- 1Department of Medicine, King Hussein Cancer Center, Amman, Jordan
- 2School of Medicine, University of Jordan, Amman, Jordan
- 3Department of Cell Therapy & Applied Genomic, King Hussein Cancer Center, Amman, Jordan
- 4Department of Internal Medicine, Istishari Hospital, Amman, Jordan
Purpose: Contrary to BRCA pathogenic variants, recommendations for management of variants of uncertain significance (VUS) are not clear and focus more on the patient’s family and personal history of cancer. Local and regional data on VUS are scarce. In this paper, we study patterns and frequency of VUS among breast cancer patients undergoing genetic testing.
Patients and Methods: Patients with breast cancer at high risk for pathogenic variants, as per the National Comprehensive Cancer Network (NCCN) guidelines, were tested at reference laboratories. Related surgical interventions were reviewed.
Results: Among a group of 1,197 patients with breast cancer who underwent genetic testing and counseling, 110 (9.2%) had VUS; most (n = 79, 71.8%) were in BRCA2. Median age (range) was 39 (25–66) years with 65 (59.1%) patients who were 40 years or younger at diagnosis. Among 103 patients with non-metastatic disease, 48 (46.6%) had breast-conserving surgery (BCS) while only 5 (4.9%) had bilateral mastectomies; all were due to bilateral disease and not prophylactic. VUS diagnosis was known prior to initial surgery in 34 (33.0%) patients; 11 (32.4%) of them had BCS only. Over the study period, only one VUS variant was upgraded to “likely positive.” The recent introduction of multiple-gene panel testing had resulted in a surge in VUS rate (22.2%) in genes other than BRCA1 or BRCA2, like PALB2, CHEK2, and ATM.
Conclusions: Rates of VUS are relatively high and increasing, mostly in non-BRCA1 or BRCA2, and this had no impact on the therapeutic or prophylactic surgical decisions. Adherence to guidelines is extremely important to avoid unnecessary procedures.
Introduction
Breast cancer is the most common cancer among women in Jordan and worldwide (1, 2). Almost 10% of breast cancer cases are hereditary and mostly related to BRCA1 or BRCA2 gene mutations (3, 4); both were initially characterized and sequenced more than 25 years ago (5, 6) and since then have become the most thoroughly studied genes in cancer biology (7).
BRCA1 or BRCA2 mutations are associated with high penetrance rates; the estimated mean cumulative risks for breast cancer by age 70 years for BRCA1 and BRCA2 mutation carriers, as reported by a meta-analysis, were 57% (95% CI, 47% to 66%) and 49% (95% CI, 40% to 57%), respectively (8). Such patients are at a higher risk for ovarian cancer, too (9). Risk-reduction interventions, such as bilateral mastectomies and oophorectomies, are highly recommended for such patients (10).
Genetic test results are usually reported as positive (pathogenic, or likely pathogenic mutation), negative (no detected mutation), or a variant of uncertain significance (VUS). The latter is a DNA alteration in the gene sequence with unknown consequences on the gene function (11).
Contrary to pathogenic mutations, recommendations for the management of VUS are not clear and focus more on clinical factors and personal and family history of cancer—breast and ovarian in particular. Genetic variants that may or may not have clinical consequence can be confusing and anxiety-provoking to patients and physicians alike (12).
The frequency of VUS reports varies worldwide and depends on the ancestry of the population served, testing prevalence, and methodology; rates as high as 21% were reported among the African-American population (13). The increased public awareness, sparked by media reporting of celebrities, resulted in growing interest by the public about the genetic background of cancer in general, and breast cancer in particular (14, 15). Additionally, the recent linkage between triple-negative breast cancer, with or without family history, and BRCA mutations along with the recent introduction of novel targeted therapies for such a subgroup of patients have also increased the requests for genetic testing (16–19).
No data on VUS exist on Arab patients in general, and Jordanians in particular. In this study, we search for the frequency of VUS among breast cancer patients and study treatment patterns and risk-reduction interventions related to such findings.
Methods
In 2016, we started a genetic testing and genetic counseling clinic for patients with breast cancer. Patients were referred to this service if they fit one of the indications recognized by the National Comprehensive Cancer Network (NCCN) guidelines (20). Such indications include patients with early-onset breast cancer (age ≤40 years), aged ≤60 years with triple-negative disease, and those diagnosed at age 50 years or younger with one or more close relatives with pancreatic, prostate (Gleason score ≥7), or breast cancer at any age. Additionally, patients diagnosed at any age with one or more close relatives with breast cancer diagnosed at age 50 years or younger and those diagnosed at any age with two or more additional diagnoses of breast cancer at any age were also tested. Since then, the database contains records on almost 1,200 patients and data on pathogenic variants were reported earlier (21, 22).
We also utilized patients’ medical records to review clinical and pathological features of their breast cancer along with surgical interventions patients had in relation to their breast cancer and VUS identification.
