
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 23 November 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1066417
This article is part of the Research Topic Surgical Oncology in the Elderly: The State of the Art and Future Challenges View all 11 articles
 Yiqing Mao1†
Yiqing Mao1† Jiarong Lan2,3*†
Jiarong Lan2,3*†Background: We reviewed the literature to assess the prognostic ability of the geriatric nutritional risk index (GNRI) for patients with colorectal cancer (CRC) undergoing curative surgery.
Methods: The online databases of PubMed, CENTRAL, ScienceDirect, Embase, and Google Scholar were searched for articles reporting the relationship between GNRI and outcomes in CRC patients. English language studies were searched up to 28th April 2022.
Results: Ten studies with 3802 patients were included. Meta-analysis indicated that patients with low GNRI had significantly poor overall survival (HR: 2.41 95% CI: 1.72, 3.41 I2 = 68%) and disease-free survival (HR: 1.92 95% CI: 1.47, 2.49 I2 = 49%) as compared to those with high GNRI. The meta-analysis also indicated a significantly higher risk of complications with low GNRI as compared to high GNRI (HR: 1.98 95% CI: 1.40, 2.82 I2 = 0%). The results did not change on subgroup analysis based on study location, age group, GNRI cut-off, and sample size.
Conclusion: Current evidence indicates that GNRI can be a valuable prognostic indicator for CRC patients undergoing surgical intervention. Patients with low GNRI have poor overall and disease-free survival and a higher incidence of complications. Clinicians could use this simple indicator to stratify patients and formulate personalized treatment plans.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier (CRD42022328374).
Cancer has become the most common cause of mortality worldwide. Amongst the numerous subtypes, colorectal cancer(CRC) ranks the 2nd most prevalent cancer in females and 3rd most common malignancy amongst men around the globe (1). The prevalence has been high in Asian populations but a large number of patients are also being detected in Western regions and developing nations (2). CRC is known to have a predilection for the older age group as the median age of diagnosis is reported to be 67 years (3). Western data indicate that of the approximately 140,000 confirmed cases of CRC detected in 2018, around 60% were elderly with an age of >65 years. Furthermore, older adults accounted for almost 70% of mortality cases in the same period (4). Identification of modifiable risk factors for poor prognosis can aid in appropriate treatment planning and improve the long-term overall survival (OS) and disease-free survival (DFS) of CRC patients. Of the numerous risk factors identified, malnutrition has been one of the most prominent and well-defined factors associated with poor survival after CRC (5). However, there has been no consensus in the literature on how to measure malnutrition to best assess the prognosis of such patients (6). Several measurement indices like the body mass index, bodyweight loss, serum albumin levels, psoas muscle area, mini-nutritional assessment, prognostic nutritional index, and controlling nutritional status score have been used to quantify malnutrition in a cancer patient (7, 8). Since >50% of patients with gastrointestinal (GI) cancer suffer from malnutrition, there is a need for an easy to calculate and robust malnutrition indicator which has a good prognostic ability (7). The Geriatric Nutritional Risk Index (GNRI) is a simple malnutrition screening tool estimated from serum albumin levels and ideal body weight (9). It has been used in literature to assess the prognosis of patients undergoing percutaneous coronary interventions, and hemodialysis as well as for those with heart and respiratory diseases (10–13). The tool has also received attention in the field of oncology with several studies reporting its use for different cancers (14–16). Recently, Xie et al (17) have reviewed the ability of GNRI to predict the prognosis of patients with GI malignancies. In a pooled analysis of nine studies, the authors reported that patients with low GNRI had a significantly higher risk of complications and poor long-term survival as compared to those with high GNRI. An important limitation of their review was patients with different GI cancer were pooled in a single meta-analysis. Over the past few years, several authors (18–20) have reported their experience with the use of GNRI for CRC patients but there has been no consolidated review to examine the available evidence. Given this deficiency in literature, we present the results of the first systematic review and meta-analysis examining the prognostic ability of GNRI for CRC patients undergoing curative surgical resection.
