
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 31 March 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.732322
This article is part of the Research TopicWomen in Gynecological Oncology: 2021View all 34 articles
Objective: Increased risk of ovarian cancer (OC) among endometriosis patients has been proposed. However, the association between endometriosis and prognosis of OC remains controversial. This study evaluated whether endometriosis had influence on the survival outcomes of OC through a meta-analysis.
Methods: Relevant studies were retrieved from PubMed, Embase, and Web of Science databases and were evaluated using the Newcastle-Ottawa Quality Assessment Scale. Effect size was presented as hazard ratio (HR) and 95% confidence interval (CI). Heterogeneity test evaluation was performed using Cochran’s Q test and I2 statistics. Publication bias was determined using Egger’s test. Statistical analysis was performed using Stata 12.0 software.
Results: Twenty-one studies involving 38641 patients were included. For the total OC, there were significant differences in overall survival (OS) [HR (95% CI)=0.67 (0.55, 0.80), P<0.001] and progression-free survival (PFS) [HR (95% CI)=0.58 (0.42, 0.81), P=0.001] between endometriosis-associated ovarian cancer (EAOC) and non-EAOC patients in the random-effects models (P<0.05). For ovarian clear cell cancer, there were significant differences in terms of OS [HR (95% CI)=0.63 (0.48, 0.83), P=0.001] and PFS [HR (95% CI)=0.67 (0.52, 0.87), P=0.002] between EAOC and non-EAOC patients in the fixed-effects models (P>0.05). Subgroup analysis suggested no significant differences between EAOC and non-EAOC in OS and PFS in the univariate analysis per subgroup, and PFS in the American subgroup (P>0.05).
Conclusion: EAOC patients tended to have better OS and PFS than non-EAOC patients. Conducting higher quality prospective cohort studies with large sample sizes is recommended to confirm the authenticity of the current study’s results.
Systematic Review Registration: https://inplasy.com/inplasy-2022-3-0109/.
Endometriosis is a type of estrogen-dependent chronic inflammatory disease and is a common gynecological condition affecting 5-10% of reproductive-aged women in the world. It is defined as the ectopic growth of endometrial glands and stroma, causing dysmenorrhea, pelvic pain and infertility (1). Although endometriosis is benign lesion, it exhibits malignant biological behaviors similar to cancer, such as local invasion, metastasis, invasion, and easy recurrence (2), and malignant transformation of endometriosis has been proposed as early as 1925 (3). Under the influence of multiple factors, ectopic ovarian endometrial cells with malignant potential gradually change their normal ectopic endometrial cystic epithelium to atypical ectopic endometrial epithelium and invasive carcinoma, which is then called endometriosis associated ovarian cancer (EAOC). Endometriosis may be a precursor lesion to specific subtypes of OC, and prevalence studies show that ovarian clear cell cancer (OCCC) and endometrioid ovarian cancer (EOC) are predominate in women with endometriosis (3–6).
Studies have shown that patients with endometriosis are at higher risk of developing ovarian cancer (OC) (7). Endometriosis is reported to be a risk factor for epithelial OC, which is associated with a 50% increase in epithelial OC risk (8). A meta-analysis of 13 case-control studies suggested that patients with endometriosis had a significantly elevated risk of specific histological subtypes of OC, including OCCC, EOC and low-grade serous OC (9). OC is the seventh most common cancer in women (10) and the 2nd gynecologic cause of cancer deaths in women worldwide (11), with a 5-year survival rate of 47.4%. Prognosis for OC patients is directly associated with tumor stage at the time of diagnosis, ranging from approximately 90% in stage I-tumors to 25% in metastatic tumors (10).
Several studies based on meta-analyses have revealed an association between endometriosis and prognosis of OC in 2014 to 2015, but inconsistent conclusions were found among them (12–14). In recent years, additional studies have been reported the differences of the prognosis of OC patients with or without endometriosis. For example, a retrospective nationwide cohort study of 32,419 women indicated that OC patients with endometriosis had longer overall survival than those without endometriosis, even after adjusting for confounding factors (15). Li et al. indicated that patients with EAOC had longer overall survival (109.8 months) than those with non-EAOC (47.4 months) (16). However, no associations between endometriosis and prognosis of OC were found in the study of Ju et al. (17). Therefore, to obtain a more comprehensive and objective result, we performed a meta-analysis to uncover the differences in terms of progression-free survival (PFS) and overall survival (OS) within EAOC and non-EAOC patients.
According to a pre-established retrieval strategy, the relevant studies were systematically retrieved from PubMed, Embase, Web of Science databases with the retrieval time up to May 11, 2021 and without language restrictions. The search terms contained three categories: research object (“ovarian neoplasm”, “ovarian cancer”, “ovary neoplasm”, “ovary cancer”, “ovarian carcinoma”, “ovary carcinoma”), exposure factors (“endometriosis”, “endometrioses”) and outcomes (“mortality”, “survival”, “prognosis”). Two search terms in the same category are combined with “OR”, while “AND” was used between two search terms of different categories. The detailed retrieval strategies for different databases are listed in Table S1. Additionally, manual retrieval was carried out for the paper version of the relevant studies, and the references of the relevant reviews and included studies were also retrieved.
Studies were included in this meta-analysis if they met the following criteria: (1) patients who were pathologically and histologically diagnosed as epithelial ovarian cancer were included; (2) PFS or OS of OC patients with or without endometriosis were reported; (3) they were retrospective or prospective cohort studies or nested case-control studies; and (4) the crude or multivariable adjusted hazard ratio (HR) and 95% confidence interval (CI) for PFS and OS were reported. Studies were excluded from this analysis if they were: (1) non-original articles, such as reviews, conference abstracts and comments; (2) the studies that provide only a figure but not a detailed HR (95% CI) to show the results of survival analysis; (3) duplicate studies or multiple studies involving the same data, with only the one with the most complete information included. On the basis of the above selection criteria, study retrieval was carried out by two independent investigators.
