- 1Department of Urology, The First People’s Hospital of Yichang, China Three Gorges University, Yichang, Hubei, China
- 2Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3Department of General Surgery, Zhongxiang People’s Hospital, Zhongxiang, Hubei, China
- 4Department of Urology, Gushi People’s Hospital, Gushi, Henan, China
Patients after kidney transplantation have a much higher risk of developing malignant tumors than the general population. And the native kidney is an organ relatively susceptible to malignant tumors after renal transplantation. However, the simultaneous development of bilateral renal tumors is very rare; especially the bilateral native kidneys harbor different pathological types of renal cell carcinoma (RCC). We report a case of a patient who developed malignant tumors in both native kidneys nearly 19 years after renal transplantation. This patient underwent bilateral laparoscopic radical nephrectomy, and postoperative pathological examination showed clear cell RCC on the left native kidney and papillary RCC on the right one. And the early detection and surgical treatment resulted in a good prognosis. The literature related to the diagnosis and treatment of bilateral RCC after renal transplantation is also reviewed.
Introduction
Renal transplantation is considered to be the best treatment for end-stage renal disease, which greatly improves the quality of life and prognosis of patients. However, due to the influence of the long-term use of immunosuppressive agents on the human immune system, renal transplantation recipients are more prone to malignant tumors (1). Renal cell carcinoma (RCC) is the third most common malignancy in renal transplant recipients after cutaneous tumors and hematopoietic cell diseases (2). However, the simultaneous presence of bilateral renal tumors is rare, especially in transplant recipients without autosomal dominant polycystic kidney disease. And the presence of bilateral primary renal tumors with different pathological components is more rare. In this paper, we described a renal transplant recipient who developed different pathological types of RCC in bilateral native kidneys.
Case report
The patient is a 61-year-old male. He underwent allogeneic kidney transplantation due to uremia in February 2000. The transplanted kidney was placed in the right iliac fossa. After the transplantation, he was normally on immunosuppressive therapy with cyclosporine (100 mg, 2 times/day) and mycophenolate mofetil (0.5 g, 2 times/day). And he maintained regular physical examinations and the function of his transplanted kidney was normal. A few years later, he developed symptoms of hypertension and started treatment with nifedipine controlled-release tablets (20 mg per night). He was also diagnosed with type 2 diabetes and was treated regularly with synthetic human insulin every day. In November 2018, he was admitted to a local hospital for poorly controlled diabetes. During this time, he underwent a Doppler ultrasound for both the native and transplanted kidneys. It showed that the left kidney had an extremely hypoechoic mass with a regular shape and clear boundary, and irregular anechoic areas could be seen in it. The size of the mass was 46 mm × 44 mm. Then an enhanced contrast CT examination of the abdomen and pelvis was performed. It showed that the mass was located in the upper pole of the left native kidney and it was significantly enhanced unevenly in the cortical phase, and the enhancement in the medullary phase was significantly reduced. Surprisingly, the CT revealed that the patient also had a nodule in the upper pole of the right native kidney and mild enhancement was seen in the nodule in the cortical phase (Figure 1).
