- 1Department of Gastroenterology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2State Key Laboratory for the Prevention and Treatment of Esophageal Cancer, Zhengzhou University, Zhengzhou, China
- 3Academy of Medical Sciences, Zhengzhou University, Zhengzhou, China
- 4Department of Pathology, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, China
- 5Department of Administration, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
- 6Department of Nuclear Medicine, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 7Department of Nuclear Medicine, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
Colorectal cancer (CRC) is among the most commonly diagnosed gastrointestinal malignancies worldwide. It is inadequate to handle in terms of staging and restaging only based on morphological imaging modalities and serum surrogate markers. And the correct and timely staging of CRC is imperative to prognosis and management. When compared to established sequential, multimodal conventional diagnostic methods, the molecular and functional imaging 18F-FDG PET/CT shows superiorities for tailoring appropriate treatment maneuvers to each patient. This review aims to summarize the utilities of 18F-FDG PET/CT in CRC, focusing on primary staging, follow-up assessment of tumor responses and diagnostic of recurrence. In addition, we also summarize the technical considerations of PET/CT and the conventional imaging modalities in those patients who are either newly diagnosed with CRC or has already been treated from this cancer.
1. Introduction
Colorectal cancer (CRC) is the first most common and leading cause of gastrointestinal cancers (GI) worldwide, whose incidence and mortality rates accounted for 10% and 9.4%, respectively (1). Based on the statistics from International Researches Agency of Cancer (IRAC), the incidence and mortality of CRC would increase 40.1% and 43.4% by 2040 worldwide, respectively (2). Approximately 20% of CRC patients have initially diagnosed with metastases, mostly depositing in regional lymph nodes, liver, lung, and peritoneum (3). And the overall survival rate of patients is closely associated with stage at presentation; the 5-year survival rate drops drastically decreases from stage I (93%) to stage IV (8%) (4). Therefore, the precise staging of the CRC is essential for prognosis and effective therapy. The curative surgery has been remained as the “workhorse” at the early stage of cancers, and part of patients only with isolated liver metastasis (5). Nowadays, the standard of care regimens tends to be multidisciplinary, consisting of surgery, 5-fluorouracil-based chemotherapy, radiotherapy, targeted therapies, and PD-1/PD-L1 blockade biotherapy (5). Accurate pre- and postoperative staging are pivotal to tailor treatment avenues, which unequivocally improving the survival and quality of life. Variety of imaging techniques have been introduced for this purpose, with varying degree of success.
In the routine clinical practice, colonoscopy continues to be the preferred method for the diagnosis of colon cancer, because it allows tissue biopsies (6). In addition, a number of non-invasive imaging modalities, including as morphologic imaging such as computed tomography (CT), magnetic resonance imaging (MRI), and metabolic imaging such as positron emission tomography (PET), are presently used for staging colon cancer (7–9). Conventional imaging modalities, CT or MRI, are only based on the modifications of morphology of lesions, which could be invalid for cases mainly with metabolic changes. And serial elevated serum carcinoembryonic antigen (CEA) is usually coupled with false positives and false negatives (10). Recent studies showed that the combined-imaging modality is preferable for the detection and characterization of different malignant lesions including colorectum when compared with traditional imaging techniques (11). PET with flourine-18 fluorodeoxyglucose (18F-FDG PET/CT, hereafter refers as PET/CT) not only provides alternations of morphology, but also glycolytic metabolism changes based on glucose uptake, which increasing early diagnostic efficacy and visualize active tumor tissue (12). As for the molecular imaging, PET/CT represents a valuable ally for staging, therapeutic monitoring, and restaging for CRC patients (13, 14). However, the false positives were usually occurred when infection, inflammation, and other non-neoplastic conditions (15). Despite these drawbacks, PET/CT is irreplaceable in its ability to assess the abnormal metabolic activity precedes morphological change and to identify small sized malignant tumors in morphologically normal structures. This review attempts to discuss the role of PET/CT in the treatment of patients with CRC, which could pave the way for early accurate diagnosis and even individualized therapeutic strategies.
2. Evaluation of the preoperative TNM staging
Tumor-Node-Metastasis (TNM) staging system (AJCC) has provided the universal framework for optimal management of CRC patients since 1959 (16). The curative surgery is the mainstay of treatment based on accurate preoperative staging by the combination of T, N, and M indicators (Figure 1). Although the conventional imaging has served as the standard modality for this staging, PET/CT has been shown superior to evaluate the primary tumor, regional lymph nodes, and distant metastasis. Here we attempt to review and summarize the pros and cons of PET/CT in preoperative staging of CRC patients.