Data extraction was conducted independently in accordance with the form pre-designed by two investigators. The following data were extracted, including the first author’s name, region of research, year of publication, when the study subjects were recruited, subject information, including: sample size, age, histological subtypes, adjuvant treatment, outcomes, and other confounding factors. The extracted data were exchanged and reviewed, and disagreements were settled through a thorough discussion. The methodological quality evaluation for the included studies, which involved the selection, comparability, and exposure of the included study subjects, were conducted with reference to the Newcastle-Ottawa Quality Assessment Scale (NOS) (18), which includes 8 scoring items with a full score of 9. Studies with a final score ranging from 7 to 9 are regarded as high quality, while those with a final score ranging from 4 to 6 or less than 4 are regarded as medium quality or low quality, respectively.
All statistical analyses were completed using Stata 12.0 software. HR and 95% CI were utilized as effect size indicators to evaluate the differences on PFS and OS of EAOC vs. non-EAOC. Cochran’s Q test and I2 test (19) were used to assess the heterogeneity among studies. Significant heterogeneity was determined with P<0.05 or I2>50%, and a random-effects model was utilized. A fixed-effects model was utilized when no significant heterogeneity was observed (P≥0.05 and I2 ≤ 50%). The effect of region, confounding factors adjusted or not for heterogeneity, and the pooled results were evaluated with a subgroup analysis. Publication bias evaluation was conducted using Egger’s test. If there was significant publication bias, the stability of the results of the meta-analysis was evaluated using the trim-and-fill method. The stability of the results was also evaluated using the method of elimination one by one.
In total, 1931 studies, including 702 studies from PubMed, 713 studies from Embase, and 516 studies from the Web of Science database were retrieved. Among these studies, 549 duplicate studies were first removed. Of the 1382 remaining studies, 1345 irrelevant studies were excluded after title/abstract reading. After full-text reading, 16 studies were further excluded, including 11 studies without detailed HR (95% CI) to show the results, 3 reviews, and 2 studies without detailed information of PFS or OS. Finally, 21 studies (12, 15–17, 20–36) were included in the current meta-analysis (Figure 1).
A total of 38641 cases were included in these 21 studies. Table 1 shows the detailed characteristics of the 21 included studies. All the studies were retrospective cohort studies except for the study of Erzen et al. (23), which was a nested case-control study. The included patients had Stage I-IV OC in all studies except for the study of Ju et al. (17), in which the included patients had Stage I-III OC (data not shown). These studies were published from 1989 to 2021, and were conducted in 8 countries, such as China, South Korea, USA, Japan, and Italy. In addition, the ages of the included cases were not reported in the study of Katagiri et al. (24), but the ages of all the cases were mentioned in three studies (20, 21, 26). The remaining 17 studies reported the ages of cases in the EAOC and non-EAOC groups, and among these studies, significant differences were found between the cases belonging to different EAOC and non-EAOC age groups in ten studies (12, 15, 16, 22, 29, 31–35).
The methodological quality evaluation for the 21 included studies was conducted with reference to NOS, and the results are shown in Table 2. The NOS scores of the 21 studies ranged from 5 to 8 points, of which 5 points was evaluated for one study (23), 6 points for five studies (21, 22, 24, 33, 36), 7 points for six studies (16, 20, 25, 28, 29, 31) and 8 points for nine studies (12, 15, 17, 26, 27, 30, 32, 34, 35). Overall, the methodological quality of the included studies was moderate. Recall bias and confounding bias were the primary bias.
The impact of endometriosis on the prognosis of total OC was presented in Figure 2. Eighteen studies reported the pooled results for OS of patients, and significant heterogeneity among studies were observed (I2 = 41.9%, P = 0.032). A random-effects model was utilized to pool the results. As shown in Figure 2, there was a significant difference in terms of OS within EAOC and non-EAOC groups [HR (95% CI) = 0.67 (0.55, 0.80), P < 0.001], suggesting that EAOC patients had better OS than non-EAOC patients. Similarly, EAOC patients had better PFS than non-EAOC patients [HR (95% CI) = 0.58 (0.42, 0.81), P = 0.001] in the random-effects model (I2 = 53.4%, P= 0.014) (Figure 2). Subgroup analysis indicated that no differences in OS were found between the OC patients with or without endometriosis [HR (95% CI) = 0.73 (0.38, 1.39), P = 0.332] in the univariate analysis subgroup. The results of other subgroups were consistent with the total pooled results. Subgroup analysis for PFS suggested that there was a statistically significant difference in Asian (P=0.005) and multivariate analysis subgroups (P=0.001), while there was no statistically significant difference in the America (P=0.169) and univariate analysis subgroups (P=0.260). In addition, whether confounding factors were adjusted or not was found to be one of the sources of significant heterogeneity for OS (Table 3).
