- 1Department of Gastrointestinal Surgery, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, Hunan, China
- 2Department of General Surgery, Institute of Digestive Surgery of Changsha, Affiliated Changsha Hospital of Hunan Normal University, Changsha, Hunan, China
Gastrointestinal (GI) cancer is a global health problem with wide lesions and numerous cases. The increased morbidity and mortality of GI cancer is a socio-economic challenge for decades to come. Melatonin, a nature indolamine, exerts a crucial role in molecular interactions involved in multiple functional and physiological processes. Increasing evidence indicates that melatonin can modulate GI tract, decrease the occurrence of GI cancer, and enhance the sensitivity to chemoradiotherapy. However, little is known about the exact role of melatonin in anti-carcinogenesis. In this review, we discuss the action of the beneficial effects of melatonin in GI carcinogenesis. Furthermore, we compile the understanding of the role of melatonin in GI cancer, including esophageal cancer (EC), gastric cancer (GC), hepatocellular carcinoma (HCC), colorectal cancer (CRC), and pancreatic cancer (PC). In addition, the potential therapeutic application and clinical evaluation of melatonin in GI cancer are also discussed.
1. Introduction
With the development of human civilization and social progress, the average life expectancy has increased significantly. However, cancer is a huge threat to longevity, among which gastrointestinal (GI) cancer is currently one of the major causes of death, with wide lesions and numerous cases. GI cancer includes cancer of the esophagus, stomach, colorectum, liver, and pancreas according to anatomy. It has been reported that colorectal cancer (CRC) is the most fatal and common GI cancer, followed by pancreatic cancer (PC), hepatocellular carcinoma (HCC), gastric cancer (GC) and esophageal cancer (EC) (1, 2). Due to the lack of methods for early diagnosis and effective management, as well as the properties of disease recurrence and metastasis, most GI cancers have a high fatality rate. The efficacy of GI patients has been well improved through surgery, chemotherapy, radiotherapy, etc., but there are still problems in patient management. Therefore, efforts should be made to explore a novel preventative and therapeutic drug therapy to intervene and retard the progress of GI cancer.
L-tryptophan (L-Trp) is hydroxylated to 5-hydroxytryptophan (5-hydroxy Trp), then decarboxylated, acetylated to 5-hydroxytryptamine (5-HT) and N-acetyl-5-hydroxytryptamine (N-acetyl 5-HT), and finally methylated to N-acetyl-5-methoxytryptamine (also known as melatonin) (Figure 1). Melatonin is involved in many physiological functions (3, 4). Increasing evidence demonstrates melatonin exhibits antioxidant properties and is responsible for several diseases. It is a strong antioxidant that acts as a scavenger of free radicals (5). Besides, melatonin can activate antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GPX), and superoxide dismutase (SOD), thereby reducing oxidative stress (6). It has been shown that melatonin enhances the expression of antioxidative enzyme genes, protects against depletion caused by ultraviolet radiation-induced, and prevents the formation of DNA damage (6). In addition, melatonin is also known to be an inhibitor of pro-oxidative xanthine oxidase (XO), an activator of DNA repair genes, and protector of mitochondrial membranes, which protect the body from harmful compounds (7). For example, Teixeira et al. indicated that results demonstrate the antioxidant effect of melatonin is mainly corelated with the activities of enzymes such as myeloperoxidase and XO (8). Liu et al. demonstrated that melatonin could increase DNA repair capacity via activating genes involved in DNA damage responsive pathways (9).
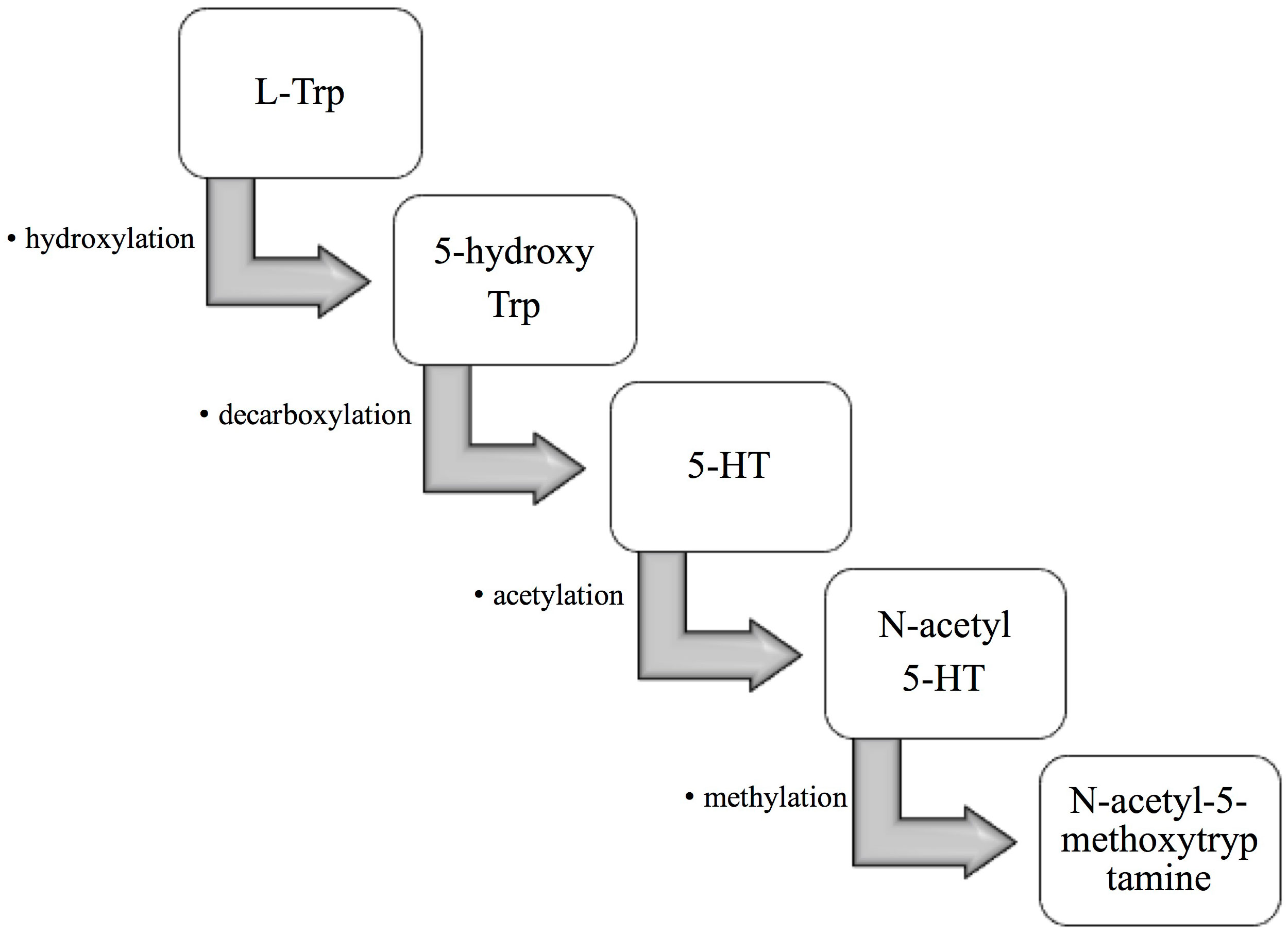
Figure 1 The formation of melatonin in the organism. First, L-tryptophan (L-Trp) is hydroxylated to 5-hydroxytryptophan (5-hydroxy Trp). 5-hydroxy Trp is then decarboxylated to 5-hydroxytryptamine (5-HT), which is then acetylated to N-acetyl-5-hydroxytryptamine (N-acetyl 5-HT). Finally, N-acetyl 5-HT is methylated to N-acetyl-5-methoxytryptamine, also known as melatonin.
