- 1Department of Urology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou City, China
- 2Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Urology, General Hospital of Eastern Theater Command, Nanjing, China
- 4Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou City, China
- 5Research Institute, GloriousMed Clinical Laboratory Co., Ltd., Shanghai, China
- 6Department of Urology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Recent studies have indicated that prostate cancer (PCa) with BRCA2 mutations is more aggressive. However, these reports mostly focused on Caucasus populations, and large-scale studies on BRCA mutations in Chinese PCa populations remain limited. Herein, we screened, from multiple centers in China, a total of 172 patients with PCa carrying BRCA1/2 germline mutations. The variant distribution and type, associated somatic variant, and frequency of the BRCA germline variants in these patients were analyzed retrospectively. We found that Chinese patients with PCa carrying BRCA1/2 germline mutations were diagnosed at an earlier age, i.e., 67 years (range, 34–89 years), and most had metastatic castration-resistant PCa (mCRPC) (54.65%, 94/172). The top three BRCA variants were frameshift, missense, and splicing variants. The overall pathogenic rates of the BRCA1 and BRCA2 variants were 17.46% (11/63) and 56.55% (82/145), respectively. Among the somatic mutations associated with BRCA2 germline mutations, the highest frequency was for FOXA1 (circulating tumor DNA [ctDNA] sequencing, 7.4%; tissue samples, 52%) and NCOR2 mutations (ctDNA sequencing, 7.4%; tissue samples, 24%); TP53 was the dominant somatic mutation associated with BRCA1 germline mutations (ctDNA sequencing, 25%; tissue samples, 17%). Ultimately, in Chinese patients, PCa with BRCA1/2 germline mutations tends to be more aggressive. Compared with BRCA1, BRCA2 has a higher frequency of germline pathogenic mutations. FOXA1, NCOR2, and TP53 somatic mutations associated with higher BRCA1/2 germline pathogenic mutations. Our description of BRCA germline mutations in the Chinese PCa patients provides more reference data for the precise diagnosis and treatment of Chinese PCa patients.
Introduction
Prostate cancer (PCa) is the highest-incidence male genitourinary system malignancy, but there are great differences in the incidence and mortality between patients in China and in other countries (1). Although PCa incidence in China is far lower than that in the western countries, it has been increasing by the year in recent years with lifestyle changes and improved cancer diagnosis levels (2). Genetics are one of the most important factors in PCa, especially in men with a family history of malignancy. Although the clinical significance of common genetic variants associated with PCa risk remains unclear, breast cancer susceptibility gene 1 (BRCA1) and BRCA2 are closely associated with PCa invasiveness and prognosis (3).
BRCA is a co-regulator of androgen receptor (AR), and the AR-mediated signaling pathway plays an important role in PCa occurrence and development. Some clinical studies have shown that patients with BRCA1 and BRCA2 mutations are more likely to have lymph node involvement or distant metastases when diagnosed, and shorter disease-free survival than patients with wild-type BRCA (4). Several large clinical studies have found that patients with metastatic castration-resistant PCa (mCRPC) with somatic or germline variants of the DNA damage repair (DDR) genes (especially BRCA1/2) may be sensitive to poly-ADP-ribose polymerase (PARP) inhibitors (PARPi) (5, 6). The PROfound phase III clinical study revealed that patients with PCa with homologous recombination repair (HRR) gene mutations can benefit from olaparib monotherapy; in particular, the risk of radiographic progression (66%) or death could be reduced in patients with BRCA1/2 and ATM mutations (7).
Therefore, it is necessary to test for BRCA mutations in patients with PCa, especially men with a family history of malignancy. Further, the consensus of Chinese experts on genetic testing for patients with PCa recommends testing for BRCA2 and BRCA1 germline mutations in patients with high-risk, locally progressive, and metastatic PCa (8). However, research data on Chinese patients with PCa carrying BRCA1/2 germline mutations are relatively scarce so far.
Active surveillance of BRCA mutation carriers is not safe, even for low-risk patients. When PCa is diagnosed in BRCA mutation carriers, radical treatment should be performed as early as possible. Currently, reports related to BRCA germline mutations in patients with PCa are mainly concentrated in foreign populations, while studies in Chinese populations are very limited. To reveal the status of BRCA germline mutations in the Chinese PCa population, 172 patients with PCa with BRCA germline mutations diagnosed at multiple centers were screened for: (1) retrospective statistical analysis of the Chinese PCa population with BRCA germline mutations in different pathological stages; (2) exploring the variant distribution and type, and the associated somatic mutations of the BRCA1/2 mutations. Ultimately, this study provides more reference data for the precise diagnosis and treatment of patients with PCa in China and can be used to guide clinical decision-making in PCa.
Methods
Patients and Samples
We conducted a retrospective study of 172 PCa patients with BRCA1/2 germline alterations (Table S1) and 312 PCa patients without BRCA germline mutations (Table S2) who underwent genomic profiling with a hybridization capture-based next-generation sequencing (NGS) assay between February 2018 and June 2020 collected from the database of GloriousMed Technology Co., Ltd. (Shanghai, China). These patients were mostly from four hospitals (Renji Hospital of Shanghai Jiaotong University School of Medicine, The First Affiliated Hospital of Wenzhou Medical University, General Hospital of Eastern Theater Command, The First Affiliated Hospital of Nanjing Medical University). The study was approved by the Committee for Ethics of the First Affiliated Hospital of Wenzhou Medical University and informed consent was obtained from each patient. We collected 123 circulating tumor DNA (ctDNA) samples from and 59 biopsied tumor tissue samples from the 172 patients (Table S1).
