- Department of Oncology, Renmin Hospital of Wuhan University, Wuhan, China
Background: Immune checkpoint inhibitors (ICIs) have made a breakthrough in the systemic treatment of patients with advanced tumors. However, little is known about their efficacy and safety in adjuvant settings after the resection of solid tumors.
Methods: We performed a meta-analysis on the efficacy and safety of programmed death 1 (PD1)/PD-1 ligand (PDL1) inhibitors in adjuvant therapy after tumor resection using Review Manager 5.3, based on published clinical studies. The outcomes included recurrence-free survival (RFS), disease-free survival (DFS), overall survival (OS), and adverse events (AEs).
Results: Eight randomized controlled trials (RCTs) were included in the analysis. The use of PD1/PDL1 inhibitors in adjuvant therapy significantly improved RFS (hazard ratio [HR] = 0.72; 95% confidence interval [CI] 0.67–0.78, p < 0.00001). However, there was no statistically significant difference in OS between PD1/PDL1 inhibitors and placebo (HR = 0.86; 95% CI 0.74–1.00, p = 0.05). Gender, age, and PDL1 status were independent predictors of RFS with PD1/PDL1 inhibitors. As for the safety analysis results, PD1/PDL1 inhibitors had a higher incidence of fatigue (risk ratio [RR] = 1.22; 95% CI 1.01–1.49, p = 0.04), nausea (RR = 1.47; 95% CI 1.11–1.94, p = 0.007), and pruritus (RR = 1.96; 95% CI 1.57–2.44, p < 0.00001). In addition, the incidence of any grade adverse events increased in the PD1/PDL1 inhibitor group (RR = 1.03; 95% CI 1.02–1.05, p < 0.0001).
Conclusions: This is the first meta-analysis on the efficacy and safety of PD1/PDL1 inhibitors in adjuvant therapy. The use of PD1/PDL1 inhibitors in adjuvant therapy could significantly reduce the recurrence rate after solid tumor resection. However, the incidence of fatigue, nausea, pruritus, and any grade AEs also increased, which should be monitored with vigilance.
1 Introduction
As per the theme of ASCO 2021, adjuvant therapy is currently the most popular research direction in oncology. Subgroups of patients with high-risk characteristics of primary tumor and regional lymph node metastasis are at an increased risk of recurrence and a poor prognosis after surgical resection (1, 2). The aim of adjuvant therapy is to eliminate minimal residual disease (MRD) after resection (3, 4). Moreover, it has been found in a variety of solid tumors to improve recurrence-free survival and improve overall survival (5–8). However, in some studies, systemic adjuvant therapy did not provide a significant survival benefit for cancer patients. For example, the STORM trial found that sorafenib in adjuvant therapy for liver cancer patients not only failed to bring survival benefits but also increased the risk of side effects and even death (9). A review by Alessandro et al. revealed that the efficacy of systemic adjuvant therapy for resected biliary tract cancer remains controversial (10). From this perspective, the choice of adjuvant therapy for cancer patients needs to be further explored.
Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, have greatly changed the treatment pattern of several types of advanced and metastatic solid tumors in the past decade, and most importantly, they have achieved significant results (11–13). The results of the ASCO-PACIFIC study in 2021 showed that durvalumab was associated with a higher 5-year recurrence-free survival (RFS) (HR = 0.55; 95% CI 0.45–0.68) and 5-year OS (HR = 0.72; 95% CI, 0.59–0.89) compared to the placebo group in patients after concurrent chemoradiation with unresectable stage III non-small cell lung cancer (NSCLC). All the above evidence indicates that immune checkpoint inhibitors (ICIs) have great potential as an adjuvant therapy strategy for high-risk recurrence tumors. Currently, many large-scale randomized controlled trials (RCTs) (14–18) have focused on the role of ICIs in adjuvant therapy. Some studies have revealed the superiority of ICI as an adjuvant therapy for cancer patients, while others indicated the opposite. Therefore, based on all the clinical study data published thus far, including the latest results of the 2021 ASCO conference, we conducted a systematic review and meta-analysis to provide a higher level of evidence-based medical recommendations on this clinical issue.
2 Materials and Methods
2.1 Search Strategy and Study Selection
A systematic literature search was conducted using the following databases: EMBASE, MEDLINE (PubMed), and Web of Science to identify eligible articles published before June 2021. The search terms mainly included adjuvant therapy, PD1 inhibitors, PDL1 inhibitors, immune checkpoints, and cancer. Details of the retrieval strategy are provided in the Supplementary Material.
The included studies were selected based on the following criteria (1): study type: randomized controlled trials (RCTs) (2); participants: patients with solid tumors that were histologically confirmed (3); experimental group: PD1/PDL1 inhibitors were used in adjuvant therapy after surgical resection; control group: placebo or drugs other than PD1/PDL1 inhibitors were used in adjuvant therapy; and (4) outcomes: overall survival (OS), recurrence-free survival (RFS), disease-free survival (DFS), and drug safety. The exclusion criteria were as follows (1): the number of patients <20, non-RCTs (2), insufficient data to estimate, and (3) non-English translation.
2.2 Data Extraction
We extracted the following information from each study: name of first author, year of publication, type of tumor, phase of trials, experimental group and control group, number of patients, hazard ratios (HRs) and confidence intervals (CIs) for outcomes (OS, RFS, and DFS), and the number of patients with adverse events.
2.3 Quality Assessment
Review Manager 5.3 was used to evaluate the quality of the included studies. The evaluation items included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Each item was evaluated and resulted as being either high risk, low risk, or unclear.
2.4 Statistical Analysis
Review Manager 5.3 was used to analyze the data. In this meta-analysis, HRs and their 95% CIs for outcomes (RFS, DFS, and OS) were used to calculate the pooled results. For dichotomous outcomes, the number of events and total patients in the experimental and control groups were extracted and used to calculate the risk ratio (RR). Differences were considered statistically significant at p < 0.05. The I2 statistic was used to evaluate the heterogeneity across the included studies. If I2 > 50%, it was considered that there was significant heterogeneity across the studies, and the random-effects model was selected. Otherwise, the fixed-effects model was selected. The source of heterogeneity was analyzed using subgroup and sensitivity analyses. Publication bias was assessed using funnel plots.