Melatonin can be produced by diverse tissues including pineal gland, GI tract, testes, retina, and lymphocytes (10). Melatonin receptors are G-protein coupled receptors, which can be divided into melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2) according to their different affinity (11). A large lines of evidence have indicated that melatonin is a vital regulator of circadian and seasonal rhythms (12, 13). Given that melatonin is a “jack-of-all-grades”, it is not surprising that it affects the progression of GI cancer. For example, Parent et al. demonstrated that night shifts may affect cancer risk by inhibiting melatonin release (14). Wang et al. confirmed that melatonin is associated with the GC metastasis and poor prognosis (15). However, the mechanisms and roles by which melatonin can modulate the GI carcinogenesis are elusive. Therefore, we compile the research progress of melatonin and GI cancer (Figure 2), including EC, GC, HCC, PC, and CRC, to provide theoretical basis and ideas for further revealing melatonin and GI cancer. Moreover, the potential therapeutic application and clinical evaluation of melatonin in GI cancer are also discussed.

Figure 2 The effects of melatonin on various gastrointestinal cancer. This figure shows the examples of several common gastrointestinal cancer (including EC, GC, HCC, PC, and CRC) where melatonin exhibits protective effects EC, esophageal cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; PC, pancreatic cancer; CRC, colorectal cancer.
2. The mechanism of action of melatonin in GI carcinogenesis
Circadian rhythm disturbance is closely related to the occurrence and development of cancer. Studies have shown that melatonin, normally up-regulated at night, helps to stabilize metabolic rhythm, thus involving cancer progression (16, 17). Melatonin exerts its anti-carcinogenesis role through various ways, including promoting cancer cell apoptosis, inhibiting proliferation, regulating angiogenesis and metastasis, modulating immunity, and involving several oncogenic signaling pathways (Figure 3). However, the intracellular signaling pathway of melatonin has not been clearly defined.
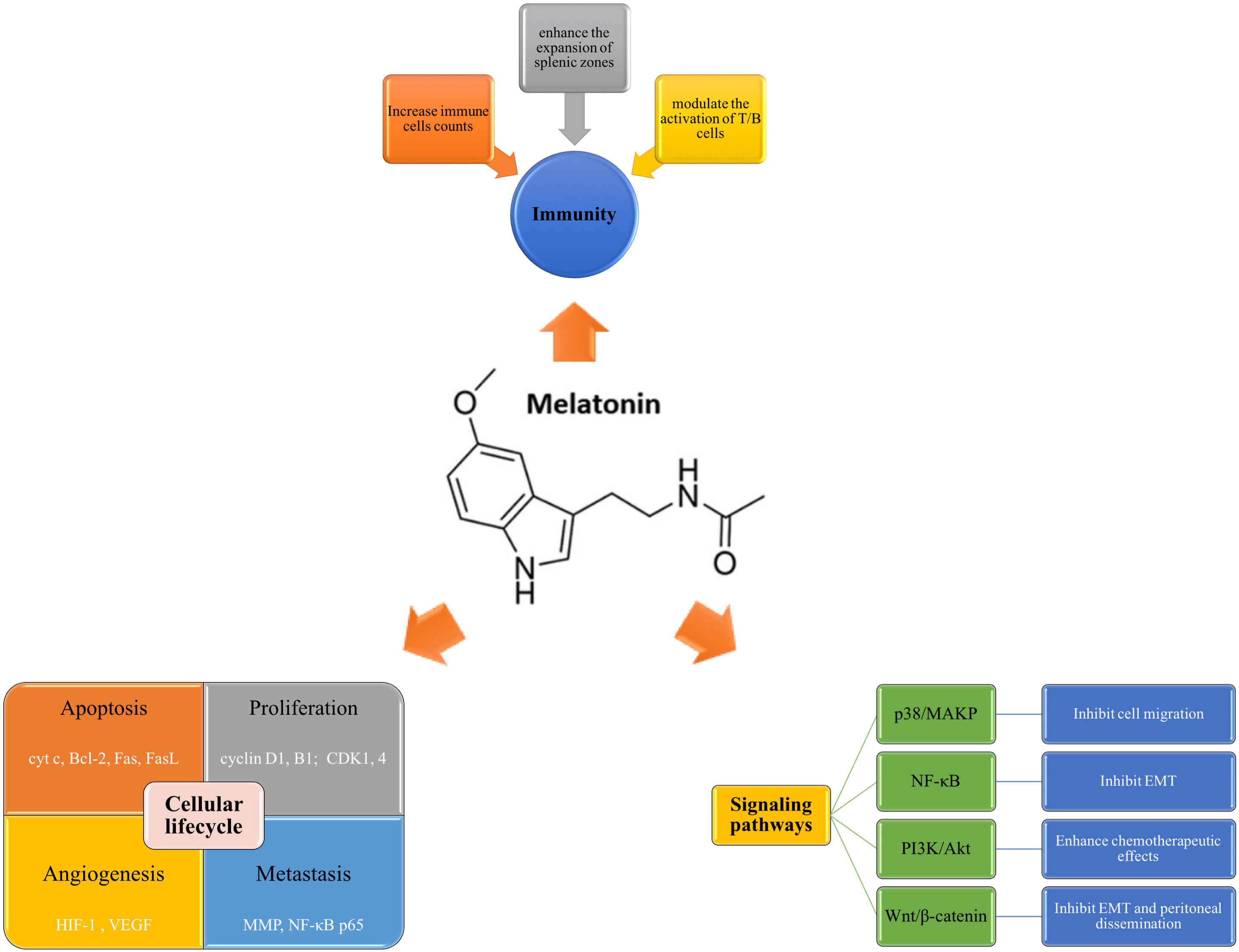
Figure 3 Mechanisms of melatonin in GI carcinogenesis. Melatonin plays a role in anti-carcinogenesis mainly through the following ways, including modulating cellular lifecycle, regulating immunity function, and involving several oncogenic signaling pathways. Melatonin can induce cell apoptosis via regulating multiple genes (cyt c, Bcl-2, Fas) and inhibit proliferation by arresting cancer cell cycle (cyclin D1, cyclin B1, CDK1, CDK 4). Moreover, it can also influence the angiogenesis and metastasis by modulating HIF-1, VEGF, MMP, etc. Secondly, melatonin is a regulator of immunity. It mediate the immune function mainly through increasing the counts of immune cells, enhancing the expansion of splenic zones, and activating the function of T/B cells. Thirdly, melatonin can inhibit carcinogenesis through specific signaling pathways, such as p38/MAPK, NF-κB, PI3K/Akt, and Wnt/β-catenin. Bcl-2, B-cell lymphoma-2; cyt c, cytochrome c; EMT, epithelial-mesenchymal transition; FasL, Fas ligand; HIF-1, hypoxia-inducible factor 1; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinases; NF-κB, nuclear factor-kappa B; PI3K, phosphoinositide 3 Kinase; VEGF, vascular endothelial growth factor.
2.1. Melatonin and cellular lifecycle
Cell proliferation, differentiation, senescence and apoptosis are structural and functional bases of organism growth, development, aging and death, respectively (18). Cells deviate from the normal lifecycle due to internal or external factors, which may lead to the occurrence of cancer. It has been reported that melatonin act as a loyal defender against GI cancer through regulating cellular apoptosis, proliferation, metastasis, and angiogenesis. With the relevant cumulative findings, herein we investigate the action and role of melatonin in GI carcinogenesis.