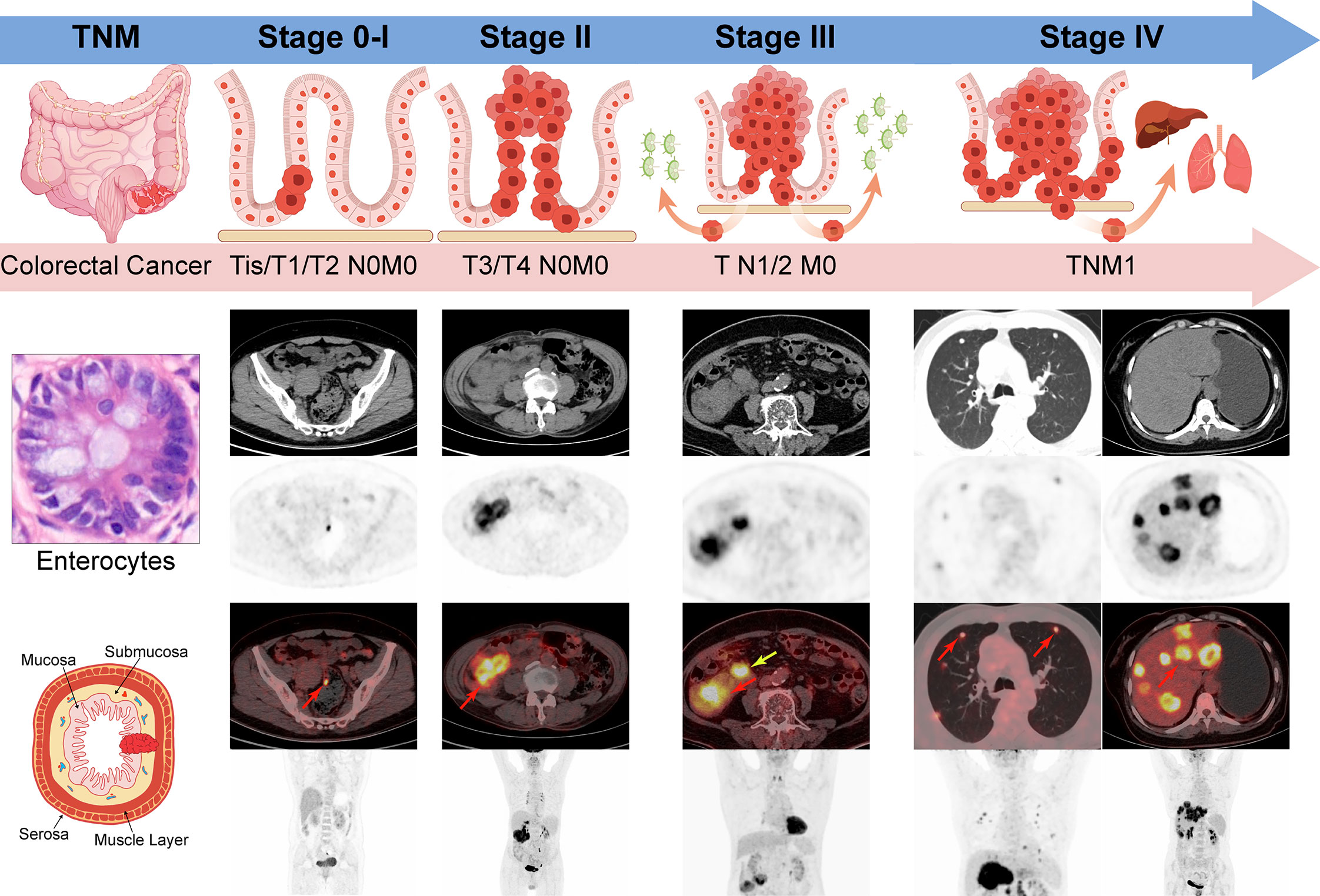
Figure 1 TNM stage of Colorectal cancer by AJCC 8th edition. Stage 0-I: Tumors spread within muscle layers without lymph nodes or distant deposits (Tis/T1/T2N0M0); Stage II: Tumors spread all layers or attached to nearby tissues without lymph nodes or distant deposits (T3/4N0M0); Stage III: Any T stage with the involvement of regional lymph nodes (TN1/2M0); Stage IV: Any T or N stage with distant metastasis (TNM1); Red arrow: the primary or metastatic tumors; yellow arrow: the metastatic lymph nodes.
2.1. T-staging: The infiltration extent of primary tumor
Accurate preoperative evaluation of T-staging aids in selecting the related surgery approaches (17). Although limited spatial resolution of PET/CT confines its clinical application, there are still several strengths regarding preoperative assessment of T-staging. A meta-analysis including 2283 CRC patients from 28 studies shown the excellent performance for preoperative T-staging by PET/CT. The pooled specificity and AUC were 99% and 96%, respectively, which was significantly superior to CT (18). Other study demonstrated that the accurate preoperative T-staging was 94.3% except for only two tumors overestimation (19). As for the obstructive CRC, preoperative PET/CT colonography shows the particular advantages. Nagata K et al. demonstrated that PET/CT colonography could recognized all 13 primary CRC and 2 synchronous lesions proximal to the obstruction, while missed by other conventional imaging and optical colonoscopy more or less (20). Indeed, all lesions removed by single-stage procedure plays a vital role in the favorable outcomes of patients.
2.2. N-staging: Involvement of regional lymph nodes
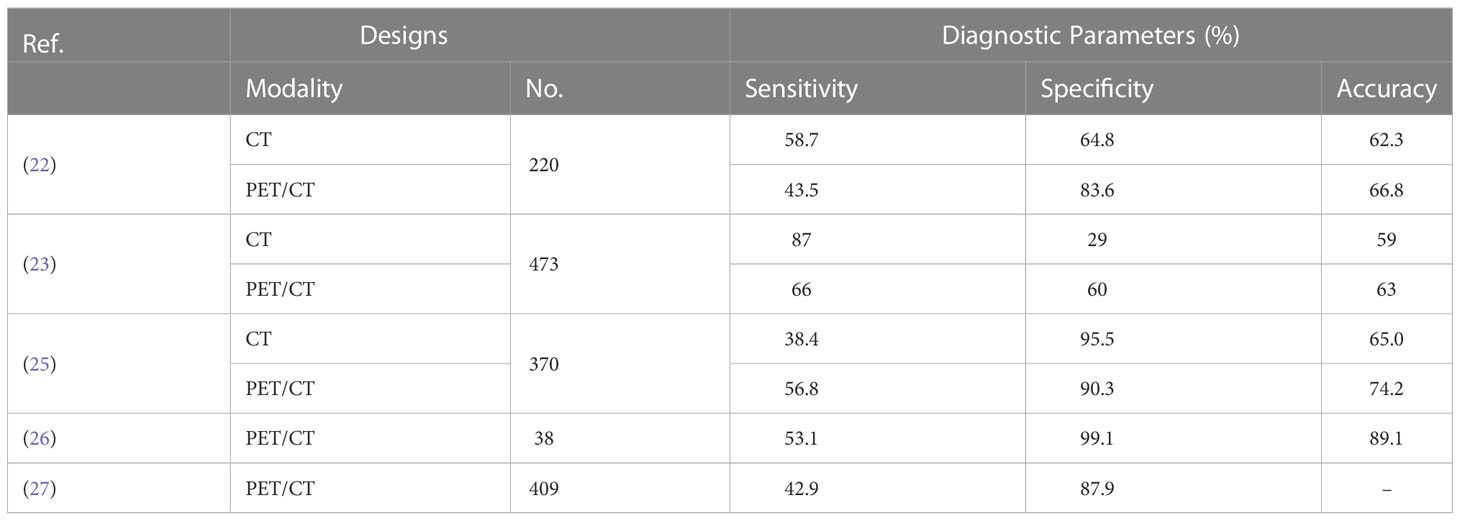
Metastatic lymphadenopathy is indicated as a risk factor of recurrence and dismal survival in patients with CRC, which highlighting the merits of accurate detection and preoperative staging (21). The diagnosis of metastatic lymph nodes is generally based on a collection of morphological and density readout from the anatomic imaging techniques (CT & MRI). However, a plethora of positive lymph nodes tend to be not detected abnormality because of low density, small diameter, regular form and so on (22). Indeed, the overall accuracy of evaluation of N-staging by contrast enhanced CT (ceCT) has been reported merely from 59% to 71% (23, 24). Therefore, the alternative molecular imaging combined the morphological and functional techniques holds the promise for improving the diagnostic performance concerning metastatic deposits of CRC patients. Here we list several studies of the outstanding performance of PET/CT for this fields (Table 1) (22, 25–28).
