- Department of Pathology, West China Hospital, Sichuan University, Chengdu, China
Introduction: Among those tumors with consistent USP6 rearrangement, some arise from soft tissue and show bone metaplasia, including myositis ossificans (MO), fibro-osseous pseudotumor of digits (FOPD), soft tissue aneurysmal bone cyst (ST-ABC) and fasciitis ossificans (FO). These lesions are easily confused with malignancies because they show a rapid growth rate and brisk mitoses. Here, we aim to clarify the clinicopathologic and genetic characteristics of this entity and analyze the correlations among the different subtypes in one of the largest cohorts.
Materials and Methods: The clinicopathologic features of 73 cases of MO, FOPD, ST-ABC and FO diagnosed at West China Hospital, Sichuan University from January 2010 to December 2021 were retrospectively analyzed. Forty-three undecalcified samples were analyzed by systematic genetic studies, including fluorescence in situ hybridization (FISH), reverse transcription polymerase chain reaction (RT-PCR), Sanger sequencing and next-generation-based sequencing were performed.
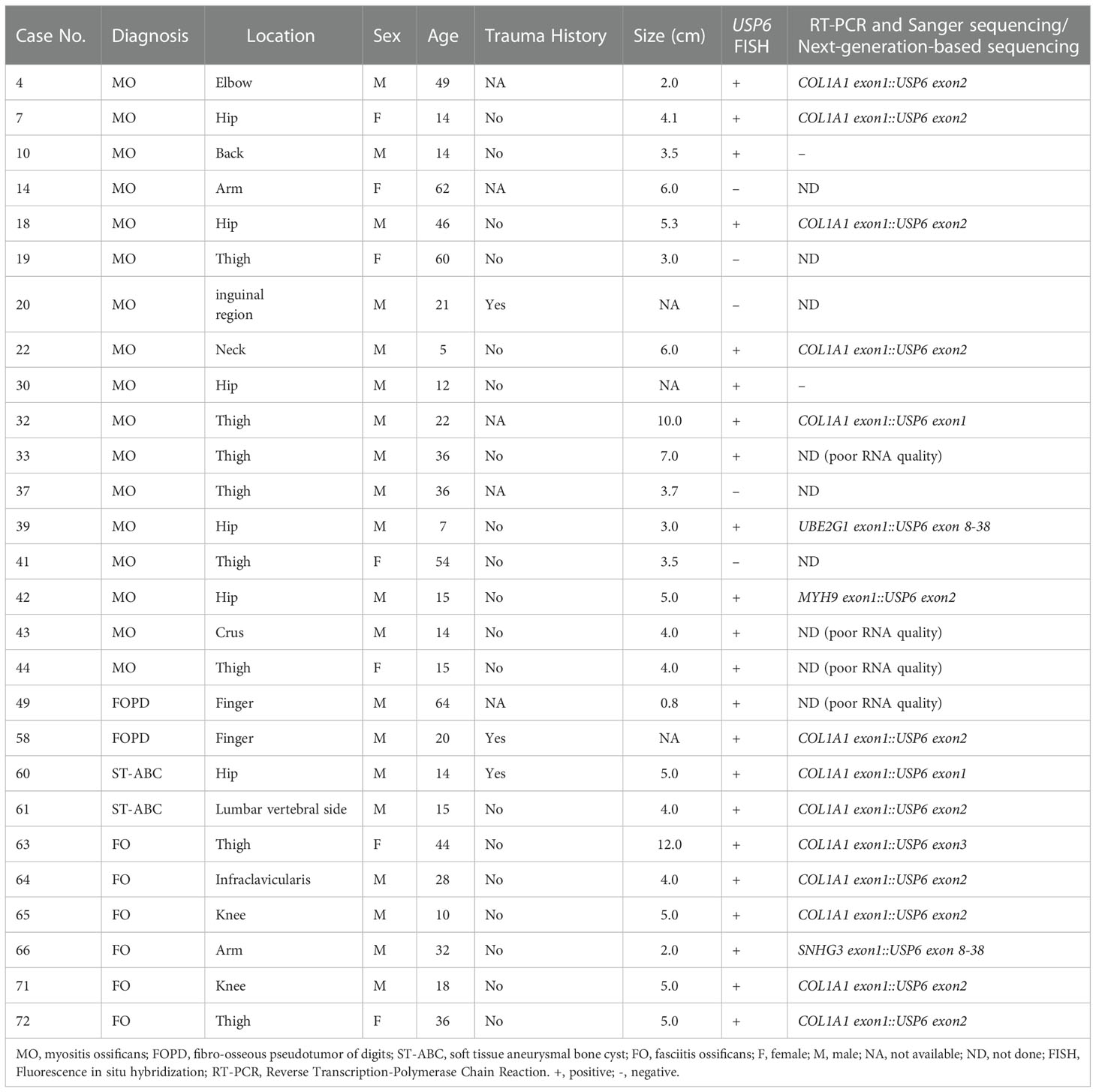
Results: This series included 40 males and 33 females aged 2 to 80 years old (median: 31 years). FOPD occurred in extremal soft tissue, while lower extremities (38/58, 65.5%) were the most commonly involved lesions in the other three subgroups. Histologically, proliferative myofibroblasts/fibroblasts with varying degrees of osteoid tissue were present. Fluorescence in situ hybridization (FISH) results indicated that 22 cases (22/27, 81.5%) were positive for USP6 rearrangement, and 5 cases were negative. Among those cases with positive FISH results, 18 underwent reverse transcription-polymerase chain reaction (RT-PCR) detection that successfully detected common USP6 fusion types. Thirteen cases showed COL1A1::USP6 fusion, one showed MYH9::USP6 fusion, and 4 were negative for common fusion types. Next-generation-based sequencing technology was performed on two lesions with negative RT–PCR results and novel fusion partners SNHG3 and UBE2G1 were discovered.
Conclusions: Our findings revealed that COL1A1 is the most common fusion partner in this entity, unlike primary aneurysmal bone cysts and nodular fasciitis. Notably, we believed that FO may demonstrate more similar clinicopathologic and genetic manifestations with MO/FOPD and ST-ABC instead of nodular fasciitis for involving lower limbs most frequently and showing recurrent COL1A1::USP6 fusion. Additionally, this study also found two novel USP6 fusion partners, which further expanded our knowledge of this neoplastic spectrum.
Introduction
The ubiquitin-specific protease 6 gene (USP6), also known as Tre-2, encodes chromosome 17p13.2 (1). In 2004, Oliveira and colleagues found recurrent USP6 rearrangement in primary aneurysmal bone cysts (ABCs) (63%) (2). Subsequent studies confirmed that CDH11 was the most common fusion partner for USP6 in primary ABC (2, 3). In 2011, Erickson-Johnson et al. recognized recurrent USP6 rearrangement and the most common fusion partner MYH9 in nodular fasciitis (NF) (4). Thereafter, these two entities were included as USP6-associated neoplasms (1). In recent years, a growing number of molecular genetic studies (including research from our group) have expanded the families of USP6-associated neoplasms. The family also includes (1) variants of NF: cranial fasciitis (CF), intravascular fasciitis (IVF) and fasciitis ossificans (FO); and (2) other spindle cell neoplastic lesions: fibroma of tendon sheath (FTS), benign infiltrative myofibroblastic neoplasms, myositis ossificans (MO), fibro-osseous pseudotumor of digits (FOPD) and soft tissue aneurysmal bone cyst (ST-ABC) (5–14).
Notably, USP6-associated neoplasms are characterized by proliferative myofibroblasts/fibroblasts with or without metaplasia of osteoid components. In this family, many tumors are classic pseudosarcomatoid lesions, which are easily confused with malignancies in the diagnostic process, especially for those with bone metaplasia. Our group has previously conducted some related studies on NF and its variants (some results have been published) (11, 15). This study will focus on USP6-associated soft tissue tumors with bone metaplasia.
USP6-associated soft tissue tumors with bone metaplasia predominantly consist of MO, FOPD, ST-ABC and FO. MO and FOPD have been previously recognized as tumors belonging to the same spectrum from the perspective of morphology, and recent studies have further confirmed the conclusion from the perspective of genetics for identifying consistent USP6 rearrangements in both MO and FOPDs (8, 9). Recent research has revealed that the most common fusion partner in MO and FOPD is COL1A1 (10), which is different from the primary ABC and NF. Recently, clinicopathological features and genetic changes similar to MO/FOPD have been identified in ST-ABC, while the 2020 WHO classification of soft tissue and bone tumors and some recent researchers have suggested that rare ST-ABC, MO and FOPD should be classified into the same subclass of USP6-associated tumors (5, 16). Notably, FO also showed ossifying components in morphology, but there are only a few studies with a small sample size on these tumors, Further studies are needed to determine whether there is a closer affinity between FO and MO, FOPD, and ST-ABC.
Based on the aforementioned background, this study will include one of the largest cohorts of MO, FOPD, ST-ABC, and FO cases to further clarify the clinicopathologic and genetic characteristics of this entity and analyze the correlations and differences among different subtypes.
Materials and methods
Case selection
This study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (No. 793, 2021). A SNOMED search of the hospital surgical pathology files from January 2010 to December 2021 identified 73 USP6-associated soft tissue tumors with bone metaplasia, which included 44 MO cases, 15 FOPD cases, 12 FO cases and 2 ST-ABC cases. Clinical, pathological, and follow-up information was collected from clinical records and pathology reports. Follow-up information was collected by telephone interviews. The follow-up duration was calculated from the date of the first surgery to the date of recurrence, death, or last follow-up.
Histologic review
Hematoxylin and eosin-stained and immunohistochemically stained slides were obtained from the surgical and pathological bank of the hospital, and all cases were reviewed independently by 2 experienced pathologists (Hongying Zhang and Xianliang Zhang) with expertise in soft tissue and bone tumor pathology and 1 general surgical pathologist (Yahan Zhang).
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was conducted on formalin-fixed, paraffin-embedded (FFPE) tissue sections using a USP6 break-apart probe (Lbp Medicine Science & Technology, Guangzhou, China) following the manufacturer-provided instructions for 43 cases (including 24 MOs, 6 FOPDs, 11 FOs and 2 ST-ABCs) with available undecalcified tissues. Thirty decalcified cases did not perform FISH because the strong acid decalcification method routinely used would damage the sample DNA. Tumor samples were evaluated by two pathologists in 100 neoplastic cells in a blind fashion using an Olympus BX53 fluorescence microscope (Japan). A red-green split signal pattern was considered positive for USP6 gene rearrangement if the distance between the green and red signals was greater than the diameter of any two signals. A case was considered positive for USP6 rearrangement when 10% or more counted cells showed red-green split signals.
