- Department of Dermatology and National Center for Tumor Diseases (NCT), University Hospital Heidelberg, Heidelberg, Germany
The liver is the most preferential initial site of metastasis for uveal melanoma (mUM), and this preference is associated with rapid mortality in mUM patients. Despite the significant clinical benefits of Immune checkpoint inhibitors (ICIs) in metastatic cutaneous melanoma patients, ICIs have shown little to no benefit in mUM patients. A potential reason for this inefficiency of ICI could be partly devoted to the involvement of the liver itself, thanks to its rich source of growth factors and immunosuppressive microenvironment. Uveal melanoma cells show increased expression of a transmembrane protein called cMET, which is known as the sole receptor for the Hepatocyte growth factor (HGF). Hyperactivation of cMET by HGF contributes to mUM development, and the liver, being the major source of HGF, may partially explain the metastasis of uveal melanoma cells to the liver. In addition, cMET/HGF signaling has also been shown to mediate resistance to ICI treatment, directly and indirectly, involving tumor and immune cell populations. Therefore, targeting the cMET/HGF interaction may enhance the efficacy of immunotherapeutic regimes for mUM patients. Hence in this minireview, we will discuss the rationale for combining cMET inhibitors/antibodies with leading immune checkpoint inhibitors for treating mUM. We will also briefly highlight the challenges and opportunities in targeting cMET in mUM.
1. Introduction
Melanocytes have functional roles in light absorption, regulation of oxidative stress, and the immune system (1). Compared to skin melanocytes, melanocytes in the eye evolved with distinct biologic characteristics, gene expression profiles, and immune regulatory pathways (2–6). Uveal melanoma (UM) is a rare but deadliest cancer that develops from the melanocytes in the uveal tract of the eye (7). The most intriguing features of UM include 90% of tumors harboring a mutation in GNA11 and GNAQ genes and its extraordinary preference to spread and colonize in the liver. As a result, approximately 90-95% of metastatic UM tumors will develop liver metastasis (LmUM), unlike metastatic cutaneous melanoma, which is only observed in approximately 20% of patients clinically (8–10). This preference is not only associated with rapid mortality with a survival expectancy of less than a year but also confers resistance to many anti-cancer therapies, including immunotherapy (11). Immunotherapy with immune checkpoint inhibitors (ICIs) such as anti-cytotoxic T-lymphocyte associated protein 4 (anti-CTLA4) and anti-programmed cell death protein 1 (anti-PD1) reverse the exhausted anti-tumor immune cell responses and has given a tremendous hope in terms of survival for metastatic cutaneous melanoma (mCM) patients in the clinic (12, 13). However, the same cannot be stated for metastatic uveal melanoma (mUM) patients (14). This inefficiency can be partly due to the involvement of the liver itself, as the ICI treatment has also been shown to be less efficient in mCM patients with liver metastasis (15, 16).
The preferential spreading of UM to the liver cannot be explained just by the anatomical connection but by biological attraction and immunosuppressive microenvironment of the liver that can protect the UM growth in this distant organ once spread. Molecular communication seems pivotal as UMs overexpress specific protein receptors for which the growth factors derived from the liver may serve as ligands, hence acting as chemoattractants that direct UM to the liver (17). Moreover, as the liver interacts with various foreign antigens, it tightly regulates immune responses to avoid unwanted immune reactions, which UM can exploit to promote metastasis (18). In vivo, the CD27+CD11b- immature natural killer cells in the liver were shown to promote the development of melanoma metastasis to the liver (19). Reduced infiltration of PD1+ and CTLA4+ T cells was observed in mice with liver metastasized melanoma compared to mice with only subcutaneous melanoma, suggesting that liver metastasis may also regulate systemic immune responses (20). Besides, direct evidence of tumors from melanoma patients with liver metastases has revealed reduced infiltration of CD8+ T cells and PD1+ T cells but increased infiltrations of TIM3+ T cells. Together these data suggest that tumors metastasized to the liver may experience a unique microenvironment (21). Throughout this process, strategies targeting immune suppression with ICIs alone seem insufficient for handling mUM; hence we may have to explore and evolve treatment combinations that can potentially target tumor signaling pathways involved in LmUM development along with immune-enhancing regimes. In this regard, the hepatocyte growth factor receptor (cMET) signaling pathway has been extensively studied and reviewed for its role in developing LmUM and may potentially enhance ICI treatment.
cMET is a transmembrane protein composed of α and β subunits connected by disulfide bonds at the N-terminus. The α subunit remains extracellular, whereas the membrane-spanning β subunit consists of extracellular, transmembrane, and an intracellular tyrosine kinase domain at the C-terminus (22). It is usually expressed in a wide range of cells, including immune cells such as neutrophils, and is involved in cellular processes like survival and migration. So far, there are four ligands reported for the cMET receptor, hepatokines such as hepatocyte growth factor (HGF), leukocyte cell-derived chemotaxin-2 (LECT2), decorin, a small leucine-rich proteoglycan, and a bacterial surface protein called Internalin B (IntlB) from Listeria Monocytogenes which is needed for pathogen entry into the host cell (23–26). All four proteins are known to bind and interact with the extracellular region of cMET but with different binding affinities. The binding of HGF or IntlB results in cMET phosphorylation and activation of signaling pathways that regulate cell proliferation, epithelial-to-mesenchymal transition (EMT), and anti-apoptotic effects. In contrast, LECT2 and decorins can counter the HGF and Intl B signaling (23, 26). For example, LECT 2 binding inhibits cMET phosphorylation and Raf1/ERK signaling, which is responsible for EMT transition, and its absence promotes EMT in tumoral hepatocytes (27, 28). Similarly, the binding of cMET to decorins expressed in most extracellular matrices suppresses intracellular beta-catenin levels and inhibits cMET-mediated cell migration and growth (29). cMET/decorin binding also promotes rapid intracellular degradation of cMET via the recruitment of the E3 ubiquitin ligase c-Cbl (25).
