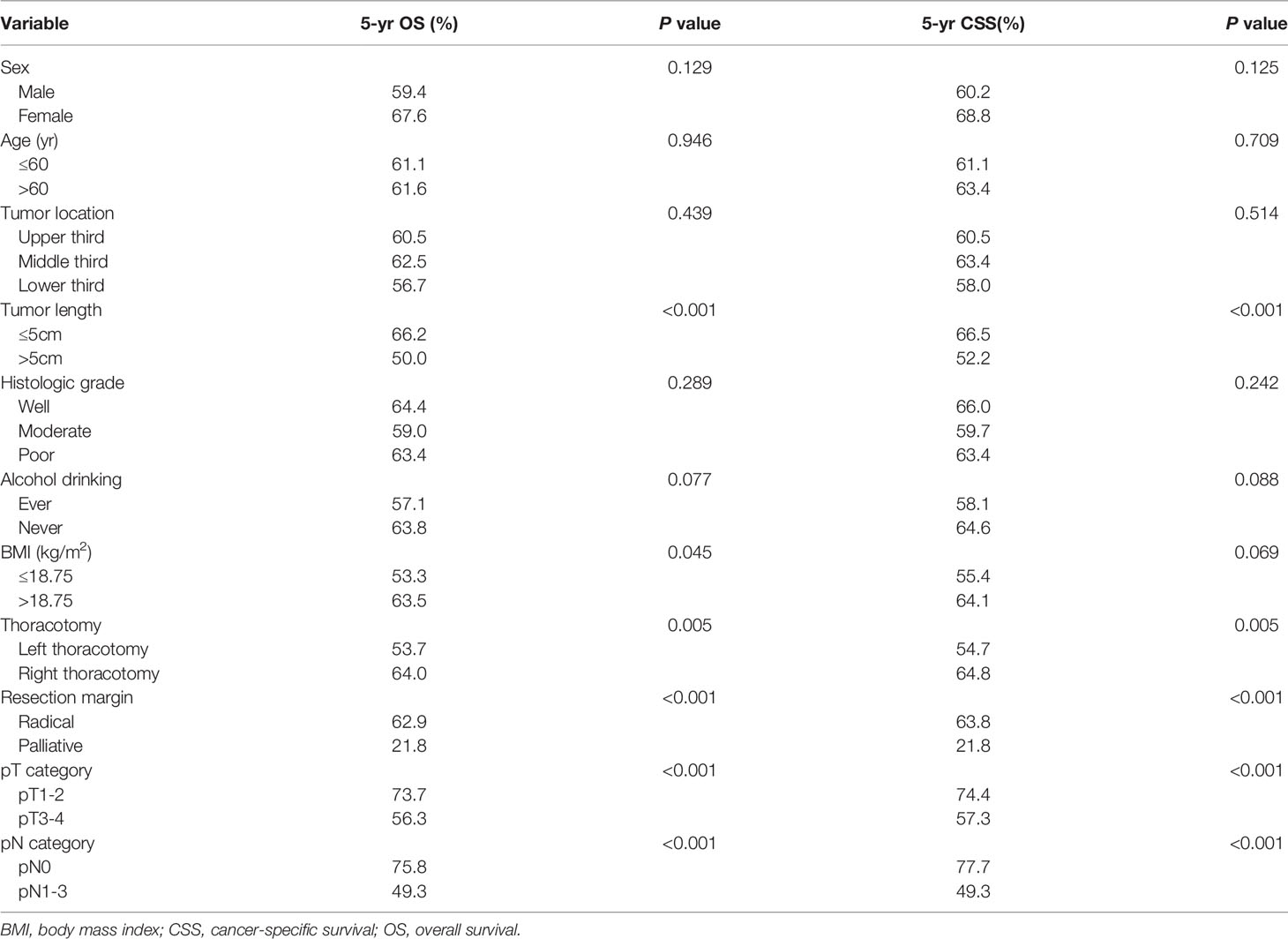
Table 2 Univariate analysis in regard to overall survival and cancer-specific survival according to clinicopathological factors.
In multivariate analysis for the entire group, only thoracotomy, resection margin, and pN category were found to be independent predictors for OS and CSS (Table 3). BMI and alcohol consumption were not independent prognostic factors for the entire group.
