
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 04 January 2023
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1064851
Uterine cervical Müllerian adenosarcoma (MA), a rare malignant tumor of the female reproductive organs, is composed of a benign epithelium and a low-grade malignant stromal component. Because few studies have investigated the clinical management of MA, misdiagnosis often occur. Therefore, we proposed an optimal course of clinical management for patients with MA. MA is possibly a malignant transformation of the cystadenofibroma. In this study, we present a case of a 46-year-old woman who presented with symptoms of MA of the uterine cervix, such as metrorrhagia and a cyst in the cervical canals, after transvaginal excision of the left ovarian mucinous cystadenofibroma.
Müllerian adenosarcoma (MA) is a malignant tumor composed of benign epithelial glands and a malignant sarcomatous stromal component. MA was first identified by Clement and Scully in 1974 (1). MA is a rare tumor accounting for approximately 2% of all instances of adenosarcomas of the female reproductive organs. In addition, MA can be found in different locations of the uterine corpus (2). Contrary to most cervical malignant tumors, no association with the human papillomavirus (HPV) has been reported (3). MA occurs in postmenopausal and young women; however, the average age of patients with MA is lower than that of patients with uterine corpus tumors. The symptoms of cervical MA include vaginal bleeding or a cervical mass/polyp, which can be detected during routine gynecological examination (4). However, the origin of cervical MA is unclear. Coverage of these cases is scant in the case report literature, and few studies have investigated the occurrence, diagnosis, and clinical management of MA; thus, misdiagnosis often occur. In this study, we report a case of a 46-year-old premenopausal woman with cervical MA after transvaginal excision of the left ovarian mucinous cystadenofibroma. A previous study proposed that MA is a malignant transformation of the cystadenofibroma (5). We corroborated the finding of the previous study and speculated that the transvaginal excision of the ovarian tumor may lead to cervical MA. According to our review of the literature, this is the first reported case of cervical adenosarcoma arising from a benign ovarian cystadenofibroma.
We treated a 46-year-old married woman who presented with a cervical cyst that had been growing for 2 years with irregular vaginal bleeding for the past 6 months in our hospital. She underwent a transvaginal excision of the left ovarian cyst in 2005. The postoperative pathology results of the left ovarian tumor showed a mucinous cystadenofibroma. Subsequently, the patient underwent a laparoscopic excision of the left adnexal pseudocyst about 10 cm and pelvic adhesion lysis in 2007. The postoperative pathology results indicated left adnexa in the fibrous connective tissue.
The examination results showed that the diameter of the mass in the cervix was 4 cm and an enlarged uterus, the size of 8-week’s gestation (8.9 × 4.4 × 3.4 cm). Ultrasonography revealed a cystic-solid mass in the cervix measuring 9.1 × 8.1 × 5.8 cm (Figure 1) and a solid-cystic mass measuring 3.6 × 2.9 × 2.7 cm in the posterior uterine wall. The test for HPV 66 was positive (6), and a biopsy of the colposcopy revealed cervicitis. The computed tomography (CT) scan showed that tumor did not extend posteriorly to the bladder or rectum and the pelvic para-aortic lymph nodes were not enlarged. Abdominal/pelvic ultrasound and chest radiography indicated no abnormalities. Total laparoscopic hysterectomy and right salpingo-oophorectomy were performed on the basis of the patient’s age, ultrasound results, HPV status, and past surgery history. We chose a watch-and-wait strategy to observe the patient. The patient is currently disease-free after a 48-month follow-up period.