Reverse transcription-polymerase chain reaction and Sanger sequencing
Total RNA was extracted using the miRNeasy FFPE Kit (Qiagen, Hilden, Germany), and the concentration and quality of RNA were measured using NanoDrop Microvolume Spectrophotometers (Thermo Fisher Scientific, Massachusetts, USA). cDNA was synthesized using the PrimeScript RT reagent kit (Takara, Tokyo, Japan). All polymerase chain reactions (PCRs) were performed for 40 cycles using a TB Green™ Premix Ex Taq™ II kit (Takara, Tokyo, Japan) with the following cycle conditions: denaturation at 94°C for 40 s, annealing at 50~60°C for 30 s, and extension at 72°C for 30 s using primers for commonly reported USP6 fusion genes, including MYH9::USP6, CDH11::USP6, COL1A1::USP6, SEC31A::USP6, RUNX2::USP6, PAFAH1B1::USP6, PPP6R3::USP6 and COL1A2::USP6 (Supplemental Table 1). Ten microliters of each amplified product were subjected to 2% agarose gel electrophoresis and photographed by a Bio-Rad imager for visualization. Sanger sequencing was performed to verify the positive reverse transcription-polymerase chain reaction (RT-PCR) products.
Next-generation-based technology
The FFPE tissue was sent to the sequencing core (242 gene DNA panels, Yousu™, OrigiMed, Shanghai, China). DNA was extracted from FFPE tissues using the QIAamp DNA FFPE Tissue Kit according to the manufacturer’s protocol (Qiagen, Hilden, Germany). A total of 0.5 μg of DNA per sample was applied as input for the DNA library preparations. Assays were performed using an Illumina MiSeq Platform (Illumina, San Diego, CA, USA) following the manufacturer’s recommendations.
Results
Clinical findings
This subgroup included 40 males and 33 females with diagnosed ages ranging from 2 to 80 years old (median: 31 years). Most cases manifested as a painful mass or swelling lesion, and the duration of these symptoms was 0.2 to 96 months (median: 1 month) in 66 patients with available data. Injury information was available for 48 patients, among which 14 (29.2%) had a history of trauma at the lesion site. Except for FOPD occurring in the soft tissues of the extremities (12 fingers and 3 toes), the lower limbs (38/58, 65.5%) were the most commonly involved sites, followed by the upper limbs (9/58, 15.5%), trunk (9/58, 15.5%), and head and neck (2/58, 3.5%) in MO, ST-ABC and FO. The range of maximum tumor diameter in 59 cases with available information was 0.5 cm to 20 cm (median: 5.0 cm).
Imaging data were available for 38 cases. MO was often in the deep muscle, FOPD was predominantly located in the subcutaneous soft tissue of the fingers and toes, ST-ABC was located in the deep soft tissue, and FO was essentially confined to the subcutaneous fat layer. Tumor boundary information was obtained in 30 cases, and most of them (21/30, 70.0%) were well circumscribed on imaging. MRI is mainly manifested as a mass with an abnormal signal in the intramuscular or subcutaneous tissues. CT and/or X-ray usually showed a slightly low-density mass in the soft tissue, with mostly high-density calcification in or around the mass (15/25, 60.0%) (Figures 1A–D).

Figure 1 Imaging findings of USP6-associated soft tissue tumors with osteoid tissue. (A) CT showed a soft tissue density mass in the muscle, with well-defined boundaries and circumferentially circular density (arrow) (Case 33). (B) X-rays demonstrated a soft tissue mass in the right finger with calcification (arrow) (Case 54). (C) CT showed mixed-density shadows with clear boundaries around the right hip (arrow) (Case 60). (D) MRI showed mixed signals in the subcutaneous fat layer of the left thigh, with clear boundaries (arrow) (Case 63).
Pathological findings
Histologically, the basic lesions of this entity are myofibroblast/fibroblast proliferation with varying degrees of osteoid components. The myofibroblasts/fibroblasts may show mild atypia. Osteoblasts were often found around the mature and braided bone, and multinucleated giant cells were shown in some areas. Some (29/71, 40.1%) lesions were accompanied by cartilage metaplasia. Varying numbers of mitoses with no atypia can be observed (0~20/10 HPFs). In those MO lesions, most cases demonstrated an ill-defined intramuscular mass. Microscopically, a zonation pattern, noted as transitions from the central immature osteoid component gradually to the peripheral mature trabecular bone, can be observed in most cases (Figure 2A). Part of MO cases (34.1%, 15/44) showed NF-like morphology in some foci, among which myofibroblasts/fibroblasts arranged as cell culture pattern, varying degree of myxoid change, extravasated erythrocytes, infiltrating inflammatory cells (Figure 2B). Extremely rare cases displayed extensive and prominent NF-like morphology (Figure 2C) and focal osteoid components with zonation patterns (Figure 2D). In terms of FOPD, the morphology was similar to MO; however, osteoid components in most FOPD cases showed a haphazard pattern instead of an obvious zonation pattern (Figures 2E, F). Most FO had classical NF-like morphology (10/12, 83.8%), and osteoid tissue of different maturation degrees were distributed haphazardly in tumors (Figure 3A). Extremely rare FO cases showed local infiltration and reverse zonal distribution that is similar to osteosarcoma (Figure 3B). The hyperplastic myofibroblasts/fibroblasts cells showed mild atypia, and immature osteoid tissue was scattered among them (Figure 3C). Besides, brisk mitosis with no atypia could be observed (Figure 3D). ST-ABC featured blood-filled cyst formation (Figure 3E), and MO-like morphology with a zonation structure was also found in some regions of one ST-ABC in this group (Figure 3F).
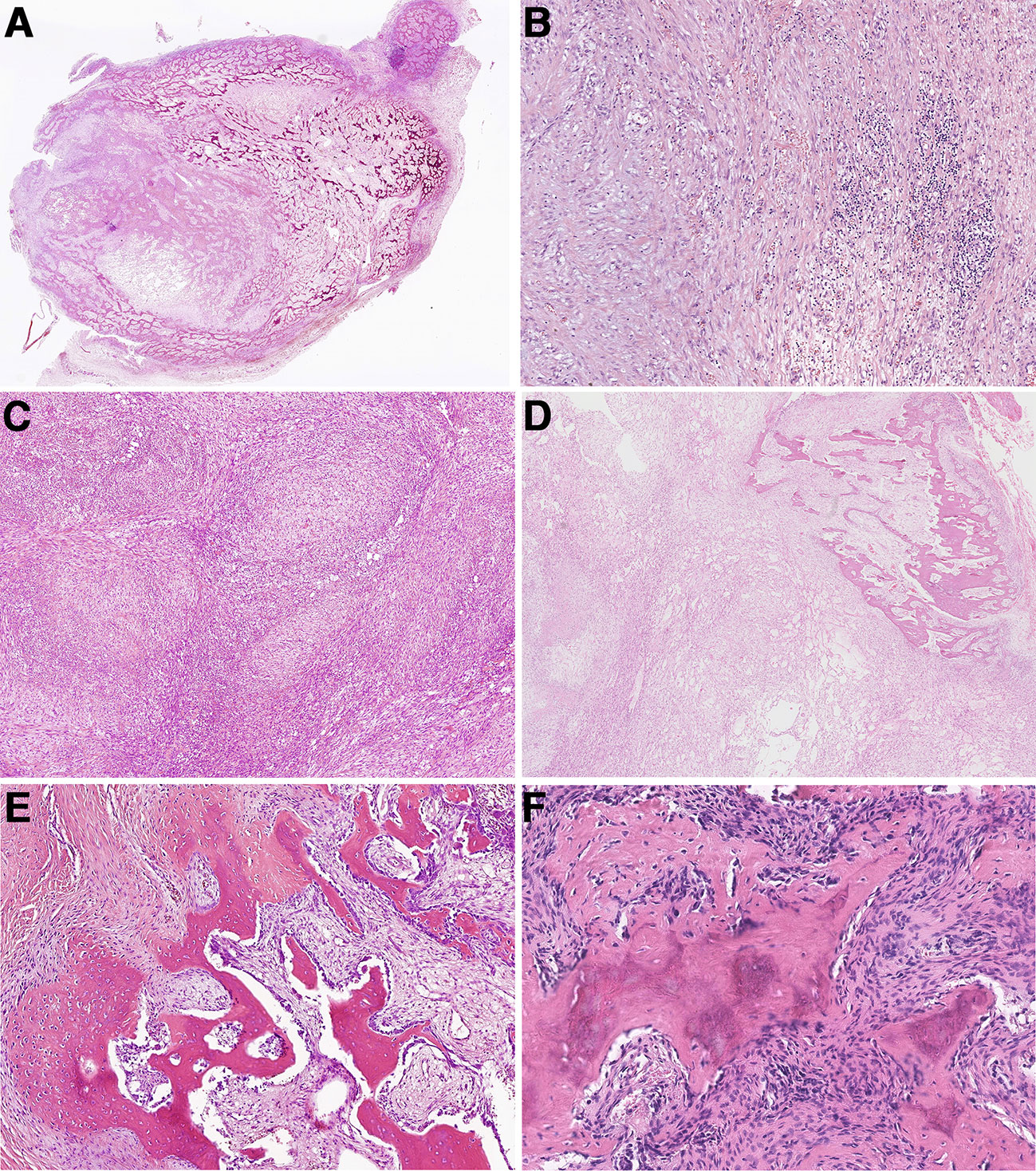
Figure 2 Histopathologic characteristics of myositis ossificans and fibro-osseous pseudotumors of digits. (A) Most myositis ossificans cases were well circumscribed with a typical zonation pattern (Case 33). (B) Myofibroblasts/fibroblasts were arranged in bundles in the myxoid stroma, accompanied by extravasated red blood cells and chronic inflammatory cells, showing NF-like morphology (Case 33). (C) Extensive NF-like morphology was observed in one myositis ossificans case (Case 42). (D) The tumor formed obvious zonal distribution focally (Case 42). (E) Osteoid components were mature and disorderly in fibro-osseous pseudotumors of digits (Case 46). (F) Immature osteoid components were observed scattered among the proliferative myofibroblast/fibroblast (Case 57).