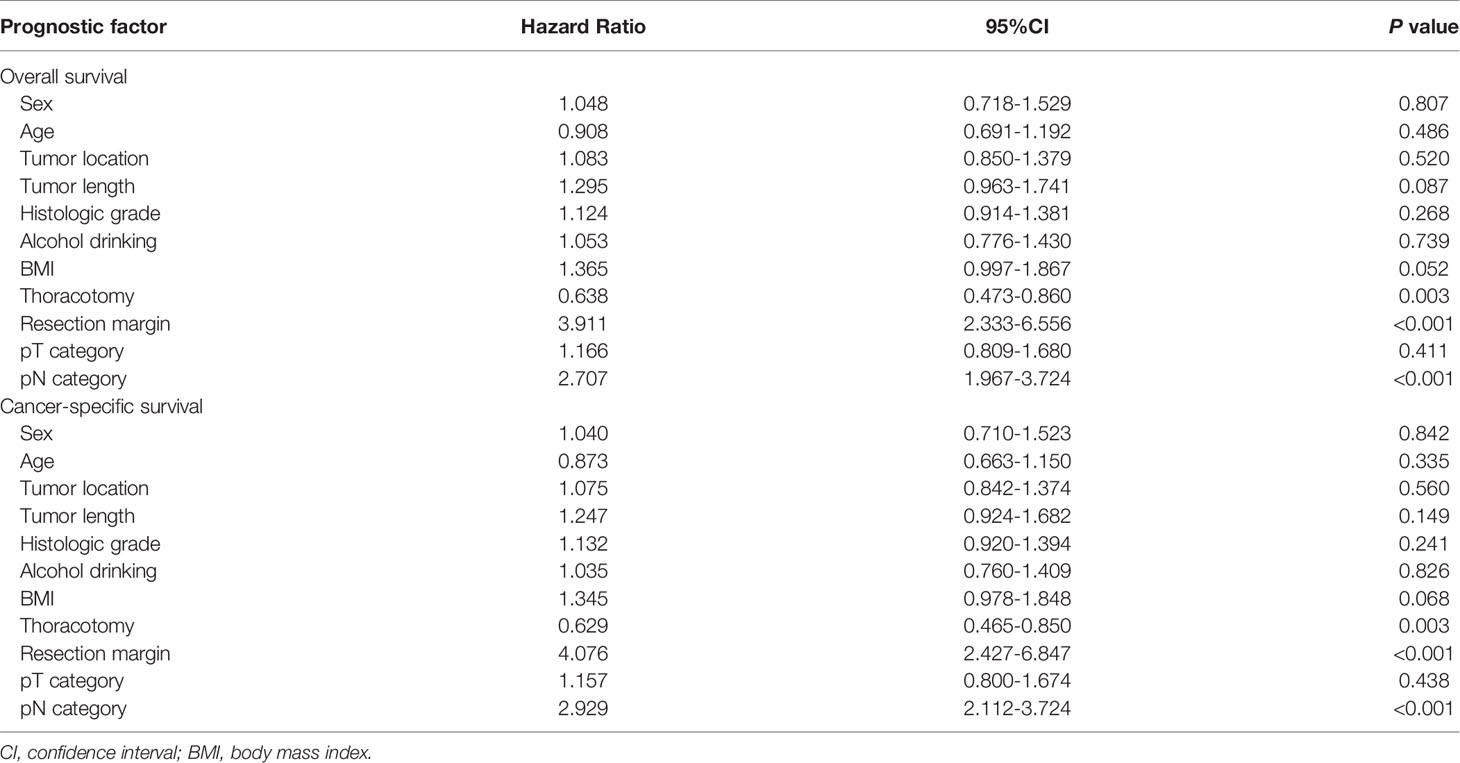
Table 3 Multivariate analysis in regard to overall survival and cancer-specific survival of the 620 patients with esophageal squamous cell carcinoma.