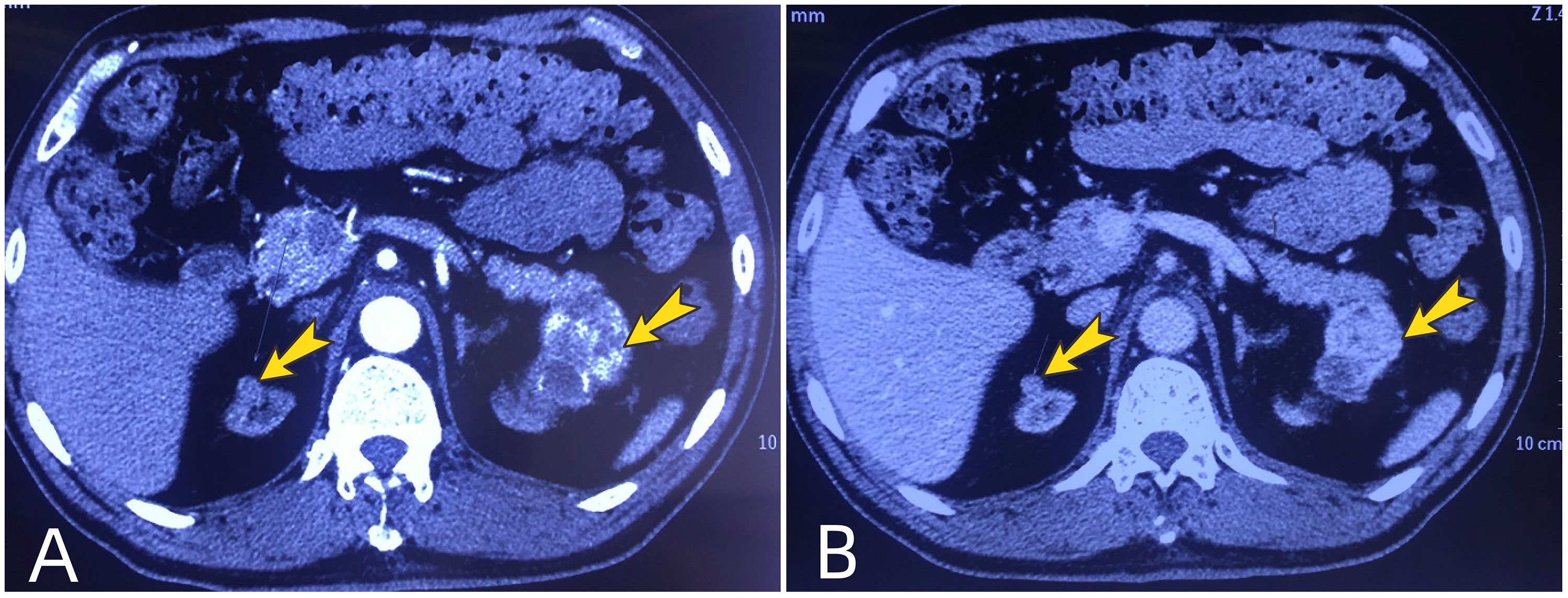
Figure 1 Preoperative renal enhanced computed tomography. Arrows show the tumors in the upper poles of bilateral native kidneys. (A) the tumors are enhanced in the cortical phase; (B) The enhancement of tumors is markedly reduced in the medullary phase, showing the performance of “fast in and fast out”.
He was soon referred to our department with the diagnosis of bilateral native renal tumors. At the time of admission, he was in generally good condition. His height was 164 cm, and his body weight was 67 kg. His body temperature was 36.6°C and he has a heart rate of 80 beats per minute. His blood pressure was 189/104mmHg. A previous surgical scar was visible on the right lower abdomen. There was no percussion pain in the native kidney area, no tenderness in the ureteral area, and no abdominal mass was touched. No other significant positive signs were found. White blood cell count was 5.11×10^9/L, hemoglobin was 136.0 g/L, serum creatinine was 97 umol/L (reference values: 59 - 104 umol/L), eGFR was 72.3 ml/min/1.73m^2, fasting blood glucose was 6.55 mmol/L, urine protein was 2+, urine glucose was 1+. Chest X-ray and ECG showed no significant abnormalities. After proper preoperative preparation, the patient underwent bilateral laparoscopic retroperitoneal radical nephrectomy. The procedure was smooth and took 250 minutes. Postoperative gross specimens showed that both kidneys had marked atrophy of the renal cortex with a thickness of about 1 cm; a 43 mm × 40 mm gray-yellow and gray-red mass in the upper pole of the left kidney, with a cystic cavity in it; a gray-white nodule with a diameter of 12 mm in the upper pole of the right kidney, the cut surface was gray-white and showed a thin papillary shape. No signs of tumor invasion of the renal pelvis and hilar vessels were found in both kidneys. No lymph nodes were palpated in the hilar region. Pathological diagnosis (Figure 2): (left native kidney) clear cell RCC, WHO grade 1, T1bN0M0; (right native kidney) type 1 papillary RCC with papillary adenoma formation, Fuhrman grade 1, T1aN0M0. No tumor invasion of perirenal fat, renal sinus, or renal collecting system was found. No hilar lymph node metastasis was detected. The serum creatinine was 127 umol/L on the first postoperative day and it decreased to 112 umol/L on the fourth postoperative day. He recovered uneventfully and was discharged one week after surgery. During the one-year follow-up, he was alive without RCC recurrence and metastasis.