DNA Sequencing and Bioinformatics
The samples underwent NGS testing at GloriousMed Clinical Laboratory Co., Ltd. Cell-free DNA (cfDNA, from plasma), tumor formalin-fixed, paraffin-embedded (FFPE) DNA, and genomic DNA (gDNA, from white blood cells) were extracted according to standard procedures using a QIAamp Circulating Nucleic Acid Kit (Qiagen), QIAamp DNA FFPE Tissue Kit (Qiagen), and Blood Genomic DNA Mini Kit (cwbiotech), respectively. From each sample, 200–500 ng FFPE DNA, 20–100 ng cfDNA, or 500 ng gDNA were used for library preparation and quantification according to KAPA HyperPrep protocols (KAPA). The genes’ coding regions were captured using custom-designed DNA enrichment panels (50/66/620/642 panels). For analysis, we focused on the common 50 genes (Supplementary Table 2). Library pools (5–6) were hybridized to the capture panel according to standard procedures. Then, the libraries were purified and quantified using AMPure XP (Beckman Coulter) and a Qubit™ dsDNA HS Assay Kit (Thermo Fisher Scientific). The final libraries were sequenced on Illumina NextSeq 500 (75-bp paired-end reads [PE75]) or NovaSeq 6000 (PE150) instruments.
Quality Control and Variant Calling
The raw data were trimmed using Trimmomatic (9). Then, the reads were aligned with the human reference genome (hg19) using Burrows-Wheeler Aligner (10). Duplicated reads were removed using Picard (http://broadinstitute.github.io/picard/). Mapped reads were realigned to the genome using Genome Analysis Tool Kit (GATK) (11). Germline mutations were called using GATK’s HaplotypeCaller (11) with a paired workflow. Variants were then annotated using ANNOVAR (12) and an in-house-developed code. The human identity concordance of the paired samples was verified using an in-house script. Germline mutations considered deleterious (frameshift insertions, nonsense/stop-gains, splice site variants, deletions, or reported as pathogenic or likely pathogenic in the ClinVar database) were included for analysis. Here, “pathogenic alterations” includes pathogenic or likely pathogenic alterations; “non-pathogenic” represents variants of uncertain significance (VUS).
Statistical Analysis
The assessment of clinical characteristics between different cohorts, including age at diagnosis, Gleason score, et al., were based on the Wilcoxon rank sum test. Graphpad Prism V8 (GraphPad Software, Inc.) and R v3.6.1 (www.R-project.org) were used for data analysis. A two-sided P value <0.05 was considered significant.
Results
Analysis of the Patients’ Characteristics
The 172 patients with BRCA germline mutations comprised patients diagnosed with PCa (NA), localized prostate cancer (LAPC), metastatic hormone-sensitive prostate cancer (mHSPC), or mCRPC (Table 1). Castration resistance was defined according to the European Association of Urology (EAU) Guidelines on Prostate Cancer (2021 edition). Significant difference was found in median age between those with and without BRCA1 mutation (69 years; range, 53–89 years vs. 66 years; range, 44–98 years, p < 0.05), but not in BRCA2 mutation(65.5 years; range, 34–85 years vs. 66 years; range, 44–98 years, p > 0.05). Overall, there was no significant difference in PSA value (13.6; range, 0–1000 vs. 0; range, 0–5000, p > 0.05) and Gleason score (p > 0.05) between the patients with and without BRCA mutation. The baseline comparison between the patients with pathogenic and non-pathogenic BRCA1/2 mutations was performed, significant difference was found in median age between them (65 years; range, 34-82 years vs. 67.5 years; range, 53-89 years, p < 0.05). Similarly, there was no significant difference in PSA value (6.95; range, 0–1000 vs. 10.85; range, 0–905, p > 0.05) and Gleason score (p > 0.05) between the patients with pathogenic and non-pathogenic BRCA1/2 mutations.

Table 1 Summary of clinical characteristics and a comparison between patients with BRCA1/2 mutation and without BRCA1/2 mutation (wild).
Frequency and Comparative Analysis of Patients With BRCA Germline Mutations
The frequency distribution of BRCA1/2 germline pathogenic and non-pathogenic (VUS) mutations differed significantly (Figure 1). BRCA1 germline mutations were mainly VUS (27.91%, 48/172) while BRCA2 germline mutations were mainly pathogenic (41.86%, 72/172). The frequency of BRCA2 germline mutations in our cohort (69.19%, 121/172) was much higher than that of BRCA1 germline mutations (32.56%, 57/172) (Table 1). Based on pathological stage, BRCA1 and BRCA2 mutations were mainly found in mCRPC, especially BRCA2, with a frequency of 16.86% (29/172) and 37.79% (65/172), respectively. While it was similar between LAPC and mHSPC, BRCA1 was 5.81% (10/172) and 6.4% (11/172), BRCA2 was 12.21% (21/172) and 9.88% (17/172).