Meanwhile, considering its significant role in cell proliferation and migration, it is not surprising to see cMET upregulation in cancer cells, but interestingly the threshold of its overexpression is way higher in mUM compared to other tumors, including mCM (30). Loss of cMET negative regulators, gene amplification, or germline mutations in exon 14 of cMET may result in such altered gene expression in tumors (31–33). The high-affinity binding of liver-derived HGF ligand to cMET can drive LmUM (23). In vitro, cMET/HGF signaling induces FAK/MAPK/STAT signaling pathways and regulates tumor cell growth invasion. cMET/HGF signaling also induces AKT/mTOR signaling pathways that activate E3 ubiquitin ligase MDM2, inhibiting apoptosis. Whereas cMET inhibition or downregulation of cMET suppresses UM proliferation and migration by inhibiting these pathways in vitro and in vivo (34). Consistent with its role in migration, liver metastatic lesions from UM patients have shown increased expression of cMET compared to primary tumors (35). In addition, over-expression of cMET correlates with high-risk parameters in UM and indicates a poor prognosis (36). cMET signaling is also involved in tumor resistance to many cancer treatments (37–40). Therefore, targeting cMET is a promising area of cancer drug development, and cMET targeting approaches are currently being investigated in clinical trials for various cancers, including mUM.
2. cMET inhibitors in clinical trials for advanced cancer patients, including mUM
So far, many strategies to target cMET, including immunotherapeutic approaches such as cMET-specific CAR T cells, cMET neutralizing antibodies, and small molecule inhibitors, are already being tested in clinical trials of solid tumors (Figure 1). Some have already demonstrated impressive therapeutic efficacy in the clinical setting. Intra- tumoral injection of cMET targeting CAR T cells was well tolerated in breast cancer patients, resulting in tumor necrosis and loss of cMET immunoreactivity (41). At the same time, a wide range of cMET small molecule inhibitors are currently being investigated in clinical trials for several cancers, including mUM. cMET inhibitors can be classified as selective inhibitors that only target cMET and non-selective inhibitors that target cMET along with other pathways such as VEGF, ALK, etc. Both have shown significant anti-tumor activity in a wide range of cancers and manageable safety profiles in the clinic. Tepotinib, a highly selective oral cMET inhibitor, was well tolerated, has shown promising efficacy in advanced hepatocellular carcinoma patients with cMET overexpression, and is currently being investigated for other cMET-deregulated cancers (42, 43). Tivantinib, another selective cMET inhibitor in combination with Sorafenib, a multi-kinase inhibitor, has also shown promising results in clinical trials of cancer patients, including advanced melanoma with a 63% of disease control rate. Interestingly, in this study, cMET overexpression was noted in only 29% of patients, and all patients with increased cMET expression have been shown to respond to the treatment (44). Meanwhile, two clinical trials were registered or are currently ongoing with cMET inhibitors for mUM. Among them, a phase II clinical trial including heavily pre-treated mUM patients who received a combination of Crizotinib, a non-selective cMET inhibitor, and Darovasertib has shown to achieve a 100% disease control rate and 31% response rate according to the preliminary reports from the investigators (45). Treatment-related adverse events (AEs), including 54% of grade 1 or 2 and 27% of patients with grade 3, were reported. In line with these findings, a reanalysis of a discontinued phase 2 trial revealed that cabozantinib, a multi-kinase inhibitor that targets cMET along with AXL, and VEGFR2, displayed anti-tumor activity with 61% disease control rate and a median PFS of 4.8 months in mUM patients (46). Although the current clinical trials targeting cMET are conducted mostly in combination with other kinase inhibitors making it hard to interpret the sole efficacy of cMET inhibition, collectively, these data suggest that cMET inhibitors are safe and can show anti-tumor activity in mUM patients.
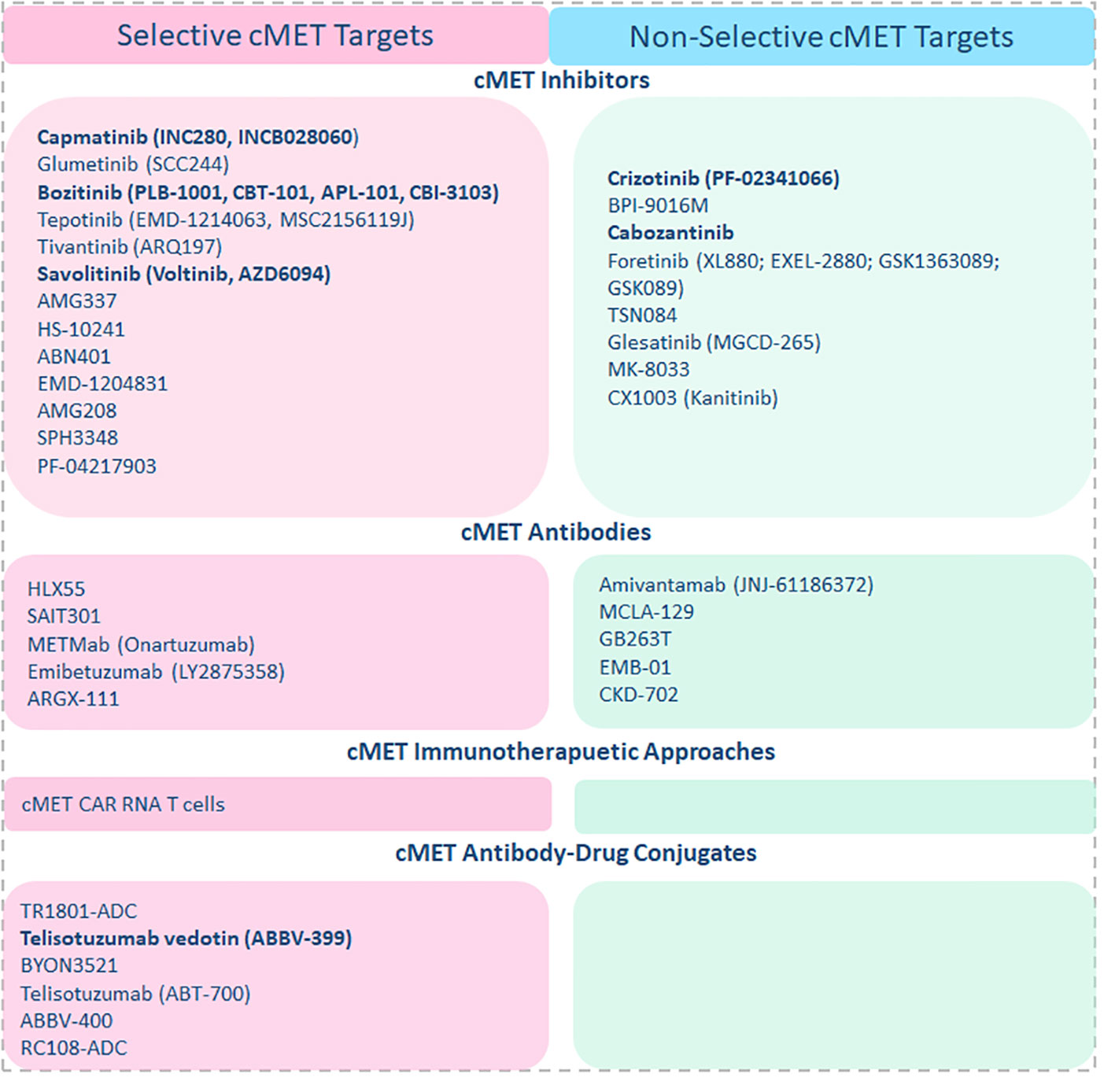
