
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 30 November 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1058329
This article is part of the Research TopicAdvances and Controversies in Skull Base Tumors: Implication for Diagnosis, Treatment and ManagementView all 19 articles
 Min Kyun Na1†
Min Kyun Na1† Bohyoung Jang2†
Bohyoung Jang2† Kyu-Sun Choi1*
Kyu-Sun Choi1* Tae Ho Lim3
Tae Ho Lim3 Wonhee Kim4
Wonhee Kim4 Youngsuk Cho4
Youngsuk Cho4 Hyun-Goo Shin3
Hyun-Goo Shin3 Chiwon Ahn5
Chiwon Ahn5 Jae Guk Kim4
Jae Guk Kim4 Juncheol Lee3
Juncheol Lee3 Sae Min Kwon6
Sae Min Kwon6 Heekyung Lee3
Heekyung Lee3Introduction: The transcranial approach (TCA) has historically been used to remove craniopharyngiomas. Although the extended endoscopic endonasal approach (EEA) to these tumors has been more commonly accepted in the recent two decades, there is debate over whether this approach leads to better outcomes. The goal of this systematic review and meta-analysis was to more comprehensively understand the benefits and limitations of these two approaches in craniopharyngioma resection based on comparative studies.
Methods: We conducted a systematic literature search in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses recommendations using MEDLINE, EMBASE, and the Cochrane Library. A total of 448 articles were screened. Data were extracted and analyzed using proportional meta-analysis. Eight comparative studies satisfied the inclusion criteria. The extent of resection, visual outcomes, and postoperative complications such as endocrine dysfunction and cerebrospinal fluid (CSF) leakage were compared.
Results and discussion: Eight studies, involving 376 patients, were included. Resection by EEA led to a greater rate of gross total resection (GTR) (odds ratio [OR], 2.42; p = 0.02; seven studies) with an incidence of 61.3% vs. 50.5% and a higher likelihood of visual improvement (OR, 3.22; p < 0.0001; six studies). However, TCA resulted in a higher likelihood of visual deterioration (OR, 3.68; p = 0.002; seven studies), and was related, though not significantly, to panhypopituitarism (OR, 1.39; p = 0.34; eight studies) and diabetes insipidus (OR, 1.14; p = 0.58; seven studies). Although TCA showed significantly lower likelihoods of CSF leakage (OR, 0.26; 95% confidence interval [CI], 0.10–0.71; p = 0.008; eight studies) compared to EEA, there was no significant difference in meningitis (OR, 0.92; 95% CI, 0.20–4.25; p = 0.91; six studies) between the two approaches. When both approaches can completely resect the tumor, EEA outperforms TCA in terms of GTR rate and visual outcomes, with favorable results in complications other than CSF leakage, such as panhypopituitarism and diabetes insipidus. Although knowledge of and competence in traditional microsurgery and endoscopic surgery are essential in surgical decision-making for craniopharyngioma treatment, when both approaches are feasible, EEA is associated with favorable surgical outcomes.
Systematic review registration: http://www.crd.york.ac.uk/PROSPERO/, identifier CRD42021234801.
Craniopharyngiomas are calcified embryonic tumors originating from the pituitary gland’s anterior lobe, from epithelial remnants of squamous cell rests of Rathke’s pouch (1, 2). Although craniopharyngiomas are histologically benign (World Health Organization grade I), their complete resection without neurological injury is challenging due to the tumor location (suprasellar, often superiorly extending into the third ventricle) and their relation to critical neurovascular structures, such as the pituitary gland, hypothalamus, infundibulum, ophthalmological systems, internal carotid artery and its branches, anterior cerebral artery-anterior communicating artery complex, basilar artery and its branches, and brain stem (3, 4). Symptoms are often related to surrounding structural compression or infiltration and may include visual disturbance, especially bitemporal hemianopsia, endocrine dysfunction, headache, and hydrocephalus (5).
