- 1Urology & Nephrology Center, Department of Urology, Zhejiang Provincial People’s Hospital, Hangzhou, Zhejiang, China
- 2The 2nd Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 3Zhejiang Provincial People’s Hospital, Qingdao University, Shandong, Qingdao, China
- 4Department of Urology, Quzhou People’s Hospital, Quzhou, Zhejiang, China
- 5Department of Urology, Jiaxing Second People’s Hospital, Jiaxing, Zhejiang, China
Introduction: Intraductal carcinoma of the prostate (IDC-P) is a special pathological type of prostate cancer that is highly aggressive with poor prognostic outcomes.
Objective: To establish an effective predictive model for predicting IDC-P.
Methods: Data for 3185 patients diagnosed with prostate cancer at three medical centers in China from October 2012 to April 2022 were retrospectively analyzed. One cohort (G cohort) consisting of 2384 patients from Zhejiang Provincial People’s Hospital was selected for construction (Ga cohort) and internal validate (Gb cohort)of the model. Another cohort (I cohort) with 344 patients from Quzhou People’s Hospital and 430 patients from Jiaxing Second People’s Hospital was used for external validation. Univariate and multivariate binary logistic regression analyses were performed to identify the independent predictors. Then, the selected predictors were then used to establish the predictive nomogram. The apparent performance of the model was evaluated via externally validated. Decision curve analysis was also performed to assess the clinical utility of the developed model.
Results: Univariate and multivariate logistic regression analyses showed that alkaline phosphatase (ALP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), prostate specific antigen (PSA) and lactate dehydrogenase were independent predictors of IDC-P. Therefore, a predictive nomogram of IDC-P was constructed. The nomogram had a good discriminatory power (AUC = 0.794). Internal validation (AUC = 0.819)and external validation (AUC = 0.903) also revealed a good predictive ability. Calibration curves showed good agreement between the predicted and observed incidences of IDC-P.
Conclusion: We developed a clinical predictive model composed of alkaline phosphatase (ALP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), prostate specific antigen (PSA) and lactate dehydrogenase (LDH) with a high precision and universality. This model provides a novel calculator for predicting the diagnosis of IDC-P and different treatment options for patients at an early stage.
Introduction
Globally, clinical incidences for prostate cancer (PCa) are increasing at a rate of 4% to 6% per year, and it accounts for 7.3% of all cancer cases. In over one-half (112 of 185) of the countries of the world, it is the most frequently diagnosed cancer among men (1). Intraductal carcinoma of the prostate (IDC-P) is a biologically aggressive form of PCa that is characterized by the proliferation of malignant cells within prostatic ducts and acini (2, 3). Clinically, IDC-P has poor prognostic outcomes, including higher pathological stage, high Gleason score, larger tumor size, and a high risk of lymphatic metastasis. Moreover, compared to the conventional prostatic acinar adenocarcinoma (PAA), IDC-P has a high risk of clinical biochemical recurrence, metastasis, as well as a poorer progression-free survival and overall survival outcomes (4, 5). The current therapeutic options for IDC-P include neoadjuvant endocrine therapy, postoperative chemotherapy consolidation, and radiotherapy among others (4–6).
Pathologically, IDC-P is often accompanied by other types of prostate cancer (7–10), including prostate adenocarcinoma and squamous cell carcinoma of the prostate. In addition, it has highly comparable histological characteristics with high Gleason-classified prostate malignant tumors. Since it is difficult to directly diagnose IDP-C using a prostatic biopsy, it can easily be misdiagnosed clinically. Patients who are diagnosed with distant metastases or with serious underlying diseases often do not have the opportunity for surgeries (11, 12). As a result, determination of the pathology of many patients by paraffin sections is challenging. Therefore, an early and accurate diagnosis of IDC-P is crucial.
Hematological parameters include clinical indicators that are easily accessible. They can be used to detect the contents of various ions, sugars, lipids, proteins, various metabolites of enzymes, and hormones in the blood. These indices reflect the condition of the internal environment of the body. The relationship between blood biochemical parameters and IDC-P is yet to be elucidated (13–17). We constructed a predictive model that is based on biochemical indicators to assist in the diagnosis of IDC-P diagnosis.
Materials and methods
Study design and participants
This was a multi-center retrospective study involving 3185 PCa patients from three independent regional medical centers in China was conducted. Data for 2384 patients had been admitted to Zhejiang Provincial People’s Hospital from October 2012 to April 2022 were collected, labeled as the G cohort, and divided into two groups for construction of the nomogram (Ga cohort; n=1556) and for internal validation (Gb cohort; n=828).
In addition, data for 344 patients who had been admitted to Quzhou People’s Hospital from April 2014 to April 2022 and 430 patients who had been admitted to Jiaxing Second Hospital from June 2013 to April 2022 were collected and labeled as the I cohort, which was used for external validation. This study was approved by the institutional ethics committee(QT2022375; Ethics Approval of Quzhou People’s Hospital No. 77, 2022; JXEY-2022HXHZ040), Each participant was required to sign an informed consent before their involvement.