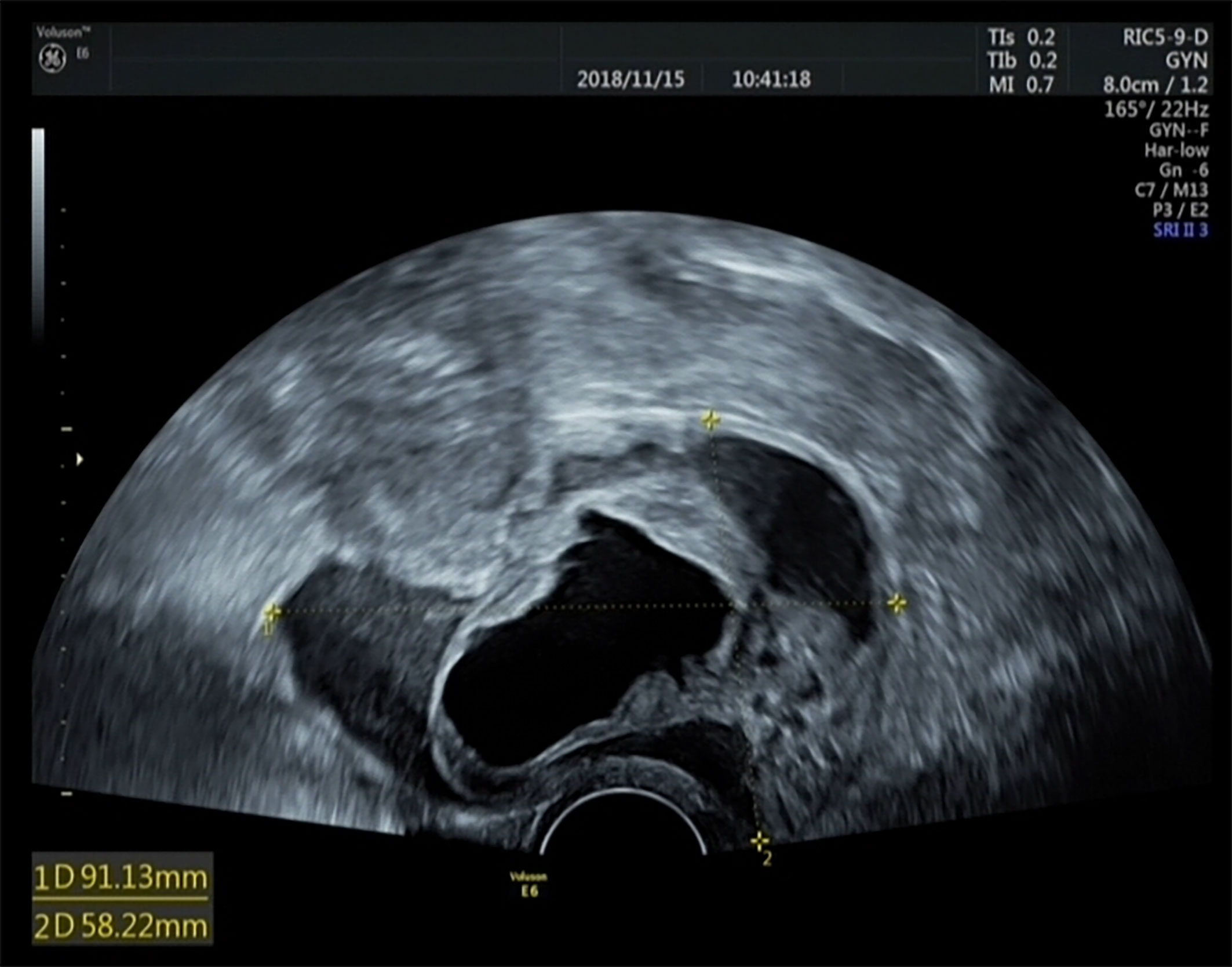
Figure 1 Ultrasound results of the tumor. Müllerian adenosarcoma of the uterine cervix is a cystic-solid mass measuring 9.1 × 8.1 × 5.8 cm.
The specimen of total laparoscopic hysterectomy with right salpingo-oophorectomy was received for histopathological examination. On gross pathological examination, a multilocular cystic mass measuring 5.0 × 4.8 × 4.5 cm was observed in the cervix. The cut surface of the tumor was a mucosal surface. A 1.5-cm myoma was observed in the myometrium of the posterior uterine wall. The endometrial cavity was free of tumors. The right ovary and the right fallopian tube were normal. On microscopic examination, the mass was found to be benign or mildly atypical Müllerian glands and low-grade malignant stroma (Figure 2). Benign and atypical endocervical glands were uniformly distributed within the tumor, and most of them had a cystic appearance. The glands were covered with a cellular stroma that formed periglandular cuffs and intraluminal polyploid projections. The stromal component focally resembled a low-grade stromal sarcoma covering 20% of the tumor area. In the low-grade sarcomatous areas, the mitotic rate was low (3 mitotic figures/10 high power fields (HPFs) (Figure 3) (7). An immunohistochemical staining analysis of various markers revealed that P16 was partially positive; Ki67 (15%), ER, PR, MUC1, CK7, CA125, and Pax8 were positive; and P53, CDX2, MUC5, CK20, and CEA were negative. Myoma was observed in the myometrium of the posterior uterine wall; however, the right ovary and the right fallopian tube were free of tumors. On the basis of these histopathological features, MA was diagnosed in accordance with the International Federation of Gynecology and Obstetrics criteria (8).
