- 1Department of Pathology, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, China
- 2Department of Nephrolgy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3Department of Orthopedics, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, China
- 4Central Catheter Room, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, China
- 5Department of Research Management, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, China
Introduction: The impact of different types of reconstruction, including tissue reconstruction, implant reconstruction and combined reconstruction, on patient survival were not illustrated completely. We tried to investigate the impact of patient survival between different types of reconstruction.
Methods: We enrolled 6271 patients with tumors in the central and nipple portion of breast cancer from the Surveillance, Epidemiology, and End Results database. Factors associated with survival were identified by Cox regression analyses. The mortality rates per 1,000 person-years were calculated and compared. Survival curves were produced by Kaplan-Meier analyses using log-rank tests and cox proportional hazards regression quantified the risk of survival.
Results: Reconstructive types, region, insurance, race, marial status, grade, stage, ER status, PR status, HER-2 status and chemotherapy were significant prognostic factors associated with breast cancer-specific survival. The breast cancer mortality rates per 1,000 person-years for patients with tissue, implant and combined group were 26.01,21.54 and 19.83 which showed a downward trend. The HR of implant and combined reconstruction adjusted for demographic, pathological, and therapeutic data was 0.82 (95% CI: 0.67-1.00, p=0.052) and 0.73(95% CI:0.55-0.97, p=0.03) compared with tissue reconstruction.
Conclusion: Breast cancer-related mortality between implant reconstruction and autologous tissue reconstruction showed no significantly different, but the risk of BCSS of compound reconstruction was lower than tissue reconstruction.
Introduction
Breast cancer is the most common cause of female malignant tumor-related death worldwide, and its morbidity and mortality are on the rise (1, 2). In addition to survival after surgery, chemotherapy and radiotherapy (3), aesthetic outcomes and quality of life after breast cancer treatment is also a consideration by breast surgeons and plastic surgeons today (4–7).
Reconstruction mitigated the psychosocial and physical consequences of mastectomy by restoring the breast mound. Of the 252,710 women estimated to have been diagnosed with invasive breast cancer in the United States in 2017, more than a third of patients with early-stage disease would opt for mastectomy as their primary surgical procedure. For these women, plastic surgeons may opt for reconstruction (8, 9).
The types of breast reconstruction mainly include implant breast reconstruction, autologous breast reconstruction and compound reconstruction. The current data focus on the potential risk of complications and long-term satisfaction among patients with different types of breast reconstruction, and help future patients understand the existing breast reconstruction options. This decision is based on expected satisfaction with the breast and quality of life. However, there is rare studies on the impact of different types of reconstruction, including compound reconstruction, on patient survival. Here, we try to conduct on this issue using a large sample of data.
Methods
Data source and cohort ascertainment
