- 1Division of Hematology and Oncology, Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan
- 2Department of Cosmetic Science, Chia-Nan University of Pharmacy and Science, Tainan, Taiwan
- 3Department of Medical Research, Chi Mei Medical Center, Tainan, Taiwan
- 4Department of Information Management, Southern Taiwan University of Science and Technology, Tainan, Taiwan
- 5Division of Colorectal Surgery, Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan
- 6Department of Medical Laboratory Science and Biotechnology, Chung Hwa University of Medical Technology, Tainan, Taiwan
- 7Department of Radiation Oncology, Chi Mei Medical Center, Tainan, Taiwan
- 8Department of Pharmacy, Chia-Nan University of Pharmacy and Science, Tainan, Taiwan
Background: For rectal cancer, it remains unclear how to incorporate tumor response to neoadjuvant chemoradiotherapy (nCRT) when deciding whether to give adjuvant chemotherapy. In this study, we aim to determinate the survival benefit of adjuvant chemotherapy for rectal cancer patients with good response (ypT0-2N0) after nCRT and surgery.
Methods: The study cohort included 720 rectal cancer patients who had good response (ypT0-2N0) after nCRT and surgery, who did or did not receive adjuvant chemotherapy between January 2007 and December 2017, from the Taiwan Cancer Registry and National Health Insurance Research database. The Kaplan–Meier method, log-rank tests, and Cox regression analysis were performed to investigate the effect of adjuvant chemotherapy on 5-year overall survival (OS) and disease-free survival (DFS).
Results: Of 720 patients, 368 (51.1%) received adjuvant chemotherapy and 352 (48.9%) did not. Patients who received adjuvant chemotherapy were more likely to be female, younger (≤ 65), with advanced clinical T (3-4)/N (1-2) classification and ypT2 classification. No significant difference in 5-year OS (p=0.681) or DFS (p=0.942) were observed by receipt of adjuvant chemotherapy or not. Multivariable analysis revealed adjuvant chemotherapy was not associated with better OS (adjusted hazard ratio [aHR], 1.03; 95% Confidence Interval [CI], 0.88-1.21) or DFS (aHR, 1.05; 95% CI, 0.89-1.24). Stratified analysis for OS and DFS found no significant protective effect in the use of adjuvant chemotherapy, even for those with advanced clinical T or N classification.
Conclusion: Adjuvant chemotherapy may be omitted in rectal cancer patients with good response (ypT0-2N0) after nCRT and surgery.
Background
Although mortality has steadily decreased since 1990, colorectal cancer remains one of the most frequent cancer-related death in the US (1). In addition, the incidence of colorectal cancer under the age of 50 increased from 1992 to 2012 at a rate of 2.1% annually, and continues to rise (2). According to the Taiwan Cancer Registry (TCR) database in recent two decades, male and female young-onset rectal cancer incidence rates rose from 4.0 to 8.3 and 3.8 to 6.4 per 100,000 (3).
Although radical resection is the cornerstone of management in rectal cancer, radiotherapy and chemotherapy has emerged as an important component of curative therapy, because local recurrence is more common in those types than with colon primaries (4, 5). Treatment for locally advanced rectal cancer (T3-4N0 or T1-4N1-2) consists of neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision and adjuvant chemotherapy with fluorouracil and oxaliplatin. The use of nCRT promotes greater sphincter preservation and facilitates tumor downstaging (6, 7). Most importantly, about 15% of these patients have a pathologic complete response (defined as ypT0N0), which is associated with an excellent long-term survival outcome (8, 9). A further 20% of patients downstage to ypT1/T2N0 (10). However, up to a third of contemporary patients who undergo surgical resection of rectal cancer patients still ultimately develop metastatic disease (11). Adjuvant chemotherapy after nCRT and resection has thus been proposed as a potential method of alleviating micrometastasis, hence reducing recurrence and enhancing survival. Currently, the use of adjuvant chemotherapy in rectal cancer patients remains controversial (12–14). National Comprehensive Cancer Network (NCCN) guidelines state that pre-treatment staging, not surgical pathology, should be used to guide decisions for adjuvant chemotherapy. It is unclear how to incorporate tumor response to nCRT when deciding to provide adjuvant chemotherapy; this uncertainty is reflected in the poor compliance with NCCN guidelines for adjuvant chemotherapy administration (15).
In this study, we sought to address the impact of adjuvant chemotherapy on survival in rectal cancer patients with good response (ypT0-2N0) after nCRT and surgery, by conducting an analysis from a large cohort of patients from the TCR and the National Health Insurance Research Database (NHIRD).
Materials and methods
Ethics approval and informed consent
The study was approved by the Ethics Committee of the Institutional Review Board of Chi Mei Medical Center (IRB: 10707–012). Informed consent was not obtained because the IRB waived the need for individual informed consent, as no personally identifiable information were used. This study had a non-interventional retrospective design, no human subjects used and all data were analyzed anonymously.