For detection of regional lymph node metastasis, a retrospective study including 370 CRC patients showed superiority of PET/CT in specificity compared with CT (83.6% vs. 64.8%, p = 0.000), while inferiority in sensitivity (43.5% vs. 58.7%, p = 0.029) (25). Consistently, Kwak et al. also reported higher specificity of PET/CT, while the comparable sensitivity compared to CT (28). Absence of functional information from CT has been recognized as the main drawback responsible for the lower specificity, while the slightly higher sensitivity could be due to the limited spatial resolution of PET/CT (29). Besides, there are still several studies indicated the possible reasons of PET/CT fail to detect the metastatic lymph nodes (1): the interference from physiological uptake by bowel and bladder (2); the proximity to the primary tumor so as to interfere the diagnosis of regional lymph node (14, 30).
2.3. M-staging: Distant metastases
Life expectancy of CRC patients declines to 71% when local regional lymph nodes involved, while plummet to 17% in the case of distant metastases (31). Therefore, the clinical significance of tumor staging is particularly regarding the assessment of distant metastases. Although PET/CT has been suggested utilized in few circumstances, including metastatic synchronous adenocarcinoma, and metachronous metastases with elevated serial CEA but negative colonoscopy or CT, various clinical trials pertaining to PET/CT have shown advantages for M-staging. Given that liver metastasis is the main site of advanced CRC patients, ineligibility for curative hepatic resection is based on extrahepatic deposits except for few resectable lung metastases. Ruers et al. demonstrated that PET/CT could reduce the futile laparotomies concerning liver metastasis from 45% to 28%, which decreasing unnecessary procedures and economic burden to a large extent (32). Other retrospective study based on Korean population reported that PET/CT was superior to CT in specificity (94% vs. 87%) and accuracy (93% vs. 86%), which was accounted for the specific metabolic changes by the former (33). Therefore, more clinical trials pertaining to PET/CT should be performed in order to pave the way for clinical utilization.
3. Assessment of the response to targeted therapies
The developments of targeted therapies were renewed and have flourished in the past two decades (34). Recent applications of such treatments in CRC have been exemplified by the approval of oral kinase inhibitors or monoclonal antibodies, e.g., anti-angiogenesis (VEGF) and targeting epidermal growth factor receptor (EGFR). However, given only part of patients respond to these targeted therapies, it has been highly recommended to early stratify patients in order to reduce unnecessary toxicity and economic burden. Generally, the anatomic imaging modalities based on RECIST criteria, CT or MRI, have been utilized to monitor the response of cytoreductive or cytotoxic chemotherapy, while shows inappropriate for evaluating the cytostatic effect by the above-mentioned targeted therapy (35, 36). It is a common knowledge that the alternations of glucose metabolism within tumor precede the morphological changes by several weeks (37). Within this framework, we review the most widely accepted PET tracer and the only licensed biomarker, 18F-FDG (38), for assessing the responses to targeted therapies for patients with CRC.