3 Results
3.1 Study Characteristics
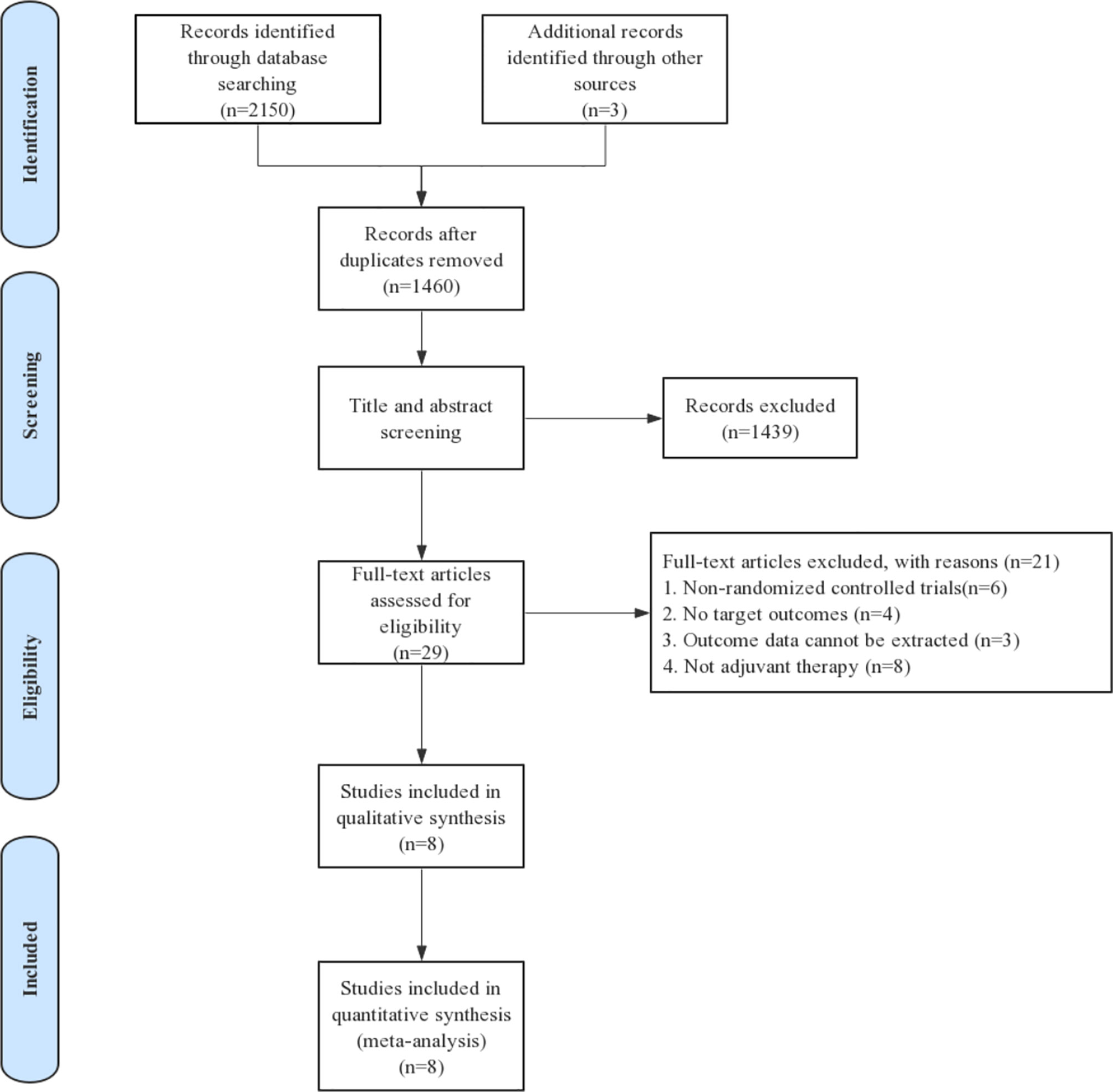
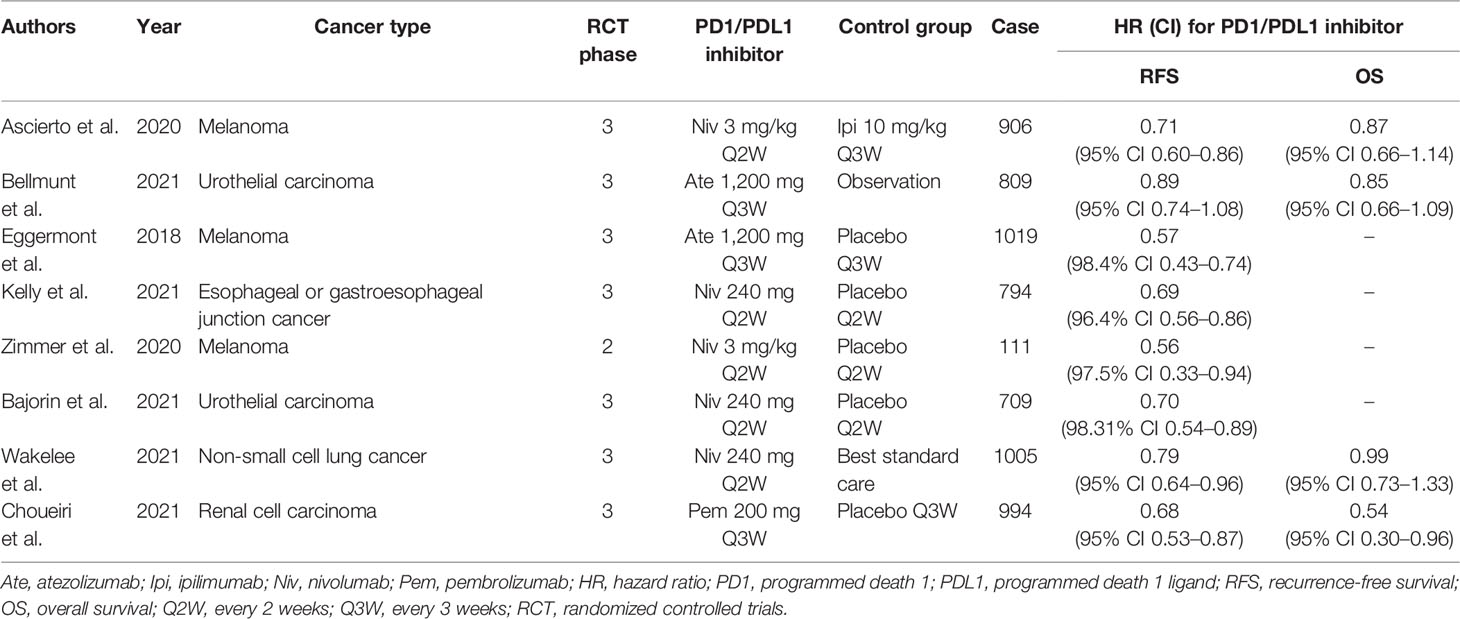
Eligible studies were identified and selected as shown in Figure 1. In total, 2,153 articles were initially evaluated, and 1,460 studies were eligible after exclusion of duplicates. The abstracts and titles of these studies were reviewed, and 1,439 studies were excluded. After an abstract review, we identified 29 articles for full manuscript review, and 21 of these articles were excluded for the reasons delineated in Figure 1. Finally, eight RCTs involving more than 6,000 patients were included in our study. Of the tumor types, three studies were conducted on melanoma, one study on esophageal cancer or gastroesophageal junction cancer, one study on NSCLC, one study on renal cell carcinoma, and two studies on urothelial carcinoma. The characteristics of each study are summarized in Table 1.
