- 1Breast Surgery Division, Department of Surgery, Montefiore Medical Center, Montefiore Einstein Center for Cancer Care, Bronx, NY, United States
- 2Department of Surgery, Advocate Medical Group, Oak Lawn, IL, United States
- 3Department of Plastic Surgery, Northwell Health System, New Hyde Park, NY, United States
- 4Department of Surgery, San Martino Hospital, University of Genoa, Genova, Italy
- 5Department of Surgery, Los Angeles Center for Women’s Health, Los Angeles, CA, United States
- 6Department of Plastic Surgery, Cleveland Clinic, Cleveland, OH, United States
- 7Department of Breast Surgery, Imperial College, London, United Kingdom
- 8Department of Surgery, University of Texas Medical Branch(UTMB) Cancer Center, Galveston, TX, United States
- 9Department of Surgery, University Hospital Cleveland Medical Center, Cleveland, OH, United States
Background: Breast cancer- related lymphedema (BCRL) affects about 3 to 5 million patients worldwide, with about 20,000 per year in the United States. As breast cancer mortality is declining due to improved diagnostics and treatments, the long-term effects of treatment for BCRL need to be addressed.
Methods: The American Society of Breast Surgeons Lymphatic Surgery Working Group conducted a large review of the literature in order to develop guidelines on BCRL prevention and treatment. This was a comprehensive but not systematic review of the literature. This was inclusive of recent randomized controlled trials, meta-analyses, and reviews evaluating the prevention and treatment of BCRL. There were 25 randomized clinical trials, 13 systemic reviews and meta-analyses, and 87 observational studies included.
Results: The findings of our review are detailed in the paper, with each guideline being analyzed with the most recent data that the group found evidence of to suggest these recommendations.
Conclusions: Prevention and treatment of BCRL involve a multidisciplinary team. Early detection, before clinically apparent, is crucial to prevent irreversible lymphedema. Awareness of risk factors and appropriate practice adjustments to reduce the risk aids are crucial to decrease the progression of lymphedema. The treatment can be costly, time- consuming, and not always effective, and therefore, the overall goal should be prevention.
1. Introduction
Breast cancer mortality is decreasing due to improved diagnostics and treatments. As survival is increased, the long-term sequelae of treatment and impaired quality of life become relevant. Breast cancer- related lymphedema (BCRL) affects about 3 to 5 million patients worldwide, with about 20,000 per year in the United States. BCRL rates range from 2% to 77% (1) based on the type of local–regional and systemic therapies.
BCRL results from disruption of the lymphatic system. This prevents adequate drainage, allowing lymph fluid to accumulate in the interstitial space (2–4). BCRL has been defined as a 2- cm increase in limb circumference, a 200- mm increase in limb volume, or a 5% to 10% change in limb volume compared to the unaffected arm (4–6).
Diagnosing pre-clinical lymphedema can be challenging and requires preoperative assessment and surveillance at standardized intervals (4, 7–10). There are several methods to detect and monitor lymphedema. There are no head-to-head comparison trials comparing all techniques (11). Increasingly, data and guidelines support the use of prospective surveillance programs to detect BCRL in its subclinical phase (12–15). Ideally, screening programs should use tools that are objective and reproducible.
Several risk factors have been identified for the development of BCRL. Risk factors are subdivided into patient-specific and treatment-specific risk factors. Patient- specific factors include body mass index (BMI) at the time of diagnosis, subclinical edema, and cellulitis on the side of treatment (4). The independent treatment- related risk factors for BCRL include lymph node surgery (16–20) and regional lymph node radiation (RLNR) (21–24).
Prevention of BCRL can be treatment specific. Decreasing the amount of axillary surgery in certain circumstances, mapping out the upper extremity lymphatics during surgery, and decreasing the amount of nodal radiation in appropriate cases can aid in the prevention.
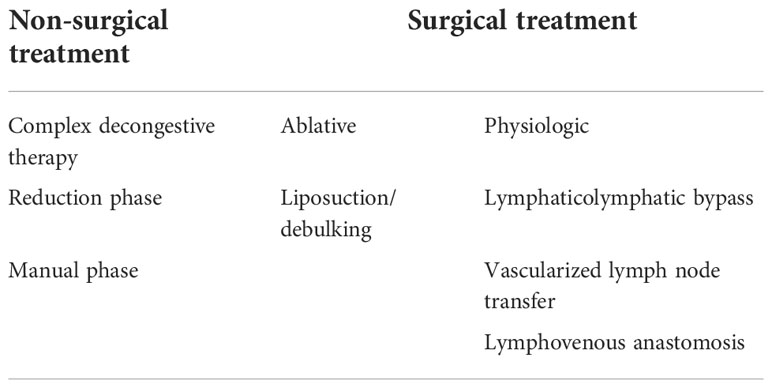
The treatment of BCRL is non- surgical management and surgical management (25). The mainstay of non-operative treatment is complex decongestive therapy (CDT). Surgical management is another option, especially in patients who have no response to non-invasive treatment. The two surgical strategies are ablative and physiologic procedures.
Emerging evidence regarding biomarkers assesses the biological effects of physical exercise on breast cancer survivors (26). This allows for a more tailored rehabilitation plan based on the patient’s characteristics. Furthermore, evidence shows that surgical prehabilitation in women with breast cancer is feasible and well- received (27).
It is imperative to identify patients at high risk for developing BCRL so they can be monitored and treated before chronic, irreversible BCRL occurs. Early detection and treatment of subclinical BCRL can prevent progression to its chronic stage, thus eliminating morbidity and the need for more intensive, costly treatments (4, 12–15).
2. Methods
The American Society of Breast Surgeons (ASBrS) built a lymphatic surgery working group to develop guidelines on BCRL. During this process, the group proposed important factors regarding assessment, surveillance, risk factors, and prevention including decreased axillary surgery, decreased radiation to the axilla, and operative techniques to assist in the prevention and treatment including non-operative and operative. This review is a reflection of this process with articles selected based on the topics proposed as guidelines. While not a systemic review, we sought to identify high- quality research to support these guidelines. This was inclusive of recent randomized controlled trials, meta-analyses, and reviews evaluating the prevention and treatment of BCRL. There were 25 randomized clinical trials, 13 systemic reviews and meta-analyses, and 87 observational studies included.
3. Results
The findings of our review are detailed in the discussion of the paper. With each guideline being analyzed with the most recent data, the group found evidence to suggest these recommendations.
4. Discussion
4.1. Pathophysiology
BCRL results from disruption of the lymphatic system. This prevents adequate drainage that allows lymph fluid to accumulate in the interstitial space (2–4). First, it is important to differentiate between lymphedema and lymphatic insufficiency. Patients with lymphatic insufficiency seen by indocyanine green (ICG) lymphangiography may not have clinically appreciable lymphedema due to compensation by remaining lymphatics. However, these patients do have impaired lymphatic function and are therefore at risk for developing clinical lymphedema.
BCRL has been defined as a 2- cm increase in limb circumference, a 200- mm increase in limb volume, or a 5% to 10% change in limb volume as compared to the unaffected arm (4–6). The four stages of lymphedema based on the International Society of Lymphology are described as follows: Stage 0 is subclinical with no visible changes. Stage 1 is mild and soft edema pits, has no dermal fibrosis, and subsides with limb elevation. Stage 2 is moderate with loss of elasticity and evolution of dermal fibrosis and no decrease in swelling with arm elevation. Stage 3 is chronic and irreversible (4).
4.2. Assessment and surveillance
Diagnosing pre-clinical lymphedema can be challenging and requires preoperative assessment and surveillance at the standardized interval (4, 7–10).