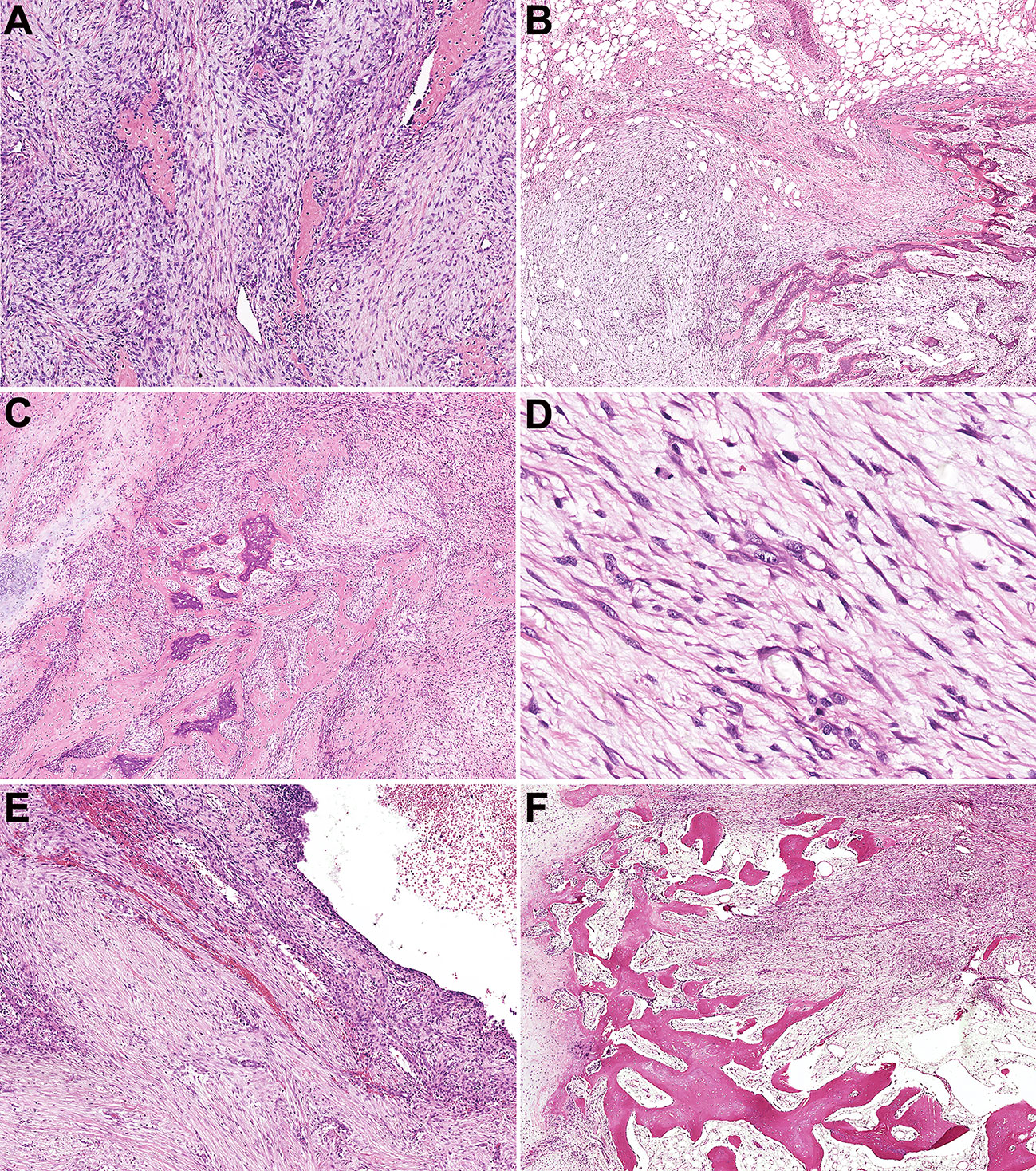
Figure 3 Histopathologic characteristics of fasciitis ossificans and soft tissue aneurysmal bone cyst. (A) Osteoid tissues were distributed disorderly in an NF-like background in fasciitis ossificans (Case 66). (B) In one fasciitis ossificans case, the tumor was locally invasive with a reverse zonation structure. (Case 63). (C) Myofibroblasts/fibroblasts cells were hyperplasia with mild atypia, and immature osteoid tissue was scattered among them. (Case 63). (D) Mitotic figures with no atypia were presented in myofibroblasts/fibroblasts (Case 63). (E) Soft tissue aneurysmal bone cyst showed a cystic cavity with hyperplastic myofibroblasts/fibroblasts and focal osteoid tissue in the cystic septum (Case 60). (F) In one soft tissue aneurysmal bone case, MO-like zone structure was observed and bone shell was formed around the tumor (Case 60).
Pertinent immunohistochemical markers were performed for diagnosis in some cases. Smooth muscle actin (SMA) showed diffuse expression in all detected cases (21/21, 100%), six cases exhibited MSA expression (6/8, 75.0%), and expression of SATB2 was present in the bone metaplasia area (8/8, 100%). Lacked expression of desmin in 19 cases (19/20, 95.0%). S-100 protein (16/16, 100%), β-catenin (8/8, 100%) and MDM2 (8/8,100%) expression were consistently absent. The MIB-I positive index ranged from 1 to 30%.
Genetic findings
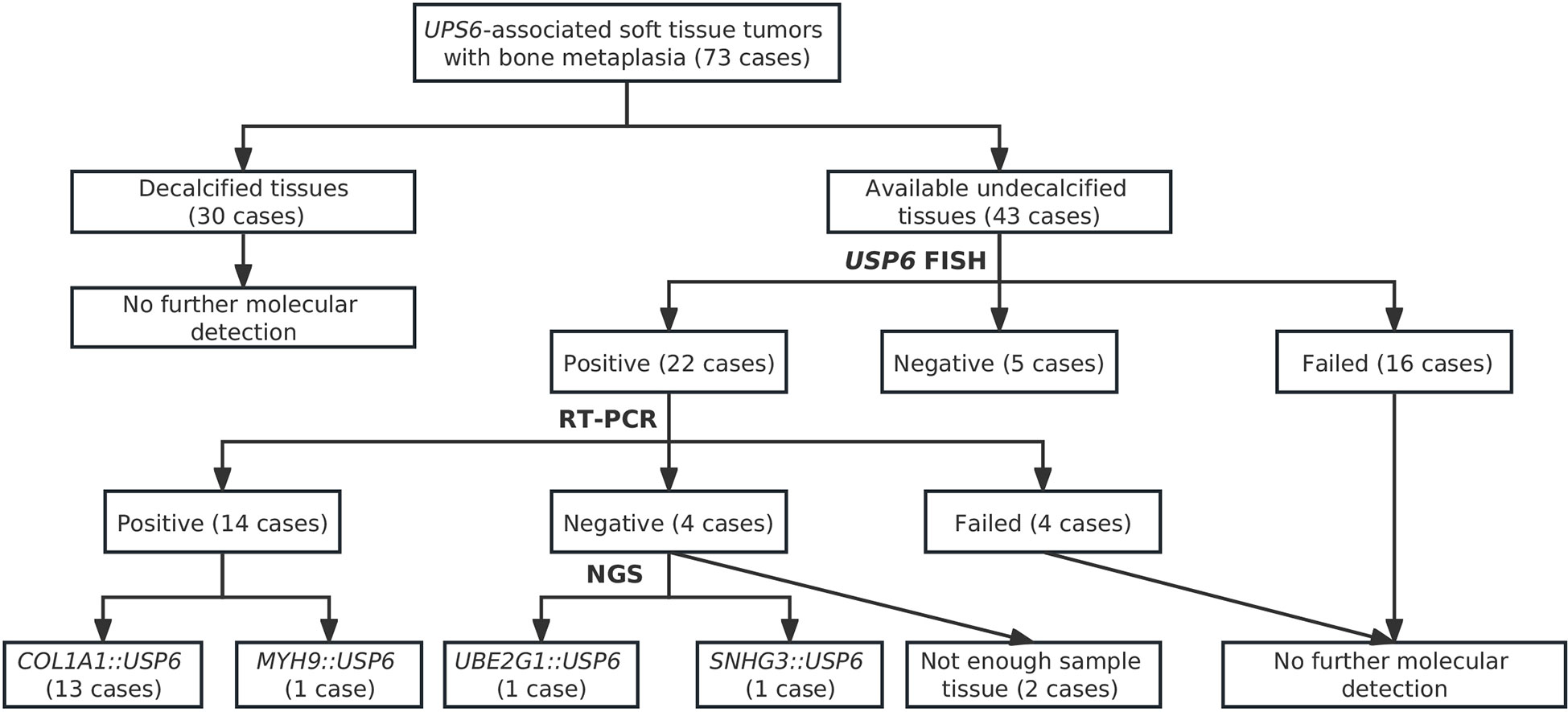
In current studies, FISH has been performed on tissues in 43 cases with available undecalcified tissues; 27 cases, including 17 MOs, 2 FOPDs, 2 ST-ABCs, and 6 FOs, were successfully detected, and 16 of them failed due to poor DNA quality or tissue falling. Among these cases with interpretable results, FISH showed rearrangements of USP6 in 20%~60% of neoplastic cells in 22 cases (81.5%) (Figures 4A–D), and 5 cases were negative for USP6 rearrangements (Table 1). The positive rates for USP6 rearrangement among cases of MO, FOPD, ST-ABC and FO were 70.6% (12/17), 100.0% (2/2), 100% (2/2) and 100.0% (6/6), respectively.