Survival Analysis Stratified by Alcohol Drinking Status
In univariate analysis for ever drinkers, age, tumor length, BMI, thoracotomy, resection margin, pT category, and pN category were found to be significantly correlated with OS and CSS (P<0.05). The 5-year OS and CSS for patients with low BMI were 46.1% and 46.1%, respectively, which were significantly lower than those for patients with high BMI (60.1% and 61.4%; P<0.05, Figure 1). In multivariate analysis for ever drinkers, low BMI was significantly correlated with worse OS (hazard ratio [HR] = 1.690; 95% confidence interval [CI], 1.037 to 2.756; P=0.035) and CSS (HR = 1.763; 95% CI, 1.077 to 2.886; P=0.024) than high BMI (Table 4).
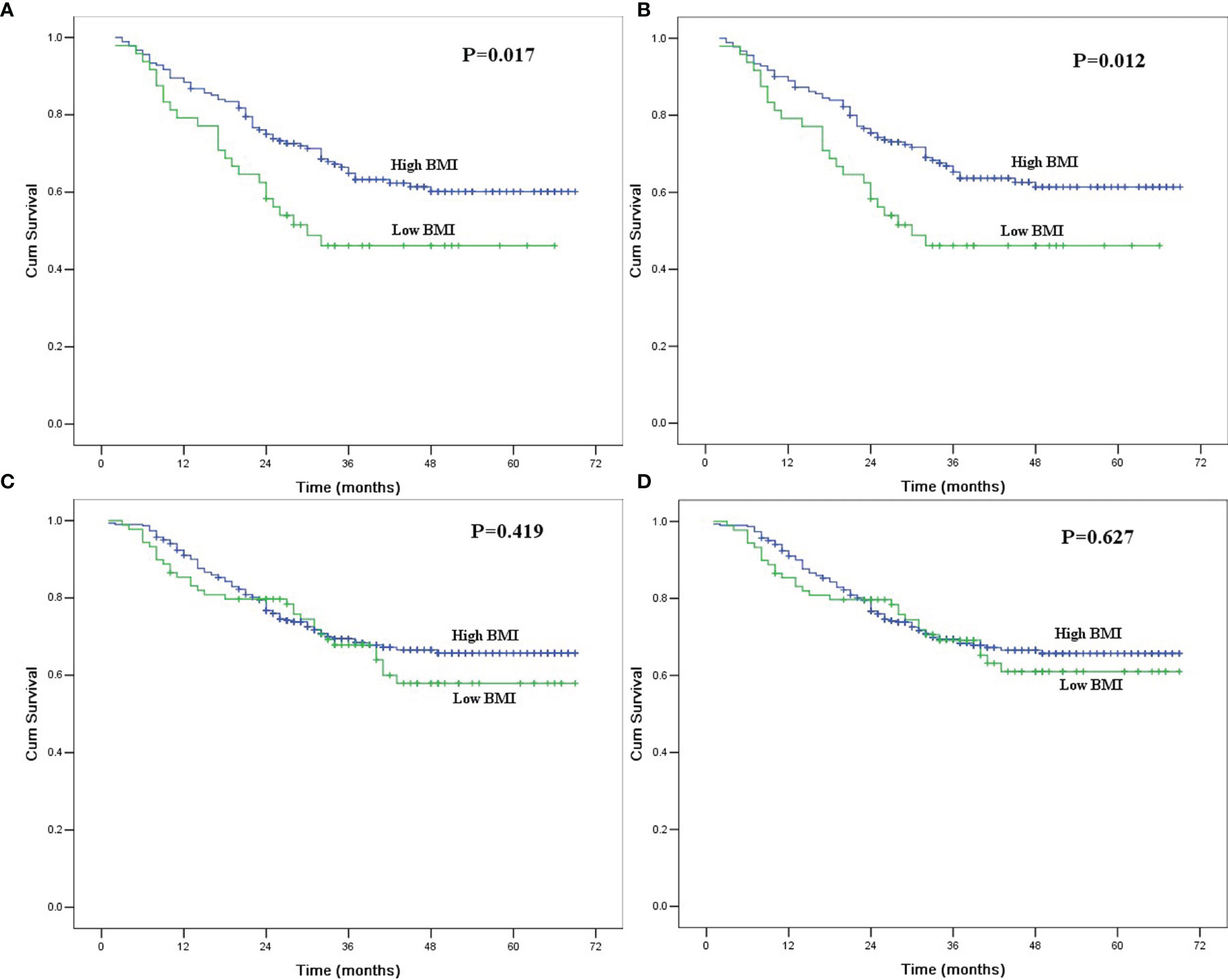
Figure 1 (A) Overall survival in ever drinkers with esophageal squamous cell carcinoma after esophagectomy according to body mass index. The difference was significant (P=0.017). (B) Cancer-specific survival in ever drinkers with esophageal squamous cell carcinoma after esophagectomy according to body mass index. The difference was significant (P=0.012). (C) Overall survival in never drinkers with esophageal squamous cell carcinoma after esophagectomy according to body mass index. The difference was not significant (P=0.419). (D) Cancer-specific survival in never drinkers with esophageal squamous cell carcinoma after esophagectomy according to body mass index. The difference was significant (P=0.627).