Apoptosis, also known as programmed cell death (PCD), is a physiologic cell death that is distinct from necrosis. It is an active “suicide” extinction process after cells are stimulated by certain signals. Recent in-depth studies on biological cell death pathways have shown that the attenuation of apoptosis is closely related to the formation of GI cancer (19). There is increasing evidence that melatonin promotes PCD in GI cancer (20). Cytochrome c (cyt c) is release into the cytoplasm when the cells are stimulated, and triggers an enzymatic cascade that leads to apoptosis (21). The B-cell lymphoma-2 (Bcl-2) family mediates the intrinsic apoptosis pathway with both anti-apoptotic and pro-apoptotic effects (22). Fas, a transmembrane protein, binds to Fas ligand (FasL) to initiate the transduction of apoptotic signals and induce apoptosis (23). Mechanistically, melatonin can modulate the expression of multiple genes associated with apoptosis, such as cytosolic cyt c, Bcl-2, Fas, etc (20, 24).
Rampant proliferation is another important characteristics of all cancers. The carcinogenesis is the result of the imbalance between cell proliferation and apoptosis. It has been reported that melatonin exerts an obvious anti-proliferation effect via arresting cancer cell cycle (25). For example, Liu et al. proved that melatonin attenuated the expression of cyclin D1 and CDK4 in G1 phase, and cyclin B1 and CDK1 in G2/M phase of human osteosarcoma cells (26). Moreover, the anti-proliferative efficacy of melatonin have been demonstrated in GC and HCC cell lines (27).
Additionally, increasing evidence indicates that melatonin is also involved in angiogenesis and metastasis in GI cancer. Cancer angiogenesis is known to be an important feature of metastasis responsible for cancer death. Melatonin has the capacity to reduce the migration and neovascularization of cancer cells. For instance, Wang et al. showed that melatonin suppressed IL-1β-induced lung metastasis of GC by downregulating the expression of matrix metalloproteinases (MMP), and nuclear factor-kappa B (NF-κB) p65 (28). Moreover, melatonin can also inhibits cancer angiogenesis via attenuating hypoxia-inducible factor (HIF)-1 and decreasing vascular endothelial growth factor (VEGF) expression (29).
2.2. Melatonin and immune function
Interactions between immune cells and cancer cells exert a crucial role in cancer development (30). Since MT are widely present in immune system, melatonin is involved in regulating immune function. Studies have shown that melatonin increases the cells of the innate immunity, such as neutrophil, macrophages, and lymphocytes counts (31). Luo et al. demonstrated that melatonin modulated the activation of T/B cells, thus playing a key role in stabilizing immune balance (32). Besides, Liu et al. indicated that melatonin inhibited GC cell growth by down-regulating the expressions of CD4 (+) and CD25 (+) regulatory T cells (Tregs) and Forkhead box p3 (Foxp3) in GC (33). Moreover, melatonin is also proved to enhance the expansion of splenic zones (34). In addition, it has been shown that melatonin participates in the regulation of cytokine production (35).
2.3. Melatonin and signaling pathways
Signaling pathways are intertwined into networks in physiological processes of systemic organs throughout the body and play important roles in human health and disease. The disruption of multiple signaling pathways is closely involved in the progression of diverse cancers. It has been indicated that melatonin could regulate the mediators in oncogenic signaling pathways, such as NF-κB, phosphoinositide 3 Kinase (PI3K)/Akt, p38/mitogen-activated protein kinase (MAPK), and Wnt/β-catenin axis (36–38). For example, Liu et al. reported that melatonin decreased Rho−associated protein kinase (ROCK) expression via p38/MAPK signaling pathway, thus inhibiting the migration of CRC cells (39). The transdifferentiation of epithelial cells into motile mesenchymal cells, known as epithelial-mesenchymal transition (EMT), is a process that enhances the invasiveness and anti-apoptotic capabilities of cancer cells (40). Wang et al. proved that melatonin suppressed EMT in GC via attenuation of IL−1β/NF−κB/MMP2/MMP9 axis (41).
3. The role of melatonin in GI cancer
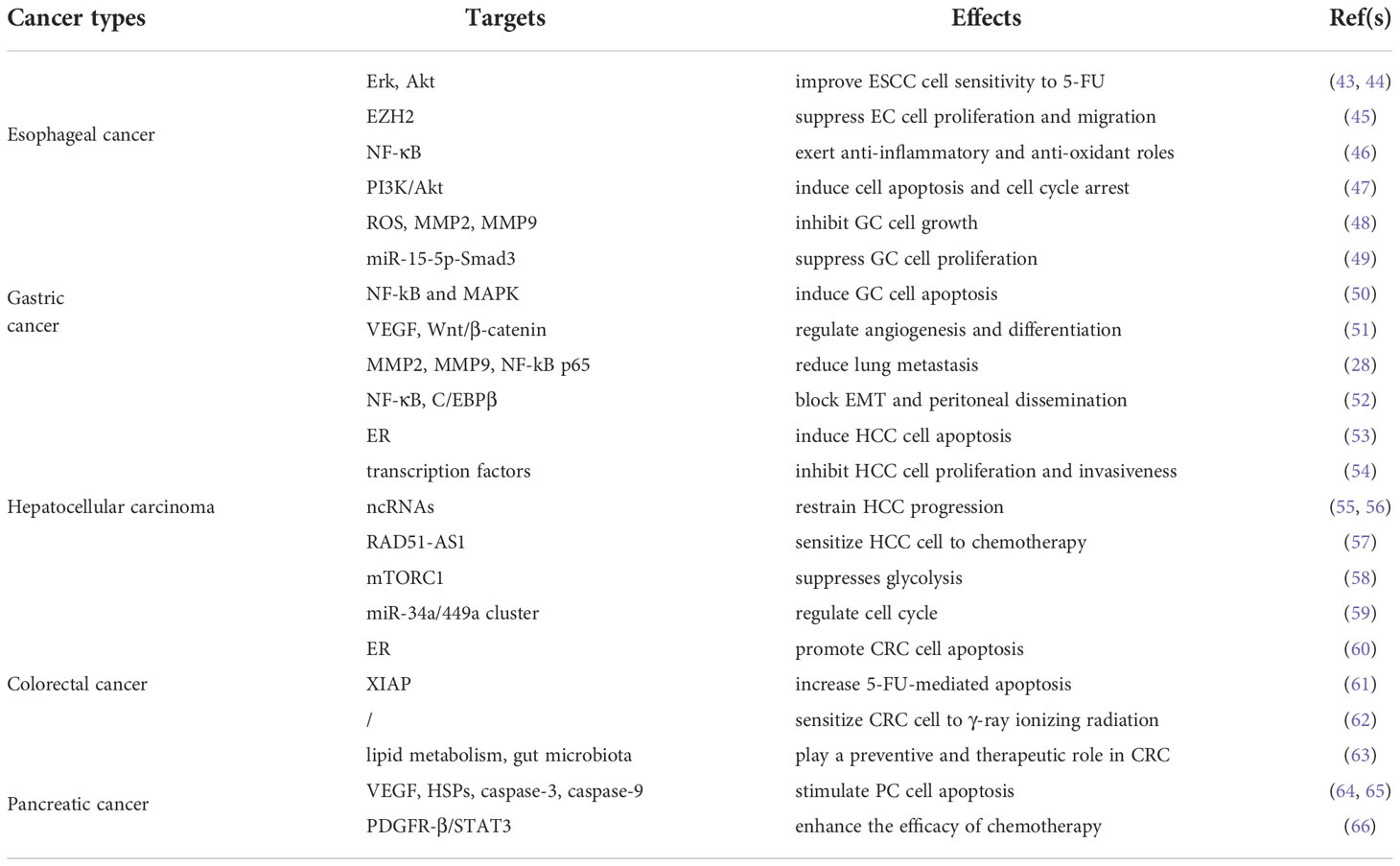
Despite tremendous scientific breakthroughs in understanding the mechanistic properties of GI cancer, therapeutic efficacy remains very limited. Increasing evidence demonstrates that melatonin has positive protective effects against both endogenous stimuli (acid and pepsin) and exogenous insults (alcohol and stress) affecting the GI tract (42). There are numerous studies assessing the roles of melatonin on health and disease. Melatonin has been seen as an adjunctive treatment for advanced cancer because of its anti-inflammatory and anti-oxidant effects. In this part, we discuss the role of melatonin in GI cancer, including EC, GC, HCC, CRC, and PC, respectively (Table 1).