The Surveillance, Epidemiology and End Results (SEER) database contains demographic, pathological and treatment characteristics on cancer patients which collected and provided by the National Cancer Institute (https://seer.cancer.gov/), and is used as the source of data for our study. We recruited breast cancer patients from SEER database by using code C50.0 (Nipple) and C50.1 (Central portion of breast) from the International Classification of Diseases for Oncology.
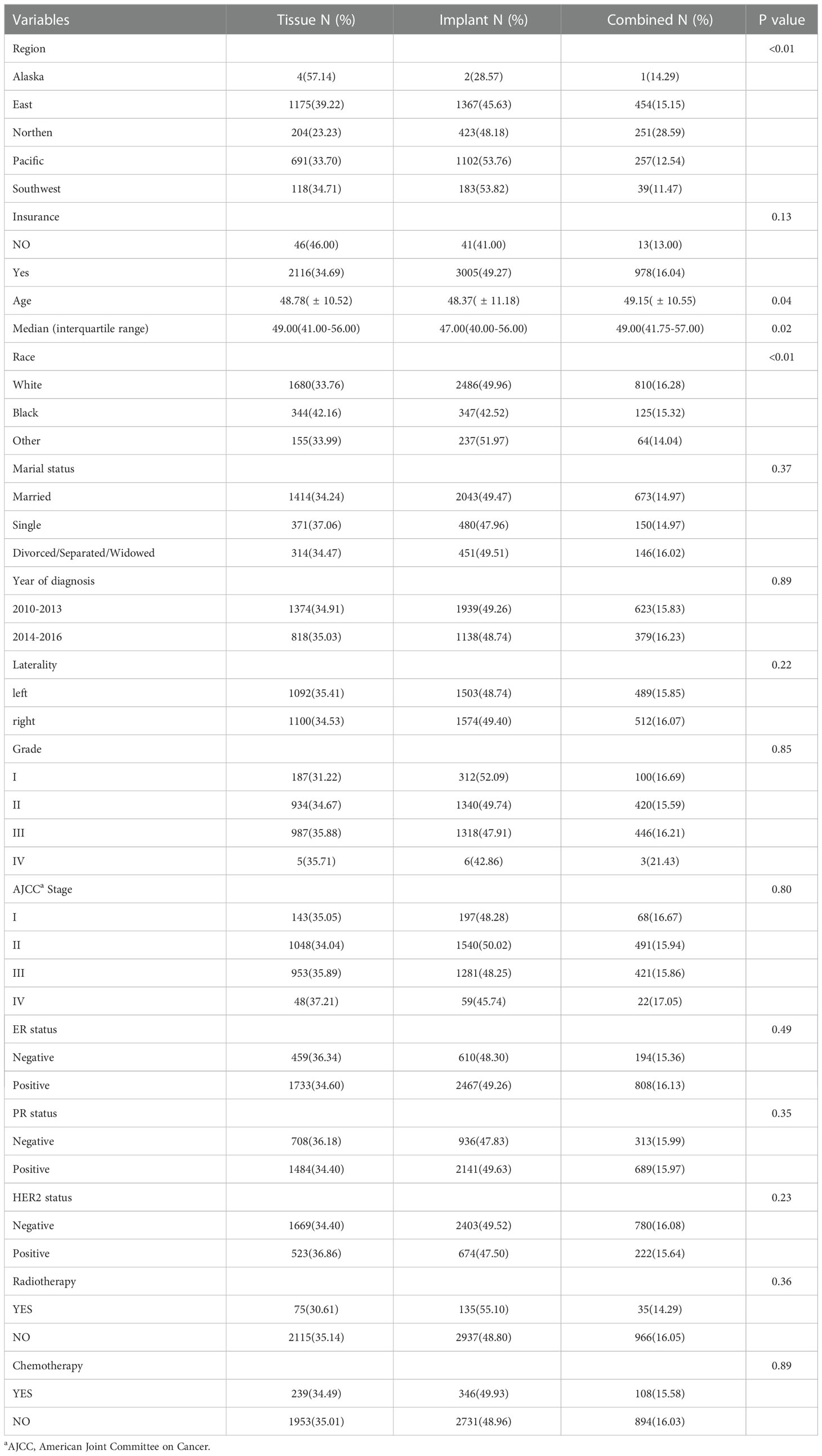
The following demographic information and clinical characteristics were collected: age at diagnosis (2010-2016), region, insurance, year of diagnosis, sex, race, marial status, laterality, extension, grade, AJCC Stage, ER status, PR status, HER2 status, radiotherapy, chemotherapy, and reconstructive method. To ensure the accuracy of the results, user missing value was performed for missing or unknown data.
Statistical analysis
Quantitative variables are expressed as median (interquartile range), while categorical variables are presented as percentages. We used univariate Cox regression analysis to investigate clinicopathological factors associated with breast cancer-specific mortality, and compared breast cancer-specific and all-cause mortality rates per 1000 person-years in patients with different types of reconstruction. Kaplan-Meier analysis was performed using log-rank test to evaluate the difference in survival risk among different risk factors. Finally, the effects of different groups on specific and all-cause mortality were assessed using a multivariate Cox regression analysis, adjusted for demographic, pathological, and therapeutic characteristics. All p-values were two-sided, with p < 0.05 considered statistically significant. Data extraction was processed by official software “SEERStat” version 8.3.7. Statistical analyses were performed using SPSS version 24.0 (IBM Corp., Armonk, NY), GraphPad Prism version 7 (GraphPad Software Inc., La Jolla, CA) and Stata/SE version 15 (Stata Corp., College Station, TX).
Results
General characteristics of the study population
Of the 6271 patients with tumors in the central and nipple portion of breast cancer, tissue reconstructive group with insurance occupied 2116(34.69%), implant and combined reconstructive group 3005(49.27) and 978(16.04), (p=0.13) respectively. Median age were 49.00, 47.00 and 49.00 (p=0.02) in tissue, implant and combined reconstructive group, respectively. According to the TNM-8th system, 143 patients (35.05%) with stage I, 1048 (34.04%) with stage II, 953 (35.89%) in stage III and 48 (37.21%) in stage IV were placed in group with tissue reconstruction, respectively; 197 patients (48.28%) with stage I, 1540 (50.02%) with stage II, 1281 (48.25%) in stage III and 59 (45.74%) in stage IV were placed in group with implant reconstruction, respectively; in addition, 68 patients (16.67%) with stage I, 491(15.94%) with stage II, 421(15.86%) in stage III and 22 (17.05%) in stage IV were placed in group with implant reconstruction, respectively. Other demographic, clinicopathological and therapy characteristics are presented in Table 1.
Clinicopathological factors associated with breast cancer specific survival and overall survival
In the univariate Cox regression analysis, reconstructive types, region, insurance, race, marial status, grade, stage, ER status, PR status, HER-2 status and chemotherapy were significant prognostic factors of BCSS (all, p < 0.05), age, year of diagnosis, laterality and radiotherapy did not show significant difference of BCSS (Table 2). Univariate Cox analyses of the factors associated with OS showed similar results (Table 2).
