
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 05 January 2023
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1070838
This article is part of the Research TopicCase Reports in Genitourinary Oncology : 2022View all 38 articles
 Marina Valeri1,2
Marina Valeri1,2 Leocadia Dore1,2
Leocadia Dore1,2 Noemi Rudini2
Noemi Rudini2 Miriam Cieri2
Miriam Cieri2 Grazia Maria Elefante2
Grazia Maria Elefante2 Alberto Saita3
Alberto Saita3 Alexia Bertuzzi4
Alexia Bertuzzi4 Piergiuseppe Colombo1,2*
Piergiuseppe Colombo1,2*Ewing sarcoma (ES) is the second most common malignant bone tumor in children and has also been described in adults with highly aggressive behavior. ES belongs to the small round blue cell tumor family and presents the distinctive translocation of FET-ETS family genes (85% with EWSR1), generating gene fusions. Extraskeletal ES mainly occurs in soft tissues; the urogenital tract is rarely affected, and ureteral localization is an exceptional event with only 4 cases described in the literature. Here we report the first Italian case of primary ES of the ureter, a 24-year-old young man with lower back pain and a narrowed left ureteral lumen on CT scan. ES of the urogenital tract is an almost unique condition with a nonspecific clinical presentation and a challenging diagnosis for pathologists. We encourage awareness of these exceptional events in the differential diagnosis of ureteral lesions in young patients.
Ewing’s sarcoma (ES) is a malignant neoplasm with highly aggressive behavior (1). It is the second most common malignant bone tumor in children, but less frequent cases have been described in older patients, often affecting extraskeletal sites (2).
ES is characterized and defined by the presence of non-random chromosomal translocations—detectable by fluorescence in situ hybridization (FISH)—producing fusion genes that involve one member of the FET family of genes and a member of the E26 transformation-specific (ETS) family (EWSR1–FLI1 in 85%–90% of cases), encoding aberrant transcription factors (3). Histologically, classical ES consists of a proliferation of uniformly small round cells with inconspicuous nucleoli and scant clear to pale cytoplasm, with a sheet-like growth pattern (4). Tumor cells usually show strong and diffuse expression of CD99 and NKX2.2 (5). The current management of ES includes neoadjuvant chemotherapy followed by surgical resection and/or radiotherapy and adjuvant chemotherapy. Systemic treatment is usually composed of multiagent chemotherapy cycles of vincristine, doxorubicin, and cyclophosphamide (VDC) alternating with cycles of ifosfamide and etoposide (IE) (6).
ES usually affects bone, but it can also localize in extraskeletal sites, most commonly in soft tissues (12% of cases) (1). Urogenital tract involvement is a rare event, most frequently arising in the kidney, followed by the bladder and the prostate (7–11). As for the ureter, a primary ES is an exceptional finding: to our knowledge, there are no more than four cases described in the literature (12–15).
Here we report the fifth case of primary ureteral ES and the first in Italy as a unique manifestation of the disease.
A 24-year-old male presented to our institution with a 6-month history of left lower back pain. Five years before, he underwent surgery for varicocele and had a history of prostatitis. No relevant family medical history was documented. Abdominal US and CT scans of the urological tract with contrast medium were performed with the finding of a narrowed left ureteral lumen and ipsilateral hydronephrosis (Figure 1). Cytology was negative for malignancy, and subsequent diagnostic ureteroscopy revealed a polypoid fibrotic lesion, but a biopsy was not feasible. To resolve the obstruction and understand the nature of the lesion, the patient underwent a left distal segmental ureterectomy with intraoperative evaluation of the specimen.