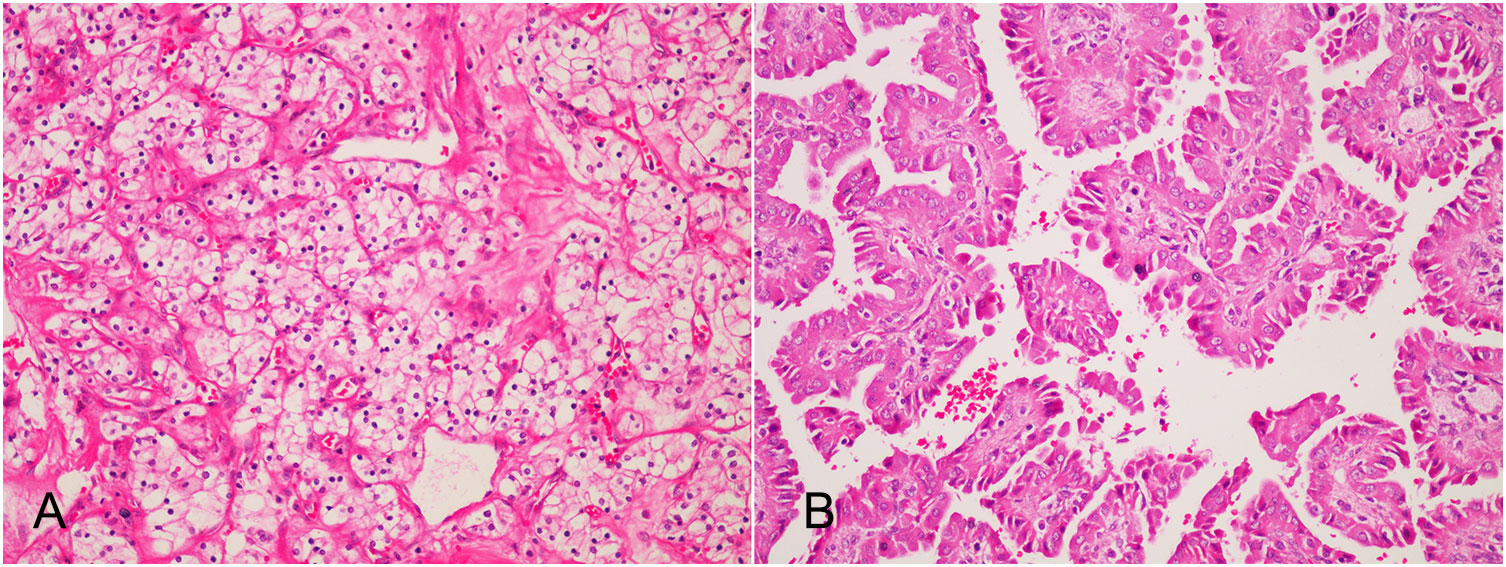
Figure 2 Postoperative pathology (HE×200): (A) The tumor in the left native kidney is clear cell renal cell carcinoma. The cancer cells are transparent and arranged in empty nests, and thin-walled sinusoidal vessels form a reticular septum; (B) The tumor in the left native kidney is type I papillary renal cell carcinoma of the right native kidney. Papillary structures formed by fibrovascular axis with foamy macrophages.
Literature review and discussion
Kidney transplantation improves survival and quality of life for patients with end-stage renal disease and is less expensive than countless dialysis sessions, making it the preferred form of kidney replacement therapy (3, 4). Nevertheless, transplant recipients are at higher risk of developing malignancies and the renal cancer incidence is 6.8 times higher than that of the normal population due to long-term immunosuppression (5, 6). A recent meta-analysis showed that the overall estimated incidence of RCC was 0.7% in kidney transplant recipients (2). In addition, most renal cancer occurs in the native kidney, and only 9% of the tumors occur in the allograft itself (7). Clear cell carcinoma is the most common pathological type of RCC. However, the incidence of papillary RCC has increased among kidney transplant recipients. Some studies show that the incidence of papillary RCC after renal transplantation is up to 30% (8). The simultaneous occurrence of malignant tumors in bilateral native kidneys is rare, especially in patients without acquired cystic kidney disease. We identified only 13 cases in the published literature that have been shown in Table 1 (9–18). The majority of these patients were male. The time interval between diagnosis and kidney transplantation ranged from 1 to 18.8 years, with a median time of 10.1 years. The case we report has the longest time interval. As can be seen from Table 1, unlike the conventional sporadic cases in which clear cell RCC is the predominant pathological type, the proportion of papillary RCC is markedly higher in native renal tumors after renal transplantation. As with patients with bilateral renal tumors in sporadic tumors, the pathological type of bilateral tumors were consistent in most patients. Interestingly, the case we present has clear cell RCC in the left native kidney and papillary RCC in the contralateral side, which is very rare.
