- 1Department of Neurosurgery, La Pitié-Salpêtrière University Hospital, Assistance Publique-Hôpitaux de Paris, Paris, France
- 2Université Paris Cité, Institut Cochin, CNRS, INSERM, Paris, France
- 3Department of Neurosurgery, Neurocenter of Southern Switzerland, Lugano, Switzerland
- 4Department of Neurosurgery, Centre Hospitalier Universitaire (CHU) de Liège, Bat B35, Domaine Universitaire du Sart-Tilman, Liège, Belgium
Introduction: Effective strategies are required to ensure optimal management of the crucial closure step in endoscopic pituitary surgery. Many surgical techniques have been reported but no significant consensus has been defined.
Methods: Between January 2006 and March 2022, 3015 adult patients with pituitary adenomas were operated on by a single expert neurosurgical team, using a mononostril endoscopic endonasal approach. Based of preoperative risk factors of and operative findings, a detailed closure strategy was used. Body mass index >40, sellar floor lysis, number of surgeries>2, large skull base destruction, prior radiotherapy were considered as preoperative risk factors for closure failure. All patients treated with an expanded endonasal approach were excluded.
Results: Patients were mostly women (F/M ratio: 1.4) with a median age of 50 (range: 18 –89). Intraoperative CSF leak requiring specific surgical management was observed in 319/3015 (10.6%) of patients. If intraoperative leak occurred, patients with predictive risk factors were managed using a Foley balloon catheter in case of sellar floor lysis or BMI>40 and a multilayer repair strategy with a vascularized nasoseptal flap in other cases. Postoperative CSF leak occurred in 29/3015 (1%) of patients, while meningitis occurred in 24/3015 (0.8%) of patients. In patients with intraoperative leak, closure management failed in 11/319 (3.4%) of cases.
Conclusion: Based on our significant 16-year experience, our surgical management is reliable and easy to follow. With a planned and stepwise strategy, the closure step can be optimized and tailored to each patient with a very low failure rate.
Introduction
Pituitary adenomas account for 15% of all intracranial neoplasms, making them the third most common pathology (1). Pituitary adenomas, recently renamed as pituitary neuroendocrine tumors (PitNETs) in the new classifications (2–4) are usually benign tumors, with a broad spectrum of biological and pathological characteristics (5–7). Surgery represents the first-line treatment for most pituitary adenomas (corticotroph, somatotroph and non-functional), except for most prolactinomas which are currently treated with dopamine agonists (8).
The transsphenoidal approach is the gold standard of surgical route for pituitary surgery (9–11), while the transcranial approach is considered only as a second-line surgical option, in well-selected patients with rare tumors extending anteriorly in the subfrontal area, laterally in the temporal fossa or encompassing the vessels (12, 13). The microscopic technique, initially developed by Cushing and successively taken forward by Dott, Guiot and Hardy (14–16), was progressively abandoned in most centers of excellence, leading to the transition towards the endoscopic technique in the late 1990s (10, 17–19). Nowadays, the endoscopic endonasal transsphenoidal approach is the mainstay in many centers worldwide, given its advantages in terms of quality of vision, tumor resection, endocrine outcome, sinus-nasal morbidity and length of hospital stay (17, 20, 21).
Despite advances in closure techniques, postoperative CSF (cerebrospinal fluid) leak remains the most common complication of the endoscopic endonasal transsphenoidal approach, occurring in around 10% of patients and requiring a specific second surgery. The rate of CSF leak-related meningitis is observed in approximately 5% of patients, increasing the length of hospital stay and medical costs (22, 23). Thus, a reconstruction strategy of the skull-base defect should be anticipated during preoperative planning and an accurate analysis of preoperative risk factors associated with CSF leak is essential. In previous studies, many preoperative risk factors have been reported such as BMI (body mass index) > 30, multiple surgeries, tumor size, extension and invasiveness, prior treatment with radiotherapy (24–29). In all these patients, the closure strategy should be rigorously considered before the sellar surgical step, in order to limit avoidable reconstruction failures (24, 30) and to modulate it according to the flow of the CSF leak observed during surgery (31–34).
Today, several protocols to reduce postoperative CSF leak have been proposed (33–40) but there is no consensus on the best closure strategy after endoscopic pituitary surgery. Moreover, a significant heterogeneity in the outcomes was reported (41). From the analysis of the first 1000 patients operated on with a mononostril endoscopic endonasal transsellar approach, we reported in 2014 a postoperative CSK leak rate <1% (18). Based on our substantial additional experience, we developed a gradual closure strategy that integrates individual preoperative risks of postoperative CSF leak with the operative findings.
The objective of the present study is to analyze the results of our closure strategy from a consecutive cohort of 3015 patients with pituitary adenomas, operated on by the same two senior expert neurosurgeons (S.G, B.B). The philosophy of this major surgical step is emphasized, highlighting the need to plan the closure step before entering the operative room.
Materials and methods
Patients
This is a French observational cohort of 3015 consecutive adult patients with pituitary adenomas operated on between January 2006 and March 2022.
Inclusion criteria were: (1) diagnosis of adenoma confirmed on histological examination; (2) adenoma patients treated with a mononostril endoscopic endonasal transsphenoidal approach, as described in the “endoscopic endonasal transsphenoidal surgery” section; (3) patients eligible for surgery selected at a multidisciplinary meeting with an endocrinologist, a neurosurgeon and a radiologist; (4) dedicated pituitary MRI performed for each patient before surgery. Exclusion criteria were: (1) adenoma patients treated with an expanded endoscopic approach - such as transtuberculum or transplanum approach; (2) patients under 18 years of age.
Prior medical therapy with dopamine agonists or somatostatin analogues was not considered an exclusion criterion. All patients with prior medical therapy were included in this series.
Predictive factors for closure failure
On the basis of previous studies (24, 25, 27–29, 42, 43), preoperative clinical and radiological assessment identified the following variables as preoperative risk factors for closure failure: severe obesity, number of surgeries > 2, focal sellar floor lysis, large skull base destruction due to invasive or giant pituitary adenomas and history of prior radiation therapy.
Intraoperative CSF leaks are often complex to treat in obese patients because of higher intracranial pressure (29). Furthermore, as previously published, the risk of symptomatic intracranial hypertension increases with increasing BMI. In patients with BMI>40, the risk of induced vision loss is well known (44). Based on this data, we have considered that patients with BMI>40 may be exposed to a higher risk of postoperative CSF leak due to increased intracranial pressure. Thus, this critical value was considered a risk factor of closure failure.
Endoscopic endonasal transsphenoidal surgery
The same two senior neurosurgeons (S.G, B.B) operated on all patients via a mononostril endoscopic endonasal transsphenoidal approach, as recently described by the present team (20). The patient was placed in a semi-sitting position. Care was taken to avoid any compression points. During patient positioning, the right thigh was prepared for musculoaponeurotic graft whenever needed. The head was deflected back by 30° to prevent jugular compression. 0° and 30° optic endoscopes (Karl Storz) were used. After lateralization of the middle turbinate, the mucosa of the anterior part of the sphenoid bone was coagulated with a luxation of the septum. The sellar floor was opened and the dura incised. The adenoma was removed for pathological analysis using standard curettes, dissecting instruments, and suction.
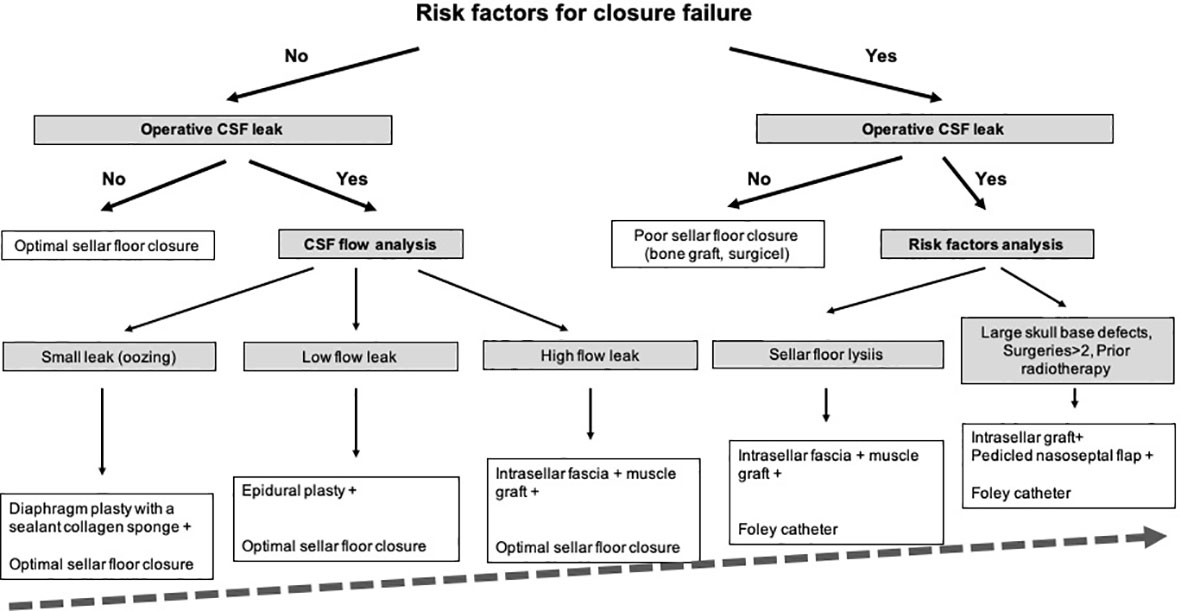
The crucial closure step was anticipated during the approach: the main objective was to preserve an optimal epidural space when the sellar floor was opened and the tumor was removed, in order to reconstruct an optimal sellar floor during the closure step. The sellar floor was opened is such a way that the bone opening was larger than the dural opening, in order to preserve the epidural space (Figure 1).