Eligible patients were identified during their routine oncology clinic visits or during the weekly breast multidisciplinary team (MDT) meetings. A detailed three-generation family history was also obtained by a genetic counselor or one of the investigators. Ten milliliters of peripheral blood samples was obtained for DNA extraction. BRCA1 and BRCA2 sequencing was performed at one of two reference laboratories: Leeds Cancer Center, Leeds, United Kingdom, and Myriad Genetics Laboratory, Salt Lake City, USA. In November 2019, we expanded our genetic testing to include a panel of 20 genes (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MSH2, MSH6, NBN, NF1, PALB2, PMS2, PTEN, RAD51C, RAD51D, STK11, TP53) and samples were tested at Invitae, San Francisco, USA. Consent for genetic testing was obtained following a detailed explanation and interview by a trained genetic counselor. The study was approved by King Hussein Cancer Center’s Institutional Review Board (IRB).
Statistical Analysis
Descriptive data of clinical and pathological characteristics of patients were collected, tabulated, and described by ranges, medians, and percentages (%). The chi-square test was used to compare the proportion of VUS according to age (≤40 versus >40), triple-negative status, and family history, in which a p-value of ≤ 0.05 was considered significant. Relatives diagnosed with breast cancer and tested after the index case in the family were not enrolled and were excluded from the analysis. The Fisher exact test was used to compare the type of surgical management; modified radical mastectomy (MRM) vs. BCS according to timing of genetic testing; and before surgery or after surgery. Analyses were conducted using Minitab® Statistical Software version 18 (2017), Minitab Inc., State College, USA.
Results
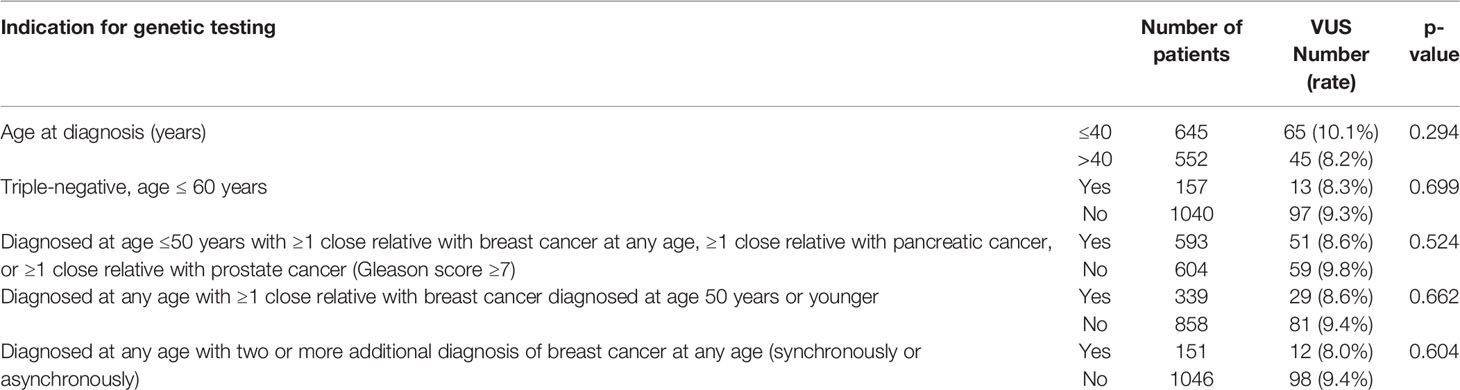
Between May 2016 and May 2020, a total of 1,197 patients were tested for BRCA1 and BRCA2 mutations and were included in our genetic testing database, and all are Jordanians. Patients were referred for testing as per the NCCN guidelines (20). The most common indication for testing were age ≤ 40 years (n = 645, 53.9%) and positive family history of breast, pancreatic, or high-grade prostate cancer (n = 593, 49.5%). Positive/likely positive variants were detected in 143 (12.0%) patients.
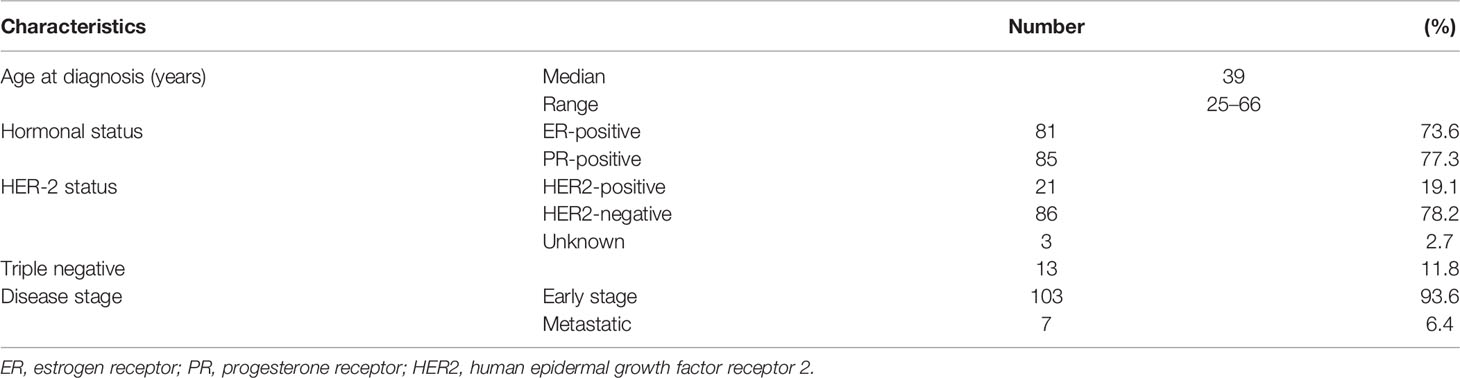
Among the whole group, 110 (9.2%) patients had VUS in BRCA1 or BRCA2 genes and are the subject of this study. Except for one patient, all were women and the median age (range) was 39 (25–66) years with 65 (59.1%) who were 40 years or younger at diagnosis. At the time of genetic counseling and testing, all patients had current or recent history of breast cancer; details of patients’ characteristics are presented in Table 1.
