- 1Department of Orthopedic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Orthopedics Research Institute of Zhejiang University, Hangzhou, China
- 3Key Laboratory of Motor System Disease Research and Precision Therapy of Zhejiang Province, Hangzhou, China
- 4Department of Orthopedics, Ningbo No.6 Hospital, Ningbo, China
- 5Department of Orthopedic Oncology, Affiliated Hangzhou Cancer Hospital, Zhejiang University School of Medicine, Hangzhou, China
Purpose: The purpose of this study is to reveal the clinicopathological features and identify risk factors of prognosis among patients with pancreatic cancer bone metastasis (PCBM).
Patients and Methods: Patients with PCBM were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database between 2010 and 2016. Independent predictors for survival of those patients were determined by the univariate and multivariate Cox regression analysis. Forest plots were drawn by GraphPad 8.0.1 and used to visually display the results of multivariate analysis.
Results: We identified 2072 eligible PCBM patients, of which 839 patients (40.5%) were female. Patients with age >60 years accounted for 70.6%. Multivariable Cox regression analysis indicated that age, pathological type, chemotherapy, liver metastasis, lung metastasis, and marital status were independent prognostic factors for both overall survival (OS) and cancer-specific survival (CSS). Kaplan–Meier survival curves showed that for patients with PCBM, age ≤60 years, non-ductal adenocarcinoma type, chemotherapy, no liver metastasis, no lung metastasis, and married status were correlated with increased survival. This population-based study showed that 1-year OS and CSS were 13.6% and 13.7%, respectively.
Conclusion: The present study identified six independent predictors of prognosis in PCBM, including age, pathological type, chemotherapy, liver metastasis, lung metastasis, and marital status. Knowledge of these survival predictors is helpful for clinicians to accelerate clinical decision process and design personalized treatment for patients with PCBM.
Introduction
Pancreatic cancer (PC) is a highly aggressive and metastatic malignancy, characterized by a high mortality. It has an extremely poor survival, with 5-year survival rate of less than 8% (1–3). Even if the diagnosis and treatment technology of PC improve, its prognosis has improved marginally over the past decades (4). The majority of PC patients develop metastasis either at the time of initial diagnosis or after initial diagnosis, which posts a new challenge for clinicians (3). He et al. (5) reported that liver and peritoneum metastases accounted for 45.1% and 49.9% of metastatic PC patients, respectively, which may be due to their anatomical sites (6). Other less common sites are the lung (11.4%), brain (0.4%), and bone (3.8%) (5). Although some previous studies focused on liver or lung metastasis of PC (7–9), studies on bone metastasis are scarce. The prevalence of bone metastasis in PC patients ranges from 5% to 20% (10, 11). Bone metastasis can result in skeletal related events (SREs), including hypercalcemia, bone pain, pathological fractures, spinal cord compression, and radiotherapy or surgery to the bone, which not only decreases the quality of life but also contributes to an unfavorable prognosis (12–14). Additionally, most bone metastases caused by pancreatic cancer are lytic lesions (15).
Previous studies reported that age, gender, tumor size, tumor location, histological type, T and N stages, histologic differentiation, radiotherapy, and chemotherapy were independent prognostic factors for PC (16, 17). Liu et al. (18) reported that age, gender, tumor size, alanine aminotransferase level (ALT) and CA19-9 were correlated with distant metastasis. Current therapeutic options for PC included surgical resection, radiotherapy, chemotherapy, immunotherapy, and et al. (19). However, there are few studies on the treatment of metastatic PC. To our knowledge, there are few studies reporting the pancreatic cancer bone metastasis (PCBM). Therefore, the present study was conducted to define the clinicopathological features and identify independent survival predictors of PCBM. To obtain insight into PCBM, we conducted a large-scale study by analyzing data of PCBM from the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and Methods
Patient Selection
Data of patients diagnosed with PCBM, were extracted from the SEER*Stat version 8.3.9 software between 2010 and 2016. The SEER database collects malignant cancer information from 18 population-based cancer registries, representing approximately a third of the U.S. population (20). As the SEER database is public available and contains no patient-identified information, ethnic approval is not needed.
We first used the case-listing session to select the Site recode ICD-O-3/WHO 2008 “Pancreas”. Meanwhile, we set the SEER Combined Mets at DX-bone (2010+) to be YES. Thus, a total of 2842 patients with PCBM were enrolled. 764 patients diagnosed not by pathology were excluded. Additionally, six patients where the cancer was found upon autopsy or necropsy were excluded. Figure 1 showed the flow chart for selection of study population. Variables obtained from the SEER database were race, gender, age at diagnosis, primary tumor site, pathological type, tumor size, surgery, radiotherapy, chemotherapy, organ metastases, marital status, vital status, survival time, and cause of death. Surgery or radiotherapy in the present study refers to treatment for primary tumor site. Overall survival (OS) was defined as the time from the date of diagnosis to the time of death from any cause (21, 22). Cancer-specific survival (CSS) was defined as the time from the date of diagnosis to the date of death from PC (21, 22).
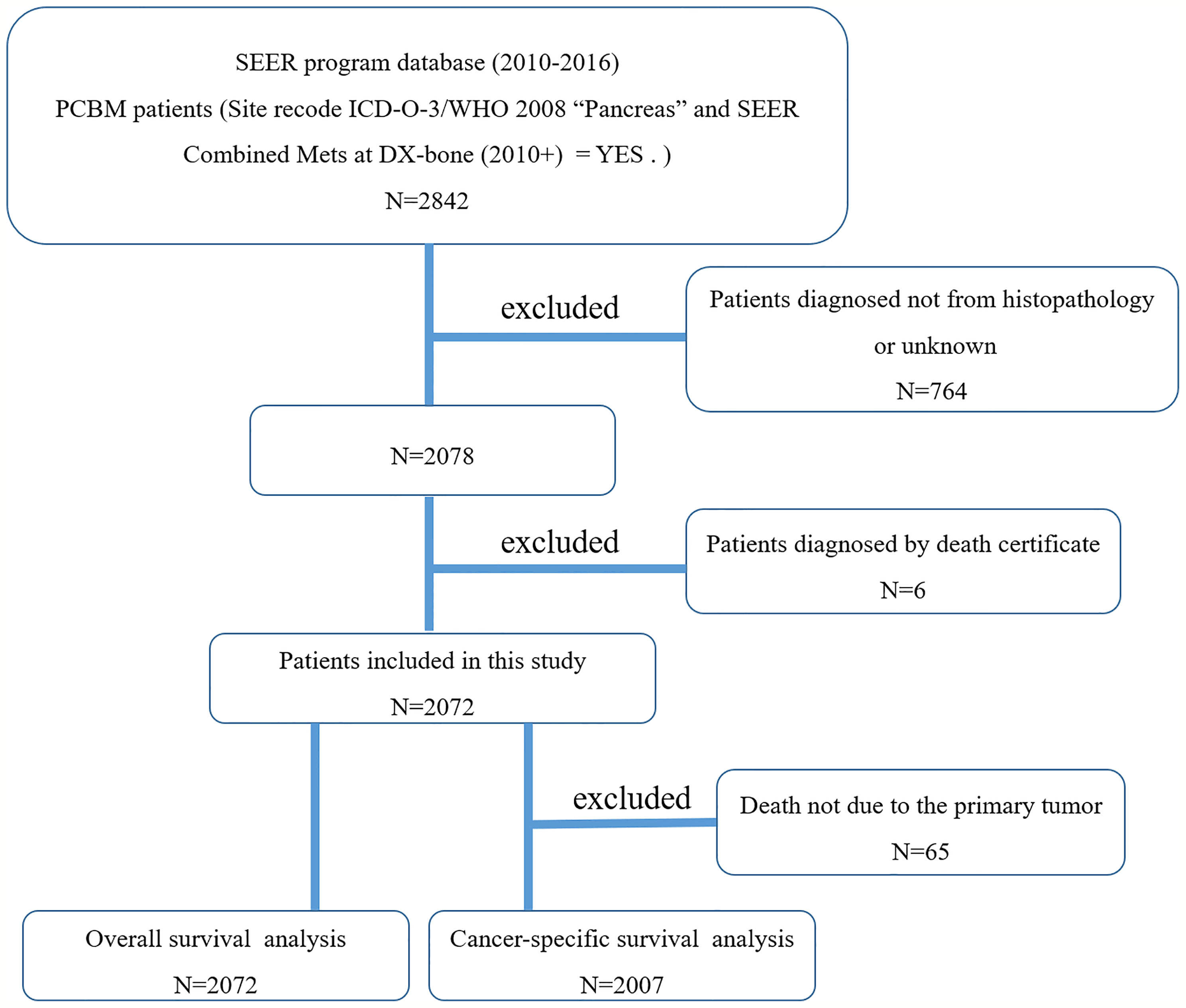
Figure 1 The flow chart for selection of study population. (SEER, Surveillance, Epidemiology, and End Results; ICD-O-3, international classification of diseases for oncology, 3rd edition; PCBM, pancreatic cancer bone metastasis).