3.2 Risk of Bias
All included studies were RCTs; therefore, the overall risk of bias was relatively low. The quality evaluation results of the included studies are shown in Figures 2A, B.

Figure 2 Risk of bias assessment at the study level. (A) Risk of bias graph: review authors’ judgment about each risk of bias item presented as percentages across all included full reported studies. (B) Risk of bias summary: review authors’ judgment about each risk of bias item for each included study.
3.3 Analysis of Efficacy Outcomes
3.3.1 Recurrence-Free Survival
Overall, eight trials on the RFS of patients receiving ICIs in adjuvant therapy involving 6,347 patients were reviewed. The pooled results revealed that the use of PD1/PDL1 inhibitors in adjuvant immunotherapy can significantly reduce the risk of recurrence after tumor resection (HR = 0.72; 95% CI 0.67–0.78, p < 0.00001) (Figure 3A). The study by Bellmunt et al. was a source of heterogeneity (I2 = 31%), in which atezolizumab was the experimental arm. The source of heterogeneity could be that it was the only study in which the experimental group was a PDL1 inhibitor. In the gender subgroup analysis, both men and women could obtain RFS benefits from adjuvant therapy with PD1/PDL1 inhibitors. HR was 0.74 (95% CI 0.67–0.82, p < 0.00001) and 0.72 (95% CI 0.62–0.84, p < 0.0001), respectively (Figure 3B). In the age subgroup analysis, longer RFS could be obtained from the adjuvant treatment of PD1/PDL1 inhibitors for those aged <65 years (HR = 0.71; 95% CI 0.63–0.79, p < 0.00001) or older than 65 years (HR = 0.82; 95% CI 0.71–0.94, p = 0.005) (Figure 3C). In the PDL1 status subgroup analysis, the use of PD1/PDL1 inhibitors in adjuvant therapy compared with placebo reduced the risk of disease recurrence in the subgroups with <5% or ≥5% PDL1 status (Figure 3D).
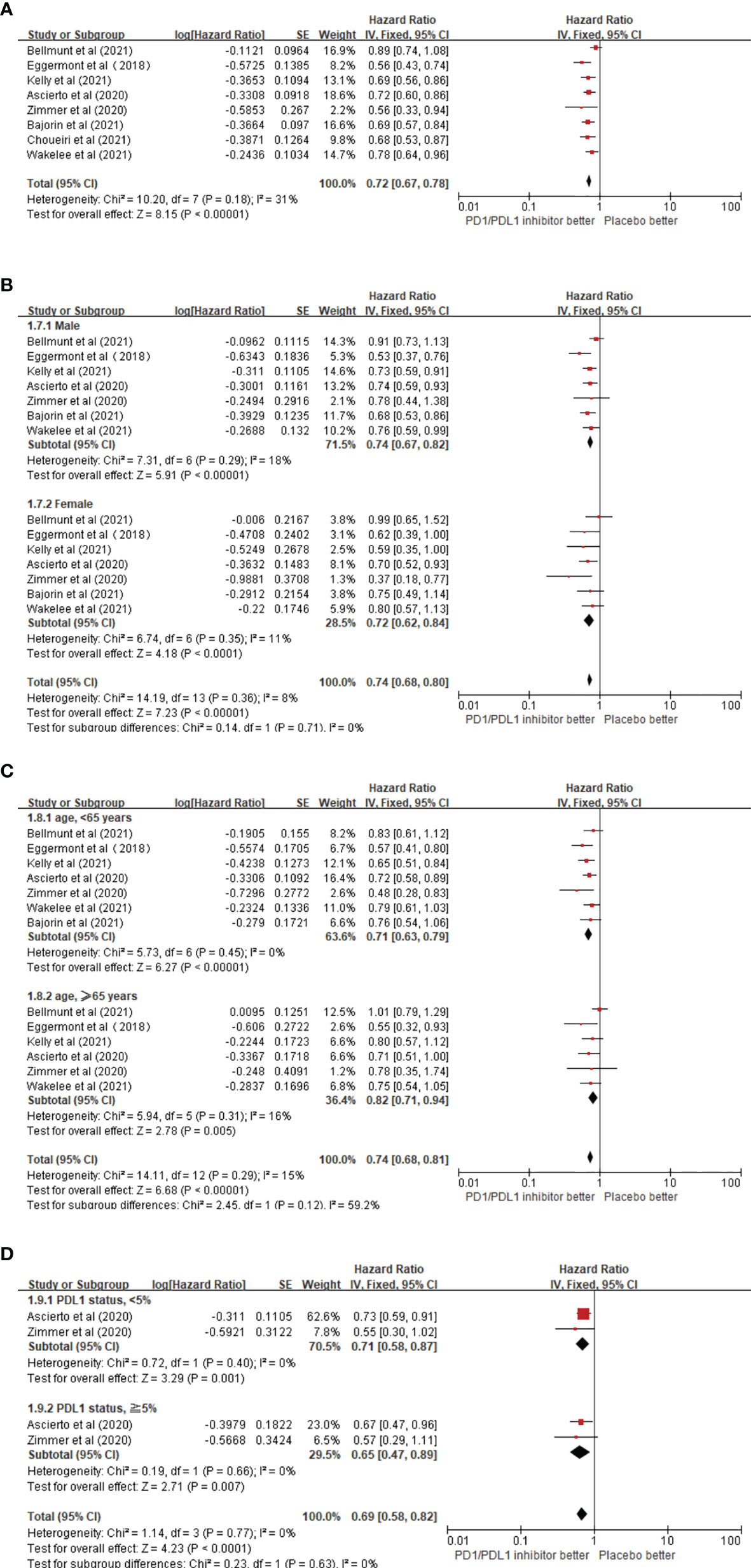
Figure 3 (A) Forest plots of the fixed-effects meta-analysis for the effects of PD1/PDL1 inhibitors on RFS. (B) Forest plots of the fixed-effects meta-analysis for the effects of PD1/PDL1 inhibitors on PFS in gender. (C) Forest plots of the fixed-effects meta-analysis for the effects of PD1/PDL1 inhibitors on RFS in different age group. (D) Forest plots of the fixed-effects meta-analysis for the effects of PD1/PDL1 inhibitors on RFS in different PDL1 status.