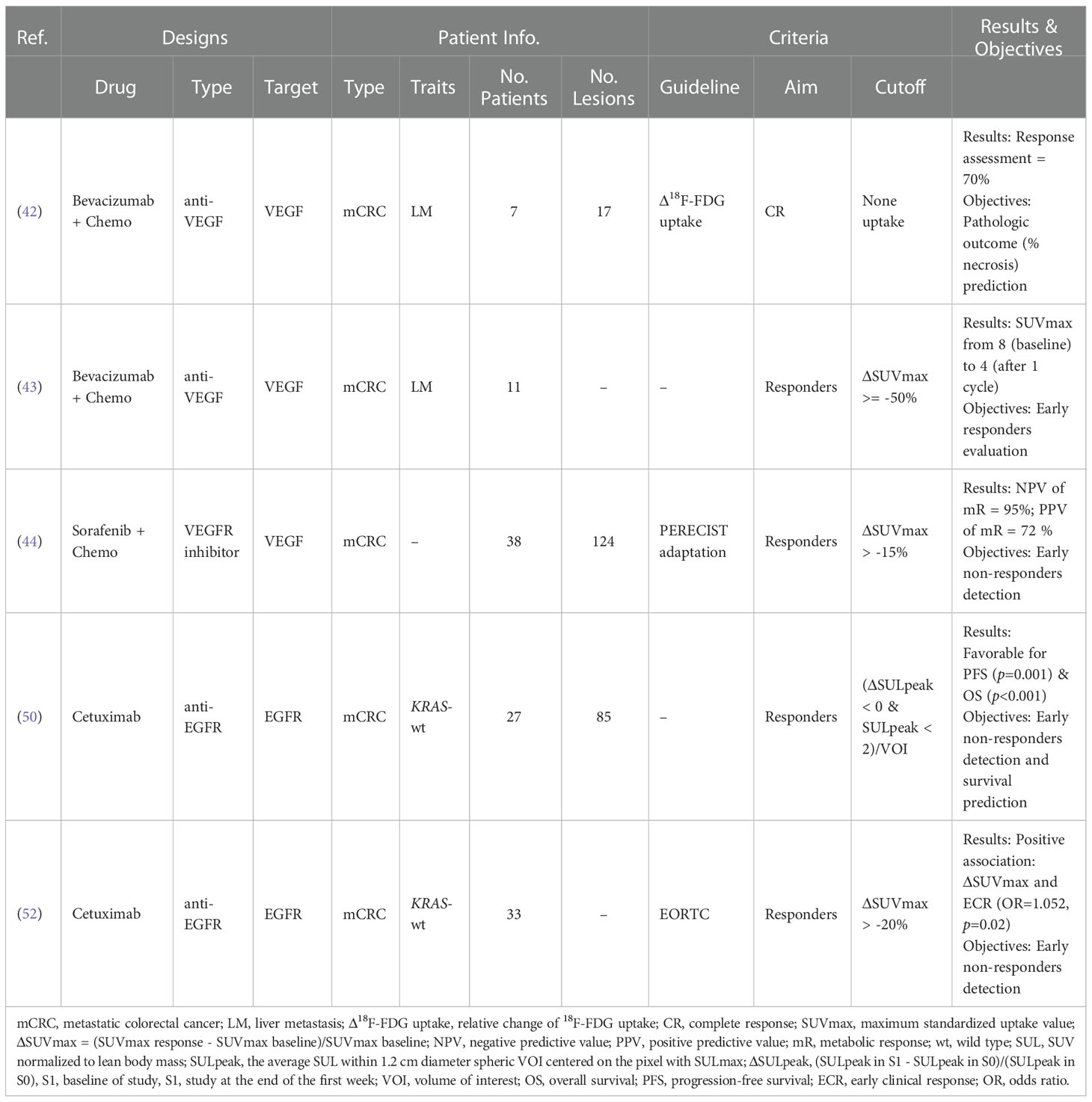
Vascular endothelial growth factor (VEGF) targeted therapy disrupts tumor vasculature as well as inhibits angiogenesis, which have been exemplified by the first approved bevacizumab (Avastin) for metastatic colon cancer (mCRC) (39, 40). The semi-quantitative analysis of 18F-FDG uptake compared to normal liver was demonstrated to be instrumental for pathologic response prediction to bevacizumab in mCRC (41). Because of the quantitative trait of PET, quantitative measurement for early treatment response shows more attraction. Standardized uptake value (SUV) is the widely accepted quantitative metric for assessing treatment response in clinical PET/CT utility (Figure 2) (42). For example, the reduction of maximum SUV (SUVmax) more than 50% showed predictive value for early response to Bevacizumab in mCRC patients, with the larger the decline, the greater the efficacy (43). And the relative decrease of SUVmax more than 15% presented highly predictive for non-responders detection after one cycle of Sorafenib combined with capecitabine in mCRC patients (44).
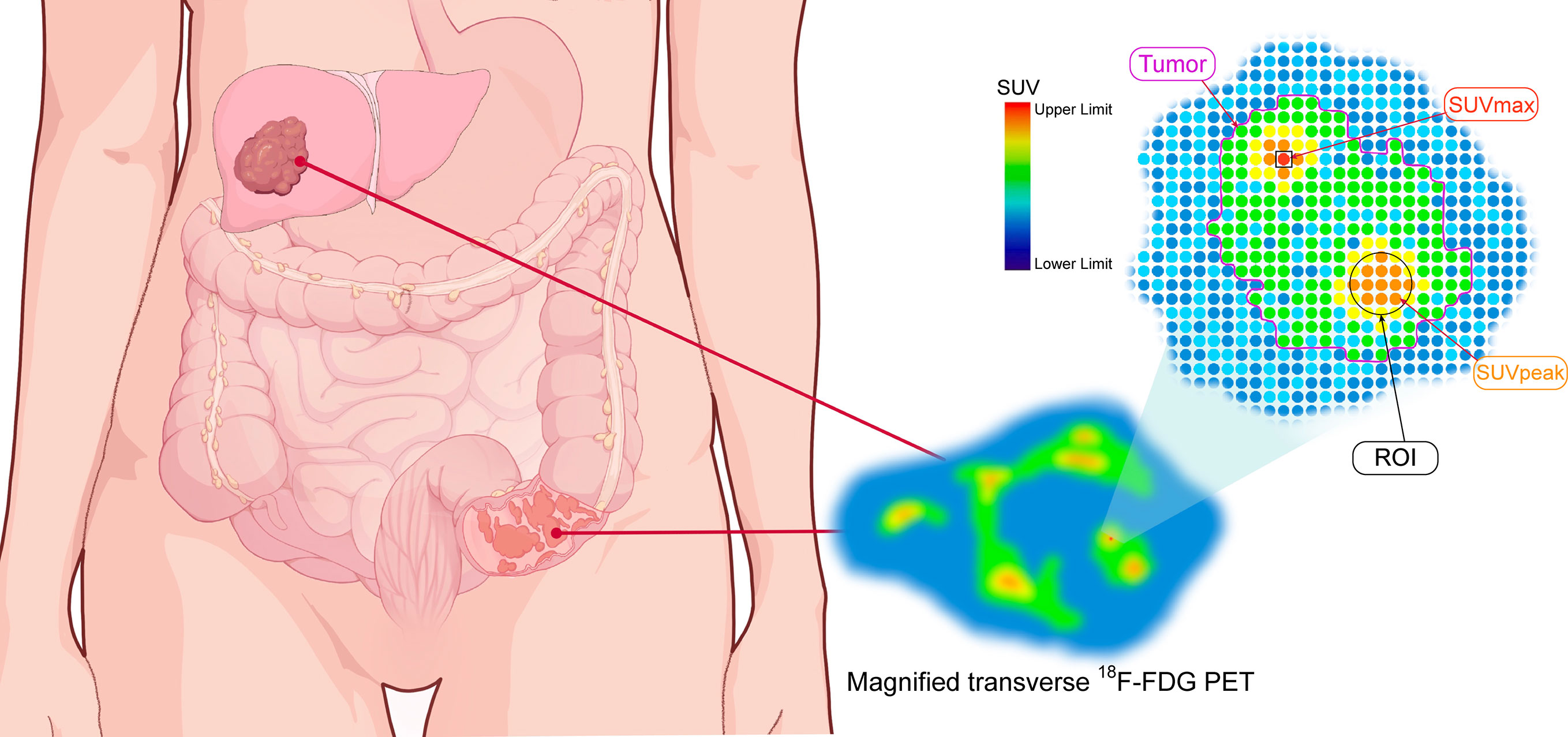
Figure 2 The schematic diagram of quantitative parameters of PET. The magnified transverse 18F-FDG PET image of radiotracer uptake in tumor (purple outline). SUVmax: maximum standardized uptake value (red spot within black square); ROI: region of interest (black circle); SUVpeak: the average SUV obtained from a 1mL sphere within the tumor.
Dysregulations of EGFR pathway have been extensively involved in carcinogenesis of CRC, such as metastasis, proliferation, and resistant to apoptosis (45–47). The cetuximab-based regimens targeting EGFR signaling cascade is clinically utilized as the third-line therapy for mCRC patients without KRAS mutation (48). The PET Response Criteria in Solid Tumors (PERECIST) criterion was proposed for assessing the early therapy response by the relative change of SULpeak, i.e., peak SUV normalized to lean body mass (SUL) in a spherical 1 cm3 volume of interest (VOI) (49). Although treatment response using PERECIST predicted survival parameters (PFS & OS) at the end of cetuximab-based therapy (4 week) for mCRC, the innovative principle based on any eligible VOI more than 2 could ahead of this schedule after one week (50). And the early metabolic response based on the reduction of SUVmax more than 20%, fitted with EORTC criteria (51), could serve as the surrogate parameter for early clinical response under cetuximab in CRC patients (52). All cases reviewed here are summarized in Table 2.
Therefore, detection of non-responders at early treatment stage could spare patients from the exposure of unnecessary toxicity and heavy economic burden.
4. Restaging of colorectal cancer
Most of CRC patients diagnosed at advanced staged would be suffered from recurrences, including liver, lung, and peritoneum metastases (21, 53). And serum markers and imaging modalities are utilized as the main examinations for those detection. Despite ceCT remains the clinical guideline recommendation, PET/CT is suggested in the workup of recurrent CRC patients with metachronous metastases, or elevated serial CEA but negative conventional imaging modalities and optical colonoscopy. Besides, PET/CT also presents the unique strengths for those kinds of scenarios.