Statistical Methods
Univariate and multivariable Cox regression models were used to identify independent predictors of OS and CSS. Meanwhile, hazard ratio (HR) and their 95% confidence interval (95% CIs) were presented in both univariate and multivariate analysis. Kaplan–Meier survival curves were applied to compare the differences among these groups by the log-rank test. Chi-square test is used to compare the rates of two groups. SPSS 21.0 software was used to conduct all above statistical tests and risk factors of p<0.05 were considered as significant predictors. Additionally, we drew Forest plots by GraphPad 8.0.1 to visually display the results of multivariate analysis.
Results
Clinicopathologic Characteristics
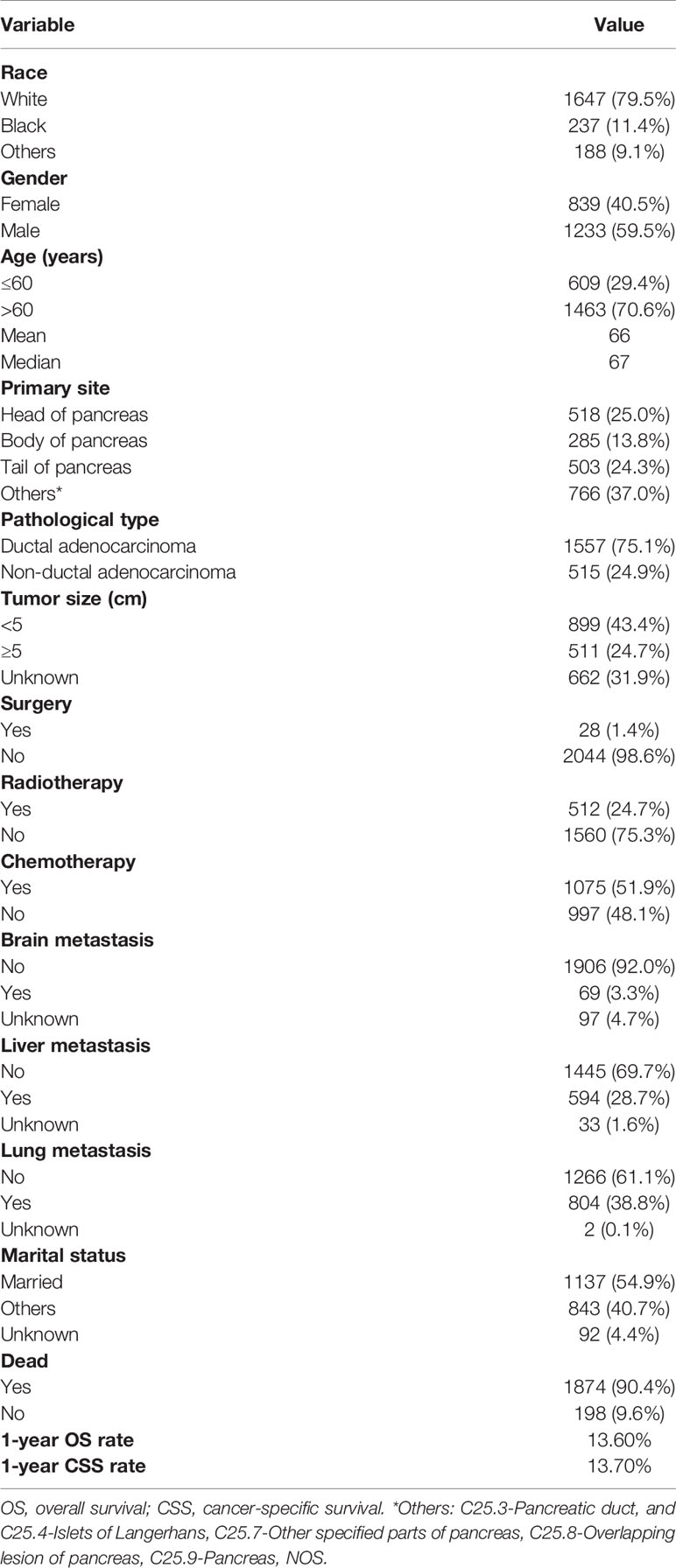
We identified 2072 eligible patients with PCBM. The baseline characteristics of all patients in the current study were summarized in the Table 1. Among the population, 79.5%, 11.4%, and 9.1% of patients were white, black, and other races, respectively. More than half of the patients (n=1233, 59.5%) were males. As for age, more patients with age > 60 years (n=1463, 70.6%) were observed. There were 518(25.0%), 285(13.8%), and 503(24.3%) of cases located in head of pancreas, body of pancreas, and tail of pancreas, respectively. Ductal adenocarcinoma accounted for 75.1% of all pathological type. 899(43.4%) of patients presented with tumor size <5 cm, and 511(24.7%) of patients presented with tumor size ≥ 5 cm. Regarding the treatment, more than half of the patients (51.9%) received chemotherapy, while only 24.7% and 1.4% of patients received radiotherapy and surgery, respectively. As for visceral metastasis, only 3.3% of patients presented with brain metastasis, while 28.7% and 38.8% of patients developed liver and lung metastases, respectively. In addition, more than half of the patients (n=1233, 59.5%) got married. The 1-year OS and CSS rates of the population were 13.6% and 13.7%, respectively.