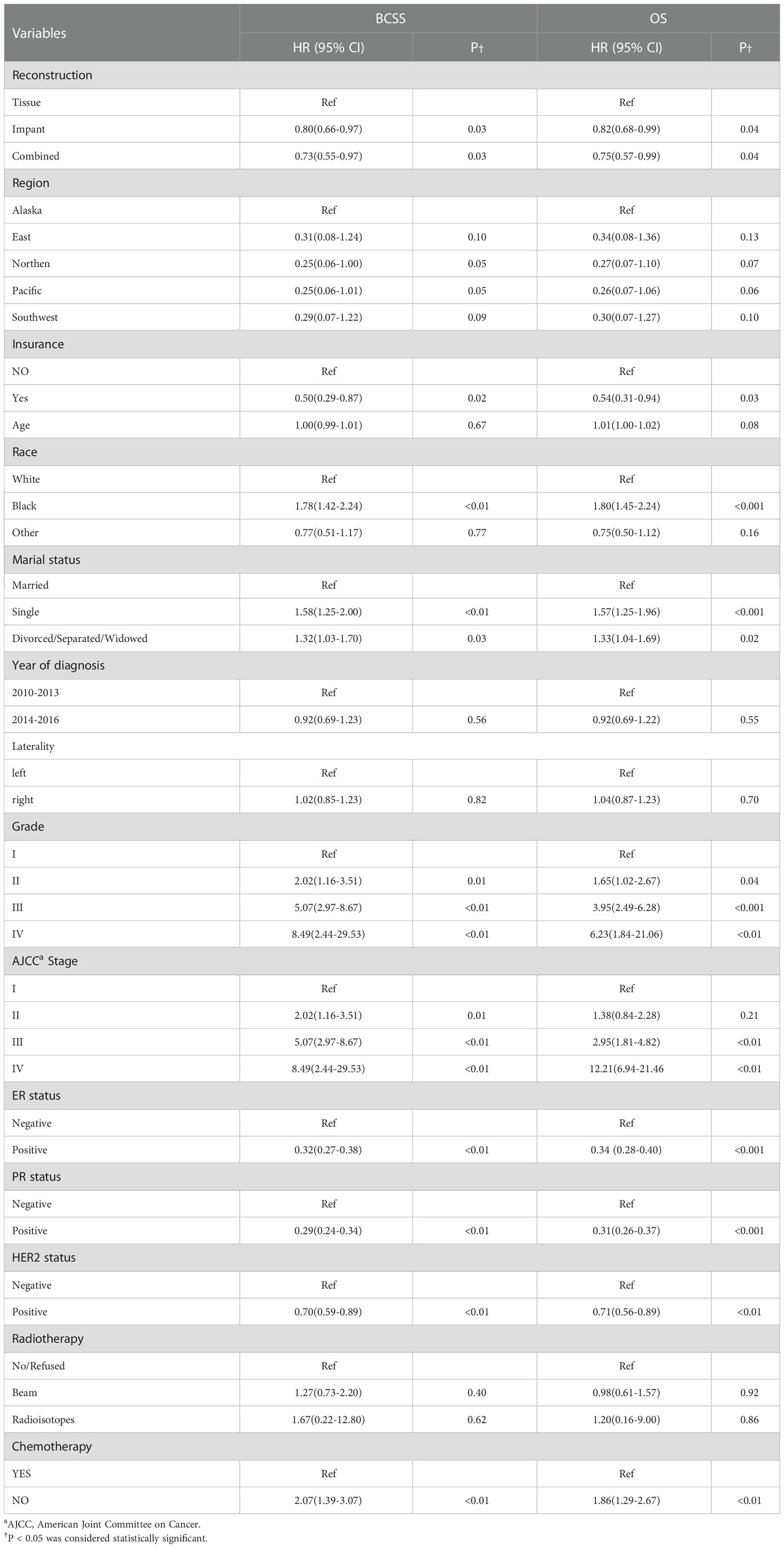
Table 2 Univariable Cox proportional hazard regression model of breast cancer-specific survival (BCSS) and overall survival (OS).
Breast cancer mortality and overall mortality rates per 1,000 person-years
During the follow-up till December 2016, the BCM rates per 1,000 person-years for patients with tissue, implant and combined group were 24.55,19.80 and 18.15. Moreover, the breast cancer mortality rates per 1,000 person-years for patients with tissue, implant and combined group were 26.01,21.54 and 19.83, all groups showed a downward trend (Table 3).
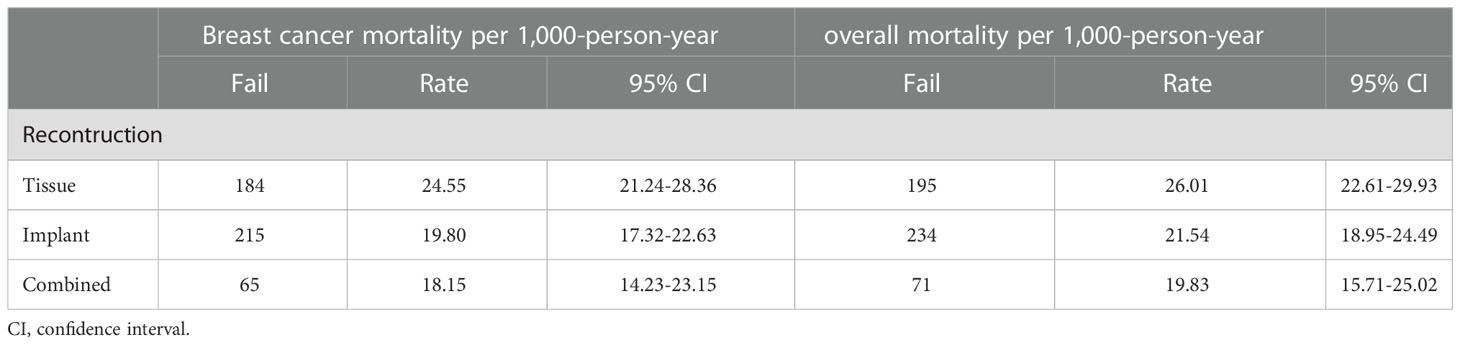
Table 3 Comparison of breast cancer and overall mortality per 1,000-person-year between different reconstruction types.