Distinction of recurrent lesions from postoperative scar tissues is the important superiority of PET/CT. FDG uptake within recurrences could significantly reduce false positives and false negatives as well (54). The sensitivity and specificity of serum surrogate marker CEA has been demonstrated elevated when combined with PET/CT. One of the retrospective studies including 112 patients showed the early detection of recurrent CRC was 71% compared with 55% of CT when CEA level greater than 13 ng/mL (55). Actually, the level of CEA less than 5 ng/mL also presented excellent performance by PET/CT (56). Besides, the diagnostic accuracy of PET/CT for recurrent cases with elevated CEA was moderate (65-75%) in the case of negative findings by conventional imaging modalities (57). Moreover, with the aim of assess the impact of PET/CT in tailoring management in CRC patients with proven or suspected recurrence, and further to evaluate the impact of different management on the prognosis of patients. Scott et al. reported that PET/CT modified the therapeutic strategies of 65.6% CRC patients with symptomatic or residual lesions suggestive of recurrence. And the treatment maneuvers of 43.9% patients with resectable metastatic deposits in lung or liver have also been changed. Besides, the additional lesions of those patients showed progressive disease in 60.5% and 65.9% in the above-mentioned two groups of patients compared with conventional imaging techniques (36.2% & 39.2%), which indicated the value of PET/CT regarding the stratification of patients into curative or palliative avenues (58). In addition, there are also plenty of studies demonstrated that the outstanding performance for recurrent lesions by PET/CT in comparison with CT or MRI, which was generally accounted for the combination with functional imaging (59–61).
5. Conclusion and perspective
Effective and precise pre- and postoperative staging is essential for the best and individualized management of CRC patients, especially for those with metastatic lesions. Nowadays, the conventional imaging modalities are still the mainstay for staging processes, while insensitivity to the monitor the treatment response of targeted therapy. Given that the single trait of morphological imaging techniques, the additional function of metabolic evaluation by PET/CT shows particular advantages, including early detection for tumors, sensitivity to the treatment response to cytostatic drugs, and evaluation numerous body regions in a single process. This makes PET/CT particularly helpful in the staging of patients to exclude the presence of distant metastases as well as in the restaging. However, more research is required to standardize the ideal PET/CT timing in relation to the chosen therapy (immunotherapy or chemotherapy). And there is still a dearth of information regarding the diagnostic potency of PET/CT in the evaluation of CRC. Given that anatomic imaging techniques are superior to detect small lesions because of the high resolution, the combined molecular and morphological imaging PET/CT would broaden its clinical utility. In conclusion, PET/CT adds value to the diagnosis of metastatic lesions and may aid patients with CRC in selecting more appropriate treatment modalities.