Data source and study cohort
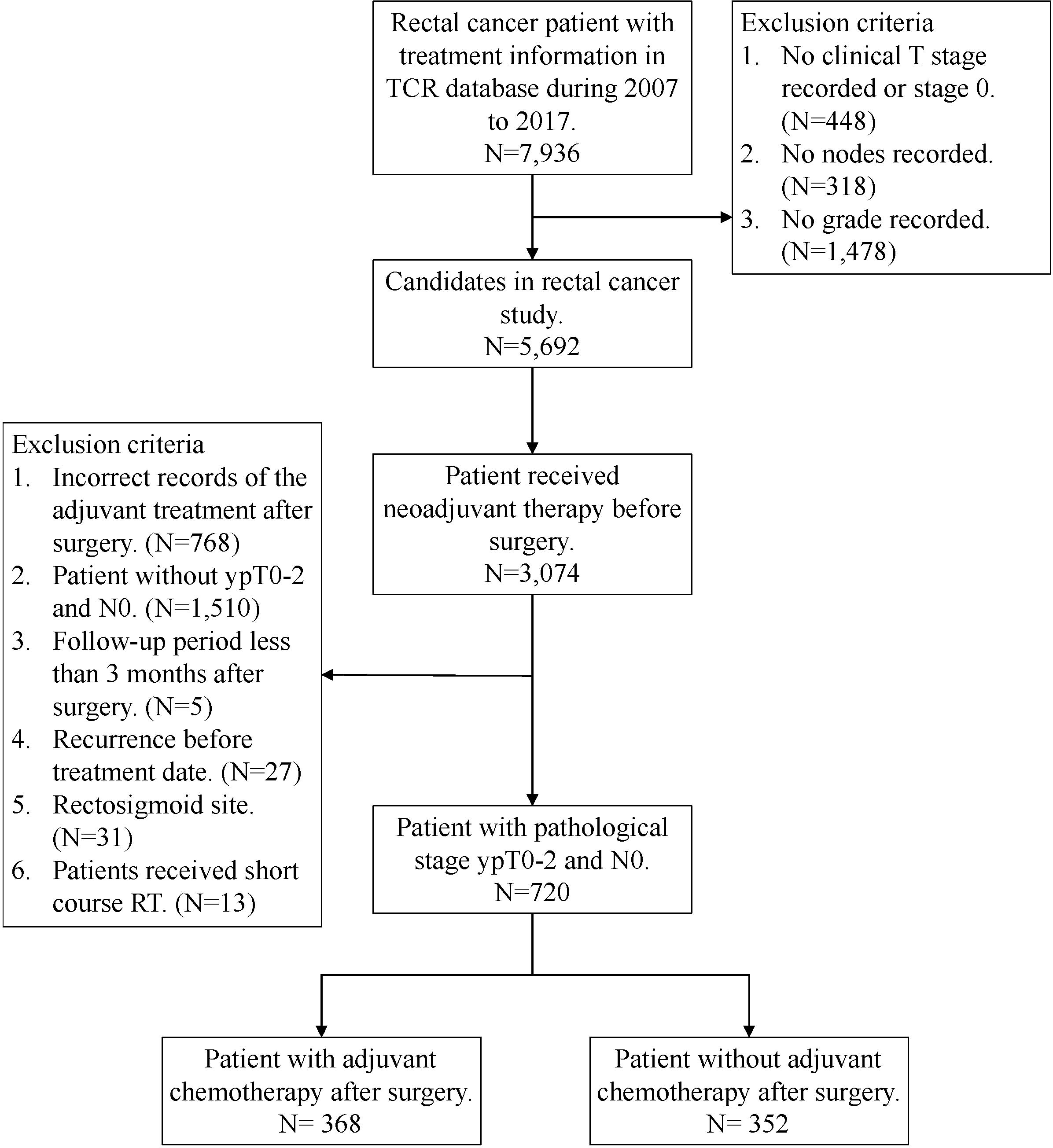
We obtained data from the TCR and NHIRD, which cover more than 95% of the cancer cases in Taiwan. The TCR also has documented excellent data quality and completeness (16). The rectal cancer patients who underwent nCRT and surgical resection with or without adjuvant chemotherapy between January 2007 and December 2017 were included. Follow-up was completed on December 31, 2018. Exclusion criteria included: 1) a history of cancer or metastatic disease; 2) no clear coding on follow-up and treatment; or 3) patients received short course radiation. Rectal cancer diagnosis were defined by the International Classification of Disease for Oncology, third edition (ICD-O-3) codes for location: rectum (code C20.9); and histologic type: adenocarcinoma (codes 8140, 8210, 8261, and 8263), mucinous adenocarcinoma (code 8480), or signet ring cell carcinoma (code 8490). These patients were all staged according to the 7th edition American Joint Committee on Cancer (AJCC) classification system. The variables from the TCR database used for analysis included age, gender, histology type, grade, stage, margin status, lymph node yield, comorbid conditions and cancer-related treatment. Here, Charlson Comorbidity Index (CCI) score were used to grade the severity of comorbid conditions (17). Finally, a total of 720 rectal cancer patients with good response “ ypT0-2N0 “ after nCRT and surgery were extracted (Figure 1).
Statistical analysis
In this study, all statistical analyses were performed using SAS 9.4 for Windows (SAS Institute, Inc., Cary, NC, USA) and Kaplan-Meier curves were plotted using STATA (version 12; Stata Corp., College Station, TX, USA). A p value ≤ 0.05 was considered statistically significant. The distribution difference between ypT0-2N0 rectal cancer patients treated with and without adjuvant chemotherapy was estimated using Pearson’s chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables. The primary endpoints were the 5-year overall survival (OS) and disease-free survival (DFS) rates and were calculated via Kaplan-Meier method, comparing by log-rank statistics. The risk was presented as hazard ratios (HRs) with 95% confidence intervals (CIs) and calculated using the Cox proportional hazards model for factors associated with survival. We also performed stratified survival analyses for important prognostic characteristics such as age, cT/cN/ypN classification.
Results
Between 2007 and 2017, a total of 720 rectal cancer patients were selected for analysis, including 368 patients receiving adjuvant chemotherapy and 352 patients without adjuvant chemotherapy (Figure 1). Baseline clinicopathological characteristics are summarized in Table 1. Among these patients, 498 were male (69.2%) and 222 were female (30.8%). The mean age at diagnosis was 61 ± 11 years, and the median (Q1-Q3) follow‐up time was 4.22 years. The information of neoadjuvant treatment is summarized in Supplementary Table 1. The median (Q1-Q3) total dose of radiotherapy was 50.4 Gy in 27 fractions. Concurrent, neoadjuvant chemotherapy regimens included fluorouracil (5-FU), leucovorin, capecitabine, oxaliplatin and UFUR. Patients who received adjuvant chemotherapy were more likely to be female, younger (≤ 65), with advanced clinical T (3-4)/N (1-2) classification and ypT2 classification. The median (Q1-Q3) timing of adjuvant chemotherapy started after operation were 36 days. Adjuvant chemotherapy regimens used as follows: Leucovorin, Fluorouracil (5-FU), UFUR, oxaliplatin and capecitabine. Most patients (88.59%) received combined regimens instead of mono-therapy.