3.1. The role of melatonin in esophageal cancer
Esophageal cancer (EC) refers to malignant tumors of esophageal epithelial origin, which is mainly manifested as choking sensation when swallowing food, foreign body sensation, retrosternal pain, or obvious dysphagia (67). It is one of the most common gastrointestinal cancer with regional variations in morbidity and mortality. The two major subtypes of EC are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Studies have shown that smoking, alcohol consumption, obesity, aging, male sex, Barrett’s esophagus, and gastroesophageal reflux disease (GERD) consist of the potential risk factors for EC (68, 69).
Melatonin has been shown to afford esophago-protection via diverse mechanisms. Firstly, it enhances tumor cell sensitivity to chemotherapy drugs. In 2016, Lu et al. demonstrated that melatonin improved ESCC cell sensitivity to 5-fluorouracil (5-FU) via suppressing the Erk and Akt pathway (43). Furthermore, Zhang et al. proved that melatonin significantly enhanced 5-FU-mediated suppression of esophageal cancer cell proliferation and migration by regulating enhancer of zeste homolog 2 (EZH2) (45). Histone methyltransferase EZH2, a catalytic component of polycomb repressive complex 2 (PRC2), is highly expressed in the development of esophageal cancer (70). Secondly, melatonin exerts anti-inflammatory and anti-oxidant effects in EC. NF-κB induces the expression of multiple genes through the activation of stimulating factors and produces multiple cytokines involved in inflammatory responses. Gu et al. revealed that melatonin inhibited cell invasion via down-regulating the NF-κB signaling pathway in EC (46). Thirdly, melatonin is involved in the regulation of cell cycle. It has been reported that melatonin causes cell cycle arrest and affects the percentage of abnormalities in different phase (47).
3.2. The role of melatonin in gastric cancer
Gastric cancer (GC) is a predominant malignancy with the second leading cause of cancer death worldwide (71). The lifestyle, genetics, helicobacter pylori (H. pylori) infection, and epigenetics are potential risk factors for GC (72). H. pylori is a major cause of gastric carcinogenesis by enhancing the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), and inducing local ulceration and inflammation (73). According to the pathological types, GC can be divided into adenocarcinoma, signet ring cell carcinoma, adenosquamous carcinoma, medullary carcinoma and undifferentiated cell carcinoma, among which adenocarcinoma is the most common. Gastroscopy is the preferred method for GC, and gastric biopsy is the “gold standard” for the diagnosis of GC.
Growing evidence demonstrates that melatonin exerts gastroprotective effects in GC through multiple mechanisms, including increasing blood flow, reducing inflammation, scavenging free radicals, and inhibiting MMP (41, 74, 75). On the one hand, melatonin has been shown to play a role in affecting the growth of GC itself. Liu et al. revealed that melatonin inhibited GC cell progression via the NF-kB signaling pathway (48). They proved that melatonin suppressed cell growth by directly reducing ROS production, while indirectly decreasing the level of MMP2 and MMP9 in cancer-associated fibroblasts (CAFs) in gastric cancer cells. Moreover, melatonin was reported to inhibit the proliferation of GC cells via modulating miR-15-5p-small mothers against decapentaplegic homolog 3 (Smad3) pathway (49). Additionally, it has been demonstrated that melatonin induces GC cell apoptosis via regulating diverse signaling pathways (76, 77). For example, Li et al. noted that melatonin stimulated GC cell apoptosis by mediating NF-kB and MAPK signaling pathways (50). Apart from mentioned above, the effects of melatonin on angiogenesis and differentiation are also involved in the progression of GC (51). On the other hand, melatonin has also been proven to be effective in reducing metastasis exacerbations. There is increasing evidence that GC migration is significantly reduced following melatonin treatment. In a study, decreased lung metastasis in GC after melatonin treatment was associated with the downregulation of MMP2, MMP9, and NF-kB p65 (28). Wu et al. indicated that melatonin blocked EMT and peritoneal dissemination through NF-κB cleavage and calpin-mediated C/EBPβ (52). Besides, melatonin was reported to inhibit the migration of GC via remodeling tight junction (78).
3.3. The role of melatonin in hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is a major global health challenge and the fourth leading cause of cancer-related death worldwide. HCC is prevalent to spread in the liver through the portal vein system, forming intrahepatic metastasis, and also easy to form tumor thrombus in the portal vein, causing the manifestation of portal hypertension (79). A commonly used tumor marker for HCC is alpha-fetoprotein (AFP), especially when it is significantly elevated, high vigilance should be exercised. For people with hepatitis infection, liver cirrhosis and family history of liver cancer, regular screening for HCC should be carried out for early detection, diagnosis and treatment (80).
Emerging evidence demonstrates that melatonin exerts anti-cancer activity on HCC. It is worth noting that melatonin reverses apoptosis resistance and activates both intrinsic and extrinsic pathways of apoptosis in HCC (54). Zha et al. found that melatonin stimulates endoplasmic reticulum (ER) stress-induced apoptosis in HCC (53). Moreover, melatonin is involved in the regulation of HCC by modulating a variety of transcription factors and related pathways to inhibit cell proliferation and invasiveness (54). In addition, melatonin has been proven to restrain HCC progression via regulating non-coding RNAs (ncRNAs), such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) (55, 56). Besides, melatonin could sensitize HCC cell to chemotherapy (57). Recent studies have shown that melatonin suppresses glycolysis in HCC cells by down-regulating mitochondrial respiration and mammalian target of rapamycin complex 1 (mTORC1) activity (58).
3.4. The role of melatonin in colorectal cancer
Colorectal cancer (CRC) is considered as the second most common cancer worldwide and the incidence of CRC increases with age (81). Adenocarcinoma is the most common pathological type of CRC. In recent years, the morbidity and mortality of colorectal cancer have shown an increasing trend, which should be paid more attention to. Chemoradiotherapy is the last-line treatment for advanced CRC with significant side effects. Therefore, it is urgent to explore an anti-cancer agent in CRC.
Melatonin holds promise as an adjunctive treatment for advanced CRC. The entero-endocrine (EE) cells in GI tract mucosa are the main source of intestinal melatonin (82). Increasing evidence evaluates the effects and safety of melatonin. It has been reported that melatonin is associated with various health outcomes in heterogeneous populations (83). Mechanically, it has been reported that melatonin prevent and delay the progression of CRC by suppressing the proliferation and inducing apoptosis of CRC cells. Ji et al. demonstrated that melatonin regulated the miR-34a/449a cluster, thus influencing the cell cycle in CRC (59). Yun et al. noted that melatonin promoted CRC cell apoptosis via superoxide-mediated ER stress (60). Besides, melatonin promotes chemotherapeutic drug-mediated apoptosis of CRC cells by enhancing oxidative stress (61). In addition to its role in sensitivity to chemotherapeutic agents, melatonin also sensitizes human CRC cells to γ-ray ionizing radiation both in vitro and in vivo (62). Interestingly, studies have pointed out that melatonin plays a preventive and therapeutic role in CRC by regulating lipid metabolism and gut microbiota (63). Menadione, a synthetic form of vitamin K, is known to stimulate an increase in intracellular ROS and alter the oxidative status of cancer cells (84). Collin et al. confirmed that menadione plus melatonin on Caco-2 cells could reduce cell proliferation, induce reactive nitrogen species formation, enhance superoxide anion content, and increase catalase activity, suggesting the potential as adjuvant therapy for CRC acting on different oncogenic pathways (85). Kvietkauskas et al. designed a study to explore the combined role of melatonin and glycine in CRC liver metastasis (86). They found that supplementation with melatonin and glycine reduce CRC liver metastasis growth by acting as natural antiangiogenic molecules.