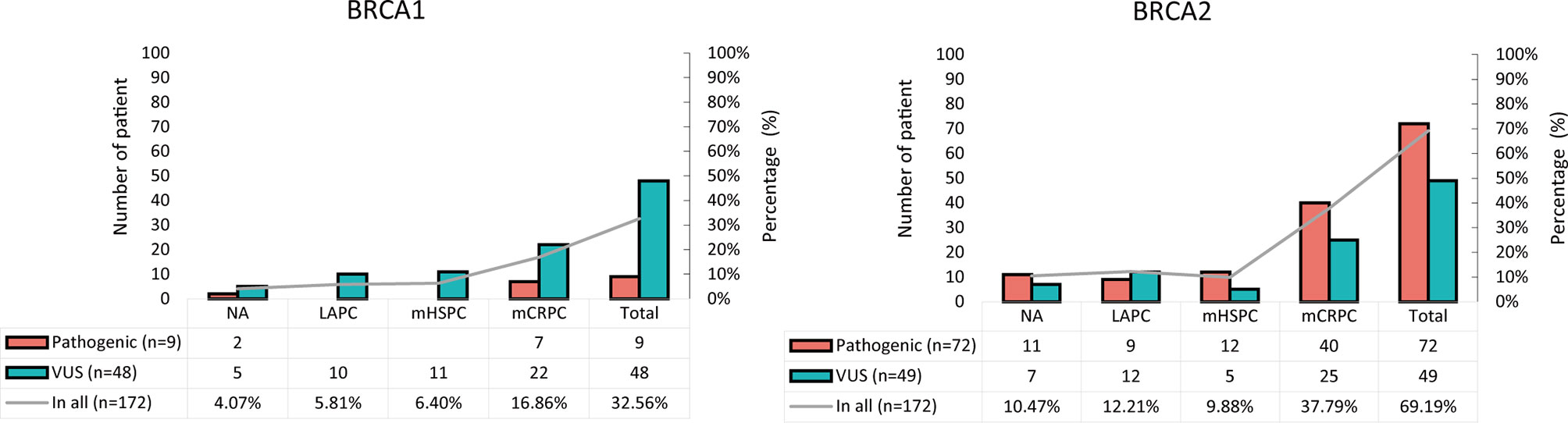
Figure 1 Frequency distribution of pathogenic and non-pathogenic BRCA1 and BRCA2 mutations in patients with PCa. VUS, variants of uncertain significance.
Genetic Distribution of BRCA1/2 Variants
The overall analysis showed that the BRCA1/2 variants were distributed in most exon regions of the BRCA1 and BRCA2 genes, and no new hot spot variants were found (Figure 2 and Figure S1). A total of 208 BRCA germline variants were identified: 63 and 145 in BRCA1 and BRCA2, respectively. c.2726A>T (p.N9091) was the most frequently mutated variant (3.37%, 7/208). Most of the variants occurred only once (58.65%, 122/208); 41.35% (86/208) of the variants with >1 occurrence were mainly distributed in mCRPC (55/86). Among the 63 BRCA1 variants, c.2726.4>T (p.N9091) was the most common (7/63), while c.5722-5723DELCT (p.L1908FS) was the most common (5/145) among the 145 BRCA2 variants.
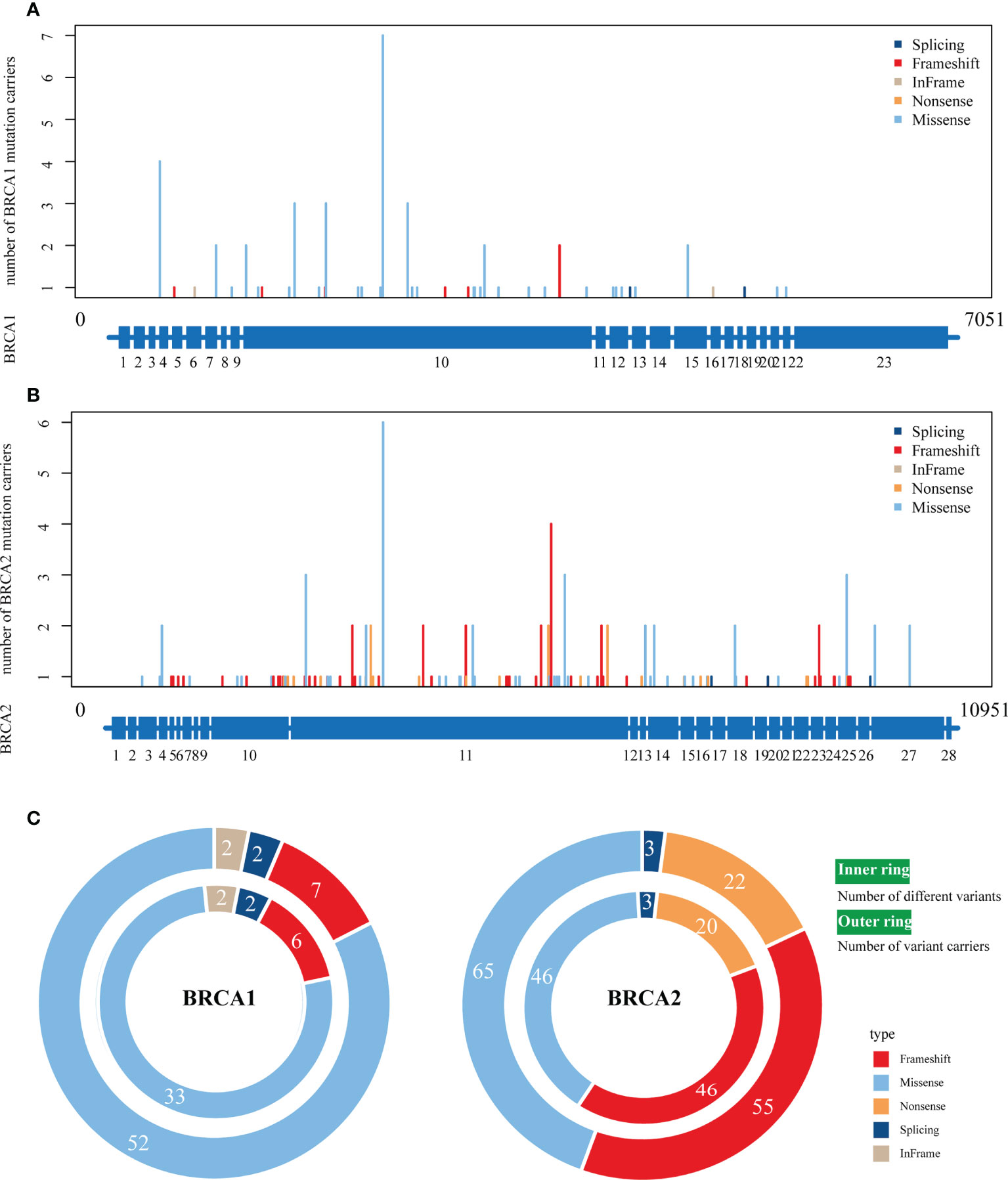
Figure 2 The distribution of various BRCA1/2 variant types on full‐length BRCA1/2 exons. (A, B), The distribution of four variant types on BRCA1 (A) and BRCA2 (B). The scheme of exons (blue bar at the bottom of each panel) is shown as the reference. (C) The number of different variants (inner ring) and number of variant carriers (outer ring). Colors represent different variant types.