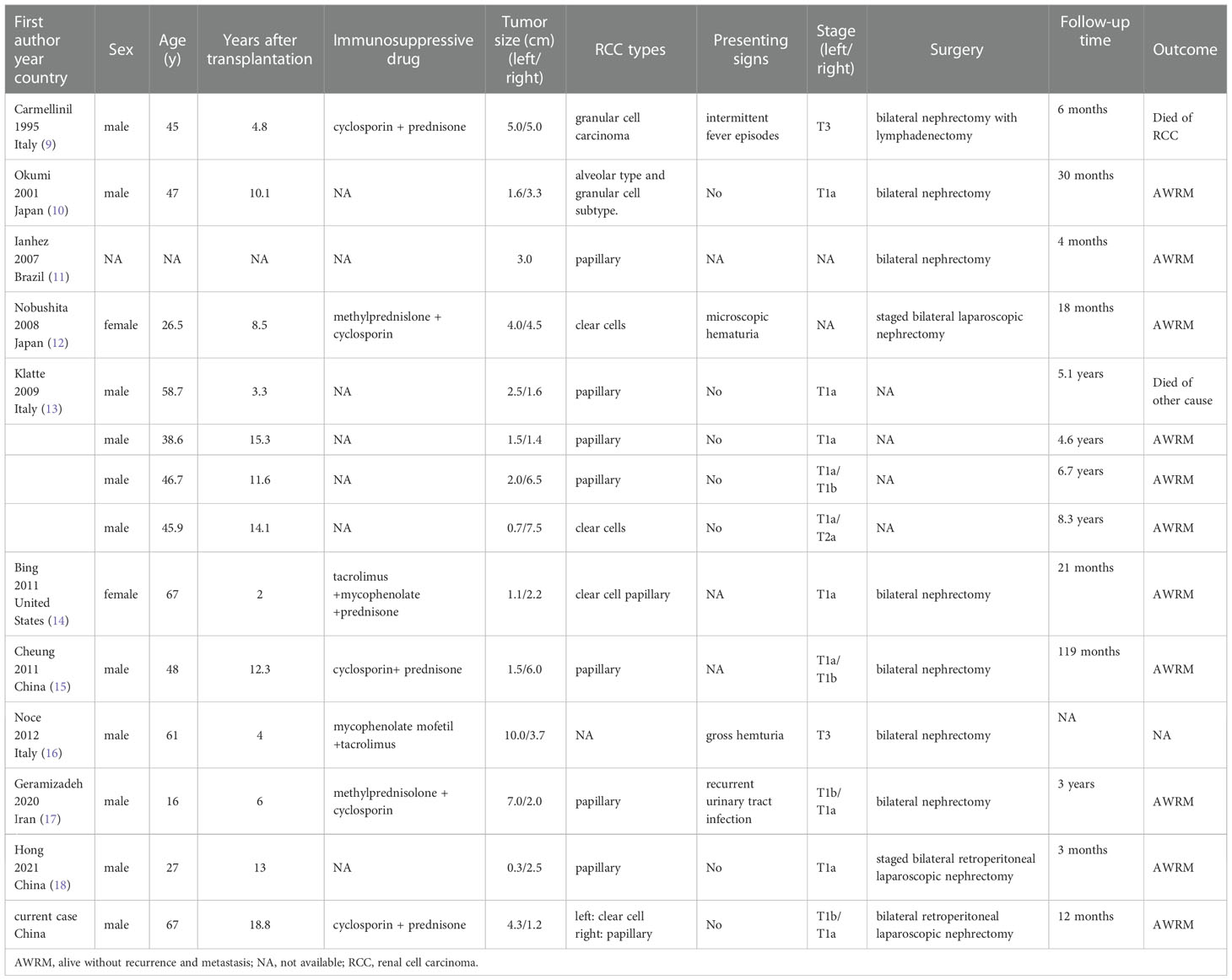
Table 1 Published cases developing renal cell carcinoma in bilateral native kidneys after kidney transplantation.