Figure 1 cMET-targeting approaches and agents in clinical trials are listed accordingly, and cMET-targeting agents currently being investigated in combination with ICI regimes are highlighted in the figure.
Nevertheless, similar to the fate of other small molecule inhibitors, the mUM may acquire resistance under cMET inhibitors. Besides, the constant TCR signaling or the highly immunosuppressive tumor microenvironment may soon lead to the exhaustion of cMET inhibition-induced anti-tumor immune responses (47). Therefore, even though the exact mechanisms of their additive activity are yet to be proven in mUM, there is a strong rationale for cMET inhibitors to evolve with ICI regimes. To this point, most of the successful attempts of ICI usage in cancers like liver cancer were demonstrated in combination or when pre-treated with small molecule tyrosine kinase inhibitors, suggesting the potential of cMET inhibitors when evolved in combination with ICIs in mUM patients (48, 49).
3. cMET inhibitors in combination with Immunotherapy
Anti-PD1 monotherapy has shown minimal success in mUM patients, with responses ranging from 0-7% with a median PFS of 2-3 months and OS limited to approximately one year (50, 51). Increased hepatic tumor load and intrinsic tumor factors such as low tumor mutational burden, PD1/PDL1 expression levels, and increased presence of alternative immunosuppressive molecules like Lymphocyte-activation gene-3 (LAG3) in mUM compared to mCM may explain such inefficacy (52–55). Interestingly, the cMET pathway is also involved in resistance to anti-PD1 treatment and associated with almost all factors mentioned above, strengthening the rationale for combination treatment (Figure 2). cMET expression correlates with liver metastasis of UM and tumor mutational burden in lung cancer patients, and cMET/HGF signaling has been shown to upregulate indoleamine-2,3-dioxygenase (IDO) 1, a highly immunosuppressive molecule involved in resistance to immunotherapy (35, 56–58). cMET inhibition stops tumor growth and invasion and can improve anti-PD1 response by involving immune cells. High-dose crizotinib induces immunogenicity in tumor cells and increases the expression of PD1, LAG3, and PDL1, in a mouse model for lung cancer, therefore preparing the tumors for immunotherapy with anti-PD1 antibodies (59). In addition, neutrophils can play a significant role in the promotion of tumor growth and liver metastasis, and their increased presence in the blood or tumor is another factor known to contribute to anti-PD1 resistance (60–63). In vivo, HGF/cMET signaling drives neutrophils from bone marrow to the TME, where they acquire immunosuppressive properties in T cell inflamed tissues and suppress immunotherapy-induced CD8+ T cell expansion and effector function. Meanwhile, concomitant inhibition of cMET and PD1 blocked the infiltration of bone marrow-derived immunosuppressive neutrophils into the tumor and led the way for the anti-tumor efficacy of PD1 inhibitors in mouse tumor models (64). Accordingly, a novel dual inhibitor of cMET and PD1 has shown superior anti-tumor efficacy with solid anti-proliferative and anti-metastatic effects compared to monotherapy with PD1 inhibitors alone in tumor cell lines and in a mouse model with liver cancer (65). Besides, several clinical trials are currently evaluating the safety and efficacy of cMET inhibitors in combination with ICIs for advanced cancer patients. A clinical phase I/II combination therapy with Cabozantinib and Nivolumab in advanced liver cancer patients reported a disease control rate of 81%, a response rate of 17%, and a median PFS of 5.5 months. However, a triple combination therapy including Cabozantinib, Ipilimumab, and Nivolumab has shown higher response rates but had to be discontinued due to increased treatment-related toxicities (66). Likewise, this combination showed a better response rate, delayed disease progression, and extended patient survival in treatment-naive metastatic renal cell carcinoma, another cMET-deregulated tumor (48). Collectively, these data suggest that a concomitant combination of cMET inhibitors and ICIs, or a sequential combination of cMET inhibitors followed by ICIs, may improve responses to cancer immunotherapy on multiple fronts in mUM patients.