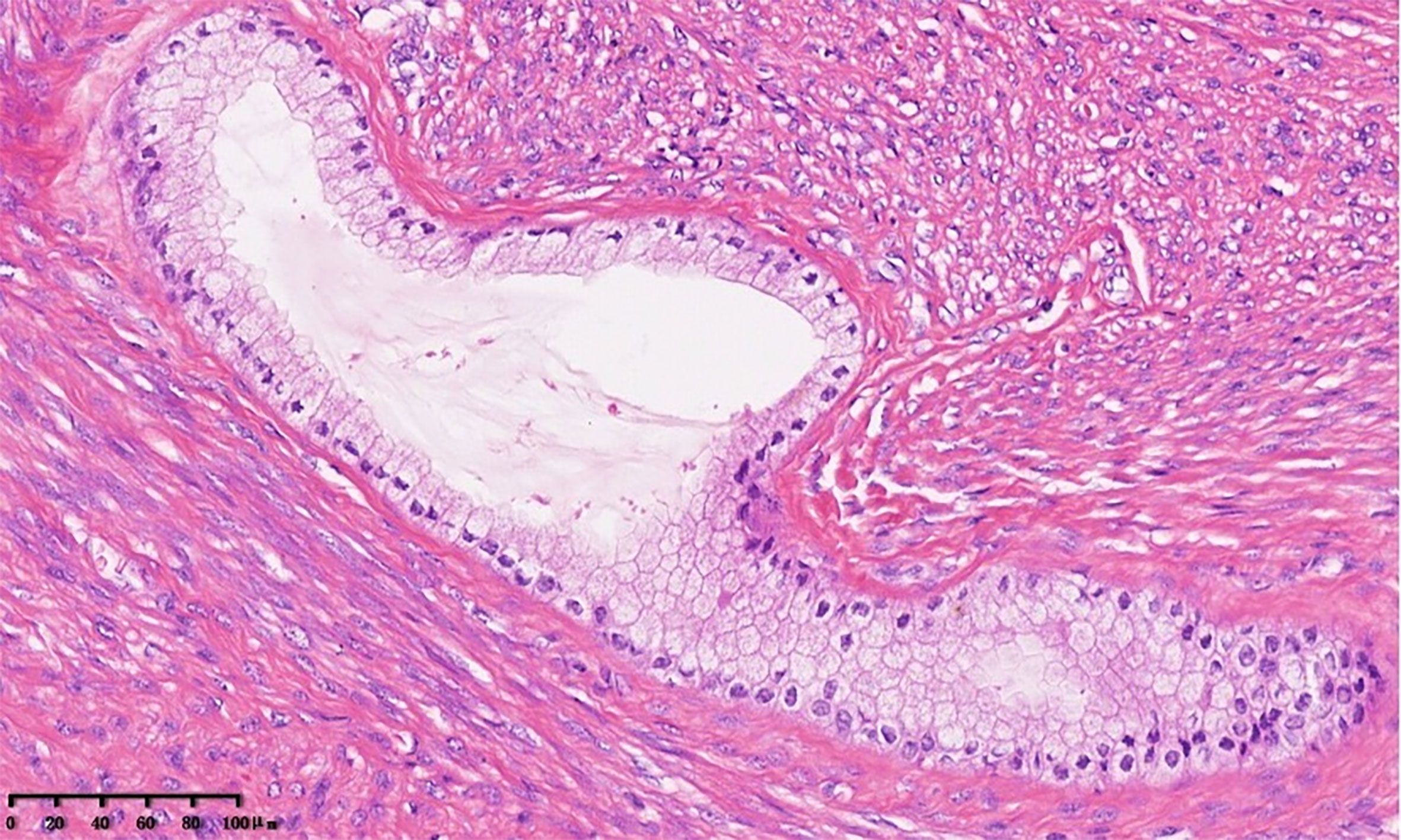
Figure 2 Müllerian adenosarcoma of the uterine cervix. The tumor shows benign columnar glands and low-grade endometrial stromal sarcoma of the cervix. The glands were surrounded by a cellular stroma that formed periglandular cuffs (×20).