The exact reason for the increased risk of RCC after kidney transplantation is uncertain but is thought to be related to immunosuppression and loss of immunosurveillance (19). Risk factors for developing RCC after kidney transplantation include longer dialysis time before transplantation, history of acquired cystic kidney disease, smoking, male sex, older age, and hypertension (2, 20, 21). It is appropriate to perform screening of kidney tumors in post-transplant recipients, as they are prone to develop renal tumors, and early staged RCC often has no obvious symptoms. Ultrasound could play an important role in the detection of renal masses (22). European Association of Urology guidelines and some authors recommend an annual abdominal ultrasound screening to achieve an early diagnosis and a good prognosis (23, 24). In the case we present, the right kidney tumor was not detected at first by ultrasound. Therefore, computed tomography and/or magnetic resonance imaging are often needed as further confirmatory examinations, especially for patients with the renal cystic disease (25).
Localized bilateral native renal tumors can be treated with bilateral radical nephrectomy. Under appropriate circumstances, bilateral nephrectomy can be safely accomplished in a single procedure as in our case[13]. We performed a bilateral retroperitoneal laparoscopic nephrectomy for this patient and the operation is uneventful. Compare with the transperitoneal approach, the retroperitoneal approach is faster and allows extra-peritoneal dissection (26). During the operation, attention should be paid to the shape of the atrophic kidney and the anatomical changes of the renal pedicle blood vessels (27). The prognosis of localized native kidney RCC after nephrectomy is good (23). As shown in Table 1, most of the recipients with localized tumors were alive and without evidence of recurrence and metastasis during the follow-up period.
The treatment of advanced or metastatic RCC in renal transplant recipients can be challenging and the prognosis is relatively poor (28). Immunosuppression is a risk factor for tumorigenesis and progression, but interruptions and reductions in immunosuppressive treatment bring the risk of immunologic graft loss (29). There is currently no consensus on this dilemma. Reduction of immunosuppression drug dose and/or switch to mammalian target of rapamycin inhibitor, graftectomy, and complete withdrawal of immunosuppression, immune checkpoint inhibitors, or tyrosine kinase inhibitor are all possible options (30–32). Shared decision-making involving the urologist, oncologist, transplant specialist, and patient is essential to develop a rational treatment strategy.
In summary, transplant recipients are at higher risk of developing RCC, but simultaneous development of bilateral native kidney tumors is rare. Regular screening can be helpful in early diagnosis. Localized bilateral native renal tumors can be treated with bilateral radical nephrectomy and may have a good prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HH and CY conceived the project. XY and CY wrote the original draft.AS, ZZ and HH constructed the figure, revised the manuscript and performed the literature review. JY and XG assisted in the clinical case analysis and treatment, and literature review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Piana A, Andras I, Diana P, Verri P, Gallioli A, Campi R, et al. Small renal masses in kidney transplantation: Overview of clinical impact and management in donors and recipients. Asian J Urol (2022) 9(3):208–14. doi: 10.1016/j.ajur.2022.06.001
2. Chewcharat A, Thongprayoon C, Bathini T, Aeddula NR, Boonpheng B, Kaewput W, et al. Incidence and mortality of renal cell carcinoma after kidney transplantation: A meta-analysis. J Clin Med (2019) 8(4):530. doi: 10.3390/jcm8040530
3. Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant (2011) 11(10):2093–109. doi: 10.1111/j.1600-6143.2011.03686.x
4. Hao X, Lai W, Xia X, Xu J, Wu Y, Lv C, et al. Transplant or dialysis: What's the better choice for rcc-induced esrd patients? a 20-year analysis of Optn/Unos data. Front Oncol (2022) 12:955771. doi: 10.3389/fonc.2022.955771
5. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with Hiv/Aids compared with immunosuppressed transplant recipients: A meta-analysis. Lancet (2007) 370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2
6. Engels EA. Epidemiologic perspectives on immunosuppressed populations and the immunosurveillance and immunocontainment of cancer. Am J Transplant (2019) 19(12):3223–32. doi: 10.1111/ajt.15495
7. Moris D, Kakavia K, Argyrou C, Garmpis N, Bokos J, Vernadakis S, et al. De novo renal cell carcinoma of native kidneys in renal transplant recipients: A single-center experience. Anticancer Res (2017) 37(2):773–9. doi: 10.21873/anticanres.11376
8. Abbas M, Patzel M, Thurn A, Brinkmann OA, Bettendorf O. Incidental occurrence of papillary renal cell carcinoma in the native kidney with autosomal dominant polycystic kidney disease after renal transplantation: A case report. Mol Clin Oncol (2021) 15(5):223. doi: 10.3892/mco.2021.2386
9. Carmellini M, Romagnoli J, Rizzo G, Marchetti A, Mosca F. Bilateral renal-cell carcinoma of the native kidneys after renal-transplantation. Oncol Rep (1995) 2(3):435–7.