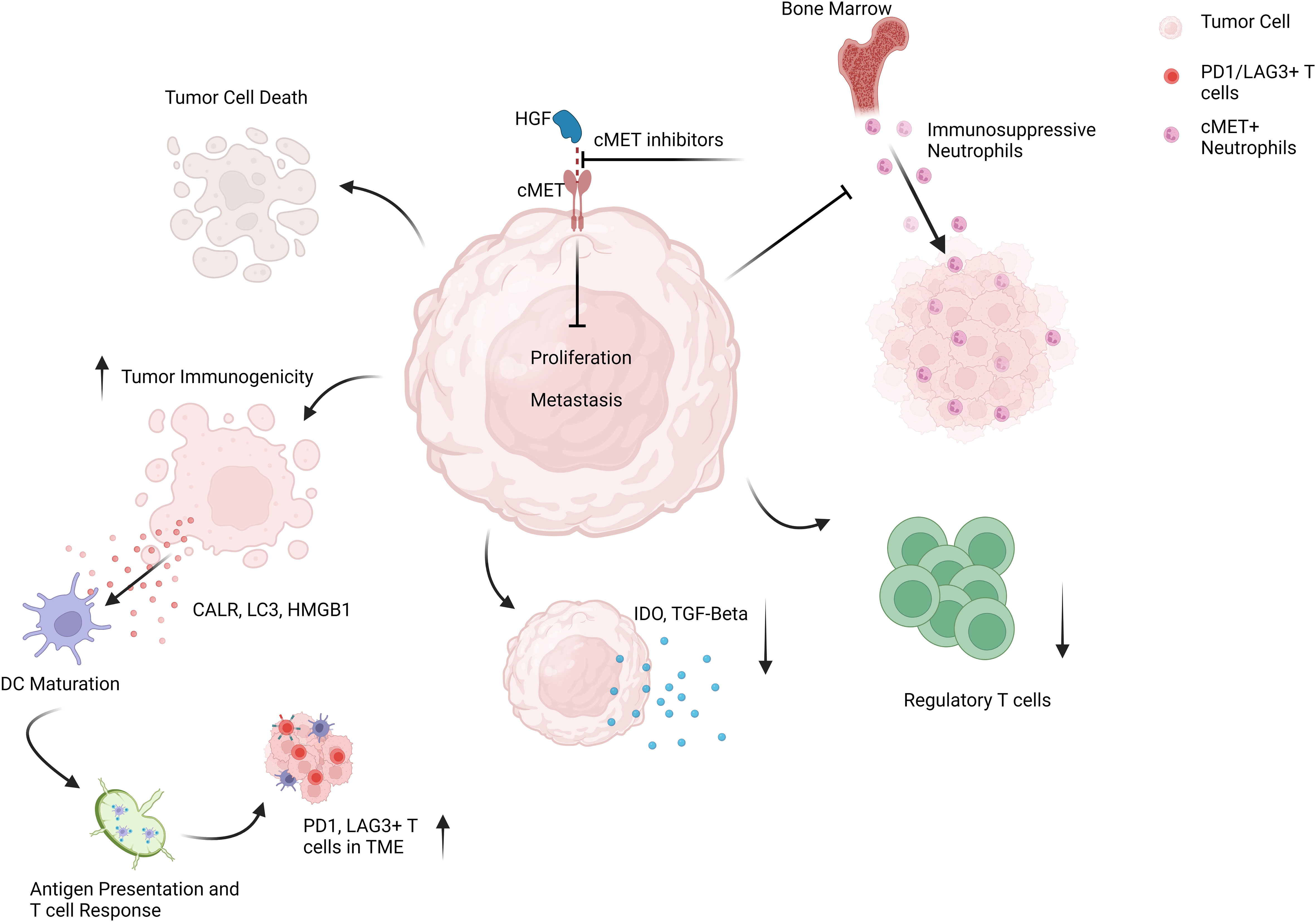
Figure 2 Schematic representation of targeting cMET pathway in mUM patients to enhance the anti-tumor efficacy of ICI regimes.
Like PD1, LAG3 is also an inhibitory receptor that controls T-cell tolerance and is the next most promising immune target after PD1 (67). It is particularly promising for mUM because it functions as an alternative pathway for PD1, and LAG3-positive T cells are more abundant in mUM TME than CTLA4 or PD1-positive T cells (54, 55). Furthermore, LAG3 is highly expressed in monosomy of chromosome 3 (M3) UM tumors and, similar to cMET, correlates with high-risk histopathological parameters (68). Accordingly, increased expression of LAG3 in TILs linked to reduced disease-free survival in mUM patients (68). Relatlimab, an anti-LAG3 antibody, has already shown promising clinical benefits in mCM patients in combination with the PD1 antibody nivolumab (69), and clinical trials are currently ongoing to determine the efficacy of relatlimab plus nivolumab in mUM patients (NCT04552223); therefore, a combination of cMET and LAG3 inhibitors theoretically offers another potential strategy for mUM. However, further studies are required to understand the relationship between these two proteins in mUM tumors and the consequences of blocking them together. Similarly, tebentafusp is the most promising soluble TCR molecule offering survival benefits in mUM patients with HLA-positive tumors (70). Even though patients progressing under tebentafusp have a survival benefit, patients responding to treatment have better overall survival. However, response rates are low, and strategies to increase efficacy through combination therapies are of great interest. cMET inhibitors can increase the immunogenicity of tumors and may further lead to an additive benefit for tebentafusp (59).