3.5. The role of melatonin in pancreatic cancer
Pancreatic cancer (PC) is the source of an increasing number of cancer-related deaths with a low survival rate. The high mortality rate of PC is likely to be associated with the absence of early symptoms and therefore delayed diagnosis, as well as high resistance to chemoradiotherapy. PC is classified as resectable, borderline, locally advanced and metastatic with greatly varied treatment among them (87). The sensitivity of computed tomography (CT) and magnetic resonance imaging (MRI) in detecting PC was as high as 96% and 93.5%, respectively (88). Despite significant advances in the treatment of PC, including improved surgical techniques and refined adjuvant and neoadjuvant therapies, the incidence and mortality of PC have not decreased significantly worldwide. We therefore emphasize the historical perspective of PC treatment, highlight the prevention strategies, and identify more integrated approaches.
Growing evidence supports the therapeutic benefits of melatonin in the management of PC. Studies have shown that a variety of inflammatory pathways are closely related to the pathological process of PC (89). Melatonin stimulates cancer cell apoptosis by acting as an inflammatory inhibitor, an oxidative stress modulator, a VEGF inhibitor, a heat shock proteins (HSPs) inhibitor, etc. (64, 65). Firstly, it protects pancreatic tissue from inflammatory damage and oxidative stress via activating anti-oxidant enzymes and scavenging ROS and RNS. Secondly, melatonin decreases endothelial cells proliferation and reduces angiogenesis by inhibiting VEGF. Thirdly, high concentration of melatonin regulate Bax/Bcl protein balance, thus stimulating the expression of caspase-3 and caspase-9, while low level of melatonin produce anti-apoptotic HSPs, such as HSP27 and HSP90, thereby preventing the activation of caspase-3. Moreover, it has been found that melatonin is involved in enhancing the efficacy of chemotherapy and decreasing side effects. Fang et al. found that melatonin and sorafenib synergistically inhibited PC through MR and PDGFR-β/STAT3 signal pathway (66). Melatonin was also proved to enhance the chemosensitivity to gemcitabine in PC (90). The metabolites of L-Trp and melatonin are called kynuramines, of which N1-acetyl-5-methoxy-kynuramine (AMK) and N1-acetyl-N1-formyl-5-methoxykynuramine (AFMK) are the best known melatonin derivatives (91). It has been shown that melatonin precursor L-Trp and the melatonin derivatives kynuramines, may be related to the physiological and functional failure of the pancreas, leading to the impairment of pancreatic function and anti-cancer ability (92).
4. The safety evaluation of melatonin in GI cancer
Melatonin is an endogenous molecule that has its own metabolic pathway in the body. The biological half-life is short and falls to the physiological level of normal after 7~8 hours of oral administration. Therefore, it is a relatively safe substance for clinical use in humans. The underlying mechanisms of melatonin in vitro, such as cell apoptosis, cell proliferation, immune function, and signaling pathways, have largely been validated in vivo studies. For clinical application, a large number of researches have further examined the role of melatonin in GI cancer in vivo. On the one hand, many studies have explored the effects of melatonin on GI cancer in animal models. For example, Winczyk et al. constructed an animal model of colon cancer in mice (93). They found an increase of apoptotic cells in cancers treated with melatonin, further confirming the pro-apoptotic efficacy of melatonin on murine colon cancer cells. On the other hand, the clinical trials have also been conducted to evaluate the safety and efficacy of melatonin in GI cancer. It is well known that melatonin’s sleep-inducing effects have been widely used clinically (94). Recent clinical studies have shown that melatonin can improve the survival rate of patients with GI cancers, increase the sensitivity to chemotherapeutic agents, and reduce the side effects of chemoradiotherapy (38). For example, Kouhi et al. conducted a double-blind controlled study in 60 patients with rectal cancer, in which the experimental group received 20 mg melatonin a day and the control group treated with placebo (95). Their subsequent study found that radiotherapy induced less severe reductions in blood cell counts in patients treated with melatonin, suggesting a role for melatonin in reversing the adverse effects of radiation. The above studies all indicate that melatonin can be utilized for the treatment of GI cancer. However, the appropriate dosage and optimal duration of melatonin still require extensive studies to specifically validate the effects of melatonin on GI cancer progression in the future.
5. Conclusions and perspectives
Melatonin, a natural indolamine, is produced by a variety of tissues and is involved in the mediation of physiological functions. Given that MT are widely distributed in many organs and tissues of the body, it is not surprising that it is called a “jack-of-all-grades”. GI cells can not only secrete melatonin, but also have MT on them, thus exerting important protective and regulatory roles. Studies have shown that melatonin boosts the GI immune system, regulates fecal moisture, slows intestinal peristalsis, and protects the GI tract from digestive enzymes and stomach acid. Emerging evidence proves that melatonin affects the progression of GI cancer. Despite our understanding of how melatonin exerts its anti-cancer effects is expanding, much remains to be studied. There are many challenges in translation to therapeutic applications in GI cancer, such as safety assessment and bioavailability. Future researches should continue to focus on the communication between melatonin metabolism and the development and progression of GI cancer.