Univariate Cox Regression Analysis
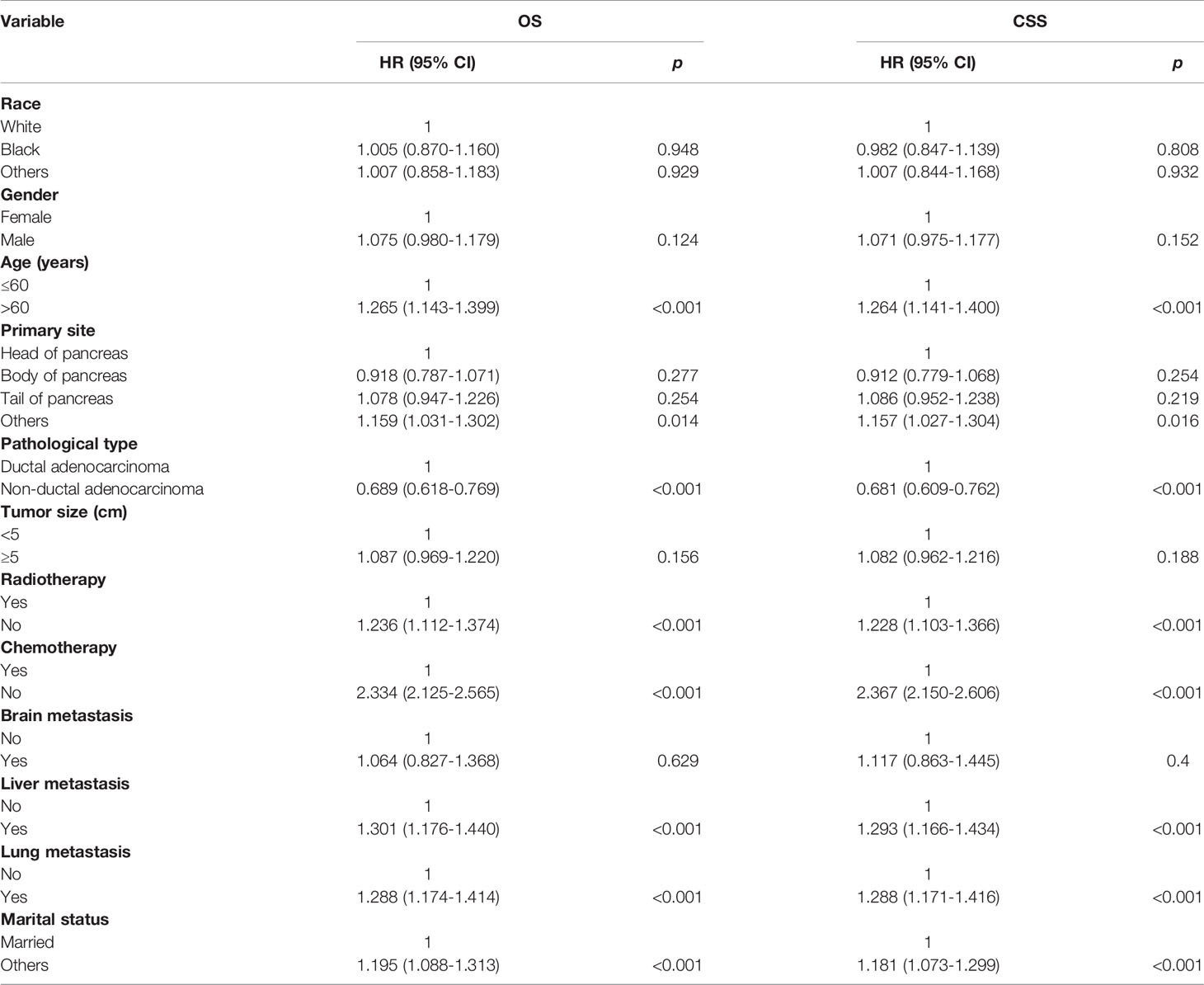
Potential risk factors for the OS and CSS by univariate analysis are summarized in Table 2. The results showed that age (>60 years vs. ≤60 years, HR=1.265; 95% CI, 1.143-1.399; p<0.001), primary site (Body of pancreas vs. Head of pancreas, HR=0.918, 95% CI, 0.787-1.071, p=0.277; Tail of pancreas vs. Head of pancreas, HR=1.078, 95% CI, 0.947-1.226, p=0.254; Others vs. Head of pancreas, HR=1.159, 95% CI, 1.031-1.302, p=0.014), pathological type (Non-ductal adenocarcinoma vs. Ductal adenocarcinoma, HR=0.689; 95% CI, 0.618-0.769; p<0.001), local radiotherapy (No vs. Yes, HR=1.236; 95% CI, 1.112-1.374; p<0.001), systemic chemotherapy (No vs. Yes, HR=2.334; 95% CI, 2.125-2.565; p<0.001), liver metastasis (Yes vs. No, HR=1.301; 95% CI, 1.176-1.440; p<0.001), lung metastasis (Yes vs. No, HR=1.288; 95% CI, 1.174-1.414; p<0.001), and marital status(Others vs. Married, HR=1.195; 95% CI, 1.088-1.313; p<0.001), were significantly associated with OS. In terms of CSS, age (>60 years vs. ≤60 years, HR=1.264; 95% CI, 1.141-1.400; p<0.001), primary site (Body of pancreas vs. Head of pancreas, HR=0.912, 95% CI, 0.779-1.068, p=0.254; Tail of pancreas vs. Head of pancreas, HR=1.086, 95% CI, 0.952-1.238, p=0.219; Others vs. Head of pancreas, HR=1.157, 95% CI, 1.027-1.304, p=0.016), pathological type (Non-ductal adenocarcinoma vs. Ductal adenocarcinoma, HR=0.681; 95% CI, 0.609-0.762; p<0.001), local radiotherapy (No vs. Yes, HR=1.228; 95% CI, 1.103-1.366; p<0.001), systemic chemotherapy (No vs. Yes, HR=2.367; 95% CI, 2.150-2.606; p<0.001), liver metastasis (Yes vs. No, HR=1.293; 95% CI, 1.166-1.434; p<0.001), lung metastasis (Yes vs. No, HR=1.288; 95% CI, 1.171-1.416; p<0.001), and marital status(Others vs. Married, HR=1.181; 95% CI, 1.073-1.299; p<0.001), were significant predictors.