4. Challenges & opportunities
Although there are abundant possibilities for combining cMET inhibitors with ICIs, several important tasks lie ahead to move the combination treatment from the lab to the clinic. Firstly, there is a need to explore the most selective and active cMET inhibitor with less off-target toxicities and high anti-tumor activity and then to evaluate the best ICI agent (anti-PD1 or/and anti-LAG3) to combine for mUM treatment. Although anti-PD1 antibodies are standard care, given the strong rationale for targeting LAG3 in mUM, evaluating the combination efficacy of cMET inhibitors and anti-LAG3 antibodies in mUM patients is highly demanding. Bispecific antibodies targeting anti-cMET/PD1 or anti-cMET/LAG3 are also promising developments for mUM. Secondly, further preclinical research aiming to design the best sequence of combination treatments, cMET inhibition followed by ICI or a concomitant inhibition, should be investigated thoroughly to integrate the combination treatments effectively in well-designed clinical trials. Consequently, a crucial task is also to identify the patients who may benefit from such combination treatment strategies. Identifying cMET overexpressed tumors may serve this purpose. However, the scarcity of tissue material and the tumor heterogeneity might make it challenging to identify the cMET-positive tumors and to define a cut-off for its overexpression. Systemic biomarkers can overcome these limitations and serve as a non-invasive material to investigate predictive biomarkers. In this regard, higher concentrations of soluble cMET, a cleaved product of membrane cMET, have been detected in the blood and may indicate the overexpression of cMET in tumors from mUM patients. Accordingly, increased concentrations of soluble cMET have been shown to correlate with worse survival in mUM patients suggesting its potential in identifying cMET tumors (71). Likewise, the abundance of circulating cMET-positive tumor cells captured using novel cMET-based Ferrofluid also be an alternative method (72). Finally, cMET-based PET CT has also been developed recently and is currently being tested in renal cell carcinoma patients; adopting such screening procedures for mUM patients can identify the mUM patients with cMET-positive tumors (73).
5. Conclusions
Enhancing the efficacy of ICI treatment for mUM patients is highly demanding. The major challenge here is the involvement of the liver in mUM patients. Targeting signaling pathways involved in liver metastasis may improve the anti-tumor efficacy of ICIs. Inhibition of cMET, a transmembrane protein highly involved in LmUM progression, induces tumor cell death and inhibits signaling pathways in tumor resistance. In addition, cMET inhibition also increases tumors’ immunogenicity and blocks immune-regulating neutrophils’ infiltration into TME, therefore, sensitizing the tumors to ICIs. Accordingly, ICIs combined with cMET inhibitors are currently being investigated in clinical trials for other cancer types and have already shown promising results in patients with liver cancer. Therefore, applying the knowledge from these studies and developing combined treatment strategies, including cMET inhibitors and ICIs for mUM patients, may have tremendous clinical potential. While the benefit of such combination therapy may sound substantial in terms of clinical benefits, several issues must be addressed to adapt this approach safely and efficiently for mUM patients. Selecting a potential cMET targeting agent or approach, identifying predictive biomarkers, and the best treatment sequence for combination should be thoroughly investigated. Besides, as combination therapies are highly associated with increased toxicity compared to monotherapies, the mechanism underlying the synergistic effect must be clarified in preclinical models. Hence, further research efforts are urgently required to optimize the development and delivery of cMET-ICI combinations, and it is highly anticipated that future studies on this combinational approach will lead to survival benefits for mUM patients.
Author contributions
DM and JH conceived and designed the overall study. DM prepared the original manuscript. JH supervised, reviewed and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
For the publication fee, we acknowledge the financial support of Deutsche Forschungsgemeinschaft within the funding program “Open Access Publikationskosten”and Heidelberg University. Graphical representation created using Biorender.
Conflict of interest
JH received a scientific support from BMS, Sunpharma, and Sanofi to the institution; honoraria for talks from Amgen, BMS, GSK, Immunocore, Novartis, Sanofi, Pierre Fabre, Sunpharma; advisory board member for MSD, GSK, Pierre Fabre, Sun Pharma, BMS, Immunocore, Novartis, and Philogen to the institution; travel grants from BMS, and Sunpharma; Clinical trial center for BioNTech, BMS, Genmab, Genetech/Roche, Idera Immunocore, IO-Biotech, Iovance, Nektar, Novartis, Philogen, Pierre Fabre, Regeneron, Replimune, and Sanofi to the institution.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mochizuki M, Sugita S, Kamoi K. Immunological homeostasis of the eye. Prog Retin Eye Res (2013) 33:10–27. doi: 10.1016/j.preteyeres.2012.10.002