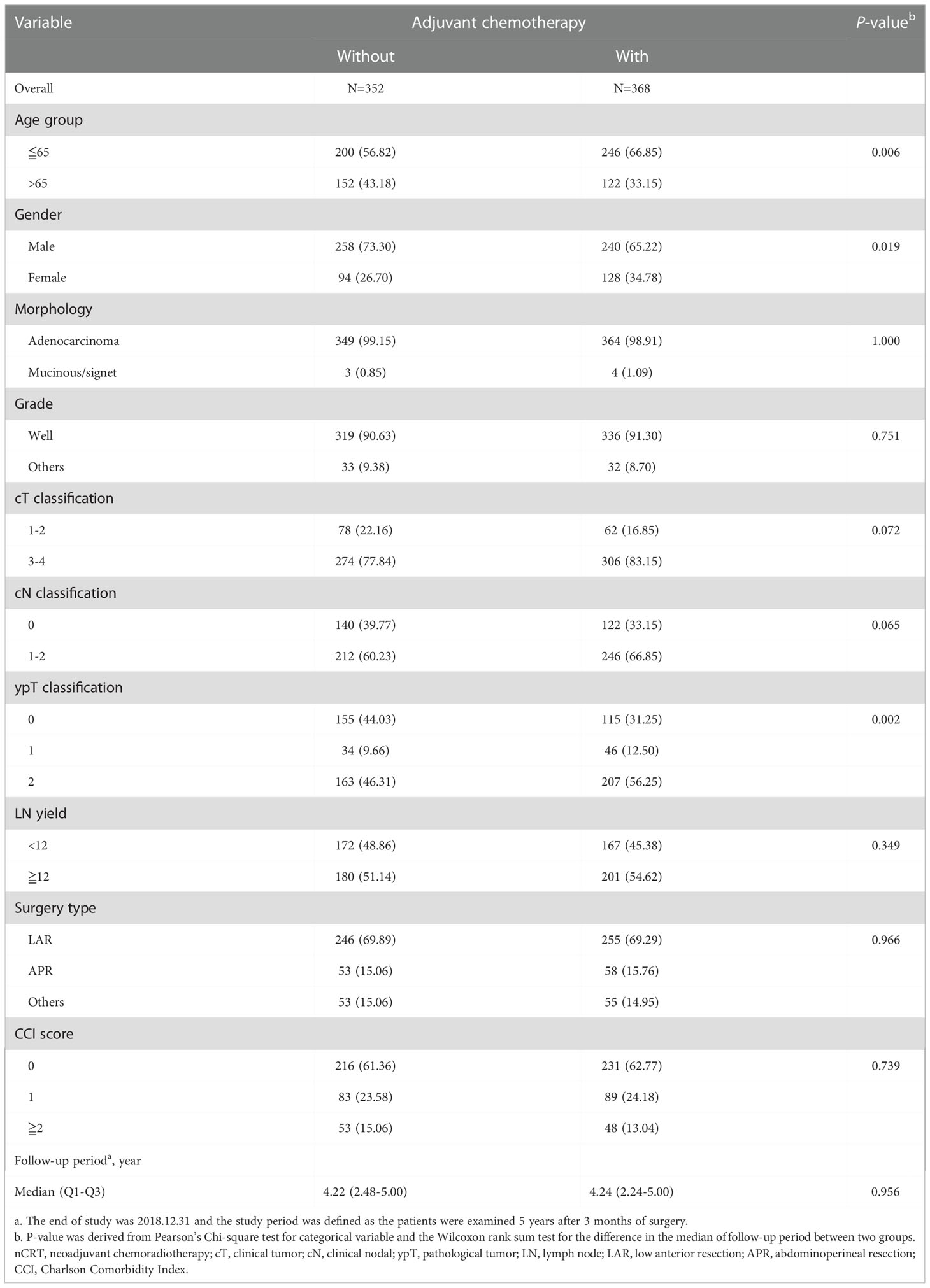
Table 1 Clinicopathological information of rectal cancer patients with good response (ypT0-2N0) after nCRT and surgery, n=720.
Kaplan-Meier survival curves were generated to compare the 5-year OS and DFS rates by receipt of adjuvant chemotherapy or not. As presented in Figure 2, there was no significant difference in 5-year OS (p=0.681) or DFS (p=0.942) between those who received adjuvant chemotherapy and those who did not. After adjustment for confounders (Table 2), multivariable analysis indicated that adjuvant chemotherapy was not significantly associated with better 5-year OS (adjusted hazard ratio [aHR], 1.03; 95% Confidence Interval [CI], 0.88-1.21) or DFS (aHR, 1.05; 95% CI, 0.89-1.224). Further stratified analysis for 5-year OS and DFS found no significant protective role in the use of adjuvant chemotherapy for these “good response” rectal cancer patients, even for those with advanced cT or cN classification (Table 2). In this study, we selected ypT1-2N0 patients without clinical metastases and there are some early stage (cT1-2N0) included into our analysis. As shown in Table 3, comparisons by different clinical and pathological T and N classifications revealed that the use of adjuvant chemotherapy provided limited survival benefit compared to not using it. Regarding to different adjuvant chemotherapy regimens, the related 5-year OS and DFS rate were presented in Table 4.

Figure 2 Probability of overall survival (A) and disease-free survival (B) in rectal cancer patients with good response (ypT0-2N0) after nCRT and surgery, n=720.