Multivariate Cox Regression Analysis
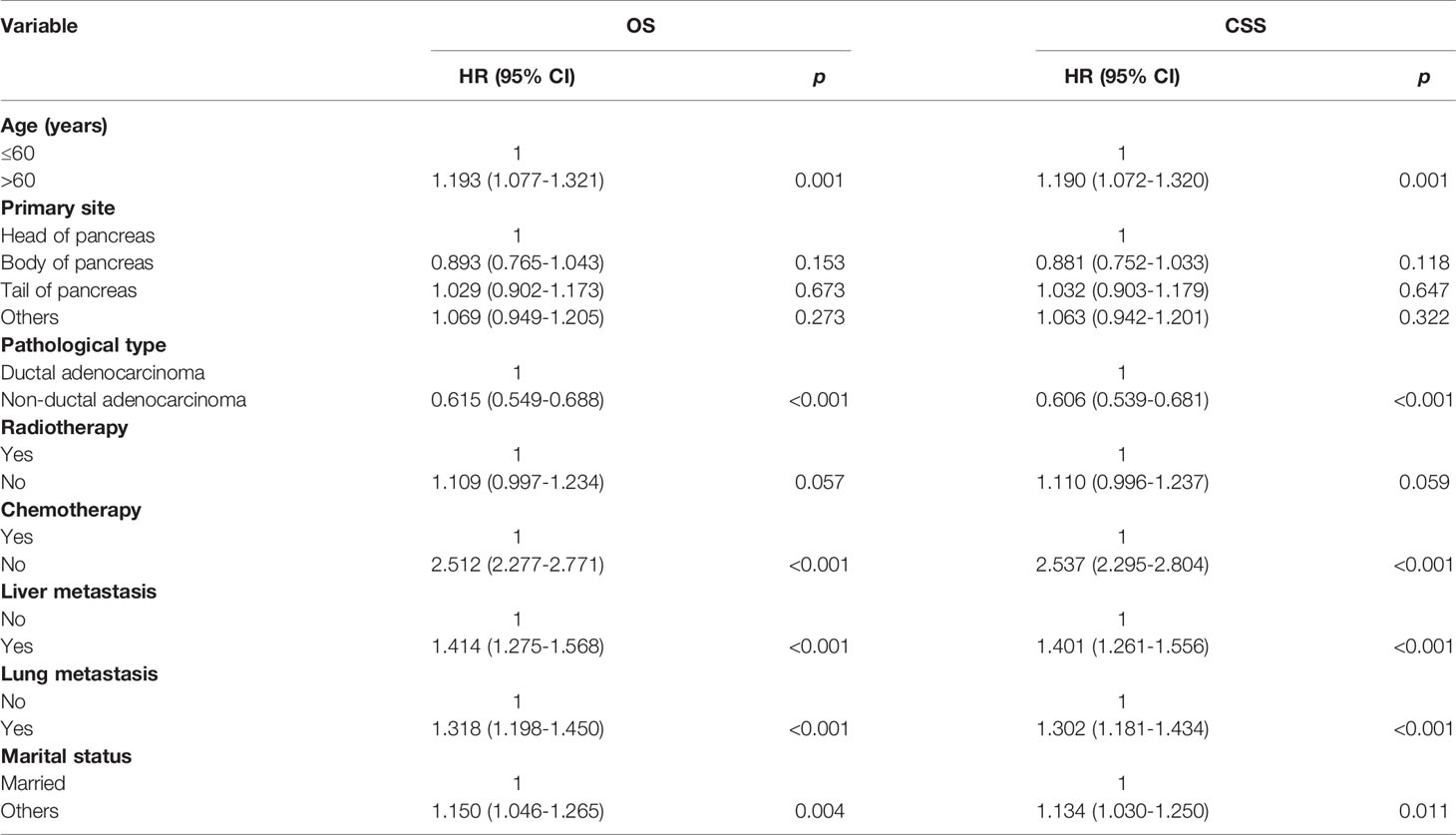
Multivariate analysis of the OS and CSS among patients with PCBM presented in Table 3 and Figure 2. Multivariable Cox regression analysis indicated that age (>60 years vs. ≤60 years, HR=1.193; 95% CI, 1.077-1.321; p<0.001), pathological type (Non-ductal adenocarcinoma vs. Ductal adenocarcinoma, HR=0.615; 95% CI, 0.549-0.688; p<0.001), systemic chemotherapy (No vs. Yes, HR=2.512; 95% CI, 2.277-2.771; p<0.001), liver metastasis (Yes vs. No, HR=1.414; 95% CI, 1.275-1.568; p<0.001), lung metastasis (Yes vs. No, HR=1.318; 95% CI, 1.198-1.450; p<0.001), and marital status(Others vs. Married, HR=1.150; 95% CI, 1.046-1.265; p=0.004) were independent prognostic factors for OS. In terms of CSS, age (>60 years vs. ≤60 years, HR=1.190; 95% CI, 1.072-1.320; p<0.001), pathological type (Non-ductal adenocarcinoma vs. Ductal adenocarcinoma, HR=0.606; 95% CI, 0.539-0.681; p<0.001), systemic chemotherapy (No vs. Yes, HR=2.537; 95% CI, 2.295-2.804; p<0.001), liver metastasis (Yes vs. No, HR=1.401; 95% CI, 1.261-1.556; p<0.001), lung metastasis (Yes vs. No, HR=1.302; 95% CI, 1.181-1.434; p<0.001), and marital status(Others vs. Married, HR=1.134; 95% CI, 1.030-1.250; p=0.011) were independent risk factors. Additionally, we drew Kaplan–Meier survival curves to visually show these independent prognostic factors. Patients with age ≤ 60 years were significantly associated with better outcomes (Figure 3). Patients with ductal adenocarcinoma type exhibited worse survival outcomes (Figure 4). Chemotherapy had a positive effect on prolonging the life of patients (Figure 5). Liver and lung metastases were associated with poor survival in patients with PCBM (Figure 6). Notably, longer OS and CSS were observed in married patients (Figure 7). The survival curves of patients in different groups decreased with time. In the first 20 months, the survival curve of patients in each group dropped rapidly.
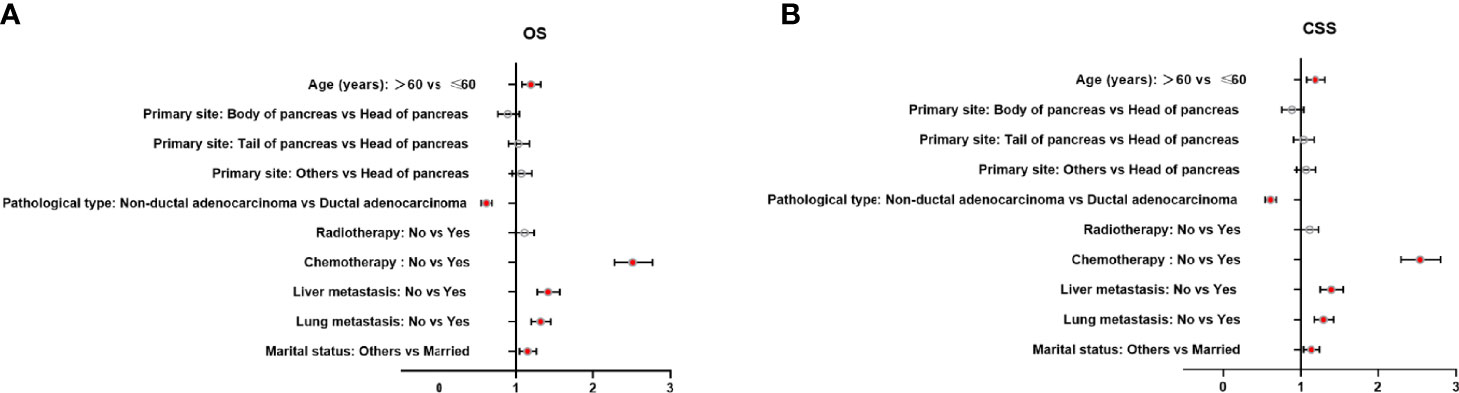
Figure 2 Forest plots of the predictors of OS (A) and CSS (B) in patients with PCBM. (PCBM, pancreatic cancer bone metastasis; OS, overall survival; CSS, cancer-specific survival).