The review was pre-registered on PROSPERO (No CRD42022328374). The PRISMA recommendations were used during the reporting of the review (21). A detailed search on the online databases of PubMed, CENTRAL, ScienceDirect, Embase, and Google Scholar was conducted for articles reporting the prognostic ability of GNRI for CRC patients. The search was last done on 28th April 2022. Two reviewers were independently involved in the search which was restricted to English-language publications only. The search terms were; “colorectal cancer”, “rectal cancer”, “geriatric nutritional risk index”, “prognosis”, “nutrition”, and “survival”. The search was conducted by combining the search terms with Boolean operators “OR” and “AND”. Details can be found in Supplementary Table 1. The search results combined for initial titles and abstract screening. Only studies relevant to the review were extracted and matched against the eligibility criteria. The entire procedure involved two reviewers working independently.
The eligibility criteria were all types of studies reporting the relationship between GNRI and outcomes of CRC patients undergoing curative resections. The outcomes were OS, DFS, and/or complications. We excluded studies 1) not reporting data for CRC patients separately 2) not on patients undergoing surgical intervention 3) not reporting any of the relevant outcomes 4) studies with duplicate data. If there were two studies from the same center conducted during the same period the article with the largest sample was to be included.
In the final stage, the full-text articles were screened based on the eligibility criteria, and those fulfilling the same were included. Any differences in study selection were resolved by discussion. Lastly, we also hand-searched the reference list of included studies and previous reviews to look for any missed articles.
Using an Excel spreadsheet the following data were extracted from the included studies: Details of study authors, publication year, study location, study type, inclusion criteria, the cut-off for GNRI, sample size, age, gender, carcinoembryonic antigen (CEA) levels, location of cancer (colon or rectal), tumor invasion, presence of lymph node metastasis, use of adjuvant therapy, follow-up and outcomes. The outcomes assessed in the review were OS, DFS, and complications. We assessed the risk of bias using the Newcastle-Ottawa scale (NOS) (22).
The prognostic ability of GNRI was reported as multivariable-adjusted hazard ratios (HR) by most studies. These were extracted and combined in a random-effects model to calculate the total effect size as HR with 95% confidence intervals (CI). We assessed inter-study heterogeneity using the I2 statistic. Publication bias was examined by visual inspection of funnel plots and a sensitivity analysis was also performed. Sub-group analysis was carried out based on study location (Japanese vs non-Japanese), age group included (≥65 years, ≥75 years, and others), GNRI cut-off (98 and others), and sample size (>300 and <300). Results were reported in tabular format. Funnel plots, sensitivity analysis, and subgroup analysis was not conducted for complication rates due to limited data. For studies not reporting outcomes as adjusted ratios, a descriptive analysis was undertaken. The data analysis was conducted using “Review Manager” (RevMan, version 5.3; Nordic Cochrane Centre [Cochrane Collaboration], Copenhagen, Denmark; 2014).
The initial search resulted in 5499 articles (Figure 1). After deduplication, 2328 articles were screened by the reviewers. 25 of these were selected for further analysis. Finally, ten studies were deemed eligible for inclusion in the review (18–20, 23–29).
All included studies were retrospective observational studies conducted in Asian countries (Table 1). Most of them were carried out in the Japanese population. One was from Taiwan and another study was from China. The number of participants in the studies ranged from 80 to 1206. The total pooled sample size was 3802 patients. Most studies included all elderly patients with CRC undergoing curative resection. However, there were some exceptions. One study included patients only with locally advanced rectal cancer, while another included individuals only with stage Tis/T1 CRC undergoing endoscopic submucosal dissection, and one study included those with CRC liver metastasis. The percentage of male patients ranged from 44 to 79.6% in the included studies while the proportion of patients with high CEA ranged from 28.9 to 48.8%. The distribution of colon and rectal cancer varied across included studies. Details on tumor invasion, lymph node metastasis, and the use of adjuvant therapy were not reported by all studies. All studies had a follow-up of more than 1 year. The NOS score ranged from 7 to 8.
Eight studies reported data on OS. Meta-analysis indicated that patients with low GNRI had significantly poor OS as compared to those with high GNRI (HR: 2.41 95% CI: 1.72, 3.41 I2 = 68%) (Figure 2). The results remained the same on the sequential exclusion of studies during the sensitivity analysis. We did not note any publication bias on the visual inspection of the funnel plot (Figure 3).