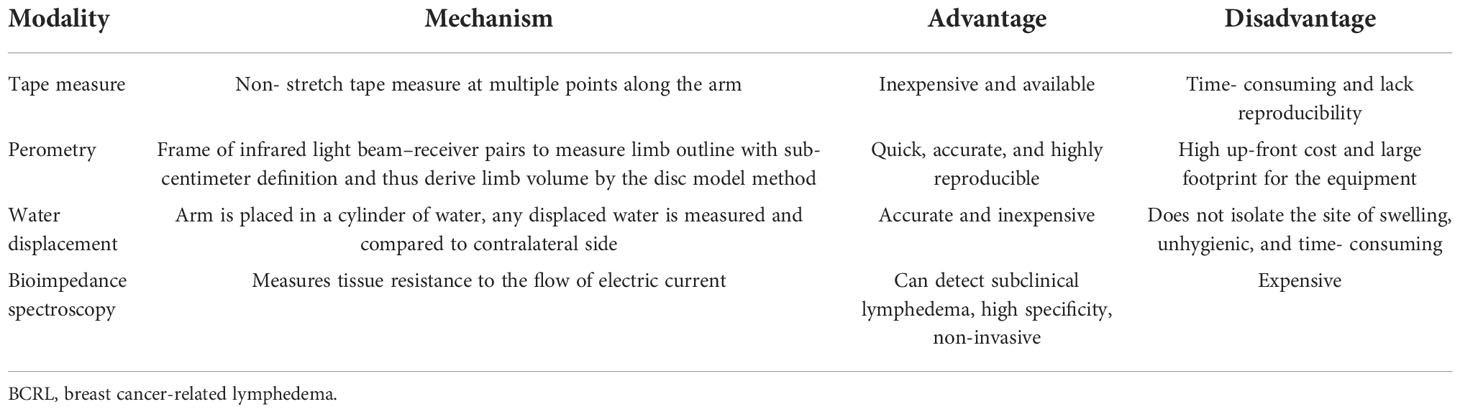
There are several methods to detect and monitor lymphedema. There are no head-to-head comparison trials comparing all techniques (11). Recently, a randomized clinical trial compared bioimpedance spectroscopy (BIS) and tape measurement for BCRL surveillance. They found that BIS provides a more precise identification of patients likely to benefit from early compression intervention (28). The advantages and disadvantages of measures of BCRL are listed in Table 1 (29).
Circumferential sequential arm measurements are an acceptable assessment method. This involves a non-stretch tape measure at multiple points along the arm (30). This is an inexpensive and easily accessible method; however, it is time- consuming and can be inconsistent with various users (31), leading to inaccurate measurements. Specificity can be as low as 73% (32). It also detects a late stage of lymphedema (4, 33, 34).
Perometry is a measurement option utilizing infrared light to measure limb volume (35). The patient places each arm into a large frame that sends infrared light beams inward while a computer calculates limb volume using a disc model. The benefits include being quick, accurate, and highly reproducible; however, it comes at a high up-front cost and a large footprint for the equipment (36). Compared to a tape measure, it has a smaller standard error with a narrower confidence interval (4, 37).
The water displacement technique is accurate and inexpensive. It is performed by placing each arm in a large cylinder of water and comparing the displaced water to the other arm. Disadvantages include that it does not isolate the site of swelling, is time- consuming and unhygienic, and requires a strict protocol (4, 31).
BIS can detect subclinical lymphedema using a non-invasive tool that measures tissue resistance to the flow of electric current to determine the quantity of extracellular fluid. A scanning device passes a small, painless electrical current through the limb and measures resistance to the current. Measurements are taken at multiple locations on the arm. The specificity is 80%–99% (12, 38–40). Advantages include fast detection and limited need for staff and space (38, 41–43). Limitations are the expense, approximately $5,000 for the equipment and $12,000 annually for data storage. The data for BIS have been encouraging. Soran et al. showed that monitoring with BIS reduces the incidence of subclinical BCRL from 36.4% to 4.4% (12). Similarly, Whitworth et al. demonstrated that 3% of patients had unresolved clinically significant BCRL when BIS was used as surveillance, which is lower than reported in contemporary studies (4, 44, 45).
Subjective measures for BCRL include patient- reported outcomes (PROs). Swelling, pain, heaviness, aching, numbness, stiffness, and impaired arm mobility are the most common PROs (46–49). Data suggest that PROs should be evaluated at intervals for 2–6 years after treatment (4, 23, 46).
Increasingly, data and guidelines support the use of prospective surveillance programs to detect BCRL in its subclinical phase (12–15). Ideally, screening programs should use tools that are objective and reproducible. An initial preoperative measurement should be obtained followed by surveillance, including regularly scheduled postoperative measurements, every 3 months for the first 2 years and then every 6 months for 3–5 years for all patients undergoing axillary surgery (4, 29).
4.3. Reduction of risk
Several risk factors have been identified for the development of BCRL. Risk factors are subdivided into patient-specific and treatment-specific risk factors. Patient- specific factors include BMI at the time of diagnosis, subclinical edema, and cellulitis on the side of treatment (4). The independent treatment- related risk factors for BCRL include lymph node surgery (16–20) and RLNR (21–24) (Table 2).
4.4. Patient- specific factors
BMI has been widely reported to be associated with BCRL (50–52). The risk of lymphatic dysfunction increases with elevated BMI and is almost universal once BMI exceeds 60. Individuals with a BMI greater than 30 kg/m2 are more likely to develop secondary upper extremity lymphedema after breast cancer treatment (52, 53). Obesity has a negative impact on lymphatic density in subcutaneous tissue, lymphatic endothelial cell proliferation, lymphatic leakiness, collecting-vessel pumping capacity, and clearance of macromolecules (54). Helyer et al. (52) compared patients with a BMI greater than 30 kg/m2 to patients with BMI less than 25 kg/m2, finding an odds ratio of 2.93 of developing BCRL in the higher BMI cohort (4).
Subclinical edema is a risk factor for BCRL. Specht et al. reported on the correlation between subclinical swelling and BCRL. They found that early on, both small and large volume increases were associated with increased BCRL (55). Studies like these emphasize the utility of early screening for limb volume increases (22, 24).
Cellulitis is a known risk factor for lymphedema. A large prospective cohort study by Ferguson et al. conducted a large prospective study proving this fact, showing a mean difference in arm volume by about 3% in those with cellulitis (18, 19, 56).
Avoidance of ipsilateral venipuncture, blood pressure readings, and air travel has been considered imperative in BCRL prevention (4). This basis is anecdotal and derived from a small number of patients (57, 58). Many studies have actually demonstrated that these practices are not inclined to greatly influence the development of lymphedema (4, 19, 22, 59). Ferguson et al. reported on over 3,000 patients who were screened prospectively for lymphedema using a perometer. At each measurement, patients reported the number of blood draws, injections, blood pressure measurements, trauma, and flights taken. There was no significant association between volume change and any of these variables among this cohort. It has been reported that flying increases the development of lymphedema due to changes in cabin air pressure. However, it is unclear if the reported lymphedema was preexisting or influenced by other factors in these studies (60). Kilbreath et al. evaluated changes in arm volume after short- and long- distance flights and found no change in arm volume as measured by BIS (61). These practices can burden patients with added anxiety and avoidance strategies (19, 22, 59).
However, while avoidance of these activities may not mitigate the development of lymphatic insufficiency, they may avoid hastening the onset or exacerbation of clinically evident lymphedema. The National Lymphedema Network position paper advises the following for risk reduction: if required to have venipuncture, ask to use a non-lymphedema limb if possible. Use an uninvolved or not at-risk extremity when taking blood pressure, and request a hand blood pressure measurement if possible. Patients can make an informed personal decision regarding compression garments during air travel; regardless, it is important to move around, exercise the at-risk body part, and maintain good hydration during air travel (62). Therefore, patients should not be overly cautioned against these things if they are necessary for medical (IV and injections) or personal (travel) reasons but should try to avoid using the affected extremity if not necessary.
4.5. Oncologic treatment- specific factors
At this time, there are inconclusive results identifying a direct correlation between taxane- based chemotherapy and BCRL. Taxane- based chemotherapy is the conventional treatment for breast cancer and can significantly improve progression-free survival and overall survival of patients (63, 64). Taxane- based chemotherapy can cause fluid retention in extremities. There has been some correlation discussed in the literature; however, it is inconsistent. Swaroop believed that taxane- based chemotherapy did not increase the risk of BCRL (65), whereas Cariati et al. showed there was a correlation (66).