Among the patients with VUS, 13 (11.8%) had triple-negative disease while 12 (10.9%) others had bilateral or two or more unilateral primary breast cancers. Family history of breast, ovarian, or pancreatic cancers, in at least one close relative, was identified in 51 (46.4%) patients. Most VUS (n = 79, 71.8%) were in BRCA2, and 18 (16.4%) had two (n = 14) or 3 (n = 4) different variants. Rates of VUS were not different in relation to age, presence of triple-negative disease, family history of breast, ovarian, or prostate cancers, and those with bilateral or more than one unilateral breast cancers (Table 2).
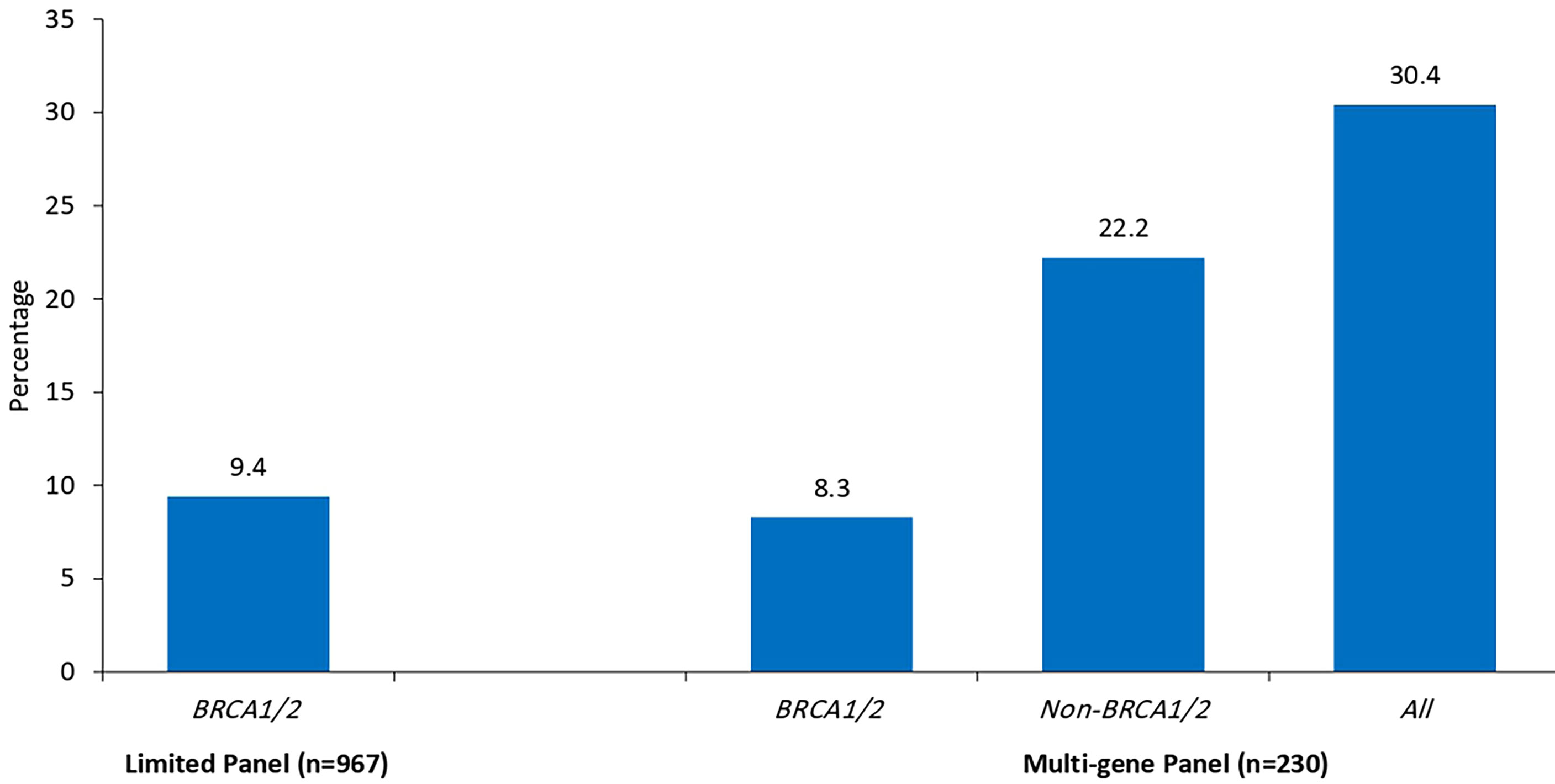
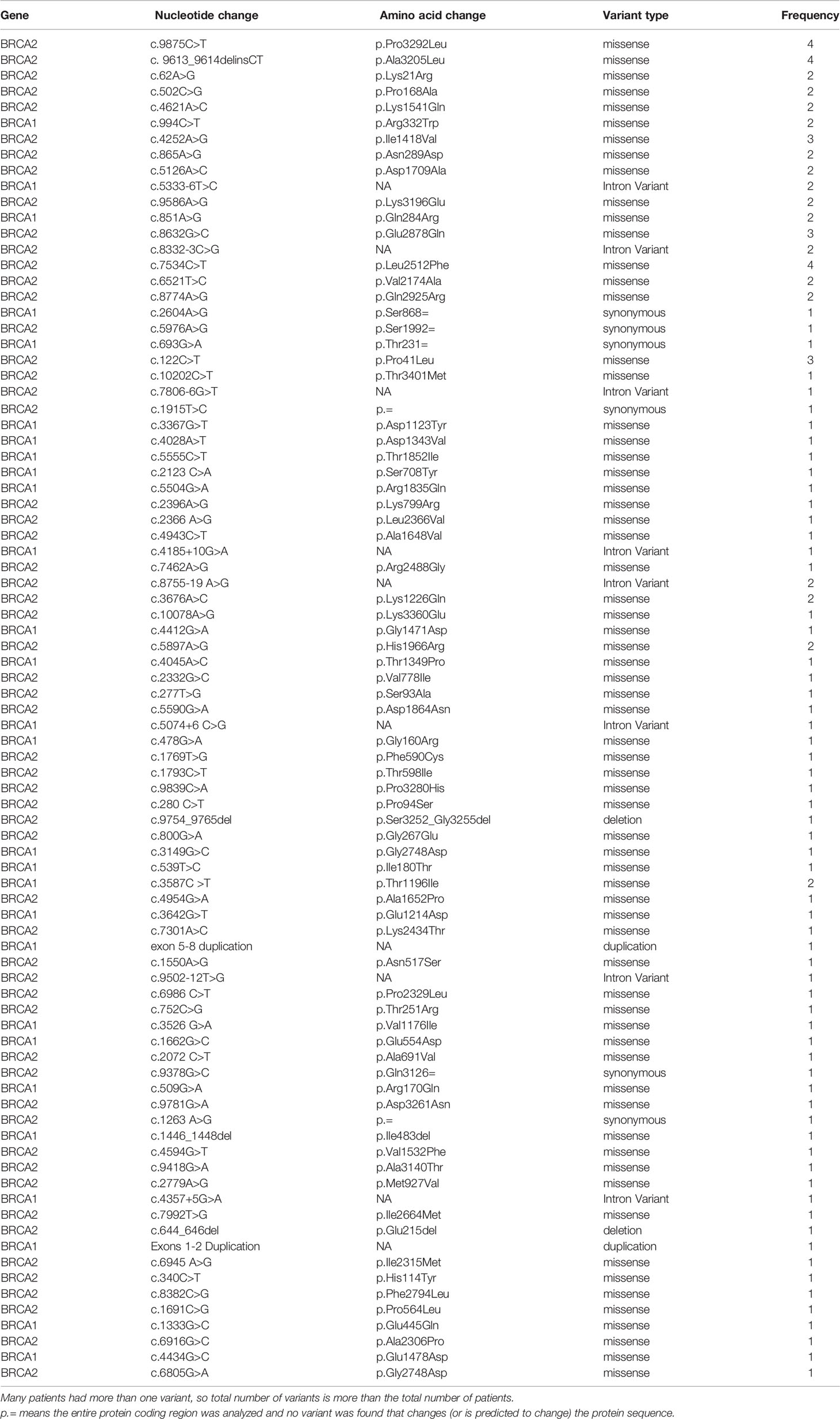
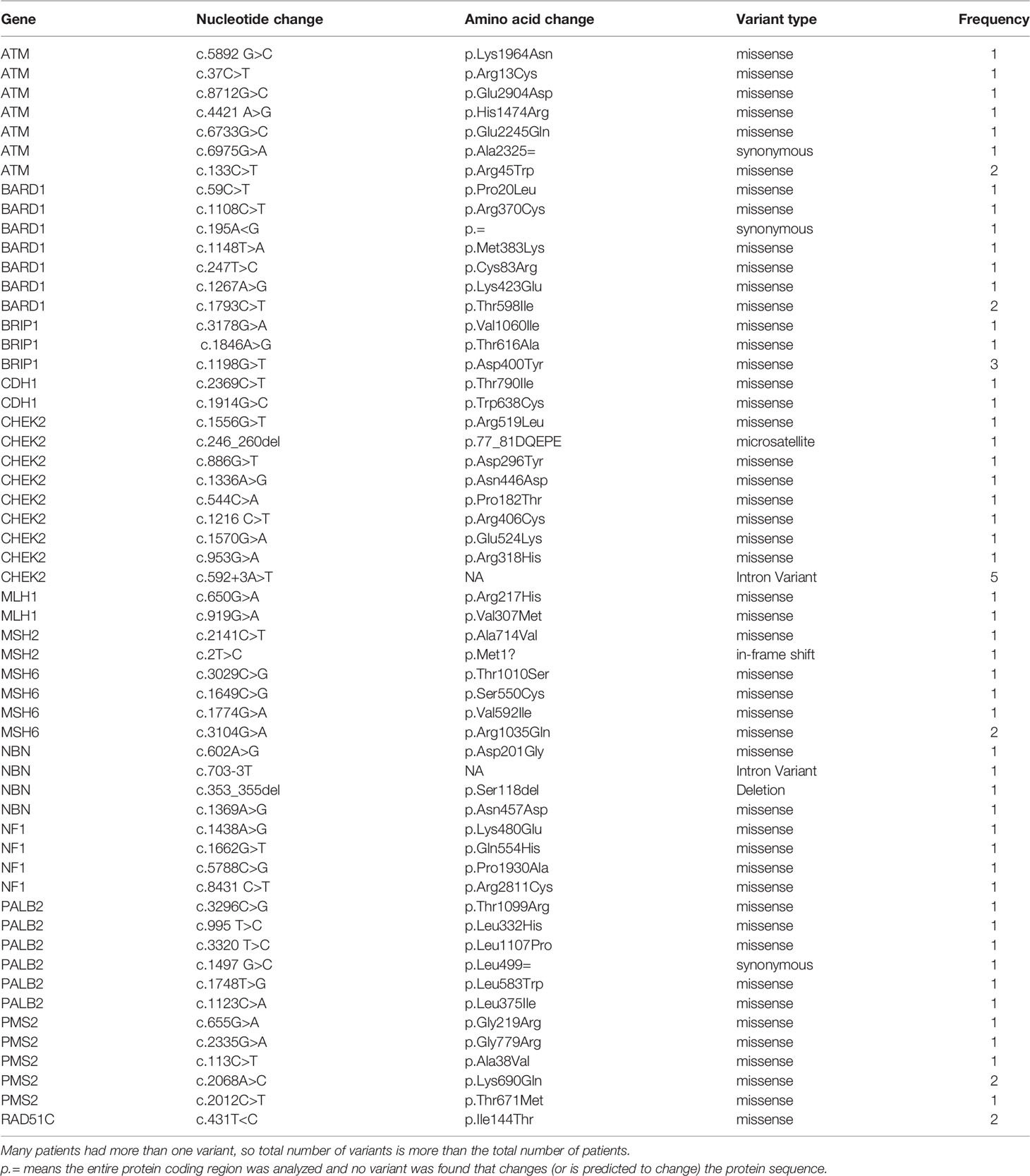