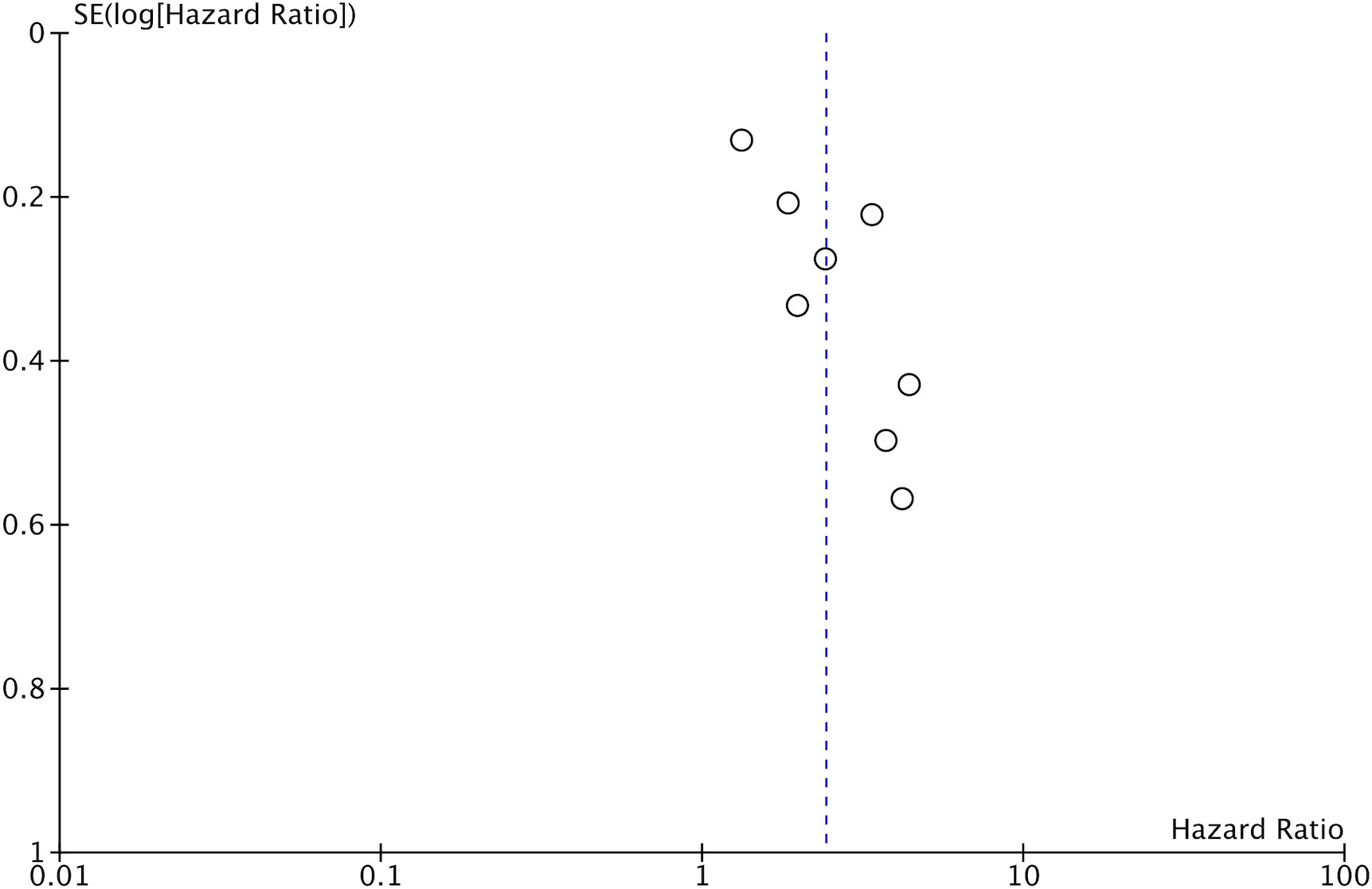
Figure 3 Funnel plot for the meta-analysis on the prognostic ability of GNRI for OS in CRC patients.
Data on DFS was available only from six studies. On pooled analysis, we noted that low GNRI was a significant predictor of poor DFS in CRC patients (HR: 1.92 95% CI: 1.47, 2.49 I2 = 49%) (Figure 4). There was no change in the significance of the results on sensitivity analysis. There was no evidence of publication bias noted on the funnel plot (Figure 5).