The primary aims of treatment include tumor elimination; functional outcomes, such as visual, pituitary, and hypothalamic functions; favorable cognitive outcome; and quality of life. Traditionally, the transcranial approach (TCA) has been used to successfully remove these craniopharyngiomas. TCA procedures include the classical craniotomies such as pterional, orbitozygomatic, bifrontal interhemispheric, unilateral subfrontal, and supraorbital approaches (6). However, these approaches have a higher risk of visual impairment, stroke, and other neurologic complications from brain retraction and neurovascular structure manipulation (7, 8). Recently, it was shown that removing craniopharyngiomas in the retrochiasmatic space that extended superiorly into the third ventricle could be accomplished successfully using the purely extended endoscopic endonasal approach (EEA) through the transplanum transtuberculum corridor. Through a subchiasmatic corridor, this approach provides a wide surgical view and allows direct access to tumors without brain retraction and neurovascular structure manipulation (4). However, TCA has surgical advantages over EEA since it avoids damage to the nasal canal or traversing a contaminated field and provides a larger view of lateral tumor extension. The European Association of Neurosurgical Societies recommended the use of TCA for craniopharyngiomas presenting lateral extensions or that are purely intraventricular, whereas the use of EEA was recommended for purely intrasellar craniopharyngiomas (9).
The approach chosen is determined based on the tumor’s location, pathology, consistency, and proximity to the pituitary stalk and optic chiasm; involvement of the third ventricle; history of prior surgeries; and the surgeon’s inclination based on experience and feasibility. Although both approaches are expected to remain feasible options for the treatment of craniopharyngiomas based on presentation, a growing number of case-series reports have provided evidence indicating specific surgical complications that are unique to each approach. Although previous meta-analyses have analyzed each approach (10, 11), comparative studies between TCA and EEA on this topic are rare. Comparative studies provide precise clinical descriptions that can be compared in a meta-analysis. To our knowledge, no meta-analysis has dealt with cranipharyngioma outcomes. Thus, the goal of this systematic review and meta-analysis was to collect all currently accessible evidence, including solely comparative studies, and determine whether there are any differences in clinical outcomes between TCA and EEA used to treat craniopharyngiomas.
The guidelines of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) (12) and the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines were used in our investigation (13). The protocol was registered at http://www.crd.york.ac.uk/PROSPERO/ (CRD42021234801).
We developed a question that was based on population, intervention, comparison, and outcome (PICO). We conducted a critical evaluation based on the literature search and compiled the qualifying studies; their results were subsequently analyzed in a meta-analysis. The PICO question was as follows: Do patients with craniopharyngioma (population) treated surgically by EEA (intervention) compared to those treated by TCA (comparator) differ in surgical outcomes (outcome)?
Two expert reviewers (M. Na and B. Jang) conducted a literature search on July 28, 2020. The search included the MEDLINE and EMBASE databases via the Ovid interface, as well as the Cochrane library with no language restriction. Additionally, we manually searched the references of qualified studies to identify relevant research on July 30, 2022.
The following Medical Subject Headings terms were used to search all comparative studies in all logical permutations: “craniopharyngioma,” and; “transcranial,” or “craniotomy,” and “endoscopic,” or “endonasal” (Supplementary Table 1). We incorporated all publications that described prospective or retrospective cohort studies that addressed our PICO question.
All studies from the literature search were registered into a reference management software, Endnote X8 (Clarivate Analytics, Philadelphia, United States). Two reviewers (M. Na and B. Jang) separately selected the studies based on predefined selection criteria after checking the title, abstract, and type of each article.
The exclusion criteria were as follows: inappropriate control in comparative studies, < 15 patients in the study, irrelevant results, duplicate data, letters, comments, editorials, case reports, reviews, or meta-analyses, and animal studies. After comparing the title, authors, and year of publication of all studies, we eliminated duplicate articles. On disagreement between the two reviewers, a third reviewer (K. Choi) intervened, and disagreements were debated until a consensus was reached. The full text of eligible publications was obtained after ineligible abstracts were removed and subjected to rigorous screening using the same inclusion and exclusion criteria.