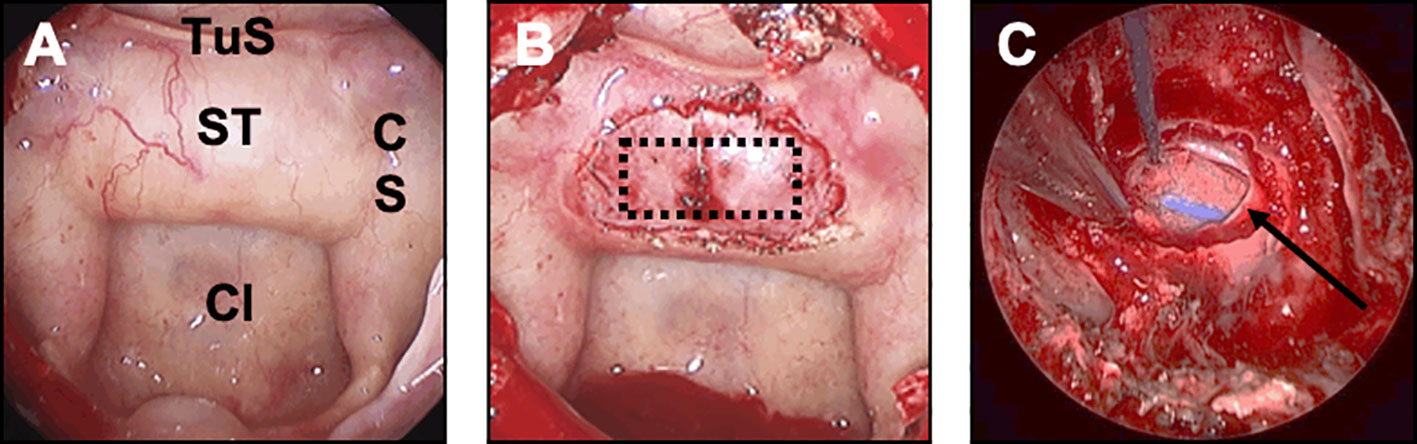
Figure 1 Preparation of the closure step during the endoscopic approach. This figure illustrates the concept that closure should be anticipated and planned for at each surgical step. (A) Sphenoid step. The main classic landmarks are identified: sella turcica (ST), tuberculum sellae (TuS), clivus (Cl), cavernous sinus (CS). (B) Sellar step. After opening the sellar floor, the dura has been carefully exposed and respected. The optimal rectangle shaped-dural opening is provided (dotted black rectangle). Note that the bone opening is oversized compared to the dural opening, in order to preserve the epidural space. (C) Sellar step after tumor resection. Note that the epidural space has been respected between the sellar floor and the dura mater (black arrow) for optimal closure.
CSF leak evaluation
Once tumor was resected, the neurosurgeon had to determine the existence and intensity of a possible CSF leak. If a CSF leak was observed, the degree of CSF flow was assessed, as proposed by Esposito et al. (33). CSF leak was classified as follows: small leak without obvious diaphragm defect, defined as diaphragm oozing; low-flow leak with focal diaphragm defect; high-flow leak with large diaphragm or dural defect.
Closure stage: General considerations
The substantial experience gained since 2006 has allowed us to gradually establish a specific stepwise surgical strategy, based on a rigorous analysis of preoperative risk factors for closure failure and operative findings. The proposed closure strategy comes from a retrospective analysis of the authors’ experience gained during the study period. Our current strategy used since 2014 for graded closure strategy in pituitary surgery has been provided in Figure 2.
Indeed, some nuances have been added from our initial operative technique. Until 2014 (n=1397 patients), a 5-day external lumbar drainage could be decided as a second surgical option in combination with the intrasellar muscular graft, in rare patients with failure of closure management. Since 2014 (n=1618 patients), external lumbar drainage has been abandoned and replaced by the Foley Catheter technique: a saline-inflate Foley catheter was applied inside the sphenoid sinus in order to constitute an abutment against the intrasellar muscular graft (in case of sellar floor lysis or BMI>40) or against the double pedicled nasoseptal flap (in case of number of surgeries > 2, large skull base destruction, giant tumors or prior radiotherapy). This change essentially resulted from an objective to improve clinical tolerance, to reduce the risk of complications due to lumbar drainage and to treat more complex patients with more complex adenomas.
If possible, no material was placed in the intrasellar compartment, so as not to interfere with the interpretation of the postoperative MRI and not to impact on tumor resection in any subsequent surgery. In the absence of CSK leak, an optimal standard bone sellar floor reconstruction was achieved, using an autologous bone from a sphenoid septation designed in a quadrangular shape and positioned by four corners in the epidural space (Figure 3). In case of patients with strong risk factors of postoperative CSF leak (large skull base destruction due to invasive or giant pituitary adenomas, number of surgeries >2, history of prior radiotherapy), a multilayer reconstruction strategy was decided, using the double pedicled nasoseptal flap, as previously reported (45, 46).
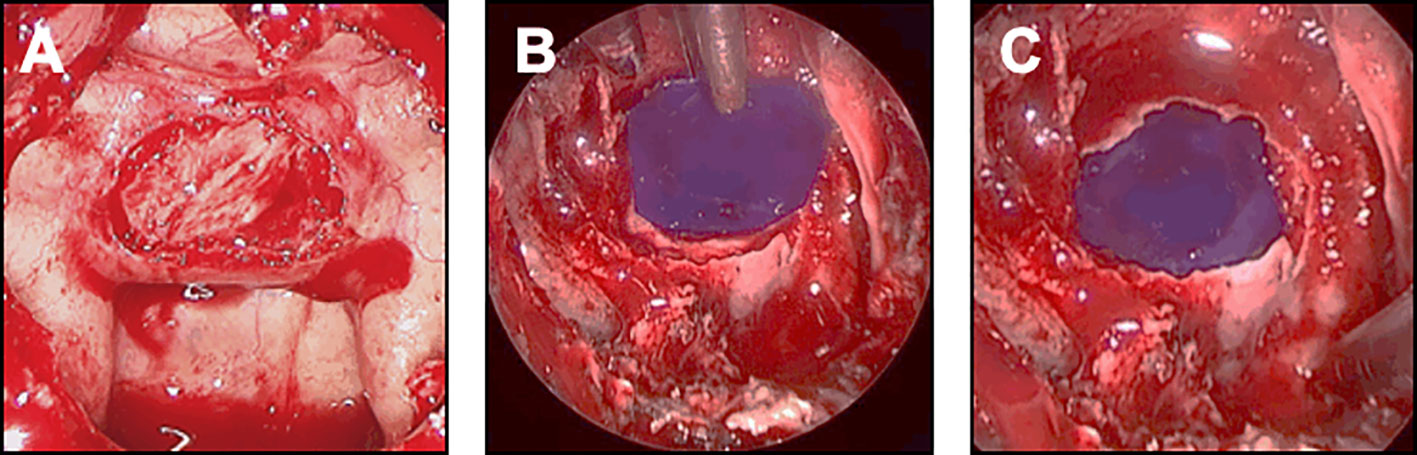
Figure 3 Closure strategy in patients with no CSF leak and no risk factor. For these patients, an optimal sellar floor closure should be achieved. (A) Sellar reconstruction with a bone graft. The piece of bone has been removed from the sphenoid rostrum or from a sphenoid septation, accurately designed and positioned in the previously prepared epidural space. (B, C) Sellar reconstruction with a synthetic polydioxanone (PDS) plate. (B) A synthetic polydioxanone (PDS) plate (in blue) has been shaped according to the sellar floor opening and introduced in the sphenoid sinus. The positioning always starts with the introduction of the two lower edges, the plate being held by a surgical forceps. (C) The two upper edges have been embedded so that the entire PDS plate was positioned in the epidural space.
Closure strategy
i. Patients with no risk factors of closure failure
In the absence of CSK leak, optimal bone sellar floor reconstruction was performed, as detailed above. In case of intraoperative CSF leak, the choice of the closure depended on the intensity of flow and the location of the leak.
In case of small leak due to diaphragm oozing, a collagen sponge coated with the human coagulation factors fibrinogen and thrombin (TachoSil®) was used. The sealant matrix was positioned in the intrasellar compartment, deployed, centered on the dural defect and applied against the diaphragm with the help of forceps holding a cottonoid and a suction tube (Figure 4A). The sellar floor was reconstructed and some biological glue was applied in the sphenoid sinus.