In the variant type analysis, frameshift, missense, and splicing were the common BRCA1 and BRCA2 variants. Frameshift and missense were the most advantageous variants, occurring in 90.7% (132/145) and 80% (50/63) of BRCA1 and BRCA2 variants, respectively. The difference was that in-frame and nonsense variants only appeared in BRCA2 and BRCA1, respectively.
Statistical Analysis of BRCA Variants
Here, we report the distribution of the pathogenic and non-pathogenic variants in the major exons of BRCA1 and BRCA2 (Figures 3A, B). There were 54.55% (6/11) and 69.51% (57/82) pathogenic variants in BRCA1 exon 10 and BRCA2 exon 10/11, respectively. The number of variants per exon was normalized according to the exon length (Figures 3C, D). Exon 4 and exon 13 had the most variants in BRCA1 and BRCA2, respectively. Exon 4 and exon 5 had the most pathogenic variants in BRCA1 and BRCA2, respectively. Among all variants, the overall pathogenic rates for BRCA1 and BRCA2 were 17.46% (11/63) and 56.55% (82/145), respectively (Figures 3E, F). The frameshift variants were pathogenic both in BRCA1 and BRCA2. Similarly, missense variants also showed the same trend in BRCA1 and BRCA2. The difference was that splicing variants were non-pathogenic and pathogenic in BRCA1 and BRCA2, respectively.
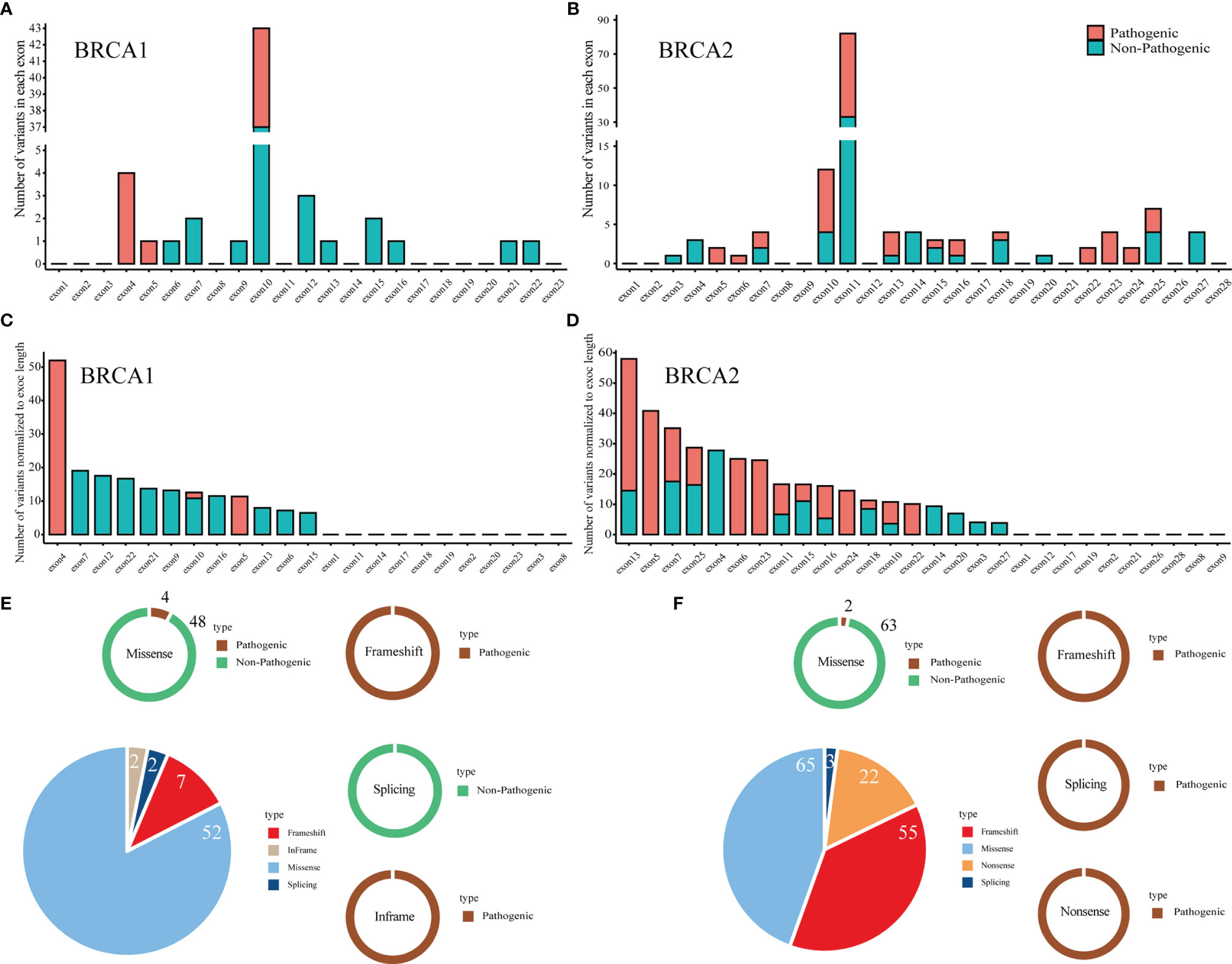
Figure 3 Interpretation of pathogenicity and distribution of pathogenic and non-pathogenic variants in full-length BRCA1/2 genes. (A, B) The number of variants in each exon of the BRCA1 (A) and BRCA2 (B) gene. (C, D), The number of variants normalized to exon length for BRCA1 (C) and BRCA2 (D). (E, F) The relative ratio of each type of pathogenicity in each type of variant in BRCA1 (E) and BRCA2 (F).