4.6. De-escalating axillary surgery
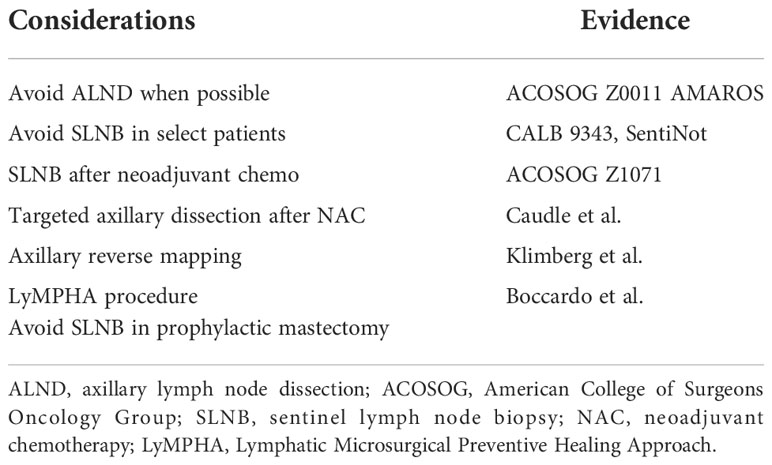
Axillary nodal status remains among the most important variables for both prognosis and adjuvant therapy when treating breast cancer (67–69). With the increased utilization of sentinel lymph node biopsy (SLNB) and data supporting the de-escalation of axillary surgery, axillary lymph node dissection (ALND) has been avoided more commonly (Table 3). The data from the American College of Surgeons Oncology Group (ACOSOG) Z0011 and AMAROS have driven this trend (70, 71). These two studies demonstrate that ALND can be avoided in certain cases. ACOSOG Z0011 randomized patients with clinically node- negative disease who underwent a lumpectomy and had two or fewer positive sentinel nodes to undergo completion axillary dissection versus no further surgery. At 10 years, the overall survival, disease- free survival, and local recurrence rates were equivalent (70). AMAROS randomized patients with T1–T2 patients undergoing lumpectomy or mastectomy, with clinically negative nodes, to receive completion dissection or axillary radiotherapy in the case of a positive sentinel node and found comparable local control, with less morbidity in the radiotherapy group (71). This is likely due to the fact that during surgery, lymph nodes are removed including the lymphatics, and this removal has a greater increase in lymphedema than leaving the anatomy intact and providing radiation.
There has not been conclusive evidence to define the number of retrieved lymph nodes to correlate with lymphedema. Vicini et al. (43) demonstrated a trend of increased lymphedema when four or more lymph nodes were retrieved; however, this was not statistically significant. Engel et al. (72) demonstrated that retrieval of 10 or more lymph nodes was significantly associated with lymphedema. However, there is no consensus regarding the number and lymphedema. In a recent study of 936 patients, there was no association between the number of nodes retrieved and lymphedema (73).
These findings have motivated the consideration of eliminating unnecessary lymph node analysis in certain circumstances. As part of Choosing Wisely Campaign, which encourages doctors and patients to question commonly used tests and treatments, the ASBrS recommended against the use of routine ALND in patients undergoing lumpectomy (74). In addition, the Choosing Wisely guidelines and the Society of Surgical Oncology recommended against routine use of SLNB in women over 70 with hormone receptor- positive HER2- negative disease. Hughes et al. showed that the risk of axillary recurrence was low among women who did not undergo axillary surgery. Furthermore, there was no difference in breast cancer- specific mortality in those who underwent axillary surgery and those without axillary surgery in women over the age of 70 (75, 76).
Patients undergoing prophylactic mastectomy, with proper imaging, such as MRI, do not need sentinel node biopsy.
To aid in this avoidance, the SentiNot study evaluated injection with superparamagnetic iron oxide nanoparticles for patients with ductal carcinoma in situ (DCIS) to minimize unnecessary SLNB. Patients who were found to have the invasive disease could go on to have SLNB even after a mastectomy, as this technique allows SLNB to be performed several weeks after injection. They found that 78.3% of patients with DCIS avoided SLNB (77).
Node- positive patients who have shown a good clinical response after neoadjuvant chemotherapy can avoid ALND in certain circumstances. This has been shown to be oncologically safe. The ACOSOG Z1071 (78) tested if SLNB could accurately assess nodal response after neoadjuvant chemotherapy (NAC) (4). They evaluated over 600 patients who were initially node- positive and received chemotherapy, followed by both SLNB and ALND. They found the false- negative rate (FNR) to be 12.6% by identifying cancer in nodes other than the sentinel node. The use of a dual tracer increased the likelihood of finding a node and lowered the FNR. In addition, the FNR improved as the number of removed SLNs increased: 31%, 21%, and 9.1% when one node, two nodes, or three nodes were removed, respectively (4). The sentinel node biopsy following neoadjuvant chemotherapy (SN FNAC) (79) and GANEA2 (80) study found similar results.
Caudle et al. (81, 82) localized a clipped node with iodine-125 radioactive seed to increase the accuracy of SLNB after neoadjuvant therapy. This technique decreased the false- negative rate, with the lowest FNR reported at 2%. Of note, 23% of clipped nodes were not the sentinel node, highlighting the importance of localization of the clipped node. Of note, these patients receive radiation therapy (XRT). This technique can also be performed using an injection of carbon particles (83), or ICG, and the use of other localization devices (84). Simons et al. reported on a prospective registry trial using magnetic seeds to locate clipped nodes and had 100% seed placement and removal (85). Miller et al. found similar success with magnetic seed for lymph node localization (86).
With reliable SLNB after NAC, ALND can be avoided in patients with a good clinical response.
4.7. Surgical prevention
4.7.1. Axillary reverse mapping
Axillary reverse mapping (ARM) is a technique used to distinguish the lymphatic drainage of the arm from that of the breast. It identifies upper extremity lymphatics coursing through the axilla to minimize unnecessary disruption of the arm lymphatics while performing lymph node surgery (87–89). ARM maps the (UE) lymphatics with blue dye while using technetium-99 in the breast, allowing for differentiation of lymphatics draining the breast (hot) and the UE (blue) (4). Variations in UE lymphatic drainage patterns exist and may coalesce or cross over with those draining the breast.
There are three factors to consider with ARM: feasibility, prevention of BCRL, and oncologic safety. A large phase 2, single- arm trial with 654 patients evaluated the feasibility of ARM. They reported the identification of blue UE lymphatics in 29% of SLNB patients and 72% of ALND patients (90–98). Boneti et al. identified blue in 39% of those patients having SLNB and in 81% of patients having ALND (92). Wijaya et al. performed a review and meta-analysis of 29 studies with 4,954 patients combined. They found the feasibility to be 37% in SLNB and 82% in ALND (99). The larger percentage of identification in ALND is due to the larger operative field and a greater area of dissection, aiding in visibility.
In terms of the prevention of BCRL, Tummel et al. prospectively reported on patients in a single study. They found that the rate of BCRL after SLNB with ARM was 0.8% and ALND with ARM was 6.5% with 26 months follow- up (98). In the meta-analysis by Wijaya et al., they found that the BCRL rate was 2% in those with SLNB and ARM and 14% with ALND and ARM (99).
In an analysis of five randomized controlled trials evaluating ALND versus ALND with ARM, all five studies favor ALND + ARM for lower BCRL rates (100).
In a small fraction of patients, the ARM (blue) node will also be the sentinel lymph node (SLN). This can call into question oncologic safety. Tummel et al. (93) found that crossover SLN (hot) and blue were seen in 3.8% of SLNB procedures. Crossover happened in 5.6% of those undergoing ALND. In the subset of patients in which an identified blue lymphatic was transected, there was an overall lymphedema rate of 18.7% when not re-anastomosed compared to 0% when re-anastomosed at 14 months of follow-up (98). Of note, the axillary recurrence rate was 0.2% in those with SLNB and 1.4% in those with ALND.
Yuan et al. described the identification and Preservation of the Arm lymphatic system (DEPART) technique, which uses ICG injected intradermally to map out the course of the arm lymphatics. They identified arm nodes using methylene blue and then injected the node with ICG to flow along several lymphatic channels. They randomized patients to ALND alone versus ALND with DEPART. They found arm nodes in 83.2% of patients and subsequent nodes in 558 (97.4%) patients. The lymphedema rate was 3.3% in the study group vs. 15.3% in the ALND alone group (101).