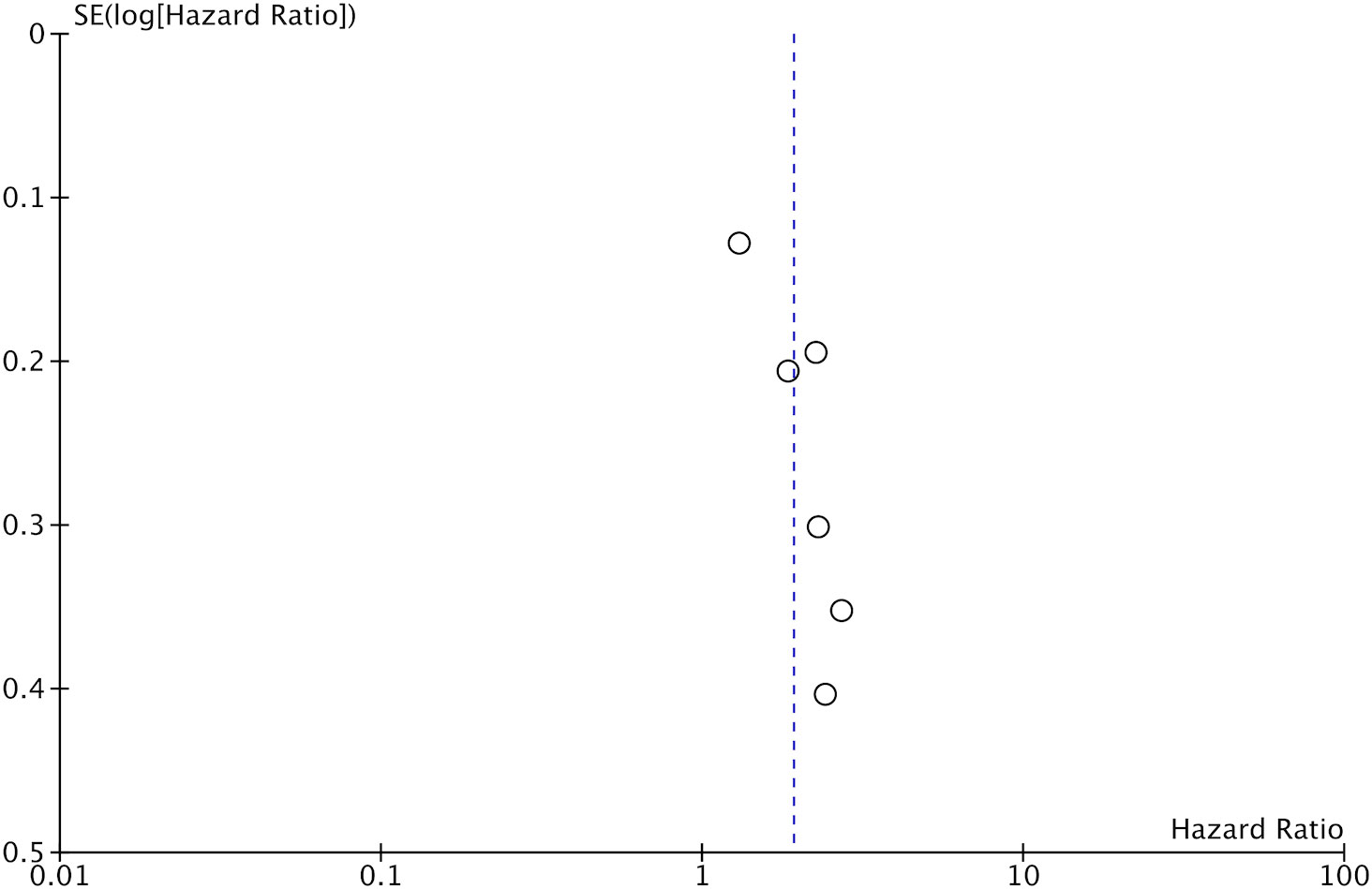
Figure 5 Funnel plot for the meta-analysis on the prognostic ability of GNRI for DFS in CRC patients.