3.3.2 Overall Survival
Regarding OS benefits, a total of four trials on the OS of patients receiving ICIs as adjuvant therapy involving 3,714 patients were reviewed. The pooled results showed that there was no statistical difference in OS benefit between the PD1/PDL1 inhibitor arm and the placebo arm in adjuvant therapy (HR = 0.86; 95% CI 0.74–1.00, p = 0.05) (Figure 4).
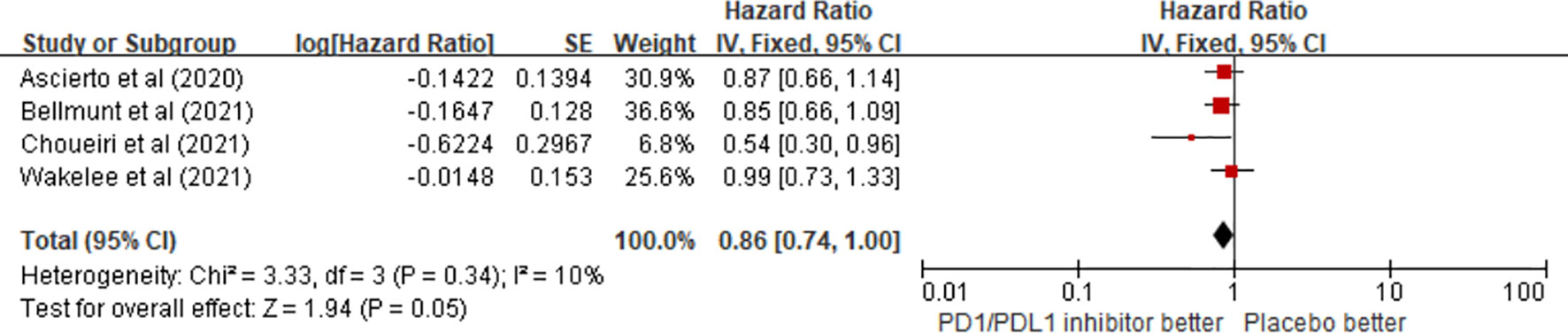
Figure 4 Forest plots of the fixed-effects meta-analysis for the effects of PD1/PDL1 inhibitors on OS.
3.4 Analysis of Safety Outcomes
3.4.1 Any Grade Adverse Events
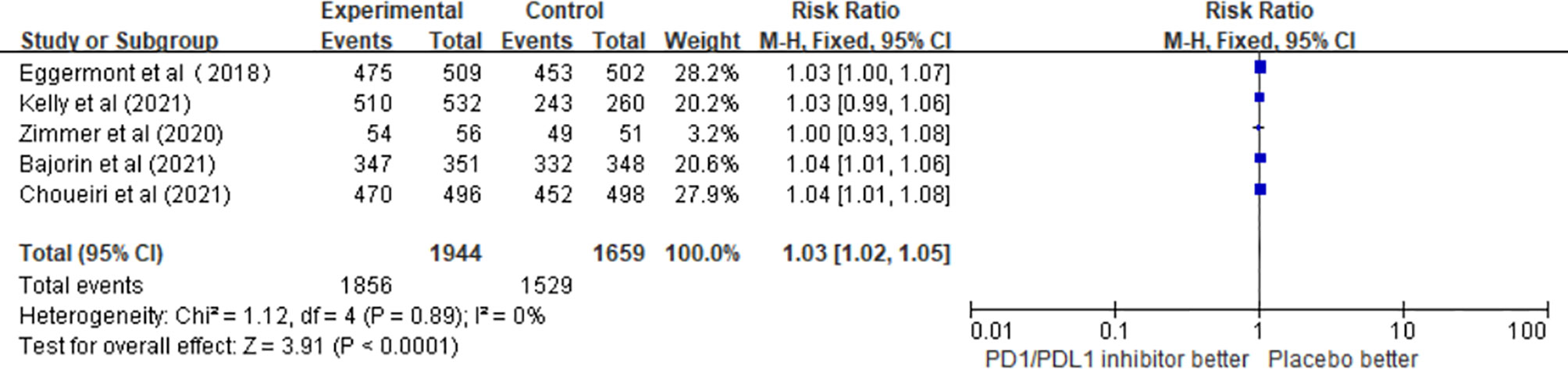
A total of five studies involving 3,603 patients confirmed the safety of PD1/PDL1 inhibitors. The pooled results revealed that the risk of any grade adverse events (AEs) was significantly higher in the adjuvant therapy with PD1/PDL1 inhibitors than in the control group (RR = 1.03; 95% CI 1.02–1.05, p < 0.0001) (Figure 5).
3.4.2 Subgroup Analysis of Any Grade Adverse Event
The results of this meta-analysis showed that the incidence of fatigue (RR = 1.22; 95% CI 1.01–1.49, p = 0.04), nausea (RR = 1.47; 95% CI 1.11–1.94, p = 0.007), and pruritus (RR = 1.96; 95% CI 1.57–2.44, p < 0.00001) in patients who received PD1/PDL1 inhibitors in adjuvant therapy was significantly higher than that in the control group. However, there was no significant difference in the incidence of diarrhea (RR = 1.27; 95% CI 0.96–1.68, p = 0.09) (Figure 6).