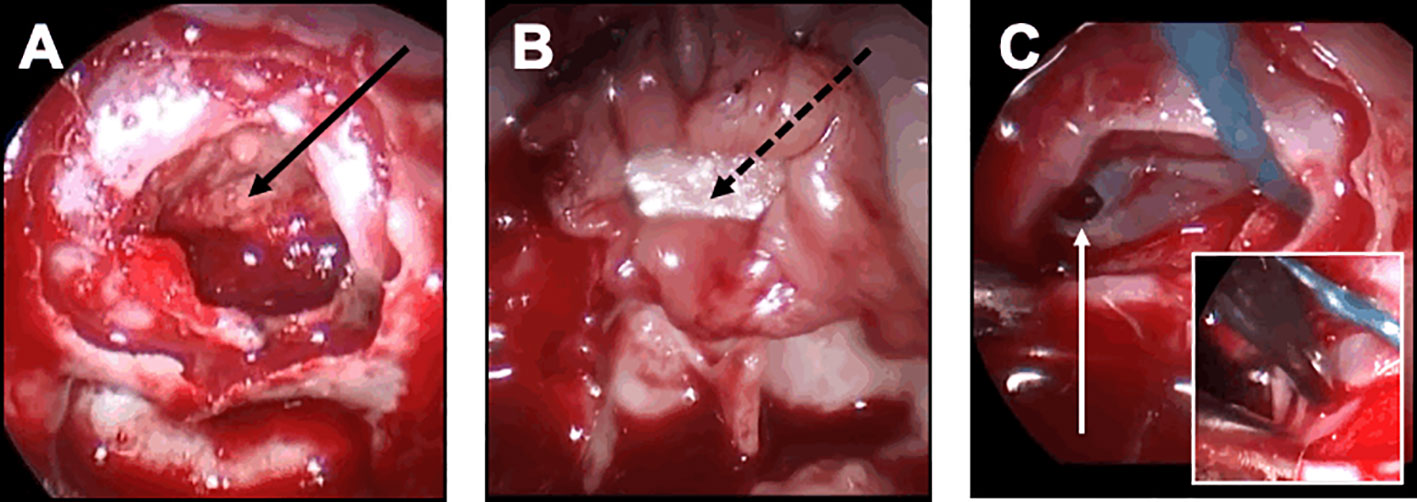
Figure 4 Closure strategy in patients with CSF leak and no risk factor. For these patients, the sella floor should be reconstructed and biological glue should be applied after the sealing has been achieved. (A, B) Minimal and diffuse flow diaphragm leak. (A) A sealant collagen sponge coated with the human coagulation factors fibrinogen and thrombin (black arrow) has been designed, positioned in the intrasellar compartment, deployed, centered on the dural defect and applied against the diaphragm with the help of a forceps holding a cottonoid and a suction tube. (B) An epidural duraplasty has been performed, using the mucosa of the right middle turbinate. After the mucosa has been embedded in the epidural space with a bone graft (black dotted arrow), the mucosa acts as a seal. (C) Focal low-flow leak. A unique focal defect is visualized on the right side of the diaphragm (white arrow). The sealing is achieved after coagulation of the dural defect using a bipolar forceps (insert).
In case of focal low-flow leak, an epidural duraplasty was performed, using a dural substitute. After a right middle turbinectomy was performed, the mucosa of the turbinate was removed away from the bone. The mucosal graft from the middle turbinate was positioned in the epidural space, with the support of a bone graft or a PDS plate embedded in an epidural fashion (Figures 4A, B). With this technique, the mucosa should act as a seal. As previously described, biological glue was applied. In rare cases of focal diaphragm defect with a distended diaphragm bulging into the intrasellar space, watertight closure could be achieved by coagulating the edges of the transdiaphragmatic orifice with bipolar forceps (Figure 4C).
In case of diffuse of high-flow leak, an intrasellar packing technique was decided. Fascia and muscle grafts were taken from the right thigh. The fascia was introduced into the sella and applied superiorly to cover the entire defect, in order to recreate a new diaphragm. The muscle graft was then positioned within the intrasellar compartment. As previously described, the sellar floor was reconstructed with bone graft or PDS plate and biological glue was applied.
ii. Patients with risk factors of closure failure
BMI>40 with intact sellar floor, no intraoperative leak
A standard closure was performed, as previously described.
Focal sellar floor lysis and BMI<40, no intraoperative leak
A poor sellar closure was usually achieved, using a bone graft or PDS plate positioned by only two or three corners in the epidural space
Focal sellar floor lysis and BMI>40, no intraoperative leak
An additional Foley catheter technique was decided in order to avoid the migration of the poor sellar reconstruction. A two-way Foley balloon catheter technique was used: the balloon stent was positioned inside the sphenoid sinus against the sella turcica to reinforce the reconstruction and counter the effects of graft migration. Usually, we used a Foley urinary catheter from 8 to 12 French filled up with saline solution, inflating it to be in contact with the graft. The catheter was left in place for 4 to 5 days.
Focal sellar floor lysis and/or BMI>40, intraoperative leak
All patients were treated with an intrasellar fascia and muscle graft, combined with an additional Foley balloon catheter. Biological glue was always applied. Repeated lumbar punctures were performed on day 1 and day 2 after surgery
Large skull base destruction and/or number of surgeries >2 and/or prior radiotherapy
In complex cases of patients with strong risk factors of closure failure, the following strategy was decided, regardless the occurrence of an intraoperative leak: a multilayer repair strategy was chosen with combined intrasellar fascia and muscle grafts, optimal epidural closure if possible, and double pedicled mucosal nasoseptal flap (Figure 5). Each left and right mucosal flap was elevated from each side of the nasal bone septum and pedicled on the sphenopalatine artery. The pedicled flap was applied against the sella turcica and held in place with a Foley catheter for 5 days. Biological glue was always applied. Repeated lumbar punctures were performed on day 1 and day 2 after surgery. This multilayer strategy was also decided in case of patients with BMI>40.
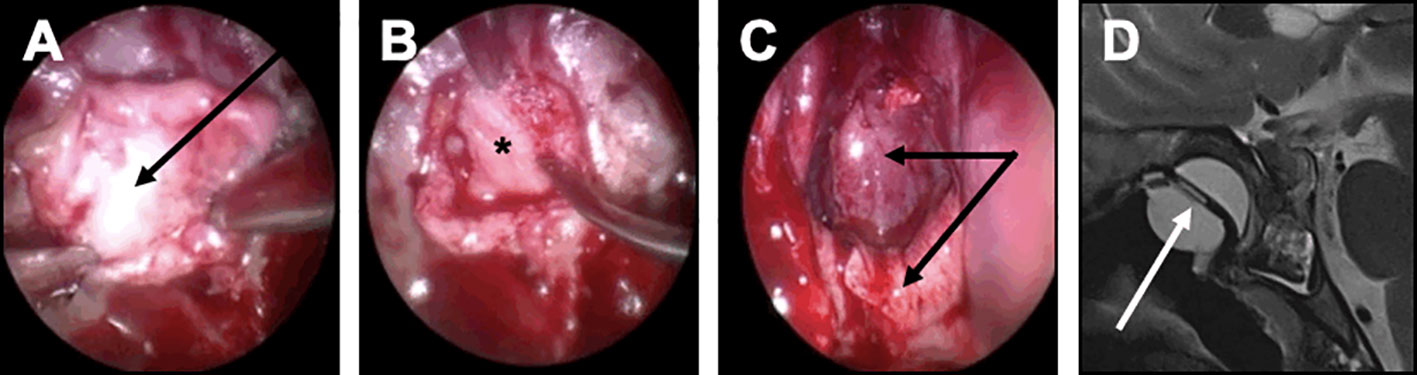
Figure 5 Closure strategy in patients with a risk factor. For these patients, a Foley urinary catheter is usually necessary and more complex multilayer closure strategies using a pedicled mucosal nasoseptal flap should be discussed. (A-D) Complex multilayer closure strategy for a patient with a recurrent pituitary adenoma treated with multiple surgery and radiotherapy. (A) After a muscle graft has been introduced in the intrasellar compartment, an autologous fascia lata graft has been positioned anterior to the sella turcica (black arrow). (B) After the fascia lata has been embedded in the epidural space with a bone graft (black asterisk), the primary sealing is obtained. (C) A vascularized mucosal nasoseptal flap is positioned and deployed in the sphenoid sinus (double black arrow), maintained using a Foley urinary catheter for 5 days. (D) Postoperative MRI showing the multilayer reconstruction and the Foley catheter (white arrow).
Closure evaluation
Closure was considered as achieved if no postoperative CSF leak was observed 2 months after surgery. Failure of closure management was diagnosed when a postoperative CSF leak occurred despite the closure strategy applied because of an intraoperative CSF leak during the first procedure, requiring a second surgery.
Data collection
The following variables were collected for each patient before, during, and after surgery.
i. Before surgery: age; sex; preoperative risk factors for closure failure.
ii. During surgery: intraoperative CSF leak; flow intensity and location of intraoperative CSF; type of closure strategy.
iii. After surgery: postoperative CSF leak; type of closure strategy; meningitis with bacteriological analysis if available.
Statistical analysis
Statistical analysis was performed using R statistical software (version 3.6.3). Descriptive statistics used median (range) for quantitative variables and raw numbers (%) for categorical variables.
Results
Characteristics of patients selected for surgery
The characteristics of 3015 patients operated on for an adenoma are provided in Table 1. Patients were mostly women (F/M ratio: 1.4), with a median age of 50 (range: 18 to 89). Hormone excess was observed in 1970/3015 (65.3%) of patients. Corticotroph adenomas with Cushing’s disease, somatotroph adenomas with acromegaly, lactotroph adenoma, thyrotroph adenoma and non-secreting adenomas were diagnosed in 822/3015 (27.3%), 768/3015 (25.5%), 331/3015 (11%), 49/3015 (1.6%) and 1045/3015 (34.6%) of patients respectively.