Only three studies assessed the prognostic ability of GNRI for predicting complications. Meta-analysis indicated a significantly higher risk of complications with low GNRI as compared to high GNRI (HR: 1.98 95% CI: 1.40, 2.82 I2 = 0%) (Figure 6).
The results of subgroup analysis for OS and DFS are reported in Table 2. We noted that subgroup analysis for the outcome OS based on study location, GNRI cut-off, and sample size did not change the significance of the results. However, GNRI was not predictive of OS on a pooled analysis of two studies including only those with ≥75 years of age. The results of the outcome DFS did not change on subgroup analysis based on study location, age group, GNRI cut-off, and sample size.
Only one study by Kataoka et al (25) did not report outcomes as adjusted ratios and hence could not be included in the meta-analysis. In their study, the authors used propensity score matching to compare data of patients with low and high GNRI (cut-off 98). Patients with low GNRI had significantly poor OS (p=0.002), DFS (p=0.006), and a higher rate of complications (p=0.001) as compared to those with high GNRI.
Cancer patients have a high prevalence of malnutrition and muscle wasting which is known to negatively affect survival and increase the length of hospital stays. Indeed, the catabolic and physiological impact of cancer cachexia escalates the nutritional and energy requirement of the individual but it is seldom met due to inadequate dietary intake and reduced physical activity (5). Malnutrition is further exacerbated in GI malignancies due to additional factors like malabsorption, obstructive syndrome, and diarrhea (7). Research has shown that malnutrition is unexpectedly high in patients with GI malignancies with clinicians recognizing only 1 out of 4 patients with malnutrition leading to inadequate pretreatment nutritional support and poor outcomes. This illustrates the fact that nutritional screening is of utmost importance even when the patient shows no overt signs of malnutrition (30). One of the limitations of various nutritional screening tools is their varying sensitivity and specificities. Ideally, the screening tool should be simple, brief, inexpensive, with high sensitivity and good specificity (31).
In this context, the GNRI was developed by Bouillanne et al. in 2005 as a simple tool to predict outcomes in elderly patients using albumin and body weight data (9). Since then the tool has been used to predict outcomes in a variety of patients (10–13). Several meta-analysis studies have reported the predictability of GNRI for various cancer subtypes. Wang et al (32) in a meta-analysis of eight studies have shown that low GNRI is associated with poor OS (HR: 1.99, 95% CI: 1.68-2.35) and DFS (HR = 2.34, 95% CI: 1.11-4.95) in patients with non-small cell lung cancer. In another recent meta-analysis, Yu et al (33) compiled data from 14 studies and noted that low pretreatment GNRI predicted poor OS (HR = 1.47, 95% CI: 1.33-1.63) and DFS (HR = 1.69, 95% CI: 1.24-2.31) in patients with esophageal cancer. Individual studies have shown that GNRI could predict outcomes in patients with head and neck cancer, renal cancer, pancreatic cancer, and gastric cancer (14, 16, 34, 35). However, since each cancer subtype is different, it is important that the predictability of GNRI is established for CRC as well.
We conducted a detailed literature search to recognize ten studies with a total of 3802 cancer patients undergoing curative surgical resection for CRC. This provided a more homogenous group of patients undergoing the same primary treatment unlike the previous meta-analysis wherein patients with different GI malignancies undergoing different treatments were included (17). On pooled analysis, it was seen that patients with low GNRI had a 2.4 times increased risk of the poor OS as compared to those with high GNRI. Secondly, patients with low GNRI had 1.9 times increased risk of recurrence as compared to those with high GNRI. We also noted that low GNRI was significantly associated with higher rates of complications, albeit with only three studies in the meta-analysis. On examination of all three forest plots of our meta-analysis, it can be seen that the direction of the result was consistent across all studies only with varying 95% CI. None of the studies noted a non-significant association between low GNRI and outcomes in CRC patients. There was little evidence of publication bias and the survival results maintained their significance on sensitivity analysis. The results were robust and thereby increase the validity of our conclusions.
An important limitation of the meta-analysis was the moderate heterogeneity in the meta-analysis of OS and DFS. This could be due to several known and unknown variables like sample size, study location, patient demographics, baseline stage of CRC, treatment protocols, and cut-off used for GNRI. Based on the availability of data we divided the studies into separate groups based on sample size, study location, age group included, and the cut-off for GNRI only to note no change in the significance of the results. The exception was the outcome of OS in the subgroup of studies including only patients aged ≥75years. The overall effect size was 2.08 with a 95% CI of 0.84, 5.17. The non-significant results could be due to the small number of studies in the analysis as the 95% CI was wide with the lower end very close to 1 and the upper end indicating a 5 times increased risk of poor OS.