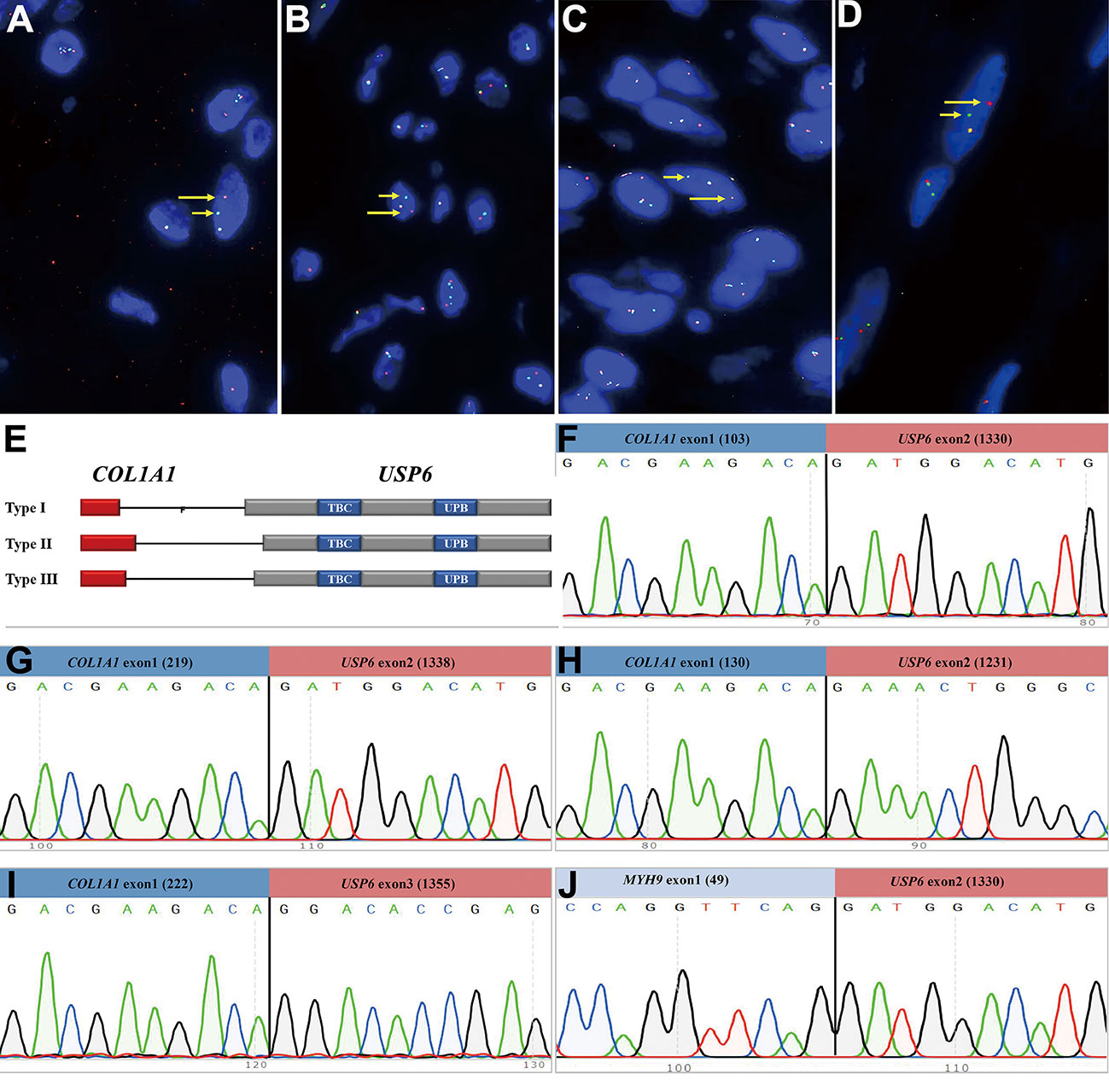
Figure 4 Findings of break-apart USP6 fluorescence in situ hybridization and reverse transcription polymerase chain reaction for identifying common USP6 fusion partners. (A) Break-apart USP6 FISH showed split red (long arrow) and green (short arrow) signals in myositis ossificans (Case 42). (B) USP6 FISH showed split red (long arrow) and green (short arrow) signals in fibro-osseous pseudotumor of digits (Case 49). (C) USP6 FISH showed split red (long arrow) and green (short arrow) signals in soft tissue aneurysmal bone cyst (No. 60). (D) USP6 FISH showed split red (long arrow) and green (short arrow) signals in fasciitis ossificans (No. 63). (E) Diagram showing the overall structure of COL1A1::USP6 fusion genes. (F) One myositis ossificans (Case 22) showed the nucleotide (103, NM_000088) of COL1A1 exon 1 fused with a nucleotide (1330, NM_0004505) of USP6 exon 2 (type I). (G) One fasciitis ossificans (Case 71) showed the last nucleotide (219, NM_000088) of COL1A1 exon 1 fused with a nucleotide (1338, NM_0004505) of USP6 exon 2 (type II). (H) One myositis ossificans (Case 32) showed the last nucleotide (130, NM_000088) of COL1A1 exon 1 fused with a nucleotide (1231, NM_0004505) of USP6 exon 1 (type III). (I) One fasciitis ossificans (Case 63) showed a nucleotide (222, NM_000088) of COL1A1 exon 1 fused with a nucleotide (1355, NM_0004505) of USP6 exon 3. (J) One myositis ossificans (Case 42) showed the last nucleotide (49, NM_000088) of MYH9 exon 1 fused with a nucleotide (1330, NM_0004505) of USP6 exon 2.
Among those 22 patients with positive USP6 rearrangements, RT–PCR was successfully performed in 18 cases (3 MOs and 1 FOPD with poor RNA quality failed testing); 13 cases (72.2%), including 5 MOs, 1 FOPD, 2 ST-ABCs and 5 FOs, were positive for COL1A1::USP6 fusion, and one case with MO was positive for MYH9::USP6 fusion. For COL1A1::USP6 fusion types (Figure 4E), 1 MO, 1 FOPD and 3 FO cases were found with fusion transcript type 1 (nucleotide 103, NM_000088 exon 1; nucleotide 1330, NM_0004505 exon 2) (Figure 4F), 3 MO, 1 ST-ABC and 1 FO cases were found with fusion type II (nucleotide 219, NM_000088 exon 1; nucleotide 1338, NM_0004505 exon 2) (Figure 4G), and 1 MO and 1 ST-ABC demonstrated fusion transcript type III (nucleotide 130 exon 1, NM_000088; nucleotide 1231, NM_0004505 exon 1) (Figure 4H). One FO case was found with a special transcript type with COL1A1 (nucleotide 222, NM_000088 exon 1) and USP6 (nucleotide 1355, NM_0004505 exon 3) fusion (Figure 4I). Additionally, one MO case showed fusion of exon 1 (nucleotide 49, NM_000088) of USP6 and exon 2 (nucleotide 1330, NM_0004505) of MYH9 instead of any transcript type of COL1A1::USP6 fusion (Figure 4J).
Four USP6-rearranged lesions were negative for COL1A1::USP6, and only two of them (1 MO and 1 FO) underwent next-generation-based sequencing technology, while the tissues of the other two cases were not sufficient to perform a further study. A novel fusion of UBE2G1 (exon 1, NM_003342) and USP6 (exon 8-38, NM_001304284) was identified in one MO case (Case 39) (Figure 5A), while a novel fusion of SNHG3 (exon 1, NR_002909) and USP6 (exon 8-38, NM_001304284) was demonstrated in one FO case (Case 66) (Figure 5B). The presence of the fusion at the DNA level was further validated by RT–PCR and Sanger sequencing using corresponding primers (UBE2G1-F: 5’-AGGCTGGTCTTGAACTCCTGA-3’and USP6-R: 5’-CGTGTGTGTTGCTTCTCTGGC-3’; SNHG3-F: 5’-TCTTAGTGGAGACGGGGTTTC-3’ and USP6-R: 5’-AGCTAGAGGATCATGTGCGGA-3’). The process and results of genetic testing are summarized in Figure 6.
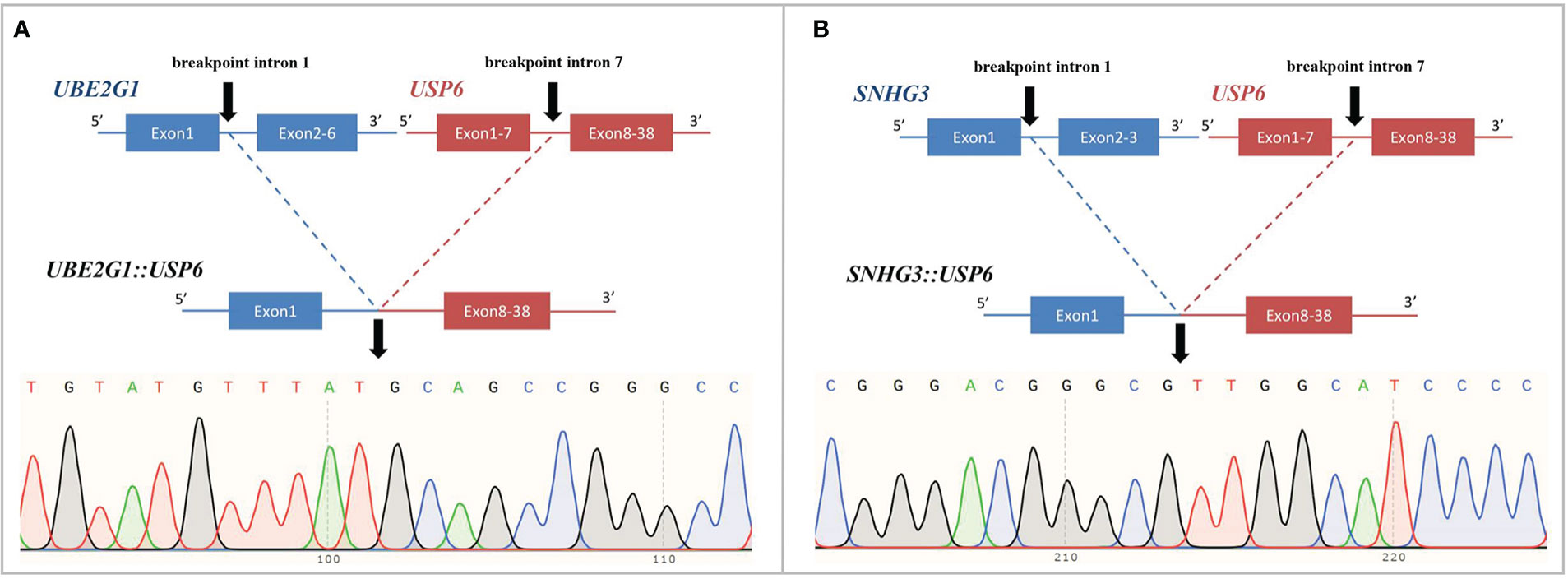
Figure 5 Novel fusion partners identified in USP6-associated soft tissue tumors with bone metaplasia. (A) NGS-based technology showing detection of UBE2G1 (exon1):: USP6 (exon8-38) fusion and breakpoint information between the two genes. Sanger sequencing analysis confirmed the presence of UBE2G1::USP6 fusion. (B) NGS-based technology showing detection of SNHG3 (exon1)::USP6 (exon8-38) fusion and breakpoint information between the two genes. Sanger sequencing analysis confirmed the presence of SNHG3::USP6 fusion.