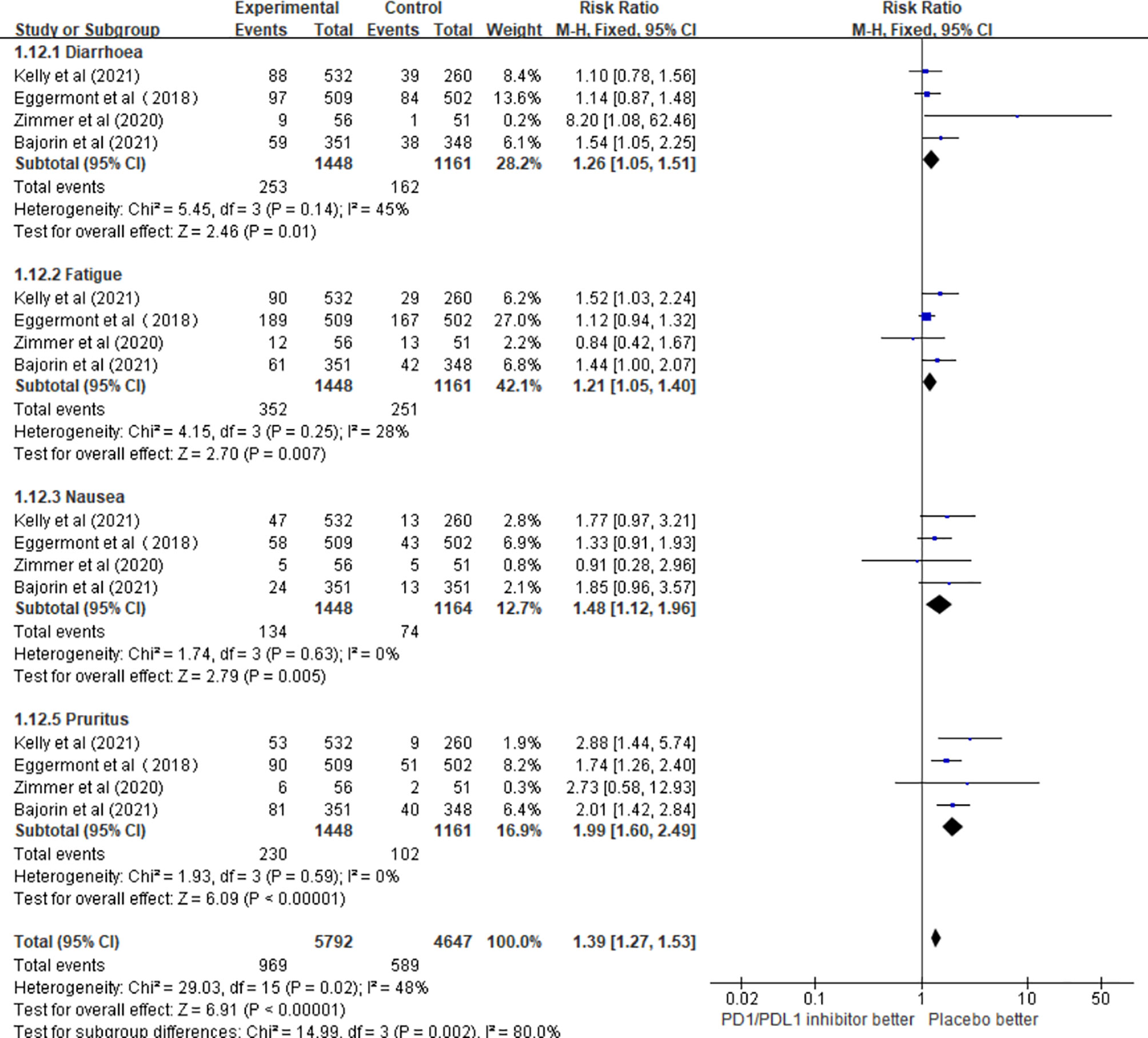
Figure 6 The incidence of different adverse events in the PD1/PDL1 inhibitors groups and placebo groups.
4 Publication Bias
Funnel plot analysis neither indicated apparent publication bias affecting the HRs for RFS and OS nor showed apparent publication bias on RRs of any adverse events (Figure 7).
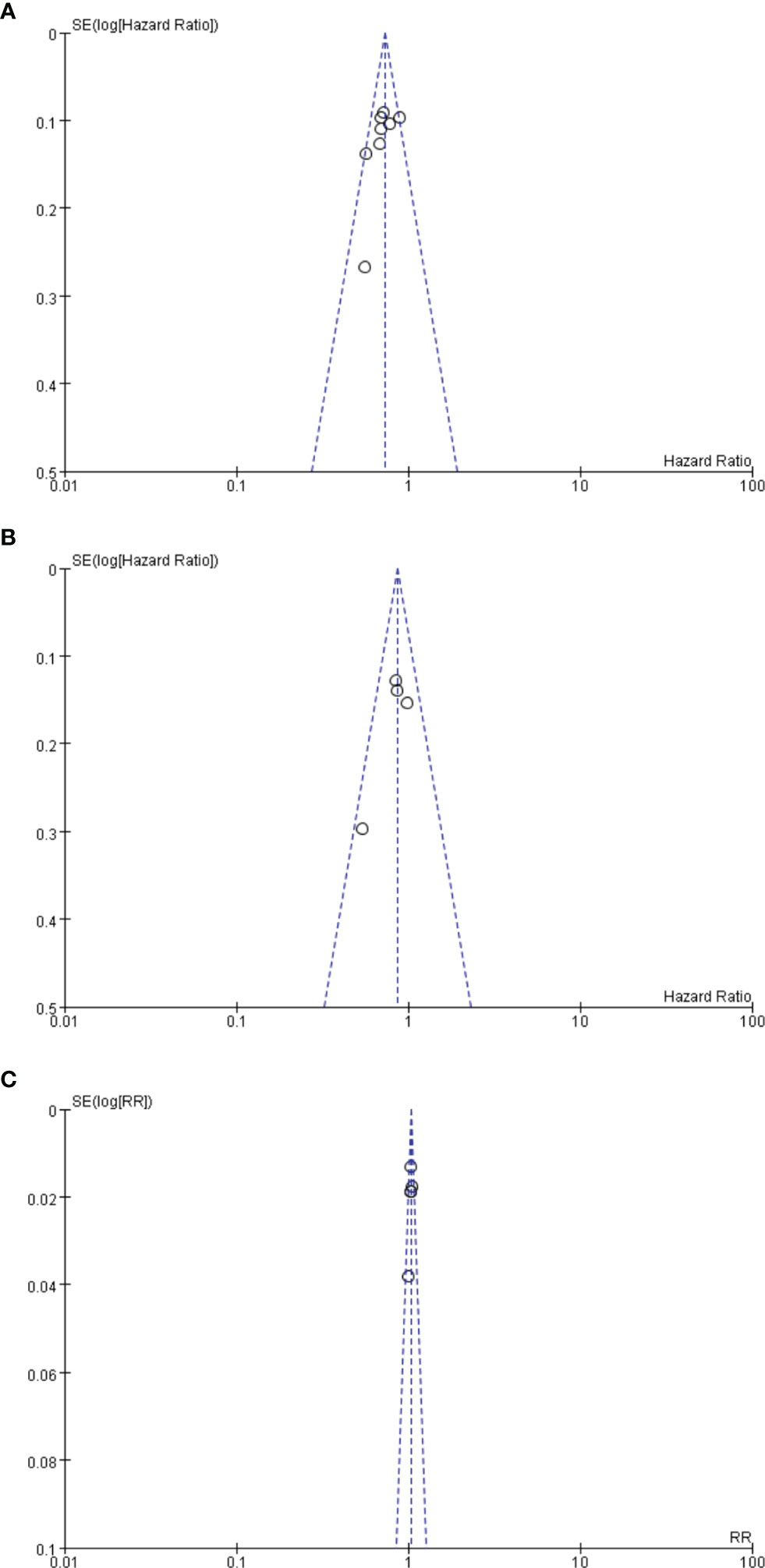
Figure 7 (A) Funnel plot analysis of potential publication bias for RFS. (B) Funnel plot analysis of potential publication bias for OS. (C) Funnel plot analysis of potential publication bias for any advent events.