Baseline data collection and processing
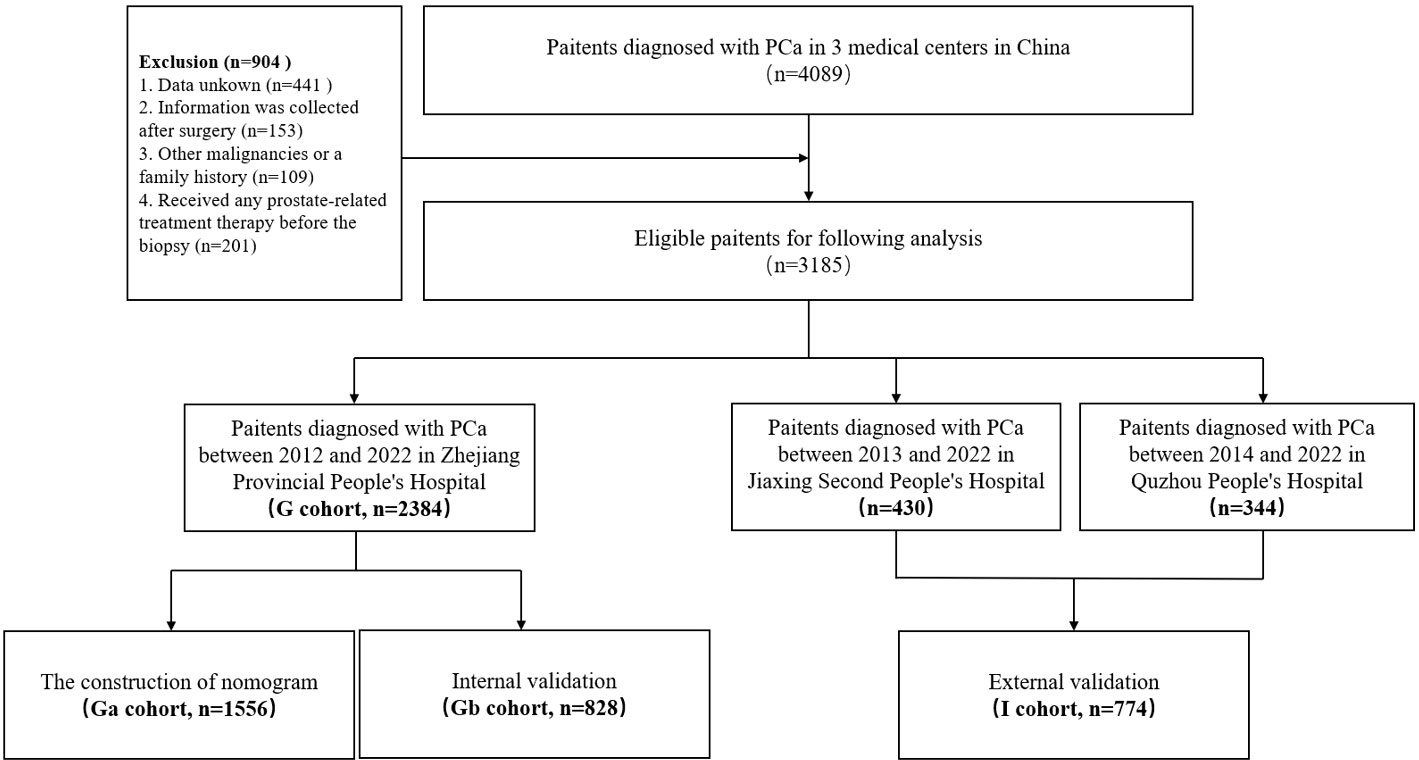
The baseline clinicopathologic information, including age, body mass index (BMI, kg/m2), personal history (smoking, drinking, heart disease, hypertension, and diabetes), biochemical indicators (albumin, globulin, glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, gamma-glutamyltransferase, alkaline phosphatase, total bilirubin, bile acids, glucse, urine creatinine, uric acid, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, lactate dehydrogenase), and prostate specific antigen (PSA) were collected. All patient information (including biochemical information) was sent to the Zhejiang Provincial People’s Hospital for reviewing and grouping (S-Table III). A set of inclusion and exclusion criteria was formulated to screen eligible patients in the three medical centers. (1) Only patients with PCa (confirmed by biopsy pathology and postoperative paraffin biopsy results). (2) Enrolled cases with complete baseline clinicopathologic information; patients with any missing values were excluded. (3) Patient information, apart from pathological information, were collected before surgery. (4) Patients with a history of other malignancies or a family history of PCa were excluded. (5) Patients who had not received any prostate-related treatment therapy before the biopsy (Figure 1).
Prostate biopsy and pathology
The final results were based on fndings from paraffin pathology. All disputed pathological results were sent to the Zhejiang Provincial People’s Hospital Pathological center for secondary pathological results review and immunohistochemical confirmation. Finally, patients whose pathological findings were not confirmatory were excluded from the study.
A positive result was defined as IDC-P or IDC-P accompanied by any other pathological type of PCa. Other pathological types of PCa, such as prostate adenocarcinoma and squamous cell carcinoma of the prostate, were considered negative outcomes.
Model construction, validation, and statistical analysis
The G cohort was divided into two cohorts: Ga cohort was used to construct the model, while the Gb was used for internal validation. The I cohort of patients from the two other centers was used for external validation.