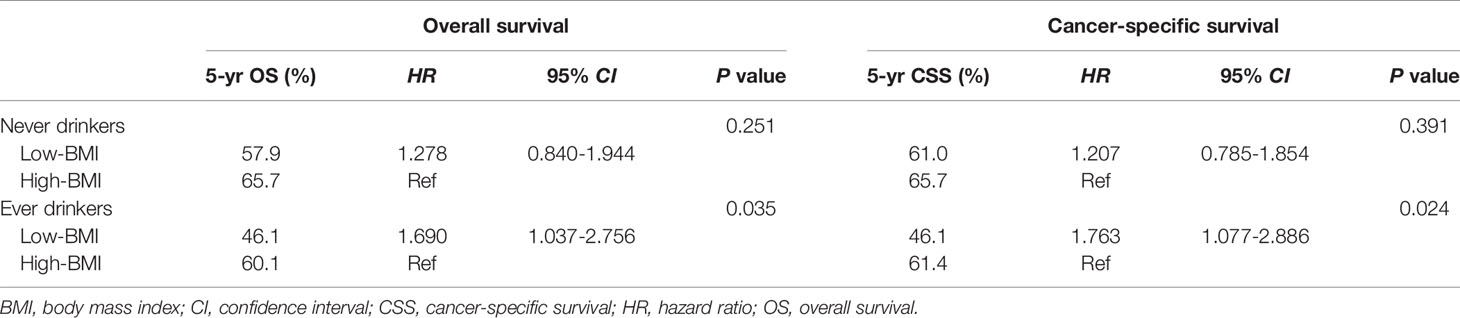
Table 4 Overall survival and cancer-specific survival according to body mass index stratified by alcohol drinking status in multivariable models adjusted for other clinicopathological factors.
In univariate analysis for never drinkers, tumor location, tumor length, thoracotomy, resection margin, pT category, and pN category were significantly correlated with OS and CSS (P<0.05). The 5-year OS and CSS for patients with low BMI were 57.9% and 61.0%, respectively, which were not significantly different from those for patients with high BMI (65.7% and 65.7%; P>0.05, Figure 1). In multivariate analysis for never drinkers, low BMI was not an independent predictor for worse OS (HR = 1.278; 95% CI, 0.840 to 1.944; P=0.251) or CSS (HR = 1.207; 95% CI, 0.785 to 1.854; P=0.391) compared with high BMI (Table 4).
Discussion
Low BMI has been recognized as an epidemiological risk factor for ESCC (7). However, the prognostic value of BMI in ESCC patients is still controversial. Sun et al. (10) evaluated the data of 502 ESCC patients from Southern China and found that the 5-year OS rate of 25.2% for patients with BMI <18.5 was significantly lower than those of 46.1% and 48.1% for patients with BMI ≥ 18.5 to 24.9 kg/m2 and BMI ≥ 25.0 kg/m2 (P<0.001). Another study from Japan by Kamachi et al. (28) found that ESCC patients with BMI <18.5 kg/m2 had significantly worse OS and disease-free survival. However, Hasegawa et al. (12) found that OS and relapse-free survival were not significantly different in ESCC patients with different BMI levels. Duan et al. (14) found that high BMI worsened the long-term survival of ESCC patients after esophagectomy. The reasons for these inconsistent results may be attributed to the different cutoff values for BMI, the different number of patients and confounding factors, such as sex, age, smoking status (14, 29), or perhaps alcohol drinking status.