MA is a mixed epithelial and mesenchymal tumor with benign or mildly atypical glandular and malignant stromal cells. In total, 71%, 15%, 12%, and 2% of cases had MA occurring in the uterine corpus, ovaries, pelvis, and uterine cervix, respectively (9). The etiology of MA remains unknown because of its rarity. According to previous studies, MA has not been associated with HPV infection. However, in our study, the patients tested positive for HPV 66. Patients with MA vary widely in age but are often younger than patients with uterine corpus tumors. A report on primary cervical adenosarcomas was published by Jones and Lefkowitz (10). In their study covering 12 cases and a review of 12 articles, the average age of patients was 31 years (11–65 years), with one-third under the age of 15 years, and the tumor size varied from 1.0 to 4.5 cm. In most of the cases reviewed, patients presented with abnormal vaginal bleeding, abdominal or pelvic pain, and a cervical mass/polyp. In our study, the woman was of the reproductive age and presented with vaginal bleeding as her major symptom.
Mixed Müllerian tumors are classified into adenomyomas, adenofibromas, adenosarcomas, and carcinosarcomas (malignant Müllerian mixed tumors) on the basis of the composition of benign or malignant epithelial and mesenchymal elements of Müllerian origin. MA is a low-grade neoplasm along the spectrum of mixed Müllerian tumors, with adenofibromas at the beginning and carcinosarcomas at the end (11). Some tumors currently classified as adenofibromas on the basis of their low mitotic count and lack of nuclear atypia are well-differentiated adenosarcomas. Indeed, adenofibroma from adenosarcoma are difficult to distinguish, and the two tumors have a somewhat similar appearance at low magnification. As a result, a confirmed diagnosis of adenofibroma cannot be made on curetted material, and a hysterectomy is required to ensure adequate sampling and exclude adenosarcoma. The most helpful criterion for distinguishing adenosarcoma from adenofibroma is the frequency of mitotic figures found in the stroma. Mitotic activity greater than 1 per 10 HPFs warrants a diagnosis of adenosarcoma (8). The practical approach is to classify as adenosarcoma any biphasic tumor with atypical hypercellular stroma, periglandular stromal cuffing, and one or more mitosis per 10 HPFs (7). Immunohistochemistry may have no utility in differentiating adenosarcoma from benign masses. In addition, previous studies have reported that a patient had an extrauterine pelvic Müllerian adenosarcoma that recurred on multiple occasions and was originally diagnosed as a benign lesion (12, 13). Thus, caution is needed in the initial classification of such benign lesions as adenofibromas. In this study, the patient underwent pelvic surgeries, one in 2005 and another one in 2007. In 2005, she underwent a transvaginal excision of the left ovarian cyst, which revealed a mucinous cystadenofibroma. A previous study proposed that MA is a malignant transformation of the cystadenofibroma (5). In our study, we propose that adenofibromas serve as precursors of Müllerian adenosarcoma in some cases and that transvaginal surgery may promote the occurrence of cervical MA. Transvaginal surgery is a minimally invasive surgery and is currently the preferred treatment for symptomatic ovarian cysts because of its three advantages: less postoperative pain, fewer postoperative complications (i.e., incisional hernia), and improved cosmetic satisfaction. However, despite the popularity of minimally invasive surgery, preoperative evaluation of the ovarian mass is necessary (14).