Two reviewers (M. Na and B. Jang) independently extracted the pertinent patient data from the included studies. Disagreements between the reviewers were discussed till a consensus was reached. The following variables were extracted: the first author’s name, country, year of publication, study design, inclusion period, number of patients, type of TCA, tumor size, preoperative symptoms, and operative outcomes (extent of resection [EOR], visual outcome, hormonal outcome, complication outcomes: endocrine disorders, cerebrospinal fluid [CSF] leak, and others).
The methodological quality of eight selected studies was examined separately by two reviewers (M. Na and B. Jang) who were blinded to the authorship and journal using the Risk of Bias Assessment Tool for Non-randomized Studies (14). Unresolved differences among reviewers were addressed through discussion or review by the third author.
For each relevant outcome, the mean difference and odds ratio (OR) were utilized as summary statistics. The results of interest were described as forest plots; the weighted mean difference or OR, 95% confidence interval (CI), and relative weightings were represented by the middle of the square, horizontal line, and relative size of the square, respectively. A random-effects model was utilized to estimate pooled outcome measures from individual data of included studies. I2 statistics were used to determine the proportion of discrepancies between studies, with values of 25%, 50%, and 75% deemed as low, moderate, and high, respectively (15).
We used Review Manager version 5.4.1 (Cochrane Collaboration, Oxford, UK) to perform the statistical analysis for both main and sub-group analyses, and a P-value < 0.05 was considered statistically significant. Additionally, meta-regression was performed to analyze the gross total resection (GTR) rate in endocrinologic complication trends using web-r (http://www.web-r.org).
Our literature search yielded eight eligible studies. On scanning the database, 447 records were found, and an additional study was identified from another source (Figure 1); 318 studies were assessed for eligibility after 130 duplicates were removed. Following this, 295 studies were eliminated after evaluating both titles and abstracts because of irrelevance to our study, leaving 23 potentially relevant studies. The full-text articles of these 23 studies were then obtained. We excluded 15 studies including systematic reviews (n = 9), those with irrelevant outcomes (n = 3), and non-comparative studies (n = 3), leaving eight studies (376 patients) to be included in the final meta-analysis (3, 4, 16–21).
The eight retrospective observational studies (OS) were published between 2008 and 2020 with an enrollment period that ranged from 2000–2019 (Table 1). All tumor resections were performed in a single institution in all studies. When reported, the overall cohort’s mean age was 43.0 years, with a higher proportion of women (52%). The TCA group included a variety of methods, including pterional, orbitozygomatic, supraorbital, subfrontal, and transcallosal approaches. Although the descriptions in each article varied, intrasellar and significant laterally extended lesions were excluded, and tumors amenable to both approaches were included (Table 1). Therefore, we excluded 25 intrasellar tumors in one study (4) to reduce differences in inclusion criteria between the studies. Finally, the total number of patients was 401 and 376 in the systematic review and meta-analysis, respectively. Among 376 patients, 212 (56%) and 164 (44%) were surgically resected using EEA and TCA, respectively (Table 2). The most frequent presenting symptoms were visual disturbance (78%), hypopituitarism (48%), and headache (33%) (Figure 2).