5 Discussion
Immune checkpoint inhibitors have been widely used in patients with several types of advanced and metastatic solid tumors and have achieved significant OS benefits (19–21). At present, adjuvant therapy is the theme of the ASCO Conference in 2021 and has become the focus of the current oncology therapy field. Several large clinical studies have focused on the adjuvant therapy of ICIs, but the conclusions have been incongruent (22–24). To the best of our knowledge, this is the first systematic review and meta-analysis on the efficacy and safety of PD1/PDL1 inhibitors in the adjuvant treatment of solid tumors after solid tumor resection.
The pooled results of this meta-analysis showed that PD1/PDL1 inhibitors were effective as an adjuvant therapy for tumors. This is consistent with the conclusions of previous studies that explored the efficacy of CTLA-4 inhibitors in adjuvant therapy. The phase III EORTC 18071 trial (25, 26) demonstrated that ipilimumab significantly improved 3-year RFS (HR = 0.75; 95% CI 0.64–0.90, p = 0.0013) after complete resection of stage III melanoma compared to placebo. This study led to the approval of ipilimumab for stage III melanoma after resection in 2015 (27) and was the first immune checkpoint inhibitor approved for adjuvant therapy. In the NCT02523313 phase trial, patients with resected stage IV melanoma with no evidence of disease receiving nivolumab plus ipilimumab in adjuvant therapy had significantly longer RFS (HR = 0.23; 97.5% CI 0.12–0.45, p < 0.0001) than those in the placebo group (16).
Our pooled results also revealed that in the subgroup analysis, patients younger than and older than 65 years could benefit from PD1/PDL1 inhibitors. In patients older than 65 years, PD1/PDL1 inhibitors reduced the risk of recurrence by 18%, and a greater benefit was observed in patients younger than 65 years. This is inconsistent with the conclusion of the EORTC-18071 trial, which revealed that there was no significant difference between the ipilimumab and placebo groups in RFS benefits for patients older than 65 years of age (25). In patients with PDL1 status ≥5%, a 35% reduction in recurrence risk was observed in the PD1/PDL1 inhibitor arm, and comparable results were observed in patients with PDL1 < 5%. This is consistent with the conclusions of two previous clinical trials. In the NCT02362594 trial (28) confirming the efficacy of pembrolizumab in the adjuvant therapy of stage III melanoma and the phase III NCT02743494 trial (15) exploring the role of nivolumab in adjuvant therapy for esophageal or gastroesophageal junction cancer, RFS benefit was observed in patients receiving pembrolizumab or nivolumab regardless of whether PDL1 expression was >1% or ≤1%. However, this is inconsistent with the results of the IMvigor010 trial, which revealed that regardless of the expression status of PDL1, atezolizumab did not improve DFS compared with placebo (14). The tumor types involved in our meta-analysis included melanoma, urothelial carcinoma, renal cancer, NSCLC, and esophageal or gastroesophageal junction cancer. There are currently many ongoing clinical trials exploring the efficacy of ICIs in adjuvant therapy for various tumor types. For example, the NCT02196961 trial is ongoing to explore the efficacy of ipilimumab or nivolumab in the adjuvant therapy of Merkel cell carcinoma (27), and the efficacy and safety of pembrolizumab are being confirmed for stage III or IV melanoma after resection in the phase III clinical trials SWOG S1404 (3, 27).
In addition, our results showed that PD1/PDL1 inhibitors did not improve OS in adjuvant therapy, which might be explained by the following reasons. In the study by Ascierto et al. (17), both the experimental and control groups were ICIs (nivolumab versus ipilimumab). In addition, effective immunotherapy or targeted therapy was subsequently used, leading to possible inherent crossover. In the study by Bellmunt et al. (14), the OS data were not complete because it was still in follow-up, and the use of ICIs in the late control group may have affected the OS. However, in the 2021 ASCO-Pacific study, durvalumab significantly improved OS in patients with unresectable stage III NSCLC following concurrent chemoradiotherapy. The difference in OS benefit may be due to the difference in efficacy between concurrent chemoradiotherapy and tumor resection.
The results of this meta-analysis revealed an increased risk of any grade AEs, fatigue, nausea, and pruritus in adjuvant therapy with PD1/PDL1 inhibitors relative to placebo, which is consistent with the safety results in advanced and metastatic cancer patients receiving PD1/PDL1 inhibitors (29–32). Therefore, these findings should be noted during the use of PD1/PDL1 inhibitors in the adjuvant therapy of solid tumors.
Tumor cells can escape the immune system by activating the T-cell suppression pathway, which is the immune checkpoint pathway. One of the most important pathways is the PD1 pathway (33–35). PD1 is expressed on the surface of T cells in the tumor microenvironment and binds to two ligands (PDL1 and PDL2), resulting in inactivation of the T cells’ tumor-specific immune response, thus allowing the tumor to progress (36–38). PD1/PDL1 inhibitors are antagonists targeting PD1 or PDL1 sites. Therefore, the use of these two drugs will activate the immune response of T cells to tumors (39, 40), thereby inhibiting the growth of tumor cells. MRD is usually present after resection of solid tumors, which is the main cause of tumor recurrence (39, 41). Tumor load is greatly reduced after tumor resection; thus, immune cells are more likely to come into contact with the remaining tumor cells and kill them. PD1/PDL1 inhibitors have the potential to eliminate MRD and thus may reduce the risk of recurrence in patients after tumor resection. In addition, for patients that are in poor physical condition during the perioperative period, clinicians may opt to use an immunotherapy with a lower incidence of adverse events compared to radiotherapy or chemotherapy.
We performed the first meta-analysis of the efficacy and safety of PD1/PDL1 inhibitors in adjuvant therapy. Apart from nivolumab in melanoma, no ICIs have been approved for adjuvant therapy, and the results of our meta-analysis may provide evidence for new clinical applications of ICIs in the future and opens a new avenue for systemic adjuvant therapy. At present, the study content of this topic cannot be applied to clinical practice, which needs to be verified by many large randomized clinical trials in the future. In addition, clinical decision-making requires a reasonable balance between the efficacy and toxicity of PD1/PDL1 inhibitors in adjuvant therapy. For future research on this topic, we think that the following aspects can be expanded on. First, to better play the role of PD1/PDL1 inhibitors in adjuvant therapy, we need to select appropriate patients, namely, the applicable population. Second, the specific regimen and dose selection of PD1/PDL1 inhibitors in adjuvant therapy still need to be further explored. Third, the efficacy predictors of PD1/PDL1 inhibitors in adjuvant therapy for cancer patients need to be explored and updated, such as blood indicators, which have guiding significance for when to stop and whether to continue using drugs. Fourth, the application of PD1/PDL1 inhibitors in combination with other therapies such as targeted therapy or radiotherapy in adjuvant therapy may also be a new breakthrough point in the future.