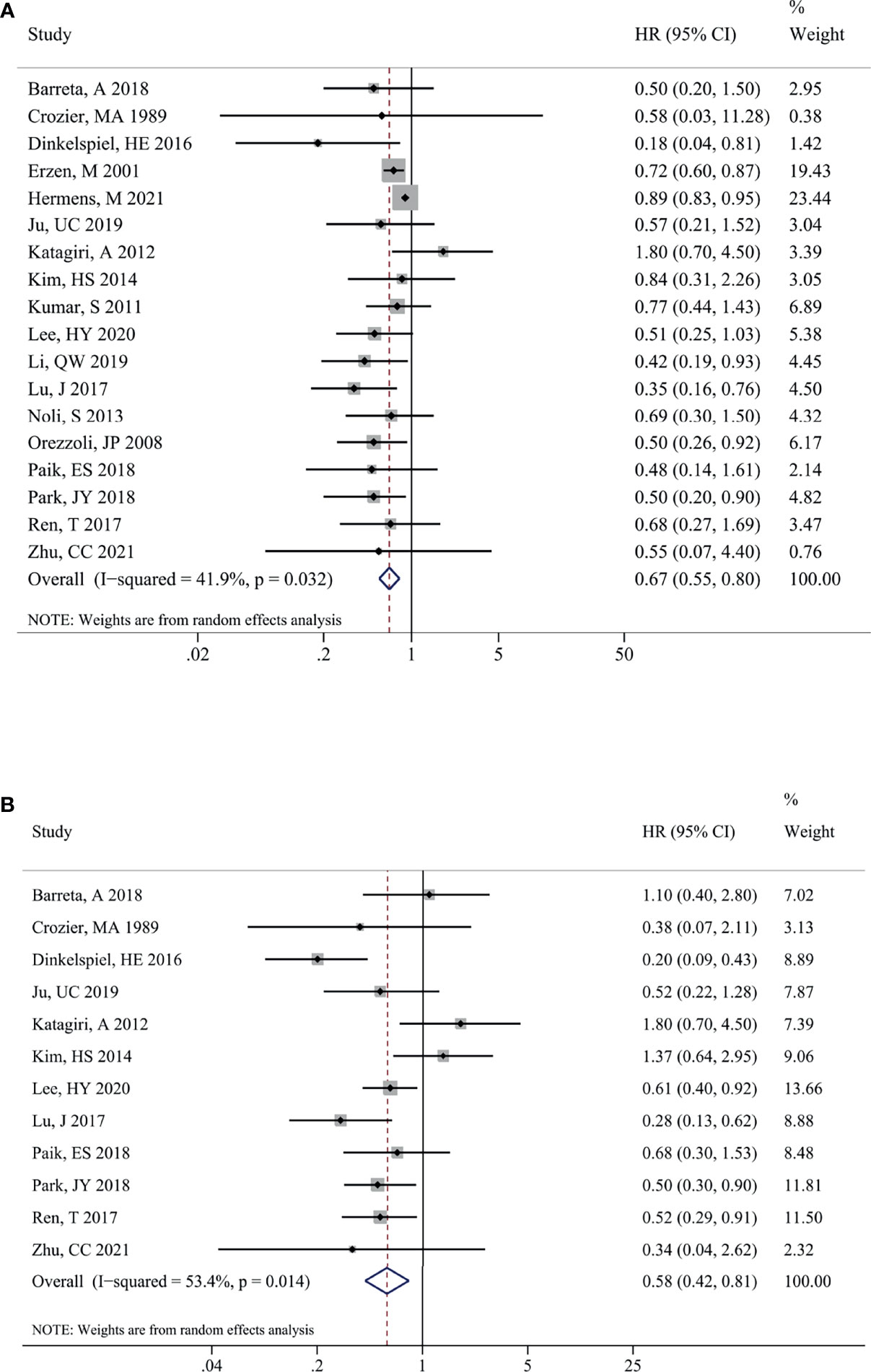
Figure 2 Impact of endometriosis on the prognosis of total OC. Forest plots showing the pooled results for endometriosis on overall survival (A) and progression-free survival (B) of total OC patients. OC, ovarian cancer.
The impact of endometriosis on OCCC prognosis is presented in Figure 3. There was no significant heterogeneity between the studies for OS (I2 = 0.0%, P = 0.589) and PFS (I2 = 40.2%, P = 0.123) therefore, fixed-effects models were used. Similarly, EAOC patients were associated with a better OS [HR (95% CI) = 0.63 (0.48, 0.83), P = 0.001] (Figure 3) and PFS [HR (95% CI) = 0.67 (0.52, 0.87), P = 0.002] (Figure 3) in comparison with non-EAOC patients. Subgroup analysis did not detect any significant difference in OS between the OCCC patients with or without endometriosis in the Europe [HR (95% CI) = 0.68 (0.28, 1.67), P =0.339] and univariate analysis subgroups [HR (95% CI) = 1.38 (0.61, 3.11), P =0.444]. The results of other subgroups were consistent with the total pooled results. Subgroup analysis for PFS suggested that there was statistical significance in the Asian (P=0.003) and multivariate analysis subgroups (P=0.001), while there was no statistical significance in the America (P=0.270) and univariate analysis subgroups (P=0.892) (Table 4).