Treatment and follow-up
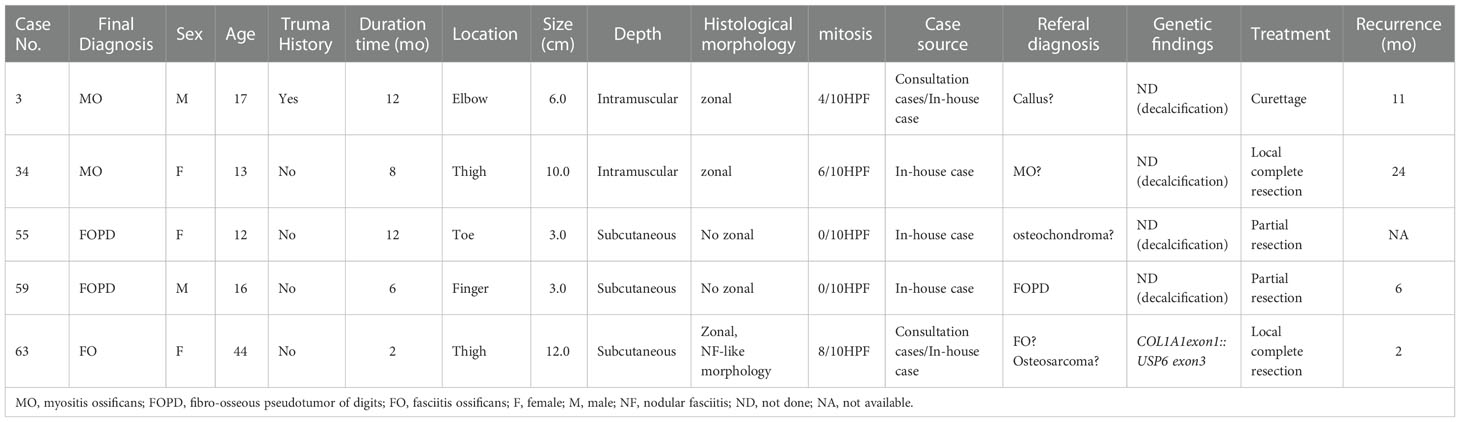
Treatment information was obtained in 63 cases. Sixteen patients underwent biopsy, 41 patients underwent local complete resection, 3 patients underwent wide resection, 2 patients underwent partial mass resection, and 1 patient underwent curettage. Follow-up information was obtained for 36 patients (4 patients underwent biopsy, and 32 patients underwent resection), and the follow-up times ranged from 1 to 127 months (median, 26 months). Four patients survived with tumors after the biopsy without tumor progression. Twenty-nine cases showed no tumor progression after biopsy or surgery, and 5 cases (5/34, 14.7%) experienced postoperative recurrence, including 1 MO with curettage, 2 FOPDs with partial mass resection, 1 MO and 1 FO with complete resection (Table 2).
Details of the clinicopathological and genetic characteristics of different subtypes of USP6-associated soft tissue tumors with bone metaplasia are shown in Supplemental Table 2.
Discussion
USP6-associated soft tissue tumors with bone metaplasia are a subgroup of myofibroblasts/fibroblastic proliferative lesions with metaplastic osteoid components, mainly including MO, FOPD, ST-ABC and FO. This group of neoplasms is not clinically common, but its histology shows pseudosarcomatous changes with the formation of an osteoid component, making it susceptible to misdiagnosis as a malignancy.
In our cohort, there were 73 cases of USP6-associated soft tissue tumors with bone metaplasia, including 44 MOs, 15 FOPDs, 2 ST-ABCs and 12 FOs. As we know, this is one of the largest groups for exploring the clinicopathologic and molecular characteristics of this entity. The diagnosis age in this group was wide, ranging from 2 to 80 years old (median: 31 years). Consistent with previous reports, these tumors can occur in all age groups, but they are more common in young adults between 20~40 years old (5, 16, 17). Fourteen patients (14/48, 29.2%) had a clear history of trauma, but the history of repeated minor trauma might be neglected. In this group, except for FOPD, the lesion sites of the other subtypes were mainly in the lower limbs. The predisposing site of our group was different from that of traditional NF, which more often involves the upper limbs and trunk (18). Notably, FO is currently classified as a special subtype of NF. However, according to the results of our study, we found that the most common site of FO was the lower limbs (9/12, 75%) instead of the upper limbs and trunk. Three FOs were also reported in the latest study, of which 2 cases were identified in the lower extremities, which was consistent with our findings (5). As for imaging examinations in our study, the results were consistent with those reported in previous literature (5, 9, 10, 16, 19).
Histologically, this group showed hyperplastic myofibroblasts/fibroblasts and osteoid tissues with different degrees of maturity. The histological morphology of MO/FOPD and ST-ABC in the present study were essentially consistent with the description in the literature (16, 17, 20). There have been few case reports of FO, and the deposition pattern of osteoid tissues is mainly described as having a haphazard distribution similar to that of FOPD. However, MO-like zonal deposition pattern was observed in 50% of FO cases in our group, which indicated that there was evident histological overlap between FO and the other three subtypes of USP6-associated soft tissue tumors with bone metaplasia.
At present, soft tissue tumors in osteoid tissues have been successively confirmed to be USP6-associated tumors. Studies have suggested that the positive rate of USP6 rearrangement/fusion in MO/FOPD, ST-ABC and FO ranges from 16.7% to 100% (5, 8–10, 17, 19, 21). However, it should be emphasized that the overall sample size of existing studies is relatively small, with only 4 studies with more than 10 cases (11 cases (8), 12 cases (10), 12 cases (21), 14 cases (5)). Our study had the largest number of cases in a single center, and the positive rate of USP6 rearrangement detected by FISH in this group of cases was 81.5% (22/27). In recent years, an increasing number of studies have focused on the fusion partners of these tumors and the relationship between them. Our study suggested that COL1A1 (13/18, 72.2%) was the most frequent fusion partner in MO/FOPD, ST-ABC and FO, which was consistent with previous studies (67.7%~100%) and differed from classical primary ABC and NF (5, 10). Notably, despite the presence of significant ossification, FO is currently classified as a variant of NF. Although it has also been reported that COL1A1 was found to be the fusion partner in a few NFs (22), COL1A1 was the unique USP6 fusion partner of FO in this study, which was consistent with the findings of Wang and colleagues (5). Meanwhile, we found that the most common site of FO was the lower limbs instead of the upper limbs of NF, suggesting that FO seemed to be more closely related to MO/FOPD and ST-ABC. Notably, in USP6-associated neoplasms, although an increasing number of partner genes have been identified, there is a preference for these partner genes in different tumor subtypes (5–7, 10, 14, 15, 17, 19, 23–35) (Figure 7A). Intriguingly, according to our study and previous literature, almost all of the USP6-associated neoplasms with bone metaplasia adopt COL1A1 as the fusion partner, including MO, FOPD, ST-ABC and FO (5, 10, 17, 19, 25). As reported, COL1A1 encodes the pro-alpha1 chain of type I collagen, which is associated with osteogenesis imperfecta and osteoporosis (36). It should be noted that the fusion of COL1A1 and USP6 still retained the whole USP6 coding sequence of the open reading frame, namely, the fusion of the partner gene exon1 and the USP6 gene exon1 or/and exon2. Although this fusion is expected to lead to the high expression of USP6 or be the main mechanism of USP6 gene-mediated tumor pathogenesis, the high percentage of COL1A1 as a fusion partner in these tumor subtypes still likely has a potential association with the osteoid formation on the histology, but further research is needed. In addition, individual USP6 fusion partners that overlap between these tumor subtypes have been identified (3, 7, 11, 15, 22, 35–39) (Figure 7B). Although such cases are limited, the possibility of some crossover or even transition between USP6-associated neoplasms cannot be ruled out.
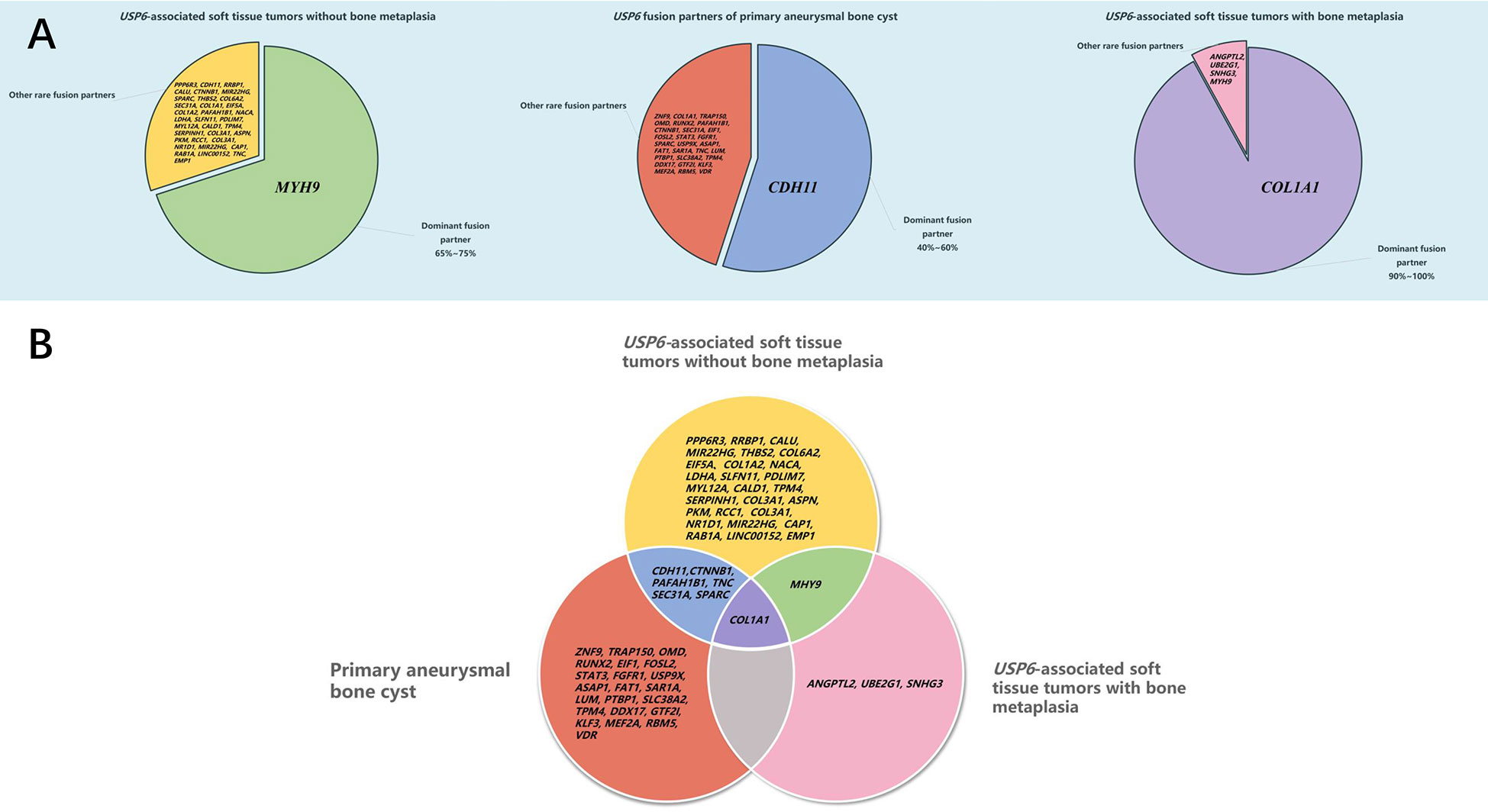
Figure 7 Distribution of USP6 fusion partners in USP6-associated tumors. (A) Distribution of the USP6 fusion gene in different USP6-associated tumors. (B) Overlap of the USP6 fusion gene in different USP6-associated tumors.