2. Thomas AJ, Erickson CA. The making of a melanocyte: The specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res (2008) 21(6):598–610. doi: 10.1111/j.1755-148X.2008.00506.x
3. Aoki H, Yamada Y, Hara A, Kunisada T. Two distinct types of mouse melanocyte: Differential signaling requirement for the maintenance of non-cutaneous and dermal versus epidermal melanocytes. Development (2009) 136(15):2511–21. doi: 10.1242/dev.037168
4. Cichorek M, Wachulska M, Stasiewicz A. Heterogeneity of neural crest-derived melanocytes. cent eur j biol (2013) 8:315–30 doi: https://doi.org/10.2478/s11535-013-0141-1
5. Colombo S, Berlin I, Delmas Véronique, Larue L. Classical and Nonclassical Melanocytes in Vertebrates. In Melanins and Melanosomes (2011) Chapter 2, Book Editors: J. Borovanský and P.A. Riley. https://doi.org/10.1002/9783527636150.ch2
6. Hu DN. Regulation of growth and melanogenesis of uveal melanocytes. Pigment Cell Res (2000) 13 Suppl 8:81–6. doi: 10.1034/j.1600-0749.13.s8.15.x
7. Kaliki S, Shields CL. Uveal melanoma: Relatively rare but deadly cancer. Eye (Lond) (2017) 31(2):241–57. doi: 10.1038/eye.2016.275
8. Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the coms trials for treatment of choroidal melanoma: Collaborative ocular melanoma study group report no. 26. Arch Ophthalmol (2005) 123(12):1639–43. doi: 10.1001/archopht.123.12.1639
9. Feldman ED, Pingpank JF, Alexander HR Jr. Regional treatment options for patients with ocular melanoma metastatic to the liver. Ann Surg Oncol (2004) 11(3):290–7. doi: 10.1245/aso.2004.07.004
10. Leiter U, Meier F, Schittek B, Garbe C. The natural course of cutaneous melanoma. J Surg Oncol (2004) 86(4):172–8. doi: 10.1002/jso.20079
11. Kath R, Hayungs J, Bornfeld N, Sauerwein W, Höffken K, Seeber S. Prognosis and treatment of disseminated uveal melanoma. Cancer (1993) 72(7):2219–23. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j
12. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (Checkmate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/s1470-2045(18)30700-9
13. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
14. Schank TE, Hassel JC. Immunotherapies for the treatment of uveal melanoma-history and future. Cancers (Basel) (2019) 11(8):1048 doi: 10.3390/cancers11081048
15. Pires da Silva I, Lo S, Quek C, Gonzalez M, Carlino MS, Long GV, et al. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-Pd-1 therapy. Cancer (2020) 126(1):86–97. doi: 10.1002/cncr.32522
16. Waninger JJ, Ma VT, Journey S, Skvarce J, Chopra Z, Tezel A, et al. Validation of the American joint committee on cancer eighth edition staging of patients with metastatic cutaneous melanoma treated with immune checkpoint inhibitors. JAMA Netw Open (2021) 4(3):e210980. doi: 10.1001/jamanetworkopen.2021.0980
17. Bakalian S, Marshall JC, Logan P, Faingold D, Maloney S, Di Cesare S, et al. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res (2008) 14(4):951–6. doi: 10.1158/1078-0432.Ccr-06-2630
18. Zheng M, Tian Z. Liver-mediated adaptive immune tolerance. Front Immunol (2019) 10:2525. doi: 10.3389/fimmu.2019.02525
19. Ballas ZK, Buchta CM, Rosean TR, Heusel JW, Shey MR. Role of nk cell subsets in organ-specific murine melanoma metastasis. PloS One (2013) 8(6):e65599. doi: 10.1371/journal.pone.0065599
20. Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol (2020) 5(52):eaba0759 doi: 10.1126/sciimmunol.aba0759
21. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-Pd-1 monoclonal antibody in patients with melanoma and nsclc. Cancer Immunol Res (2017) 5(5):417–24. doi: 10.1158/2326-6066.CIR-16-0325
22. Uchikawa E, Chen Z, Xiao GY, Zhang X, Bai XC. Structural basis of the activation of c-met receptor. Nat Commun (2021) 12(1):4074. doi: 10.1038/s41467-021-24367-3
23. Tanaka R, Terai M, Londin E, Sato T. The role of Hgf/Met signaling in metastatic uveal melanoma. Cancers (Basel) (2021) 13(21):5457. doi: 10.3390/cancers13215457
24. Zhu S, Bennett S, Li Y, Liu M, Xu J. The molecular structure and role of Lect2 or chm-ii in arthritis, cancer, and other diseases. J Cell Physiol (2022) 237(1):480–8. doi: 10.1002/jcp.30593
25. Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, et al. Decorin is a novel antagonistic ligand of the met receptor. J Cell Biol (2009) 185(4):743–54. doi: 10.1083/jcb.200901129
26. Shen Y, Naujokas M, Park M, Ireton K. Inib-dependent internalization of listeria is mediated by the met receptor tyrosine kinase. Cell (2000) 103(3):501–10. doi: 10.1016/s0092-8674(00)00141-0
27. Chen CK, Yang CY, Hua KT, Ho MC, Johansson G, Jeng YM, et al. Leukocyte cell-derived chemotaxin 2 antagonizes met receptor activation to suppress hepatocellular carcinoma vascular invasion by protein tyrosine phosphatase 1b recruitment. Hepatology (2014) 59(3):974–85. doi: 10.1002/hep.26738
28. Shirasaki T, Yamagoe S, Shimakami T, Murai K, Imamura R, Ishii KA, et al. Leukocyte cell-derived chemotaxin 2 is an antiviral regulator acting through the proto-oncogene met. Nat Commun (2022) 13(1):3176. doi: 10.1038/s41467-022-30879-3
29. Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes met receptor activity and down-regulates {Beta}-catenin and myc levels. J Biol Chem (2010) 285(53):42075–85. doi: 10.1074/jbc.M110.172841