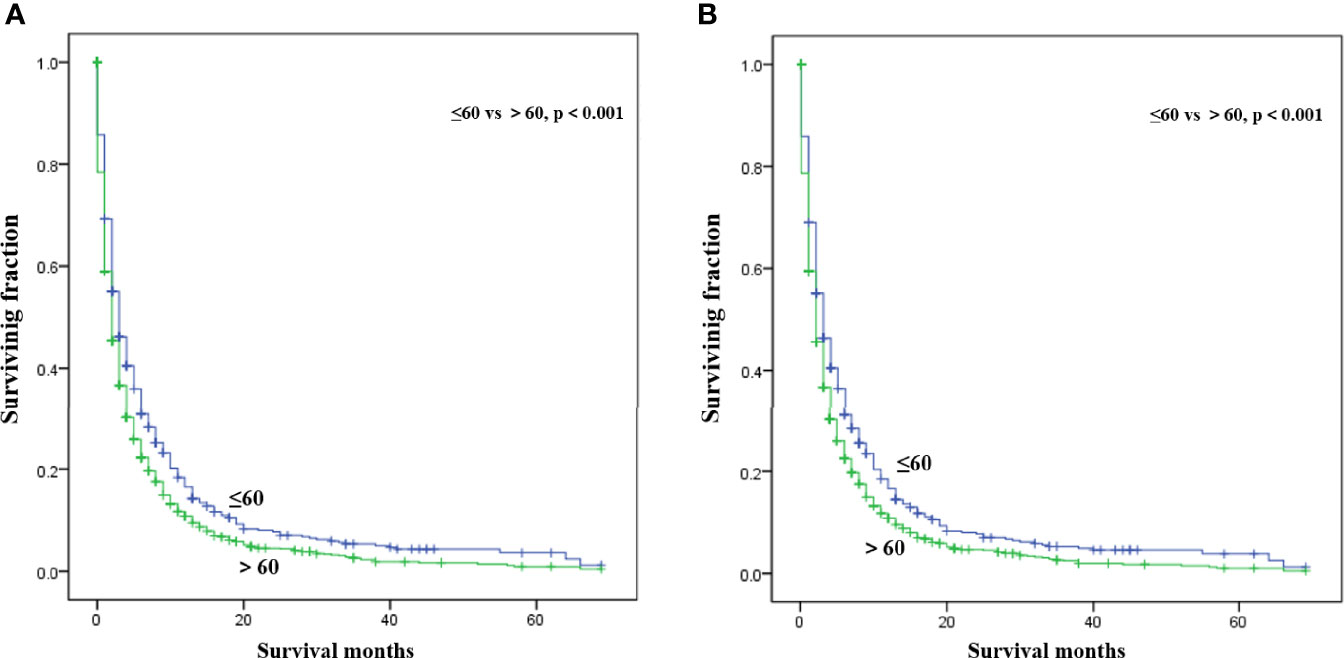
Figure 3 Kaplan-Meier survival curves for estimating OS (A) and CSS (B) in patients with PCBM based on age.
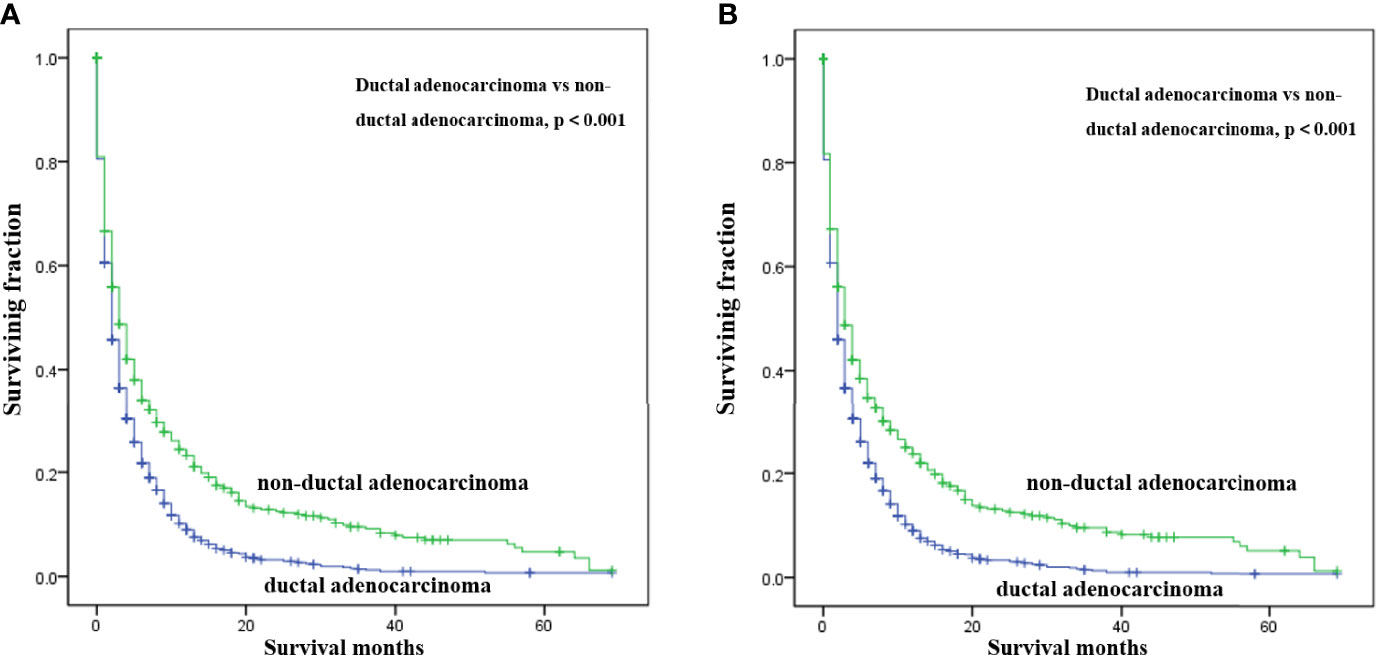
Figure 4 Kaplan-Meier survival curves for estimating OS (A) and CSS (B) in patients with PCBM based on pathological type.

Figure 5 Kaplan-Meier survival curves for estimating OS (A) and CSS (B) in patients with PCBM based on chemotherapy.