Our meta-analysis has several limitations. First, many ongoing clinical trials have not yet been completed. Second, due to the diversity of cancer types and adjuvant treatment options and the limited number of included studies, we were unable to conduct a subgroup analysis on the various cancer types and treatment options. This was one of the sources of the heterogeneity in the study results. In the future, more studies on the use of PD1/PDL1 inhibitors in the adjuvant treatment of cancer patients will be conducted, and this will give the study greater statistical significance. Third, although the heterogeneity between the results of each analysis was not particularly significant, the study of Bellmunt et al. was the main source of heterogeneity after the heterogeneity test, which may be related to the PDL1 inhibitor in the experimental group. This suggests that there may be great heterogeneity between the efficacy and safety of PD1 and PDL1 inhibitors, and more studies are needed to confirm this.
6 Conclusion
Overall, the results of our meta-analysis revealed that the use of PD1/PDL1 inhibitors in adjuvant therapy was associated with better RFS compared to controls. Men or women older than or younger than 65 years of age can benefit from PD1/PDL1 inhibitors. Moreover, regardless of the expression status of PDL1, PD1/PDL1 inhibitors can reduce the risk of recurrence. However, the use of PD1/PDL1 inhibitors in adjuvant therapy also increases the risk of adverse events such as fatigue, nausea, and pruritus. Our results provide a reference for the application of PD1/PDL1 inhibitors as adjuvant therapy for solid tumors. However, more studies are needed to demonstrate the efficacy and safety of PD1/PDL1 inhibitors in adjuvant therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization, YW, MP. Methodology, YJ, ZX. Software, JyW. Formal analysis, JsW, XyC. Writing—original draft preparation, YJ, JyW, PW, XC. Writing—review and editing, YW. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81770169), National Natural Science Foundation of China (81802980), and National Natural Science Foundation of China (81102024).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.732814/full#supplementary-material
References
1. Thomas D, Bello DM. Adjuvant Immunotherapy for Melanoma. J Surg Oncol (2021) 123(3):789–97. doi: 10.1002/jso.26329
2. Watanabe SI, Nakagawa K, Suzuki K, Takamochi K, Ito H, Okami J, et al. Neoadjuvant and Adjuvant Therapy for Stage III non-Small Cell Lung Cancer. Jpn J Clin Oncol (2017) 47(12):1112–8. doi: 10.1093/jjco/hyx147
3. Agha A, Tarhini AA. Adjuvant Therapy for Melanoma. Curr Oncol Rep (2017) 19(5):36. doi: 10.1007/s11912-017-0594-5
4. Testori AAE, Ribero S, Indini A, Mandalà M. Adjuvant Treatment of Melanoma: Recent Developments and Future Perspectives. Am J Clin Dermatol (2019) 20(6):817–27. doi: 10.1007/s40257-019-00456-4
5. Cockrell C, Teague J, Axelrod DE. Prevention of Colon Cancer Recurrence From Minimal Residual Disease: Computer Optimized Dose Schedules of Intermittent Apoptotic Adjuvant Therapy. JCO Clin Cancer Inform (2020) 4:514–20. doi: 10.1200/CCI.20.00016
6. Funck-Brentano E, Malissen N, Roger A, Lebbé C, Deilhes F, Frénard C, et al. Which Adjuvant Treatment for Patients With BRAF(V600)-Mutant Cutaneous Melanoma? Ann Dermatol Venereol (2021) 148(3):145–55. doi: 10.1016/j.annder.2020.11.006
7. Huang SM, Chen YC, Chen WY, Yang LY, Tsan DL, Tsang NM, et al. Optimal Timing for Postsurgical Adjuvant Therapy in Patients With Gastric Cancer: A Propensity Score Matching Study. J Cancer (2019) 10(2):332–40. doi: 10.7150/jca.27753
8. Cohen JV, Buchbinder EI. The Evolution of Adjuvant Therapy for Melanoma. Curr Oncol Rep (2019) 21(12):106. doi: 10.1007/s11912-019-0858-3
9. Rizzo A, Ricci AD, Brandi G. Systemic Adjuvant Treatment in Hepatocellular Carcinoma: Tempted to do Something Rather Than Nothing. Future Oncol (2020) 16(32):2587–9. doi: 10.2217/fon-2020-0669
10. Rizzo A, Brandi G. Pitfalls, Challenges, and Updates in Adjuvant Systemic Treatment for Resected Biliary Tract Cancer. Expert Rev Gastroenterol Hepatol (2021) 15(5):547–54. doi: 10.1080/17474124.2021.1890031
11. Kon E, Benhar I. Immune Checkpoint Inhibitor Combinations: Current Efforts and Important Aspects for Success. Drug Resist Update (2019) 45:13–29. doi: 10.1016/j.drup.2019.07.004
12. Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
13. Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc (2019) 94(8):1623–40. doi: 10.1016/j.mayocp.2019.01.013
14. Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant Atezolizumab Versus Observation in Muscle-Invasive Urothelial Carcinoma (IMvigor010): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2021) 22(4):525–37. doi: 10.1016/S1470-2045(21)00004-8
15. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
16. Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, et al. Adjuvant Nivolumab Plus Ipilimumab or Nivolumab Monotherapy Versus Placebo in Patients With Resected Stage IV Melanoma With No Evidence of Disease (IMMUNED): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet (2020) 395(10236):1558–68. doi: 10.1016/S0140-6736(20)30417-7
17. Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, et al. Adjuvant Nivolumab Versus Ipilimumab in Resected Stage IIIB–C and Stage IV Melanoma (CheckMate 238): 4-Year Results From a Multicentre, Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2020) 21(11):1465–77. doi: 10.1016/S1470-2045(20)30494-0
18. Brooker RC, Schache AG, Sacco JJ. NICO Phase II Clinical Trial - Focus on an Emerging Immunotherapy Strategy for the Adjuvant Treatment of Locally-Advanced Oral Cancers. Br J Oral Maxillofac Surg (2021) 59(8):959–62. doi: 10.1016/j.bjoms.2020.08.059