10. Okumi M, Matsuoka Y, Tsukikawa M, Fujimoto N, Itoh K. A case of renal cell carcinoma arising in bilateral original kidneys after failure of transplant graft function. Hinyokika Kiyo (2001) 47(9):637–40.
11. Ianhez LE, Lucon M, Nahas WC, Sabbaga E, Saldanha LB, Lucon AM, et al. Renal cell carcinoma in renal transplant patients. Urology (2007) 69(3):462–4. doi: 10.1016/j.urology.2006.11.007
12. Nobushita T, Hara N, Nakagawa Y, Saito K, Nishiyama T, Takahashi K. Renal cell carcinomas arising from the allograft and bilateral native kidneys. Int J Urol (2008) 15(2):175–7. doi: 10.1111/j.1442-2042.2007.01941.x
13. Klatte T, Seitz C, Waldert M, de Martino M, Kikic Z, Böhmig GA, et al. Features and outcomes of renal cell carcinoma of native kidneys in renal transplant recipients. BJU Int (2010) 105(9):1260–5. doi: 10.1111/j.1464-410X.2009.08941.x
14. Bing Z, Tomaszewski JE. Clear cell papillary renal cell carcinoma in the bilateral native kidneys after 2 Years of renal transplantation: Report of a case and review of the literature. Case Rep Transplant (2011) 2011:387645. doi: 10.1155/2011/387645
15. Cheung CY, Lam MF, Lee KC, Chan GS, Chan KW, Chau KF, et al. Renal cell carcinoma of native kidney in Chinese renal transplant recipients: A report of 12 cases and a review of the literature. Int Urol Nephrol (2011) 43(3):675–80. doi: 10.1007/s11255-011-9912-2
16. Noce A, Iaria G, Durante O, Sforza D, Canale MP, Di Villahermosa SM, et al. Bilateral native kidney neoplasia detected by ultrasound in functionning renal allograft recipient. Arch Ital Urol Androl (2012) 84(4):253–5.