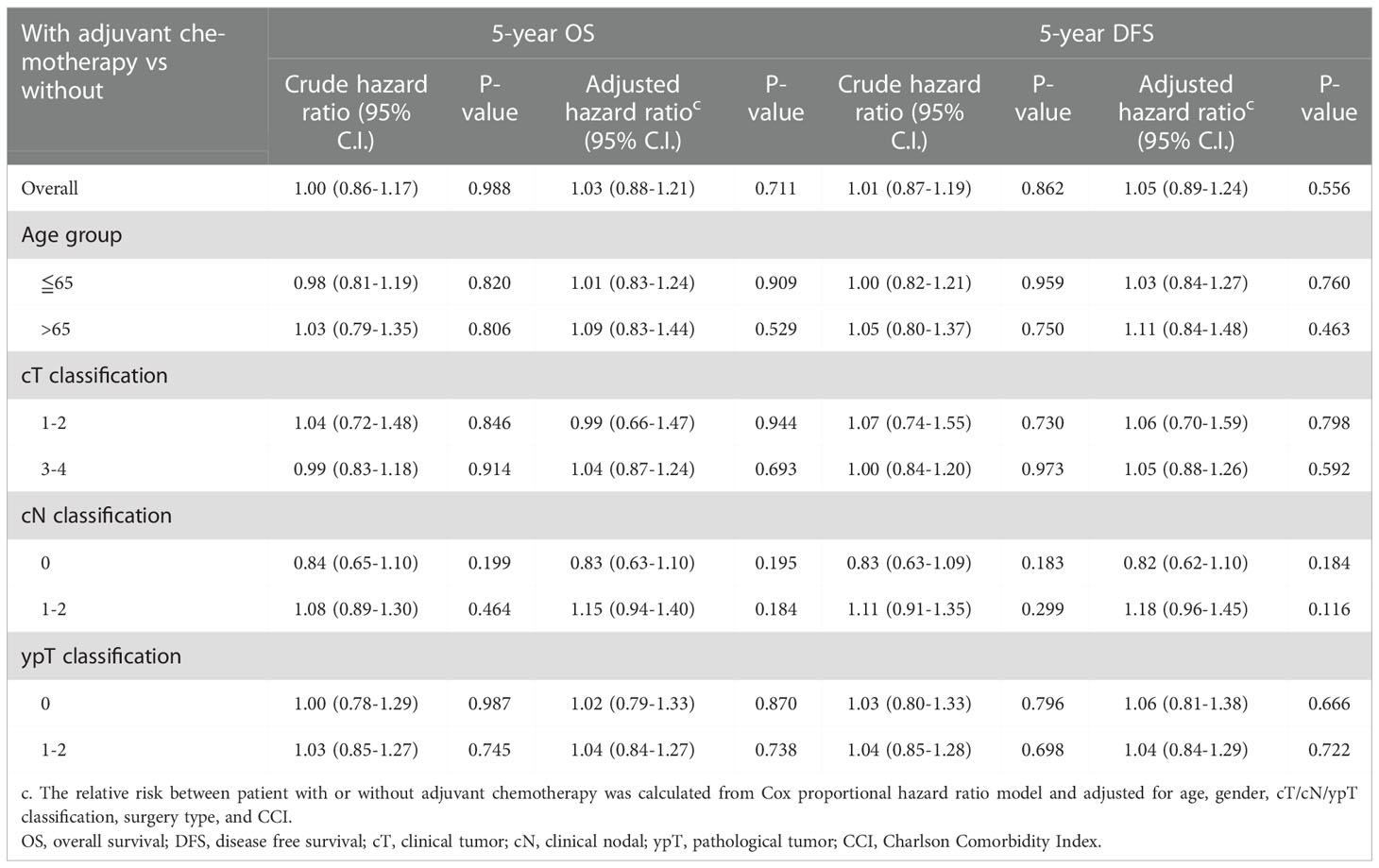
Table 2 Overall and stratified analysis of the use of adjuvant chemotherapy for 5-year OS and DFS in rectal cancer patients with good response (ypT0-2N0) after nCRT and surgery, n=720.
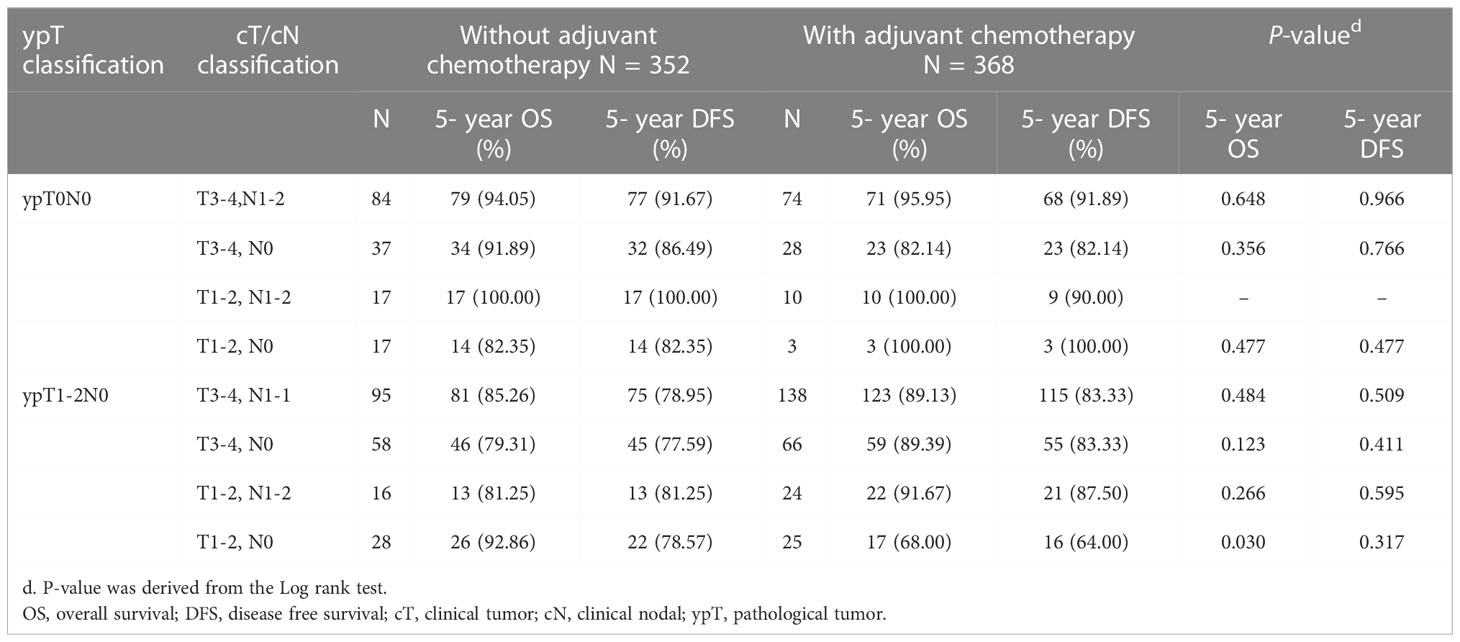
Table 3 5-year OS and DFS comparison by different clinicopathological stage and the use of adjuvant chemotherapy of study patients, n =720.