Somatic Mutation Analysis of Patients With BRCA Germline Pathogenic Mutations
We identified somatic alterations in the AR pathway genes, DDR pathway genes, and tumor suppressor genes (TP53/RB1) in the patients (Figure 4). AR (26%, 32/123), TP53 (20%, 25/123), FOXA1 (15%, 18/123), NCOR2 (12%, 15/123), and PTEN (10%, 12/123) were the top five somatic mutation genes associated with ctDNA sequencing. In the tissue samples, the top seven somatic mutation genes were FOXA1 (34%, 20/59), TP53 (15%, 9/59), AR (15%, 9/59), NCOR2 (14%, 8/59), FANCA (12%, 7/59), RB1 (12%, 7/59), and SPOP (10%, 6/59). Among the somatic mutations associated with BRCA2 germline mutations, the most frequent were FOXA1 (ctDNA sequencing, 7.4% [4/54]; tissue samples, 52% [11/21) and NCOR2 (ctDNA sequencing, 7.4% [4/54]; tissue samples, 24% [5/21]) mutations; TP53 was the dominant somatic mutation associated with BRCA1 germline mutations (ctDNA sequencing, 25% [1/4]; tissue samples, 17% [1/6]).
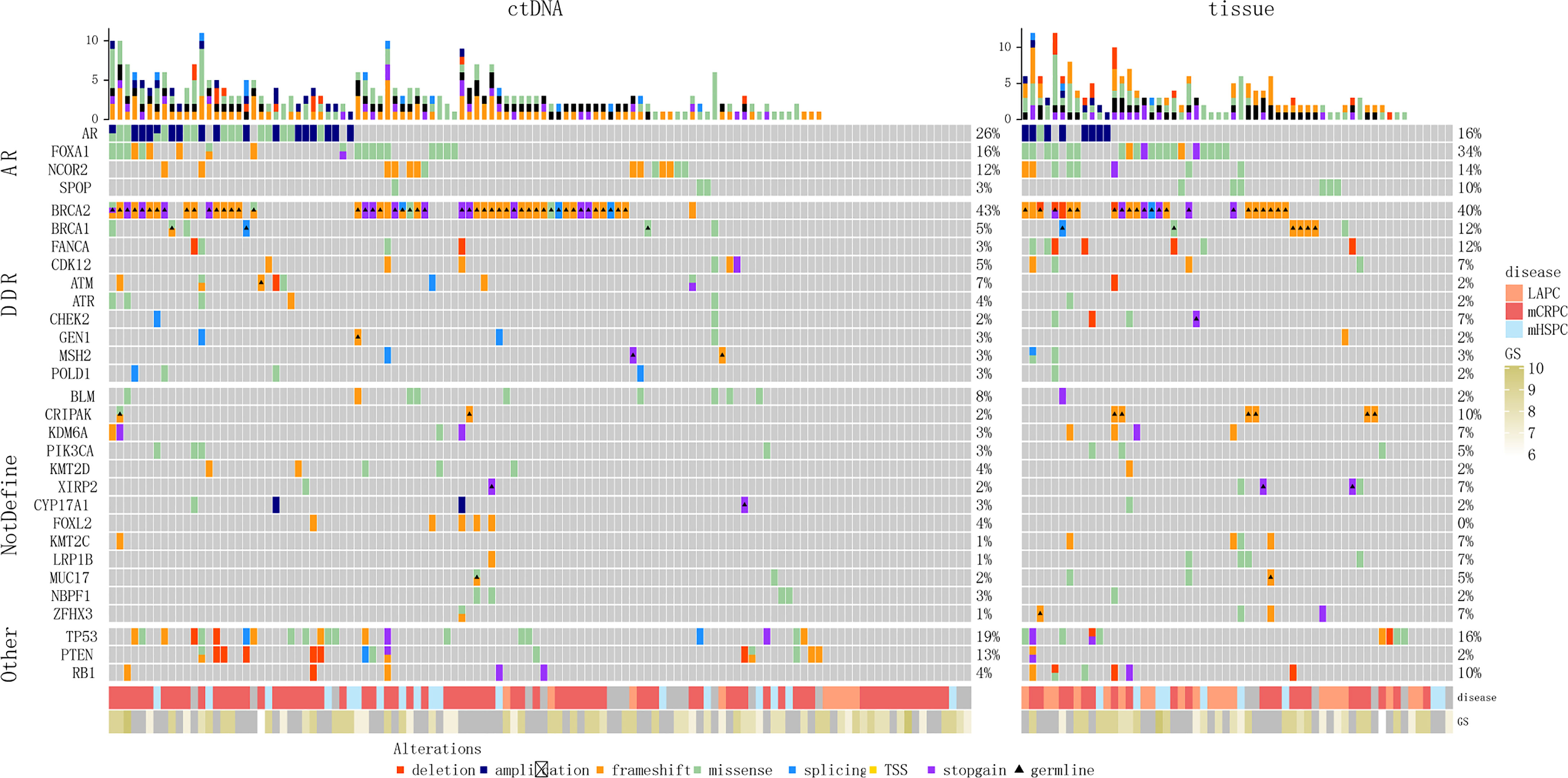
Figure 4 Genomic landscape of patients with PCa with BRCA germline pathogenic mutations. (A) Blood samples. (B) Tissue samples. TSS, translation start site.