The management of adenofibroma and adenosarcoma is assumed to be similar and includes a hysterectomy. However, the optimal course of therapy for adenosarcomas is also uncertain. This uncertainty may arise from the fact that adenosarcoma appears in the early stages of reproductive life, and its potential for malignancy remains poorly understood. Most authors have recommended hysterectomy accompanied by bilateral salpingo-oophorectomy because treatment plans are made by analogy with treatments for common diseases for which the optimal treatment has already been determined (15). Although salpingo-oophorectomy is generally recommended, the strength of the evidence for or against ovarian conservation remains insufficient (16). In our study, because the patient had previously undergone laparoscopic left adnexectomy, a total laparoscopic hysterectomy with right salpingo-oophorectomy was performed. Local excision has been curative in rare cases and can be performed in young patients with pedunculated cervical tumors and uninvolved stalks, which allows the conservation of reproductive function (17). However, postoperative recurrences after 5 years are not unusual (18). In a series of 100 cases, Clement and Scully (7) reported that the recurrence rate of MA after surgery was 23.9% and that one-third of the recurrences occurred after 5 years. Therefore, a long-term follow-up period of more than 5 years is necessary for adequate surveillance.
The prognosis of MA is characterized by invasion of the cervical wall and sarcomatous overgrowth (SO). Although MA is regarded as less aggressive and has lower malignancy potential, some unfavorable prognostic factors have been reported, such as a high mitotic rate and the presence of heterologous elements, deep myometrial invasion, necrosis, and extrauterine spread. However, the presence of myometrial invasion and SO has been demonstrated to be associated with poor prognosis, increased risk of postoperative recurrence, and fatal outcome (7, 19). Fortunately, in our study, the patient was diagnosed with MA.
We conducted a Medline search for articles on MA published in English between 1976 and 2022 using “Müllerian adenosarcoma, uterine cervix” or “adenosarcoma, uterine cervix” or “Müllerian adenosarcoma, cervix” or “adenosarcoma, cervix” as keywords and selected papers reporting data on premenopausal women. The clinical characteristics of these patients are summarized in Table 1. We can conclude from the table that cervical MAs are rare tumors and tend to appear more often in younger women. Most patients presented with vaginal or cervical masses and vaginal bleeding. Hysterectomy with salpingo-oophorectomy or fertility-sparing surgery was performed depending on the patient’s age, bearing requirement, and stage of the disease. Kanayama et al. (36) reported that normal fertility is not affected after conservative surgery. Myometrial invasion and SO are major prognostic factors. Therapies should be planned based on a patient’s condition. Recurrences may occur late; therefore, long-term follow-up is necessary. To our knowledge, this is the first reported case of cervical adenosarcoma arising from a benign ovarian cystadenofibroma, furthermore, more studies are recommended to clarify it.