If the GNRI has to be incorporated into clinical practice, a well-established cut-off is needed to segregate patients into low and high GNRI groups. Most of the studies in our review as well as in literature (15, 33) have used the cut-off of 98 for classifying patients into those with low and high GNRI. Nevertheless, there has been no consensus and other cut-offs have been used by studies based on receiver operating curve analysis of population-specific data. In our subgroup analysis, we noted that the results were the same for studies using a cut-off of 98 or any other for assessing the prognosis of CRC patients. Future studies should focus on GNRI cut-off in different populations to arrive at a common figure for clinical practice.
The good prognostic ability of GNRI could be due to its combined use of two important markers of malnutrition: albumin and body weight (9). Low serum albumin levels have been congruous with malnutrition and hypoalbuminemia has been associated with poor wound healing, infections, and reduced survival in cancer patients. Serum albumin has an immunomodulatory role with low levels leading to reduced cell-mediated immunity against cancer cells (5). Hypoalbuminemia causes reduced macrophage activation and granuloma formation which may promote surgical site infections and other complications in CRC patients (6). Hu et al (5) in a study on 30676 CRC patients have found a statistically significant association between low albumin levels and postoperative complications like venous thromboembolism, surgical site infections, pneumonia, septic shock, prolonged ventilator use, blood transfusion, return to the operating room, stroke, and re-intubation in CRC patients. Secondly, the GNRI uses a ratio of current body weight to ideal body weight as a marker of the body mass index (BMI) of the patients. Cancer patients with low BMI are at an increased risk of poor survival (36). Thus, it can be postulated that the combination of albumin and body weight increases the ability of the GNRI to predict prognosis in cancer patients.
There are several strengths to our review. It is the first meta-analysis to aggregate evidence on the role of GNRI in predicting outcomes in CRC patients. We attempted to include a homogenous population of patients undergoing surgical intervention. The validity of the results was tested by sensitivity and different subgroup analyses.
Nevertheless, there are some limitations as well. Not all studies provided data for all three outcomes. Hence, the number of studies in the meta-analysis was less than 10. Secondly, not all studies were of large sample size and this may have reduced the power of our analysis. Thirdly, there was some heterogeneity in the study population included in the studies. Some included only patients with T1 stage while another included patients with liver metastasis. The effect of such variation could be assessed only by sensitivity analysis and not by subgroup analysis. Fourthly, all studies were on Asian populations with most studies from Japan. Thus the results cannot be generalized to other populations.
Current evidence indicates that GNRI can be a valuable prognostic indicator for CRC patients undergoing surgical intervention. Patients with low GNRI have poor OS, DFS, and a higher incidence of complications. Clinicians could use this simple indicator to stratify patients and formulate personalized treatment plans. Further studies with a larger sample size are required to validate the results in non-Asian populations and obtain the most optimal cut-off to predict outcomes.