Since we started genetic testing 4 years ago, we have not witnessed significant changes in VUS rates. The classification of variants was upgraded from VUS to likely positive in only one patient (BRCA1 exon 5–8 duplications). The recent introduction of multiple-gene panel testing resulted in a surge of VUS rates in genes other than BRCA1 or BRCA2. Among the whole group, a total of 230 patients were tested using the recently introduced multigene panels, and positive/likely positive variants were detected in 27 (11.7%) patients; 14 (51.9%) were in genes other than BRCA1 and BRCA2. Additionally, VUS variants in BRCA1 or BRCA2 genes were detected in 19 (8.3%) patients while 51 (22.2%) others had VUS in many genes other than BRCA1 or BRCA2; 19 (37.3%) of them had 2 (n = 16) or 3 (n = 3) various VUS. The most common mutations encountered were in PALB2, CHEK2, ATM, BARD1, NBN, and NF1. Figure 1 details VUS rates using the limited two-gene panel (BRCA1 and BRCA2) versus a multigene (20-gene) panel. Variant types and classification encountered are detailed in supplementary tables (Table 3 for BRCA1/2 variants and Table 4 for non-BRCA1/2 variants).
Among 103 patients with non-metastatic disease, only 5 (4.9%) had bilateral mastectomies; all were due to bilateral disease and not prophylactic. Breast-conserving surgery (BCS) was performed on 48 (46.6%). Genetic testing results (VUS) were known prior to initial surgery in 34 (33.0%) patients; 11 (32.4%) of them had BCS. Rates of MRM and BCS were not different among patients with known VUS prior to the surgery and those without (Figure 2) . Only 1 patient of the 103 patients with non-metastatic disease underwent prophylactic oophorectomy.