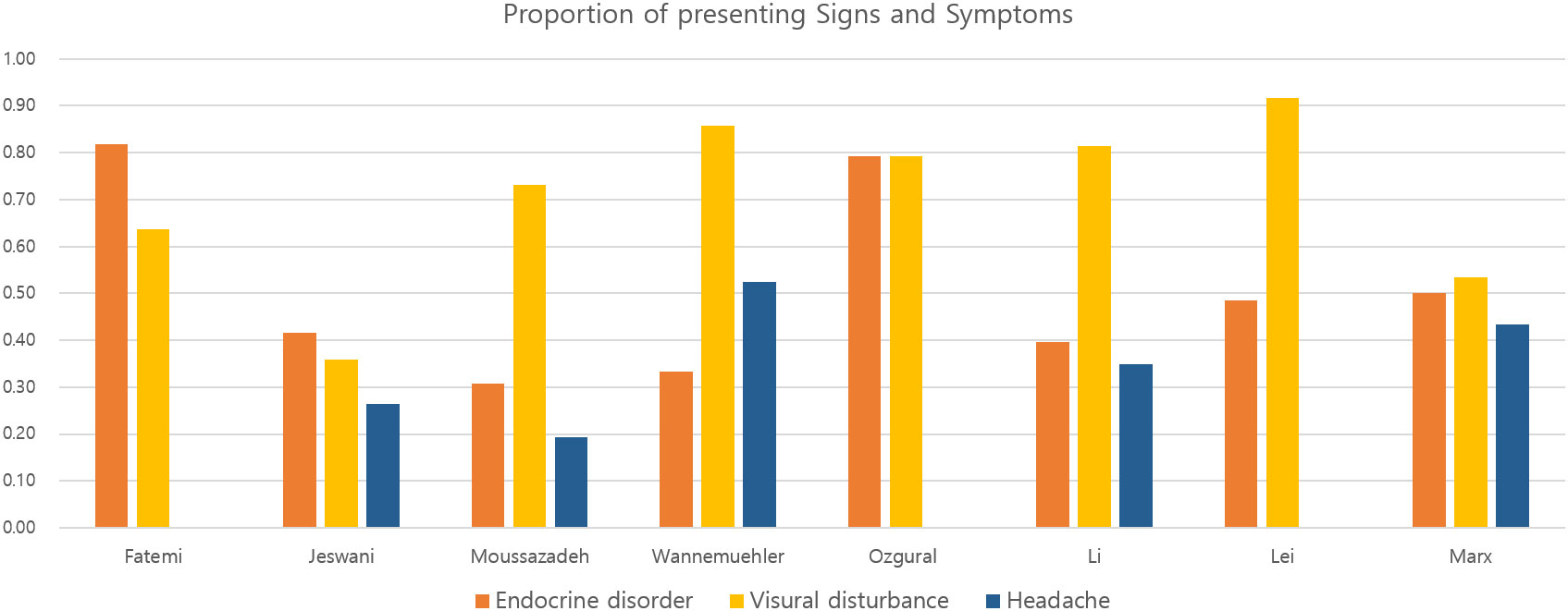
Figure 2 Bar plots representing the proportion of signs and symptoms in patients with craniopharyngioma in the included studies.
EOR was assessed in seven studies. We defined GTR as an event, and EEA demonstrated significantly higher likelihood of GTR (OR, 2.42; 95% CI, 1.13–5.17; p = 0.02; I2 = 35%). The incidence of GTR was 80/130 (61.5%) and 149/193 (77.2%) in TCA and EEA, respectively (Figure 3).
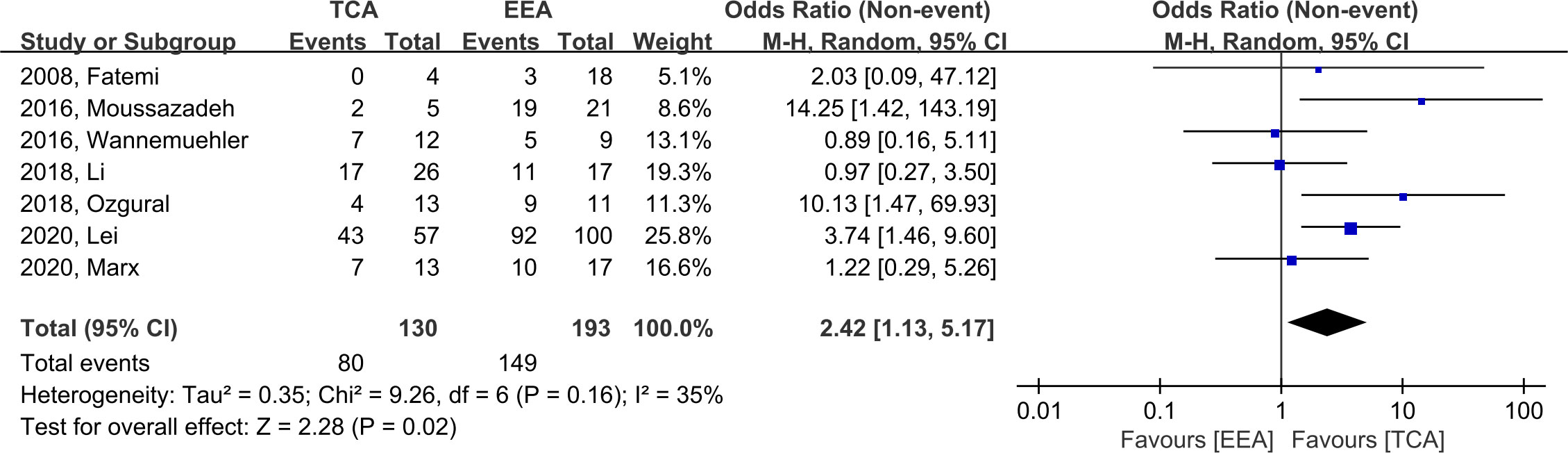
Figure 3 Forest plot comparing odd ratios (ORs) of extent of resection following TCA vs. EEA in craniopharyngioma patients. TCA, transcranial approach; EEA, endoscopic endonasal approach; CI, confidence interval; SD, standard deviation.
When compared to TCA, EEA demonstrated significantly higher likelihood of visual improvement (OR, 3.22; CI, 1.87–5.53; p < 0.0001; I2 = 0%; six studies); the incidence of visual improvement was 34/104 (32.7%) and 108/178 (60.7%) in TCA and EEA, respectively (Figure 4A). When utilizing TCA compared to EEA, there was a significantly higher likelihood of