Alcohol consumption is one of the leading risk factors for cancer development and cancer death globally (30) and has been shown to affect BMI in the general population (19–21). A previous study found that alcohol abuse raised the risk of developing ESCC synergistically with low BMI (31). Therefore, it may be reasonable to assume that the prognostic value of BMI in patients with ESCC may be impacted by their alcohol drinking status. However, no previous studies have investigated the potential effect of the interaction between BMI and alcohol consumption on the prognosis of patients with ESCC. In our current study, we evaluated the prognostic value of BMI in a large cohort of ESCC patients who underwent surgical resection when stratified by alcohol drinking status. We found that BMI was a prognostic factor only for ever drinkers but not for never drinkers. After adjusting for other clinicopathological factors, patients with low BMI still had worse OS (HR = 1.690; 95% CI, 1.037 to 2.756; P=0.035) and CSS (HR = 1.763; 95% CI, 1.077 to 2.886; P=0.024) than patients with high BMI. However, in never drinkers, the 5-year OS and CSS were not significantly different between patients with low and high BMI in univariate and multivariate analyses. These results highlight the importance of alcohol drinking status in the prognostic prediction of BMI in ESCC patients and, for the first time, reveal that low BMI adversely impacts survival only in ever drinkers with ESCC but not in never drinkers.
The mechanism underlying the effect of the interaction between low BMI and alcohol consumption on the outcomes of ESCC patients is still not clear. We speculate that there are several possible explanations. First, malnutrition reflected in low BMI may be associated with a compromised immune system, which can lead to the development and early metastasis of cancer (32, 33). Immune cells in the tumor microenvironment may play an important role in regulating tumor progression (34). Malnourished patients have reduced lymphocyte function and cellular immunity, which leads to easier cancer development and metastasis (35). Chronic alcohol abuse also reduces cell-mediated immunity and lymphocyte numbers (36), which might enhance the adverse impact of malnutrition on the prognosis of cancer patients. Second, both ethanol and its metabolite acetaldehyde are carcinogenic to humans, which may lead to cancer development and metastasis (37). These effects may further be amplified by malnutrition, which is usually associated with insufficiency of micronutrients from improper maintenance of antioxidants and immune functions (31). Third, the presence of malnutrition might decrease the tolerance and response to treatment, which might also lead to the poor prognosis of cancer patients (38, 39). These effects may be amplified by alcohol consumption, as a previous study also showed that patients who consumed alcohol had poorer response rates to chemotherapy, smaller radiation doses, and less multimodality treatment (40). Based on these theories, whether a nutritional intervention and alcohol drinking cessation will improve the prognosis of these patients also needs to be investigated.
The strengths of our study are the relatively large patient cohort and homogeneity in histopathology and treatment. All of our patients chose esophagectomy as their initial treatment and were histopathologically diagnosed with ESCC. We excluded patients who received neoadjuvant therapy to avoid potential treatment-related BMI changes. Nevertheless, our study also has several limitations. First, it was a retrospective study from a single center, which undermined its power. Second, most of the patients who consumed alcohol in this study were male patients, with only one patient who consumed alcohol being female. Thus, we are unable to elucidate the interaction between low BMI and alcohol consumption in female patients with ESCC. Third, we did not consider the synergistic effect between alcohol and tobacco consumption in this study, as a previous study found that the prognostic value of BMI may be impacted by smoking status in patients with ESCC, and alcohol consumption and smoking are highly correlated behaviors (29). Further studies with larger cohorts are needed to confirm our results and investigate the mechanism underlying the effect of the interaction between low BMI and alcohol consumption on the prognosis of patients with ESCC.
In conclusion, our study showed that low BMI was independently associated with worse survival only in ever drinkers with ESCC but not in never drinkers. Further studies should be conducted to evaluate our findings and elucidate the mechanism underlying the effect of the interaction between BMI and alcohol consumption on the prognosis of patients with ESCC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of Shantou University Medical College Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-pC designed the research and wrote part of the paper. S-bC analyzed the data and wrote part of the paper. D-tL wrote part of the paper. All authors contributed to the article and approved the submitted version.