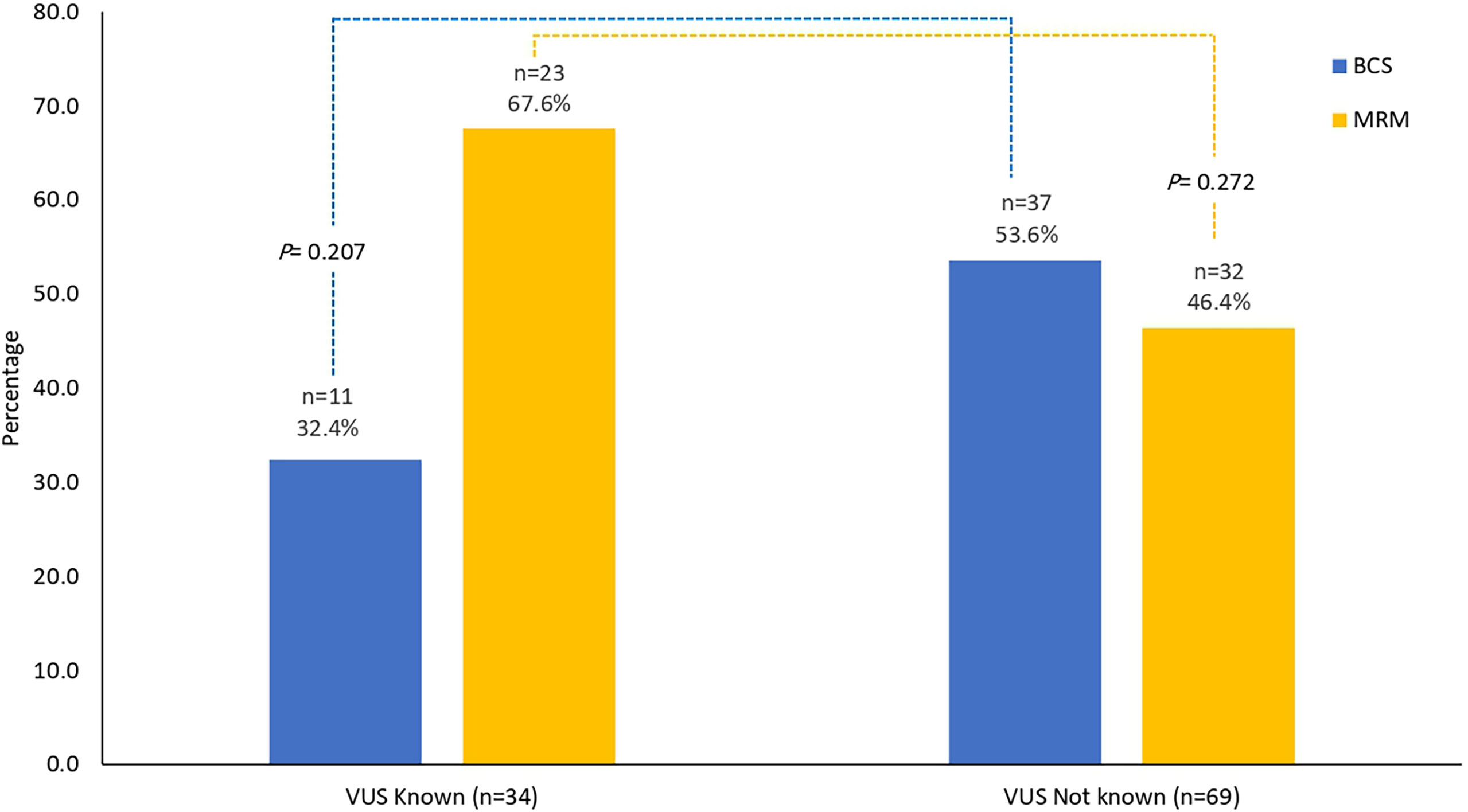
Figure 2 Surgical interventions. Surgical interventions among patients with results of VUS known prior to surgery (n = 34) and those without (n = 69). No difference in the rates of MRM (p = 0.272) or BCS (p = 0.207). MRM, modified radical mastectomy; BCS, breast-conserving surgery; VUS, variants of uncertain significance.
Discussion
To our knowledge, this is the first study in Jordan and the region addressing the pattern and frequency of VUS among “Arab” patients who underwent genetic testing following a diagnosis of breast cancer. Our rate of VUS is relatively high, with no indication of getting lower with time. Obviously, the rate of VUS depends on the ancestry of patients as well as the frequency and methodology of testing. Our population is obviously very different from other widely studied ethnic groups, where the rates of VUS are getting significantly lower. In a study conducted by Myriad Genetic Laboratories, Salt Lake City, based on over 20 years of experience and over a million samples tested, the VUS identification rate in both BRCA1 and BRCA2 had significantly decreased over the years to rates around 2.0% (23). However, such rates continued to be significantly higher in certain ethnic groups of African, Asian, or Middle Eastern descent (23).
The wider adoption and availability of genetic testing technology, including multiple-gene panels and whole-genome sequencing and at more affordable cost, are expected to keep the rates of VUS even higher especially in nations with diverse ethnicity (24). Such high rates of VUS will likely put lots of pressure on both patients and physicians on risk perception and medical management. This highlights the need to improve our counseling tools and communication skills to better explain these results as misinterpretation of such results can lead to misinformation given to patients, inappropriate medical decisions, and psychological distress for patients and their families (25, 26). Adherence to guidelines is extremely important to avoid unnecessary procedures.
Current international guidelines discourage using VUS results to make major medical decisions like risk-reducing mastectomy or oophorectomy. Instead, more emphasis was put on using patients’ personal history of cancer and close family members to rationalize risk-reducing surgeries (27). Such directions were clearly demonstrated in our patients addressed in this study; none of our patients had risk-reducing surgeries based on VUS findings. Fears of mismanaging such patients are always there as the level of experience of healthcare providers varies significantly. In one study, a survey was sent to 800 United Kingdom (UK) breast cancer specialists to collect data on VUS general knowledge; results interpretation and communication were based on two hypothetical genetics reports. Although 95% of the specialists referred patients for BRCA genetic testing, 71% were not sure about the clinical implications of the test reports presented and 12% never received any genetics training. Better communication of VUS results was reported by specialists when patients under discussion had a positive family history and management guidance was included in the genetic test report. More than a third (39%) did not know how to communicate results to patients with no family history and in cases where reports lacked management guidance (28).