Further, there were some differences in the blood and tissue profiles, so we conducted consistency analysis on mutation data from 10 patients using both tissue and matched blood samples (Figure S2 and Table S3). Most of the 10 patients had a relatively high degree of consistency between the mutations in the tissue and matched blood samples (e.g. Patients #2, 6, 9). However, the sample size (10 patients) was small, which limited consistent comparison of the occurrence frequency of the related genes in specific tissue and blood pathways.
Discussion
Differences in BRCA Germline Mutations in Different Populations
Research targeting BRCA1/2 mutations has received increasing attention in recent years, in part because of the success of PARPi in clinical studies. While these studies were mostly focused on foreign populations, there have been few studies on Chinese populations. Studying BRCA1/2 mutations in the Chinese PCa population will enable more comprehensive understanding of BRCA1/2 mutations in this population, and further insightful analysis of the characteristics of these mutations will ultimately provide a more optimal treatment plan for patients.
Although our cohort was smaller than that in another prospective study of BRCA1 and BRCA2 germline pathogenic mutations in a Chinese population (172 vs. 316) (13), our study has a larger Chinese cohort with BRCA1 and BRCA2 germline pathogenic mutations (9 vs. 2, 72 vs. 20). The BRCA2 germline mutation carriers in the present study were at an earlier age, i.e., 67 years (range, 34–89 years), which was similar to previous reports of patients with PCa with BRCA2 mutations having an earlier age of diagnosis (14). Furthermore, most of the clinical stages were concentrated in the mCRPC stage and had high Gleason scores, and the frequency of BRCA1 and BRCA2 germline mutations during the metastatic PCa (MPC) stage (mHSPC and mCRPC) was higher than that in the localized stage. These results confirm that BRCA1/2 mutation carriers are more likely to have lymph node involvement and distant metastases (15). BRCA-positive PCa populations often have higher Gleason scores (≥8) and higher tumor-node-metastasis (TNM) stage (15). These findings could provide more comprehensive evidence for novel endocrine therapy treatments for PCa. New endocrine therapy has a better effect on BRCA mutation carriers compared with non-carriers in the mCRPC population, and PCa populations with BRCA1 or BRCA2 mutations could benefit from abiraterone or enzalutamide treatment (16).
Analysis of BRCA Variants
Understanding the distribution of pathogenic variants in BRCA1/2 in key domains (exons or introns) and the role of each specific variant is of great significance for PCa treatment. Here, BRCA1/2 variant analysis revealed no distinct hotspot mutation. In patients with PCa, the BRCA2 gene has a higher risk of mutations in the c.756-c.1000 and c.7914+ regions (17, 18). However, our results yield no similar conclusions: the frequency of BRCA2 mutation was 16.55% (24/145) in the c.7914+ region, and was 8.97% (13/145) in the in c.756-c.1000 region.
Notably, each variant type showed different characteristics. Here, the top three BRCA variants were frameshift, missense, and splicing variants. Meta-analyses investigating the presence of BRCA genes in patients with cancer found that the missense variant was the most frequent in patients with BRCA1 and BRCA2 variants (19). However, our results show that the frameshift variant was the most frequent in BRCA2 variant carriers, and that all of the variants were pathogenic. Therefore, it is necessary to develop new specific tests for exon or intron–exon boundaries for more accurate PCa clinical diagnosis and treatment.
Differences Between BRCA1 and BRCA2 Mutations
We collected BRCA1/2 gene germline mutation data from the Chinese PCa population, and found that the BRCA2 mutation frequency (69.19%) was much higher than that of BRCA1 (32.56%), and that most mutations occurred at the MPC (mHSPC and mCRPC) stage. This suggests that there might be different tumor gene expression patterns in BRCA2. This is similar to previous studies reporting that the characteristics of BRCA2 mutated tumors were more similar to those of mCRPC than of LAPC (20–22). In 6,902 men with BRCA1 or BRCA2 mutations who developed cancer, especially breast, prostate, and pancreatic cancer, and multiple primary tumors, there was an association with a higher rate of BRCA2 mutations (23). Moreover, clinical trial data (TRITON2 and PROfound) have shown that patients with BRCA2 mutations could benefit more from PARPi than those with BRCA1 mutations (24). These results suggest that patients with PCa with BRCA2 mutations might receive higher prognostic benefit than BRCA1 carriers.
There is an association between patients with PCa with BRCA1/2 mutations carrying other mutations (e.g., TP53) with poorer prognosis and PARPi sensitivity (25). The TRITON2 study observed 62% and 42% BRCA1 and BRCA2 mutation carriers with TP53 mutations, respectively (26). The cBioPortal database, which contains publicly available genomic information, shows that harmful TP53 mutations are more common in patients with PCa carrying BRCA1 mutations than in patients carrying BRCA2 mutations (39% vs. 23%) (26). In the present study, ctDNA sequencing showed that the TP53 mutation frequency in the somatic mutant along with BRCA1 germline mutations was much higher than that of BRCA2 germline mutation (25% [1/4] and 5.6% [3/54]). In tissue sequencing, the frequency was 17% (1/6) and 5% (1/21), respectively.