visual deterioration (OR, 3.68; CI, 1.60–8.49; p = 0.002; I2 = 0%; seven studies), with an incidence of 20/138 (14.5%) and 9/195 (4.6%) in TCA and EEA, respectively (Figure 4B).

Figure 4 Forest plots comparing odd ratios (ORs) of visual outcomes following TCA vs. EEA in craniopharyngioma patients. (A) Visual improvement and (B) Visual deterioration. TCA, transcranial approach; EEA, endoscopic endonasal approach; CI, confidence interval; SD, standard deviation.
There was no significant difference between TCA and EEA with respect to panhypopituitarism (OR, 1.37; 95% CI, 0.56–3.33; p = 0.49; I2 = 46%; six studies), with an incidence of 55/103 (53.4%) and 43/94 (45.7%), respectively. In terms of diabetes insipidus (DI), there was no significant difference between TCA and EEA (OR, 1.18; 95% CI, 0.74–1.89; p = 0.48; I2 = 0; six studies), with an incidence of 71/147 (48.3%) and 73/183 (39.9%), respectively (Figure 5).
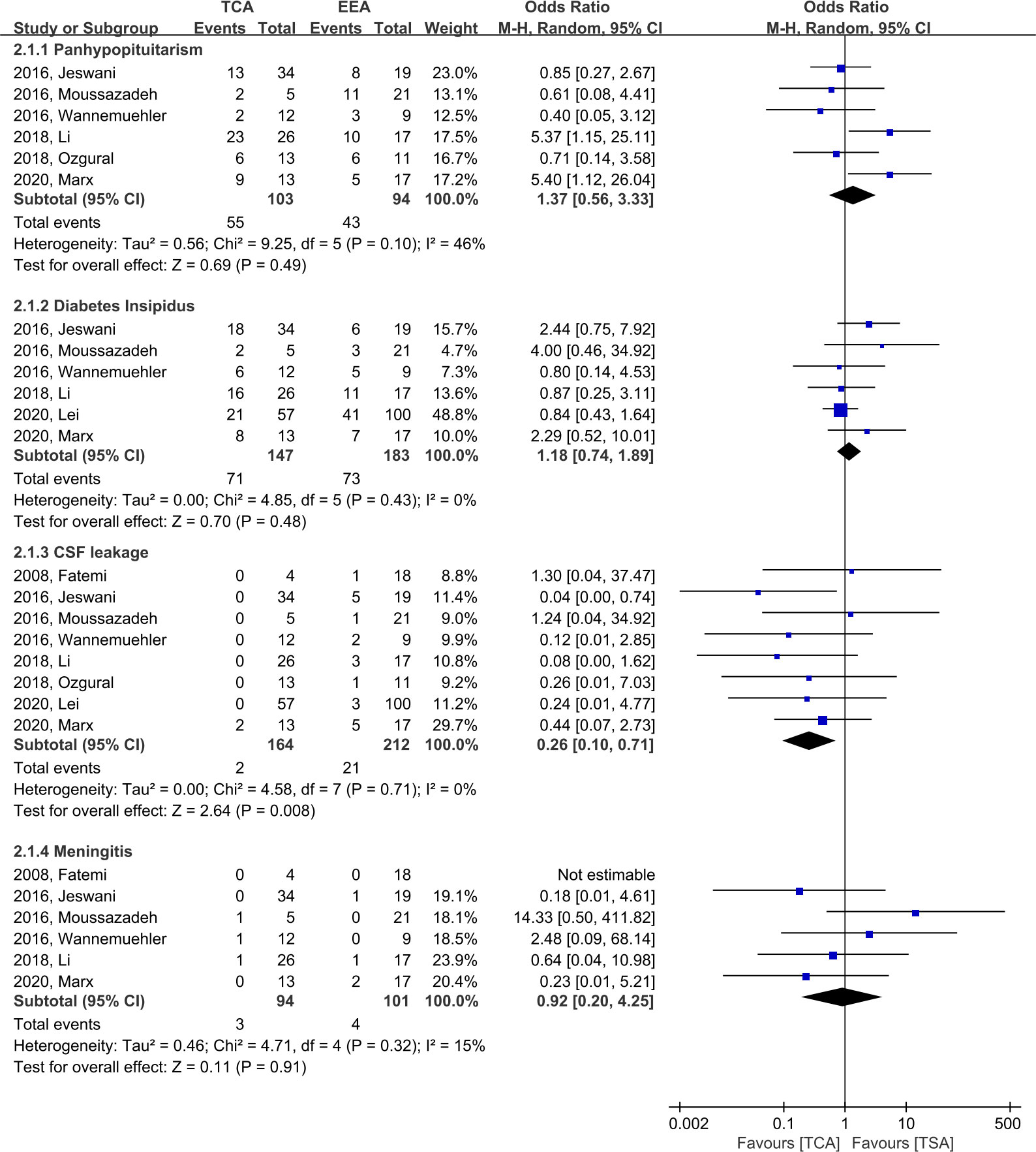