Hazard ratios of different subgroups for BCSS
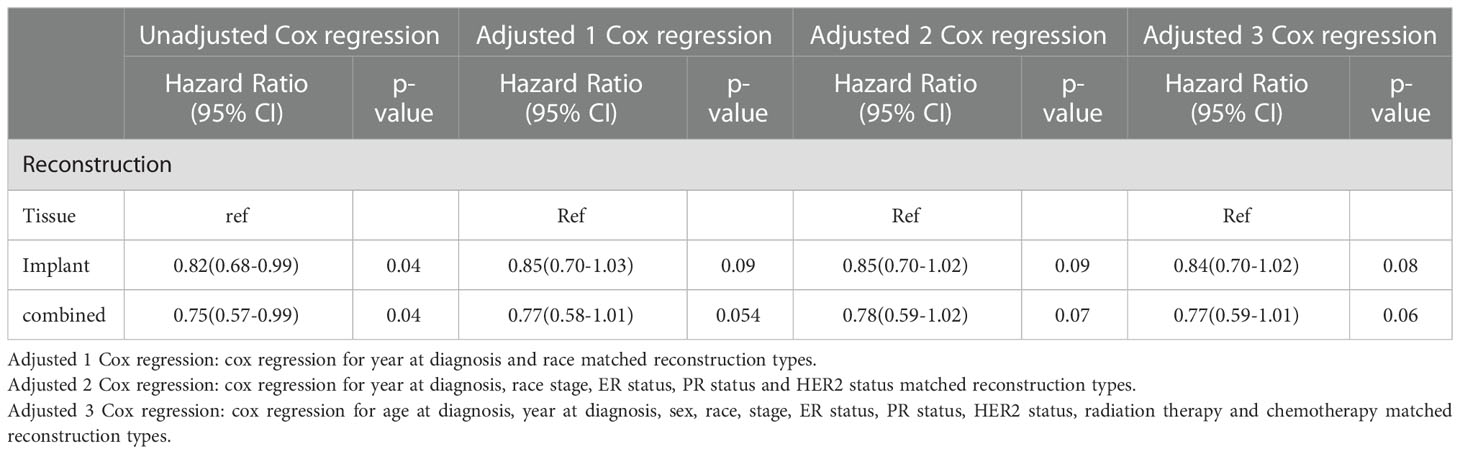
The HRs for BCSS of the group with tissue reconstruction compared with the other groups are displayed in Table 4. The unadjusted HR of the group with implant reconstruction was 0.83 (95% CI: 0.68-1.01, p=0.56) compared with group with tissue reconstruction; The HR adjusted for demographic data was 0.83(95% CI: 0.68-1.00, p=0.06). The HR adjusted for demographic and pathological data was 0.84(95% CI: 0.69-1.03, p=0.10). The HR adjusted for demographic, pathological, and therapeutic data was 0.82 (95% CI: 0.67-1.00, p=0.052). The unadjusted HR of the group with combined reconstruction was 0.74(95% CI: 0.56-0.99, p=0.04) compared with group with tissue reconstruction; As to the group with combined reconstruction, the Cox regression HRs for adjusted 1, adjusted 2, and adjusted 3 models were 0.75(95% CI:0.57-0.99), p=0.05, 0.73(95% CI:0.55-0.97), p=0.03, and 0.75(95% CI:0.56-0.99), p=0.04, respectively.
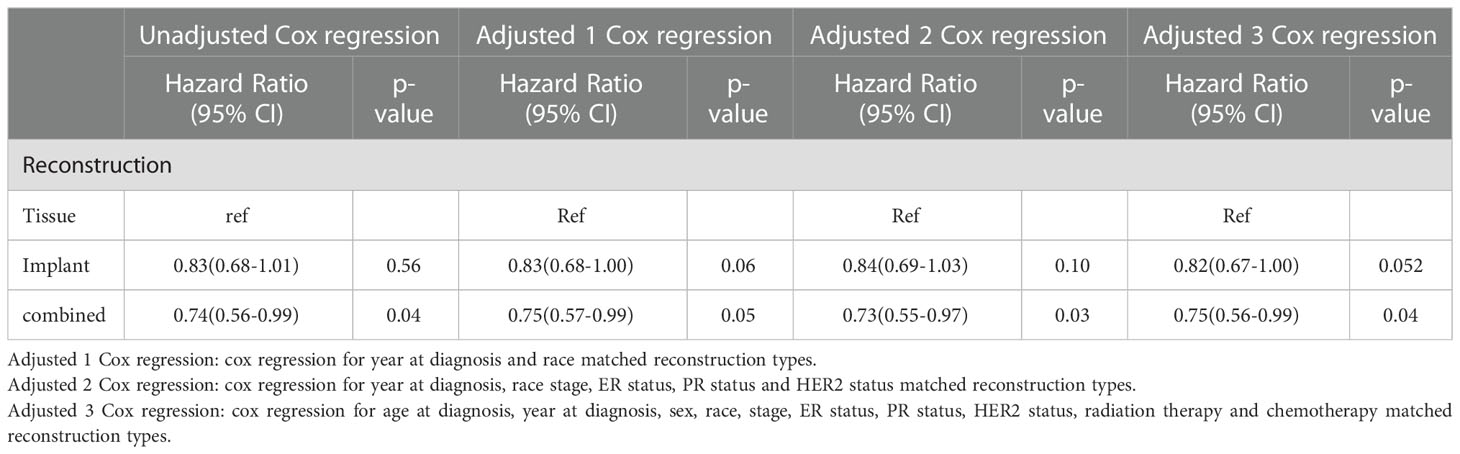
Table 4 Hazard ratios of different reconstruction types for the cancer specific mortality of breast cancer.