Tools to help physicians better manage these patients exist but are underutilized. Collaborative efforts among commercial labs and academic institutions to study and reclassify VUS from multigene testing resulted in the establishment of an online registry called “Prospective Registry of Multiplex Testing (PROMPT)”; patients, physicians, and institutions can register and follow (29). A similar online regional registry should help our patients, genetic counselors, and physicians better deal with this problem.
Conclusions
Despite significant decline in VUS rates reported in Western societies, our rate continues to be relatively high and increasing. Our knowledge of VUS had not significantly impacted therapeutic or prophylactic surgical decisions. Such decisions were dictated by patients’ personal and family history. Adherence to guidelines is extremely important to avoid unnecessary procedures.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the King Hussein Cancer Center Institutional Review Board (IRB). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Conception and design: HA-R. Provision of study materials or patients: LA, FT, RA, and MA. Collection and assembly of data: LA, FT, RA, HA, SE, and MA. Data analysis and interpretation: HA-R, RB, SE, HA, and RA-R. Manuscript writing: HA-R and RB. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abdel-Razeq H, Mansour A, Jaddan D. Breast Cancer Care in Jordan. JCO Global Oncol (2020) 6:260–8. doi: 10.1200/JGO.19.00279
2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
3. Couch F, Shimelis H, Hu C, Hart S, Polley E, Na J, et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol (2017) 3:1190–6. doi: 10.1001/jamaoncol.2017.0424
4. Larsen M, Thomassen M, Gerdes A, Kruse T. Hereditary Breast Cancer: Clinical, Pathological and Molecular Characteristics. Breast Cancer: Basic Clin Res (2014) 8:145–55. doi: 10.4137/BCBCR.S18715
5. Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the Breast Cancer Susceptibility Gene BRCA2. Nature (1995) 378:789–92. doi: 10.1038/378789a0
6. Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A Strong Candidate for the Breast and Ovarian Cancer Susceptibility Gene BRCA1. Science (1994) 266:66–71. doi: 10.1126/science.7545954
7. Tung N, Lin N, Kidd J, Allen B, Singh N, Wenstrup R, et al. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J Clin Oncol (2016) 34:1460–8. doi: 10.1200/JCO.2015.65.0747
8. Chen S, Parmigiani G. Meta-Analysis of BRCA1 and BRCA2 Penetrance. J Clin Oncol (2007) 25:1329–33. doi: 10.1200/JCO.2006.09.1066
9. Mavaddat N, Peock S, Frost D, Platter R, Fineberg E, Evans D, et al. Cancer Risks for BRCA1 and BRCA2 Mutation Carriers: Results From Prospective Analysis of EMBRACE. Journal of National Cancer Institute. J Natl Cancer Inst (2013) 105:812–22. doi: 10.1093/jnci/djt095
10. Collins J, Isaacs C. Management of Breast Cancer Risk in BRCA1/2 Mutation Carriers Who are Unaffected With Cancer. Breast J (2020) 26:1520–7. doi: 10.1111/tbj.13970
11. Monteiro A, Bouwman P, Kousholt A, Eccles D, Millot G, Masson J, et al. Variants of Uncertain Clinical Significance in Hereditary Breast and Ovarian Cancer Genes: Best Practices in Functional Analysis for Clinical Annotation. J Med Genet (2020) 57:509–18. doi: 10.1136/jmedgenet-2019-106368
12. Richter S, Haroun I, Graham T, Eisen A, Kiss A, Warner E. Variants of Unknown Significance in BRCA Testing: Impact on Risk Perception, Worry, Prevention and Counseling. Ann Oncol (2013) 24:viii69–74. doi: 10.1093/annonc/mdt312
13. Ready K, Gutierrez-Barrera A, Amos C, Meric-Bernstam F, Lu K, Hortobagyi G, et al. Cancer Risk Management Decisions of Women With BRCA1 or BRCA2 Variants of Uncertain Significance. Breast J (2011) 17:210–2. doi: 10.1111/j.1524-4741.2010.01055.x
14. Evans D, Barwell J, Eccles D, Collins A, Izatt L, Jacobs C, et al. The Angelina Jolie Effect: How High Celebrity Profile can Have a Major Impact on Provision of Cancer Related Services. Breast Cancer Res (2014) 16:442. doi: 10.1186/s13058-014-0442-6
15. Nabi H, Dorval M, Chiquette J, Simard J. Increased Use of BRCA Mutation Test in Unaffected Women Over the Period 2004–2014 in the U.S.: Further Evidence of the “Angelina Jolie Effect”? Am J Prev Med (2017) 53:e195–6. doi: 10.1016/j.amepre.2017.05.016
16. Lips E, Benard-Slagter A, Opdam M, Scheerman C, Wesseling J, Hogervorst F, et al. BRCAness digitalMLPA Profiling Predicts Benefit of Intensified Platinum-Based Chemotherapy in Triple-Negative and Luminal-Type Breast Cancer. Breast Cancer Res (2020) 22:79. doi: 10.1186/s13058-020-01313-7
17. Paluch-Shimon S, Evron E. Targeting DNA Repair in Breast Cancer. Breast (2019) 47:33–42. doi: 10.1016/j.breast.2019.06.007
18. Robert M, Patsouris A, Frenel J, Gourmelon C, Augereau P, Campone M. Emerging PARP Inhibitors for Treating Breast Cancer. Expert Opin Emerging Drugs (2018) 23:211–21. doi: 10.1080/14728214.2018.1527900
19. Tutt A, Tovey H, Cheang M, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-Mutated and Triple-Negative Breast Cancer BRCAness Subgroups: The TNT Trial. Nat Med (2018) 24:628–37. doi: 10.1038/s41591-018-0009-7
20. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancers, in: NCCN Clinical Practice Guidelines in Oncology. Available at: https://www.nccn.org/professionals/physician_gls/default.aspxdetection (Accessed 01 July 2020).