17. Geramizadeh B, Keshavarz P, Kashkooe A, Ariafar A, Salehipour M. Bilateral renal cell carcinoma of the native kidneys in a 16-Year-Old boy: Report of a rare case and review of the literature. Urologia (2020) 87(3):115–8. doi: 10.1177/0391560319887323
18. Hong P, Tian X, Zhao X, Yang F, Liu Z, Lu M, et al. Bilateral papillary renal cell carcinoma following kidney transplantation: A case report. J Peking Univ Health Sci (2021) 53(4):811–3. doi: 10.19723/j.issn.1671-167x.2021.04.033
19. D'Arcy ME, Castenson D, Lynch CF, Kahn AR, Morton LM, Shiels MS, et al. Risk of rare cancers among solid organ transplant recipients. J Natl Cancer Inst (2021) 113(2):199–207. doi: 10.1093/jnci/djaa078
20. Goh A, Vathsala A. Native renal cysts and dialysis duration are risk factors for renal cell carcinoma in renal transplant recipients. Am J Transplant (2011) 11(1):86–92. doi: 10.1111/j.1600-6143.2010.03303.x
21. Hickman LA, Sawinski D, Guzzo T, Locke JE. Urologic malignancies in kidney transplantation. Am J Transplant (2018) 18(1):13–22. doi: 10.1111/ajt.14533
22. Roussel E, Campi R, Amparore D, Bertolo R, Carbonara U, Erdem S, et al. Expanding the role of ultrasound for the characterization of renal masses. J Clin Med (2022) 11(4):1112. doi: 10.3390/jcm11041112
23. Vegso G, Toronyi E, Hajdu M, Piros L, Gorog D, Deak PA, et al. Renal cell carcinoma of the native kidney: A frequent tumor after kidney transplantation with favorable prognosis in case of early diagnosis. Transplant Proc (2011) 43(4):1261–3. doi: 10.1016/j.transproceed.2011.03.068
24. Rodriguez Faba O, Boissier R, Budde K, Figueiredo A, Taylor CF, Hevia V, et al. European Association of urology guidelines on renal transplantation: Update 2018. Eur Urol Focus (2018) 4(2):208–15. doi: 10.1016/j.euf.2018.07.014
25. Park KH, Yoon JA, Kim HS, Kim H, Park SK, Kim YH, et al. Clinical features and outcomes in kidney transplant recipients with renal cell carcinoma: A single-center study. Kidney Res Clin Pract (2019) 38(4):517–24. doi: 10.23876/j.krcp.19.078
26. Fan X, Xu K, Lin T, Liu H, Yin Z, Dong W, et al. Comparison of transperitoneal and retroperitoneal laparoscopic nephrectomy for renal cell carcinoma: A systematic review and meta-analysis. BJU Int (2013) 111(4):611–21. doi: 10.1111/j.1464-410X.2012.11598.x
27. Vegso G, Toronyi E, Deak PA, Doros A, Langer RM. Detection and management of renal cell carcinoma in the renal allograft. Int Urol Nephrol (2013) 45(1):93–8. doi: 10.1007/s11255-012-0274-1
28. Garcia Alvarez T, Mazuecos Blanca A, Navas Garcia N, Calle Garcia L, Vallejos Roca E, Moreno Salazar A, et al. Early diagnosis and treatment of renal cell carcinoma of native kidney in kidney transplantation. Nefrologia (2011) 31(5):567–72. doi: 10.3265/Nefrologia.pre2011.Jun.10929
29. Leveridge M, Musquera M, Evans A, Cardella C, Pei Y, Jewett M, et al. Renal cell carcinoma in the native and allograft kidneys of renal transplant recipients. J Urol (2011) 186(1):219–23. doi: 10.1016/j.juro.2011.03.032
30. Suso-Palau D, Chavarriaga J, Usubillaga F, Asprilla J, Micolta L, Urrego M. Metastatic renal cell carcinoma of the native kidney in a renal transplant recipient: Revisiting the era of tyrosine kinase inhibitors - case report. Urol Case Rep (2022) 43:102082. doi: 10.1016/j.eucr.2022.102082
31. Dahle DO, Skauby M, Langberg CW, Brabrand K, Wessel N, Midtvedt K. Renal cell carcinoma and kidney transplantation: A narrative review. Transplantation (2022) 106(1):e52–63. doi: 10.1097/TP.0000000000003762
Keywords: renal cell carcinoma, kidney transplantation, bilateral renal tumors, native kidney, clear cell, papillary, nephrectomy
Citation: Yi C, You X, Sha A, Zhang Z, Yu J, Guo X and Hu H (2023) Renal cell carcinoma of different pathological types in bilateral native kidneys of a kidney transplant recipient: A case report and literature review. Front. Oncol. 12:1112343. doi: 10.3389/fonc.2022.1112343
Received: 30 November 2022; Accepted: 28 December 2022;
Published: 16 January 2023.
Edited by:
Haoran Liu, Stanford University, United StatesReviewed by:
Aleksandr Shulyak, National Academy of Medical Sciences of Ukraine, UkraineKehua Jiang, Guizhou Provincial People’s Hospital, China
Zhaowei Zhu, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2023 Yi, You, Sha, Zhang, Yu, Guo and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henglong Hu, aHVoZW5nbG9uZ0BodXN0LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Cheng Yi1,2†
Cheng Yi1,2† Xiangyun You
Xiangyun You Henglong Hu
Henglong Hu