19. Katz H, Biglow L, Alsharedi M. Immune Checkpoint Inhibitors in Locally Advanced, Unresectable, and Metastatic Upper Gastrointestinal Malignancies. J Gastrointest Cancer (2020) 51(2):611–9. doi: 10.1007/s12029-019-00243-8
20. Wu Z, Man S, Sun R, Li Z, Wu Y, Zuo D. Recent Advances and Challenges of Immune Checkpoint Inhibitors in Immunotherapy of non-Small Cell Lung Cancer. Int Immunopharmacol (2020) 85:106613. doi: 10.1016/j.intimp.2020.106613
21. Franzin R, Netti GS, Spadaccino F, Porta C, Gesualdo L, Stallone G, et al. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where do We Stand? Front Immunol (2020) 11:574271. doi: 10.3389/fimmu.2020.574271
22. Bajorin DF, Witjes JA, Gschwend J, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med (2021) 384(22):2102–14. doi: 10.1056/NEJMoa2034442
23. Wakelee HA, Altorki NK, Zhou C, Csőszi T, Vynnychenko IO, Goloborodko O, et al. IMpower010: Primary Results of a Phase III Global Study of Atezolizumab Versus Best Supportive Care After Adjuvant Chemotherapy in Resected Stage IB-IIIA non-Small Cell Lung Cancer (NSCLC). JCO (2021) 39(15_suppl):8500–. doi: 10.1200/JCO.2021.39.15_suppl.8500
24. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y, et al. Pembrolizumab Versus Placebo as Post-Nephrectomy Adjuvant Therapy for Patients With Renal Cell Carcinoma: Randomized, Double-Blind, Phase III KEYNOTE-564 Study. JCO (2021) 39(18_suppl):LBA5–. doi: 10.1200/JCO.2021.39.15_suppl.LBA5
25. Eggermont AMM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant Ipilimumab Versus Placebo After Complete Resection of Stage III Melanoma: Long-Term Follow-Up Results of the European Organisation for Research and Treatment of Cancer 18071 Double-Blind Phase 3 Randomised Trial. Eur J Cancer (2019) 119:1–10. doi: 10.1016/j.ejca.2019.07.001
26. Hemstock M, Amadi A, Kupas K, Roskell N, Kotapati S, Gooden K, et al. Indirect Treatment Comparison of Nivolumab Versus Placebo for the Adjuvant Treatment of Melanoma. Eur J Cancer (2020) 132:176–86. doi: 10.1016/j.ejca.2020.03.011
27. Moujaess E, Haddad FG, Eid R, Kourie HR. The Emerging Use of Immune Checkpoint Blockade in the Adjuvant Setting for Solid Tumors: A Review. Immunotherapy (2019) 11(16):1409–22. doi: 10.2217/imt-2019-0087
28. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab Versus Placebo in Resected Stage III Melanoma. N Engl J Med (2018) 378(19):1789–801. doi: 10.1056/NEJMoa1802357
29. Zhai Y, Ye X, Hu F, Xu J, Guo X, Zhuang Y, et al. Endocrine Toxicity of Immune Checkpoint Inhibitors: A Real-World Study Leveraging US Food and Drug Administration Adverse Events Reporting System. J Immunother Cancer (2019) 7(1):286. doi: 10.1186/s40425-019-0754-2
30. Li L, Li G, Rao B, Dong AH, Liang W, Zhu JX, et al. Landscape of Immune Checkpoint Inhibitor-Related Adverse Events in Chinese Population. Sci Rep (2020) 10(1):15567. doi: 10.1038/s41598-020-72649-5
31. Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol (2016) 34(26):3119–25. doi: 10.1200/JCO.2016.67.9761
32. Chai QQ, Du JY, Zhu J, Wu B. The Differences in the Safety and Tolerability of Immune Checkpoint Inhibitors as Treatment for non-Small Cell Lung Cancer and Melanoma: Network Meta-Analysis and Systematic Review. Front Pharmacol (2019) 10:1260. doi: 10.3389/fphar.2019.01260
33. Sharpe AH, Pauken KE. The Diverse Functions of the PD1 Inhibitory Pathway. Nat Rev Immunol (2018) 18(3):153–67. doi: 10.1038/nri.2017.108
34. Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 Pathway From Discovery to Clinical Implementation. Front Immunol (2016) 7:550. doi: 10.3389/fimmu.2016.00550
35. Han Y, Liu D, Li L. PD-1/PD-L1 Pathway: Current Research in Cancer. Am J Cancer Res (2020) 10(3):727–42.
36. Carreau NA, Pavlick AC. Nivolumab and Ipilimumab: Immunotherapy for Treatment of Malignant Melanoma. Future Oncol (2019) 15(4):349–58. doi: 10.2217/fon-2018-0607
37. de Mello RA, Veloso AF, Esrom Catarina P, Nadine S, Antoniou G. Potential Role of Immunotherapy in Advanced non-Small-Cell Lung Cancer. Onco Targets Ther (2017) 10:21–30. doi: 10.2147/OTT.S90459
38. Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 Pathway. Sci Adv (2020) 6(38). doi: 10.1126/sciadv.abd2712
39. Levi J, Lam T, Goth SR, Yaghoubi S, Bates J, Ren G, et al. Imaging of Activated T Cells as an Early Predictor of Immune Response to Anti-PD-1 Therapy. Cancer Res (2019) 79(13):3455–65. doi: 10.1158/0008-5472.CAN-19-0267
40. Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity (2018) 48(3):434–52. doi: 10.1016/j.immuni.2018.03.014
Keywords: human cancers, immune checkpoint inhibitor, PD1, PDL1, adjuvant therapy, meta-analysis
Citation: Jin Y, Wei J, Weng Y, Feng J, Xu Z, Wang P, Cui X, Chen X, Wang J and Peng M (2022) Adjuvant Therapy With PD1/PDL1 Inhibitors for Human Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 12:732814. doi: 10.3389/fonc.2022.732814
Received: 29 June 2021; Accepted: 02 February 2022;
Published: 25 February 2022.
Edited by:
Franz Rödel, University Hospital Frankfurt, GermanyReviewed by:
Alessandro Rizzo, National Cancer Institute Foundation (IRCCS), ItalyChunxia Su, Shanghai Pulmonary Hospital, China
Copyright © 2022 Jin, Wei, Weng, Feng, Xu, Wang, Cui, Chen, Wang and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Peng, bXBlbmczMjBAd2h1LmVkdS5jbg==
†These authors share first authorship
 Yao Jin
Yao Jin Jiayan Wei
Jiayan Wei Yiming Weng
Yiming Weng Jia Feng
Jia Feng Zexi Xu
Zexi Xu Peiwei Wang
Peiwei Wang Xue Cui
Xue Cui Xinyi Chen
Xinyi Chen Min Peng
Min Peng