Future work includes the Alliance trial A221702, a randomized prospective phase III trial that studies how well axillary reverse mapping works in preventing lymphedema in patients with breast cancer undergoing axillary lymph node dissection. Patients will be randomized to ARM versus no ARM. This randomized trial will help establish the change in practice as needed based on the evidence found.
4.7.2. Lymphatic microsurgical preventive healing approach
A logical progression from ARM is the Lymphatic Microsurgical Preventive Healing Approach (LyMPHA) procedure. LyMPHA provides a preventative lymphovenous anastomosis. During an axillary node dissection, if the malignant node is draining the arm, a lympha-vascular anastomosis can be performed (4). In a randomized study in 2011, Boccardo et al. compared LyMPHA in 46 patients undergoing complete ALND. At 6 months of follow-up, they showed that one (4.34%) patient with LyMPHA developed lymphedema versus seven (30.43%) control patients (102). Four- year follow- up demonstrated minimal lymphedema, with only 3/74 (4%) having BCRL (103). Feldman et al. reported a lymphedema rate in three of 24 (12.5%) undergoing LyMPHA compared to four of eight (50%) who did not have the procedure. They concluded that LyMPHA is feasible, safe, and effective for the primary prevention of BCRL (4, 104).
Simplified Lymphatic Microsurgical Preventing Healing Approach (S-LyMPHA) is a simplified modification of LyMPHA. During the ALND, transected blue lymphatic channels are identified and invaginated into a cut end of a vein and secured in place by suture. The microscope is not needed and can be performed by the operating surgeon. Of 380 patients who underwent ALND, there was a significantly lower rate of BCRL (105).
Johnson et al. recently described the benefits of LyMPHA in those patients undergoing ALND and nodal radiation. They performed a literature review and included 19 studies. The pooled cumulative incidence of lymphedema was 33.4% in those undergoing ALND and RLNR versus 10.3% in those undergoing ALND, RLNR, and LyMPHA (p = 0.004) (106).
ARM and LyMPHA help prevent BCRL by identifying nodes draining the arm, as well as establishing a reconnection for the disrupted lymphatics. Further randomized multicenter trials would need to be conducted to establish more long-term data.
4.7.3. Minimize radiation to prevent lymphedema
For patients who undergo lumpectomy, radiation therapy is usually recommended. Traditionally whole breast radiation is recommended; however, there are data that show that in patients with early- stage, favorable breast cancer, partial breast irradiation (PBI) is safe and effective (4). PBI can be delivered as an external beam radiation therapy (EBRT), as targeted intraoperative radiation (TARGIT-IORT), as a balloon catheter, or as interstitial catheter brachytherapy. There have been two large prospective randomized trials comparing whole breast radiation to both forms of IORT, electron and 50-kV photons, showing low local recurrence rates and excellent overall survival outcomes (107, 108).
Vaidya et al. (109) randomized patients to an external beam of whole breast radiotherapy or TARGIT-IORT, a one-time dose immediately after lumpectomy in the operating room. They have found no statistical difference for local recurrence-free survival, mastectomy-free survival, distant disease-free survival, overall survival, and breast cancer mortality. They found that patients getting IORT compared to EBRT reported less general pain and arm symptoms as well as better overall functioning. The TARGIT-A trial has not yet reported specifically on breast cancer- related lymphedema. We hypothesize that since RLNR is avoided with TARGIT-IORT, the BCRL rate will likely be lower compared with that of EBRT. Interestingly, a review of the radiation fields used in the ACOSOG Z0011 trial found that in approximately half the patients, the radiation fields were within 2 cm of the humeral head, likely causing substantial incidental axillary irradiation (4, 110). Lymphedema rates from TARGIT-A would be valuable data to be able to identify ways to prevent lymphedema with more directed radiotherapy.
Obi et al. (111) reported outcomes and acute toxicities from a single institution with intraoperative radiation. They found the rate of arm lymphedema was 0.5% (1/201) with a median follow- up of 23 months.
Warren et al. (24) conducted a prospective cohort study of 1,476 breast cancer patients using volume measurements with a perometer to assess the impact of various radiation techniques on rates of BCRL. They found that the 2-year cumulative incidence of BCRL was 6.8% at 25.4 months. The cumulative incidence defined by the type of radiation was as follows: 3.0% no radiation, 3.1% breast or chest wall alone, 21.9% supraclavicular (SC), and 21.1% with SC and posterior axillary boost. Interestingly, they treated 6% of patients with PBI. In a univariate analysis for factors with risk of lymphedema, those who underwent PBI only had a hazard ratio of 0.38; however, this was not statistically significant due to the low numbers of patients. This demonstrates the potential benefit of PBI in avoiding RLNR and thus decreasing the risk of BCRL (4). This has been suggested in other studies as well (112).
The omission of radiation has come into acceptance by older patients. CALGB 9343 randomized women older than 70 years with T1N0M0 ER-positive breast cancer who received lumpectomy and tamoxifen to radiation or no radiation. Among these two cohorts, there were no differences in cancer-specific survival, overall survival, or breast preservation rates. Not surprisingly, there was a statistical difference in locoregional control 98% versus 90% in the tamoxifen- only group (75). They concluded that women over the age of 70 with favorable, small estrogen- responsive tumors could avoid radiation if they commit to taking endocrine therapy (4).
By finding ways to minimize and possibly avoid radiation, BCRL rates can be improved.
4.8. Lymphedema treatment
4.8.1. Non-surgical management of arm lymphedema
For patients in which lymphedema has been established, there are both non-operative and operative management options (Table 4). The mainstay of non-operative treatment is CDT. This is the universal first -line therapy. There are two phases consisting of reduction and maintenance. The reduction phase includes manual lymph drainage, sequential gradient pump, exercises, low-stretch bandages, and skin care. The maintenance phase consists of compression garments, exercises, and skin care.
There has not been one strategy identified as the most beneficial. A randomized control trial compared complex decongestive therapy over standard compressive therapy reporting no significant difference in the percent volume reduction of the arm at up to 52 weeks. In addition, the PROs of quality of life (QoL) did not differ (4, 113). Compression garments have been shown to prevent progression in subclinical BCRL as well as reduce arm volume (15, 114, 115). Manual lymphatic drainage is important for volume reduction. It has been shown to be beneficial to patients with mild-to- moderate BCRL in conjunction with compression bandaging (116).
Physical therapy by lymphedema- trained therapists has been shown to be more beneficial than just patient education and physical therapy alone (14, 117). Exercise with aerobic and resistance exercises does not incite or exacerbate BCRL (118–122). The data suggest that monitored management with trained physical therapists is better than self-directed treatment (123).
Early detection and intervention are integral parts of preventing the progression of BCRL. A randomized trial from Madrid enrolled 120 patients who underwent ALND and had no evidence of BCRL. Patients were randomized to a program of manual lymphatic drainage, massage, and exercise or BCRL education alone. At 1 year follow- up, 7% of patients in the intervention arm developed BCRL, compared to 25% in the education- only group (14). An additional study randomized 65 women who underwent ALND to prospective monitoring and treatment with physiotherapy versus surveillance. At 2 years, the incidence of BCRL was 11% in the early intervention group compared to 30% in surveillance alone (117). This data support the recommendation that early detection and monitored treatment are beneficial in preventing progression (4).
4.8.2. Surgical treatment of arm lymphedema
Surgical management is another option, especially in patients who have no response to non-invasive treatment. The two surgical strategies are ablative and physiologic procedures.
Ablative procedures remove edematous tissue, thus reducing limb volume. Liposuction or suction-assisted protein lipectomy (SAPL) is preferred over debulking procedures that require skin grafting. Liposuction/SAPL has been shown to have significant volume reductions (124–126). However, compression garments must be worn continuously to maintain the decreased volume (125, 126).
Physiologic procedures work by rebuilding and directing axillary lymphatic drainage. This is performed with re-anastomosis of lymphatics to distal tissue or veins.
Procedures utilizing distal tissues generally involve lymphatic grafts or vascularized flaps containing lymphatic soft tissue. The lymphaticolymphatic bypass procedure involves an anastomosis between healthy lymphatic tissue of the lower extremity to the affected arm’s axillary lymphatics and supraclavicular lymphatics. This has been shown to produce long-term patency and reduce upper extremity volume (127, 128).