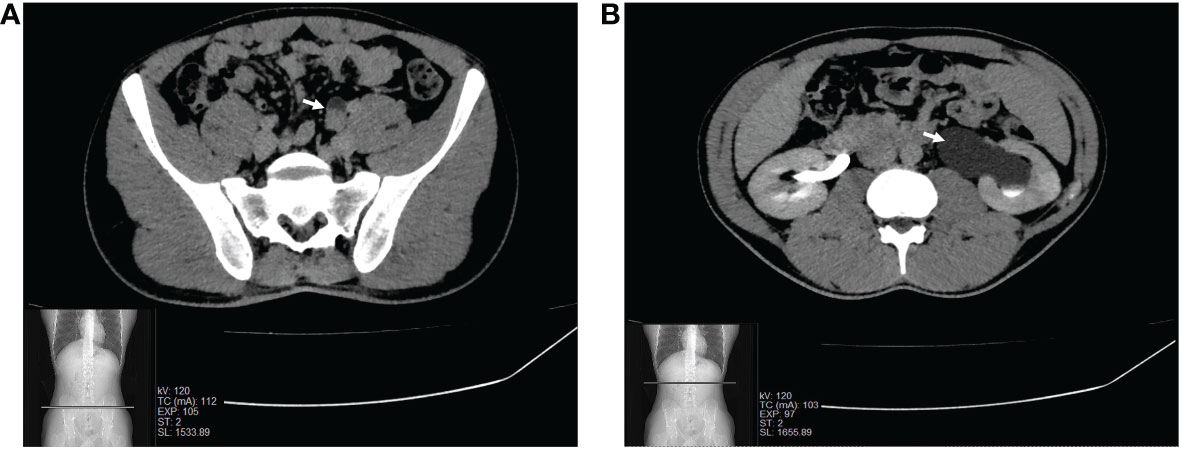
Figure 1 CT scan of the urological tract scan showing the narrowed lumen of the left pelvic ureter (A) and ipsilateral hydronephrosis (B).
Frozen sections showed a proliferation of round to ovoid epithelioid cells, arranged in solid sheets, with nuclear atypia and eosinophilic cytoplasm (Figure 2A). These findings, together with the clinical presentation, initially suggested a urothelial carcinoma. Therefore, robot-assisted uretero-ureteral termino-terminal anastomosis and ureteral double-J stenting were performed.
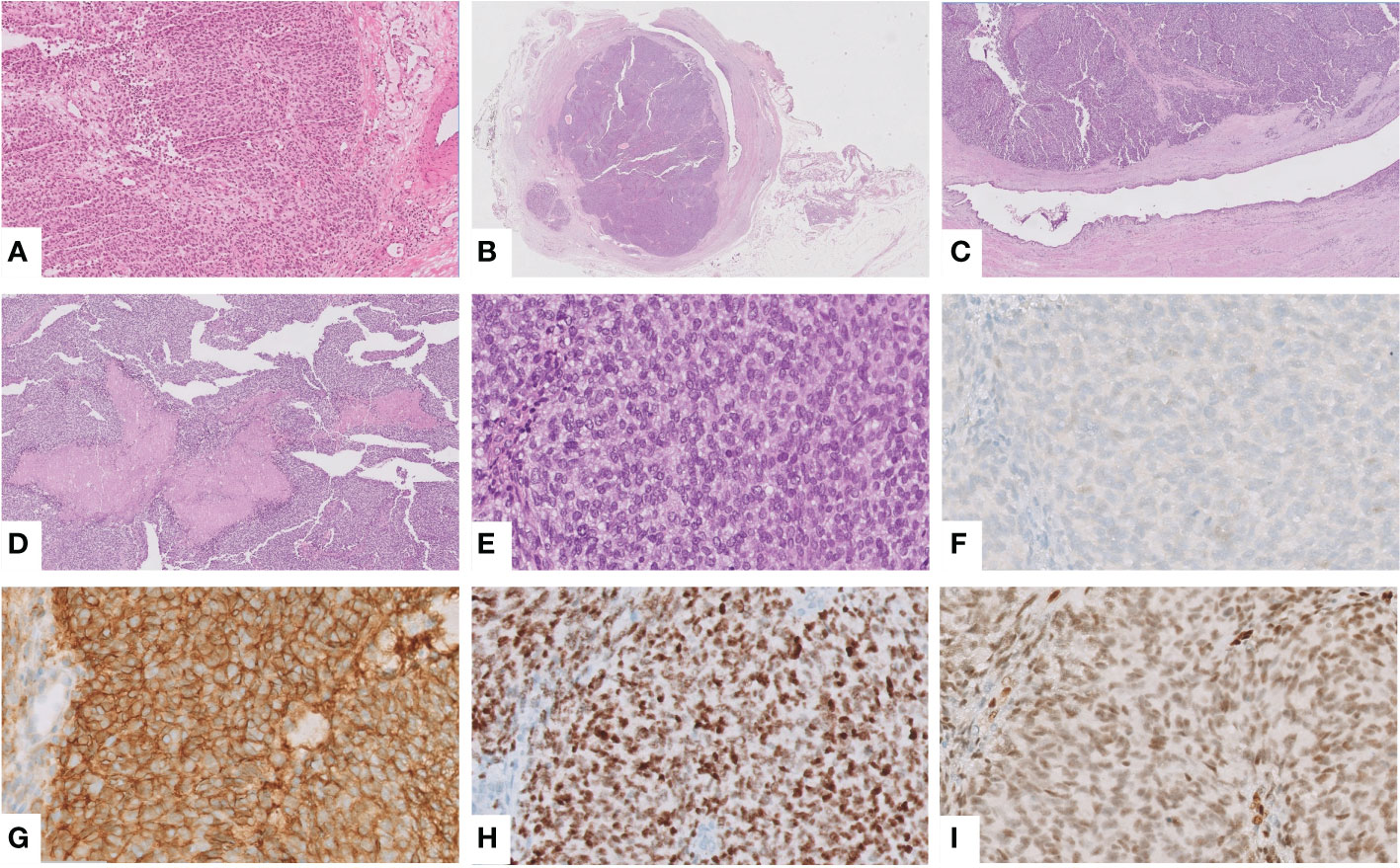
Figure 2 Intraoperative frozen section showing a solid proliferation of round to ovoid epithelioid cells, with atypical nuclei and eosinophilic cytoplasm, suggesting a urothelial carcinoma (hematoxylin and eosin (H&E), ×10) (A). An obstructing mass was found in the ureteral wall (H&E, ×0.25) (B), which corresponded to a solid growth with spared overlining urothelium (H&E, ×2) (C) and foci of necrosis (H&E, ×5) (D). Notably, the tumor was composed of round to spindle, monotonous, small cells with the peculiar intracytoplasmic glycogen (H&E, ×40) (E). Immunohistochemically, the tumor was negative for GATA3 (F) but showed the characteristic positivity for CD99 (G), NKX2.2 (H) and FLI1, the suspected and most common partner of translocation, confirmed at NGS analysis (I).
Grossly, an obstructing grayish mass of 2 cm was found in the ureteral wall. Histological examination demonstrated solid growth of round to spindle cells, monotonous, small-cell malignant proliferation, and foci of necrosis. Remarkably, the overlying urothelium was spared (Figures 2B–E). To confirm the urothelial nature of the neoplasm, an immunohistochemical stain for GATA3 was performed, with a negative result (Figure 2F). These features prompted us to consider a non-urothelial tumor and to rule out small, round, blue-cell tumors. Indeed, an epithelial neoplasm was excluded by immunonegativity for panCK; tumor cells were positive for CD99, FLI1, NKX2.2, synaptophysin (only focal), and negative for myogenin, ERG, and WT1 (Figures 2G–I). To detect EWSR1 