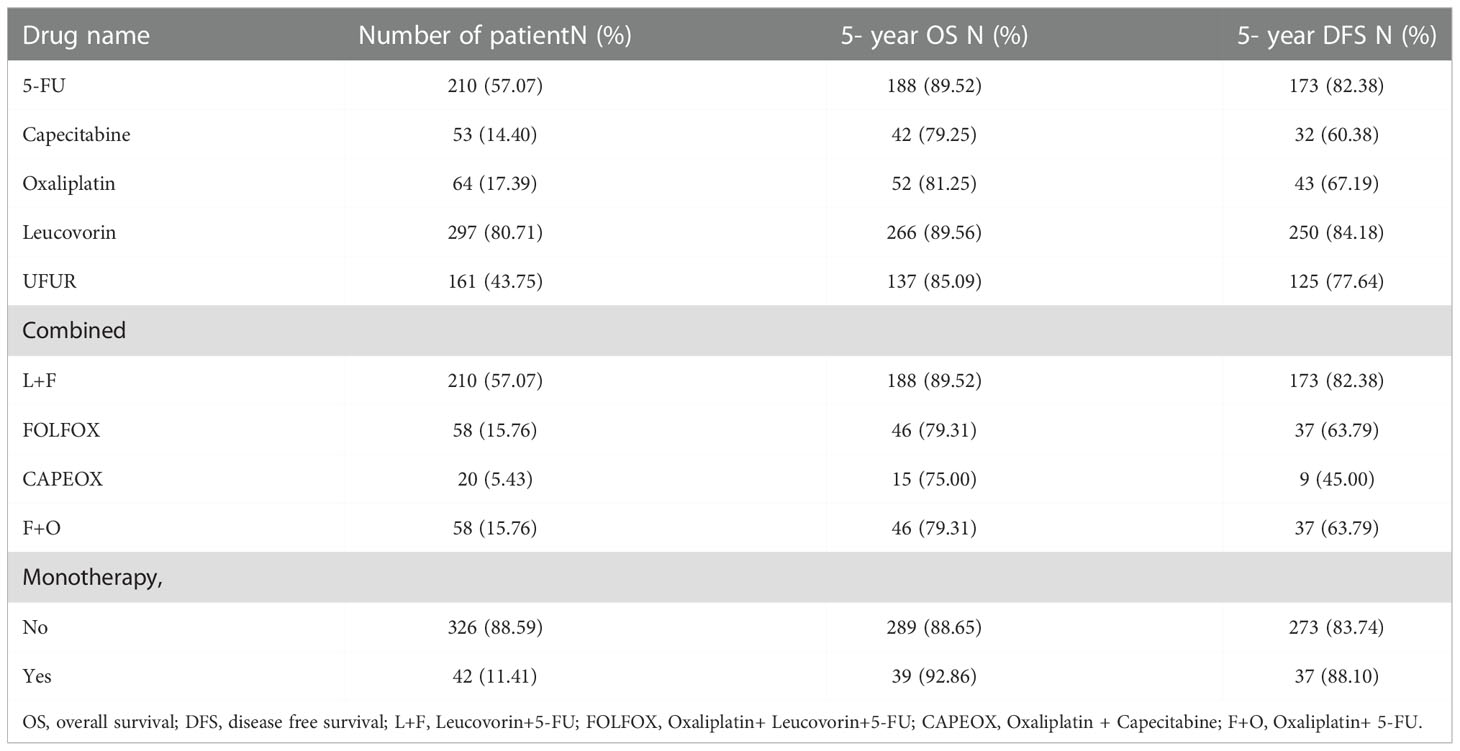
Table 4 The distribution and 5-year survival among different adjuvant chemotherapy regimens groups, n =368.
Discussion
Treatment for clinical stage II or III rectal cancer patients consists of nCRT followed by total mesorectal excision and adjuvant chemotherapy with fluorouracil and oxaliplatin. However, the benefit of adjuvant chemotherapy for downstaged or good response (ypT0-2N0) rectal cancer after nCRT remains inconsistent (12–14). In this nationwide, population-based, cohort study, our results found no significant difference in 5-year OS or DFS between those who received adjuvant chemotherapy and those who did not. Moreover, there was no significantly difference in 5-year survival even in those with advanced cT or cN classification.