Another major surgical treatment involves vascularized lymph node transfer (VLNT). A free flap of lymph node tissue is harvested with its vascular supply from a donor site and introduced to the affected limb. Blood supply is anastomosed from the lymph node flap’s blood vessels to the native axillary blood vessels (129, 130). There has been a reduction in limb volume following this procedure, circumference differentiation of 7.3%, and a reduction rate of 40.4% at a mean follow- up of 39.1 months. Furthermore, by mapping the lymphatic drainage of the donor site, selective removal of lymph nodes can be achieved to decrease the risk of lymphedema in the donor site (131).
A combination of SAL and VLNT has been shown to have a statistically significant reduction in arm circumference (132). Emerging evidence regarding microvascular breast reconstruction and lymph node transfer for post- mastectomy patients has been shown to relieve the effects of lymphedema after surgery (133).
Lastly, lymphatic venous anastomosis (LVA) uses proximal tissue instead of grafts to reestablish lymphatic drainage. Multiple lymphatic vessels are anastomosed to venules, allowing lymphatic drainage into the venous system. Chang et al. performed 89 LVAs in women with upper extremity lymphedema. Symptom improvement was reported in 96% of patients, and the mean volume reduction was 42% overall (134, 135).
There are no randomized clinical trials yet to fully compare and evaluate the benefits of these surgical procedures.
4.9. Study limitations
This study does not provide a systemic review of the literature regarding the prevention and treatment of BCRL. It provides a comprehensive review, giving evidence for guidelines being developed by the ASBrS. The bias is related to the expert panel selecting the topics to generate the guidelines.
5. Conclusion
BCRL is a challenging consequence of breast cancer treatment. Various methods of surveillance, early detection, avoidance of practices, and treatment adjustments can improve the outcomes.
Author contributions
MM: substantial contributions to acquisition of data and involved in drafting and revision of the manuscript. AG, MS and FB: substantial contributions in revising the manuscript. DH, RD, PT, SK and JD: equal contributions to acquisition of data and in the revision of the manuscript. SF: substantial contributions to data acquisition, involved in drafting and revising the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shah C, Arthur D, Riutta J, Whitworth P, Vicini FA. Breast-cancer related lymphedema: a review of procedure-specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J (2012) 18(4):357–61. doi: 10.1111/j.1524-4741.2012.01252.x
2. Chowdhry M, Rozen WM, Griffiths M. Lymphatic mapping and preoperative imaging in the management of post-mastectomy lymphoedema. Gland Surg (2016) 5(2):187–96. doi: 10.3978
3. Hespe GE NM, Mehrara BJ. Pathophysiology of lymphedema. lymphadema presentation, diagnosis and treatment. Switzerland: Springer (2015).
4. McEvoy MP, Ravetch E, Patel G, Fox J, Feldman S. Prevention of breast cancer-related lymphedema. Clin Breast Cancer (2021) 21(2):128–42. doi: 10.1016/j.clbc.2021.02.009
5. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med (2003) 349(6):546–53. doi: 10.1056/NEJMoa012782
6. McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol (2008) 26(32):5213–9. doi: 10.1200/JCO.2008.16.3725
7. Executive C. The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the international society of lymphology. Lymphology (2016) 49(4):170–84.
8. Armer JM, Hulett JM, Bernas M, Ostby P, Stewart BR, Cormier JN. Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr Breast Cancer Rep (2013) 5(2):134–44. doi: 10.1007/s12609-013-0105-0
9. Ostby PL, Armer JM, Dale PS, Van Loo MJ, Wilbanks CL, Stewart BR. Surveillance recommendations in reducing risk of and optimally managing breast cancer-related lymphedema. J Pers Med (2014) 4(3):424–47. doi: 10.3390/jpm4030424
10. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: Clinical measures of pain. Rehabil Oncol (2014) 32(1):13–21. doi: 10.1097/01893697-201432010-00004
11. . Supplement to the NLN position breast cancer screening: screening and early detection of breast cancer-related lymphedema. California: National Lymphedema Network (2012).
12. Soran A, Ozmen T, McGuire KP, Diego EJ, McAuliffe PF, Bonaventura M, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol (2014) 12(4):289–94. doi: 10.1089/lrb.2014.0035
13. Laidley A, Anglin B. The impact of l-Dex((R)) measurements in assessing breast cancer-related lymphedema as part of routine clinical practice. Front Oncol (2016) 6:192. doi: 10.3389/fonc.2016.00192
14. Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, Prieto Merino D, Mayoral del Moral O, Cerezo Tellez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ (2010) 340:b5396. doi: 10.1136
15. Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer (2008) 112(12):2809–19. doi: 10.1002/cncr.23494
16. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol (2013) 14(6):500–15. doi: 10.1016/S1470-2045(13)70076-7
17. Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol (2009) 16(7):1959–72. doi: 10.1245/s10434-009-0452-2
18. Asdourian MS, Skolny MN, Brunelle C, Seward CE, Salama L, Taghian AG. Precautions for breast cancer-related lymphoedema: risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol (2016) 17(9):e392–405. doi: 10.1016/S1470-2045(16)30204-2
19. Ferguson CM, Swaroop MN, Horick N, Skolny MN, Miller CL, Jammallo LS, et al. Impact of ipsilateral blood draws, injections, blood pressure measurements, and air travel on the risk of lymphedema for patients treated for breast cancer. J Clin Oncol (2016) 34(7):691–8. doi: 10.1200/JCO.2015.61.5948
20. McLaughlin SA, Wright MJ, Morris KT, Sampson MR, Brockway JP, Hurley KE, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol (2008) 26(32):5220–6. doi: 10.1200/JCO.2008.16.3766
21. Shaitelman SF, Chiang YJ, Griffin KD, DeSnyder SM, Smith BD, Schaverien MV, et al. Radiation therapy targets and the risk of breast cancer-related lymphedema: a systematic review and network meta-analysis. Breast Cancer Res Treat (2017) 162(2):201–15. doi: 10.1007/s10549-016-4089-0
22. Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Ung OA, Dylke ES, et al. Risk factors for lymphoedema in women with breast cancer: A large prospective cohort. Breast (2016) 28:29–36. doi: 10.1016/j.breast.2016.04.011
23. Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment–a nationwide study of prevalence and associated factors. Breast (2010) 19(6):506–15. doi: 10.1016/j.breast.2010.05.015
24. Warren LE, Miller CL, Horick N, Skolny MN, Jammallo LS, Sadek BT, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys (2014) 88(3):565–71. doi: 10.1016/j.ijrobp.2013.11.232
25. de Sire A, Losco L, Lippi L, Spadoni D, Kaciulyte J, Sert G, et al. Surgical treatment and rehabilitation strategies for upper and lower extremity lymphedema: A comprehensive review. Medicina (Kaunas) (2022) 58(7):1–14. doi: 10.3390/medicina58070954
26. Invernizzi M, Lippi L, Folli A, Turco A, Zattoni L, Maconi A, et al. Integrating molecular biomarkers in breast cancer rehabilitation. what is the current evidence? a systematic review of randomized controlled trials. Front Mol Biosci (2022) 9:930361. doi: 10.3389/fmolb.2022.930361
27. Brahmbhatt P, Sabiston CM, Lopez C, Chang E, Goodman J, Jones J, et al. Feasibility of prehabilitation prior to breast cancer surgery: A mixed-methods study. Front Oncol (2020) 10:571091. doi: 10.3389/fonc.2020.571091
28. Ridner SH, Dietrich MS, Boyages J, Koelmeyer L, Elder E, Hughes TM, et al. A comparison of bioimpedance spectroscopy or tape measure triggered compression intervention in chronic breast cancer lymphedema prevention. Lymphat Res Biol (2022). doi: 10.1089/lrb.2021.0084
29. McLaughlin SA, Staley AC, Vicini F, Thiruchelvam P, Hutchison NA, Mendez J, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema: Recommendations from a multidisciplinary expert ASBrS panel : Part 1: Definitions, assessments, education, and future directions. Ann Surg Oncol (2017) 24(10):2818–26. doi: 10.1245/s10434-017-5982-4
30. Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology (2010) 43(3):118–27.