Publicly available datasets were analyzed in this study. The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YM and JL conceived and designed the study. YM and JL were involved in literature search and data collection. YM analyzed the data. JL wrote the paper. and JL reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1066417/full#supplementary-material
1. Pérez-Escalante E, Cariño-Cortés R, Fernández-Martínez E, Ortiz MI, Muñoz-Pérez VM, Sánchez-Crisóstomo I, et al. Colorectal cancer: Causes and evidence of chemopreventive treatments. Curr Pharm Biotechnol (2018) 19:1135–55. doi: 10.2174/1389201020666181226112712
2. Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer (2018) 119:785–92. doi: 10.1038/S41416-018-0264-X
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/CAAC.21492
4. Papamichael D, Hernandez P, Mistry R, Xenophontos E, Kakani C. Adjuvant chemotherapy in patients with colorectal cancer. is there a role in the older adult? Eur J Surg Oncol (2020) 46:363–8. doi: 10.1016/J.EJSO.2020.01.002
5. Hu WH, Eisenstein S, Parry L, Ramamoorthy S. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J (2019) 18:33. doi: 10.1186/S12937-019-0458-Y
6. Hu WH, Cajas-Monson LC, Eisenstein S, Parry L, Cosman B, Ramamoorthy S. Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS-NSQIP. Nutr J (2015) 14:91. doi: 10.1186/S12937-015-0081-5
7. Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg (2015) 152 Suppl 1:S3–7. doi: 10.1016/S1878-7886(15)30003-5
8. Kubota T, Shoda K, Konishi H, Okamoto K, Otsuji E. Nutrition update in gastric cancer surgery. Ann Gastroenterol Surg (2020) 4:360–8. doi: 10.1002/AGS3.12351
9. Bouillanne O, Morineau G, Dupant C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr (2005) 82:777–83. doi: 10.1093/AJCN/82.4.777
10. Takahashi H, Ito Y, Ishii H, Aoyama T, Kamoi D, Kasuga H, et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol (2014) 64:32–6. doi: 10.1016/J.JJCC.2013.10.018
11. Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol (2017) 119:1740–5. doi: 10.1016/J.AMJCARD.2017.02.051
12. Li H, Cen K, Sun W, Feng B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res (2021) 33:1477–86. doi: 10.1007/S40520-020-01656-3
13. Matsumura T, Mitani Y, Oki Y, Fujimoto Y, Ohira M, Kaneko H, et al. Comparison of geriatric nutritional risk index scores on physical performance among elderly patients with chronic obstructive pulmonary disease. Heart Lung (2015) 44:534–8. doi: 10.1016/J.HRTLNG.2015.08.004
14. Sakamoto T, Yagyu T, Uchinaka E, Miyatani K, Hanaki T, Kihara K, et al. The prognostic significance of combined geriatric nutritional risk index and psoas muscle volume in older patients with pancreatic cancer. BMC Cancer (2021) 21:342. doi: 10.1186/S12885-021-08094-Y
15. Matsuura S, Morikawa K, Ito Y, Kubota T, Ichijo K, Mochizuki E, et al. The geriatric nutritional risk index and prognostic nutritional index predict the overall survival of advanced non-small cell lung cancer patients. Nutr Cancer (2021) 74:1606–13. doi: 10.1080/01635581.2021.1960387
16. Nakayama M, Gosho M, Adachi M, Ii R, Matsumoto S, Miyamoto H, et al. The geriatric nutritional risk index as a prognostic factor in patients with advanced head and neck cancer. Laryngoscope (2021) 131:E151–6. doi: 10.1002/LARY.28587
17. Xie H, Tang S, Wei L, Gan J. Geriatric nutritional risk index as a predictor of complications and long-term outcomes in patients with gastrointestinal malignancy: a systematic review and meta-analysis. Cancer Cell Int (2020) 20:530. doi: 10.1186/S12935-020-01628-7
18. Iguchi T, Sugimachi K, Mano Y, Motomura T, Sugiyama M, Ota M, et al. Prognostic impact of geriatric nutritional risk index in patients with synchronous colorectal liver metastasis. Anticancer Res (2020) 40:4165–71. doi: 10.21873/ANTICANRES.14416
19. Sasaki M, Miyoshi N, Fujino S, Ogino T, Takahashi H, Uemura M, et al. The geriatric nutritional risk index predicts postoperative complications and prognosis in elderly patients with colorectal cancer after curative surgery. Sci Rep (2020) 10:10744. doi: 10.1038/S41598-020-67285-Y
20. Ide S, Okugawa Y, Omura Y, Yamamoto A, Ichikawa T, Kitajima T, et al. Geriatric nutritional risk index predicts cancer prognosis in patients with local advanced rectal cancer undergoing chemoradiotherapy followed by curative surgery. World J Surg Oncol (2021) 19:34. doi: 10.1186/S12957-021-02139-Z
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
22. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed October 30, 2020).