Baseline characteristics of patients are presented as means ± standard deviation (SD), interquartile range (IQR), range, number, and proportions. Univariate binary logistic regression analysis method was performed to compare the different variables. Variables with p ≤ 0.05 were entered into the stepwise (forward: conditional) multivariate logistic regression analysis model. Then, the odds ratios (OR) and 95% confidence interval (95% CI) were calculated. Variables with p ≤ 0.05 in the multivariate analysis were used to establish the nomogram. The performance of the nomogram was validated using the Gb and I cohort via the bootstrap method. Discrimination and calibration were assessed for model validation, respectively (18). Discrimination was measured using C-statistics, which is equal to the area under the curve (AUC) calculated by plotting the receiver operating characteristic (ROC) curve (19). Calibration was measured by drawing calibration curves. Statistical analysis was performed using SPSS version 26.0 and R version 4.1.1. P ≤ 0.05 was the threshold for statistically significance.
Decision curve analysis (DCA)
In addition to providing urologists with a quantitative nomogram for diagnosis of IDC-P, a DCA curve was constructed to predict the probability of IDC-P. The clinical net benefits of our model at different threshold probabilities were quantified using the R software.
Results
Patient characteristics
The clinical characteristics for all study participants are shown in Table 1. A patient selection must strictly adhere to the above-mentioned inclusion and exclusion criteria. A total of 1556 and 828 patients were respectively included in the Ga and Gb cohorts while 774 patients were included in the I cohort respectively. The IDP-C positive rates for the Ga, Gb and I cohorts was 8.9%, 8.9% and 8.1%, respectively (Table 1). Standard values for all variables were defined by Zhejiang Provincial People’s Hospital, the main unit of all research centers, and are shown in the S-Table III. Based on the standard value, each variable is divided into high-group, standard group and low-group. In univariate analysis, the standard group was the control group while analyzing OR values (S-Table 3).
A comparison of between-group differences between the internal and external validation cohorts did not reveal significant differences (S-Table I).
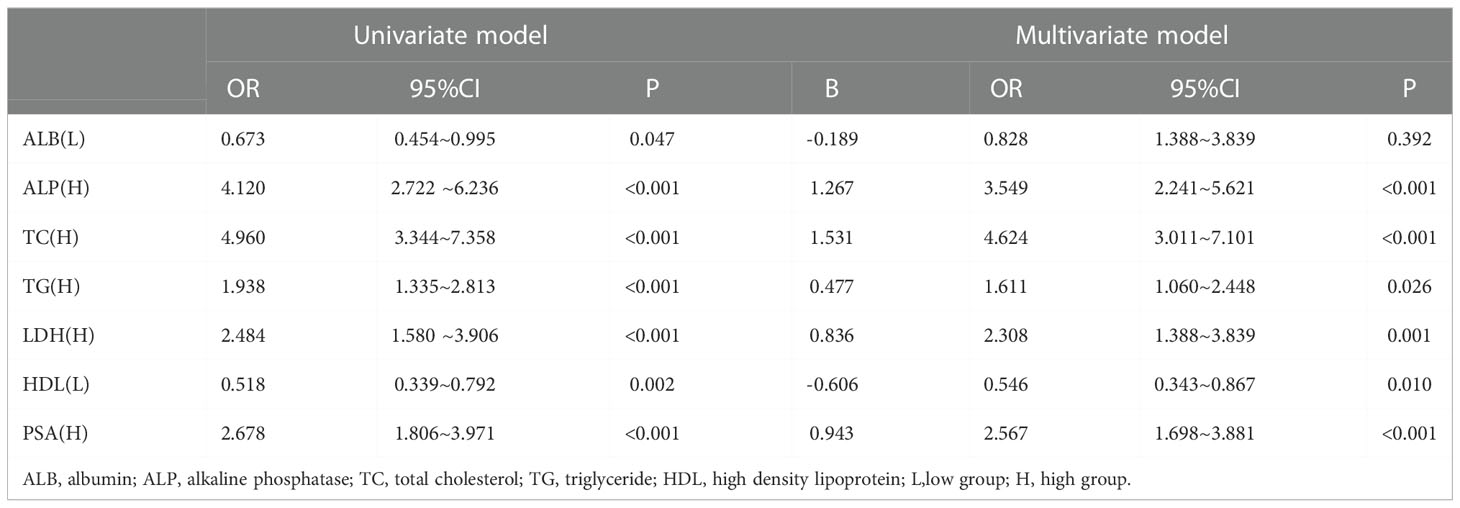
Establishment of the nomogram
Binary logistic regression analysis was performed to screen the predictors of PCa in the Ga cohort. Univariate analysis revealed that low ALB (OR: 0.637, 95% CI: 0.454~0.995; P = 0.047), high ALP (OR: 4.120, 95% CI: 2.722 ~6.236; P<0.001), high TC (OR: 4.960, 95% CI: 3.344~7.358; P<0.001), high TG (OR: 1.938, 95% CI: 1.335~2.813; P<0.001), high LDH (OR: 2.484, 95% CI: 1.580 ~3.906; P<0.001), low HDL (OR: 0.518, 95% CI: 0.339~0.792; P = 0.002), high LDH (OR: 2.484, 95% CI: 1.580~3.906; P<0.001) and high PSA (OR: 2.678, 95% CI: 1.806~3.971; P<0.001) were significantly associated with the diagnosis of IDC-P. (S-Table II) Variables that were significantly associated with diagnosis of IDC-P in univariate analysis were subjected to multivariate analysis. It was found that ALP, TC, TG, HDL, LDH and PSA were independent predictors of IDC-P (Table 2). Therefore, a predictive model containing ALP, TC, TG, HDL, LDH and PSA was established (Figure 2).