31. McLaughlin SA, DeSnyder SM, Klimberg S, Alatriste M, Boccardo F, Smith ML, et al. Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema, recommendations from an expert panel: Part 2: Preventive and therapeutic options. Ann Surg Oncol (2017) 24(10):2827–35. doi: 10.1245/s10434-017-5964-6
32. Lopez Penha TR, Slangen JJ, Heuts EM, Voogd AC, Von Meyenfeldt MF. Prevalence of lymphoedema more than five years after breast cancer treatment. Eur J Surg Oncol (2011) 37(12):1059–63. doi: 10.1016/j.ejso.2011.09.001
34. National lymphedema network . Available at: http://www.nationallymphedemanetwork.org.
35. Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg (1996) 12(4):412–7. doi: 10.1016/S1078-5884(96)80005-0
36. Sun F, Hall A, Tighe MP, Brunelle CL, Sayegh HE, Gillespie TC, et al. Perometry versus simulated circumferential tape measurement for the detection of breast cancer-related lymphedema. Breast Cancer Res Treat (2018) 172(1):83–91. doi: 10.1007/s10549-018-4902-z
37. Sharkey AR, King SW, Kuo RY, Bickerton SB, Ramsden AJ, Furniss D. Measuring limb volume: Accuracy and reliability of tape measurement versus perometer measurement. Lymphat Res Biol (2018) 16(2):182–6. doi: 10.1089/lrb.2017.0039
38. Cornish BH, Chapman M, Hirst C, Mirolo B, Bunce IH, Ward LC, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology (2001) 34(1):2–11.
39. Bundred NJ, Stockton C, Keeley V, Riches K, Ashcroft L, Evans A, et al. Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat (2015) 151(1):121–9. doi: 10.1007/s10549-015-3357-8
40. Fu MR, Cleland CM, Guth AA, Kayal M, Haber J, Cartwright F, et al. L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. Lymphology (2013) 46(2):85–96. doi: 10.1200/jco.2013.31.26_suppl.12
41. Gaw R, Box R, Cornish B. Bioimpedance in the assessment of unilateral lymphedema of a limb: the optimal frequency. Lymphat Res Biol (2011) 9(2):93–9. doi: 10.1089/lrb.2010.0020
42. Shah C. Bioimpedance spectroscopy in the detection of breast cancer-related lymphedema: An ounce of prevention. Breast J (2019) 25(6):1323–5. doi: 10.1111/tbj.13618
43. Vicini F, Shah C, Lyden M, Whitworth P. Bioelectrical impedance for detecting and monitoring patients for the development of upper limb lymphedema in the clinic. Clin Breast Cancer (2012) 12(2):133–7. doi: 10.1016/j.clbc.2012.01.004
44. Whitworth PW, Shah C, Vicini F, Cooper A. Preventing breast cancer-related lymphedema in high-risk patients: The impact of a structured surveillance protocol using bioimpedance spectroscopy. Front Oncol (2018) 8:197. doi: 10.3389/fonc.2018.00197
45. Whitworth PW, Cooper A. Reducing chronic breast cancer-related lymphedema utilizing a program of prospective surveillance with bioimpedance spectroscopy. Breast J (2018) 24(1):62–5. doi: 10.1111/tbj.12939
46. Bulley C, Gaal S, Coutts F, Blyth C, Jack W, Chetty U, et al. Comparison of breast cancer-related lymphedema (upper limb swelling) prevalence estimated using objective and subjective criteria and relationship with quality of life. BioMed Res Int (2013) 2013:807569. doi: 10.1155/2013/807569
47. Ridner SH, Dietrich MS. Development and validation of the lymphedema symptom and intensity survey-arm. Support Care Cancer (2015) 23(10):3103–12. doi: 10.1007/s00520-015-2684-y
48. Bulley C, Coutts F, Blyth C, Jack W, Chetty U, Barber M, et al. A morbidity screening tool for identifying fatigue, pain, upper limb dysfunction and lymphedema after breast cancer treatment: a validity study. Eur J Oncol Nurs (2014) 18(2):218–27. doi: 10.1016/j.ejon.2013.10.006
49. Fu MR, Axelrod D, Cleland CM, Qiu Z, Guth AA, Kleinman R, et al. Symptom report in detecting breast cancer-related lymphedema. Breast Cancer (Dove Med Press) (2015) 7:345–52. doi: 10.2147/BCTT.S87854
50. Zou L, Liu FH, Shen PP, Hu Y, Liu XQ, Xu YY, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer (2018) 25(3):309–14. doi: 10.1007/s12282-018-0830-3
51. Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, et al. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomarkers Prev (2010) 19(11):2734–46. doi: 10.1158/1055-9965.EPI-09-1245
52. Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J (2010) 16(1):48–54. doi: 10.1111/j.1524-4741.2009.00855.x
53. Werner RS, McCormick B, Petrek J, Cox L, Cirrincione C, Gray JR, et al. Arm edema in conservatively managed breast cancer: obesity is a major predictive factor. Radiology (1991) 180(1):177–84. doi: 10.1148/radiology.180.1.2052688
54. Sudduth CL, Greene AK. Lymphedema and obesity. Cold Spring Harb Perspect Med (2022) 12(5). doi: 10.1101/cshperspect.a041176
55. Specht MC, Miller CL, Russell TA, Horick N, Skolny MN, O'Toole JA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat (2013) 140(3):485–94. doi: 10.1007/s10549-013-2655-2
56. Sayegh HE, Asdourian MS, Swaroop MN, Brunelle CL, Skolny MN, Salama L, et al. Diagnostic methods, risk factors, prevention, and management of breast cancer-related lymphedema: Past, present, and future directions. Curr Breast Cancer Rep (2017) 9(2):111–21. doi: 10.1007/s12609-017-0237-8
57. Brennan MJ. Lymphedema following the surgical treatment of breast cancer: a review of pathophysiology and treatment. J Pain Symptom Manage (1992) 7(2):110–6. doi: 10.1016/0885-3924(92)90122-X
58. Smith J. The practice of venepuncture in lymphoedema. Eur J Cancer Care (Engl) (1998) 7(2):97–8. doi: 10.1046/j.1365-2354.1998.00089.x
59. Showalter SL, Brown JC, Cheville AL, Fisher CS, Sataloff D, Schmitz KH. Lifestyle risk factors associated with arm swelling among women with breast cancer. Ann Surg Oncol (2013) 20(3):842–9. doi: 10.1245/s10434-012-2631-9
60. Graham PH. Compression prophylaxis may increase the potential for flight-associated lymphoedema after breast cancer treatment. Breast (2002) 11(1):66–71. doi: 10.1054/brst.2001.0370
61. Kilbreath SL, Ward LC, Lane K, McNeely M, Dylke ES, Refshauge KM, et al. Effect of air travel on lymphedema risk in women with history of breast cancer. Breast Cancer Res Treat (2010) 120(3):649–54. doi: 10.1007/s10549-010-0793-3
62. Network NL. Position paper - risk reduction practices. NY, NY: National Lymphedema Network (2012).
63. Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol (2003) 21(6):976–83. doi: 10.1200/JCO.2003.02.063
64. Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med (2010) 363(23):2200–10. doi: 10.1056/NEJMoa0910320
65. Swaroop MN, Ferguson CM, Horick NK, Skolny MN, Miller CL, Jammallo LS, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: results from a large prospective cohort. Breast Cancer Res Treat (2015) 151(2):393–403. doi: 10.1007/s10549-015-3408-1
66. Cariati M, Bains SK, Grootendorst MR, Suyoi A, Peters AM, Mortimer P, et al. Adjuvant taxanes and the development of breast cancer-related arm lymphoedema. Br J Surg (2015) 102(9):1071–8. doi: 10.1002/bjs.9846
67. Fisher B, Slack NH. Number of lymph nodes examined and the prognosis of breast carcinoma. Surg Gynecol Obstet (1970) 131(1):79–88.