23. Tang S, Xie H, Kuang J, Gao F, Gan J, Ou H. The value of geriatric nutritional risk index in evaluating postoperative complication risk and long-term prognosis in elderly colorectal cancer patients. Cancer Manag Res (2020) 12:165–75. doi: 10.2147/CMAR.S234688
24. Kato M, Hayashi Y, Fukuda H, Yamaguchi S, Inoue T, Ogiyama H, et al. Geriatric nutritional risk index as a prognostic indicator in elderly patients with early colorectal cancer undergoing endoscopic submucosal dissection. Dig Endosc (2022) 34:569–78. doi: 10.1111/DEN.14090
25. Kataoka M, Hirano Y, Ishii T, Ishikawa S, Kataoka A, Fujii T, et al. Prognostic utility of geriatric nutritional risk index after curative resection of colorectal cancer: A propensity score-matched study. Cancer Diagnosis Progn (2021) 1:479–84. doi: 10.21873/cdp.10064
26. Liao CK, Chern YJ, Hsu YJ, Lin YC, Yu YL, Chiang JM, et al. The clinical utility of the geriatric nutritional risk index in predicting postoperative complications and long-term survival in elderly patients with colorectal cancer after curative surgery. Cancers (Basel) (2021) 13:5852. doi: 10.3390/CANCERS13225852
27. Doi S, Migita K, Ueno M, Yasuda S, Aoki S, Fujimoto K, et al. The prognostic significance of the geriatric nutritional risk index in colorectal cancer patients. Nutr Cancer (2022) 74:2838–45. doi: 10.1080/01635581.2022.2036768
28. Hayama T, Hashiguchi Y, Ozawa T, Watanabe M, Fukushima Y, Shimada R, et al. The preoperative geriatric nutritional risk index (GNRI) is an independent prognostic factor in elderly patients underwent curative resection for colorectal cancer. Sci Rep (2022) 12:3682. doi: 10.1038/S41598-022-07540-6
29. Yagyu T, Yamamoto M, Tanio A, Hara K, Sugezawa K, Uejima C, et al. Risk factors for recurrence in elderly patients with stage II colorectal cancer: a multicenter retrospective study. BMC Cancer (2022) 22:390. doi: 10.1186/S12885-022-09501-8
30. Attar A, Malka D, Sabaté JM, Bonnetain F, Lecomte T, Aparicio T, et al. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross-sectional multicenter study. Nutr Cancer (2012) 64:535–42. doi: 10.1080/01635581.2012.670743
31. Håkonsen SJ, Pedersen PU, Bath-Hextall F, Kirkpatrick P. Diagnostic test accuracy of nutritional tools used to identify undernutrition in patients with colorectal cancer: a systematic review. JBI Database Syst Rev Implement Rep (2015) 13:141–87. doi: 10.11124/JBISRIR-2015-1673
32. Wang H, Li C, Yang R, Jin J, Liu D, Li W. Prognostic value of the geriatric nutritional risk index in non-small cell lung cancer patients: A systematic review and meta-analysis. Front Oncol (2022) 11:794862. doi: 10.3389/FONC.2021.794862
33. Yu J, Zhang W, Wang C, Hu Y. The prognostic value of pretreatment geriatric nutritional risk index in esophageal cancer: A meta-analysis. Nutr Cancer (2022) 74:3202–10. doi: 10.1080/01635581.2022.2069273
34. Kang HW, Seo SP, Kim WT, Yun SJ, Lee SC, Kim WJ, et al. A low geriatric nutritional risk index is associated with aggressive pathologic characteristics and poor survival after nephrectomy in clear renal cell carcinoma: A multicenter retrospective study. Nutr Cancer (2020) 72:88–97. doi: 10.1080/01635581.2019.1621357
35. Furuke H, Matsubara D, Kubota T, Kiuchi J, Kubo H, Ohashi T, et al. Geriatric nutritional risk index predicts poor prognosis of patients after curative surgery for gastric cancer. Cancer Diagnosis Progn (2021) 1:43–52. doi: 10.21873/cdp.10007
Keywords: nutrition, prognosis, survival, complications, cancer
Citation: Mao Y and Lan J (2022) Prognostic value of the geriatric nutritional index in colorectal cancer patients undergoing surgical intervention: A systematic review and meta-analysis. Front. Oncol. 12:1066417. doi: 10.3389/fonc.2022.1066417
Received: 17 October 2022; Accepted: 08 November 2022;
Published: 23 November 2022.
Edited by:
Cosimo Sperti, University of Padua, ItalyCopyright © 2022 Mao and Lan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiarong Lan, c2R3YXRlcnNAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.