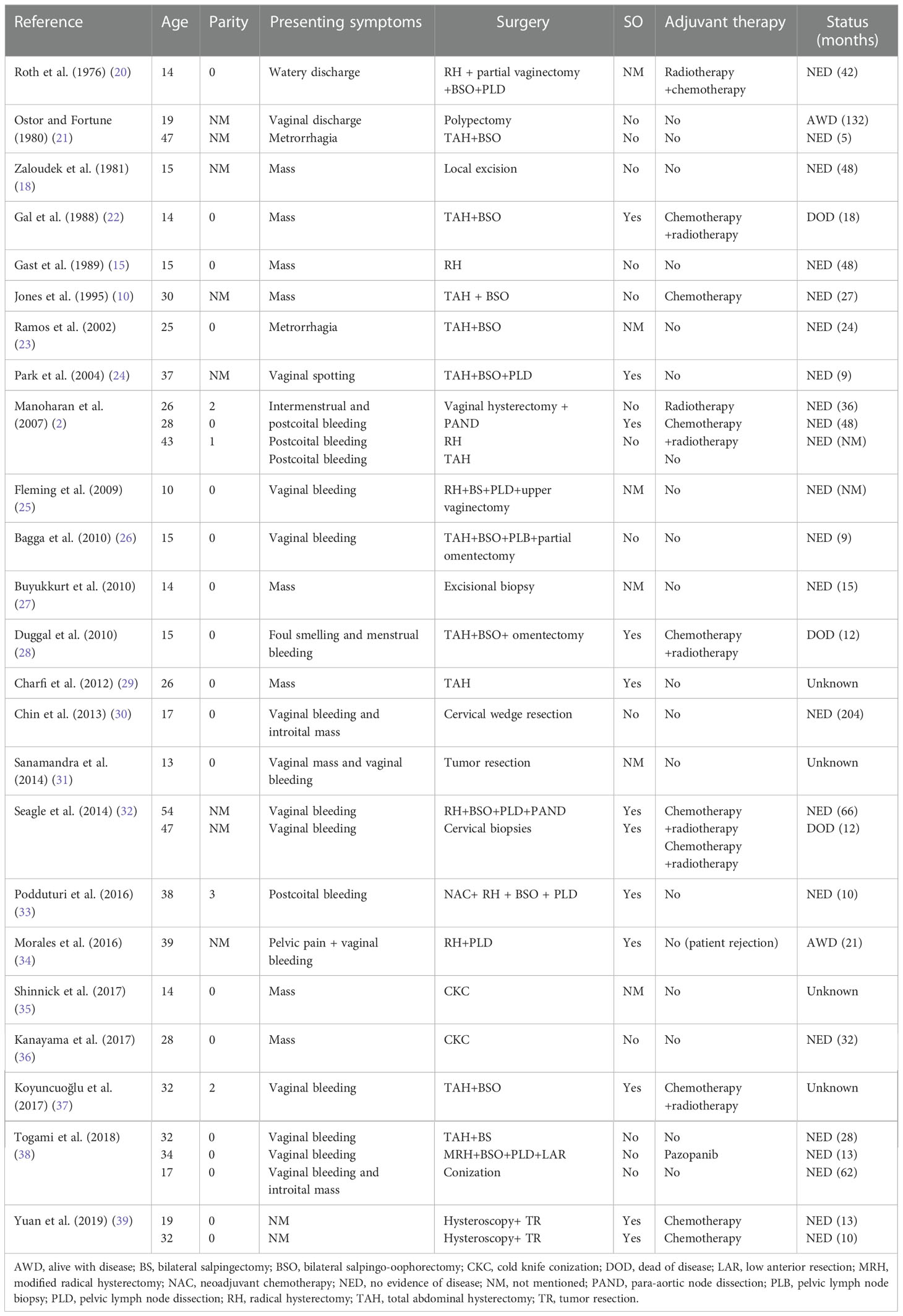
Table 1 Clinicopathologic characteristics of Müllerian adenosarcoma of the cervix in chronological order.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Peking University First Hospital’s ethical review committee. The ethics committee waived the requirement of written informed consent for participation.
XZ: conceptualization and writing-original draft. CP: data analysis. YH: writing-original draft. YZ: conceptualization, writing-reviewing and editing. All authors contributed to the article and approved the submitted version.
The study is supported by The National Natural Science Foundation of China (grant number 82002746).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature (2011) 474:609–15. doi: 10.1038/nature10166
2. Manoharan M, Azmi MA, Soosay G, Mould T, Weekes AR. Mullerian adenosarcoma of uterine cervix: Report of three cases and review of literature. Gynecol Oncol (2007) 105:256–60. doi: 10.1016/j.ygyno.2006.12.029
3. Mumba E, Ali H, Turton D, Cooper K, Grayson W. Human papillomaviruses do not play an aetiological role in müllerian adenosarcomas of the uterine cervix. J Clin Pathol (2008) 61:1041–4. doi: 10.1136/jcp.2008.056614
4. Gallardo A, Prat J. Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol (2009) 33:278–88. doi: 10.1097/PAS.0b013e318181a80d
6. Giannini A, Bogani G, Vizza E, Chiantera V, Laganà AS, Muzii L, et al. Advances on prevention and screening of gynecologic tumors: Are we stepping forward? Healthcare (Basel) (2022) 10. doi: 10.3390/healthcare10091605
7. Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol (1990) 21:363–81. doi: 10.1016/0046-8177(90)90198-e
8. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO committee on gynecologic oncology. Int J Gynaecol Obstet (2000) 70:209–62. doi: 10.1016/S0020-7292(00)90001-8
9. Arend R, Bagaria M, Lewin SN, Sun X, Deutsch I, Burke WM, et al. Long-term outcome and natural history of uterine adenosarcomas. Gynecol Oncol (2010) 119:305–8. doi: 10.1016/j.ygyno.2010.07.001
10. Jones MW, Lefkowitz M. Adenosarcoma of the uterine cervix: a clinicopathological study of 12 cases. Int J Gynecol Pathol (1995) 14:223–9. doi: 10.1097/00004347-199507000-00005
11. D'Angelo E, Prat J. Pathology of mixed müllerian tumours. Best Pract Res Clin Obstet Gynaecol (2011) 25:705–18. doi: 10.1016/j.bpobgyn.2011.05.010
12. Mahoney AD, Waisman J, Zeldis LJ. Adenomyoma: a precursor of extrauterine müllerian adenosarcoma? Arch Pathol Lab Med (1977) 101:579–84.