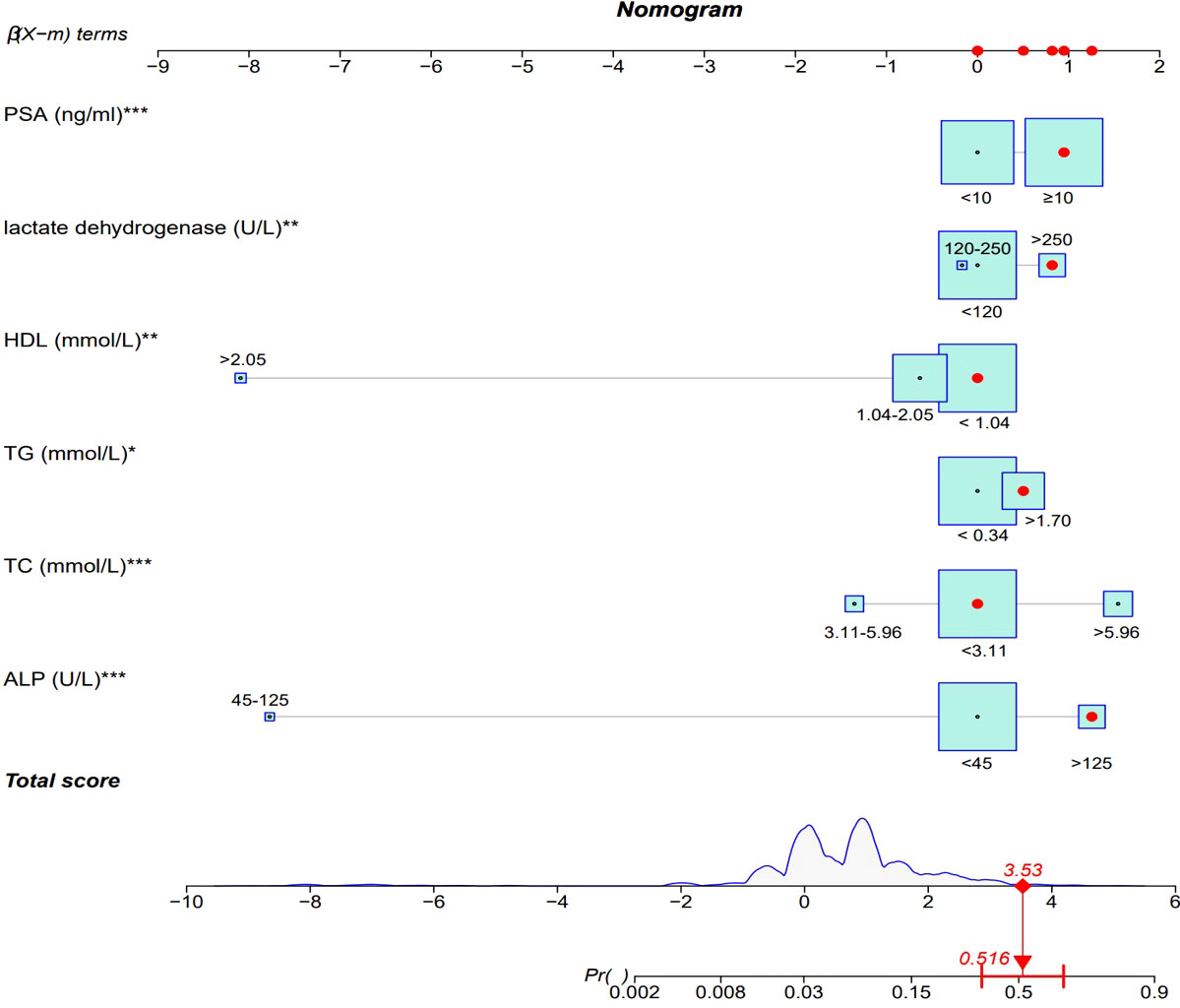
Figure 2 ALP, alkaline phosphatase; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; PSA, prostate specific antigen.
Model verification revealed that it had excellent reproducibility (AUC=0.794). Moreover, both internal and external validations revealed that the predictive nomogram had a good discriminative power (AUCGb = 0.819; AUCI=0.903). Calibration curves showed good agreements between the predicted and observed incidence of IDC-P in the three cohorts (Figure 3).