Author contributions
YS, XM, and JJZ formulated the design of this study; YS and XM wrote the manuscript; MW and CL revised the draft; JYZ drew the illustrations and figures; ZX assessed the histopathology of CRC. All authors contributed to the article and approved the submitted version.
Acknowledgments
We express our apology to the authors whose study could not be cited for the limited spaces. We would like to thanks Saif Ullah for his assistance in revising the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer (2021). doi: 10.1002/ijc.33588
2. Ferlay J, Laversanne M, Ervik M, Lam F, Colombet M, Mery L, et al. Global cancer observatory. In: Cancer tomorrow Lyon. France: International Agency for Research on Cancer (2020). Available at: https://gco.iarc.fr/tomorrow.
3. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol (2014) 25 Suppl 3::iii1–9. doi: 10.1093/annonc/mdu260
4. Díaz-Tasende J. Colorectal cancer screening and survival. Rev Espanola Enfermedades Digestivas Organo Oficial la Sociedad Espanola Patologia Digestiva (2018) 110(11):681–3. doi: 10.17235/reed.2018.5870/2018
5. Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 1.2017, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN (2017) 15(3):370–98. doi: 10.6004/jnccn.2017.0036
6. Zhang Q, Shen Y, Xu J, Gao P, Bing H. Clear colonoscopy as a surveillance tool in the prediction and reduction of advanced neoplasms: A randomized controlled trial. Surg Endosc (2021) 35(8):4501–10. doi: 10.1007/s00464-020-07964-z
7. Olsen ASF, Gundestrup AK, Kleif J, Thanon T, Bertelsen CA. Accuracy of preoperative staging with multidetector computed tomography in colon cancer. Colorectal Dis Off J Assoc Coloproctol Great Britain Ireland (2021) 23(3):680–8. doi: 10.1111/codi.15415
8. Chen SH, Miles K, Taylor SA, Ganeshan B, Rodriquez M, Fraioli F, et al. Fdg-Pet/Ct in colorectal cancer: Potential for vascular-metabolic imaging to provide markers of prognosis. Eur J Nucl Med Mol Imag (2021) 49(1):371–84. doi: 10.1007/s00259-021-05318-y
9. Nerad E, Lambregts DM, Kersten EL, Maas M, Bakers FC, van den Bosch HC, et al. Mri for local staging of colon cancer: Can mri become the optimal staging modality for patients with colon cancer? Dis Colon Rectum (2017) 60(4):385–92. doi: 10.1097/dcr.0000000000000794
10. Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest (2005) 23(4):338–51. doi: 10.1081/cnv-58878
11. Rodríguez-Fraile M, Cózar-Santiago MP, Sabaté-Llobera A, Caresia-Aróztegui AP, Delgado Bolton RC, Orcajo-Rincon J, et al. Fdg Pet/Ct in colorectal cancer. Rev Espanola Medicina Nucl e Imagen Mol (2020) 39(1):57–66. doi: 10.1016/j.remn.2019.09.009
12. Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol (2011) 38(1):55–69. doi: 10.1053/j.seminoncol.2010.11.012
13. Almuhaideb A, Papathanasiou N, Bomanji J. 18f-fdg Pet/Ct imaging in oncology. Ann Saudi Med (2011) 31(1):3–13. doi: 10.4103/0256-4947.75771
14. Delbeke D, Martin WH. Pet and pet-ct for evaluation of colorectal carcinoma. Semin Nucl Med (2004) 34(3):209–23. doi: 10.1053/j.semnuclmed.2004.03.006
15. Hess S, Hansson SH, Pedersen KT, Basu S, Høilund-Carlsen PF. Fdg-Pet/Ct in infectious and inflammatory diseases. PET Clinics (2014) 9(4):497–519. doi: 10.1016/j.cpet.2014.07.002
16. Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the ajcc cancer staging manual and the future of tnm. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
17. Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for cancer of the colon and rectum (Jsccr) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol (2018) 23(1):1–34. doi: 10.1007/s10147-017-1101-6
18. Ye Y, Liu T, Lu L, Wang G, Wang M, Li J, et al. Pre-operative tnm staging of primary colorectal cancer by (18)F-fdg pet-ct or pet: A meta-analysis including 2283 patients. Int J Clin Exp Med (2015) 8(11):21773–85.