We also found that, except TP53, FOXA1 and NCOR2, along with BRCA1 germline mutations, were more frequent than BRCA2 germline mutations. Tumors with FOXA1 mutations accompanied by higher Gleason scores, shorter biochemical relapse time, and faster metastatic disease progression (27). NCOR2 could interact with AR, thereby inhibiting the transcriptional activity of AR (28, 29). Recent studies have also found that patients with FOXA1 and NCOR2 mutations had poor prognosis (22). This evidence suggests that FOXA1 and NCOR2 somatic mutations may affect disease progression in patients with BRCA germline mutation. However, our results lack follow-up information for the patients, and the relationship between FOXA1 and NCOR2 mutations and the prognosis of patients carrying BRCA germline mutations should be explored in the future.
Our study has some limitations. Our data contain many variants of VUS (53.85%, 112/208), which prevents elucidation of the pathogenicity of some mutations, consequently delaying the selection of appropriate therapies. Therefore, new database updates or more information mining of mutations are needed. In addition, the panel we used can only capture exon regions and may have missed some meaningful intron mutations.
Conclusions
Our results reveal that PCa with BRCA2 germline mutations is highly aggressive in Chinese patients. The frequency of BRCA1 and BRCA2 germline mutations was significantly different, FOXA1, NCOR2, and TP53 somatic mutations associated with higher BRCA1/2 germline pathogenic mutations. Our results suggest that early genetic testing should be actively recommended for patients with PCa family inheritance, which could provide more accurate data support for them to obtain better treatment.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data in the article relates to Chinese human genetic data, so it is not disclosed. Requests to access these datasets should be directed toZ3lsY2hlbjBAcXEuY29t.
Ethics Statement
The studies involving human participants were reviewed and approved by The First Affiliated hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conception and design: WC, WX, SX, BD, and ZY. Acquisition of data (acquired and managed patients, provided facilities, etc.): WX, SX, HH, QL, YL, PZ, BD, and ZY. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, and computational analysis): TL, YZ, JW, and YY. Writing, review, and/or revision of the manuscript: TL, JW, and YY. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): WX, SX, HH, QL, and BD. Study supervision: BD and ZY. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Zhejiang Provincial Natural Science Foundation of China (No. LY20H160013), Zhejiang Provincial Traditional Chinese Medicine Scientific Research Fund (No. 2022ZB215), Zhejiang Province Medical and health science and Technology project (No. 2022KY199), Zhejiang Province Medical and health science and Technology project (No. 2020KY185), and Clinical Research Special Fund of Wu Jieping Medical Foundation (320.6750.2020-14-6).
Conflict of Interest
Authors TL, YZ, PZ, JW and YY were employed by company GloriousMed Clinical Laboratory Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.746102/full#supplementary-material
Supplementary Figure 1 | Alterations in the BRCA gene. (A) BRCA1 mutations were primarily missense and truncating. (B) BRCA2 mutations were primarily truncating, in-frame, and missense.
Supplementary Figure 2 | Concordance of mutation calls between ctDNA samples and paired tumor tissues in the 10 patients.
References
1. Khazaei Z, Jarrahi AM, Momenabadi V, Ghor F, Goodarzi E. Global Cancer Statistics 2018: Globocan Estimates Of Incidence And Mortality Worldwide Stomach Cancers And Their Relationship With The Human Development Index (HDI). Adv Hum Biol (2019) 9(3):e1257. doi: 10.4103/2321-8568.262891
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
3. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. doi: 10.1002/ijc.29210
4. Gallagher DJ, Cronin AM, Milowsky MI, Morris MJ, Bhatia J, Scardino PT, et al. Germline BRCA Mutation Does Not Prevent Response to Taxane-Based Therapy for the Treatment of Castration-Resistant Prostate Cancer. BJU Int (2012) 109(5):713–9. doi: 10.1111/j.1464-410X.2011.10292.x
5. Pomerantz MM, Spisak S, Jia L, Cronin AM, Csabai I, Ledet E, et al. The Association Between Germline BRCA2 Variants and Sensitivity to Platinum-Based Chemotherapy Among Men With Metastatic Prostate Cancer. Cancer (2017) 123(18):3532–9. doi: 10.1002/cncr.30808
6. Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA1/2 Mutation. J Clin Oncol (2015) 33(3):244–50. doi: 10.1200/JCO.2014.56.2728
7. Di Lorenzo G, Autorino R. Re: Olaparib for Metastatic Castration-Resistant Prostate Cancer. Eur Urol (2020) 78(5):767–8. doi: 10.1016/j.eururo.2020.06.011
8. Zhu Y. Chinese Expert Consensus on Genomic Testing of Prostate Cancer Patients (the 2020 Edition). China Oncol (2020) 30(07):551–60. doi: 10.19401/j.cnki.1007-3639.2020.07.011