13. Chen ZW, Saad RS. Ovarian adenosarcoma arising from benign cystadenoma and associated intraoperative consultation pitfalls. Int J Gynecol Pathol (2010) 29:415–8. doi: 10.1097/PGP.0b013e3181d90bca
14. Giannini A, D'Oria O, Chiantera V, Margioula-Siarkou C, Di Donna MC, Terzic S, et al. Minimally invasive surgery for cervical cancer: Should we look beyond squamous cell carcinoma? J Invest Surg (2022) 35:1602–3. doi: 10.1080/08941939.2022.2075495
15. Gast MJ, Radkins LV, Jacobs AJ, Gersell D. Mullerian adenosarcoma of the cervix with heterologous elements: diagnostic and therapeutic approach. Gynecol Oncol (1989) 32:381–4. doi: 10.1016/0090-8258(89)90646-x
16. Michener CM, Simon NL. Ovarian conservation in a woman of reproductive age with müllerian adenosarcoma. Gynecol Oncol (2001) 83:424–7. doi: 10.1006/gyno.2001.6398
17. Chen KT. Rhabdomyosarcomatous uterine adenosarcoma. Int J Gynecol Pathol (1985) 4:146–52. doi: 10.1097/00004347-198506000-00006
18. Zaloudek CJ, Norris HJ. Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases. Cancer (1981) 48:354–66. doi: 10.1002/1097-0142(19810715)48:2<354::aid-cncr2820480222>3.0.co;2-q
19. Clement PB. Müllerian adenosarcomas of the uterus with sarcomatous overgrowth. a clinicopathological analysis of 10 cases. Am J Surg Pathol (1989) 13:28–38. doi: 10.1097/00000478-198901000-00004
20. Roth LM, Pride GL, Sharma HM. Müllerian adenosarcoma of the uterine cervix with heterologous elements: a light and electron microscopic study. Cancer (1976) 37:1725–36. doi: 10.1002/1097-0142(197604)37:4<1725::aid-cncr2820370418>3.0.co;2-1
21. Ostör AG, Fortune DW. Benign and low grade variants of mixed müllerian tumour of the uterus. Histopathology (1980) 4:369–82. doi: 10.1111/j.1365-2559.1980.tb02932.x
22. Gal D, Kerner H, Beck D, Peretz BA, Eyal A, Paldi E. Mullerian adenosarcoma of the uterine cervix. Gynecol Oncol (1988) 31:445–53. doi: 10.1016/s0090-8258(88)80030-1
23. Ramos P, Ruiz A, Carabias E, Piñero I, Garzon A, Alvarez I. Müllerian adenosarcoma of the cervix with heterologous elements: report of a case and review of the literature. Gynecol Oncol (2002) 84:161–6. doi: 10.1006/gyno.2001.6423
24. Park HM, Park MH, Kim YJ, Chun SH, Ahn JJ, Kim CI, et al. Mullerian adenosarcoma with sarcomatous overgrowth of the cervix presenting as cervical polyp: a case report and review of the literature. Int J Gynecol Cancer (2004) 14:1024–9. doi: 10.1111/j.1048-891X.2004.014546.x
25. Fleming NA, Hopkins L, de Nanassy J, Senterman M, Black AY. Mullerian adenosarcoma of the cervix in a 10-year-old girl: case report and review of the literature. J Pediatr Adolesc Gynecol (2009) 22:e45–51. doi: 10.1016/j.jpag.2008.06.001
26. Bagga R, Keepanasseril A, Srinivasan R, Dey P, Gainder S, Saha SC, et al. Adenosarcoma of the uterine cervix with heterologous elements: a case report and review of literature. Arch Gynecol Obstet (2010) 281:669–75. doi: 10.1007/s00404-009-1200-3
27. Buyukkurt S, Guzel AB, Gumurdulu D, Vardar MA, Zeren H, Sucu M. Mullerian adenosarcoma of the uterine cervix in an adolescent girl. J Pediatr Adolesc Gynecol (2010) 23:e13–5. doi: 10.1016/j.jpag.2009.05.008