Funding
The Medical Scientific Research Foundation of Guangdong Province of China (B2019070).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank American Journal Experts (www.aje.cn) for English language editing.
References
1. Rustgi AK, El-Serag HB. Esophageal Carcinoma. N Engl J Med (2014) 371(26):2499–509. doi: 10.1056/NEJMra1314530
2. Caccialanza R, Pedrazzoli P, Cereda E, Gavazzi C, Pinto C, Paccagnella A, et al. Nutritional Support in Cancer Patients: A Position Paper From the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J Cancer (2016) 7(2):131–5. doi: 10.7150/jca.13818
3. Shahjehan F, Merchea A, Cochuyt JJ, Li Z, Colibaseanu DT, Kasi PM. Body Mass Index and Long-Term Outcomes in Patients With Colorectal Cancer. Front Oncol (2018) 8:620. doi: 10.3389/fonc.2018.00620
4. Gama RR, Song Y, Zhang Q, Brown MC, Wang J, Habbous S, et al. Body Mass Index and Prognosis in Patients With Head and Neck Cancer. Head Neck (2017) 39(6):1226–33. doi: 10.1002/hed.24760
5. Juszczyk K, Kang S, Putnis S, Winn R, Chen J, Aghmesheh M, et al. High body mass index is Associated With an Increased Overall Survival in Rectal Cancer. J Gastrointest Oncol (2020) 11(4):626–32. doi: 10.21037/jgo-20-48
6. Guo Q, Burgess S, Turman C, Bolla MK, Wang Q, Lush M, et al. Body Mass Index and Breast Cancer Survival: A Mendelian Randomization Analysis. Int J Epidemiol (2017) 46(6):1814–22. doi: 10.1093/ije/dyx131
7. Smith M, Zhou M, Whitlock G, Yang G, Offer A, Hui G, et al. Esophageal Cancer and Body Mass Index: Results From a Prospective Study of 220,000 Men in China and a Meta-Analysis of Published Studies. Int J Cancer (2008) 122(7):1604–10. doi: 10.1002/ijc.23198
8. Deng HY, Qin CL, Qiu XM, Zhou QH. Does High Body Mass Index Have Any Impact on Survival of Patients Undergoing Oesophagectomy for Oesophageal Cancer? Interact Cardiovasc Thorac Surg (2018) 26(4):693–5. doi: 10.1093/icvts/ivx403
9. Ren C, Cai XY, Qiu MZ, Wang DS, Wang FH, Luo HY, et al. Impact of Body Mass Index on Survival of Esophageal Squamous Carcinoma Patients in Southern China. J Thorac Dis (2015) 7(3):337–45. doi: 10.3978/j.issn.2072-1439.2014.10.12
10. Sun P, Zhang F, Chen C, An X, Li YH, Wang FH, et al. Comparison of the Prognostic Values of Various Nutritional Parameters in Patients With Esophageal Squamous Cell Carcinoma From Southern China. J Thorac Dis (2013) 5(4):484–91. doi: 10.3978/j.issn.2072-1439.2013.08.38
11. Zhang HL, Yang YS, Duan JN, Shang QX, He SL, Gu YM, et al. Prognostic Value of Preoperative Weight Loss-Adjusted Body Mass Index on Survival After Esophagectomy for Esophageal Squamous Cell Carcinoma. World J Gastroenterol (2020) 26(8):839–49. doi: 10.3748/wjg.v26.i8.839
12. Hasegawa T, Kubo N, Ohira M, Sakurai K, Toyokawa T, Yamashita Y, et al. Impact of Body Mass Index on Surgical Outcomes After Esophagectomy for Patients With Esophageal Squamous Cell Carcinoma. J Gastrointest Surg (2015) 19(2):226–33. doi: 10.1007/s11605-014-2686-y
13. Miao L, Chen H, JXiang J, Zhang Y. A High Body Mass Index in Esophageal Cancer Patients is Not Associated With Adverse Outcomes Following Esophagectomy. J Cancer Res Clin Oncol (2015) 141(5):941–50. doi: 10.1007/s00432-014-1878-x