Notably, although COL1A1 was the most frequent fusion partner in this entity, we still identified MYH9::USP6 fusion in one case of MO (Case 42). At present, after thoroughly reviewing the English literature, MHY9 has not been reported as a USP6 fusion partner in MO/FOPD, ST-ABC and FO. As reported, MYH9::USP6 fusion is the most frequent fusion type in NF (including variants of NF) and FTS in USP6-associated tumors (4, 5, 40). After carefully reviewing the slides of this lesion, we found that it was an intramuscular mass and demonstrated a prominent zonation pattern in focal areas (Figure 2D) and obvious NF-like morphology in some foci (Figure 2C), which indicated there are some overlapping histological features as well as molecular findings among different subtypes of USP6-associated neoplasms, for example, as the major fusion type in the primary ABC, CDH11::USP6 also can be detected in the NF, but how they work in building the bridge between them are still not clear and needs to be further studied in a larger cohort.
In this study, we also identified a novel ubiquitin-conjugating enzyme E2 G1 (UBE2G1)::USP6 gene fusion in one case of MO (Case 39) and a novel small nuclear protein RNA host gene 3 (SNHG3)::USP6 fusion in one case of FO (Case 66). To our knowledge, fusions of these two genes with USP6 have not been previously reported. UBE2G1 is located on chromosome 17p13.2; it encodes a member of the E2 ubiquitin-conjugating enzyme family and catalyzes the covalent attachment of ubiquitin to other proteins (41). SNHG3 is located on chromosome 1p35.3, which belongs to a group of long noncoding RNAs associated with multiple cancers and is dysregulated in multiple cancers (42). Recent studies have shown that SNHG3 expression is higher in many tumors, such as osteosarcoma, breast cancer and hepatocellular carcinoma (42). Similar to previous findings (4, 43), the entire coding region of USP6 was preserved in these two rearrangements, which likely leads to activating USP6 transcription and subsequent neoplastic processes.
Notably, except for MYH9, UBE2G1 and SNHG3, which were discovered in our study, the unusual USP6 partner ANGPTL2 has also been identified in one MO-like ST-ABC (17). However, despite the presence of uncommon fusion types, no peculiar clinicopathologic findings were identified in these 4 cases. Whether the novel fusions may be related to the clinicopathological features of these tumors remains to be further investigated. Additionally, according to many reported studies, in most cases of USP6-associated tumors, the fusion site of USP6 was exon 1 or/and exon 2 (4, 5, 10). In the current study, we found rare fusion sites of the USP6 gene, which were exon 3 and exon 8 of USP6 (NM_001304284). In addition, a previous study by our team also reported an equally rare case of atypical NF in children at the fusion site of exon 9 of USP6 (NM_001304284) (15). However, whether these rare fusion sites are related to clinicopathologic characteristics still needs further study.
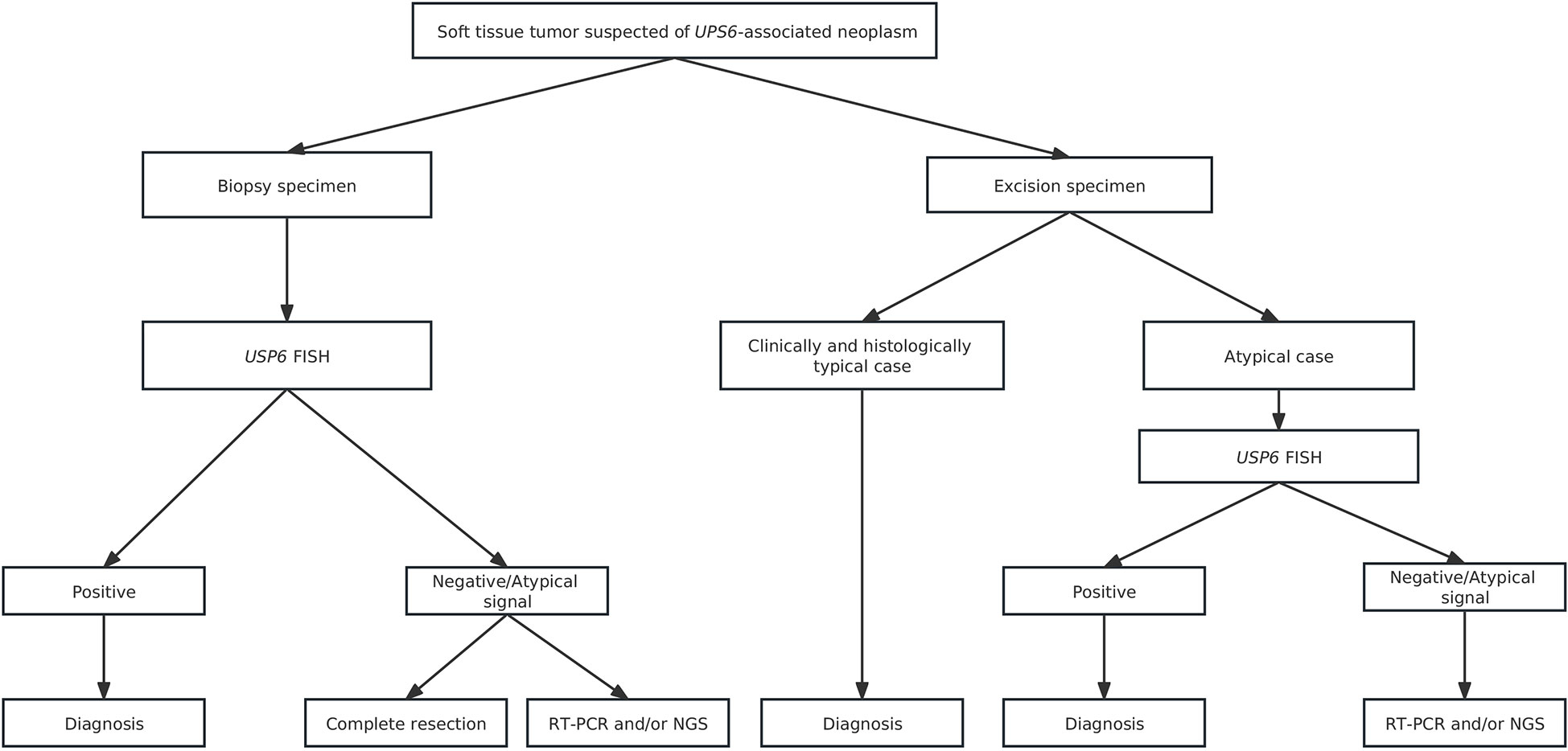
For differential diagnosis, USP6-associated soft tissue tumors with bone metaplasia are extremely easily confounded with extraskeletal osteosarcoma, especially in MO cases. Ten of 33 consultation cases were initially considered extraskeletal osteosarcoma in the local hospital. Clinically, extraskeletal osteosarcoma more commonly occurs in middle-aged and elderly populations and often lacks an injury history. Histologically, osteoid components of extraskeletal osteosarcoma are often arranged in a reverse zonation pattern. Obvious cell atypia with pathologic mitosis can be present among neoplastic cells (44). In addition, USP6-associated soft tissue tumors with bone metaplasia need to be differentiated from other osteogenic sarcomas, such as malignant peripheral nerve sheath tumors with heterogeneous bone differentiation and dedifferentiated liposarcomas with heterogeneous bone differentiation. Malignant peripheral nerve sheath tumors is usually a rare, high-grade sarcoma with high morphological heterogeneity. The tumor cells are long and fusiform with wavy, curved, comma-shaped or asymmetrically ovoid nuclei, the chromatin is usually uniform or dense, and pathological mitosis is easy to observe. Complete loss of H3K27me3 along with SUZ12 and EED gene deletions are frequently seen in this entity (45), which are invaluable in the diagnosis of challenging cases. For dedifferentiated liposarcomas with heterogeneous bone differentiation, except for dedifferentiated bone elements, well-differentiated liposarcomatous components are characteristically present. More importantly, consistent MDM2 and/or CDK4 amplification is present in dedifferentiated liposarcomas (46), which is absent in USP6-associated soft tissue tumors with bone metaplasia. In addition to those malignant tumors, USP6-associated soft tissue tumors with bone metaplasia should also be differentiated from some benign lesions, including proliferative fasciitis/proliferative myositis, bizarre parosteal ostochondromatous proliferation (Nora’s lesion) and subungual exostosis. Proliferative fasciitis/proliferative myositis is a type of myofibroblast/fibroblast proliferative disease that occurs in the subcutaneous fascia and muscle. It is characterized by a scattered distribution of ganglion-like cells, interstitial mucinous degeneration and collagenization in the background (47). In addition, no USP6 rearrangement was identified in proliferative fasciitis/proliferative myositis, indicating that proliferative fasciitis/proliferative myositis is a real reparative lesion instead of a neoplastic change. Nora’s lesion often presents bizarre, enlarged nuclei and stroma with a characteristically basophilic tinctorial quality. Additionally, genetic changes with t(1;17) (q32; q21) and inv (7) and inv (6) were recurrently identified in Nora’s lesion and were not commonly seen in FOPD, MO and FO (48). FOPD should also be distinguished from subungual exostosis, as it is more prone to involve digits. Unlike FOPD, subungual exostosis commonly presents as a lesion with an irregular bone-cartilage interface with enlarged, atypical and binucleated chondrocytes histologically and harbors a t(X;6) (q24-q26; q15-q25) change genetically (49). In clinical practice, molecular testing is usually unnecessary for clinically and histologically typical cases. However, in challenging cases, USP6 FISH, RT-PCR and/or NGS may be used to confirm the diagnosis. We recommend the diagnostic algorithm shown in the figure to reduce misdiagnosis as much as possible (Figure 8).