Our study offers a number of advantages over earlier studies from single institutions or several national datasets. First, we obtained data from the TCR and the NHIRD (18). This database covered more than 95% cancer cases in Taiwan. The follow-up period was long and the patient number was enough (N=764) to make our results convincing. Additionally, we included individuals with rectal cancer diagnosed between 2007 and 2018, which increased the relevance of our study. Second, this database had comprehensive information on patient characteristics, clinical staging, pathological staging, surgical methods and comorbidities enabling us to conduct in-depth analyses on the actual impact of adjuvant chemotherapy.
Adjuvant chemotherapy is recommended as standard treatment for those with high risk stage II and stage III colon cancer (19, 20). However, the precise benefit of adjuvant chemotherapy in rectal cancer remains unclear. According to NCCN guidelines, whether or not to give adjuvant chemotherapy depends on the pre-treatment clinical staging. The guidelines suggest all patients who underwent nCRT for locally advanced (T3/4 or node-positive) non-metastatic rectal cancer receive four months of adjuvant chemotherapy, regardless of the pathologic findings at the time of resection. However, in terms of the evidence level, four randomized phase III trials explored the benefit of adjuvant chemotherapy following nCRT for rectal cancer (6, 11, 21–23). None of the four found any advantage in the use of adjuvant chemotherapy, either in terms of recurrence rate or OS. However, all of these trials were flawed. For example, in the European Organisation for Research and Treatment of Cancer (EORTC) trial 22921 and the cooperative Italian study, the adjuvant chemotherapy regimen consisted of four to six cycles of postoperative bolus fluorouracil plus leucovorin, which was not consistent with the current standard regimen (24, 25). In these two studies, the rate of adherence to adjuvant chemotherapy was poor (43% in the EORTC 22921 study and 72% in the cooperative Italian study). In the Dutch colorectal PROCTOR/SCRIPT trials, the adjuvant chemotherapy regimens included fluorouracil/leucovorin or capecitabine (21). However, the trial did not reach full accrual. The adjuvant chemotherapy regimen in the United Kingdom phase III Chronicle trial was capecitabine plus oxaliplatin (XELOX), which was the current standard chemotherapy regimen (22). Unfortunately, the study was also closed prematurely due to poor accrual. A meta-analysis of individual patient data from all four of these trials concluded that fluorouracil-based chemotherapy did not improve OS, DFS, or distant recurrence rates (26). Another systematic review published in 2017 identified eight phase III trials and one randomized phase II trial comparing adjuvant chemotherapy with observation in patients with non-metastatic rectal cancer treated with nCRT. The authors reported that the data were not robust enough to warrant routine use of adjuvant therapy in this population (27). However, other meta-analyses have come to the opposite conclusion. A systematic review of the scientific literature from 1975 until March 2011 quantitatively summarized the available evidence regarding the impact of adjuvant chemotherapy on the survival of patients with surgically resectable rectal cancer (28). The authors supported the use of 5-FU based postoperative adjuvant chemotherapy, but available data did not allow them to define whether the efficacy of this treatment was greatest for one specific TNM stage. Consequently, conclusive data on the benefits of adjuvant therapy in rectal cancer patients remains lacking.
Our study focused on the benefit of adjuvant chemotherapy for patients with good response (ypT0-2N0) to nCRT and surgery. Patients who achieve a pathologic complete response after preoperative therapy have excellent outcomes (10). These findings raise concerns regarding the possibility of overtreatment in this group, when adding adjuvant chemotherapy. Other database studies have also failed to discover a significant benefit to adjuvant chemotherapy in this setting (29–31). However, three similar observational studies using the National Cancer Database, which looked only at patients achieving pathologic complete response, found that adjuvant chemotherapy did improve survival in this favorable subgroup (32–34).
Our study demonstrated that adjuvant chemotherapy is not beneficial for those rectal patients with good response to nCRT and surgery, even those with advanced clinical stage disease. However, our study showed that patients with clinical nodal positive status had worse OS and DSS. This finding implies that clinical stage might be a prognostic factor rather than a predictive factor. There are some potential explanations for the conflicting results, which are also the common limitations of nationwide cancer registry database analysis, including ours. First, it is challenging to accurately assess clinical stage, since the imaging tools used to determine stage differ by hospital. Some patients could be over-staged, which makes adjuvant chemotherapy appear to be of no benefit in this group. Second, the presence of perineural and extramural venous invasion, particularly after preoperative irradiation, is a significant negative prognostic factor for local recurrence, metastatic disease, and OS (35). However, this information was not recorded in our database. Third, due to treatment-related toxicity, patients receiving adjuvant chemotherapy may face interruption or dose reduction during the course of treatment. However, we could not assess the compliance with the administration of adjuvant chemotherapy (including prescribed dose, and cycles) (36, 37). Future work including the information about the compliance with the administration of chemotherapy may help us to investigate in depth about the role of adjuvant chemotherapy on survival. However, we could know the stop of neoadjuvant CRT course from the coding of total radiation dose. We found 20 (2.7%) patients received less than 45Gy during their neoadjuvant CRT. Fourth, patients with different molecular profiles, such as microsatellite instability, KRAS mutation, or BRAF mutation, may vary in their prognostic profiles and sensitivity to chemotherapeutic and biological agents. The microsatellite instability status also influences the decision to provide adjuvant chemotherapy, but these molecular profiles were not available for our analysis (38). Finally, following nCRT and surgery, patients with a pathologic complete response or a clinically significant downstage to ypT1/T2N0 often have a good prognosis and are unlikely to benefit from additional adjuvant chemotherapy. Although no markers were available in our cancer registry database, we defined the good response from the pathological stage (yp) which is generally accepted and used. However, the response should be a dynamic process and is better evaluated by tumor regression grade (39).
Questions remain regarding how much downstaging is predictive of further benefit from adjuvant therapy. It is possible that adjuvant chemotherapy may not benefit patients at the two extremes of pathologic response: patients with good response and those with poor or minimal pathologic response to nCRT. Perhaps the intermediate group may benefit from further adjuvant chemotherapy. However, identifying this group remains challenging and more prospective studies are needed before this occurs.