translocation, FISH was performed on 4-mm-thick formalin-fixed, paraffin-embedded (FFPE) tumor sections. Vysis LSI EWSR1 (22q12) Dual Color Break Apart Rearrangement Probe (Abbott Molecular, Abbott Park, IL), a mixture of two probes that map the 5’ side of the EWSR1 gene (labeled in SpectrumOrange) and the 3’ side of the EWSR1 gene (labeled in SpectrumGreen), has been used. Signals were evaluated in at least 100 tumor nuclei per specimen. EWSR1 translocation was detected in 80% of cells in the tumor area (Figure 3A). Eventually, primary ureteral ES was diagnosed. Subsequently, Archer Pan Solid Tumor v2 next-generation sequence analysis (NGS) was performed. Archer Data Analysis Software 6.0 confirmed FLI-1 as the translocation partner (Figure 3B).

Figure 3 FISH with an EWSR1 Dual Color Break Apart Rearrangement Probe detected EWSR1 translocation in 80% of tumor cells as orange and green spitting signals (white arrows) (A). Pan Solid Tumor v2 NGS analysis revealed the gene fusion involving exon 7 of EWSR1 and exon 6 of FLI, detected by a gene specific primer (GSP2) specific for EWSR1 (B).
Post-surgical CT total body and PET scan did not show any distant metastases. After a definitive diagnosis and multidisciplinary tumor board, systemic multiagent adjuvant chemotherapy was administered with four cycles of vincristine (2 mg), doxorubicin (37.5 mg/m2), and cyclophosphamide (600 mg/m2) (VDC) alternating with five cycles of ifosfamide (3 g/m2) and etoposide (150 mg/m2) (IE). Subsequently, the patient underwent radicalization surgery with a partial ureterectomy and surrounding soft tissue resection, without residual tumor. After 15 months of follow-up, the patient was alive with no sign of recurrence or metastatic disease on CT total body and with programmed maintenance chemotherapy (VC×3/IE×2).
A temporal timeline of the most relevant events is depicted in Figure 4.
ES is a small round cell sarcoma characterized by FET-ETS family gene translocations (EWSR1–FLI1 in 85%–90% of cases) (2). It is an aggressive disease usually treated with surgical excision preceded by neoadjuvant CT and followed by adjuvant CT (6).