68. Fisher B, Fisher ER, Redmond C. Ten-year results from the national surgical adjuvant breast and bowel project (NSABP) clinical trial evaluating the use of l-phenylalanine mustard (L-PAM) in the management of primary breast cancer. J Clin Oncol (1986) 4(6):929–41. doi: 10.1200/JCO.1986.4.6.929
69. Schwartz GF, Giuliano AE, Veronesi U, Consensus Conference C. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19-22, 2001, Philadelphia, Pennsylvania. Cancer (2002) 94(10):2542–51. doi: 10.1002/cncr.10539
70. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA (2017) 318(10):918–26. doi: 10.1001/jama.2017.11470
71. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol (2014) 15(12):1303–10. doi: 10.1016/S1470-2045(14)70460-7
72. Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Axilla surgery severely affects quality of life: results of a 5-year prospective study in breast cancer patients. Breast Cancer Res Treat (2003) 79(1):47–57. doi: 10.1023/A:1023330206021
73. Kim HK, Ju YW, Lee JW, Kim KE, Jung J, Kim Y, et al. Association between number of retrieved sentinel lymph nodes and breast cancer-related lymphedema. J Breast Cancer (2021) 24(1):63–74. doi: 10.4048/jbc.2021.24.e9
75. Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med (2004) 351(10):971–7. doi: 10.1056/NEJMoa040587
76. Martelli G, Miceli R, Daidone MG, Vetrella G, Cerrotta AM, Piromalli D, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol (2011) 18(1):125–33. doi: 10.1245/s10434-010-1217-7
77. Karakatsanis A, Hersi AF, Pistiolis L, Olofsson Bagge R, Lykoudis PM, Eriksson S, et al. Effect of preoperative injection of superparamagnetic iron oxide particles on rates of sentinel lymph node dissection in women undergoing surgery for ductal carcinoma in situ (SentiNot study). Br J Surg (2019) 106(6):720–8. doi: 10.1002/bjs.11110
78. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA (2013) 310(14):1455–61. doi: 10.1001/jama.2013.278932
79. Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol (2015) 33(3):258–64. doi: 10.1200/JCO.2014.55.7827
80. Classe JM, Loaec C, Gimbergues P, Alran S, de Lara CT, Dupre PF, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat (2019) 173(2):343–52. doi: 10.1007/s10549-018-5004-7
81. Caudle AS, Yang WT, Mittendorf EA, Black DM, Hwang R, Hobbs B, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg (2015) 150(2):137–43. doi: 10.1001/jamasurg.2014.1086
82. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J Clin Oncol (2016) 34(10):1072–8. doi: 10.1200/JCO.2015.64.0094
83. Allweis TM, Menes T, Rotbart N, Rapson Y, Cernik H, Bokov I, et al. Ultrasound guided tattooing of axillary lymph nodes in breast cancer patients prior to neoadjuvant therapy, and identification of tattooed nodes at the time of surgery. Eur J Surg Oncol (2020) 46(6):1041–5. doi: 10.1016/j.ejso.2019.11.501
84. Yuan L, Qi X, Zhang Y, Yang X, Zhang F, Fan L, et al. Comparison of sentinel lymph node detection performances using blue dye in conjunction with indocyanine green or radioisotope in breast cancer patients: a prospective single-center randomized study. Cancer Biol Med (2018) 15(4):452–60. doi: 10.20892
85. Simons JM, Scoggins ME, Kuerer HM, Krishnamurthy S, Yang WT, Sahin AA, et al. Prospective registry trial assessing the use of magnetic seeds to locate clipped nodes after neoadjuvant chemotherapy for breast cancer patients. Ann Surg Oncol (2021) 28(8). doi: 10.1245/s10434-020-09542-y
86. Miller ME, Patil N, Li P, Freyvogel M, Greenwalt I, Rock L, et al. Hospital system adoption of magnetic seeds for wireless breast and lymph node localization. Ann Surg Oncol (2020) 28(6). doi: 10.1245/s10434-020-09311-x
87. Klimberg VS. A new concept toward the prevention of lymphedema: axillary reverse mapping. J Surg Oncol (2008) 97(7):563–4. doi: 10.1002/jso.20905
88. Thompson M, Korourian S, Henry-Tillman R, Adkins L, Mumford S, Westbrook KC, et al. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol (2007) 14(6):1890–5. doi: 10.1245/s10434-007-9412-x
89. Boneti C, Korourian S, Bland K, Cox K, Adkins LL, Henry-Tillman RS, et al. Axillary reverse mapping: mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J Am Coll Surg (2008) 206(5):1038–42. doi: 10.1016/j.jamcollsurg.2007.12.022
90. Ahmed M, Rubio IT, Kovacs T, Klimberg VS, Douek M. Systematic review of axillary reverse mapping in breast cancer. Br J Surg (2016) 103(3):170–8. doi: 10.1002/bjs.10041
91. Beek MA, Gobardhan PD, Schoenmaeckers EJ, Klompenhouwer EG, Rutten HJ, Voogd AC, et al. Axillary reverse mapping in axillary surgery for breast cancer: an update of the current status. Breast Cancer Res Treat (2016) 158(3):421–32. doi: 10.1007/s10549-016-3920-y
92. Boneti C, Badgwell B, Robertson Y, Korourian S, Adkins L, Klimberg V, et al. Axillary reverse mapping (ARM): initial results of phase II trial in preventing lymphedema after lymphadenectomy. Minerva Ginecol (2012) 64(5):421–30.
93. Boneti C, Korourian S, Diaz Z, Santiago C, Mumford S, Adkins L, et al. Scientific impact award: Axillary reverse mapping (ARM) to identify and protect lymphatics draining the arm during axillary lymphadenectomy. Am J Surg (2009) 198(4):482–7. doi: 10.1016/j.amjsurg.2009.06.008
94. Gebruers N, Tjalma WA. Clinical feasibility of axillary reverse mapping and its influence on breast cancer related lymphedema: a systematic review. Eur J Obstet Gynecol Reprod Biol (2016) 200:117–22. doi: 10.1016/j.ejogrb.2016.03.014
95. Han C, Yang B, Zuo WS, Zheng G, Yang L, Zheng MZ. The feasibility and oncological safety of axillary reverse mapping in patients with breast cancer: A systematic review and meta-analysis of prospective studies. PloS One (2016) 11(2):e0150285. doi: 10.1371/journal.pone.0150285
96. Noguchi M, Noguchi M, Ohno Y, Morioka E, Nakano Y, Kosaka T, et al. Feasibility study of axillary reverse mapping for patients with clinically node-negative breast cancer. Eur J Surg Oncol (2016) 42(5):650–6. doi: 10.1016/j.ejso.2016.02.244
97. Ochoa D, Korourian S, Boneti C, Adkins L, Badgwell B, Klimberg VS. Axillary reverse mapping: five-year experience. Surgery (2014) 156(5):1261–8. doi: 10.1016/j.surg.2014.05.011
98. Tummel E, Ochoa D, Korourian S, Betzold R, Adkins L, McCarthy M, et al. Does axillary reverse mapping prevent lymphedema after lymphadenectomy? Ann Surg (2017) 265(5):987–92. doi: 10.1097/SLA.0000000000001778
99. Wijaya WA, Peng J, He Y, Chen J, Cen Y. Clinical application of axillary reverse mapping in patients with breast cancer: A systematic review and meta-analysis. Breast (2020) 53:189–200. doi: 10.1016/j.breast.2020.08.007
100. Guo X, Jiao D, Zhu J, Xiao H, Zhao X, Yang Y, et al. The effectiveness of axillary reverse mapping in preventing breast cancer-related lymphedema: a meta-analysis based on randomized controlled trials. Gland Surg (2021) 10(4):1447–59. doi: 10.21037/gs-21-186
101. Yuan Q, Wu G, Xiao SY, Hou J, Ren Y, Wang H, et al. Identification and preservation of arm lymphatic system in axillary dissection for breast cancer to reduce arm lymphedema events: A randomized clinical trial. Ann Surg Oncol (2019) 26(11):3446–54. doi: 10.1245/s10434-019-07569-4
102. Boccardo FM, Casabona F, Friedman D, Puglisi M, De Cian F, Ansaldi F, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol (2011) 18(9):2500–5. doi: 10.1245/s10434-011-1624-4