Author contributions
Y-QG collected literature and wrote the manuscript. F-TH summarized the table and drew the figures. C-LX and C-LL supervised the manuscript and modified the figures. G-HH and C-WC conceived the idea and supervised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Scientific Research Project of Hunan Provincial Health Commission (No. 20200453).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Murphy N, Jenab M, Gunter MJ. Adiposity and gastrointestinal cancers: Epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol (2018) 15(11):659–70. doi: 10.1038/s41575-018-0038-1
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Muñiz-Calvo S, Bisquert R, Fernández-Cruz E, García-Parrilla MC, Guillamón JM. Deciphering the melatonin metabolism in saccharomyces cerevisiae by the bioconversion of related metabolites. J Pineal Res (2019) 66(3):e12554. doi: 10.1111/jpi.12554
4. Gheban BA, Rosca IA, Crisan M. The morphological and functional characteristics of the pineal gland. Med Pharm Rep (2019) 92(3):226–34. doi: 10.15386/mpr-1235
5. Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res (2011) 51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x
6. Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2'-deoxyguanosine) in ex vivo human skin. J Pineal Res (2013) 54(3):303–12. doi: 10.1111/jpi.12018
7. Suwannakot K, Sritawan N, Prajit R, Aranarochana A, Sirichoat A, Pannangrong W, et al. Melatonin protects against the side-effects of 5-fluorouracil on hippocampal neurogenesis and ameliorates antioxidant activity in an adult rat hippocampus and prefrontal cortex. Antioxidants (Basel) (2021) 10(4). doi: 10.3390/antiox10040615
8. Teixeira A, Morfim MP, de Cordova CA, Charão CC, de Lima VR, Creczynski-Pasa TB. Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. J Pineal Res (2003) 35(4):262–8. doi: 10.1034/j.1600-079x.2003.00085.x
9. Liu R, Fu A, Hoffman AE, Zheng T, Zhu Y. Melatonin enhances DNA repair capacity possibly by affecting genes involved in DNA damage responsive pathways. BMC Cell Biol (2013) 14:1. doi: 10.1186/1471-2121-14-1
10. Shafabakhsh R, Reiter RJ, Davoodabadi A, Asemi Z. Melatonin as a potential inhibitor of colorectal cancer: Molecular mechanisms. J Cell Biochem (2019) 120(8):12216–23. doi: 10.1002/jcb.28833
11. Wongprayoon P, Govitrapong P. Melatonin receptor as a drug target for neuroprotection. Curr Mol Pharmacol (2021) 14(2):150–64. doi: 10.2174/1874467213666200421160835
12. Skeldon AC, Dijk DJ. Weekly and seasonal variation in the circadian melatonin rhythm in humans: Entrained to local clock time, social time, light exposure or sun time? J Pineal Res (2021) 71(1):e12746. doi: 10.1111/jpi.12746
13. Lanfumey L, Mongeau R, Hamon M. Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther (2013) 138(2):176–84. doi: 10.1016/j.pharmthera.2013.01.005
14. Parent M, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol (2012) 176(9):751–9. doi: 10.1093/aje/kws318
15. Wang XT, Chen CW, Zheng XM, Wang B, Zhang SX, Yao MH, et al. Expression and prognostic significance of melatonin receptor MT1 in patients with gastric adenocarcinoma. Neoplasma (2020) 67(2):415–20. doi: 10.4149/neo_2019_190220N141
16. Talib WH. Melatonin and cancer hallmarks. Molecules (2018) 23(3). doi: 10.3390/molecules23030518
17. Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci (2017) 173:94–106. doi: 10.1016/j.lfs.2017.02.008
18. Ni YQ, Liu YS. New insights into the roles and mechanisms of spermidine in aging and age-related diseases. Aging Dis (2021) 12(8):1948–63. doi: 10.14336/ad.2021.0603
19. Al-Ishaq RK, Overy AJ, Büsselberg D. Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules (2020) 10(1). doi: 10.3390/biom10010105
20. Samanta S. Melatonin: an endogenous miraculous indolamine, fights against cancer progression. J Cancer Res Clin Oncol (2020) 146(8):1893–922. doi: 10.1007/s00432-020-03292-w
21. Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol (2008) 9(7):532–42. doi: 10.1038/nrm2434
22. Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun (2018) 500(1):26–34. doi: 10.1016/j.bbrc.2017.06.190
23. Wang J, Cao Z, Wang P, Zhang X, Tang J, He Y, et al. Apoptotic extracellular vesicles ameliorate multiple myeloma by restoring fas-mediated apoptosis. ACS Nano (2021) 15(9):14360–72. doi: 10.1021/acsnano.1c03517
24. García-Santos G, Martin V, Rodríguez-Blanco J, Herrera F, Casado-Zapico S, Sánchez-Sánchez AM, et al. Fas/Fas ligand regulation mediates cell death in human ewing's sarcoma cells treated with melatonin. Br J Cancer (2012) 106(7):1288–96. doi: 10.1038/bjc.2012.66
25. Kohandel Z, Farkhondeh T, Aschner M, Samarghandian S. Molecular targets for the management of gastrointestinal cancer using melatonin, a natural endogenous body hormone. BioMed Pharmacother (2021) 140:111782. doi: 10.1016/j.biopha.2021.111782
26. Liu L, Xu Y, Reiter RJ. Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone (2013) 55(2):432–8. doi: 10.1016/j.bone.2013.02.021
27. Ao L, Li L, Sun H, Chen H, Li Y, Huang H, et al. Transcriptomic analysis on the effects of melatonin in gastrointestinal carcinomas. BMC Gastroenterol (2020) 20(1):233. doi: 10.1186/s12876-020-01383-z
28. Wang X, Wang B, Zhan W, Kang L, Zhang S, Chen C, et al. Melatonin inhibits lung metastasis of gastric cancer. Vivo BioMed Pharmacother (2019) 117:109018. doi: 10.1016/j.biopha.2019.109018
29. Park SY, Jang WJ, Yi EY, Jang JY, Jung Y, Jeong JW, et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res (2010) 48(2):178–84. doi: 10.1111/j.1600-079x.2009.00742.x
30. Pandya PH, Murray ME, Pollok KE, Renbarger JL. The immune system in cancer pathogenesis: Potential therapeutic approaches. J Immunol Res (2016) 2016:4273943. doi: 10.1155/2016/4273943
31. Calvo JR, González-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: A review. J Pineal Res (2013) 55(2):103–20. doi: 10.1111/jpi.12075
32. Luo J, Zhang Z, Sun H, Song J, Chen X, Huang J, et al. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci (2020) 242:117191. doi: 10.1016/j.lfs.2019.117191
33. Liu H, Xu L, Wei JE, Xie MR, Wang SE, Zhou RX. Role of CD4+ CD25+ regulatory T cells in melatonin-mediated inhibition of murine gastric cancer cell growth in vivo and in vitro. Anat Rec (Hoboken) (2011) 294(5):781–8. doi: 10.1002/ar.21361
34. Xiong J, Wang Z, Cao J, Dong Y, Chen Y. Melatonin mediates monochromatic light-induced proliferation of T/B lymphocytes in the spleen via the membrane receptor or nuclear receptor. Poult Sci (2020) 99(9):4294–302. doi: 10.1016/j.psj.2020.06.008
35. Cardinali DP. Melatonin and healthy aging. Vitam Horm (2021) 115:67–88. doi: 10.1016/bs.vh.2020.12.004
36. Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, et al. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog Neurobiol (2008) 85(3):335–53. doi: 10.1016/j.pneurobio.2008.04.001
37. Pourhanifeh MH, Mehrzadi S, Kamali M, Hosseinzadeh A. Melatonin and gastrointestinal cancers: Current evidence based on underlying signaling pathways. Eur J Pharmacol (2020) 886:173471. doi: 10.1016/j.ejphar.2020.173471
38. Mirza-Aghazadeh-Attari M, Mohammadzadeh A, Mostavafi S, Mihanfar A, Ghazizadeh S, Sadighparvar S, et al. Melatonin: An important anticancer agent in colorectal cancer. J Cell Physiol (2020) 235(2):804–17. doi: 10.1002/jcp.29049
39. Liu Z, Zou D, Yang X, Xue X, Zuo L, Zhou Q, et al. Melatonin inhibits colon cancer RKO cell migration by downregulating rho−associated protein kinase expression via the p38/MAPK signaling pathway. Mol Med Rep (2017) 16(6):9383–92. doi: 10.3892/mmr.2017.7836
40. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer (2013) 13(2):97–110. doi: 10.1038/nrc3447
41. Wang X, Wang B, Xie J, Hou D, Zhang H, Huang H. Melatonin inhibits epithelial−to−mesenchymal transition in gastric cancer cells via attenuation of IL−1β/NF−κB/MMP2/MMP9 signaling. Int J Mol Med (2018) 42(4):2221–8. doi: 10.3892/ijmm.2018.3788
42. Majka J, Wierdak M, Brzozowska I, Magierowski M, Szlachcic A, Wojcik D, et al. Melatonin in prevention of the sequence from reflux esophagitis to barrett's esophagus and esophageal adenocarcinoma: Experimental and clinical perspectives. Int J Mol Sci (2018) 19(7). doi: 10.3390/ijms19072033
43. Lu YX, Chen DL, Wang DS, Chen LZ, Mo HY, Sheng H, et al. Melatonin enhances sensitivity to fluorouracil in oesophageal squamous cell carcinoma through inhibition of erk and akt pathway. Cell Death Dis (2016) 7(10):e2432. doi: 10.1038/cddis.2016.330
44. Han LP, Xu CQ, Jiang CM, Li HZ, Zhao YJ, Gong YS, et al. [Myocardial polyamine metabolism and the ischemia-reperfusion injury in the rat heart]. Zhonghua Xin Xue Guan Bing Za Zhi (2008) 36(4):346–9.