19. Mainenti PP, Iodice D, Segreto S, Storto G, Magliulo M, De Palma GD, et al. Colorectal cancer and 18fdg-Pet/Ct: What about adding the T to the n parameter in loco-regional staging? World J Gastroenterol (2011) 17(11):1427–33. doi: 10.3748/wjg.v17.i11.1427
20. Nagata K, Ota Y, Okawa T, Endo S, Kudo SE. Pet/Ct colonography for the preoperative evaluation of the colon proximal to the obstructive colorectal cancer. Dis Colon Rectum (2008) 51(6):882–90. doi: 10.1007/s10350-008-9236-1
21. Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep (2016) 6:29765. doi: 10.1038/srep29765
22. Lee JY, Yoon SM, Kim JT, Kim KB, Kim MJ, Park JG, et al. Diagnostic and prognostic value of preoperative (18)F-fluorodeoxyglucose positron emission Tomography/Computed tomography for colorectal cancer: Comparison with conventional computed tomography. Intestinal Res (2017) 15(2):208–14. doi: 10.5217/ir.2017.15.2.208
23. Mainenti PP, Cirillo LC, Camera L, Persico F, Cantalupo T, Pace L, et al. Accuracy of single phase contrast enhanced multidetector ct colonography in the preoperative staging of colo-rectal cancer. Eur J Radiol (2006) 60(3):453–9. doi: 10.1016/j.ejrad.2006.08.001
24. Filippone A, Ambrosini R, Fuschi M, Marinelli T, Genovesi D, Bonomo L. Preoperative T and n staging of colorectal cancer: Accuracy of contrast-enhanced multi-detector row ct colonography–initial experience. Radiology (2004) 231(1):83–90. doi: 10.1148/radiol.2311021152
25. Sasaki K, Kawasaki H, Sato M, Koyama K, Yoshimi F, Nagai H. Impact of fluorine-18 2-Fluoro-2-Deoxy-D-Glucose uptake on preoperative positron emission Tomography/Computed tomography in the lymph nodes of patients with primary colorectal cancer. Digest Surg (2017) 34(1):60–7. doi: 10.1159/000448222
26. Kawashima K, Kato K, Tomabechi M, Matsuo M, Otsuka K, Ishida K, et al. Clinical evaluation of (18)F-fludeoxyglucose positron emission Tomography/Ct using point spread function reconstruction for nodal staging of colorectal cancer. Br J Radiol (2016) 89(1063):20150938. doi: 10.1259/bjr.20150938
27. Lu YY, Chen JH, Ding HJ, Chien CR, Lin WY, Kao CH. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18f-fdg pet or Pet/Ct. Nucl Med Commun (2012) 33(11):1127–33. doi: 10.1097/MNM.0b013e328357b2d9
28. Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS, Kim JC. Diagnostic value of fdg-Pet/Ct for lymph node metastasis of colorectal cancer. World J Surg (2012) 36(8):1898–905. doi: 10.1007/s00268-012-1575-3
29. Dahmarde H, Parooie F, Salarzaei M. Is (18)F-fdg Pet/Ct an accurate way to detect lymph node metastasis in colorectal cancer: A systematic review and meta-analysis. Contrast Media Mol Imaging (2020) 2020:5439378. doi: 10.1155/2020/5439378
30. Kantorová I, Lipská L, Bêlohlávek O, Visokai V, Trubaĉ M, Schneiderová M. Routine (18)F-fdg pet preoperative staging of colorectal cancer: Comparison with conventional staging and its impact on treatment decision making. J Nucl Med Off Publication Soc Nucl Med (2003) 44(11):1784–8.
31. Maffione AM, Rubello D, Caroli P, Colletti PM, Matteucci F. Is it time to introduce Pet/Ct in colon cancer guidelines? Clin Nucl Med (2020) 45(7):525–30. doi: 10.1097/rlu.0000000000003076
32. Ruers TJ, Wiering B, van der Sijp JR, Roumen RM, de Jong KP, Comans EF, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-fdg pet: A randomized study. J Nucl Med Off Publication Soc Nucl Med (2009) 50(7):1036–41. doi: 10.2967/jnumed.109.063040
33. Lee JY, Ock M, Jo MW, Son WS, Lee HJ, Kim SH, et al. Estimating utility weights and quality-adjusted life year loss for colorectal cancer-related health states in Korea. Sci Rep (2017) 7(1):5571. doi: 10.1038/s41598-017-06004-6
34. Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol (2018) 834:188–96. doi: 10.1016/j.ejphar.2018.07.034
35. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised recist guideline (Version 1.1). Eur J Cancer (Oxf Engl 1990) (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
36. Skougaard K, Johannesen HH, Nielsen D, Schou JV, Jensen BV, Høgdall EV, et al. Ct versus fdg-Pet/Ct response evaluation in patients with metastatic colorectal cancer treated with irinotecan and cetuximab. Cancer Med (2014) 3(5):1294–301. doi: 10.1002/cam4.271
37. Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, et al. Pet to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The municon phase ii trial. Lancet Oncol (2007) 8(9):797–805. doi: 10.1016/s1470-2045(07)70244-9
38. Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. New Engl J Med (2006) 354(5):496–507. doi: 10.1056/NEJMra050276
39. Folkman J, Klagsbrun M. Angiogenic factors. Sci (New York NY) (1987) 235(4787):442–7. doi: 10.1126/science.2432664
40. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-vegf antibody for treating cancer. Nat Rev Drug Discov (2004) 3(5):391–400. doi: 10.1038/nrd1381
41. Goshen E, Davidson T, Zwas ST, Aderka D. Pet/Ct in the evaluation of response to treatment of liver metastases from colorectal cancer with bevacizumab and irinotecan. Technol Cancer Res Treat (2006) 5(1):37–43. doi: 10.1177/153303460600500105
42. Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR (2010) 31(6):496–505. doi: 10.1053/j.sult.2010.10.001
43. Malavasi N, Bagni B, Bertolini F, Dealis C, Depenni R, Cucca M, et al. Predictive role of fluorodeoxyglucose positron emission tomography (18 ff-fdg ct-pet) in early assessment of response to bevacizumab and folfox-6 combined neoadjuvant therapy for liver metastasis (Lm) from colorectal cancer (Crc). J Clin Oncol (2008) 26(15_suppl):15094–. doi: 10.1200/jco.2008.26.15_suppl.15094