30. Shen Z, Xue W, Zheng Y, Geng Q, Wang L, Fan Z, et al. Molecular mechanism study of Hgf/C-met pathway activation and immune regulation for a tumor diagnosis model. Cancer Cell Int (2021) 21(1):374. doi: 10.1186/s12935-021-02051-2
31. Van Der Steen N, Pauwels P, Gil-Bazo I, Castañon E, Raez L, Cappuzzo F, et al. Cmet in nsclc: Can we cut off the head of the hydra? from the pathway to the resistance. Cancers (Basel) (2015) 7(2):556–73. doi: 10.3390/cancers7020556
32. Ma PC, Maulik G, Christensen J, Salgia R. C-met: Structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev (2003) 22(4):309–25. doi: 10.1023/a:1023768811842
33. Gelsomino F, Rossi G, Tiseo M. Met and small-cell lung cancer. Cancers (Basel) (2014) 6(4):2100–15. doi: 10.3390/cancers6042100
34. Surriga O, Rajasekhar VK, Ambrosini G, Dogan Y, Huang R, Schwartz GK. Crizotinib, a c-met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol Cancer Ther (2013) 12(12):2817–26. doi: 10.1158/1535-7163.Mct-13-0499
35. Gardner FP, Serie DJ, Salomao DR, Wu KJ, Markovic SN, Pulido JS, et al. C-met expression in primary and liver metastases in uveal melanoma. Melanoma Res (2014) 24(6):617–20. doi: 10.1097/cmr.0000000000000118
36. Economou MA, All-Ericsson C, Bykov V, Girnita L, Bartolazzi A, Larsson O, et al. Receptors for the liver synthesized growth factors igf-1 and Hgf/Sf in uveal melanoma: Intercorrelation and prognostic implications. Invest Ophthalmol Vis Sci (2005) 46(12):4372–5. doi: 10.1167/iovs.05-0322
37. Wang Q, Yang S, Wang K, Sun SY. Met inhibitors for targeted therapy of egfr tki-resistant lung cancer. J Hematol Oncol (2019) 12(1):63. doi: 10.1186/s13045-019-0759-9
38. Wang W, Li Q, Takeuchi S, Yamada T, Koizumi H, Nakamura T, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in egfr mutant lung cancer. Clin Cancer Res (2012) 18(6):1663–71. doi: 10.1158/1078-0432.Ccr-11-1171
39. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. Met amplification leads to gefitinib resistance in lung cancer by activating Erbb3 signaling. Science (2007) 316(5827):1039–43. doi: 10.1126/science.1141478
40. Benedettini E, Sholl LM, Peyton M, Reilly J, Ware C, Davis L, et al. Met activation in non-small cell lung cancer is associated with De novo resistance to egfr inhibitors and the development of brain metastasis. Am J Pathol (2010) 177(1):415–23. doi: 10.2353/ajpath.2010.090863
41. Tchou J, Zhao Y, Levine BL, Zhang PJ, Davis MM, Melenhorst JJ, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (Car) T cells in metastatic breast cancer. Cancer Immunol Res (2017) 5(12):1152–61. doi: 10.1158/2326-6066.CIR-17-0189
42. Decaens T, Barone C, Assenat E, Wermke M, Fasolo A, Merle P, et al. Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with met overexpression. Br J Cancer (2021) 125(2):190–9. doi: 10.1038/s41416-021-01334-9
43. Ryoo BY, Cheng AL, Ren Z, Kim TY, Pan H, Rau KM, et al. Randomised phase 1b/2 trial of tepotinib vs sorafenib in Asian patients with advanced hepatocellular carcinoma with met overexpression. Br J Cancer (2021) 125(2):200–8. doi: 10.1038/s41416-021-01380-3
44. Puzanov I, Sosman J, Santoro A, Saif MW, Goff L, Dy GK, et al. Phase 1 trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors. Invest New Drugs (2015) 33(1):159–68. doi: 10.1007/s10637-014-0167-5
45. Ideaya reports clinical data from phase 2 expansion dose of darovasertib and crizotinib synthetic lethal combination in heavily pre-treated metastatic uveal melanoma. (South San Francisco, California: IDEAYA Biosciences, Inc) (2021). Available at: https://media.ideayabio.com/2021-12-07-IDEAYA-Reports-Clinical-Data-from-Phase-2-Expansion-Dose-of-Darovasertib-and-Crizotinib-Synthetic-Lethal-Combination-in-Heavily-Pre-Treated-Metastatic-Uveal-Melanoma
46. Daud A, Kluger HM, Kurzrock R, Schimmoller F, Weitzman AL, Samuel TA, et al. Phase ii randomised discontinuation trial of the Met/Vegf receptor inhibitor cabozantinib in metastatic melanoma. Br J Cancer (2017) 116(4):432–40. doi: 10.1038/bjc.2016.419
48. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
49. Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Lam TB, et al. The 2021 updated European association of urology guidelines on renal cell carcinoma: Immune checkpoint inhibitor-based combination therapies for treatment-naive metastatic clear-cell renal cell carcinoma are standard of care. Eur Urol (2021) 80(4):393–7. doi: 10.1016/j.eururo.2021.04.042
50. Heppt MV, Heinzerling L, Kahler KC, Forschner A, Kirchberger MC, Loquai C, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined pd-1/Cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer (2017) 82:56–65. doi: 10.1016/j.ejca.2017.05.038
51. Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with pd-1 and pd-L1 antibodies. Cancer (2016) 122(21):3344–53. doi: 10.1002/cncr.30258
52. Johnson DB, Bao R, Ancell KK, Daniels AB, Wallace D, Sosman JA, et al. Response to anti-Pd-1 in uveal melanoma without high-volume liver metastasis. J Natl Compr Canc Netw (2019) 17(2):114–7. doi: 10.6004/jnccn.2018.7070
53. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to pd-1 inhibition. N Engl J Med (2017) 377(25):2500–1. doi: 10.1056/NEJMc1713444