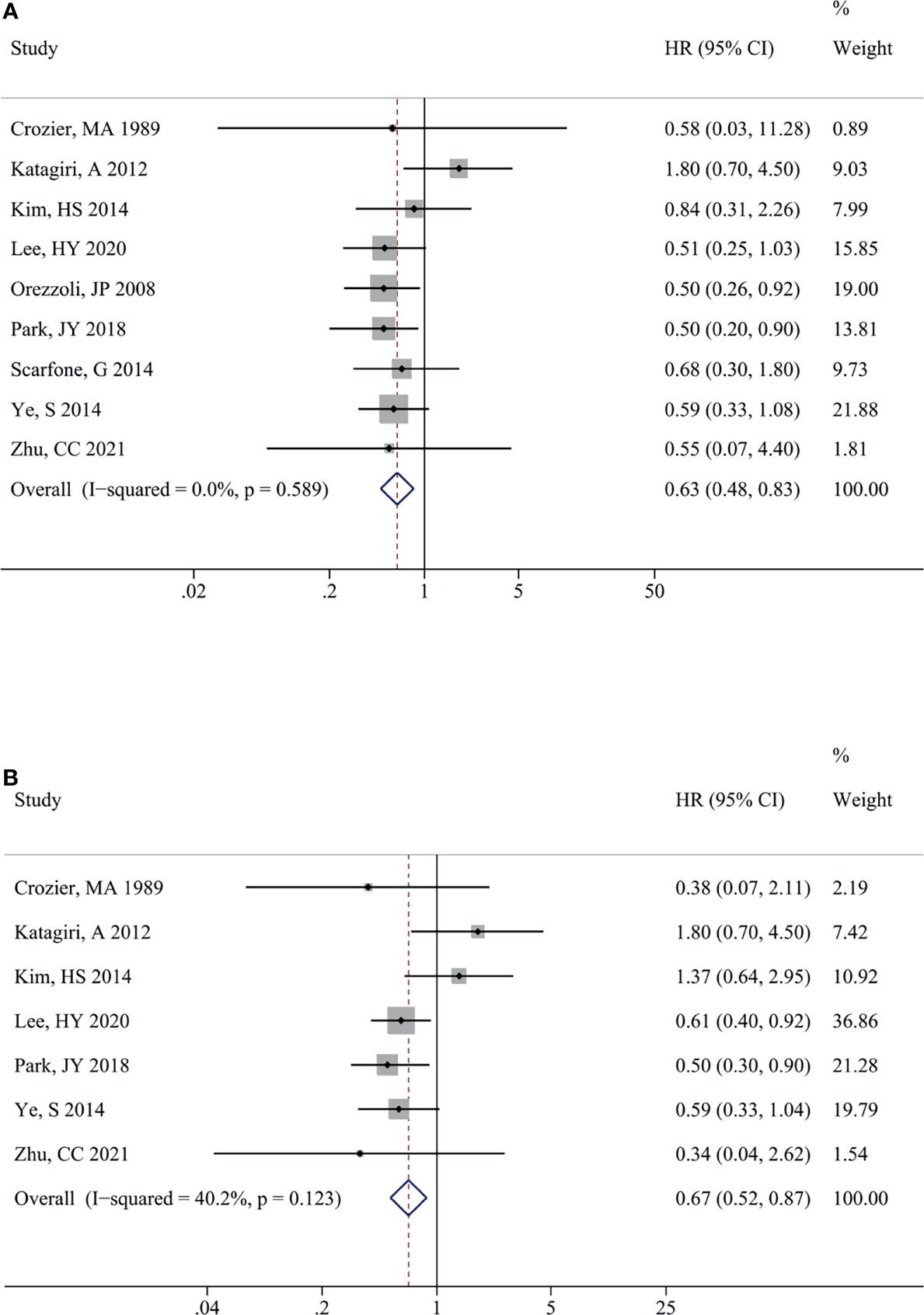
Figure 3 Impact of endometriosis on the prognosis of OCCC. Forest plots showed the pooled results for endometriosis on overall survival (A) and progression-free survival (B) of OCCC patients. OCCC, ovarian clear cell cancer.
Egger’s test found no significant publication bias among the studies for PFS (P=0.945) of total OC patients, as well as the studies for OS (P=0.523) and PFS (P=0.728) of OCCC patients. There was significant publication bias among studies that reported the OS of total OC patients (P=0.002). The stability of the pooled results of the meta-analysis was evaluated using the trim-and-fill method, and the results found a significant difference in terms of OS within EAOC and non-EAOC groups, indicating stable results.
In addition, sensitivity analysis revealed that the variation ranges of pooled results for OS [HR (95% CI) = 0.63 (0.50, 0.79) to 0.70 (0.59, 0.83), P < 0.05, Figure 4] and PFS [HR (95% CI) = 0.53 (0.39, 0.73) to 0.64 (0.47, 0.86), P < 0.05, Figure 4] of total OC