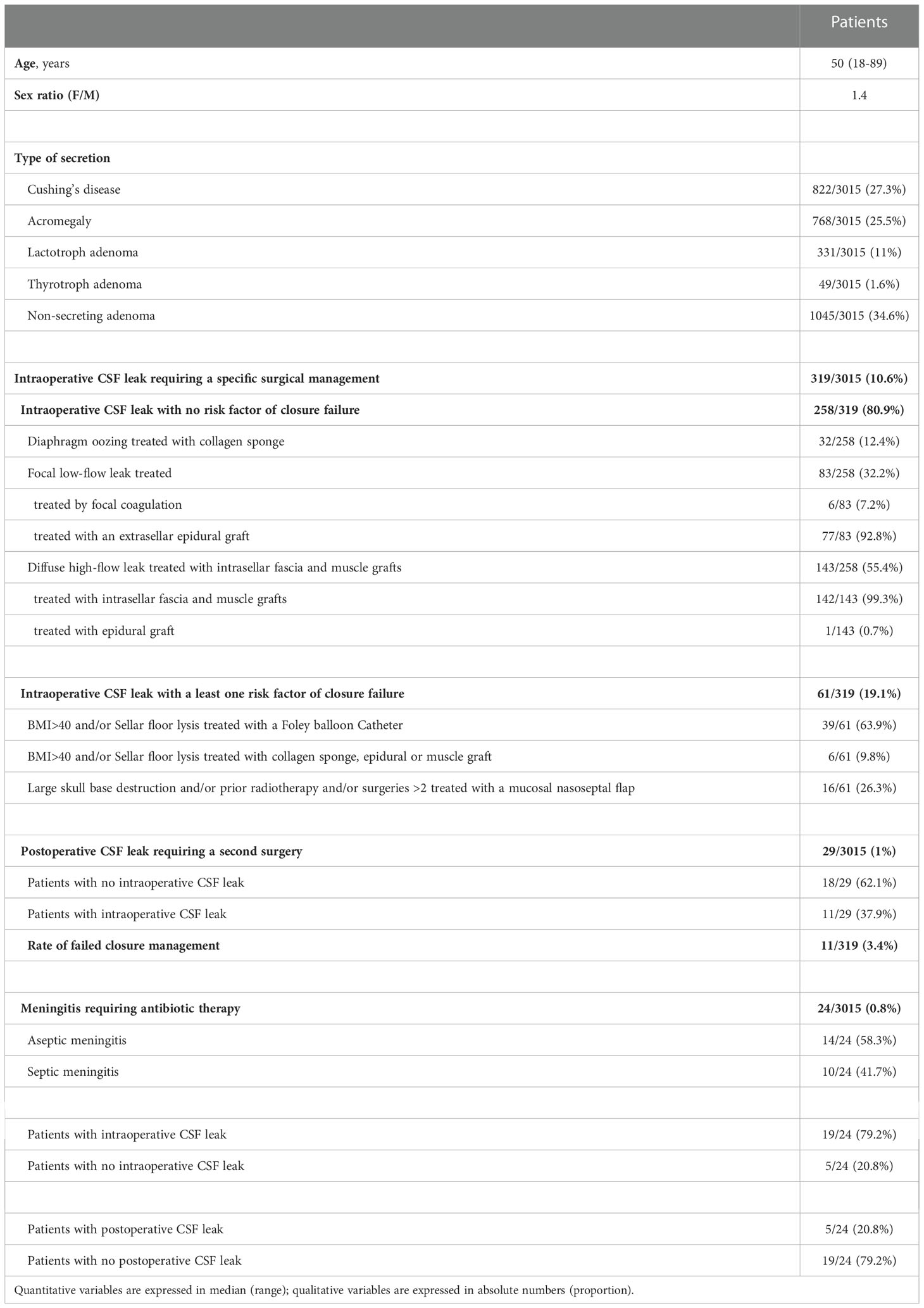
Table 1 Characteristics of 3015 adenoma patients treated with a mononostril endoscopic endonasal transsellar approach.
Intraoperative CSF leak
Intraoperative CSF leak requiring a specific surgical management was observed in 319/3015 (10.6%) of patients.
No preoperative risk factor of closure failure was noted in 258/319 (80.9%) of these patients. Diaphragm oozing was noted in 32/258 (12.4%) of patients and was treated with collagen sponge (TachoSil® patch). Focal low-flow leak was noted in 83/258 (32.2%) of patients, mainly treated with epidural graft and more rarely with bipolar coagulation. Diffuse high-flow leak was observed in 143/258 (55.4%) of patients and was treated with intrasellar fascia and muscle graft technique in the vast majority of cases.
Preoperative factors of closure failure were observed in 61/319 (19.1%) of patients. Considering BMI>40 and/or sellar floor lysis, 39/61 (63.9%) of patients were treated with the Foley-catheter technique, while 6/61 (9.8%) were treated using collagen sponge, epidural graft or muscle graft. The 16/61(26.3%) patients with large skull base destruction and/or prior radiation therapy and/or number of surgeries >2 were treated with a multilayer repair strategy with nasoseptal flaps.
Postoperative CSF leak
Postoperative CSF leak requiring a second surgery was observed in 29/3015 (1%) of patients.
Among these patients, 18/29 (62.1%) had no intraoperative CSF leak. No risk factors for closure failure were noted in these 18 patients. In 2 patients, a postoperative leak occurred 5 and 6 days after surgery, in the context of nose blowing.
In contrast, 11/29 (37.9%) of patients had a well-identified intraoperative leak requiring a dedicated closure technique, as detailed in Table 2. The majority of these 11 patients had preoperative risk factors (n=7/11, 63.6%), mostly with BMI >40. Diaphragm oozing, low-flow leak and high-flow were identified in 2/11 (18.2%), 4/11 (36.4%) and 5/11 (45.4%) of patients respectively. All these 11 patients were reoperated on, using an intrasellar fascia and muscle graft combined with external lumbar drainage in 3 patients (before 2014) and Foley-catheter or mucosal flap in 2 patients (after 2014). A permanent lumboperitoneal shunt was needed in 2 patients. No difference in postoperative leak rate was observed after the change of our surgical strategy in 2014 (5 patients identified before 2014 and 6 patients identified after 2014). Considering the 11 patients with both intra and postoperative leak and all 319 patients with intraoperative leak, the rate of failed closure was 3.4%. The closure failure was mainly due to poor analysis of intraoperative CSF flow.
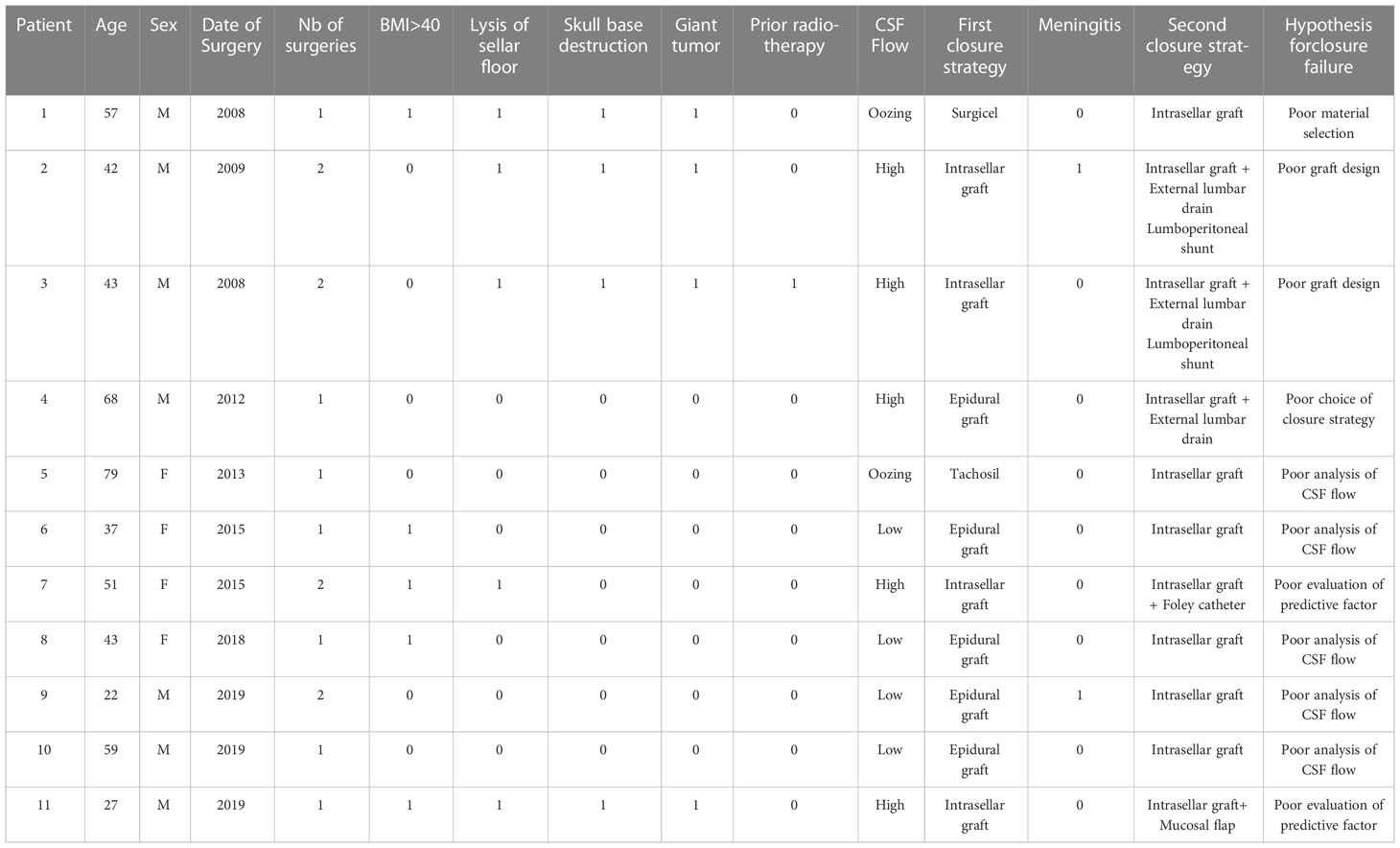
Table 2 Characteristics of the 11 patients with failed closure management despite a well-identified intraoperative CSF leak.