103. Boccardo F, Casabona F, De Cian F, Friedman D, Murelli F, Puglisi M, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery (2014) 34(6):421–4. doi: 10.1002/micr.22254
104. Feldman S, Bansil H, Ascherman J, Grant R, Borden B, Henderson P, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol (2015) 22(10):3296–301. doi: 10.1245/s10434-015-4721-y
105. Ozmen T, Lazaro M, Zhou Y, Vinyard A, Avisar E. Evaluation of simplified lymphatic microsurgical preventing healing approach (S-LYMPHA) for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection. Ann Surg (2019) 270(6):1156–60. doi: 10.1097/SLA.0000000000002827
106. Johnson AR, Kimball S, Epstein S, Recht A, Lin SJ, Lee BT, et al. Lymphedema incidence after axillary lymph node dissection: Quantifying the impact of radiation and the lymphatic microsurgical preventive healing approach. Ann Plast Surg (2019) 82(4S Suppl 3):S234–41. doi: 10.1097/SAP.0000000000001864
107. Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-a randomised trial. Lancet (2014) 383(9917):603–13. doi: 10.1016/S0140-6736(13)61950-9
108. Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol (2013) 14(13):1269–77. doi: 10.1016/S1470-2045(13)70497-2
109. Vaidya JS, Bulsara M, Baum M, Wenz F, Massarut S, Pigorsch S, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-a randomised clinical trial. BMJ (2020) 370:m2836. doi: 10.1136/bmj.m2836
110. Jagsi R, Chadha M, Moni J, Ballman K, Laurie F, Buchholz TA, et al. Radiation field design in the ACOSOG Z0011 (Alliance) trial. J Clin Oncol (2014) 32(32):3600–6. doi: 10.1200/JCO.2014.56.5838
111. Obi E, Tom MC, Manyam BV, Grobmyer SR, Al-Hilli Z, Valente S, et al. Outcomes with intraoperative radiation therapy for early-stage breast cancer. Breast J (2020) 26(3):454–7. doi: 10.1111/tbj.13574
112. Shah C, Vicini F, Beitsch P, Laidley A, Anglin B, Ridner SH, et al. The use of bioimpedance spectroscopy to monitor therapeutic intervention in patients treated for breast cancer related lymphedema. Lymphology (2013) 46(4):184–92. doi: 10.1016/j.ijrobp.2013.06.1522
113. Andersen L, Hojris I, Erlandsen M, Andersen J. Treatment of breast-cancer-related lymphedema with or without manual lymphatic drainage–a randomized study. Acta Oncol (2000) 39(3):399–405. doi: 10.1080/028418600750013186
114. McNeely ML, Magee DJ, Lees AW, Bagnall KM, Haykowsky M, Hanson J. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: a randomized controlled trial. Breast Cancer Res Treat (2004) 86(2):95–106. doi: 10.1023/B:BREA.0000032978.67677.9f
115. Dayes IS, Whelan TJ, Julian JA, Parpia S, Pritchard KI, D'Souza DP, et al. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol (2013) 31(30):3758–63. doi: 10.1200/JCO.2012.45.7192
116. Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev (2015) 2015 (5):CD003475. doi: 10.1002/14651858.CD003475.pub2
117. Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Physiotherapy after breast cancer surgery: results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res Treat (2002) 75(1):51–64. doi: 10.1023/A:1016591121762
118. Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med (2009) 361(7):664–73. doi: 10.1056/NEJMoa0810118
119. Schmitz KH, Troxel AB, Cheville A, Grant LL, Bryan CJ, Gross CR, et al. Physical activity and lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials (2009) 30(3):233–45. doi: 10.1016/j.cct.2009.01.001
120. Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. a prospective, randomized controlled trial with two years follow-up. Acta Oncol (2009) 48(8):1102–10. doi: 10.3109
121. Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol (2006) 24(18):2765–72. doi: 10.1200/JCO.2005.03.6749
122. Rogan S, Taeymans J, Luginbuehl H, Aebi M, Mahnig S, Gebruers N. Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat (2016) 159(1):1–14. doi: 10.1007/s10549-016-3919-4
123. Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol (2007) 18(4):639–46. doi: 10.1093/annonc/mdl182
124. Granzow JW, Soderberg JM, Kaji AH, Dauphine C. An effective system of surgical treatment of lymphedema. Ann Surg Oncol (2014) 21(4):1189–94. doi: 10.1245/s10434-014-3515-y
125. Greene AK, Maclellan RA. Operative treatment of lymphedema using suction-assisted lipectomy. Ann Plast Surg (2016) 77(3):337–40. doi: 10.1097/SAP.0000000000000597
126. Boyages J, Kastanias K, Koelmeyer LA, Winch CJ, Lam TC, Sherman KA, et al. Liposuction for advanced lymphedema: A multidisciplinary approach for complete reduction of arm and leg swelling. Ann Surg Oncol (2015) 22 Suppl 3:S1263–70. doi: 10.1245/s10434-015-4700-3
127. Baumeister RG, Siuda S, Bohmert H, Moser E. A microsurgical method for reconstruction of interrupted lymphatic pathways: autologous lymph-vessel transplantation for treatment of lymphedemas. Scand J Plast Reconstr Surg (1986) 20(1):141–6. doi: 10.3109
128. Baumeister RG, Mayo W, Notohamiprodjo M, Wallmichrath J, Springer S, Frick A. Microsurgical lymphatic vessel transplantation. J Reconstr Microsurg (2016) 32(1):34–41. doi: 10.1055
129. Cheng MH, Chen SC, Henry SL, Tan BK, Chia-Yu Lin M, Huang JJ. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg (2013) 131(6):1286–98. doi: 10.1097/PRS.0b013e31828bd3b3
130. Cheng MH, Huang JJ, Wu CW, Yang CY, Lin CY, Henry SL, et al. The mechanism of vascularized lymph node transfer for lymphedema: natural lymphaticovenous drainage. Plast Reconstr Surg (2014) 133(2):192e–8e. doi: 10.1097/01.prs.0000437257.78327.5b
131. Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg (2015) 135(1):277–85. doi: 10.1097/PRS.0000000000000822
132. Bolletta A, di Taranto G, Losco L, Elia R, Sert G, Ribuffo D, et al. Combined lymph node transfer and suction-assisted lipectomy in lymphedema treatment: A prospective study. Microsurgery (2022) 42(5):433–40. doi: 10.1002/micr.30855
133. Saaristo AM, Niemi TS, Viitanen TP, Tervala TV, Hartiala P, Suominen EA. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann Surg (2012) 255(3):468–73. doi: 10.1097/SLA.0b013e3182426757
134. Poumellec MA, Foissac R, Cegarra-Escolano M, Barranger E, Ihrai T. Surgical treatment of secondary lymphedema of the upper limb by stepped microsurgical lymphaticovenous anastomoses. Breast Cancer Res Treat (2017) 162(2):219–24. doi: 10.1007/s10549-017-4110-2
Keywords: breast cancer, lymphedema, breast cancer related lymphedema, axillary reverse mapping, LyMPHA, axillary surgery
Citation: McEvoy MP, Gomberawalla A, Smith M, Boccardo FM, Holmes D, Djohan R, Thiruchelvam P, Klimberg S, Dietz J and Feldman S (2022) The prevention and treatment of breast cancer- related lymphedema: A review. Front. Oncol. 12:1062472. doi: 10.3389/fonc.2022.1062472
Received: 05 October 2022; Accepted: 07 November 2022;
Published: 06 December 2022.
Edited by:
Nicola Fusco, University of Milan, ItalyReviewed by:
Marco Invernizzi, University of Eastern Piedmont, ItalyLuigi Losco, University of Salerno, Italy
Copyright © 2022 McEvoy, Gomberawalla, Smith, Boccardo, Holmes, Djohan, Thiruchelvam, Klimberg, Dietz and Feldman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maureen P. McEvoy, bW1jZXZveUBtb250ZWZpb3JlLm9yZw==
 Maureen P. McEvoy
Maureen P. McEvoy Ameer Gomberawalla2
Ameer Gomberawalla2 Mark Smith
Mark Smith Risal Djohan
Risal Djohan Paul Thiruchelvam
Paul Thiruchelvam Suzanne Klimberg
Suzanne Klimberg Jill Dietz
Jill Dietz Sheldon Feldman
Sheldon Feldman