Hazard ratios of different subgroups for OM
The HRs for OM of the group with tissue reconstruction compared with the other groups are displayed in Table 5. The unadjusted HR of the group with implant and combined reconstruction were 0.82 (95% CI: 0.68-0.99, p=0.04) and 0.75(95% CI: 0.57-0.99, p=0.04) compared with group with tissue reconstruction, respectively. The HR adjusted for demographic data with implant and combined reconstruction were 0.85(95% CI: 0.70-1.03, p=0.09) and 0.77(95% CI: 0.58-1.01, p=0.054). The HR adjusted for demographic and pathological data with implant and combined reconstruction were 0.85(95% CI: 0.70-1.02, p=0.09) and 0.78(95% CI:0.59-1.02, p=0.07). The HR adjusted for demographic, pathological, and therapeutic data with implant and combined reconstruction were 0.84 (95% CI: 0.70-1.02, p=0.08) and 0.77(95% CI: 0.59-1.01, p=0.06), respectively.
Kaplan-Meier analyses using log-rank tests
Kaplan-Meier analyses using log-rank tests showed that BCSS and OS were significantly different between the reconstructive types groups (Figures 1A, B). Meanwhile, BCSS and OS with the cases under 55 years also showed significantly different between the reconstructive types groups (Figures 2B, D). In addition, BCSS and OS with the cases with radiotherapy were significantly different between reconstructive types groups (Figures 4B, D). Prognosis of cases with different stage, over 55 years and without radiotherapy were showed no significant difference between different reconstructive types groups (Figures 2–4).
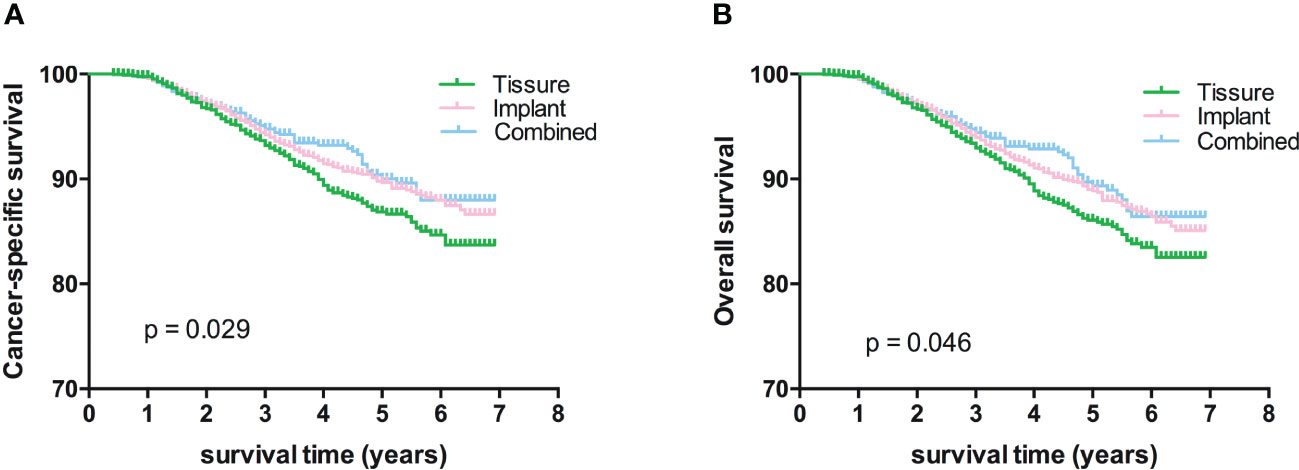
Figure 1 Kaplan-Meier analyses of CSS (A) and OS (B) among patients stratified according to reconstructive types.

Figure 2 Kaplan-Meier analyses of CSS (A, B) and OS (C, D) among patients over or under 55 years stratified according to reconstructive types.
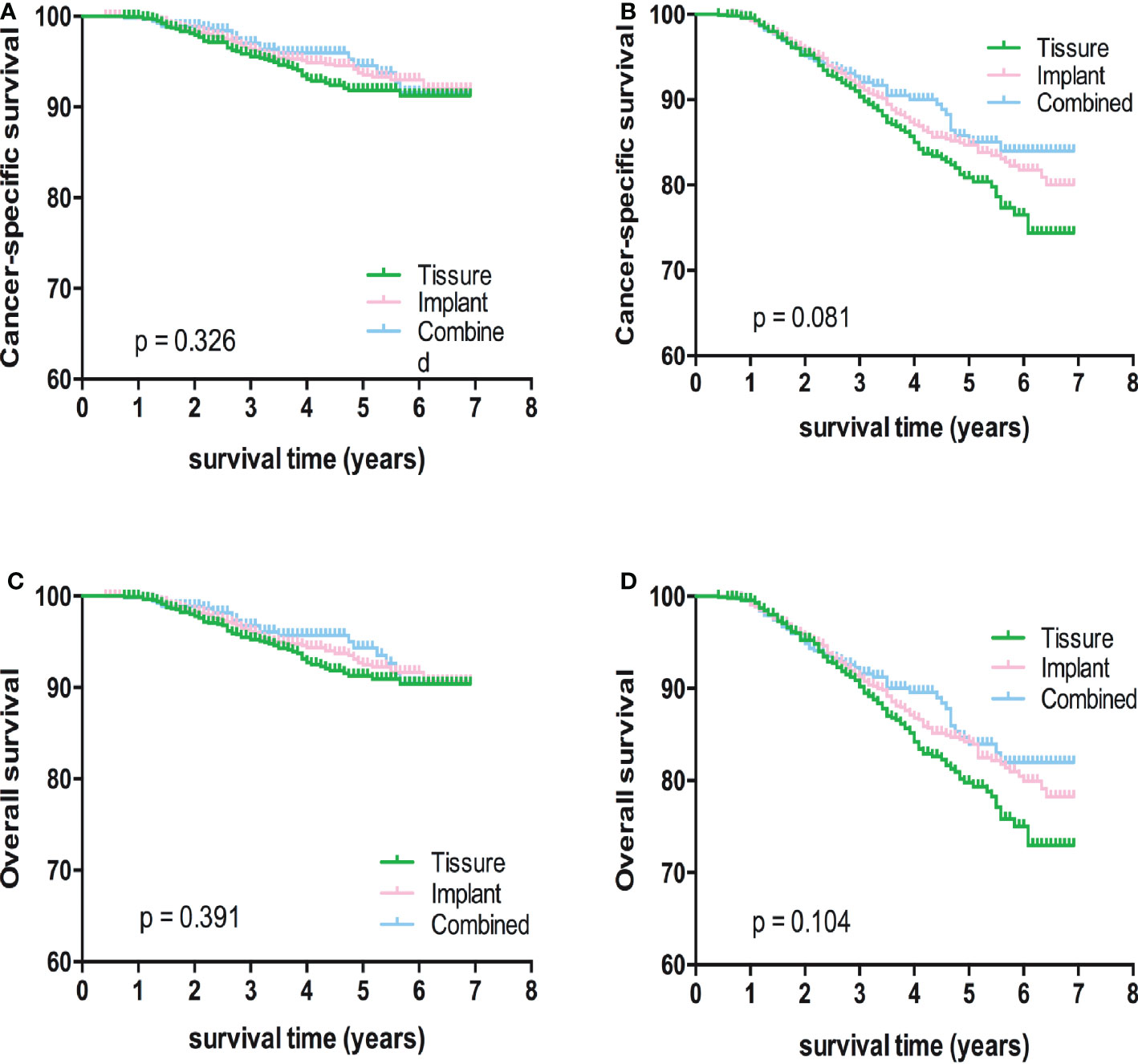
Figure 3 Kaplan-Meier analyses of CSS (A, B) and OS (C, D) among patients with I/II or III/IV stage stratified according to reconstructive types.
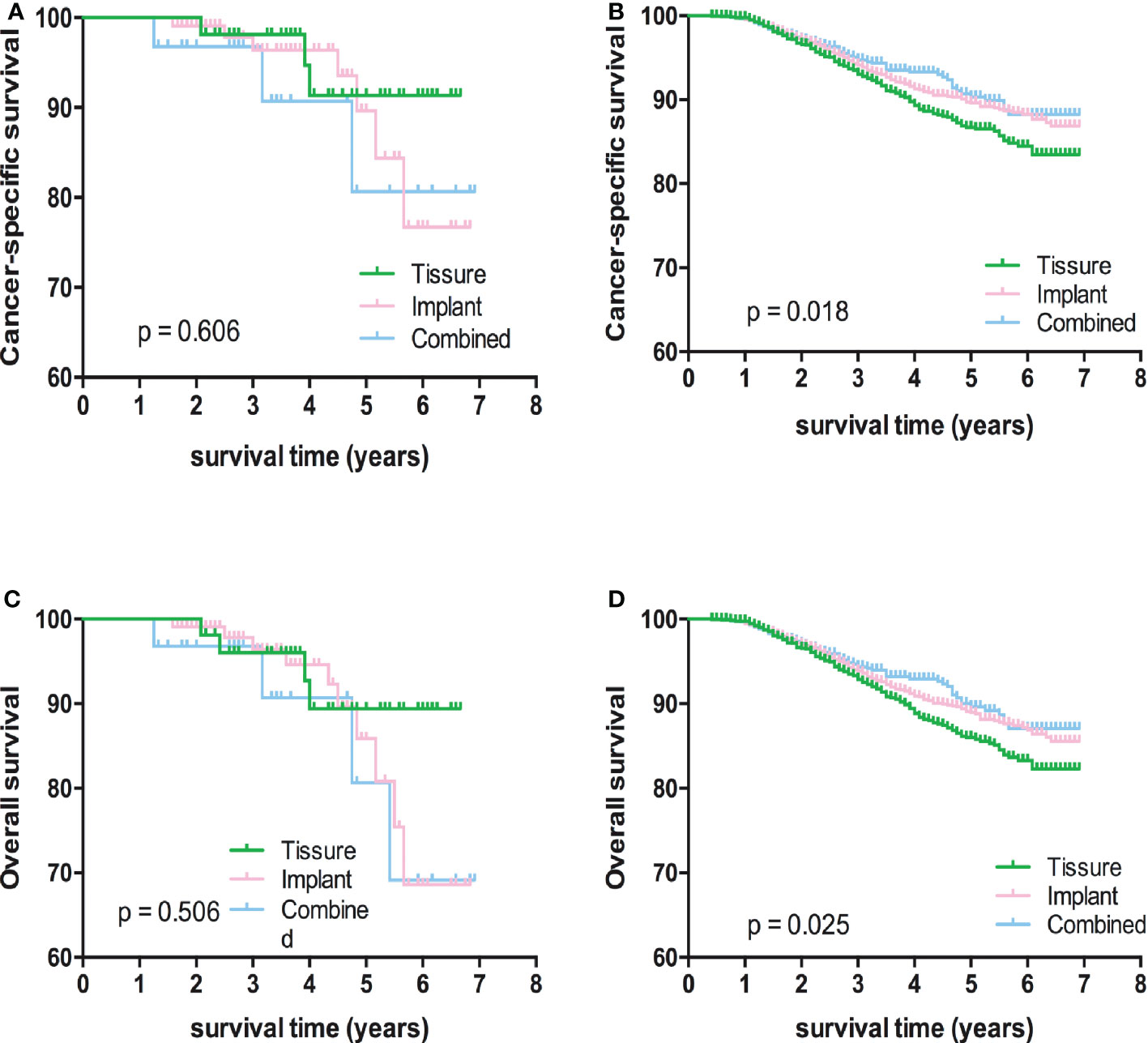
Figure 4 Kaplan-Meier analyses of CSS (A, B) and OS (C, D) among patients with or without radiotherapy stratified according to reconstructive types.