54. Qin Y, Petaccia de Macedo M, Reuben A, Forget MA, Haymaker C, Bernatchez C, et al. Parallel profiling of immune infiltrate subsets in uveal melanoma versus cutaneous melanoma unveils similarities and differences: A pilot study. Oncoimmunology (2017) 6(6):e1321187. doi: 10.1080/2162402X.2017.1321187
55. Durante MA, Rodriguez DA, Kurtenbach S, Kuznetsov JN, Sanchez MI, Decatur CL, et al. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat Commun (2020) 11(1):496. doi: 10.1038/s41467-019-14256-1
56. Sabari JK, Leonardi GC, Shu CA, Umeton R, Montecalvo J, Ni A, et al. Pd-L1 expression, tumor mutational burden, and response to immunotherapy in patients with met exon 14 altered lung cancers. Ann Oncol (2018) 29(10):2085–91. doi: 10.1093/annonc/mdy334
57. Wang D, Saga Y, Sato N, Nakamura T, Takikawa O, Mizukami H, et al. The hepatocyte growth factor antagonist Nk4 inhibits indoleamine-2,3-Dioxygenase expression Via the c-Met-Phosphatidylinositol 3-Kinase-Akt signaling pathway. Int J Oncol (2016) 48(6):2303–9. doi: 10.3892/ijo.2016.3486
58. Kocher F, Amann A, Zimmer K, Geisler S, Fuchs D, Pichler R, et al. High indoleamine-2,3-Dioxygenase 1 (Ido) activity is linked to primary resistance to immunotherapy in non-small cell lung cancer (Nsclc). Transl Lung Cancer Res (2021) 10(1):304–13. doi: 10.21037/tlcr-20-380
59. Liu P, Zhao L, Pol J, Levesque S, Petrazzuolo A, Pfirschke C, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun (2019) 10(1):1486. doi: 10.1038/s41467-019-09415-3
60. Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol (2018) 15(4):206–21. doi: 10.1038/nrgastro.2017.183
61. Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res (2016) 76(6):1367–80. doi: 10.1158/0008-5472.CAN-15-1591
62. Arasanz H, Bocanegra AI, Morilla I, Fernandez-Irigoyen J, Martinez-Aguillo M, Teijeira L, et al. Circulating low density neutrophils are associated with resistance to first line anti-Pd1/Pdl1 immunotherapy in non-small cell lung cancer. Cancers (Basel) (2022) 14(16):3846. doi: 10.3390/cancers14163846
63. Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, Postow MA, et al. High neutrophil-to-Lymphocyte ratio (Nlr) is associated with treatment failure and death in patients who have melanoma treated with pd-1 inhibitor monotherapy. Cancer (2020) 126(1):76–85. doi: 10.1002/cncr.32506
64. Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-met limit cancer immunotherapy. Immunity (2017) 47(4):789–802 e9. doi: 10.1016/j.immuni.2017.09.012
65. Yuan Q, Liang Q, Sun Z, Yuan X, Hou W, Wang Y, et al. Development of bispecific anti-C-Met/Pd-1 diabodies for the treatment of solid tumors and the effect of c-met binding affinity on efficacy. Oncoimmunology (2021) 10(1):1914954. doi: 10.1080/2162402X.2021.1914954
66. Yau T, Zagonel V, Santoro A, Acosta-Rivera M. Nivolumab (Nivo) + ipilimumab (Ipi) + cabozantinib (Cabo) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (Ahcc): Results from checkmate 040. J Clin Oncol (2020) 38(4):478. doi: 10.1200/JCO.2020.38.4_suppl.478
67. Long L, Zhang X, Chen F, Pan Q, Phiphatwatchara P, Zeng Y, et al. The promising immune checkpoint lag-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer (2018) 9(5-6):176–89. doi: 10.18632/genesandcancer.180
68. Souri Z, Wierenga APA, Kroes WGM, van der Velden PA, Verdijk RM, Eikmans M, et al. Lag3 and its ligands show increased expression in high-risk uveal melanoma. Cancers (2021) 13(17):4445 doi: 10.3390/cancers13174445
69. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med (2022) 386(1):24–34. doi: 10.1056/NEJMoa2109970
70. Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med (2021) 385(13):1196–206. doi: 10.1056/NEJMoa2103485
71. Barisione G, Fabbi M, Gino A, Queirolo P, Orgiano L, Spano L, et al. Potential role of soluble c-met as a new candidate biomarker of metastatic uveal melanoma. JAMA Ophthalmol (2015) 133(9):1013–21. doi: 10.1001/jamaophthalmol.2015.1766
72. Zhang T, Boominathan R, Foulk B, Rao C, Kemeny G, Strickler JH, et al. Development of a novel c-Met-Based ctc detection platform. Mol Cancer Res (2016) 14(6):539–47. doi: 10.1158/1541-7786.MCR-16-0011
73. Mittlmeier LM, Todica A, Gildehaus FJ, Unterrainer M, Beyer L, Brendel M, et al. (68)Ga-Emp-100 Pet/Ct-a novel ligand for visualizing c-met expression in metastatic renal cell carcinoma-first in-human biodistribution and imaging results. Eur J Nucl Med Mol Imaging (2022) 49(5):1711–20. doi: 10.1007/s00259-021-05596-6
Keywords: uveal melanoma, immunotherapy, liver metastasis, cMET signaling, combination (combined) therapy, PD1 (programmed cell death protein 1), LAG3: Lymphocyte-activation gene 3, resistance mechanism
Citation: Machiraju D and Hassel JC (2023) Targeting the cMET pathway to enhance immunotherapeutic approaches for mUM patients. Front. Oncol. 12:1068029. doi: 10.3389/fonc.2022.1068029
Received: 18 October 2022; Accepted: 28 December 2022;
Published: 24 January 2023.
Edited by:
Francesco Sabbatino, University of Salerno, ItalyReviewed by:
Beatrice Gallo, Royal Berkshire Hospital, United KingdomCopyright © 2023 Machiraju and Hassel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica C. Hassel, SmVzc2ljYS5IYXNzZWxAbWVkLnVuaS1oZWlkZWxiZXJnLmRl
 Devayani Machiraju
Devayani Machiraju Jessica C. Hassel
Jessica C. Hassel