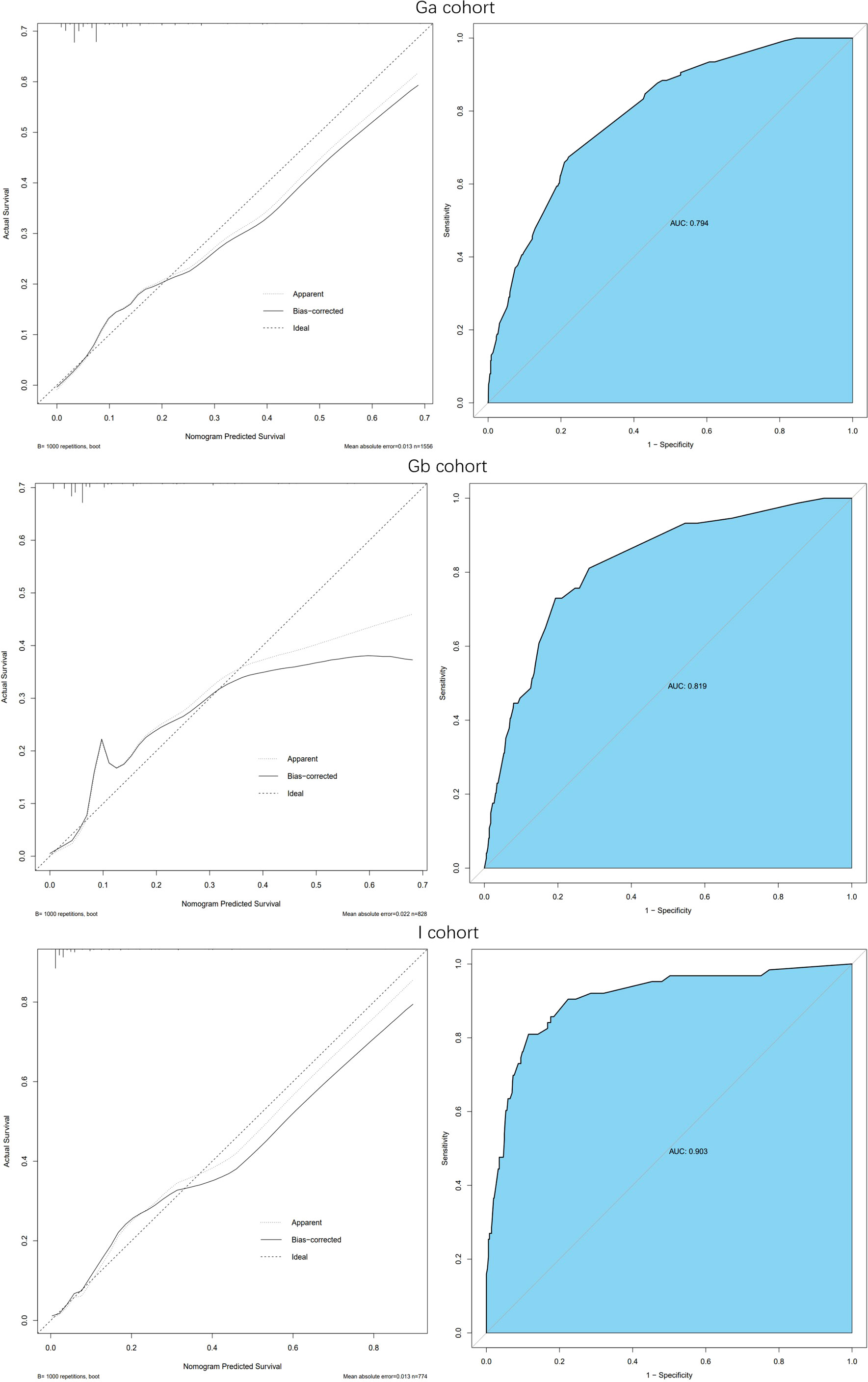
Figure 3 Calibration curve and ROC curve: Discrimination of the nomogram was evaluated by the ROC curve, AUC=0.794 in Ga cohort, AUC=0.819 in Gb cohort and AUC=0.903 in I cohort; Calibration curves illuminate the agreement between the predicted risks of IDP-C and the observed incidence of IDP-C. The dotted line represents an ideal flawless model.
Decision curve analysis
To assess the clinical usefulness of our nomogram, a DCA curve was drawn. The DCA curve showed that the detection rate of IDP-C patients was effectively increased by our prediction model, These findings imply that the model can be used for early diagnosis of IDC-P (Figure 4).