Conclusion
In summary, our results demonstrated that adjuvant chemotherapy does not improve 5-year OS or DFS in rectal cancer patients with good response after nCRT and surgery. Moreover, there is no significantly difference in 5-year OS, even in those with advanced cT or cN classification. Therefore, adjuvant chemotherapy may be omitted in these good response patients. Prospective studies that include more patients and clinicopathological variables are necessary to valid our findings into clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Study design: Y-HK, Y-TL, C-HH, C-LC, L-CC, C-JT, W-JH, Y-CC, C-CY. Data analysis: Y-HK, C-HH, Y-CC, C-CY. Manuscript writing: Y-HK, Y-TL, C-HH, C-CY. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to Health Data Science Center, National Cheng Kung University Hospital for providing administrative and technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1087778/full#supplementary-material
Abbreviations
- LARC, Locally advanced rectal cancer; nCRT, Neoadjuvant chemoradiotherapy; pCR, Pathologic complete response; NCCN, National Comprehensive Cancer Network; TCR, Taiwan Cancer Registry; NHIRD, National Health Insurance Research database; AJCC, American Joint Committee on Cancer; ICD-O-3, International Classification of Diseases for Oncology, 3rd Edition; CCI, Charlson comorbidity index; OS, Overall survival; DFS, Disease free survival; HRs, Hazard ratios; CIs, Confidence intervals.
References
1. Cronin KA, Scott S, Firth AU, Sung H, Henley SJ, Sherman RL, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer (2022) 128(24):4251–84. doi: 10.1002/cncr.34479
2. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology (2020) 158(2):341–53. doi: 10.1053/j.gastro.2019.07.055
3. Sung JJY, Chiu HM, Jung KW, Jun JK, Sekiguchi M, Matsuda T, et al. Increasing trend in young-onset colorectal cancer in Asia: More cancers in men and more rectal cancers. Am J Gastroenterol (2019) 114(2):322–9. doi: 10.14309/ajg.0000000000000133
4. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med (2004) 351(17):1731–40. doi: 10.1056/NEJMoa040694
5. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (Mrc Cr07 and ncic-ctg C016): A multicentre, randomised trial. Lancet (2009) 373(9666):811–20. doi: 10.1016/S0140-6736(09)60484-0
6. Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of Ct3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European organisation for research and treatment of cancer radiation oncology group. J Clin Oncol (2007) 25(28):4379–86. doi: 10.1200/JCO.2007.11.9685
7. Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol (2012) 30(15):1770–6. doi: 10.1200/JCO.2011.39.7901
8. Fokas E, Strobel P, Fietkau R, Ghadimi M, Liersch T, Grabenbauer GG, et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J Natl Cancer Inst (2017) 109(12). doi: 10.1093/jnci/djx095
9. Zhang JW, Cai Y, Xie XY, Hu HB, Ling JY, Wu ZH, et al. Nomogram for predicting pathological complete response and tumor downstaging in patients with locally advanced rectal cancer on the basis of a randomized clinical trial. Gastroenterol Rep (Oxf) (2020) 8(3):234–41. doi: 10.1093/gastro/goz073
10. Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol (2010) 11(9):835–44. doi: 10.1016/S1470-2045(10)70172-8
11. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the eortc 22921 randomised study. Lancet Oncol (2014) 15(2):184–90. doi: 10.1016/S1470-2045(13)70599-0
12. Chang GJ. Is there validity in propensity score-matched estimates of adjuvant chemotherapy effects for patients with rectal cancer? JAMA Oncol (2018) 4(7):921–3. doi: 10.1001/jamaoncol.2018.0227
13. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the Cao/Aro/Aio-94 trial. J Clin Oncol (2014) 32(15):1554–62. doi: 10.1200/JCO.2013.54.3769
14. Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol (2005) 23(34):8688–96. doi: 10.1200/JCO.2005.02.1329
15. Xu Z, Mohile SG, Tejani MA, Becerra AZ, Probst CP, Aquina CT, et al. Poor compliance with adjuvant chemotherapy use associated with poorer survival in patients with rectal cancer: An ncdb analysis. Cancer (2017) 123(1):52–61. doi: 10.1002/cncr.30261
16. Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ, Lai MS. Incidence and survival of adult cancer patients in Taiwan, 2002-2012. J Formos Med Assoc (2016) 115(12):1076–88. doi: 10.1016/j.jfma.2015.10.011
17. Tian Y, Xu B, Yu G, Li Y, Liu H. Age-adjusted charlson comorbidity index score as predictor of prolonged postoperative ileus in patients with colorectal cancer who underwent surgical resection. Oncotarget (2017) 8(13):20794–801. doi: 10.18632/oncotarget.15285
18. Liang YH, Shao YY, Chen HM, Cheng AL, Lai MS, Yeh KH. Irinotecan and oxaliplatin might provide equal benefit as adjuvant chemotherapy for patients with resectable synchronous colon cancer and liver-confined metastases: A nationwide database study. Anticancer Res (2017) 37(12):7095–104. doi: 10.21873/anticanres.12183
19. Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage ii and iii colon cancer: Who benefits and by how much? J Clin Oncol (2004) 22(10):1797–806. doi: 10.1200/JCO.2004.09.059
20. Quasar Collaborative G, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet (2007) 370(9604):2020–9. doi: 10.1016/S0140-6736(07)61866-2
21. Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, et al. Adjuvant chemotherapy after preoperative (Chemo)Radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol (2015) 16(2):200–7. doi: 10.1016/S1470-2045(14)71199-4
22. Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: Results of a randomised phase iii trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (Xelox) versus control. Ann Oncol (2014) 25(7):1356–62. doi: 10.1093/annonc/mdu147
23. Swets M, Kuppen PJK, Blok EJ, Gelderblom H, van de Velde CJH, Nagtegaal ID. Are pathological high-risk features in locally advanced rectal cancer a useful selection tool for adjuvant chemotherapy? Eur J Cancer (2018) 89:1–8. doi: 10.1016/j.ejca.2017.11.006
24. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med (2006) 355(11):1114–23. doi: 10.1056/NEJMoa060829
25. Sainato A, Cernusco Luna Nunzia V, Valentini V, De Paoli A, Maurizi ER, Lupattelli M, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (Larc): Long term results of a randomized trial (I-Cnr-Rt). Radiother Oncol (2014) 113(2):223–9. doi: 10.1016/j.radonc.2014.10.006
26. Breugom AJ, van Gijn W, Muller EW, Berglund A, van den Broek CBM, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (Chemo)Radiotherapy and total mesorectal excision: A Dutch colorectal cancer group (Dccg) randomized phase iii trial. Ann Oncol (2015) 26(4):696–701. doi: 10.1093/annonc/mdu560
27. Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol (2017) 18(6):e354–e63. doi: 10.1016/S1470-2045(17)30346-7
28. Petersen SH, Harling H, Kirkeby LT, Wille-Jorgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev (2012) 3):CD004078. doi: 10.1002/14651858.CD004078.pub2
29. Garlipp B, Ptok H, Benedix F, Otto R, Popp F, Ridwelski K, et al. Adjuvant treatment for resected rectal cancer: Impact of standard and intensified postoperative chemotherapy on disease-free survival in patients undergoing preoperative chemoradiation-a propensity score-matched analysis of an observational database. Langenbecks Arch Surg (2016) 401(8):1179–90. doi: 10.1007/s00423-016-1530-0
30. Hu X, Li YQ, Li QG, Ma YL, Peng JJ, Cai SJ. Adjuvant chemotherapy seemed not to have survival benefit in rectal cancer patients with yptis-2n0 after preoperative radiotherapy and surgery from a population-based propensity score analysis. Oncologist (2019) 24(6):803–11. doi: 10.1634/theoncologist.2017-0600
31. Loree JM, Kennecke HF, Lee-Ying RM, Goodwin RA, Powell ED, Tang PA, et al. Impact of postoperative adjuvant chemotherapy following long-course chemoradiotherapy in stage ii rectal cancer. Am J Clin Oncol (2018) 41(7):643–8. doi: 10.1097/COC.0000000000000342
32. Dossa F, Acuna SA, Rickles AS, Berho M, Wexner SD, Quereshy FA, et al. Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol (2018) 4(7):930–7. doi: 10.1001/jamaoncol.2017.5597
33. Polanco PM, Mokdad AA, Zhu H, Choti MA, Huerta S. Association of adjuvant chemotherapy with overall survival in patients with rectal cancer and pathologic complete response following neoadjuvant chemotherapy and resection. JAMA Oncol (2018) 4(7):938–43. doi: 10.1001/jamaoncol.2018.0231
34. Turner MC, Keenan JE, Rushing CN, Gulack BC, Nussbaum DP, Benrashid E, et al. Adjuvant chemotherapy improves survival following resection of locally advanced rectal cancer with pathologic complete response. J Gastrointest Surg (2019) 23(8):1614–22. doi: 10.1007/s11605-018-04079-8
35. Gosens MJ, Klaassen RA, Tan-Go I, Rutten HJ, Martijn H, van den Brule AJ, et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin Cancer Res (2007) 13(22 Pt 1):6617–23. doi: 10.1158/1078-0432.CCR-07-1197
36. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA (2011) 305(22):2335–42. doi: 10.1001/jama.2011.749
37. Gresham G, Cheung WY, Speers C, Woods R, Kennecke H. Time to adjuvant chemotherapy and survival outcomes among patients with stage 2 to 3 rectal cancer treated with preoperative chemoradiation. Clin Colorectal Cancer (2015) 14(1):41–5. doi: 10.1016/j.clcc.2014.11.004
38. Koenig JL, Toesca DAS, Harris JP, Tsai CJ, Haraldsdottir S, Lin AY, et al. Microsatellite instability and adjuvant chemotherapy in stage ii colon cancer. Am J Clin Oncol (2019) 42(7):573–80. doi: 10.1097/COC.0000000000000554
Keywords: rectal cancer, neoadjuvant chemoradiotherapy, surgery, adjuvant chemotherapy, survival
Citation: Kuo Y-H, Lin Y-T, Ho C-H, Chou C-L, Cheng L-C, Tsai C-J, Hong W-J, Chen Y-C and Yang C-C (2022) Adjuvant chemotherapy and survival outcomes in rectal cancer patients with good response (ypT0-2N0) after neoadjuvant chemoradiotherapy and surgery: A retrospective nationwide analysis. Front. Oncol. 12:1087778. doi: 10.3389/fonc.2022.1087778
Received: 11 November 2022; Accepted: 01 December 2022;
Published: 16 December 2022.
Edited by:
Veronika Vymetalkova, Academy of Sciences of the Czech Republic (ASCR), CzechiaReviewed by:
Beatrice Mohelnikova-Duchonova, Palacký University, CzechiaLudmila Boublikova, Charles University, Czechia
Stanislav Filip, Charles University, Czechia
Copyright © 2022 Kuo, Lin, Ho, Chou, Cheng, Tsai, Hong, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Chieh Yang, Y2xlYW5jbGVhcjA5MDVAZ21haWwuY29t
†These authors have contributed equally to this work
 Yu-Hsuan Kuo
Yu-Hsuan Kuo Yun-Tzu Lin
Yun-Tzu Lin Chung-Han Ho
Chung-Han Ho Chia-Lin Chou5,6
Chia-Lin Chou5,6 Yi-Chen Chen
Yi-Chen Chen Ching-Chieh Yang
Ching-Chieh Yang