patients were not significantly reversed. Similarly, the variation ranges of pooled results for OS [HR (95% CI) = 0.56 (0.42, 0.76) to 0.66 (0.49, 0.91), P < 0.05, Figure 5] and PFS [HR (95% CI) = 0.62 (0.47, 0.81) to 0.73 (0.55, 0.97), P < 0.05, Figure 5] of OCCC patients were not significantly reversed. These results suggest that the pooled results for all outcomes were stable and not significantly altered by any single study.
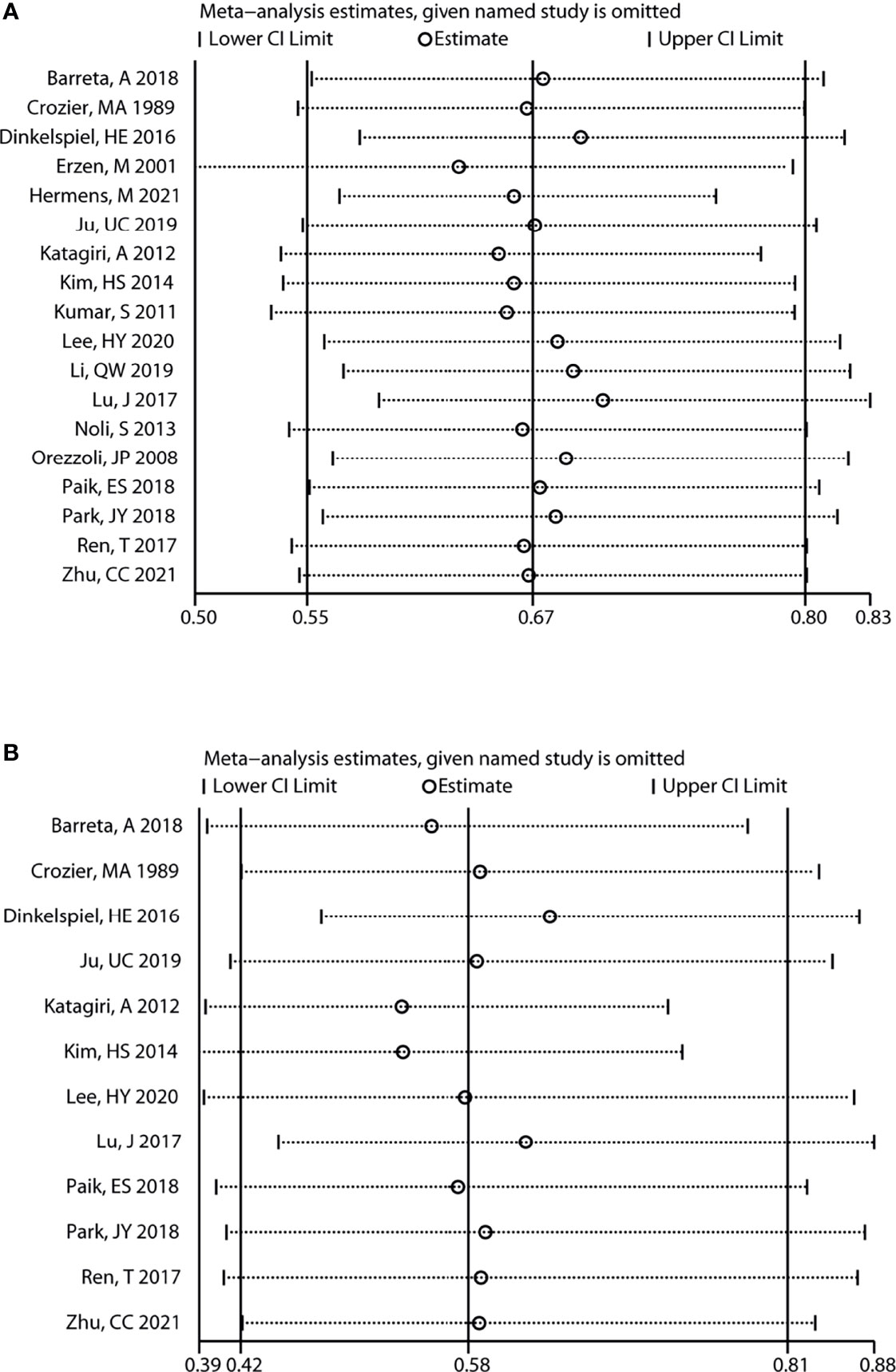
Figure 4 Sensitivity analysis for the results of total OC. Sensitivity analysis to evaluate the stability of the pooled results for the impact of endometriosis on overall survival (A) and progression-free survival (B) of total OC patients. OC, ovarian cancer.
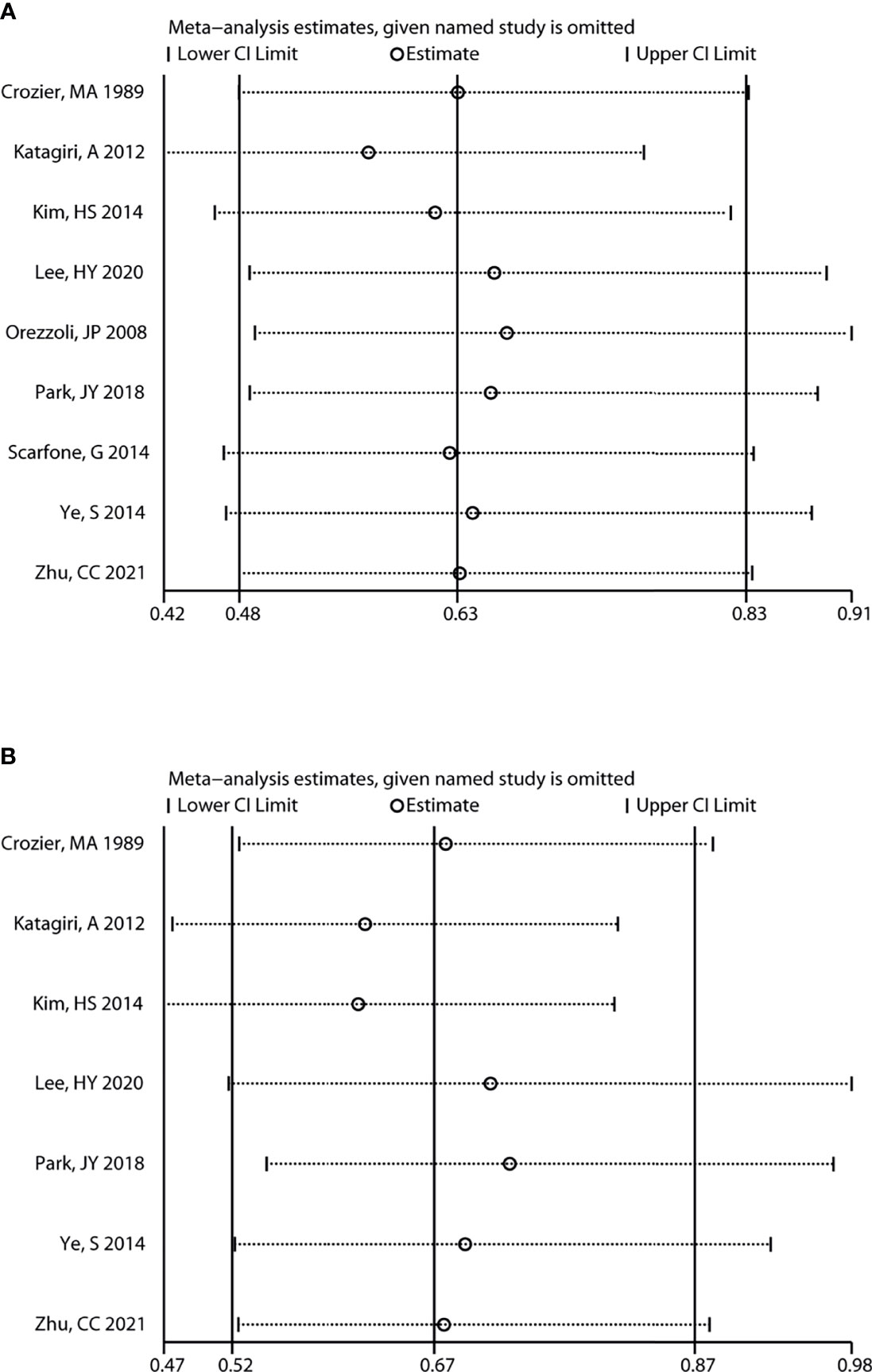
Figure 5 Sensitivity analysis for the results of OCCC. Sensitivity analysis to evaluate the stability of the pooled results for assessing the impact of endometriosis on the overall survival (A) and progression-free survival (B) of OCCC patients. OCCC, ovarian clear cell cancer.