14. Duan XF, Tang P, Shang XB, Jiang HJ, Zhao Q, Yu ZT. High Body Mass Index Worsens Survival in Patients With Esophageal Squamous Cell Carcinoma After Esophagectomy. Dig Surg (2017) 34(4):319–27. doi: 10.1159/000453044
15. Pan W, Sun Z, Xiang Y, Fang W. The Correlation Between High Body Mass Index and Survival in Patients With Esophageal Cancer After Curative Esophagectomy: Evidence From Retrospective Studies. Asia Pac J Clin Nutr (2015) 24(3):480–8. doi: 10.6133/apjcn.2015.24.3.05
16. GBD 2016 Alcohol Collaborators. Alcohol use and Burden for 195 Countries and Territories, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet (2018) 392(10152):1015–35. doi: 10.1016/S0140-6736(18)31310-2
17. Jürgen R, Shield KD, Weiderpass E. Alcohol Consumption. A Leading Risk Factor for Cancer. Chem Biol Interact (2020) 331:109280. doi: 10.1016/j.cbi.2020.109280
18. Rumgay H, Shield K, Charvat H, Ferrari P, Sornpaisarn B, Obot I, et al. Global Burden of Cancer in 2020 Attributable to Alcohol Consumption: A Population-Based Study. Lancet Oncol (2021) 22(8):1071–80. doi: 10.1016/S1470-2045(21)00279-5
19. Traversy G, Chaput JP. Alcohol Consumption and Obesity: An Update. Curr Obes Rep (2015) 4(1):122–30. doi: 10.1007/s13679-014-0129-4
20. Sayon-Orea C, Martinez-Gonzalez MA, Bes-Rastrollo M. Alcohol Consumption and Body Weight: A Systematic Review. Nutr Rev (2011) 69(8):419–31. doi: 10.1111/j.1753-4887.2011.00403.x
21. Kleiner KD, Gold MS, Frost-Pineda K, Lenz-Brunsman B, Perri MG, Jacobs WS. Body Mass Index and Alcohol Use. J Addict Dis (2004) 23(3):105–18. doi: 10.1300/J069v23n03_08
22. Lee WT, Hsiao JR, Ou CY, Huang CC, Chang CC, Tsai ST, et al. The Influence of Prediagnosis Alcohol Consumption and the Polymorphisms of Ethanol-Metabolizing Genes on the Survival of Head and Neck Cancer Patients. Cancer Epidemiol Biomarkers Prev (2019) 28(2):248–57. doi: 10.1158/1055-9965.EPI-18-0425
23. Giraldi L, Leoncini E, Pastorino R, Wünsch-Filho V, de Carvalho M, Lopez R, et al. Alcohol and Cigarette Consumption Predict Mortality in Patients With Head and Neck Cancer: A Pooled Analysis Within the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Ann Oncol (2017) 28(11):2843–51. doi: 10.1093/annonc/mdx486
24. Duffy SA, Ronis DL, McLean S, Fowler KE, Gruber SB, Wolf GT, et al. Pretreatment Health Behaviors Predict Survival Among Patients With Head and Neck Squamous Cell Carcinoma. J Clin Oncol (2009) 27(12):1969–75. doi: 10.1200/JCO.2008.18.2188
25. Ma Q, Liu W, Jia R, Long H, Zhang L, Lin P, et al. Alcohol and Survival in ESCC: Prediagnosis Alcohol Consumption and Postoperative Survival in Lymph Node-Negative Esophageal Carcinoma Patients. Oncotarget (2016) 7(25):38857–63. doi: 10.18632/oncotarget.8754
26. McCain RS, McManus DT, McQuaid S, James JA, Salto-Tellez M, Reid NB, et al. Alcohol Intake, Tobacco Smoking, and Esophageal Adenocarcinoma Survival: A Molecular Pathology Epidemiology Cohort Study. Cancer Causes Control (2020) 31(1):1–11. doi: 10.1007/s10552-019-01247-2
27. Thrift AP, Nagle CM, Fahey PP, Smithers BM, Watson DI, Whiteman DC. Predictors of Survival Among Patients Diagnosed With Adenocarcinoma of the Esophagus and Gastroesophageal Junction. Cancer Causes Control (2012) 23(4):555–64. doi: 10.1007/s10552-012-9913-1