28. Duggal R, Nijhawan R, Aggarwal N, Sikka P. Mullerian adenosarcoma (heterologous) of the cervix with sarcomatous overgrowth: a case report with review of literature. J Gynecol Oncol (2010) 21:125–8. doi: 10.3802/jgo.2010.21.2.125
29. Charfi S, Kallel R, Mnif H, Ellouze S, Dhouib M, Guermazi M, et al. Mullerian adenosaroma of the cervix with sarcomatous overgrowth and heterologous elements presenting as a recurrent cervical polyp. Case Rep Obstet Gynecol (2012) 2012:358302. doi: 10.1155/2012/358302
30. Chin PS, Chia YN, Lim YK, Yam KL. Diagnosis and management of müllerian adenosarcoma of the uterine cervix. Int J Gynaecol Obstet (2013) 121:229–32. doi: 10.1016/j.ijgo.2012.12.015
31. Sanamandra SK, Leong MY, Fortier MV. Vaginal mass in a 13-year-old girl. Ann Acad Medicine Singapore (2014) 43:127–9.
32. Seagle BL, Falter KJ 2nd, Lee SJ, Frimer M, Samuelson R, Shahabi S. Mullerian adenosarcoma of the cervix: Report of two large tumors with sarcomatous overgrowth or heterologous elements. Gynecol Oncol Case Rep (2014) 9:7–10. doi: 10.1016/j.gynor.2014.04.005
33. Podduturi V, Pinto KR. Mullerian adenosarcoma of the cervix with heterologous elements and sarcomatous overgrowth. Proc (Bayl Univ Med Cent) (2016) 29:65–7. doi: 10.1080/08998280.2016.11929364
34. Morales FD, Medina RM, Trujillo LM, Beltrán MI, Dulcey IC. Müllerian adenosarcoma of the uterine cervix with sarcomatous overgrowth: A case report of aggressive disease in a young patient. Int J Surg Case Rep (2016) 27:155–61. doi: 10.1016/j.ijscr.2016.08.044
35. Shinnick JK, Kumar N, Beffa L, Miller K, Friedman MA, Kalife E, et al. Management of low-grade cervical müllerian adenosarcoma in a 14-Year-Old girl. J Pediatr Adolesc Gynecol (2017) 30:652–4. doi: 10.1016/j.jpag.2017.05.010
36. Kanayama S, Nakamura M, Oi H, Sugimoto S, Sasaki Y, Uchiyama T, et al. Case report of successful childbearing after conservative surgery for cervical mullerian adenosarcoma. Case Rep Obstet Gynecol (2017) 2017:4187416. doi: 10.1155/2017/4187416
37. Koyuncuoğlu M, Saatli B, Yıldırım N. Ovarian metastasis of müllerian adenosarcoma of the cervix with sarcomatous overgrowth. Turk J Obstet Gynecol (2017) 14:195–8. doi: 10.4274/tjod.75735
38. Togami S, Kawamura T, Fukuda M, Yanazume S, Kamio M, Kobayashi H. Clinical management of uterine cervical mullerian adenosarcoma: A clinicopathological study of six cases and review of the literature. Taiwan J Obstet Gynecol (2018) 57:479–82. doi: 10.1016/j.tjog.2018.04.032
Keywords: Müllerian adenosarcoma, cystadenofibroma, clinicopathological characteristics, differential diagnosis, prognosis
Citation: Zhu X, Peng C, Huang Y and Zhou Y (2023) Uterine cervical Müllerian adenosarcoma possibly arising from ovarian cystadenofibroma: A case report and review of the literature. Front. Oncol. 12:1064851. doi: 10.3389/fonc.2022.1064851
Received: 08 October 2022; Accepted: 09 December 2022;
Published: 04 January 2023.
Edited by:
Paolo Scollo, Kore University of Enna, ItalyReviewed by:
Hanlin Zhu, Hangzhou Ninth People’s Hospital, ChinaCopyright © 2023 Zhu, Peng, Huang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingfang Zhou, emhvdXlmODg1M0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.