The prevalence of OC was 2.0-17.0% endometriosis patients, while the prevalence of endometriosis was 3.4-52.6% OC patients (37). A Nationwide Population-Based 14-year Cohort Study suggested that the prevalence of EOC in endometriosis patients ranged from 1.9 per 10000 in recalled endometriosis patients to 18.7 per 10000 in tissue-confirmed endometrioma (38). These data suggested that endometriosis was closely related to OC. EAOC is not a homogeneous group of malignancies and includes several histological subtypes. A previous study suggested that patients with endometriosis had specific histological subtypes of OC, including OCCC, EOC and low-grade serous OC, but not with mucinous or high-grade serous OC or borderline OC (9).
In this meta-analysis, we found that EAOC patients had a better OS in comparison to non-EAOC patients in both total OC and OCCC cohorts. Although there was significant publication bias among studies in terms of OS in the total OC cohort, the results of both the trim-and-fill method and the one-by-one elimination method suggested stability of the results of this meta-analysis. Consistent with our results, a higher OS rate in EAOC patients were also reported in the meta-analysis of Yang et al. (14) and Kim et al. (13). In addition, endometriosis-associated higher OS rates were also observed in the OCCC and mixed-subtype of OC subgroups in the meta-analysis of Yang et al. (14). However, another meta-analysis suggested that endometriosis had no influence on the OS of OCCC patients (12). Additionally, we also found that EAOC patients had better PFS than non-EAOC patients. However, no significant differences in terms of PFS within EAOC and non-EAOC patients were observed in previous meta-analyses (12–14). In these meta-analyses, less studies (4, 4 and 6 studies) in terms of PFS were included, while 12 studies were included in our study to explore the differences within EAOC and non-EAOC patients. More recent studies ranging from 2016 to 2021 were included. This might explain the reason for the different results in our meta-analysis and previous meta-analyses. For example, a recent study suggested that a significantly longer DFS was found in EAOC patients (51.9 months) than in non-EAOC patients (30.5 months) (39).
There was no significant difference in terms of PFS between EAOC and non-EAOC patients in the American subgroup in our study, suggesting that the study area might have influence on the results. Among the 21 included studies, the study of Hermens et al. (15) involved large amount of Dutch population-based cohort, and they suggested that patients with endometriosis had better OS than the patients without endometriosis. Similar results were observed in Asia and America populations in our study. Despite most of patients from the study of Hermens et al. (15), sensitivity analysis indicated that pooled results for all outcomes were stable and not significantly altered by their study. Additionally, we found that patients with endometriosis had better PFS than the patients without endometriosis in Asia population but not in America population. While PFS was not mentioned in the study of Hermens et al. (15).It was reported that OC patients with endometriosis were usually younger, and were usually diagnosed at an early stage (39). Significant differences between early stages (stages I and II, 73.8% vs. 41.8%, P < 0.00001) and low histological grades (grade I, 33.3% vs. 19.3%, P < 0.001) were found between EAOC and non-EAOC patients, suggesting a significant association of endometriosis with low tumor grades and early stage tumors (14). These might explain the better prognosis of EAOC patients, in that early-stage cancer might be a driver for better prognosis of EAOC patients rather than an association with endometriosis, as suggested in the study of Li et al. (16). However, Sharfrir et al. found a 29% decreased risk of death among OC patients who pre-diagnostic endometriosis, and association was stable after adjustment for FIGO stage, tumor histology, tumor characteristics and chemotherapy (40). Further studies should be performed to investigate the associations of endometriosis with prognosis of OC in age and stage matched studies. Women with endometriosis are more aware of physical discomfort, so they are more likely to see a doctor earlier, and may require more frequent gynecological ultrasound. This may explain the reason why OC patients with endometriosis were usually diagnosed at an early stage. On the other hand, the treatment of endometriosis involves continuous oral contraceptive use, which has been reported to reduce the risk of OC (41).
There were still some limitations in the current meta-analysis. (1) The adjuvant therapy regimens were not uniform among the included studies, which might affect the association between endometriosis and OS and PFS in OC patients. (2) The pooled results showed no statistical significance among studies that reported the crude HR, probably because that there was no significant difference in terms of OS or PFS of EAOC vs. non-EAOC in the univariate analysis of these studies and were therefore not included in the multivariate analysis. On the contrary, studies with statistical significance in univariate analysis and further implementation of multivariate analysis to correct for the influence of confounders on survival risk, endometriosis was more likely to be significantly associated with OS and PFS in OC patients. This might allow the pooled results of meta-analysis to overstate the strength of the association between endometriosis and risk of death. (3) All the included studies were retrospective studies; thus, recall and confounding biases were common and inevitable biases among them. Although multivariate analysis was performed in most studies to correct for the influence of confounding factors on the results, the inconsistency of correction factors might also affect the accuracy of the results.
In conclusion, this meta-analysis suggested that endometriosis was significantly associated with the OS and PFS of OC patients. EAOC patients tended to have longer OS and PFS than non-EAOC patients. Conducting higher quality prospective cohort studies with larger sample sizes are recommended to confirm the authenticity of the results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
PC acquired funding, collected data, conducted the statistical analysis, created tables and figures, and wrote the manuscript. C-YZ designed the study and helped in writing the manuscript. All authors contributed to the article and approved the submitted version.