28. Kamachi K, Ozawa S, Hayashi T, Kazuno A, Ito E, Makuuchi H. Impact of Body Mass Index on Postoperative Complications and Long-Term Survival in Patients With Esophageal Squamous Cell Cancer. Dis Esophagus (2016) 29(3):229–35. doi: 10.1111/dote.12327
29. Sun P, Zhang F, Chen C, Ren C, Bi XW, Yang H, et al. Prognostic Impact of Body Mass Index Stratified by Smoking Status in Patients With Esophageal Squamous Cell Carcinoma. Onco Targets Ther (2016) 9:6389–97. doi: 10.2147/OTT.S111843
30. Rehm J, Shield KD, Weiderpass E. Alcohol Consumption. A Leading Risk Factor for Cancer. Chem Biol Interact (2020) 331:109280. doi: 10.1016/j.cbi.2020.109280
31. Choi YJ, Lee DH, Han K, Yoon H, Shin CM, Park YS, et al. Joint Effects of Low Body Mass Index and Alcohol Consumption on Developing Esophageal Squamous Cell Cancer: A Korean Nationwide Population-Based Cohort Study. Asian Pac J Cancer Prev (2017) 18(7):1881–7. doi: 10.22034/APJCP.2017.18.7.1881
32. Van Cutsem E, Arends J. The Causes and Consequences of Cancer-Associated Malnutrition. Eur J Oncol Nurs (2005) 9:S51–63. doi: 10.1016/j.ejon.2005.09.007
33. Scarpa M, Cagol M, Bettini S, Alfieri R, Carraro A, Cavallin F, et al. Overweight Patients Operated on for Cancer of the Esophagus Survive Longer Than Normal-Weight Patients. J Gastrointest Surg (2013) 17(2):218–27. doi: 10.1007/s11605-012-2023-2
34. Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer Immunology–Analysis of Host and Tumor Factors for Personalized Medicine. Nat Rev Clin Oncol (2011) 8(12):711–9. doi: 10.1038/nrclinonc.2011.122
35. Morley JE, Thomas DR, Wilson MM. Cachexia: Pathophysiology and Clinical Relevance. Am J Clin Nutr (2006) 83(4):735–43. doi: 10.1093/ajcn/83.4.735
36. Cook RT. Alcohol Abuse, Alcoholism, and Damage to the Immune System–a Review. Alcohol Clin Exp Res (1998) 22(9):1927–42. doi: 10.1111/j.1530-0277.1998.tb05900.x
38. Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do Patients With Weight Loss Have a Worse Outcome When Undergoing Chemotherapy for Gastrointestinal Malignancies? Eur J Cancer (1998) 34(4):503–9. doi: 10.1016/s0959-8049(97)10090-9
39. Di Fiore F, Lecleire S, Pop D, Rigal O, Hamidou H, Paillot B, et al. Baseline Nutritional Status is Predictive of Response to Treatment and Survival in Patients Treated by Definitive Chemoradiotherapy for a Locally Advanced Esophageal Cancer. Am J Gastroenterol (2007) 102(11):2557–63. doi: 10.1111/j.1572-0241.2007.01437.x
Keywords: alcohol drinking, body mass index, esophageal neoplasm, prognosis, squamous cell carcinoma
Citation: Chen S-b, Liu D-t and Chen Y-p (2022) Prognostic Value of Body Mass Index Stratified by Alcohol Drinking Status in Patients With Esophageal Squamous Cell Carcinoma. Front. Oncol. 12:769824. doi: 10.3389/fonc.2022.769824
Received: 10 September 2021; Accepted: 31 January 2022;
Published: 17 February 2022.
Edited by:
Alberto Biondi, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Miaozhen Qiu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaBin Zheng, Fujian Medical University, China
Copyright © 2022 Chen, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-ping Chen, c3RjaGVueXBAaG90bWFpbC5jb20=
 Shao-bin Chen
Shao-bin Chen Di-tian Liu
Di-tian Liu Yu-ping Chen
Yu-ping Chen