USP6-associated soft tissue tumors with bone metaplasia are benign lesions. Currently, local resection is the most commonly used and most effective treatment. Postoperative recurrence is rare (16). Patients with incomplete resection are likely to experience recurrence. In our study, among the patients with follow-up information, 4 patients who received biopsy only survived with tumors without tumor progression; most of the patients showed no evidence for recurrence, while 5 patients (5/32, 15.6%), including 2 MOs, 2 FOPDs and 1 FO, showed recurrence after surgery. Those two FOPD patients underwent partial resection with positive margins to retain the normal function of the finger or toe, and one MO was not completely curetted, which may lead to relapses of these tumors. Another MO and FO with recurrence underwent complete local resection and showed a large tumor size of over 10.0 cm (10.0 cm and 12.0 cm). In this study, the tumor volumes of 2 cases with recurrence after complete resection were large (over 10.0 cm), among which one case also harbored a rare fusion site (Case 63) (the other case did not undergo genetic testing due to sample decalcification). Notably, the FO recurrent case (Case 63) had special histological morphology. Some areas of the tumor showed invasive growth, with the active proliferation of myofibroblasts/fibroblasts, messy distribution of osteoid tissue, and reverse distribution of focal areas similar to osteosarcoma (Figures 3B–D). Due to the large size of the tumor, osteosarcoma could not be excluded from the original diagnosis after the lesion recurrence, and the diagnosis of FO was finally confirmed by USP6 FISH test. However, whether these were related to clinical conditions (especially biological behavior) still needs to be further studied by increasing the number of cases. Given the nature of this group of tumors, if the lesion can be diagnosed in the biopsy, it may provide more treatment options for patients. However, due to the small size of biopsies, it is difficult to diagnose these tumors only by histology and immunohistochemistry. FISH detection of USP6 rearrangement will be valuable for clarifying the diagnosis, and the method of surgery can be determined according to the tumor size, location, and follow-up.
Conclusions
In summary, USP6-associated soft tissue tumors with bone metaplasia include MO, FOPD, ST-ABC and FO. Here, we explored the peculiar clinicopathologic and molecular features of this entity in one of the largest cohorts. Tumors can occur in all age groups but most often affect young adults. Different subtypes of this entity not only share overlapping clinicopathological features but also exhibit similar genetic changes, namely, consistent USP6 rearrangement and frequent COL1A1::USP6 fusion. Notably, for FO, the lower limbs are commonly involved sites, and COL1A1 is the most frequent fusion partner, suggesting that FO may be closer to MO/FOPD and ST-ABC than conventional NF. Another point we should address is that MYH9::USP6 was first identified in MO, and the novel USP6 fusion partners UBE2G1 and SNHG3 were uncovered in one case with MO and one case with FO, respectively, expanding our knowledge of USP6-associated soft tissue tumors with bone metaplasia. The prognosis of this entity is good, and local complete resection is the most effective treatment. Recurrence may be associated with incomplete resection and/or large tumor size (over 10.0 cm), and whether rare fusion sites or novel/uncommon fusion partners are correlated with clinical parameters still needs to be further studied in larger cohorts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (No. 793, 2021). Approval for waiver of informed consent was obtained for this study.
Author contributions
YZ: collected the clinicopathological data, performed the histopathological examinations, and molecular detection, and prepared the manuscript. YQ: analyzed the molecular data and prepared the manuscript. XZ: performed the histopathological examinations. XH and CC: helped with data review. MC: helped with molecular experiments. HZ: the corresponding author, was responsible for study design, histopathological and molecular examinations, and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972520) and the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2018HXFH011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1065071/full#supplementary-material
References
1. Oliveira AM, Chou MM. USP6-induced neoplasms: the biologic spectrum of aneurysmal bone cyst and nodular fasciitis. Hum Pathol (2014) 45(1):1–11. doi: 10.1016/j.humpath.2013.03.005
2. Oliveira AM, Perez-Atayde AR, Inwards CY, Medeiros F, Derr V, Hsi BL, et al. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am J Pathol (2004) 165(5):1773–80. doi: 10.1016/s0002-9440(10)63432-3
3. Guseva NV, Jaber O, Tanas MR, Stence AA, Sompallae R, Schade J, et al. Anchored multiplex PCR for targeted next-generation sequencing reveals recurrent and novel USP6 fusions and upregulation of USP6 expression in aneurysmal bone cyst. Genes Chromosomes Cancer (2017) 56(4):266–77. doi: 10.1002/gcc.22432
4. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest (2011) 91(10):1427–33. doi: 10.1038/labinvest.2011.118
5. Wang JC, Li WS, Kao YC, Lee JC, Lee PH, Huang SC, et al. Clinicopathological and molecular characterisation of USP6-rearranged soft tissue neoplasms: the evidence of genetic relatedness indicates an expanding family with variable bone-forming capacity. Histopathology (2021) 78(5):676–89. doi: 10.1111/his.14268
6. Jacquot C, Szymanska J, Nemana LJ, Steinbach LS, Horvai AE. Soft-tissue aneurysmal bone cyst with translocation t(17;17)(p13;q21) corresponding to COL1A1 and USP6 loci. Skeletal Radiol (2015) 44(11):1695–9. doi: 10.1007/s00256-015-2205-6
7. Carter JM, Wang X, Dong J, Westendorf J, Chou MM, Oliveira AM. USP6 genetic rearrangements in cellular fibroma of tendon sheath. Mod Pathol (2016) 29(8):865–9. doi: 10.1038/modpathol.2016.83
8. Bekers EM, Eijkelenboom A, Grünberg K, Roverts RC, de Rooy JWJ, van der Geest ICM, et al. Myositis ossificans - another condition with USP6 rearrangement, providing evidence of a relationship with nodular fasciitis and aneurysmal bone cyst. Ann Diagn Pathol (2018) 34:56–9. doi: 10.1016/j.anndiagpath.2018.01.006
9. Flucke U, Shepard SJ, Bekers EM, Tirabosco R, van Diest PJ, Creytens D, et al. Fibro-osseous pseudotumor of digits - expanding the spectrum of clonal transient neoplasms harboring USP6 rearrangement. Ann Diagn Pathol (2018) 35:53–5. doi: 10.1016/j.anndiagpath.2018.05.003
10. Švajdler M, Michal M, Martínek P, Ptáková N, Kinkor Z, Szépe P, et al. Fibro-osseous pseudotumor of digits and myositis ossificans show consistent COL1A1-USP6 rearrangement: a clinicopathological and genetic study of 27 cases. Hum Pathol (2019) 88:39–47. doi: 10.1016/j.humpath.2019.02.009
11. Lu Y, He X, Qiu Y, Chen H, Zhuang H, Yao J, et al. Novel CTNNB1-USP6 fusion in intravascular fasciitis of the large vein identified by next-generation sequencing. Virchows Arch (2020) 477(3):455–59. doi: 10.1007/s00428-020-02792-x
12. Salib C, Edelman M, Lilly J, Fantasia JE, Yancoskie AE. USP6 gene rearrangement by FISH analysis in cranial fasciitis: A report of three cases. Head Neck Pathol (2020) 14(1):257–61. doi: 10.1007/s12105-019-01018-0
13. Malik F, Wang L, Yu Z, Edelman MC, Miles L, Clay MR, et al. Benign infiltrative myofibroblastic neoplasms of childhood with USP6 gene rearrangement. Histopathology (2020) 77(5):760–68. doi: 10.1111/his.14182
14. Pižem J, Matjašič A, Zupan A, Luzar B, Šekoranja D, Dimnik K. Fibroma of tendon sheath is defined by a USP6 gene fusion-morphologic and molecular reappraisal of the entity. Mod Pathol (2021) 34(10):1876–88. doi: 10.1038/s41379-021-00836-4
15. Qiu Y, Peng R, Chen H, Zhuang H, He X, Zhang H. Atypical nodular fasciitis with a novel PAFAH1B1-USP6 fusion in a 22-month-old boy. Virchows Arch (2021) 479(3):623–29. doi: 10.1007/s00428-020-02961-y
16. Oliveira AM, Rosenberg AE. Myositis ossificans and fibro-osseous pseudotumour of digits. In: World health organization classification of tumors: soft tissue and bone tumours, 5th ed. France: Lyon (2020). p. 53–4.
17. Zhang L, Hwang S, Benayed R, Zhu GG, Mullaney KA, Rios KM, et al. Myositis ossificans-like soft tissue aneurysmal bone cyst: a clinical, radiological, and pathological study of seven cases with COL1A1-USP6 fusion and a novel ANGPTL2-USP6 fusion. Mod Pathol (2020) 33(8):1492–504. doi: 10.1038/s41379-020-0513-4
18. Oliveira AM, Wang J, Wang WL. Nodular fasciitis. In: World health organization classification of tumors: soft tissue and bone tumours, 5th ed. Lyon: IARC press (2020). p. 49–50.