The present study was supported by The Natural Science Foundation of Liaoning Province (grant no. 2018010551–301) and the Shengjing Hospital of China Medical University (grant no. MF95). These funding supported in data analysis of the study and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.732322/full#supplementary-material
1. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking Mechanisms, Diagnosis and Management of Endometriosis. Nat Rev Endocrinol (2019) 15(11):666–82. doi: 10.1038/s41574-019-0245-z
2. Kajiyama H, Suzuki S, Yoshihara M, Tamauchi S, Yoshikawa N, Niimi K, et al. Endometriosis and Cancer. Free Radic Biol Med (2019) 133:186–92. doi: 10.1016/j.freeradbiomed.2018.12.015
3. Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant Transformation of Endometriosis and its Clinical Significance. Fertil Steril (2014) 102(2):342–4. doi: 10.1016/j.fertnstert.2014.04.050
4. Herreros-Villanueva M, Chen CC, Tsai EM, Er TK. Endometriosis-Associated Ovarian Cancer: What Have We Learned So Far? Clin Chim Acta (2019) 493:63–72. doi: 10.1016/j.cca.2019.02.016
5. Nezhat FR, Pejovic T, Reis FM, Guo SW. The Link Between Endometriosis and Ovarian Cancer: Clinical Implications. Int J Gynecol Cancer (2014) 24(4):623–8. doi: 10.1097/IGC.0000000000000100
6. Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, Nezhat C. The Relationship of Endometriosis and Ovarian Malignancy: A Review. Fertil Steril (2008) 90(5):1559–70. doi: 10.1016/j.fertnstert.2008.08.007
7. Králíčková M, Laganà AS, Ghezzi F, Vetvicka V. Endometriosis and Risk of Ovarian Cancer: What do We Know? Arch Gynecol Obstet (2020) 301(1):1–10. doi: 10.1007/s00404-019-05358-8
8. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: Pathogenesis and Treatment. Nat Rev Endocrinol (2014) 10(5):261–75. doi: 10.1038/nrendo.2013.255
9. Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association Between Endometriosis and Risk of Histological Subtypes of Ovarian Cancer: A Pooled Analysis of Case-Control Studies. Lancet Oncol (2012) 13(4):385–94. doi: 10.1016/S1470-2045(11)70404-1
10. Stewart C, Ralyea C, Lockwood S. Ovarian Cancer: An Integrated Review. Semin Oncol Nurs (2019) 35(2):151–6. doi: 10.1016/j.soncn.2019.02.001
11. Lheureux S BM, Oza AM. Epithelial Ovarian Cancer: Evolution of Management in the Era of Precision Medicine. CA Cancer J Clin (2019) 69(4):280–304. doi: 10.3322/caac.21559
12. Kim HS, Kim MA, Lee M, Suh DH, Kim K, No JH, et al. Effect of Endometriosis on the Prognosis of Ovarian Clear Cell Carcinoma: A Two-Center Cohort Study and Meta-Analysis. Ann Surg Oncol (2015) 22(8):2738–45. doi: 10.1245/s10434-014-4319-9
13. Kim HS, Kim TH, Chung HH, Song YS. Risk and Prognosis of Ovarian Cancer in Women With Endometriosis: A Meta-Analysis. Br J Cancer (2014) 110(7):1878–90. doi: 10.1038/bjc.2014.29
14. Yang B, Wang D, Chen H, Yang F. The Association Between Endometriosis and Survival Outcomes of Ovarian Cancer: Evidence-Based on a Meta-Analysis. Niger J Clin Pract (2015) 18(5):577–83. doi: 10.4103/1119-3077.158941
15. Hermens M, van Altena AM, van der Aa M, Bulten J, van Vliet H, Siebers AG, et al. Ovarian Cancer Prognosis in Women With Endometriosis: A Retrospective Nationwide Cohort Study of 32,419 Women. Am J Obstet Gynecol (2021) 224(3):284.e1–10. doi: 10.1016/j.ajog.2020.08.056
16. Li Q, Sun Y, Zhang X, Wang L, Wu W, Wu M, et al. Endometriosis-Associated Ovarian Cancer is a Single Entity With Distinct Clinicopathological Characteristics. Cancer Biol Ther (2019) 20(7):1029–34. doi: 10.1080/15384047.2019.1595278
17. Ju UC, Kang WD, Kim SM. The Effect of Concurrent Endometriosis on the Prognosis of Women With Ovarian Clear Cell or Endometrioid Carcinoma. Int J Gynaecol Obstet (2019) 146(2):177–83. doi: 10.1002/ijgo.12861
18. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses (2014). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
20. Barreta A, Sarian L, Ferracini AC, Eloy L, Brito ABC, de Angelo Andrade L, et al. Endometriosis-Associated Ovarian Cancer: Population Characteristics and Prognosis. Int J Gynecol Cancer (2018) 28(7):1251–7. doi: 10.1097/IGC.0000000000001317
21. Crozier MA, Copeland LJ, Silva EG, Gershenson DM, Stringer CA. Clear Cell Carcinoma of the Ovary: A Study of 59 Cases. Gynecol Oncol (1989) 35(2):199–203. doi: 10.1016/0090-8258(89)90043-7
22. Dinkelspiel HE, Matrai C, Pauk S, Pierre-Louis A, Chiu YL, Gupta D, et al. Does the Presence of Endometriosis Affect Prognosis of Ovarian Cancer? Cancer Invest (2016) 34(3):148–54. doi: 10.3109/07357907.2016.1139716
23. Erzen M, Rakar S, Klancnik B, Syrjänen K. Endometriosis-Associated Ovarian Carcinoma (EAOC): An Entity Distinct From Other Ovarian Carcinomas as Suggested by a Nested Case-Control Study. Gynecol Oncol (2001) 83(1):100–8. doi: 10.1006/gyno.2001.6382