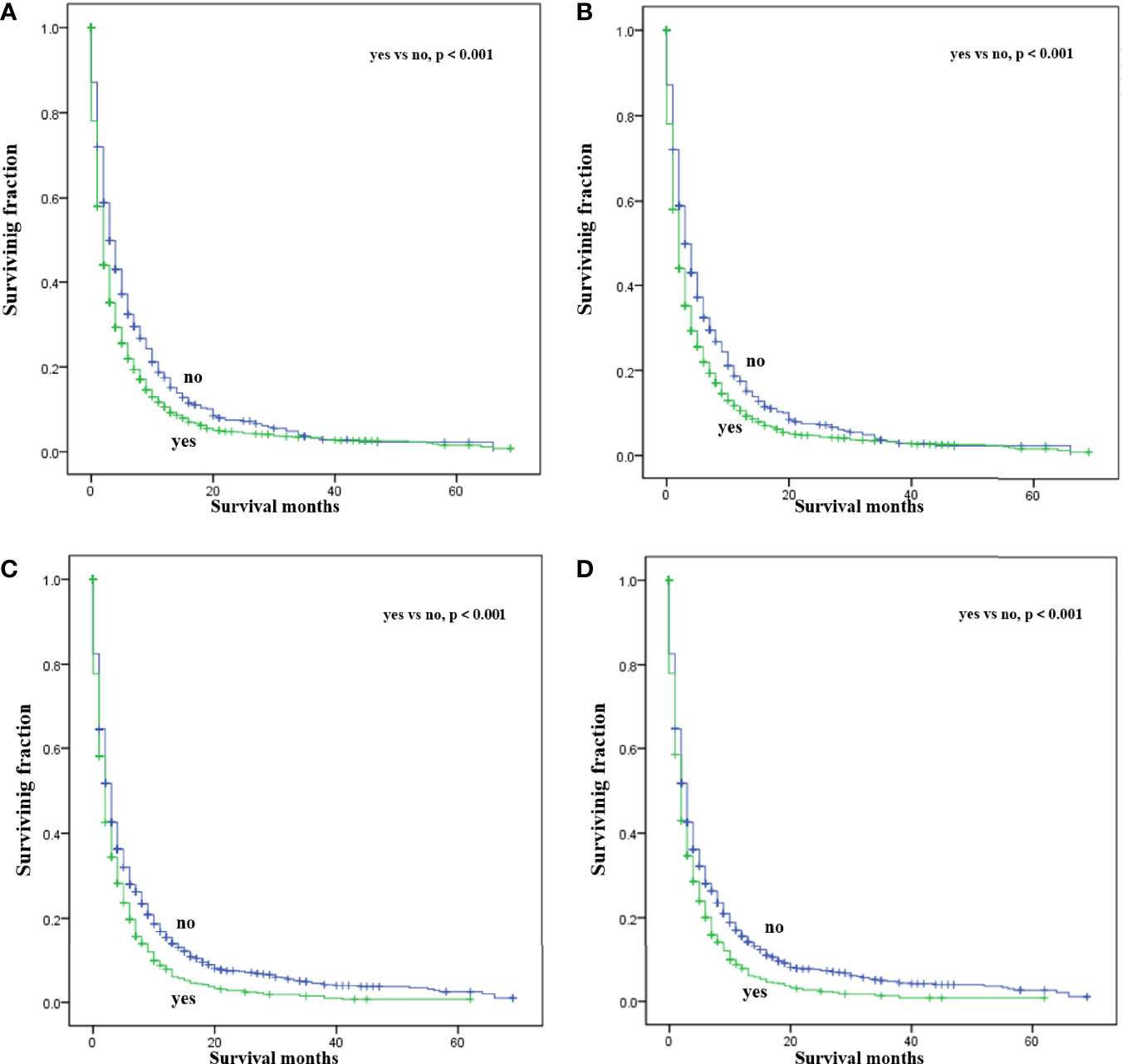
Figure 6 Kaplan-Meier survival curves for estimating OS and CSS in patients with PCBM based on liver (A, B) and lung (C, D) metastasis.
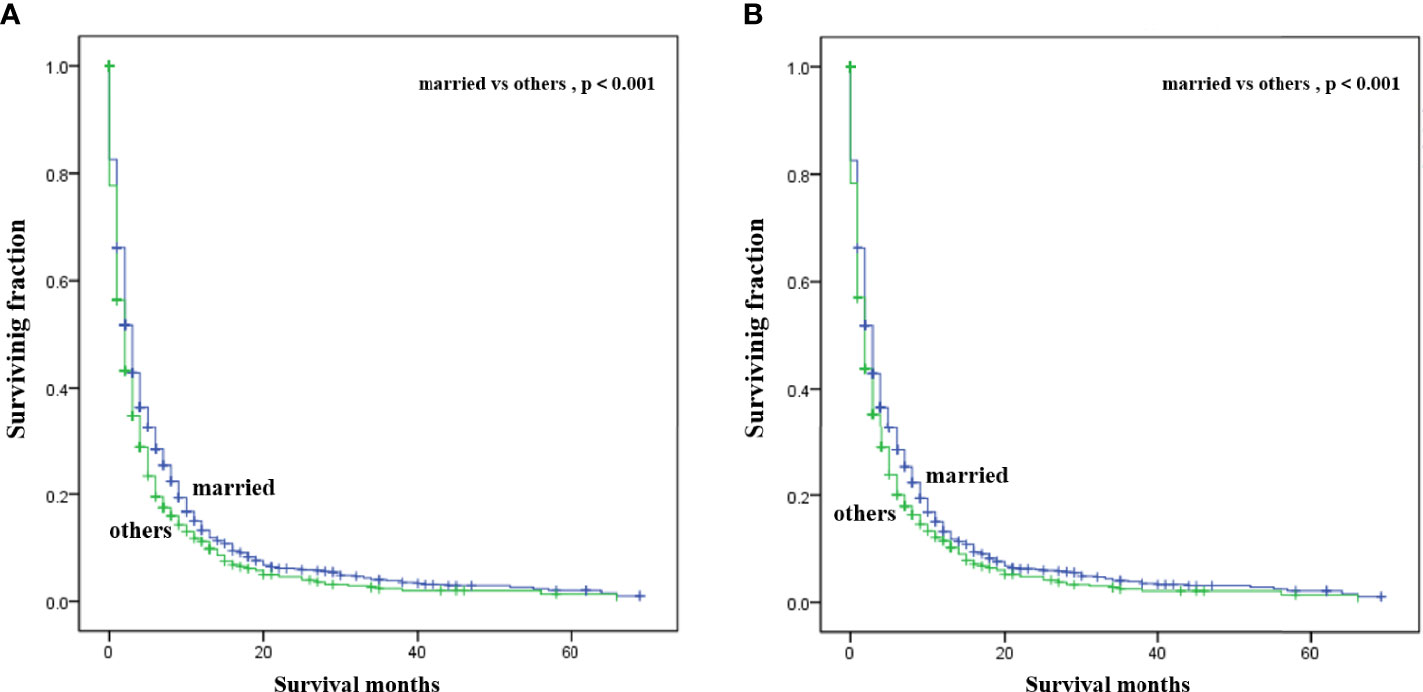
Figure 7 Kaplan-Meier survival curves for estimating OS (A) and CSS (B) in patients with PCBM based on marital status.
Discussion
Although treating PC has made remarkable progress over the past century, the prognosis remains poor mainly due to difficulties in its early diagnosis and metastasis (23). Bone metastasis represents an underappreciated site of metastasis among PC patients. As indicated in the present study, patients with PCBM experienced quite poor survival with 1-year OS and CSS rates of less than 15%. However, factors influencing their survival remain poorly understood. Previous studies of metastatic PC have been limited by small sample sizes, lack of population-based data. To our knowledge, the present study is the first population-based analysis to explore the survival predictors for patients with PCBM.
In this observational study, age at diagnosis was proved to be an independent survival predictor for patients with PCBM, which was consistent with previous findings among all PC patients (24–26). Patients over 60 years old are generally considered the elderly. Based on previous literature (27, 28), we divided the patients’ age into >60 years old and ≤60 years old for convenient analysis. Although some researchers reported tumor site was an independent survival predictor of PC (29–31), our multivariate Cox regression analysis showed that tumor site was not correlated with OS or CSS in patients with PCBM. For convenient analysis, patients’ tumor size were divided into<5 and ≥5 cm based on previous literature (21, 28). However, univariate Cox regression analysis showed tumor size was not correlated with survival. Pancreatic ductal adenocarcinomas (PDACs) account for approximately 95% of all PC pancreatic cancers (32). In our study, ductal adenocarcinoma subtype (75%) also represented most histological subtype of patients with PCBM. Moreover, ductal adenocarcinoma subtype was an important independent predictor for worse CSS and OS of patients with PCBM. Maybe non-ductal tumors have unique morphology and biology that is distinct from that of ductal neoplasms of the pancreas (33). Further researches are needed to clarify its specific mechanism. Lung or liver metastasis worsens the prognosis of patients with PCBM, which were in line with previous studies on PC (34, 35). Interestingly, better prognosis was observed in married patients, which may be associated with family social support. Similar results were supported by other studies on PC patients (36, 37). In Supplement Table 1, we found that among married patients, the proportions of white patients, male patients, patients with age>60 years, patients undergoing surgery and chemotherapy were higher.