Discussion
In this study, we tried to conduct on the impact of different types of reconstruction, including tissue reconstruction, implant reconstruction and combined reconstruction, on patient survival using SEER database, and found that group with combined reconstruction showed less Hazard Ritio:0.75(95% CI:0.56-0.99) than the tissue reconstruction group for the breast cancer specific mortality. And between tissue and implant reconstructive group, the BCSS were not significantly different.
Breast reconstruction has become common in breast cancer surgery (10–12). In the United States, breast reconstruction rates are increasing among early-stage breast cancer patients who undergo mastectomies, most of which involve implant reconstruction, as well as reconstruction of autologous tissue. And the more severe the defect, the higher the rate of reconstruction, for example, about three-quarters of patients who had bilateral mastectomies had breast reconstruction at the same time (9).
Autogenous tissue breast reconstruction is the use of a patient’s own tissue, transplanted from other parts of the body, to the breast area to restore breast volume after mastectomy. Common autologous tissue reconstruction methods include muscle flaps of rectus abdominis (TRAM), deep abdominal perforator flaps (DIEP), latissimus dorsi flap, autologous fat transplantation. Autogenous breast reconstruction is considered the gold standard by many plastic surgeons because it is softer, does not carry risks such as capsular contracture, and has a better aesthetic effect (13, 14).
Although radiation therapy increases the rate of capsular contracture and reconstruction failure of implants, implant breast reconstruction is a practical option, especially for some patients who lack autologous tissue (4, 15, 16). Regardless of the type of reconstruction, ensuring the safety of the patient’s tumor reconstruction is a top priority for clinicians and patients. In our study, there was no difference in cancer-related mortality after adjusting for all potential influencing factors between implant reconstruction and autologous tissue reconstruction, but the risk of compound reconstruction was lower. Pusic et al. reported that the satisfaction with breasts, psychosocial well-being, and sexual well-being of autologous breast reconstruction were significantly higher than that of implant reconstruction (17–19), Therefore, in this perspective, autologous breast reconstruction can be more cost-effective without affecting survival outcomes.
In patients with advanced breast cancer, postoperative radiotherapy has been shown to improve patient survival, especially in patients with positive lymph nodes (20). But it has some negative consequences for women with breast reconstruction, such as complications and reduced cosmetic results. With advances in plastic surgery and radiation therapy, clinicians are already trying to integrate radiation therapies to minimize interference with the effects of reconstruction with minimal impact on tumor survival (9). In our study based on SEER big data, we found that the rate of implant reconstruction is higher than tissue reconstruction for the patients underwent radiotherapy.
As with all large database studies, our study still has some limitations. First, genetic factors such as BRCA mutations should be considered in comparative analysis. Secondly, our data did not contain specific information about immediate reconstruction or delayed reconstruction, nipple-sparing mastectomy or not, and there was no detailed record of complications of reconstructive surgery. Finally, the population we included is based on SEER database, which mainly includes the population of North America. These may affect the generality of our systematic conclusions.
Conclusion
Breast cancer-related mortality between implant reconstruction and autologous tissue reconstruction showed no significantly different, but the risk of BCSS of compound reconstruction was lower than tissue reconstruction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
PW wrote the main manuscript text. YD and LW edited and revised the article critically. XL did the literature research, and ES collected the data. LK provided the source of data information, YY analyzed the data, and PW prepared figures. YD and PW provided the study concepts and study design. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1092506/full#supplementary-material
Abbreviations
BC, breast cancer; AJCC, American Joint Committee on Cancer; SEER, Surveillance, Epidemiology, and End Results; BCSS, breast cancer-specific survival; OS, all-cause survival; BCM, breast cancer-cause mortality; OM, all-cause mortality.