Postoperative meningitis
Meningitis occurred in 24/3015 (0.8%) of patients. Aseptic meningitis, diagnosed on the basis of fever, clinical symptoms and repeated CSF examinations (cells increase, low glucose, high protein), was found in 14/24 (58.3%) of patients, while septic meningitis with a well-documented bacteriological analysis was reported in 10/24 (41.7%) of patients. CSF examination revealed Gram-positive bacteria in 6 patients (Coagulase-negative staphylococci in 4 patients and Staphyloccocus aureus in 2 patients) and Gram-negative in 4 patients (Klebsiella pneumonia in 3 patients and Escherichia coli in 1 patient). All patients were treated with antibiotic therapy and had a favorable outcome.
Intraoperative leak was observed in 19/24 (79.2%) of patients with meningitis, while postoperative leak was observed in 5/24 (20.8%) of patients.
Discussion
This paper focuses on the evaluation of the closure step in pituitary surgery, based on a large series of 3015 adenoma patients treated with a mononostril endoscopic endonasal transsellar approach by the same expert surgical team. In PitNETs, invasion of the basal dura, cavernous sinus and/or diaphragm is encountered in up to 35% of patients (2), explaining the substantial occurrence of intraoperative leak requiring reliable watertight closure techniques. Initially the endoscopic technique was associated with high reported rates of postoperative CSF leak, ranging from 30 to 40% (47). With advances in endoscopic pituitary surgery and the development of new reconstruction techniques, optimized lower rates <10 % are currently reported (24, 40, 48–50). The present study demonstrates that our stepwise strategy is safe, reliable and effective. Applying our decision strategy, our rates of postoperative CSF leak and meningitis were 1% and 0.8% respectively, which compares favorably with the best rates previously reported (34, 51).
In pituitary surgery, the crucial step of closure should be anticipated. The neurosurgeon should keep in mind that the closure step already begins during the approach. Even if no intraoperative leak occurs, the overall concept is to preserve a good epidural space during the approach and to recreate a sellar floor whenever possible. Indeed, sellar floor closure is useful to identify bony landmarks in the event of a later second surgery and may limit the risk of postoperative CSF leak related to a secondary stall of the diaphragm during sneezing or blowing. During the approach, the sellar floor is opened is such a way that the bone opening is larger than the dural opening, in order to preserve the epidural space (34, 38, 52). When possible, an autologous bone graft is removed and preserved from the sphenoidal rostrum and/or one of a sphenoid sinus septation. If no bone graft is available, a synthetic resorbable polydioxanone (PDS) plate can be used with similar shaping and positioning (53). We choose to use the PDS material because of its stiffness, which is close to that of bone. We recommend the use of a resorbable sellar floor substitute to limit the risk of infectious complications.
In case of intraoperative CSF leak, our patients were divided into two groups on the basis of selected preoperative risk factors of closure failure (28, 29, 43). A specific gradual closure strategy was planned accordingly. Interestingly, other preoperative risk factors of closure failure have been reported recently by expert teams, such as suprasellar extension, chronic respiratory disease, type of sellar barrier, fibrous consistency, dumbbell-shape or lobulated asymmetrical configuration (42). A comprehensive analysis of all these factors should allow surgical management to be tailored to the individual patient.
In patients with no risk factor, the objective is to achieve a watertight closure and reconstruct the sellar floor. Different materials have been proposed for the reconstruction of skull-base defects. Autologous materials have been proposed, such as mucosal grafts from middle turbinate, fat grafts from abdominal region, muscle graft from lateral thigh, fascia grafts from fascia lata, lateral thigh or temporal muscle (31, 32, 48, 53–58). Heterologous biologic dural substitutes have been used, such as equine pericardium sheet (59) or human-derived acellular dermal matrix (60). Heterologous synthetic dural substitutes have also been proposed, such as polyester-silicone (61), resorbable polyglactin acid sheet (62), polytetrafluoroethylene (63) or collagen matrix (64, 65). At the end of the closure procedure, fibrin glue should is usually applied inside the sphenoidal cavity to fill the dead spaces (66–68). In our strategy, the key objective was to minimize the risk of postoperative leak while minimizing the proportion of patients treated by packing the sella or sphenoid sinus. Indeed, packing the sellar area with additional fibrous scar tissue may impact on postoperative MRI analysis and alter the quality of surgical landmarks in case of repeated surgeries. The choice of the closure technique depends on the flow intensity and on the leak location, as proposed by Conger et al. (34). In case of minimal diaphragm oozing, a simple treatment with a collagen sponge can be chosen (64, 66, 69). Collagen sponge has the great advantage to be easy to use. However, if the CSF is underestimated, this closure technique will be insufficient and a postoperative CSF leak will occur, requiring a second surgery. We therefore recommend spending a lot of time analyzing the intensity of CSF flow and adopting a safer alternative reconstruction strategy when in doubt. Focal low-flow intraoperative leak requires a stronger closure strategy: although an adequate epidural graft is more complex to perform, this strategy is well suited in this case, with a high rate of watertight closure without intrasellar packing. An epidural duraplasty can be performed, using a dural substitute, such as mucosa from a middle turbinate or fascia lata, held in place by a rigid buttress, as previously reported under different names, such as the gasket seal technique (34, 52, 70). However, the surgeon should be aware that if part of this duraplasty is positioned inside the intradural intrasellar space, the strategy will not prevent a postoperative leak. In our experience, the graft will paradoxically have a “gutter effect” with an increased risk of postoperative leak. Of note, in rare cases of focal diaphragm defect with a distended diaphragm bulging into the intrasellar space, a watertight closure can be elegantly obtained by coagulating the edges of the transdiaphragmatic orifice with bipolar forceps. In case of high-flow leak, the objective is to achieve a strong and persistent watertight closure with an intrasellar packing. Some authors recommend to use intrasellar fat and glue (33, 57, 71–75) with low rates of postoperative leak. In our experience, we prefer to use a fascia and muscle graft to achieve a two-layer intrasellar repair: the fascia is introduced into the sella and applied superiorly to cover the entire defect, in order to recreate a new diaphragm; the muscle graft is then positioned in the intrasellar compartment. Special care must be taken in the design of the muscle graft to avoid significant mass effect with compression of the optic chiasma.
In patients with risk factors, more complex strategies should be decided. In case of sellar floor lysis, bone may be missing, which is why no solid epidural buttress can be performed. Solutions with a buttress positioned within the sphenoid sinus have been developed. Some authors proposed a fat buttress to pack the sphenoid sinus (33, 57, 76, 77). The disadvantage of this technique is that the counterpressure is not applied in a targeted manner against the sellar floor and the fibrous scarring may complicate the surgical approach if further intervention is required. For these reasons, we prefer to use a Foley balloon catheter inflated within the sphenoid sinus for a few days, as previously described (78). Thus, the transient buttress is directly applied against the sella floor until the intrasellar muscle graft can no longer move. In more complex patients with giant tumors and large dural defects, a multilayer strategy with mucosal flap is usually recommended. Vascularized grafts have been a surgical revolution in preventing postoperative CSF leak, especially in patients with risk factors (24, 25, 48, 49, 79, 80). Different types of vascularized grafts have been described: unilateral or bilateral mucosal flaps originating from nasal septum or inferior turbinate; pericranium or temporal fascia flaps (45, 46, 48, 53, 54, 56, 81, 82). The choice of using vascularized flaps involves more complex skull base reconstruction techniques with multilayered closure and should balance the risk of postoperative CSF leak versus the morbidity of the flap itself (30, 83–85).
All 29 patients with postoperative CSF leak were reoperated with a dedicated upgraded closure strategy. Our management has evolved over the time. Indeed, the use of external lumbar drainage in these patients is associated with a higher risk of complication (86, 87). Of note, as previously reported, there is a lack of statistically significant improvement between patients with lumbar drains and patients with no lumbar drains and graded reconstruction strategies without lumbar drainage have been proposed (39, 88–90). Today, external lumbar drainage has been abandoned for the overwhelming majority of our patients treated with simple transsellar approach.
Postoperative CSF leak occurred in 18 patients with no intraoperative CSF leak identified. In 2 patients, the postoperative leak was obviously caused by inappropriate postoperative nose blowing. In 16 patients, the complication may have been due to intraoperative surgical misinterpretation, related to unnoticed diaphragm oozing.
Despite of our closure strategy, surgery failed in 11 patients with a well-identified intraoperative CSF leak, leading to an overall closure failure rate of 3.4%. Interestingly, most of the patients were treated before 2018, suggesting that this type of complication may decrease with endoscopic surgical experience, as previously proposed (91). Firstly, lack of experience with endoscopic techniques may have led to poor material selection or poor graft design, as previously reported (92–94). Secondly, the majority of these patients had at least one risk factor of closure failure. Thus, surgical failure was also explained by underestimated risk factors, such as severe obesity; BMI>40 is associated with increased intracranial pressure (44), which may affect the quality of closure (29). In patients with BMI>40 and focal sellar floor lysis, we now recommend the use of an additional Foley-catheter combined with repeated lumbar punctures, even if no CSF occurred during surgery. Closure failure was finally caused by poor operative conditions reducing the quality of vision and impacting on the analysis of CSF flow.