9. Bolger AM, Lohse M, Usadel B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics (2014) 30(15):2114–20. doi: 10.1093/bioinformatics/btu170
10. Li H, Durbin R. Fast and Accurate Short Read Alignment With Burrows-Wheeler Transform. Bioinformatics (2009) 25(14):1754–60. doi: 10.1093/bioinformatics/btp324
11. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing Next-Generation DNA Sequencing Data. Genome Res (2010) 20(9):1297–303. doi: 10.1101/gr.107524.110
12. Wang K, Li M, Hakonarson H. ANNOVAR: Functional Annotation of Genetic Variants From High-Throughput Sequencing Data. Nucleic Acids Res (2010) 38(16):e164–4. doi: 10.1093/nar/gkq603
13. Bratt O, Loman N. Clinical Management of Prostate Cancer in Men With BRCA Mutations. Eur Urol (2015) 68(2):194–5. doi: 10.1016/j.eururo.2014.11.005
14. Page EC, Bancroft EK, Brook MN, Assel M, Hassan Al Battat M, Thomas S, et al. Interim Results From the IMPACT Study: Evidence for Prostate-Specific Antigen Screening in BRCA2 Mutation Carriers. Eur Urol (2019) 76(6):831–42. doi: 10.1016/j.eururo.2019.08.019
15. Castro E, Goh C, Olmos D, Saunders E, Leongamornlert D, Tymrakiewicz M, et al. Germline BRCA Mutations Are Associated With Higher Risk of Nodal Involvement, Distant Metastasis, and Poor Survival Outcomes in Prostate Cancer. J Clin Oncol (2013) 31(14):1748–57. doi: 10.1200/JCO.2012.43.1882
16. Antonarakis ES, Lu C, Luber B, Liang C, Wang H, Chen Y, et al. Germline DNA-Repair Gene Mutations and Outcomes in Men With Metastatic Castration-Resistant Prostate Cancer Receiving First-Line Abiraterone and Enzalutamide. Eur Urol (2018) 74(2):218–25. doi: 10.1016/j.eururo.2018.01.035
17. Nyberg T, Frost D, Barrowdale D, Evans DG, Bancroft E, Adlard J, et al. Prostate Cancer Risk by BRCA2 Genomic Regions. Eur Urol (2020) 78(4):494–7. doi: 10.1016/j.eururo.2020.05.005
18. Patel VL, Busch EL, Friebel TM, Cronin A, Leslie G, McGuffog L, et al. Association of Genomic Domains in BRCA1 and BRCA2 With Prostate Cancer Risk and Aggressiveness. Cancer Res (2020) 80(3):624–38. doi: 10.1158/0008-5472.CAN-19-1840
19. Sun P, Li Y, Chao X, Li J, Luo R, Li M, et al. Clinical Characteristics and Prognostic Implications of BRCA-Associated Tumors in Males: A Pan-Tumor Survey. BMC Cancer (2020) 20(1):994. doi: 10.1186/s12885-020-07481-1
20. Robinson D, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell (2015) 161(5):1215–28. doi: 10.1016/j.cell.2015.05.001
21. Taylor RA, Fraser M, Livingstone J, Espiritu SM, Thorne H, Huang V, et al. Germline BRCA2 Mutations Drive Prostate Cancers With Distinct Evolutionary Trajectories. Nat Commun (2017) 8:13671. doi: 10.1038/ncomms13671
22. Armenia J, Wankowicz SAM, Liu D, Gao J, Kundra R, Reznik E, et al. The Long Tail of Oncogenic Drivers in Prostate Cancer. Nat Genet (2018) 50(5):645–51. doi: 10.1038/s41588-018-0078-z
23. Silvestri V, Leslie G, Barnes DR, CG, Agnarsson BA, Aittomaki K, et al. Characterization of the Cancer Spectrum in Men With Germline BRCA1 and BRCA2 Pathogenic Variants: Results From the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). JAMA Oncol (2020) 6(8):1218–30. doi: 10.1001/jamaoncol.2020.2134
24. Markowski MC, Antonarakis ES. BRCA1 Versus BRCA2 and PARP Inhibitor Sensitivity in Prostate Cancer: More Different Than Alike? J Clin Oncol (2020) 38(32):3735–9. doi: 10.1200/JCO.20.02246
25. Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic Correlates of Clinical Outcome in Advanced Prostate Cancer. Proc Natl Acad Sci USA (2019) 116(23):11428–36. doi: 10.1073/pnas.1902651116
26. Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol (2020) 38(32):3763–72. doi: 10.1200/JCO.20.01035
27. Adams EJ, Karthaus WR, Hoover E, Liu D, Gruet A, Zhang Z, et al. FOXA1 Mutations Alter Pioneering Activity, Differentiation and Prostate Cancer Phenotypes. Nature (2019) 571(7765):408–12. doi: 10.1038/s41586-019-1318-9
28. Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing Repression: Evolving Models of Co-Repressor Action. Nat Rev Genet (2010) 11(2):109–23. doi: 10.1038/nrg2736
Keywords: prostate cancer in China, BRCA1 and BRCA2 germline mutations, somatic mutation, BRCA variants, pathogenic rate
Citation: Chen W, Xia W, Xue S, Huang H, Lin Q, Liu Y, Liu T, Zhang Y, Zhang P, Wang J, Yang Y, Dong B and Yu Z (2022) Analysis of BRCA Germline Mutations in Chinese Prostate Cancer Patients. Front. Oncol. 12:746102. doi: 10.3389/fonc.2022.746102
Received: 23 July 2021; Accepted: 24 January 2022;
Published: 17 February 2022.
Edited by:
Susan Percy Ivy, National Cancer Institute (NCI), United StatesCopyright © 2022 Chen, Xia, Xue, Huang, Lin, Liu, Liu, Zhang, Zhang, Wang, Yang, Dong and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhixian Yu, eXV6eDUxNUAxNjMuY29t; Baijun Dong, ZG9uZ2JhaWp1bkByZW5qaS5jb20=
 Wei Chen1
Wei Chen1 Baijun Dong
Baijun Dong Zhixian Yu
Zhixian Yu