References
1. Meattini I, Becherini C, Bernini M, Bonzano E, Criscitiello C, De Rose F, et al. Breast reconstruction and radiation therapy: An Italian expert Delphi consensus statements and critical review. Cancer Treat Rev (2021) 99:102236. doi: 10.1016/j.ctrv.2021.102236
2. Sharma R. Breast cancer incidence, mortality and mortality-to-incidence ratio (MIR) are associated with human development, 1990-2016: evidence from global burden of disease study 2016. Breast Cancer (2019) 26:428–45. doi: 10.1007/s12282-018-00941-4
3. Wu ZY, Han HH, Kim HJ, Lee JW, Chung IY, Kim J, et al. A propensity score-matched comparison of recurrence outcomes after immediate implant vs autologous flap reconstruction in patients receiving neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat (2021) 187:417–25. doi: 10.1007/s10549-021-06114-w
4. Altinok AY, Bese N, Kara H, Yazar S, Unal C. The satisfaction of patients with breast cancer undergone immediate reconstruction with implant and the effect of radiotherapy. Contemp Oncol (Pozn) (2018) 22:27–30. doi: 10.5114/wo.2018.74390
5. Kooijman MML, Hage JJ, Oldenburg HSA, Stouthard JM, Woerdeman LAE. Surgical complications of skin-sparing mastectomy and immediate implant-based breast reconstruction in women concurrently treated with adjuvant chemotherapy for breast cancer. Ann Plast Surg (2021) 86:146–50. doi: 10.1097/SAP.0000000000002435
6. Hamann M, Brunnbauer M, Scheithauer H, Hamann U, Braun M, Polcher M. Quality of life in breast cancer patients and surgical results of immediate tissue expander/implant-based breast reconstruction after mastectomy. Arch Gynecol Obstet (2019) 300:409–20. doi: 10.1007/s00404-019-05201-0
7. Ogita M, Nagura N, Kawamori J, In R, Yoshida A, Yamauchi H, et al. Risk factors for complications among breast cancer patients treated with post-mastectomy radiotherapy and immediate tissue-expander/permanent implant reconstruction: a retrospective cohort study. Breast Cancer (2018) 25:167–75. doi: 10.1007/s12282-017-0808-6
8. Strach MC, Prasanna T, Kirova YM, Alran S, O'Toole S, Beith JM, et al. Optimise not compromise: The importance of a multidisciplinary breast cancer patient pathway in the era of oncoplastic and reconstructive surgery. Crit Rev Oncol Hematol (2019) 134:10–21. doi: 10.1016/j.critrevonc.2018.11.007
9. Ho AY, Hu ZI, Mehrara BJ, Wilkins EG. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol (2017) 18:e742–53. doi: 10.1016/S1470-2045(17)30617-4
10. Bhat S, Orucevic A, Woody C, Heidel RE, Bell JL. Evolving trends and influencing factors in mastectomy decisions. Am Surg (2017) 83:233–8. doi: 10.1177/000313481708300317
11. Manyam BV, Shah C, Woody NM, Reddy CA, Weller MA, Juloori A, et al. Long-term outcomes after autologous or tissue Expander/Implant-based breast reconstruction and postmastectomy radiation for breast cancer. Pract Radiat Oncol (2019) 9:e497–505. doi: 10.1016/j.prro.2019.06.008
12. Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg (2015) 150:9–16. doi: 10.1001/jamasurg.2014.2895
13. Sgarzani R, Negosanti L, Morselli PG, Vietti Michelina V, Lapalorcia LM, Cipriani R. Patient satisfaction and quality of life in DIEAP flap versus implant breast reconstruction. Surg Res Pract (2015) 2015:405163. doi: 10.1155/2015/405163
14. Rubio GA, McGee CS, Thaller SR. Autologous breast reconstruction surgery outcomes in patients with autoimmune connective tissue disease. J Plast Reconstr Aesthet Surg (2019) 72:848–62. doi: 10.1016/j.bjps.2018.12.038
15. Lohmander F, Lagergren J, Johansson H, Roy PG, Brandberg Y, Frisell J. Effect of immediate implant-based breast reconstruction after mastectomy with and without acellular dermal matrix among women with breast cancer: A randomized clinical trial. JAMA Netw Open (2021) 4:e2127806. doi: 10.1001/jamanetworkopen.2021.27806
16. McCarthy CM, Pusic AL, Disa JJ, McCormick BL, Montgomery LL, Cordeiro PG. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg (2005) 116:1642–7. doi: 10.1097/01.prs.0000187794.79464.23
17. Pusic AL, Matros E, Fine N, Buchel E, Gordillo GM, Hamill JB, et al. Patient-reported outcomes 1 year after immediate breast reconstruction: Results of the mastectomy reconstruction outcomes consortium study. J Clin Oncol (2017) 35:2499–506. doi: 10.1200/JCO.2016.69.9561
18. Nelson JA, Allen RJ Jr., Polanco T, Shamsunder M, Patel AR, McCarthy CM, et al. Long-term patient-reported outcomes following postmastectomy breast reconstruction: An 8-year examination of 3268 patients. Ann Surg (2019) 270:473–83. doi: 10.1097/SLA.0000000000003467
19. Santosa KB, Qi J, Kim HM, Hamill JB, Wilkins EG, Pusic AL. Long-term patient-reported outcomes in postmastectomy breast reconstruction. JAMA Surg (2018) 153:891–9. doi: 10.1001/jamasurg.2018.1677
20. Ebctcg, McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (2014) 383:2127–35. doi: 10.1016/S0140-6736(14)60488-8
Keywords: reconstructive types, prognosis, breast cancer, SEER, implant
Citation: Wang P, Wang L, Liang X, Si E, Yang Y, Kong L and Dong Y (2023) Reconstructive types effect the prognosis of patients with tumors in the central and nipple portion of breast cancer? An analysis based on SEER database. Front. Oncol. 12:1092506. doi: 10.3389/fonc.2022.1092506
Received: 08 November 2022; Accepted: 12 December 2022;
Published: 23 January 2023.
Edited by:
Zeming Liu, Huazhong University of Science and Technology, ChinaReviewed by:
Yan Gong, Zhongnan Hospital, Wuhan University, ChinaWen Zeng, Department of Ophthalmology, Zhongnan Hospital of Wuhan University, China
Shuntao Wang, Huazhong University of Science and Technology, China
Copyright © 2023 Wang, Wang, Liang, Si, Yang, Kong and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingfei Kong, a29uZ2xpbmdmZWkxODUzQDE2My5jb20=; Yonghui Dong, ZG9uZ3loQHp6dS5lZHUuY24=
†These authors have contributed equally to this work
 Ping Wang
Ping Wang Le Wang2†
Le Wang2† Yongguang Yang
Yongguang Yang