Our rate of postoperative meningitis was 0.8%, with a majority of aseptic meningitis. This result is in accordance with previous studies from pituitary centers of excellence (51, 95). In patients with no identified bacteria, antibiotic treatment was indicated on the basis of combined neurological symptoms (fever, meningeal syndrome) and analysis of repeated CSF examinations (cells increase, low glucose, high protein). These patients may have been overtreated. Nevertheless, all symptoms improved immediately after treatment was introduced, suggesting that a small bacterial inoculum was still present. In patients with septic meningitis, drug-sensitive Gram-positive positive organisms were predominant. All patients were treated by antibiotic therapy, with favorable outcome. Our results confirmed the data published by Jin et al. in a large retrospective study of 3242 patients (51). Interestingly, the vast majority of our patients (79.2%) with meningitis had a well-identified intraoperative leak, whereas a postoperative CSF leak was observed in only 20.8% of these patients. This result may suggest that the duration of CSF leak is not a strong predictor of postoperative meningitis. More data are needed to confirm this finding.
The main strength of this major study was the consecutive inclusion of all cases of adenoma patients treated with an endoscopic endonasal transsellar approach, reaching the substantial number of 3015 patients. Conversely, the inclusion of patients operated on by a single surgical team may be considered as a limitation. However, as mainly reported, the high expertise of surgical centers is essential (92, 96, 97). In this series, all patients were operated on by two experienced neurosurgeons treating >200 adenomas per year, after surgical indication was validated in a multidisciplinary meeting. If the expertise is not guaranteed, it can be hypothesized that outcome would become less favorable. Thus, at the beginning of the learning curve or if there is any doubt about a high-flow leak, safety should be paramount and intrasellar packing should be chosen. Due to the length of the study, our list of predictive factors is not exhaustive, which is a limitation of the present work. The main objective was to propose a graded closure strategy with excellent efficacy, based on our significant experience. Further studies with stronger decision-making paradigms, including more predictive factors, are needed in the future.
In conclusion, in pituitary surgery, the closure step should not be underestimated. By using a rigorous strategy, the postoperative leak rate can be reduced to 1% of patients. This crucial step must be planned before surgery and gently prepared during the surgical approach.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
In accordance with the French legislation, patient consent was not needed for this retrospective non-interventional study evaluating a routine care. An agreement was obtained after ethical acceptance of the study by the General Register of the Assistance Publique des Hôpitaux de Paris - Sorbonne University (registered under “CLOSPIT” Study, N°: 202211210146).
Author contributions
BB, AV, and SG contributed to conception and design of the study. BB, AJ, and AV organized the database. BB and AJ performed the statistical analysis. AV wrote the first draft of the manuscript. BB, AV, AJ, GR, and SG wrote sections of the manuscript. BB, AJ, GR, and SG contributed to interpretation of data for work. All authors contributed to manuscript revision, read, and approved the submitted version
Acknowledgments
We thank Pr Paolo Cappabianca for his thoughtful and expert comments. We thank Dr Charlotte Bellamy for her useful grammar and spelling corrections.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: A systematic review. Cancer (2004) 101:613–9. doi: 10.1002/cncr.20412
2. Trouillas J, Jaffrain-Rea M-L, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers (Basel) (2020) 12:E514. doi: 10.3390/cancers12020514
3. Asa SL, Casar-Borota O, Chanson P, Delgrange E, Earls P, Ezzat S, et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An international pituitary pathology club proposal. Endocr Relat Cancer (2017) 24:C5–8. doi: 10.1530/ERC-17-0004
4. Laws ER, Penn DL, Repetti CS. Advances and controversies in the classification and grading of pituitary tumors. J Endocrinol Invest (2019) 42:129–35. doi: 10.1007/s40618-018-0901-5
5. Dekkers OM, Karavitaki N, Pereira AM. The epidemiology of aggressive pituitary tumors (and its challenges). Rev Endocr Metab Disord (2020) 21:209–12. doi: 10.1007/s11154-020-09556-7
6. Neou M, Villa C, Armignacco R, Jouinot A, Raffin-Sanson M-L, Septier A, et al. Pangenomic classification of pituitary neuroendocrine tumors. Cancer Cell (2020) 37:123–134.e5. doi: 10.1016/j.ccell.2019.11.002
7. Vroonen L, Daly AF, Beckers A. Epidemiology and management challenges in prolactinomas. Neuroendocrinology (2019) 109:20–7. doi: 10.1159/000497746
8. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2011) 96:273–88. doi: 10.1210/jc.2010-1692
9. Cushing H. III. partial hypophysectomy for acromegaly: With remarks on the function of the hypophysis. Ann Surg (1909) 50:1002–17. doi: 10.1097/00000658-190912000-00003
10. Jane JA, Catalino MP, Laws ER. Surgical treatment of pituitary adenomas, in: Endotext (2000). South Dartmouth (MA: MDText.com, Inc. Available at: http://www.ncbi.nlm.nih.gov/books/NBK278983/ (Accessed May 9, 2021).
11. Liu JK, Weiss MH, Couldwell WT. Surgical approaches to pituitary tumors. Neurosurg Clin N Am (2003) 14:93–107. doi: 10.1016/s1042-3680(02)00033-5
12. Buchfelder M, Schlaffer S. Surgical treatment of pituitary tumours. Best Pract Res Clin Endocrinol Metab (2009) 23:677–92. doi: 10.1016/j.beem.2009.05.002
13. Youssef AS, Agazzi S, van Loveren HR. Transcranial surgery for pituitary adenomas. Neurosurgery (2005) 57:168–75. doi: 10.1227/01.neu.0000163602.05663.86
14. Guiot G, Derome P. Surgical approaches to hypophyseal adenomas. Rev Otoneuroophtalmol (1974) 46:337–46.
15. Hardy J. Transphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg (1969) 16:185–217. doi: 10.1093/neurosurgery/16.cn_suppl_1.185
16. Patel SK, Husain Q, Eloy JA, Couldwell WT, Liu JK. Norman Dott, Gerard guiot, and Jules hardy: Key players in the resurrection and preservation of transsphenoidal surgery. Neurosurg Focus (2012) 33:E6. doi: 10.3171/2012.6.FOCUS12125
17. Li A, Liu W, Cao P, Zheng Y, Bu Z, Zhou T. Endoscopic versus microscopic transsphenoidal surgery in the treatment of pituitary adenoma: A systematic review and meta-analysis. World Neurosurg (2017) 101:236–46. doi: 10.1016/j.wneu.2017.01.022
18. Gaillard S. The transition from microscopic to endoscopic transsphenoidal surgery in high-caseload neurosurgical centers: The experience of foch hospital. World Neurosurg (2014) 82:S116–120. doi: 10.1016/j.wneu.2014.07.033
19. Eseonu CI, ReFaey K, Rincon-Torroella J, Garcia O, Wand GS, Salvatori R, et al. Endoscopic versus microscopic transsphenoidal approach for pituitary adenomas: Comparison of outcomes during the transition of methods of a single surgeon. World Neurosurg (2017) 97:317–25. doi: 10.1016/j.wneu.2016.09.120
20. Baussart B, Declerck A, Gaillard S. Mononostril endoscopic endonasal approach for pituitary surgery. Acta Neurochir (Wien) (2021) 163:655–9. doi: 10.1007/s00701-020-04542-z
21. Rotenberg B, Tam S, Ryu WHA, Duggal N. Microscopic versus endoscopic pituitary surgery: a systematic review. Laryngoscope (2010) 120:1292–7. doi: 10.1002/lary.20949
22. Asemota AO, Ishii M, Brem H, Gallia GL. Comparison of complications, trends, and costs in endoscopic vs microscopic pituitary surgery: Analysis from a US health claims database. Neurosurgery (2017) 81:458–72. doi: 10.1093/neuros/nyx350
23. Naunheim MR, Sedaghat AR, Lin DT, Bleier BS, Holbrook EH, Curry WT, et al. Immediate and delayed complications following endoscopic skull base surgery. J Neurol Surg B Skull Base (2015) 76:390–6. doi: 10.1055/s-0035-1549308