ES usually affects the bone of children and young adults, but it can also arise in older patients and localize in extraskeletal sites, mainly in soft tissues (1). A review of the English-language literature revealed that the urogenital tract is rarely involved: the most common site is the kidney, with almost 200 cases described (7, 8), followed by the bladder (9), and the prostate (10, 11). Ureteral localization is an exceptional finding, with only four cases of primary ES of the ureter previously reported (12–15).
Table 1 summarizes the patients’ clinical features. As for the literature cases, two patients were males, and the median age was 39.5 years. All patients had clinical symptoms as the initial presentation of the disease. Hematuria and flank pain were the most frequent symptoms (3/4), but only in two cases where they present simultaneously. Indeed, our patient presented with lower back pain without hematuria. All patients underwent surgery, and three received adjuvant systemic therapy, like in our case. All tumors showed typical features of ES. Similarly, our case presented morphological features of the classic ES and also the characteristic immunohistochemical profile with positivity for CD99, NKX2.2, FLI1, and synaptophysin (4, 5). Our Archer Pan Solid Tumor v2 NGS analysis revealed a gene fusion involving exon 7 of EWSR1 and exon 6 of FLI, in keeping with the literature. Indeed, the most common breakpoints are exons 7 and 6 of EWSR1 and exons 7 and 5 of FLI1 (3). Interestingly, the EWSR1–FLI1 fusion was identified only in one other patient by RT-PCR (12). Only one patient had a fatal outcome. This patient was a 45-year-old male with a deferred diagnosis of a recurrent lesion of the ureter treated with segmental ureteral resection and partial cystectomy. After 7 years, he experienced pelvic recurrence and progression to metastatic disease regardless of adjuvant chemotherapy with three courses of vincristine, adriamycin, cyclophosphamide (VAC), and IE (13).
In a clinical scenario with a young patient presenting with non-specific symptoms and a ureteral mass, in the absence of other primitive tumors, like in our case, the differential diagnosis should comprise a localization of either a germ cell tumor or a lymphoma. Blood markers and imaging to detect abdominal lymphadenopathy might be helpful in this setting. For our patient, the CT scan excluded further abdominal lesions, while blood markers were not tested.
Although less frequent, a retroperitoneal soft tissue tumor should also be ruled out.
In the suspected case of primary urothelial carcinoma of the ureter, our patient underwent surgery without a previous diagnosis. This is because the lesion could not be biopsied during diagnostic ureteroscopy due to lumen fibrotic obstruction. Therefore, an intraoperative evaluation was performed on the partial ureterectomy specimen. Frozen sections were somehow misleading since they suggested a urothelial neoplasm. Indeed, the tumor showed a more epithelioid morphology with round to ovoid cells with eosinophilic cytoplasm, growing in solid sheets with a fair degree of atypia. The urothelial nature was also consistent with site and clinical presentation, although in a young patient. The results of the intraoperative evaluation prompted the urologist to perform an uretero-ureteral anastomosis without radicalization.
The clinical management after definitive diagnosis was challenging and required several multidisciplinary tumor boards to decide for a completion surgery and adjuvant chemotherapy. In daily practice, a preoperative diagnosis of ES is of paramount importance to the oncologist since these tumors—of any site—undergo standardized protocols with preoperative neoadjuvant chemotherapy (6). Indeed, the difficulty in obtaining adequate diagnostic material during ureteroscopy in our case had an actual impact.
Remarkably, due to the lack of a preoperative histological diagnosis, three of the four previously reported cases received chemotherapy only after surgery (12–14). In one patient, a 69-year-old woman, systemic therapy was not administered due to age and impaired cardiopulmonary function (15).
To the best of our knowledge, this is the fifth reported case of primary ureteral ES and the first in Italy. ES of the urogenital tract is an exceptional condition with nonspecific clinical presentation and a challenging diagnosis, with a possible impact on oncological management due to the common lack of preoperative histological information precluding neoadjuvant treatment protocols. We encourage awareness of these exceptional events in the differential diagnosis of ureteral lesions in young patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
MV and PC conceived, designed, wrote, and revised the final manuscript. LD reviewed the literature, provided figures and tables, and revised the final manuscript. MC and GE reviewed the histological slides, critically revised the work, and approved the final manuscript. NR performed molecular analyses. AS performed surgery. AB followed the patient and provided clinical data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Grünewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, De Álava E, Kovar H, et al. Ewing Sarcoma. Nat Rev Dis Prim (2018) 4(1):5. doi: 10.1038/s41572-018-0003-x
2. WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours. In: Lyon (France): International agency for research on cancer (2020). Lyon: International Agency for Research on Cancer (IARC).
3. Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature (1992) 359:162–5. doi: 10.1038/359162a0
4. Llombart-Bosch A, Machado I, Navarro S, Bertoni F, Bacchini P, Alberghini M, et al. Histological heterogeneity of ewing’s sarcoma/PNET: An immunohistochemical analysis of 415 genetically confirmed cases with clinical support. Virchows Arch (2009) 455(5):397–411. doi: 10.1007/s00428-009-0842-7
5. Machado I, Yoshida A, Morales MGN, Abrahão-Machado LF, Navarro S, Cruz J, et al. Review with novel markers facilitates precise categorization of 41 cases of diagnostically challenging, “undifferentiated small round cell tumors”. a clinicopathologic, immunophenotypic and molecular analysis. Ann Diagn Pathol (2018) 34:1–12. doi: 10.1016/j.anndiagpath.2017.11.011
6. Gaspar N, Hawkins DS, Dirksen U, Lewis IJ, Ferrari S, Le Deley MC, et al. Ewing Sarcoma: Current management and future approaches through collaboration. J Clin Oncol (2015) 33(27):3036–46. doi: 10.1200/JCO.2014.59.5256
7. Liang L, Song H, Ma B, Zhang Z, Zhu K, Li Q, et al. Renal ewing’s sarcoma/primitive neuroectodermal tumor (PNET): A case series of 7 patients and literature review. Transl Androl Urol (2021) 10(2):548–54. doi: 10.21037/tau-20-1122
8. Patra S, Trivedi P. Primary Ewing sarcoma of the kidney: A series of four cases. Malays J Pathol (2022) 44(1):93–9.
9. Nakdali Kassab B, Pérez-Seoane Ballester H, Sarrió Sanz P, Sánchez Caballero L, Gómez Garberí M, Ortiz Gorraiz M. Ewing-Like sarcoma bladder primary tumour: A case report and literature review. Urol Case Rep (2022) 44(June):19–22. doi: 10.1016/j.eucr.2022.102139
10. Esch L, Barski D, Bug R, Otto T. Prostatic sarcoma of the Ewing family in a 33-year-old male - a case report and review of the literature. Asian J Urol (2016) 3(2):103–6. doi: 10.1016/j.ajur.2015.11.007
11. Borges da Ponte C, Leitão TP, Miranda M, Polido J, Alvim C, Fernandes I, et al. Prostate Ewing Sarcoma/PNET: A case of long survival in a highly aggressive malignancy. Urology (2021) 154:e11–2. doi: 10.1016/j.urology.2021.05.006
12. Charny CK, Glick RD, Genega EM, Meyers PA, Reuter VE, La Quaglia MP. Ewing’s sarcoma/primitive neuroectodermal tumor of the ureter: A case report and review of the literature. J Pediatr Surg (2000) 35(9):1356–8. doi: 10.1053/jpsu.2000.9333
13. Huang KH, Shun CT, Huang SY, Yu HJ, Chueh SC, Chen J. Primary primitive neuroectodermal tumor of the urinary tract. J Formos Med Assoc (2006) 105(12):1008–12. doi: 10.1016/S0929-6646(09)60285-0
14. Song HC, Sun N, Zhang WP, Huang CR. Primary ewing’s sarcoma/primitive neuroectodermal tumor of the urogenital tract in children. Chin Med J (Engl) (2012) 125(5):932–6. doi: 10.3760/cma.j.issn.0366-6999.2012.05.035
Keywords: Ewing sarcoma, ureter, extraskeletal ES, EWSR1–FLI1, rare tumor, case report
Citation: Valeri M, Dore L, Rudini N, Cieri M, Elefante GM, Saita A, Bertuzzi A and Colombo P (2023) Case report: Primary Ewing sarcoma of the ureter, an exceptional finding of unique manifestation of disease. Front. Oncol. 12:1070838. doi: 10.3389/fonc.2022.1070838
Received: 15 October 2022; Accepted: 12 December 2022;
Published: 05 January 2023.
Edited by:
Haoran Liu, Stanford University, United StatesReviewed by:
Colin Moore, Moffitt Cancer Center, United StatesCopyright © 2023 Valeri, Dore, Rudini, Cieri, Elefante, Saita, Bertuzzi and Colombo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piergiuseppe Colombo, cGllcmdpdXNlcHBlLmNvbG9tYm9AaHVuaW1lZC5ldQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.