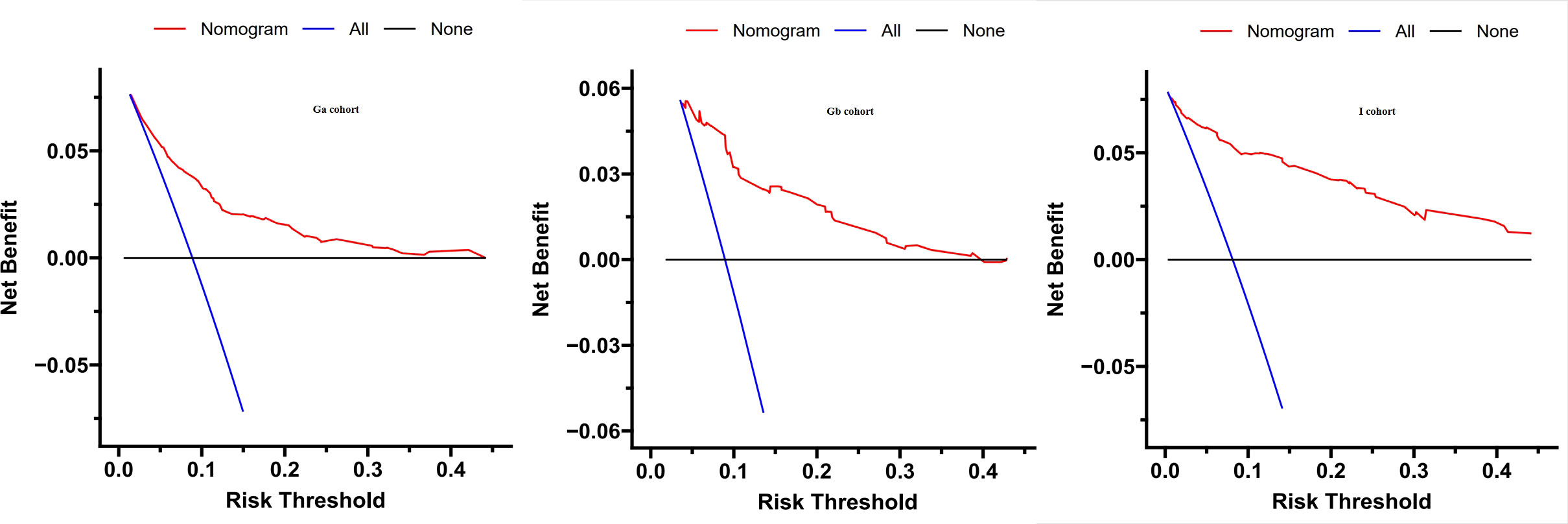
Figure 4 Decision curve analysis was prformed to estimate the clinical usefulness of the nomogram (IDP-C). The quantified net benefits were measured at different threshold probabilities. The y-axis denotes the standardized net benefit, while the x-axis denotes the threshold probabilities. The red line represents our nomogram, the blue line represents the condition that all patients have IDP-C, and the black line represents the condition that none has IDP-C.
Discussion
With almost 1.4 million new cases and 375,000 deaths worldwide, prostate cancer was the second most frequent cancer and the fifth leading cause of cancer-associated deaths among men in 2020 (1). Clinically, IDC-P is a biologically aggressive pathological form of PCa that has poor prognostic outcomes. However, IDC-P in the absence of other prostate cancer pathological types is rare on biopsy, 27 cases out of a denominator of approximately 45 000 prostate needle biopsy consults (0.06%) (20). Pathologically, IDC-P is often accompanied by prostatic acinar adenocarcinoma (PAA) (7–10).
Since the early signs and symptoms of IDC-P tend to hide and clinicians as well as pathologists lack detailed knowledge and understanding of the pathological and clinical aspects of the disease, the possibility of a missed or delayed diagnosis cannot be ruled out. The presence of IDC-P indicates a poor prognostic outcomes for PCa. Therefore, early detection of IDC-P and formulation of an effective treatment plan is of clinical significance (21–25).
Even though there are no clear treatment guidelines for IDC-P, for localized prostate cancer, whether IDC-P or other pathological types, radical prostatectomy is the best choice. However, patients with metastatic IDC-P, IDC-P in the mCRPC stage, or patients with IDC-P who have lost the opportunity for surgery are treated differently from patients with prostate adenocarcinoma. Transcriptome sequencing of tumor lesions from the same patient after neoadjuvant hormonal therapy (NHT) revealed that NHT treatment significantly inhibited AR pathway activities in adenocarcinoma, but had less effects on AR signaling activities in IDC-P. Therefore, IDC-P may be resistant to classical endocrine therapy, but is sensitive to abiraterone therapy (12, 26–28).
Compared with other pathological types of tumors, IDC-P has a higher HRD score, suggesting that IDC-P has molecular characteristics of homologous recombination deficiency. Thus, IDC-P patients are more likely to carry the BRCA2 gene or BRCA-like molecular alterations. These molecular characteristic changes suggest that PARP inhibitors, represented by olaparib, are among the therapeutic options for improving the clinical prognosis of IDC-P positive patients (29).
Given the different treatment options for IDC-P and prostate adenocarcinoma, early diagnosis of IDC-P is necessary. Various models have been developed to predict PCa outcomes in the Chinese population (30, 31). Recently, Chen et al. (32). constructed the Chinese Prostate Cancer Consortium Risk Calculator (CPCC-RC) that is based on prostate-specific antigen (PSA), age, prostate volume, free to total PSA (fPSA-to-tPSA) rate, and digital rectal examination (DRE) to predict the initial prostate biopsy. However, the prediction models for IDP-C have not been established.
In this study, the risk factors (high TC, high TG, and low HDL) are all biochemical indicators associated with obesity in our study. Therefore, we postulated that IDC-P may be associated with the obesity status of patients. Excess fat mass is characterized by low-level chronic inflammation, which results in abnormal secretion of adipokines, leading to disrupted immune responses and other metabolic irregularities (33). This state leads to decreased cancer cell apoptosis and increased cancer cell growth and migration. Thus, these multiple factors may work in tandem to create a favorable tumor microenvironment for PCa cell growth in the catheter (34). Obesity has also been associated with insulin and sex hormone levels as well as the insulin-like growth factor axis. Perhaps, in this microenvironment, PCa cells proliferate in the normal prostate catheter and transform to IDC-P (35).
This study has several limitations. First, since it involved three hospitals in Zhejiang Province, China, it may not be reproducible in less experienced medical centers or other countries. Secondly, several indicators, such as TMN stage and PI-RADS were not included due to the irretrievably missing values and variance in measuring instruments. Thirdly, although we followed a set of inclusive and exclusive criteria during data collection, bias was inevitable, especially due to the independence of each hospital, but this also shows that our model has good universality. Moreover, prospective validation is required forour model.