19. Song W, Suurmeijer AJH, Bollen SM, Cleton-Jansen AM, Bovée J, Kroon HM. Soft tissue aneurysmal bone cyst: six new cases with imaging details, molecular pathology, and review of the literature. Skeletal Radiol (2019) 48(7):1059–67. doi: 10.1007/s00256-018-3135-x
20. Nielsen GP, Fletcher CD, Smith MA, Rybak L, Rosenberg AE. Soft tissue aneurysmal bone cyst: a clinicopathologic study of five cases. Am J Surg Pathol (2002) 26(1):64–9. doi: 10.1097/00000478-200201000-00007
21. Sukov WR, Franco MF, Erickson-Johnson M, Chou MM, Unni KK, Wenger DE, et al. Frequency of USP6 rearrangements in myositis ossificans, brown tumor, and cherubism: molecular cytogenetic evidence that a subset of "myositis ossificans-like lesions" are the early phases in the formation of soft-tissue aneurysmal bone cyst. Skeletal Radiol (2008) 37(4):321–7. doi: 10.1007/s00256-007-0442-z
22. Stražar K, Šekoranja D, Matjašič A, Zupan A, Snoj Ž, Martinčič D, et al. Intraarticular nodular fasciitis-detection of USP6 gene fusions in three cases by targeted RNA sequencing. Virchows Arch (2021) 478(6):1117–24. doi: 10.1007/s00428-020-02991-6
23. Dermawan JK, Cheng YW, Tu ZJ, Meyer A, Habeeb O, Zou Y, et al. Diagnostic utility of a custom 34-gene anchored multiplex PCR-based next-generation sequencing fusion panel for the diagnosis of bone and soft tissue neoplasms with identification of novel USP6 fusion partners in aneurysmal bone cysts. Arch Pathol Lab Med (2021) 145(7):851–63. doi: 10.5858/arpa.2020-0336-OA
24. Eisenberg JM, Buckwalter VJ, Snow AN, Davick J. Cellular fibroma of tendon sheath with novel TNC-USP6 gene fusion clinically mimicking arthritis in a 7-Year-Old boy. Pediatr Dev Pathol (2022) 25(2):192–96. doi: 10.1177/10935266211043869
25. Hiemcke-Jiwa LS, van Gorp JM, Fisher C, Creytens D, van Diest PJ, Flucke U. USP6-associated neoplasms: A rapidly expanding family of lesions. Int J Surg Pathol (2020) 28(8):816–25. doi: 10.1177/1066896920938878
26. Legrand M, Jourdan ML, Tallet A, Collin C, Audard V, Larousserie F, et al. Novel partners of USP6 gene in a spectrum of bone and soft tissue lesions. Virchows Arch (2021) 479(1):147–56. doi: 10.1007/s00428-021-03047-z
27. Lenz J, Michal M, Svajdler M, Ptakova N, Lenz D, Konecna P, et al. Novel EIF5A-USP6 gene fusion in nodular fasciitis associated with unusual pathologic features: A report of a case and review of the literature. Am J Dermatopathol (2020) 42(7):539–43. doi: 10.1097/dad.0000000000001602
28. Mantilla JG, Gross JM, Liu YJ, Hoch BL, Ricciotti RW. Characterization of novel USP6 gene rearrangements in a subset of so-called cellular fibroma of tendon sheath. Mod Pathol (2021) 34(1):13–9. doi: 10.1038/s41379-020-0621-1
29. Panagopoulos I, Gorunova L, Andersen K, Lobmaier I, Lund-Iversen M, Micci F, et al. Fusion of the lumican (LUM) gene with the ubiquitin specific peptidase 6 (USP6) gene in an aneurysmal bone cyst carrying a t(12;17)(q21;p13) chromosome translocation. Cancer Genomics Proteomics (2020) 17(5):555–61. doi: 10.21873/cgp.20211
30. Papke DJ Jr., Bredella MA, Lozano-Calderon S, Oliveira AM, Lennerz J, Nielsen GP. Aneurysmal bone cyst with an unusual clinical presentation and a novel VDR-USP6 fusion. Genes Chromosomes Cancer (2021) 60(12):833–36. doi: 10.1002/gcc.22989
31. Papke DJ Jr., Oliveira AM, Chou MM, Fletcher CDM. Morphologically malignant nodular fasciitis with CALD1-USP6 fusion. Virchows Arch (2021) 479(5):1007–12. doi: 10.1007/s00428-021-03149-8
32. Rodriguez Pena MDC, Morlote D, Prieto Granada CN. Cutaneous nodular fasciitis with rare TPM4-USP6 fusion. J Cutan Pathol (2022) 49(2):196–99. doi: 10.1111/cup.14151
33. Teramura Y, Yamazaki Y, Tanaka M, Sugiura Y, Takazawa Y, Takeuchi K, et al. Case of mesenchymal tumor with the PPP6R3-USP6 fusion, possible nodular fasciitis with malignant transformation. Pathol Int (2019) 69(12):706–09. doi: 10.1111/pin.12851
34. Tomassen T, van de Ven C, Anninga J, Koelsche C, Hiemcke-Jiwa LS, Ter Horst S, et al. Nodular fasciitis with malignant morphology and a COL6A2-USP6 fusion: A case report (of a 10-year-old boy). Int J Surg Pathol (2021) 29(6):642–47. doi: 10.1177/1066896921996045
35. Warren M, Xu D, Li X. Gene fusions PAFAH1B1-USP6 and RUNX2-USP6 in aneurysmal bone cysts identified by next generation sequencing. Cancer Genet (2017) 212-213:13–8. doi: 10.1016/j.cancergen.2017.03.007
36. Oliveira AM, Perez-Atayde AR, Dal Cin P, Gebhardt MC, Chen CJ, Neff JR, et al. Aneurysmal bone cyst variant translocations upregulate USP6 transcription by promoter swapping with the ZNF9, COL1A1, TRAP150, and OMD genes. Oncogene (2005) 24(21):3419–26. doi: 10.1038/sj.onc.1208506
37. Lam SW, Cleton-Jansen AM, Cleven AHG, Ruano D, van Wezel T, Szuhai K, et al. Molecular analysis of gene fusions in bone and soft tissue tumors by anchored multiplex PCR-based targeted next-generation sequencing. J Mol Diagn (2018) 20(5):653–63. doi: 10.1016/j.jmoldx.2018.05.007
38. Parkinson B, Patton A, Rogers A, Farhadi HF, Oghumu S, Iwenofu OH. Intraneural nodular fasciitis of the femoral nerve with a unique CTNNB1::USP6 gene fusion: Apropos of a case and review of literature. Int J Surg Pathol (2022) 30(6):673–81. doi: 10.1177/10668969221080064
39. Cloutier JM, Kunder CA, Charville GW, Hosfield EM, García JJ, Brown RA, et al. Nodular fasciitis of the breast: clinicopathologic and molecular characterization with identification of novel USP6 fusion partners. Mod Pathol (2021) 34(10):1865–75. doi: 10.1038/s41379-021-00844-4
40. Patel NR, Chrisinger JSA, Demicco EG, Sarabia SF, Reuther J, Kumar E, et al. USP6 activation in nodular fasciitis by promoter-swapping gene fusions. Mod Pathol (2017) 30(11):1577–88. doi: 10.1038/modpathol.2017.78
41. Chen G, Liu T, Yu B, Wang B, Peng Q. CircRNA-UBE2G1 regulates LPS-induced osteoarthritis through miR-373/HIF-1a axis. Cell Cycle (2020) 19(13):1696–705. doi: 10.1080/15384101.2020.1772545
42. Xu B, Mei J, Ji W, Bian Z, Jiao J, Sun J, et al. LncRNA SNHG3, a potential oncogene in human cancers. Cancer Cell Int (2020) 20(1):536. doi: 10.1186/s12935-020-01608-x
43. Paulson VA, Stojanov IA, Wasman JK, Restrepo T, Cano S, Plunkitt J, et al. Recurrent and novel USP6 fusions in cranial fasciitis identified by targeted RNA sequencing. Mod Pathol (2020) 33(5):775–80. doi: 10.1038/s41379-019-0422-6
44. Yamashita K, Hameed M. Extraskeletal osteosarcoma. In: WHO classification of tumours of soft tissue and bone, 5th ed. Lyon: IARC Press (2020). p. 224–25.
45. Lee W, Teckie S, Wiesner T, Ran L, Prieto Granada CN, Lin M, et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet (2014) 46(11):1227–32. doi: 10.1038/ng.3095
46. Dei Tos AP, Marino-Enriquez A, Pedeutour F. Dedifferentiated liposarcoma. In: WHO classification of tumours of soft tissue and bone, 5th ed. Lyon: IARC Press (2020). p. 39–41.
47. Wang WL, Lazar AJ. Proliferative fasciitis/Proliferative myositis. In: WHO classification of tumours of soft tissue and bone, 5th ed. Lyon: IARC Press (2020). p. 51–2.
48. Nilsson M, Domanski HA, Mertens F, Mandahl N. Molecular cytogenetic characterization of recurrent translocation breakpoints in bizarre parosteal osteochondromatous proliferation (Nora's lesion). Hum Pathol (2004) 35(9):1063–9. doi: 10.1016/j.humpath.2004.02.008
49. Storlazzi CT, Wozniak A, Panagopoulos I, Sciot R, Mandahl N, Mertens F, et al. Rearrangement of the COL12A1 and COL4A5 genes in subungual exostosis: molecular cytogenetic delineation of the tumor-specific translocation t(X;6)(q13-14;q22). Int J Cancer (2006) 118(8):1972–6. doi: 10.1002/ijc.21586
Keywords: USP6 rearrangement, myositis ossificans, fibro-osseous pseudotumor of digits, soft tissue aneurysmal bone cyst, fasciitis ossificans, differential diagnosis, USP6-associated neoplasms
Citation: Zhang Y, Qiu Y, Zhang X, He X, Chen C, Chen M and Zhang H (2023) USP6-associated soft tissue tumors with bone metaplasia: Clinicopathologic and genetic analysis and the identification of novel USP6 fusion partners. Front. Oncol. 12:1065071. doi: 10.3389/fonc.2022.1065071
Received: 09 October 2022; Accepted: 30 December 2022;
Published: 16 January 2023.
Edited by:
Ming Zhao, Zhejiang Provincial People’s Hospital, ChinaReviewed by:
Sean R. Williamson, Cleveland Clinic, United StatesIsidro Machado, Instituto Valenciano de Oncologia, Spain
Copyright © 2023 Zhang, Qiu, Zhang, He, Chen, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongying Zhang, aHlfemhhbmdAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yahan Zhang†
Yahan Zhang† Xin He
Xin He Min Chen
Min Chen Hongying Zhang
Hongying Zhang