The impact of chemotherapy to increase survival of PCBM remains unknown. This retrospective data analysis using a large cancer database suggests that use of chemotherapy could improve survival in patients with PCBM. Additionally, chemotherapy can stabilize health-related quality of life and improve pain control among advanced PC patients (38, 39). As for radiotherapy, our study did not find its survival benefits for patients with PCBM. Surgery is the mainstay of treatment for non-metastatic PC patients (40). Due to the small number of patients with PCBM receiving surgery in the current study, we did not analyze the its effect on survival. Additionally, previous studies indicated that surgical resection of oligometastatic disease after PDAC might have benefit for prolonging survival (41–43). Thus, research efforts should focus on exploring the role of surgery in patients with PCBM in the future. In summary, this study provides support for the selection of clinical treatment for patients with PCBM.
Although the SEER database provides a large amount of clinical data, there are still many limitations in our research. First, we need to further conduct a follow-up clinical trial to verify this result. Second, the role of surgery on prognosis were not analyzed due to the small number of cases who received surgery. Finally, the performance status, lymph node metastasis, and CEA or CA19-9 levels, were not available in the SEER database, which need to be further studied.
Conclusion
Our population-based study showed that age ≤60, non-ductal adenocarcinoma type, chemotherapy, no liver metastasis, no lung metastasis, and married status were independent predictors for better OS and CSS, which may have implications for future clinical practice. However, further studies are warranted to validate these results.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZW conceived and designed the study. YR, SW, and BW collected the data. YR and SW performed the statistical analysis. YR wrote the manuscript. ZW and BW revised it. All authors read and approved the final manuscript.
Funding
This work was supported by the China Postdoctoral Science Foundation (2021M692792), National Natural Science Foundation of China (82103499), and Zhejiang Provincial Natural Science Foundation (LQ22H160040).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.759403/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA: Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Deplanque G, Demartines N. Pancreatic Cancer: Are More Chemotherapy and Surgery Needed? Lancet (Lond Engl) (2017) 389(10073):985–6. doi: 10.1016/s0140-6736(17)30126-5
3. Ayres Pereira M, Chio IIC. Metastasis in Pancreatic Ductal Adenocarcinoma: Current Standing and Methodologies. Genes (2019) 11(1):6. doi: 10.3390/genes11010006
4. Hall BR, Cannon A, Atri P, Wichman CS, Smith LM, Ganti AK, et al. Advanced Pancreatic Cancer: A Meta-Analysis of Clinical Trials Over Thirty Years. Oncotarget (2018) 9(27):19396–405. doi: 10.18632/oncotarget.25036
5. He C, Huang X, Zhang Y, Lin X, Li S. The Impact of Different Metastatic Patterns on Survival in Patients With Pancreatic Cancer. Pancreatol: Off J Int Assoc Pancreatol (IAP) [et al] (2021) 21(3):556–63. doi: 10.1016/j.pan.2021.01.014
6. Tao L, Yuan C, Ma Z, Jiang B, Xiu D. Surgical Resection of a Primary Tumor Improves Survival of Metastatic Pancreatic Cancer: A Population-Based Study. Cancer Manag Res (2017) 9:471–9. doi: 10.2147/cmar.S145722
7. Tagawa T, Ito K, Fukuzawa K, Okamoto T, Yoshimura A, Kawasaki T, et al. Surgical Resection for Pulmonary Metastasis From Pancreatic and Biliary Tract Cancer. Anticancer Res (2017) 37(3):1413–6. doi: 10.21873/anticanres.11464
8. Zhou W, Wang D, Lou W. Current Role of Surgery in Pancreatic Cancer With Synchronous Liver Metastasis. Cancer Control: J Moffitt Cancer Center (2020) 27(1):1073274820976593. doi: 10.1177/1073274820976593
9. Morimoto D, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, et al. Characteristics of Lung Metastasis as an Initial Recurrence Pattern After Curative Resection of Pancreatic Cancer. Pancreas (2020) 49(5):699–705. doi: 10.1097/mpa.0000000000001559
10. Hatfield DR, DeLand FH, Maruyama Y. Skeletal Metastases in Pancreatic Carcinoma: Study by Isotopic Bone Scanning. Oncology (1976) 33(1):44–7. doi: 10.1159/000225100
11. Borad MJ, Saadati H, Lakshmipathy A, Campbell E, Hopper P, Jameson G, et al. Skeletal Metastases in Pancreatic Cancer: A Retrospective Study and Review of the Literature. Yale J Biol Med (2009) 82(1):1–6.
12. Wu C, Jiang H, Chen J. A Systematic Review and Meta-Analysis About the Effect of Bisphosphonates on the Risk of Skeletal-Related Event in Men With Prostate Cancer. Anti-cancer Agents Med Chem (2020) 20(13):1604–12. doi: 10.2174/1871520620666200521114815
13. Hong S, Youk T, Lee SJ, Kim KM, Vajdic CM. Bone Metastasis and Skeletal-Related Events in Patients With Solid Cancer: A Korean Nationwide Health Insurance Database Study. PloS One (2020) 15(7):e0234927. doi: 10.1371/journal.pone.0234927
14. Howard LE, De Hoedt AM, Aronson WJ, Kane CJ, Amling CL, Cooperberg MR, et al. Do Skeletal-Related Events Predict Overall Survival in Men With Metastatic Castration-Resistant Prostate Cancer? Prostate Cancer Prostatic Dis (2016) 19(4):380–4. doi: 10.1038/pcan.2016.26
15. Saif MW, Galanina N, Ravage-Mass L, Kaley K, Cornfeld D, Lamb L, et al. Bone Metastasis as the Only Metastatic Site in a Patient With Pancreatic Cancer Following Distal Pancreatectomy. Case Rep Med (2010) 2010:634975. doi: 10.1155/2010/634975
16. Kang JS, Mok L, Heo JS, Han IW, Shin SH, Yoon YS, et al. Development and External Validation of Survival Prediction Model for Pancreatic Cancer Using Two Nationwide Databases: Surveillance, Epidemiology and End Results (SEER) and Korea Tumor Registry System-Biliary Pancreas (KOTUS-Bp). Gut Liver (2021) 15(6):912–21. doi: 10.5009/gnl20306
17. Zhang W, Xu L, Che X. Nomogram for Predicting the Prognoses of Patients With Pancreatic Head Cancer After Pancreaticoduodenectomy: A Population-Based Study on SEER Data. Front Oncol (2021) 11:766071:766071. doi: 10.3389/fonc.2021.766071
18. Liu X, Fu Y, Chen Q, Wu J, Gao W, Jiang K, et al. Predictors of Distant Metastasis on Exploration in Patients With Potentially Resectable Pancreatic Cancer. BMC Gastroenterol (2018) 18(1):168. doi: 10.1186/s12876-018-0891-y