Conclusion
In summary, we established a clinical predictive nomogram consisting of ALP, TC, TG, HDL, LDH and PSA with excellent reproducibility and generalizability. This model provides a novel calculator for predicting the diagnosis of IDC-P and different treatment options for patients at an early stage.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies have been performed in accordance with the Declaration of Helsinki, and the participants were reviewed and approved by the Ethics Committee of Zhejiang Provincial People’s Hospital, Quzhou People’s Hospital and Jiaxing Second People’s Hospital. All study participants were informed about the planned procedure and signed informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Author contributions
Conceptualization, YY; Data curation, LW, ZT, and QZ; Methodology, YY; Project administration, YY; Visualization, YY and WZ; Writing – original draft, YY and WZ; Writing – review & editing, YY, YB, and DZ. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank for the contributions made to this study by all patients, their partners, and families as well as all nurses and doctors engaged at Zhejiang Provincial People’s Hospital, Quzhou People’s Hospital and Jiaxing Second People’s Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1074478/full#supplementary-material
Abbreviations
MI, Body Mass Index; CAD, Coronary Atherothrombotic Disease; PSA, Prostate specific antigen; ALB, albumin; GLB, Globulin; ALT, glutamic pyruvic transaminase; AST, glutamic oxaloacetic transaminase; GGT, Gamma-glutamyltransferase; ALP, Alkaline phosphatase; Tbil, total bilirubin; BA, bile acids; GLU, glucse; UC, urine Creatinine; TC, total cholesterol; TG, Triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDH, lactate dehydrogenase.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and Male genital organs-part b: Prostate and bladder tumours. Eur Urol (2016) 70(1):106–19. doi: 10.1016/j.eururo.2016.02.028
3. Szentirmai E, Giannico GA. Intraductal carcinoma of the prostate. Pathologica (2020) 112(1):17–24. doi: 10.32074/1591-951X-5-20
4. Kimura K, Tsuzuki T, Kato M, Saito AM, Sassa N, Ishida R, et al. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate (2014) 74(6):680–7. doi: 10.1002/pros.22786
5. Dinerman BF, Khani F, Golan R, Bernstein AN, Cosiano MF, Margolis DJ, et al. Population-based study of the incidence and survival for intraductal carcinoma of the prostate. Urol Oncol (2017) 35(12):673.e9–673.e14. doi: 10.1016/j.urolonc.2017.08.015
6. Watts K, Li J, Magi-Galluzzi C, Zhou M. Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study. Histopathology (2013) 63(4):574–9. doi: 10.1111/his.12198
7. Wilcox G, Soh S, Chakraborty S, Scardino PT, Wheeler TM. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum Pathol (1998) 29(10):1119–23. doi: 10.1016/S0046-8177(98)90423-3
8. Miyai K, Divatia MK, Shen SS, Miles BJ, Ayala AG, Ro JY. Heterogeneous clinicopathological features of intraductal carcinoma of the prostate: a comparison between “precursor-like” and “regular type” lesions. Int J Clin Exp Pathol (2014) 7(5):2518–26.
9. Robinson B, Magi-Galluzzi C, Zhou M. Intraductal carcinoma of the prostate. Arch Pathol Lab Med (2012) 136(4):418–25. doi: 10.5858/arpa.2011-0519-RA
10. Shah RB, Zhou M. Atypical cribriform lesions of the prostate: clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate. Adv Anat Pathol (2012) 19(4):270–8. doi: 10.1097/PAP.0b013e31825c6c0e
11. Trudel D, Downes MR, Sykes J, Kron KJ, Trachtenberg J, van der Kwast TH. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur J Cancer (2014) 50(9):1610–6. doi: 10.1016/j.ejca.2014.03.009
12. Zhao T, Liao B, Yao J, Liu J, Huang R, Shen P, et al. Is there any prognostic impact of intraductal carcinoma of prostate in initial diagnosed aggressively metastatic prostate cancer? Prostate (2015) 75(3):225–32. doi: 10.1002/pros.22906
13. Bhindi B, Locke J, Alibhai SMH, Kulkarni GS, Margel DS, Hamilton RJ, et al. Dissecting the association between metabolic syndrome and prostate cancer risk: analysis of a large clinical cohort. Eur Urol (2015) 67(1):64–70. doi: 10.1016/j.eururo.2014.01.040
14. Hayashi N, Matsushima M, Yamamoto T, Sasaki H, Takahashi H, Egawa S. The impact of hypertriglyceridemia on prostate cancer development in patients aged ≥60 years. BJU Int (2012) 109(4):515–9. doi: 10.1111/j.1464-410X.2011.10358.x
15. Morales R, Suarez C, Ropero J, Nunez I, Planas J, PlacerClaudia J, et al. The influence of metabolic syndrome on prostate cancer risk detection and its aggressiveness. JCO (2012) 30(5_suppl):226–6. doi: 10.1200/jco.2012.30.5_suppl.226
16. Morote J, Ropero J, Planas J, Bastarós JM, Delgado G, Placer J, et al. Metabolic syndrome increases the risk of aggressive prostate cancer detection. BJU Int (2013) 111(7):1031–6. doi: 10.1111/j.1464-410X.2012.11406.x
17. Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control (2011) 22(11):1545–52. doi: 10.1007/s10552-011-9831-7
18. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur Heart J (2014) 35(29):1925–31. doi: 10.1093/eurheartj/ehu207
19. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology (2010) 21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2
20. Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod Pathol (2006) 19(12):1528–35. doi: 10.1038/modpathol.3800702
21. Robinson BD, Epstein JI. Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings. J Urol (2010) 184(4):1328–33. doi: 10.1016/j.juro.2010.06.017
22. Chen X, Yang Y, Wang W, Han B, Qi M, Geng S, et al. Prognostic significance of the presence of intraductal carcinoma of the prostate and bone metastasis in needle biopsy for prostate carcinoma patients with grade group 5. Pathol Res Pract (2020) 216(1):152693. doi: 10.1016/j.prp.2019.152693
23. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE working group on poor ovarian response definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (2011) 26(7):1616–24. doi: 10.1093/humrep/der092
24. Porter LH, Lawrence MG, Ilic D, Clouston D, Bolton DM, Frydenberg M, et al. Systematic review links the prevalence of intraductal carcinoma of the prostate to prostate cancer risk categories. Eur Urol (2017) 72(4):492–5. doi: 10.1016/j.eururo.2017.03.013
25. Friis Wang N, Skouby SO, Humaidan P, Andersen CY. Response to ovulation trigger is correlated to late follicular phase progesterone levels: A hypothesis explaining reduced reproductive outcomes caused by increased late follicular progesterone rise. Hum Reprod (2019) 34(5):942–8. doi: 10.1093/humrep/dez023
26. Zhao J, Liu J, Sun G, Zhang M, Chen J, Shen P, et al. The prognostic value of the proportion and architectural patterns of intraductal carcinoma of the prostate in patients with De novo metastatic prostate cancer. J Urol (2019) 201(4):759–68. doi: 10.1016/j.juro.2018.10.016
27. Zhao J, Shen P, Sun G, Chen N, Liu J, Tang X, et al. The prognostic implication of intraductal carcinoma of the prostate in metastatic castration-resistant prostate cancer and its potential predictive value in those treated with docetaxel or abiraterone as first-line therapy. Oncotarget (2017) 8(33):55374–83. doi: 10.18632/oncotarget.19520
28. Wang Z, Zhu S, Zhao J, Nie L, Chen X, Zhang M, et al. The heterogeneity of intraductal carcinoma of the prostate is associated with different efficacy of standard first-line therapy for patients with metastatic castration-resistant prostate cancer. Prostate (2021) 81(15):1191–201. doi: 10.1002/pros.24215
29. Zhu S, Zhao J, Nie L, Yin W, Zhang Y, Zhao F, et al. Homologous recombination deficiency (HRD) score in aggressive prostatic adenocarcinoma with or without intraductal carcinoma of the prostate (IDC-p). BMC Med (2022) 20(1):237. doi: 10.1186/s12916-022-02430-0
30. Niu XK, He WF, Zhang Y, Das SK, Li J, Xiong Y, et al. Developing a new PI- RADS V2-based nomogram for forecasting high-grade prostate cancer. Clin Radiol (2017) 72(6):458–64. doi: 10.1016/j.crad.2016.12.005
31. Li M, Chen T, Zhao W, Wei C, Li X, Duan S, et al. Radiomics prediction model for the improved diagnosis of clinically significant prostate cancer on biparametric MRI. Quant Imaging Med Surg (2020) 10(2):368–79. doi: 10.21037/qims.2019.12.06
32. Chen R, Xie L, Xue W, Ye Z, Ma L, Gao X, et al. Development and external multicenter validation of Chinese prostate cancer consortium prostate cancer risk calculator for initial prostate biopsy. Urol Oncol (2016) 34(9):416.e1–7. doi: 10.1016/j.urolonc.2016.04.004
33. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol (2016) 11:421–49. doi: 10.1146/annurev-pathol-012615-044359
34. Adesunloye BA. Mechanistic insights into the link between obesity and prostate cancer. Int J Mol Sci (2021) 22(8):3935. doi: 10.3390/ijms22083935
Keywords: PCa, IDC-P, predictive nomogram, biochemical indicators, obesity
Citation: Yang Y, Zhang W, Wan L, Tang Z, Zhang Q, Bai Y and Zhang D (2022) Construction and validation of a clinical predictive nomogram for intraductal carcinoma of the prostate based on Chinese multicenter clinical data. Front. Oncol. 12:1074478. doi: 10.3389/fonc.2022.1074478
Received: 19 October 2022; Accepted: 30 November 2022;
Published: 15 December 2022.
Edited by:
Daniel Taussky, Université de Montréal, CanadaReviewed by:
Li Ding, The Affiliated Hospital of Xuzhou Medical University, ChinaYishuo Wu, Fudan University, China
Copyright © 2022 Yang, Zhang, Wan, Tang, Zhang, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: YuChen Bai, WURCWFk5OTc0QDE2My5jb20=; DaHong Zhang, WVlLWkRIWVlLQDE2My5jb20=
 YunKai Yang
YunKai Yang Wei Zhang
Wei Zhang LiJun Wan4
LiJun Wan4 YuChen Bai
YuChen Bai DaHong Zhang
DaHong Zhang