24. Fraser S, Gardner PA, Koutourousiou M, Kubik M, Fernandez-Miranda JC, Snyderman CH, et al. Risk factors associated with postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery. J Neurosurg (2018) 128:1066–71. doi: 10.3171/2016.12.JNS1694
25. Karnezis TT, Baker AB, Soler ZM, Wise SK, Rereddy SK, Patel ZM, et al. Factors impacting cerebrospinal fluid leak rates in endoscopic sellar surgery. Int Forum Allergy Rhinol (2016) 6:1117–25. doi: 10.1002/alr.21783
26. Klatt-Cromwell CN, Thorp BD, Del Signore AG, Ebert CS, Ewend MG, Zanation AM. Reconstruction of skull base defects. Otolaryngol Clin North Am (2016) 49:107–17. doi: 10.1016/j.otc.2015.09.006
27. Patel PN, Stafford AM, Patrinely JR, Smith DK, Turner JH, Russell PT, et al. Risk factors for intraoperative and postoperative cerebrospinal fluid leaks in endoscopic transsphenoidal sellar surgery. Otolaryngol Head Neck Surg (2018) 158:952–60. doi: 10.1177/0194599818756272
28. Jahangiri A, Wagner J, Han SW, Zygourakis CC, Han SJ, Tran MT, et al. Morbidity of repeat transsphenoidal surgery assessed in more than 1000 operations. J Neurosurg (2014) 121:67–74. doi: 10.3171/2014.3.JNS131532
29. Dlouhy BJ, Madhavan K, Clinger JD, Reddy A, Dawson JD, O’Brien EK, et al. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg (2012) 116:1311–7. doi: 10.3171/2012.2.JNS111837
30. Peris-Celda M, Chaskes M, Lee DD, Kenning TJ, Pinheiro-Neto CD. Optimizing sellar reconstruction after pituitary surgery with free mucosal graft: Results from the first 50 consecutive patients. World Neurosurg (2017) 101:180–5. doi: 10.1016/j.wneu.2017.01.102
31. Mattavelli D, Schreiber A, Villaret AB, Accorona R, Turri-Zanoni M, Lambertoni A, et al. Complications and donor site morbidity of 3-layer reconstruction with iliotibial tract of the anterior skull base: Retrospective analysis of 186 patients. Head Neck (2018) 40:63–9. doi: 10.1002/hed.24931
32. Patel MR, Stadler ME, Snyderman CH, Carrau RL, Kassam AB, Germanwala AV, et al. How to choose? endoscopic skull base reconstructive options and limitations. Skull Base (2010) 20:397–404. doi: 10.1055/s-0030-1253573
33. Esposito F, Dusick JR, Fatemi N, Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper Neurosurg (Hagerstown) (2007) 60:295–303. doi: 10.1227/01.NEU.0000255354.64077.66
34. Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, Esposito F, et al. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg (2018) 130:861–75. doi: 10.3171/2017.11.JNS172141
35. Patel KS, Komotar RJ, Szentirmai O, Moussazadeh N, Raper DM, Starke RM, et al. Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg (2013) 119:661–8. doi: 10.3171/2013.4.JNS13124
36. Strickland BA, Lucas J, Harris B, Kulubya E, Bakhsheshian J, Liu C, et al. Identification and repair of intraoperative cerebrospinal fluid leaks in endonasal transsphenoidal pituitary surgery: surgical experience in a series of 1002 patients. J Neurosurg (2018) 129:425–9. doi: 10.3171/2017.4.JNS162451
37. CRANIAL Consortium. CSF rhinorrhoea after endonasal intervention to the skull base (CRANIAL) - part 1: Multicenter pilot study. World Neurosurg (2021) 149:e1077–89. doi: 10.1016/j.wneu.2020.12.171
38. Tabaee A, Anand VK, Brown SM, Lin JW, Schwartz TH. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope (2007) 117:1133–7. doi: 10.1097/MLG.0b013e31805c08c5
39. Ha C-M, Hong SD, Choi JW, Seol HJ, Nam D-H, Lee J-I, et al. Graded reconstruction strategy using a multilayer technique without lumbar drainage after endoscopic endonasal surgery. World Neurosurg (2021) 158:e451–8. doi: 10.1016/j.wneu.2021.11.003
40. Ogiwara T, Nagm A, Hasegawa T, Hanaoka Y, Ichinose S, Goto T, et al. Pitfalls of skull base reconstruction in endoscopic endonasal approach. Neurosurg Rev (2019) 42:683–9. doi: 10.1007/s10143-018-1006-5
41. Dorismond C, Santarelli GD, Thorp BD, Kimple AJ, Ebert CS, Zanation AM. Heterogeneity in outcome reporting in endoscopic endonasal skull base reconstruction: A systematic review. J Neurol Surg B Skull Base (2021) 82:506–21. doi: 10.1055/s-0040-1714108
42. Villalonga JF, Solari D, Cavallo LM, Cappabianca P, Prevedello DM, Carrau R, et al. The sellar barrier on preoperative imaging predicts intraoperative cerebrospinal fluid leak: a prospective multicenter cohort study. Pituitary (2021) 24:27–37. doi: 10.1007/s11102-020-01082-8
43. Staartjes VE, Zattra CM, Akeret K, Maldaner N, Muscas G, Bas van Niftrik CH, et al. Neural network-based identification of patients at high risk for intraoperative cerebrospinal fluid leaks in endoscopic pituitary surgery. J Neurosurg (2019), 21:1–7. doi: 10.3171/2019.4.JNS19477
44. Subramaniam S, Fletcher WA. Obesity and weight loss in idiopathic intracranial hypertension: A narrative review. J Neuroophthalmol (2017) 37:197–205. doi: 10.1097/WNO.0000000000000448
45. Baussart B, Racy E, Gaillard S. Double pedicled nasoseptal flap for skull base repair after endoscopic expanded endonasal approach. Acta Neurochir (2022) 164(4):1111–4. doi: 10.1007/s00701-021-05094-6
46. Nyquist GG, Anand VK, Singh A, Schwartz TH. Janus flap: bilateral nasoseptal flaps for anterior skull base reconstruction. Otolaryngol Head Neck Surg (2010) 142:327–31. doi: 10.1016/j.otohns.2009.12.020
47. Kassam A, Carrau RL, Snyderman CH, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus (2005) 19:E8.
48. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope (2006) 116:1882–6. doi: 10.1097/01.mlg.0000234933.37779.e4
49. Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope (2012) 122:452–9. doi: 10.1002/lary.22475
50. Schuss P, Hadjiathanasiou A, Klingmüller D, Güresir Á, Vatter H, Güresir E. Transsphenoidal pituitary surgery: Comparison of two sellar reconstruction techniques and their effect on postoperative cerebrospinal fluid leakage. Neurosurg Rev (2018) 41:1053–8. doi: 10.1007/s10143-018-0949-x
51. Jin Y, Liu X, Gao L, Guo X, Wang Q, Bao X, et al. Risk factors and microbiology of meningitis and/or bacteremia after transsphenoidal surgery for pituitary adenoma. World Neurosurg (2018) 110:e851–63. doi: 10.1016/j.wneu.2017.11.125
52. Garcia-Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg (2013) 80:563–8. doi: 10.1016/j.wneu.2011.08.034
53. Di Perna G, Penner F, Cofano F, De Marco R, Baldassarre BM, Portonero I, et al. Skull base reconstruction: A question of flow? a critical analysis of 521 endoscopic endonasal surgeries. PloS One (2021) 16:e0245119. doi: 10.1371/journal.pone.0245119
54. Hannan CJ, Kelleher E, Javadpour M. Methods of skull base repair following endoscopic endonasal tumor resection: A review. Front Oncol (2020) 10:1614. doi: 10.3389/fonc.2020.01614
55. Kimple AJ, Leight WD, Wheless SA, Zanation AM. Reducing nasal morbidity after skull base reconstruction with the nasoseptal flap: Free middle turbinate mucosal grafts. Laryngoscope (2012) 122:1920–4. doi: 10.1002/lary.23325
56. Reyes C, Mason E, Solares CA. Panorama of reconstruction of skull base defects: from traditional open to endonasal endoscopic approaches, from free grafts to microvascular flaps. Int Arch Otorhinolaryngol (2014) 18:S179–186. doi: 10.1055/s-0034-1395268
57. Roca E, Penn DL, Safain MG, Burke WT, Castlen JP, Laws ER. Abdominal fat graft for sellar reconstruction: Retrospective outcomes review and technical note. Oper Neurosurg (Hagerstown) (2019) 16:667–74. doi: 10.1093/ons/opy219
58. Sciarretta V, Mazzatenta D, Ciarpaglini R, Pasquini E, Farneti G, Frank G. Surgical repair of persisting CSF leaks following standard or extended endoscopic transsphenoidal surgery for pituitary tumor. Minim Invasive Neurosurg (2010) 53:55–9. doi: 10.1055/s-0029-1246161
59. Cavallo LM, Solari D, Somma T, Di Somma A, Chiaramonte C, Cappabianca P. Use of equine pericardium sheet (LYOMESH®) as dura mater substitute in endoscopic endonasal transsphenoidal surgery. Transl Med UniSa (2013) 7:23–8.