24. Katagiri A, Nakayama K, Rahman MT, Rahman M, Katagiri H, Nakayama N, et al. Loss of ARID1A Expression is Related to Shorter Progression-Free Survival and Chemoresistance in Ovarian Clear Cell Carcinoma. Mod Pathol (2012) 25(2):282–8. doi: 10.1038/modpathol.2011.161
25. Kumar S, Munkarah A, Arabi H, Bandyopadhyay S, Semaan A, Hayek K, et al. Prognostic Analysis of Ovarian Cancer Associated With Endometriosis. Am J Obstet Gynecol (2011) 204(1):63.e1–7. doi: 10.1016/j.ajog.2010.08.017
26. Lee HY, Hong JH, Byun JH, Kim HJ, Baek SK, Kim JY, et al. Clinical Characteristics of Clear Cell Ovarian Cancer: A Retrospective Multicenter Experience of 308 Patients in South Korea. Cancer Res Treat (2020) 52(1):277–83. doi: 10.4143/crt.2019.292
27. Lu J, Tao X, Zhou J, Lu Y, Wang Z, Liu H, et al. Improved Clinical Outcomes of Patients With Ovarian Carcinoma Arising in Endometriosis. Oncotarget (2017) 8(4):5843–52. doi: 10.18632/oncotarget.13967
28. Noli S, Cipriani S, Scarfone G, Villa A, Grossi E, Monti E, et al. Long Term Survival of Ovarian Endometriosis Associated Clear Cell and Endometrioid Ovarian Cancers. Int J Gynecol Cancer (2013) 23(2):244–8. doi: 10.1097/IGC.0b013e31827aa0bb
29. Orezzoli JP, Russell AH, Oliva E, Del Carmen MG, Eichhorn J, Fuller AF, et al. Prognostic Implication of Endometriosis in Clear Cell Carcinoma of the Ovary. Gynecol Oncol (2008) 110(3):336–44. doi: 10.1016/j.ygyno.2008.05.025
30. Paik ES, Kim TJ, Choi CH, Kim BG, Bae DS, Lee JW, et al. Clinical Outcomes of Patients With Clear Cell and Endometrioid Ovarian Cancer Arising From Endometriosis. J Gynecol Oncol (2018) 29(2):e18. doi: 10.3802/jgo.2018.29.e18
31. Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, et al. Significance of Ovarian Endometriosis on the Prognosis of Ovarian Clear Cell Carcinoma. Int J Gynecol Cancer (2018) 28(1):11–8. doi: 10.1097/IGC.0000000000001136
32. Ren T, Wang S, Sun J, Qu JM, Xiang Y, Shen K, et al. Endometriosis is the Independent Prognostic Factor for Survival in Chinese Patients With Epithelial Ovarian Carcinoma. J Ovarian Res (2017) 10(1):67. doi: 10.1186/s13048-017-0363-y
33. Scarfone G, Bergamini A, Noli S, Villa A, Cipriani S, Taccagni G, et al. Characteristics of Clear Cell Ovarian Cancer Arising From Endometriosis: A Two Center Cohort Study. Gynecol Oncol (2014) 133(3):480–4. doi: 10.1016/j.ygyno.2014.03.017
34. Wang S, Qiu L, Lang JH, Shen K, Huang HF, Pan LY, et al. Prognostic Analysis of Endometrioid Epithelial Ovarian Cancer With or Without Endometriosis: A 12-Year Cohort Study of Chinese Patients. Am J Obstet Gynecol (2013) 209(3):241.e1–9. doi: 10.1016/j.ajog.2013.05.032
35. Ye S, Yang J, You Y, Cao D, Bai H, Lang J, et al. Comparative Study of Ovarian Clear Cell Carcinoma With and Without Endometriosis in People’s Republic of China. Fertil Steril (2014) 102(6):1656–62. doi: 10.1016/j.fertnstert.2014.08.008
36. Zhu C, Zhu J, Qian L, Liu H, Shen Z, Wu D, et al. Clinical Characteristics and Prognosis of Ovarian Clear Cell Carcinoma: A 10-Year Retrospective Study. BMC Cancer (2021) 21(1):322. doi: 10.1186/s12885-021-08061-7
37. Heidemann LN, Hartwell D, Heidemann CH, Jochumsen KM. The Relation Between Endometriosis and Ovarian Cancer - a Review. Acta Obstet Gynecol Scand (2014) 93(1):20–31. doi: 10.1111/aogs.12255
38. Lee WL, Chang WH, Wang KC, Guo CY, Chou YJ, Huang N, et al. The Risk of Epithelial Ovarian Cancer of Women With Endometriosis May be Varied Greatly If Diagnostic Criteria Are Different: A Nationwide Population-Based Cohort Study. Med (Baltimore) (2015) 94(39):e1633. doi: 10.1097/MD.0000000000001633
39. Bassiouny D, El-Baz MA, Gamil TM, Shams N, Ismiil N, Dubé V, et al. Endometriosis-Associated Ovarian Cancer is a Subset With a More Favorable Outcome and Distinct Clinical-Pathologic Characteristics. Int J Gynecol Pathol (2019) 38(5):435–42. doi: 10.1097/PGP.0000000000000533
40. Shafrir AL, Babic A, Tamimi RM, Rosner BA, Tworoger SS, Terry KL, et al. Reproductive and Hormonal Factors in Relation to Survival and Platinum Resistance Among Ovarian Cancer Cases. Br J Cancer (2016) 115(11):1391–9. doi: 10.1038/bjc.2016.316
Keywords: endometriosis, ovarian cancer, prognosis, meta-analysis, overall survival, progression-free survival
Citation: Chen P and Zhang C-Y (2022) Association Between Endometriosis and Prognosis of Ovarian Cancer: An Updated Meta-Analysis. Front. Oncol. 12:732322. doi: 10.3389/fonc.2022.732322
Received: 28 June 2021; Accepted: 07 March 2022;
Published: 31 March 2022.
Edited by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyReviewed by:
Farr Nezhat, Nezhat Surgery for Gyn/Onc, United StatesCopyright © 2022 Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Yuan Zhang, emN5c2pjbXVAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.