45. Zhang M, Zhang M, Li R, Zhang R, Zhang Y. Melatonin sensitizes esophageal cancer cells to 5−fluorouracil via promotion of apoptosis by regulating EZH2 expression. Oncol Rep (2021) 45(4). doi: 10.3892/or.2021.7973
46. Gu H, Shen Q, Mei D, Yang Y, Wei R, Ni M. Melatonin inhibits TE-1 esophageal cancer cells metastasis by suppressing the NF-κB signaling pathway and decreasing MMP-9. Ann Clin Lab Sci (2020) 50(1):65–72.
47. Zhao A, Zhao K, Xia Y, Lyu J, Chen Y, Li S. Melatonin inhibits embryonic rat H9c2 cells growth through induction of apoptosis and cell cycle arrest via PI3K-AKT signaling pathway. Birth Defects Res (2021) 113(16):1171–81. doi: 10.1002/bdr2.1938
48. Liu D, Shi K, Fu M, Chen F. Melatonin indirectly decreases gastric cancer cell proliferation and invasion via effects on cancer-associated fibroblasts. Life Sci (2021) 277:119497. doi: 10.1016/j.lfs.2021.119497
49. Zhu C, Huang Q, Zhu H. Melatonin inhibits the proliferation of gastric cancer cells through regulating the miR-16-5p-Smad3 pathway. DNA Cell Biol (2018) 37(3):244–52. doi: 10.1089/dna.2017.4040
50. Li W, Wang Z, Chen Y, Wang K, Lu T, Ying F, et al. Melatonin treatment induces apoptosis through regulating the nuclear factor-κB and mitogen-activated protein kinase signaling pathways in human gastric cancer SGC7901 cells. Oncol Lett (2017) 13(4):2737–44. doi: 10.3892/ol.2017.5785
51. Asghari MH, Moloudizargari M, Ghobadi E, Fallah M, Abdollahi M. Melatonin as a multifunctional anti-cancer molecule: Implications in gastric cancer. Life Sci (2017) 185:38–45. doi: 10.1016/j.lfs.2017.07.020
52. Wu SM, Lin WY, Shen CC, Pan HC, Keh-Bin W, Chen YC, et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPβ and NFκB cleavage. J Pineal Res (2016) 60(2):142–54. doi: 10.1111/jpi.12295
53. Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F, et al. Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress-induced apoptosis. J Pineal Res (2012) 52(3):322–31. doi: 10.1111/j.1600-079X.2011.00946.x
54. Mortezaee K. Human hepatocellular carcinoma: Protection by melatonin. J Cell Physiol (2018) 233(10):6486–508. doi: 10.1002/jcp.26586
55. Wang TH, Wu CH, Yeh CT, Su SC, Hsia SM, Liang KH, et al. Melatonin suppresses hepatocellular carcinoma progression via lncRNA-CPS1-IT-mediated HIF-1α inactivation. Oncotarget (2017) 8(47):82280–93. doi: 10.18632/oncotarget.19316
56. Wang TH, Hsueh C, Chen CC, Li WS, Yeh CT, Lian JH, et al. Melatonin inhibits the progression of hepatocellular carcinoma through MicroRNA Let7i-3p mediated RAF1 reduction. Int J Mol Sci (2018) 19(9). doi: 10.3390/ijms19092687
57. Chen CC, Chen CY, Wang SH, Yeh CT, Su SC, Ueng SH, et al. Melatonin sensitizes hepatocellular carcinoma cells to chemotherapy through long non-coding RNA RAD51-AS1-Mediated suppression of DNA repair. Cancers (Basel) (2018) 10(9). doi: 10.3390/cancers10090320
58. Lee S, Byun JK, Kim NY, Jin J, Woo H, Choi YK, et al. Melatonin inhibits glycolysis in hepatocellular carcinoma cells by downregulating mitochondrial respiration and mTORC1 activity. BMB Rep (2022) 55(9):459–64. doi: 10.5483/BMBRep.2022.55.9.177
59. Ji G, Zhou W, Li X, Du J, Li X, Hao H. Melatonin inhibits proliferation and viability and promotes apoptosis in colorectal cancer cells via upregulation of the microRNA-34a/449a cluster. Mol Med Rep (2021) 23(3). doi: 10.3892/mmr.2021.11826
60. Yun CW, Kim S, Lee JH, Lee SH. Melatonin promotes apoptosis of colorectal cancer cells via superoxide-mediated ER stress by inhibiting cellular prion protein expression. Anticancer Res (2018) 38(7):3951–60. doi: 10.21873/anticanres.12681
61. Mihanfar A, Yousefi B, Ghazizadeh Darband S, Sadighparvar S, Kaviani M, Majidinia M. Melatonin increases 5-flurouracil-mediated apoptosis of colorectal cancer cells through enhancing oxidative stress and downregulating survivin and XIAP. Bioimpacts (2021) 11(4):253–61. doi: 10.34172/bi.2021.36
62. Wang Q, Sun Z, Du L, Xu C, Wang Y, Yang B, et al. Melatonin sensitizes human colorectal cancer cells to γ-ray ionizing radiation in vitro and In vivo. Int J Mol Sci (2018) 19(12). doi: 10.3390/ijms19123974
63. Pan S, Guo Y, Hong F, Xu P, Zhai Y. Therapeutic potential of melatonin in colorectal cancer: Focus on lipid metabolism and gut microbiota. Biochim Biophys Acta Mol Basis Dis (2022) 1868(1):166281. doi: 10.1016/j.bbadis.2021.166281
64. Tamtaji OR, Mirhosseini N, Reiter RJ, Behnamfar M, Asemi Z. Melatonin and pancreatic cancer: Current knowledge and future perspectives. J Cell Physiol (2019) 234(5):5372–8. doi: 10.1002/jcp.27372
65. Jaworek J, Leja-Szpak A. Melatonin influences pancreatic cancerogenesis. Histol Histopathol (2014) 29(4):423–31. doi: 10.14670/hh-29.10.423
66. Fang Z, Jung KH, Yan HH, Kim SJ, Rumman M, Park JH, et al. Melatonin synergizes with sorafenib to suppress pancreatic cancer via melatonin receptor and PDGFR-β/STAT3 pathway. Cell Physiol Biochem (2018) 47(5):1751–68. doi: 10.1159/000491058
67. Sachdeva UM, Shimonosono M, Flashner S, Cruz-Acuña R, Gabre JT, Nakagawa H. Understanding the cellular origin and progression of esophageal cancer using esophageal organoids. Cancer Lett (2021) 509:39–52. doi: 10.1016/j.canlet.2021.03.031
68. Chen C, Lin H, Xu F, Liu J, Cai Q, Yang F, et al. Risk factors associated with suicide among esophageal carcinoma patients from 1975 to 2016. Sci Rep (2021) 11(1):18766. doi: 10.1038/s41598-021-98260-w
69. Niu C, Liu Y, Wang J, Liu Y, Zhang S, Zhang Y, et al. Risk factors for esophageal squamous cell carcinoma and its histological precursor lesions in China: a multicenter cross-sectional study. BMC Cancer (2021) 21(1):1034. doi: 10.1186/s12885-021-08764-x
70. Rehman AU, Iqbal MA, Sattar RSA, Saikia S, Kashif M, Ali WM, et al. Elevated expression of RUNX3 co-expressing with EZH2 in esophageal cancer patients from India. Cancer Cell Int (2020) 20:445. doi: 10.1186/s12935-020-01534-y
71. Gong YQ, Lu TL, Hou FT, Chen CW. Antisense long non-coding RNAs in gastric cancer. Clin Chim Acta (2022) 534:128–37. doi: 10.1016/j.cca.2022.07.013
72. Abdi E, Latifi-Navid S, Zahri S, Yazdanbod A, Pourfarzi F. Risk factors predisposing to cardia gastric adenocarcinoma: Insights and new perspectives. Cancer Med (2019) 8(13):6114–26. doi: 10.1002/cam4.2497
73. Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol (2013) 10(11):643–55. doi: 10.1038/nrclinonc.2013.170
74. Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu DP, et al. Melatonin for the prevention and treatment of cancer. Oncotarget (2017) 8(24):39896–921. doi: 10.18632/oncotarget.16379
75. Bubenik GA, Blask DE, Brown GM, Maestroni GJ, Pang SF, Reiter RJ, et al. Prospects of the clinical utilization of melatonin. Biol Signals Recept (1998) 7(4):195–219. doi: 10.1159/000014545
76. Li W, Hu C, Zhong X, Wu J, Li G. Melatonin induces AGS gastric cancer cell apoptosis via regulating PERK/eIF2α and HSF1/NF-κB signaling pathway. Ann Clin Lab Sci (2022) 52(1):40–7.