60. Gaynor BG, Benveniste RJ, Lieberman S, Casiano R, Morcos JJ. Acellular dermal allograft for sellar repair after transsphenoidal approach to pituitary adenomas. J Neurol Surg B Skull Base (2013) 74:155–9. doi: 10.1055/s-0033-1338263
61. Cappabianca P, Cavallo LM, Mariniello G, de Divitiis O, Romero AD, de Divitiis E. Easy sellar reconstruction in endoscopic endonasal transsphenoidal surgery with polyester-silicone dural substitute and fibrin glue: Technical note. Neurosurgery (2001) 49:473–5. doi: 10.1097/00006123-200108000-00042
62. Yano S, Tsuiki H, Kudo M, Kai Y, Morioka M, Takeshima H, et al. Sellar repair with resorbable polyglactin acid sheet and fibrin glue in endoscopic endonasal transsphenoidal surgery. Surg Neurol (2007) 67:59–64. doi: 10.1016/j.surneu.2006.05.049
63. Sherman JH, Pouratian N, Okonkwo DO, Jane JA, Laws ER. Reconstruction of the sellar dura in transsphenoidal surgery using an expanded polytetrafluoroethylene dural substitute. Surg Neurol (2008) 69:73–6. doi: 10.1016/j.surneu.2007.07.069
64. Jiménez Zapata HD, Rodríguez Berrocal V, Vior Fernández C, Sánchez FM, García Fernández A. Sellar diaphragm reconstruction with tachosil during endoscopic endonasal surgery: Technical note. J Neurol Surg B Skull Base (2020) 81:275–9. doi: 10.1055/s-0039-1688781
65. Shahein M, Montaser AS, Barbero JMR, Maza G, Todeschini AB, Otto BA, et al. Collagen matrix with mucoperiosteum graft as an effective fatless flapless reconstruction after endoscopic pituitary adenoma resection. Oper Neurosurg (Hagerstown) (2020) 19:E573–80. doi: 10.1093/ons/opaa212
66. Esposito F, Angileri FF, Kruse P, Cavallo LM, Solari D, Esposito V, et al. Fibrin sealants in dura sealing: A systematic literature review. PloS One (2016) 11:e0151533. doi: 10.1371/journal.pone.0151533
67. Cappabianca P, Esposito F, Magro F, Cavallo LM, Solari D, Stella L, et al. Natura abhorret a vacuo–use of fibrin glue as a filler and sealant in neurosurgical “dead spaces”. Tech Acta Neurochir (Wien) (2010) 152:897–904. doi: 10.1007/s00701-009-0580-2
68. Dusick JR, Mattozo CA, Esposito F, Kelly DF. BioGlue for prevention of postoperative cerebrospinal fluid leaks in transsphenoidal surgery: A case series. Surg Neurol (2006) 66:371–6. doi: 10.1016/j.surneu.2006.06.043
69. Spitaels J, Moore J, Zaidman N, Arroteia IF, Appelboom G, Barrit S, et al. Fibrin-coated collagen fleece versus absorbable dural sealant for sellar closure after transsphenoidal pituitary surgery: a comparative study. Sci Rep (2022) 12:7998. doi: 10.1038/s41598-022-12059-x
70. Jane JA. “Gasket-seal” closure for cerebrospinal fluid leaks. World Neurosurg (2013) 80:491–2. doi: 10.1016/j.wneu.2011.10.007
71. Villalonga JF, Solari D, Guizzardi G, Scala MR, Campero A. Fat and fibrin glue: Quo vadis? Turk Neurosurg (2021) 31:238–46. doi: 10.5137/1019-5149.JTN.29712-20.2
72. Ahn S, Park J-S, Kim DH, Kim SW, Jeun S-S. Surgical experience in prevention of postoperative CSF leaks using abdominal fat grafts in endoscopic endonasal transsphenoidal surgery for pituitary adenomas. J Neurol Surg B Skull Base (2021) 82:522–7. doi: 10.1055/s-0040-1712179
73. Laws ER. Autograft fat in neurological surgery. World Neurosurg (2013) 80:489–90. doi: 10.1016/j.wneu.2012.11.006
74. Ziu M, Jimenez DF. The history of autologous fat graft use for prevention of cerebrospinal fluid rhinorrhea after transsphenoidal approaches. World Neurosurg (2013) 80:554–62. doi: 10.1016/j.wneu.2012.08.001
75. Cavallo LM, Solari D, Somma T, Cappabianca P. The 3F (Fat, flap, and flash) technique for skull base reconstruction after endoscopic endonasal suprasellar approach. World Neurosurg (2019) 126:439–46. doi: 10.1016/j.wneu.2019.03.125
76. Wei CC, Palmer JN. Planum, tubercular, sellar and clival defects. Adv Otorhinolaryngol (2013) 74:119–29. doi: 10.1159/000342288
77. Citardi MJ, Cox AJ, Bucholz RD. Acellular dermal allograft for sellar reconstruction after transsphenoidal hypophysectomy. Am J Rhinol (2000) 14:69–73. doi: 10.2500/105065800781602920
78. Cavallo LM, Messina A, Esposito F, de Divitiis O, Dal Fabbro M, de Divitiis E, et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg (2007) 107:713–20. doi: 10.3171/JNS-07/10/0713
79. Michael AP, Elbuluk O, Tsiouris AJ, Tabaee A, Kacker A, Anand VK, et al. The critical importance of a vascularized flap in preventing recurrence after endoscopic repair of spontaneous cerebrospinal fluid leaks and meningoencephaloceles. J Neurosurg (2021), 12:1–8. doi: 10.3171/2021.7.JNS211427
80. Reuter G, Bouchain O, Demanez L, Scholtes F, Martin D. Skull base reconstruction with pedicled nasoseptal flap: Technique, indications, and limitations. J Craniomaxillofac Surg (2019) 47:29–32. doi: 10.1016/j.jcms.2018.11.012
81. Boetto J, Labidi M, Watanabe K, Hanakita S, Bouazza S, Passeri T, et al. Combined nasoseptal and inferior turbinate flap for reconstruction of Large skull base defect after expanded endonasal approach: Operative technique. Oper Neurosurg (Hagerstown) (2019) 16:45–52. doi: 10.1093/ons/opy046
82. Liu JK, Schmidt RF, Choudhry OJ, Shukla PA, Eloy JA. Surgical nuances for nasoseptal flap reconstruction of cranial base defects with high-flow cerebrospinal fluid leaks after endoscopic skull base surgery. Neurosurg Focus (2012) 32:E7. doi: 10.3171/2012.5.FOCUS1255
83. Greig SR, Cooper TJ, Sommer DD, Nair S, Wright ED. Objective sinonasal functional outcomes in endoscopic anterior skull-base surgery: an evidence-based review with recommendations. Int Forum Allergy Rhinol (2016) 6:1040–6. doi: 10.1002/alr.21760
84. Shahangian A, Soler ZM, Baker A, Wise SK, Rereddy SK, Patel ZM, et al. Successful repair of intraoperative cerebrospinal fluid leaks improves outcomes in endoscopic skull base surgery. Int Forum Allergy Rhinol (2017) 7:80–6. doi: 10.1002/alr.21845
85. Sonnenburg RE, White D, Ewend MG, Senior B. Sellar reconstruction: is it necessary? Am J Rhinol (2003) 17:343–6.
86. Moza K, McMenomey SO, Delashaw JB. Indications for cerebrospinal fluid drainage and avoidance of complications. Otolaryngol Clin North Am (2005) 38:577–82. doi: 10.1016/j.otc.2005.01.001
87. Stokken J, Recinos PF, Woodard T, Sindwani R. The utility of lumbar drains in modern endoscopic skull base surgery. Curr Opin Otolaryngol Head Neck Surg (2015) 23:78–82. doi: 10.1097/MOO.0000000000000119
88. D’Anza B, Tien D, Stokken JK, Recinos PF, Woodard TR, Sindwani R. Role of lumbar drains in contemporary endonasal skull base surgery: Meta-analysis and systematic review. Am J Rhinol Allergy (2016) 30:430–5. doi: 10.2500/ajra.2016.30.4377
89. Eloy JA, Kuperan AB, Choudhry OJ, Harirchian S, Liu JK. Efficacy of the pedicled nasoseptal flap without cerebrospinal fluid (CSF) diversion for repair of skull base defects: incidence of postoperative CSF leaks. Int Forum Allergy Rhinol (2012) 2:397–401. doi: 10.1002/alr.21040
90. Mehta GU, Oldfield EH. Prevention of intraoperative cerebrospinal fluid leaks by lumbar cerebrospinal fluid drainage during surgery for pituitary macroadenomas. J Neurosurg (2012) 116:1299–303. doi: 10.3171/2012.3.JNS112160
91. Castelnuovo P, Mauri S, Locatelli D, Emanuelli E, Delù G, Giulio GD. Endoscopic repair of cerebrospinal fluid rhinorrhea: learning from our failures. Am J Rhinol (2001) 15:333–42. doi: 10.1177/194589240101500509
92. Mortini P, Nocera G, Roncelli F, Losa M, Formenti AM, Giustina A. The optimal numerosity of the referral population of pituitary tumors centers of excellence (PTCOE): A surgical perspective. Rev Endocr Metab Disord (2020) 21:527–36. doi: 10.1007/s11154-020-09564-7
93. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery (1997) 40:225–36. doi: 10.1097/00006123-199702000-00001
94. Alobaid A, Dehdashti AR. Hemostasis in skull base surgery. Otolaryngol Clin North Am (2016) 49:677–90. doi: 10.1016/j.otc.2016.02.003
95. Pagliano P, Caggiano C, Ascione T, Solari D, Di Flumeri G, Cavallo LM, et al. Characteristics of meningitis following transsphenoidal endoscopic surgery: a case series and a systematic literature review. Infection (2017) 45:841–8. doi: 10.1007/s15010-017-1056-6
96. Ciric I, Zhao J-C, Du H, Findling JW, Molitch ME, Weiss RE, et al. Transsphenoidal surgery for cushing disease: experience with 136 patients. Neurosurgery (2012) 70:70–80. doi: 10.1227/NEU.0b013e31822dda2c
Keywords: pituitary surgery, closure, skull base repair, endoscopy, strategy, nasoseptal flap
Citation: Baussart B, Venier A, Jouinot A, Reuter G and Gaillard S (2023) Closure strategy for endoscopic pituitary surgery: Experience from 3015 patients. Front. Oncol. 12:1067312. doi: 10.3389/fonc.2022.1067312
Received: 11 October 2022; Accepted: 05 December 2022;
Published: 04 January 2023.
Edited by:
Carlo Serra, University Hospital Zürich, SwitzerlandReviewed by:
Filippo Flavio Angileri, University of Messina, ItalyMing Chen, Shanghai Jiao Tong University, China
Teresa Somma, Federico II University Hospital, Italy
Copyright © 2023 Baussart, Venier, Jouinot, Reuter and Gaillard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bertrand Baussart, bertrand.baussart@aphp.fr; bertranbaussart@gmail.com
†These authors have contributed equally to this work and share first authorship
 Bertrand Baussart
Bertrand Baussart