21. Abdel-Razeq H, Abujamous L, Jadaan D. Patterns and Prevalence of Germline BRCA1 and BRCA2 Mutations Among High-Risk Breast Cancer Patients in Jordan: A Study of 500 Patients. J Oncol (2020) 2020:1–7. doi: 10.1155/2020/8362179
22. Abdel-Razeq H, Al-Omari A, Zahran F, Arun B. Germline BRCA1/BRCA2 Mutations Among High Risk Breast Cancer Patients in Jordan. BMC Cancer (2018) 18:152. doi: 10.1186/s12885-018-4079-1
23. Eggington J, Bowles K, Moyes K, Manley S, Esterling L, Sizemore S, et al. A Comprehensive Laboratory-Based Program for Classification of Variants of Uncertain Significance in Hereditary Cancer Genes. Clin Genet (2013) 86:229–37. doi: 10.1111/cge.12315
24. Welsh J, Hoskin T, Day C, Thomas A, Cogswell J, Couch F, et al. Clinical Decision-Making in Patients With Variant of Uncertain Significance in BRCA1 or BRCA2 Genes. Ann Surg Oncol (2017) 24:3067–72. doi: 10.1245/s10434-017-5959-3
25. Brierley KL, Campfield D, Ducaine W, Dohany L, Donenberg T, Shannon K, et al. Errors in Delivery of Cancer Genetics Services: Implications for Practice. Connecticut Med (2010) 74:413–23.
26. Brierley K, Blouch E, Cogswell W, Homer J, Pencarinha D, Stanislaw C, et al. Adverse Events in Cancer Genetic Testing. Cancer J (2012) 18:303–9. doi: 10.1097/PPO.0b013e3182609490
27. Morgan R, Brown A, Hamman K, Sampson J, Naik A, Massimino K. Risk Management Decisions in Women With BRCA1 and BRCA2 Mutations. Am J Surg (2018) 215:899–903. doi: 10.1016/j.amjsurg.2018.02.010
28. Eccles B, Copson E, Maishman T, Abraham J, Eccles D. Understanding of BRCA VUS Genetic Results by Breast Cancer Specialists. BMC Cancer (2015) 15:936. doi: 10.1186/s12885-015-1934-1
29. Invitae Connect Patient Insights Network. Available at: http://connect.patientcrossroads.org/?org=prompt (Accessed 15 Jan 2021).
Keywords: breast cancer, genetic testing, BRCA, variants of uncertain significance, VUS
Citation: Abdel-Razeq H, Tamimi F, Abujamous L, Abdel-Razeq R, Abunasser M, Edaily S, Abdulelah H, Khashabeh RA and Bater R (2022) Rates of Variants of Uncertain Significance Among Patients With Breast Cancer Undergoing Genetic Testing: Regional Perspectives. Front. Oncol. 12:673094. doi: 10.3389/fonc.2022.673094
Received: 26 February 2021; Accepted: 25 February 2022;
Published: 25 March 2022.
Edited by:
Alberto Farolfi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Silvia Soddu, Hospital Physiotherapy Institutes (IRCCS), ItalyGiulia Federici, Hospital Physiotherapy Institutes (IRCCS), Italy
Copyright © 2022 Abdel-Razeq, Tamimi, Abujamous, Abdel-Razeq, Abunasser, Edaily, Abdulelah, Khashabeh and Bater. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hikmat Abdel-Razeq, aGFiZGVscmF6ZXFAa2hjYy5qbw==; orcid.org/0000-0003-2833-6051
 Hikmat Abdel-Razeq
Hikmat Abdel-Razeq Faris Tamimi
Faris Tamimi Lama Abujamous
Lama Abujamous Rashid Abdel-Razeq
Rashid Abdel-Razeq Mahmoud Abunasser
Mahmoud Abunasser Sara Edaily
Sara Edaily Hazem Abdulelah
Hazem Abdulelah Razan Abu Khashabeh
Razan Abu Khashabeh Rayan Bater
Rayan Bater