77. Song J, Ma SJ, Luo JH, Zhang H, Wang RX, Liu H, et al. Melatonin induces the apoptosis and inhibits the proliferation of human gastric cancer cells via blockade of the AKT/MDM2 pathway. Oncol Rep (2018) 39(4):1975–83. doi: 10.3892/or.2018.6282
78. Wei X, Chen S, Xu Z, Jia N, Qi Y, Zhou Q, et al. Melatonin inhibits the migration of human gastric carcinoma cells at least in part by remodeling tight junction. J Cell Biochem (2019) 120(6):9781–6. doi: 10.1002/jcb.28258
79. Qiu B, Li K, Dong X, Liu FQ. Transjugular intrahepatic portosystemic shunt for portal hypertension in hepatocellular carcinoma with portal vein tumor thrombus. Cardiovasc Intervent Radiol (2017) 40(9):1372–82. doi: 10.1007/s00270-017-1655-8
80. Loomba R, Liu J, Yang HI, Lee MH, Lu SN, Wang LY, et al. Synergistic effects of family history of hepatocellular carcinoma and hepatitis b virus infection on risk for incident hepatocellular carcinoma. Clin Gastroenterol Hepatol (2013) 11(12):1636–45.e1-3. doi: 10.1016/j.cgh.2013.04.043
81. Jin K, Ren C, Liu Y, Lan H, Wang Z. An update on colorectal cancer microenvironment, epigenetic and immunotherapy. Int Immunopharmacol (2020) 89(Pt A):107041. doi: 10.1016/j.intimp.2020.107041
82. Raikhlin NT, Kvetnoy IM. Melatonin and enterochromaffine cells. Acta Histochem (1976) 55(1):19–24. doi: 10.1016/s0065-1281(76)80092-x
83. Posadzki PP, Bajpai R, Kyaw BM, Roberts NJ, Brzezinski A, Christopoulos GI, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med (2018) 16(1):18. doi: 10.1186/s12916-017-1000-8
84. Funk MI, Conde MA, Piwien-Pilipuk G, Uranga RM. Novel antiadipogenic effect of menadione in 3T3-L1 cells. Chem Biol Interact (2021) 343:109491. doi: 10.1016/j.cbi.2021.109491
85. Collin A, Kohan R, de Talamoni NT, Picotto G. Melatonin enhances anti-tumoral effects of menadione on colon cancer cells. Anticancer Agents Med Chem (2022) 22(13):2411–8. doi: 10.2174/1871520621666211207141729
86. Kvietkauskas M, Zitkute V, Leber B, Strupas K, Stiegler P, Schemmer P. Dietary melatonin and glycine decrease tumor growth through antiangiogenic activity in experimental colorectal liver metastasis. Nutrients (2021) 13(6). doi: 10.3390/nu13062035
87. Loveday BPT, Lipton L, Thomson BN. Pancreatic cancer: An update on diagnosis and management. Aust J Gen Pract (2019) 48(12):826–31. doi: 10.31128/ajgp-06-19-4957
88. Chu LC, Goggins MG, Fishman EK. Diagnosis and detection of pancreatic cancer. Cancer J (2017) 23(6):333–42. doi: 10.1097/ppo.0000000000000290
89. Geng Y, Fan J, Chen L, Zhang C, Qu C, Qian L, et al. A notch-dependent inflammatory feedback circuit between macrophages and cancer cells regulates pancreatic cancer metastasis. Cancer Res (2021) 81(1):64–76. doi: 10.1158/0008-5472.Can-20-0256
90. Leja-Szpak A, Nawrot-Porąbka K, Góralska M, Jastrzębska M, Link-Lenczowski P, Bonior J, et al. Melatonin and its metabolite N1-acetyl-N2-formyl-5-methoxykynuramine (afmk) enhance chemosensitivity to gemcitabine in pancreatic carcinoma cells (PANC-1). Pharmacol Rep (2018) 70(6):1079–88. doi: 10.1016/j.pharep.2018.05.007
91. Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res (2007) 42(1):28–42. doi: 10.1111/j.1600-079X.2006.00407.x
92. Jaworek J, Leja-Szpak A, Nawrot-Porąbka K, Szklarczyk J, Kot M, Pierzchalski P, et al. Effects of melatonin and its analogues on pancreatic inflammation, enzyme secretion, and tumorigenesis. Int J Mol Sci (2017) 18(5). doi: 10.3390/ijms18051014
93. Winczyk K, Pawlikowski M, Karasek M. Melatonin and RZR/ROR receptor ligand CGP 52608 induce apoptosis in the murine colonic cancer. J Pineal Res (2001) 31(2):179–82. doi: 10.1034/j.1600-079x.2001.310213.x
94. Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol (2018) 175(16):3190–9. doi: 10.1111/bph.14116
95. Kouhi Habibi N, Shabestani Monfared A, Ebrahimnejad Gorji K, Karimi M, Moghadamnia AA, Tourani M, et al. The protective effects of melatonin on blood cell counts of rectal cancer patients following radio-chemotherapy: a randomized controlled trial. Clin Transl Oncol (2019) 21(6):745–52. doi: 10.1007/s12094-018-1977-2
Keywords: melatonin, gastrointestinal cancer, carcinogenesis, cellular lifecycle, immunity
Citation: Gong Y-Q, Hou F-T, Xiang C-L, Li C-L, Hu G-H and Chen C-W (2022) The mechanisms and roles of melatonin in gastrointestinal cancer. Front. Oncol. 12:1066698. doi: 10.3389/fonc.2022.1066698
Received: 11 October 2022; Accepted: 29 November 2022;
Published: 15 December 2022.
Edited by:
Emilio Francesco Giunta, Università degli Studi della Campania Luigi Vanvitelli, ItalyReviewed by:
Jolanta Jaworek, Jagiellonian University Medical College, PolandMaggie Amer, Mansoura University, Egypt
Dino Hasanagic, University of Banja Luka, Bosnia and Herzegovina
Copyright © 2022 Gong, Hou, Xiang, Li, Hu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Huang Hu, aGdoMTIzNDE2QDE2My5jb20=; Chao-Wu Chen, Y2hlbmNoYW93dTBAMTYzLmNvbQ==
 Yong-Qiang Gong1
Yong-Qiang Gong1 Cheng-Long Li
Cheng-Long Li Chao-Wu Chen
Chao-Wu Chen