44. Woff E, Hendlisz A, Garcia C, Deleporte A, Delaunoit T, Maréchal R, et al. Monitoring metabolic response using fdg pet-ct during targeted therapy for metastatic colorectal cancer. Eur J Nucl Med Mol Imag (2016) 43(10):1792–801. doi: 10.1007/s00259-016-3365-x
45. Ward WH, Cook PN, Slater AM, Davies DH, Holdgate GA, Green LR. Epidermal growth factor receptor tyrosine kinase. Invest Catalytic Mechanism Structure Based Searching Discovery Potent Inhibitor Biochem Pharmacol (1994) 48(4):659–66. doi: 10.1016/0006-2952(94)90042-6
46. Huang SM, Harari PM. Epidermal growth factor receptor inhibition in cancer therapy: Biology, rationale and preliminary clinical results. Invest New Drugs (1999) 17(3):259–69. doi: 10.1023/a:1006384521198
47. Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, et al. Impact of Egfr Expression on Colorectal Cancer Patient Prognosis and Survival. Annals of oncology : official journal of the European Society for Medical Oncology (2005) 16(1):102–8. doi: 10.1093/annonc/mdi006
48. Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer (2012) 11(1):1–13. doi: 10.1016/j.clcc.2011.05.005
49. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From recist to percist: Evolving considerations for pet response criteria in solid tumors. J Nucl Med Off Publication Soc Nucl Med (2009) 50 Suppl 1(Suppl 1):122s–50s. doi: 10.2967/jnumed.108.057307
50. Liu FY, Yen TC, Wang JY, Yang TS. Early prediction by 18f-fdg Pet/Ct for progression-free survival and overall survival in patients with metastatic colorectal cancer receiving third-line cetuximab-based therapy. Clin Nucl Med (2015) 40(3):200–5. doi: 10.1097/rlu.0000000000000693
51. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18f]-fluorodeoxyglucose and positron emission tomography: Review and 1999 eortc recommendations. European organization for research and treatment of cancer (Eortc) pet study group. Eur J Cancer (Oxf Engl 1990) (1999) 35(13):1773–82. doi: 10.1016/s0959-8049(99)00229-4
52. Berger AK, Lücke S, Abel U, Haag GM, Grüllich C, Stange A, et al. Early metabolic response in sequential fdg-Pet/Ct under cetuximab is a predictive marker for clinical response in first-line metastatic colorectal cancer patients: Results of the phase ii remotux trial. Br J Cancer (2018) 119(2):170–5. doi: 10.1038/s41416-018-0152-4
53. Dromain C, Caramella C, Dartigues P, Goere D, Ducreux M, Deschamps F. Liver, lung and peritoneal metastases in colorectal cancers: Is the patient still curable? what should the radiologist know. Diagn Intervent Imag (2014) 95(5):513–23. doi: 10.1016/j.diii.2014.03.011
54. O'Connor OJ, McDermott S, Slattery J, Sahani D, Blake MA. The use of pet-ct in the assessment of patients with colorectal carcinoma. Int J Surg Oncol (2011) 2011:846512. doi: 10.1155/2011/846512
55. Huang YY, Lee PI, Liu MC, Chen CC, Huang KC, Huang AT. A general cutoff level combined with personalized dynamic change of serum carcinoembryonic antigen can suggest timely use of fdg pet for early detection of recurrent colorectal cancer. Clin Nucl Med (2015) 40(10):e465–9. doi: 10.1097/rlu.0000000000000900
56. Sanli Y, Kuyumcu S, Ozkan ZG, Kilic L, Balik E, Turkmen C, et al. The utility of fdg-Pet/Ct as an effective tool for detecting recurrent colorectal cancer regardless of serum cea levels. Ann Nucl Med (2012) 26(7):551–8. doi: 10.1007/s12149-012-0609-0
57. Kalff V, Hicks RJ, Ware RE, Hogg A, Binns D, McKenzie AF. The clinical impact of (18)F-fdg pet in patients with suspected or confirmed recurrence of colorectal cancer: A prospective study. J Nucl Med Off Publication Soc Nucl Med (2002) 43(4):492–9.
58. Scott AM, Gunawardana DH, Kelley B, Stuckey JG, Byrne AJ, Ramshaw JE, et al. Pet changes management and improves prognostic stratification in patients with recurrent colorectal cancer: Results of a multicenter prospective study. J Nucl Med Off Publication Soc Nucl Med (2008) 49(9):1451–7. doi: 10.2967/jnumed.108.051615
59. Odalovic S, Stojiljkovic M, Sobic-Saranovic D, Pandurevic S, Brajkovic L, Milosevic I, et al. Prospective study on diagnostic and prognostic significance of postoperative fdg Pet/Ct in recurrent colorectal carcinoma patients: Comparison with mri and tumor markers. Neoplasma (2017) 64(6):954–61. doi: 10.4149/neo_2017_613
60. Caglar M, Yener C, Karabulut E. Value of ct, fdg pet-ct and serum tumor markers in staging recurrent colorectal cancer. Int J Comput Assisted Radiol Surg (2015) 10(7):993–1002. doi: 10.1007/s11548-014-1115-8
Keywords: FDG PET/CT, colorectal cancer, TNM staging, treatment monitoring, restaging
Citation: Shi Y, Wang M, Zhang J, Xiang Z, Li C, Zhang J and Ma X (2022) Tailoring the clinical management of colorectal cancer by 18F-FDG PET/CT. Front. Oncol. 12:1062704. doi: 10.3389/fonc.2022.1062704
Received: 06 October 2022; Accepted: 12 December 2022;
Published: 22 December 2022.
Edited by:
Zhendong Jin, Second Military Medical University, ChinaReviewed by:
Wendi Ma, University of Miami Health System, United StatesMuhammad Azhar Nisar, Tulane University, United States
Copyright © 2022 Shi, Wang, Zhang, Xiang, Li, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Shi, ZG9jdG9yc2hpMTk5MEBnbWFpbC5jb20=; ZmNjc2hpeUB6enUuZWR1LmNu; Jingjing Zhang, emhhbmdqaW5namluZzEwN0BzaW5hLmNvbQ==; Xing Ma, ZmNjbWF4QHp6dS5lZHUuY24=
†These authors have contributed equally to this work
 Yang Shi
Yang Shi Meiqi Wang3†
Meiqi Wang3† Xing Ma
Xing Ma