19. Raufi AG, Manji GA, Chabot JA, Bates SE. Neoadjuvant Treatment for Pancreatic Cancer. Semin Oncol (2019) 46(1):19–27. doi: 10.1053/j.seminoncol.2018.12.002
20. Lin S, Liu C, Tao Z, Zhang J, Hu X. Clinicopathological Characteristics and Survival Outcomes in Breast Carcinosarcoma: A SEER Population-Based Study. Breast (Edinburgh Scotland) (2020) 49:157–64. doi: 10.1016/j.breast.2019.11.008
21. Wang Z, Cheng Y, Chen S, Shao H, Chen X, Wang Z, et al. Novel Prognostic Nomograms for Female Patients With Breast Cancer and Bone Metastasis at Presentation. Ann Trans Med (2020) 8(5):197. doi: 10.21037/atm.2020.01.37
22. Wang Z, Wu B, Zhou Y, Huang X, Pan W, Liu M, et al. Predictors of the Survival of Primary and Secondary Older Osteosarcoma Patients. J Cancer (2019) 10(19):4614–22. doi: 10.7150/jca.32627
23. Torphy RJ, Fujiwara Y, Schulick RD. Pancreatic Cancer Treatment: Better, But a Long Way to Go. Surg Today (2020) 50(10):1117–25. doi: 10.1007/s00595-020-02028-0
24. Exarchakou A, Papacleovoulou G, Rous B, Magadi W, Rachet B, Neoptolemos JP, et al. Pancreatic Cancer Incidence and Survival and the Role of Specialist Centres in Resection Rates in England, 2000 to 2014: A Population-Based Study. Pancreatol: Off J Int Assoc Pancreatol (IAP) [et al] (2020) 20(3):454–61. doi: 10.1016/j.pan.2020.01.012
25. Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Uesaka K. Impact of Patient Age on the Postoperative Survival in Pancreatic Head Cancer. Ann Surg Oncol (2017) 24(11):3220–8. doi: 10.1245/s10434-017-5994-0
26. Loveday BPT, Lipton L, Thomson BN. Pancreatic Cancer: An Update on Diagnosis and Management. Aust J Gen Pract (2019) 48(12):826–31. doi: 10.31128/ajgp-06-19-4957
27. Liu Y, Zhang P, Zhang Y, Zheng L, Xu W, Hou D, et al. Clinical Characteristics and Overall Survival Nomogram of Second Primary Malignancies After Prostate Cancer, a SEER Population-Based Study. Sci Rep (2021) 11(1):1293. doi: 10.1038/s41598-020-80534-4
28. Hu H, Wang Z, Zhang M, Niu F, Yu Q, Ren Y, et al. Clinicopathological Characteristics and Prognosis in Endometrial Cancer With Bone Metastasis: A SEER-Based Study of 584 Women. Front Oncol (2021) 11:694718:694718. doi: 10.3389/fonc.2021.694718
29. Zheng Z, Wang M, Tan C, Chen Y, Ping J, Wang R, et al. Disparities in Survival by Stage After Surgery Between Pancreatic Head and Body/Tail in Patients With Nonmetastatic Pancreatic Cancer. PloS One (2019) 14(12):e0226726. doi: 10.1371/journal.pone.0226726
30. Yadav S, Sharma P, Zakalik D. Comparison of Demographics, Tumor Characteristics, and Survival Between Pancreatic Adenocarcinomas and Pancreatic Neuroendocrine Tumors: A Population-Based Study. Am J Clin Oncol (2018) 41(5):485–91. doi: 10.1097/coc.0000000000000305
31. Chen J, Yang Y, Liu Y, Kan H. Prognosis Analysis of Patients With Pancreatic Neuroendocrine Tumors After Surgical Resection and the Application of Enucleation. World J Surg Oncol (2021) 19(1):11. doi: 10.1186/s12957-020-02115-z
32. Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, et al. Multidisciplinary Standards of Care and Recent Progress in Pancreatic Ductal Adenocarcinoma. CA: Cancer J Clin (2020) 70(5):375–403. doi: 10.3322/caac.21626
33. Dhillon J. Non-Ductal Tumors of the Pancreas. Monogr Clin Cytol (2020) 26:92–108. doi: 10.1159/000455737
34. Lianyuan T, Deyu L, Haibo Y, Yadong D, Guanjing T. Clinical Features and Prognostic Factors of Elderly Patients With Metastatic Pancreatic Cancer: A Population-Based Study. Aging (2021) 13(5):7133–46. doi: 10.18632/aging.202570
35. Ni X, Li D, Dai S, Pan H, Sun H, Ao J, et al. Development and Evaluation of Nomograms to Predict the Cancer-Specific Mortality and Overall Mortality of Patients With Hepatocellular Carcinoma. BioMed Res Int (2021) 2021:1658403. doi: 10.1155/2021/1658403
36. Zhou H, Zhang Y, Song Y, Tan W, Qiu Z, Li S, et al. Marital Status is an Independent Prognostic Factor for Pancreatic Neuroendocrine Tumors Patients: An Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database. Clinics Res Hepatol Gastroenterol (2017) 41(4):476–86. doi: 10.1016/j.clinre.2017.02.008
37. Song W, Miao DL, Chen L. Nomogram for Predicting Survival in Patients With Pancreatic Cancer. OncoTargets Ther (2018) 11:539–45. doi: 10.2147/ott.S154599
38. Kristensen A, Vagnildhaug OM, Grønberg BH, Kaasa S, Laird B, Solheim TS. Does Chemotherapy Improve Health-Related Quality of Life in Advanced Pancreatic Cancer? A Systematic Review. Crit Rev Oncol Hematol (2016) 99:286–98. doi: 10.1016/j.critrevonc.2016.01.006
39. Laquente B, Macarulla T, Bugés C, Martín M, García C, Pericay C, et al. Quality of Life of Patients With Metastatic Pancreatic Adenocarcinoma Initiating First-Line Chemotherapy in Routine Practice. BMC Palliat Care (2020) 19(1):103. doi: 10.1186/s12904-020-00610-4
40. Heinrich S, Lang H. Neoadjuvant Therapy of Pancreatic Cancer: Definitions and Benefits. Int J Mol Sci (2017) 18(8):1622. doi: 10.3390/ijms18081622
41. Ilmer M, Schiergens TS, Renz BW, Schneider C, Sargut M, Waligora R, et al. Oligometastatic Pulmonary Metastasis in Pancreatic Cancer Patients: Safety and Outcome of Resection. Surg Oncol (2019) 31:16–21. doi: 10.1016/j.suronc.2019.08.010
42. Lopez-Lopez V, Robles-Campos R, López-Conesa A, Brusadin R, Carbonel G, Gomez-Ruiz A, et al. Surgical Resection of Liver Metastasis in Pancreatic and Periampullary Carcinoma. Minerva Chir (2019) 74(3):253–62. doi: 10.23736/s0026-4733.18.07972-5
Keywords: pancreatic cancer, bone metastasis, prognosis, risk factor, clinicopathological features
Citation: Ren Y, Wang S, Wu B and Wang Z (2022) Clinicopathological Features, Prognostic Factors and Survival in Patients With Pancreatic Cancer Bone Metastasis. Front. Oncol. 12:759403. doi: 10.3389/fonc.2022.759403
Received: 16 August 2021; Accepted: 19 January 2022;
Published: 09 February 2022.
Edited by:
Nadia M. Hamdy, Ain Shams University, EgyptReviewed by:
Bethany A. Kerr, Wake Forest School of Medicine, United StatesHideo Baba, Kumamoto University, Japan
Copyright © 2022 Ren, Wang, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhan Wang, d2FuZ3poYW5oekB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Ying Ren
Ying Ren Shicheng Wang
Shicheng Wang Bo Wu
Bo Wu Zhan Wang
Zhan Wang