Figure 5 Forest plots comparing odd ratios (ORs) of complications following TCA vs. EEA in craniopharyngioma patients. TCA, transcranial approach; EEA, endoscopic endonasal approach; CI, confidence interval; SD, standard deviation; CSF, cerebrospinal fluid.
When compared to EEA, TCA demonstrated a significantly lower likelihood of CSF leakage (OR, 0.26; 95% CI, 0.10–0.71; p = 0.008; I2 = 0%; eight studies), with an incidence of 2/164 (1.2%) and 21/212 (9.9%) in TCA and EEA, respectively (Figure 5). When EEA patients were divided into two groups according to the start date of the study period, the CSF leakage rate was reduced from 16.7% (14/84, five studies) before 2010 to 5.5% (7/128, three studies) after 2010 (Supplementary Figure 1). In terms of meningitis, there was no significant difference between TCA and EEA (OR, 0.92; 95% CI, 0.20–4.25; p = 0.91; I2 = 15%; six studies), with an incidence of 3/94 (3.2%) and 4/101 (4.0%), respectively (Figure 5).
Compared to EEA, TCA showed higher linear association between GTR and occurrence of panhypopituitarism (slope, 0.98; p = 0.048 vs. slope, 0.4; p=0.062) (Figure 6A). There was a linear association between GTR and occurrence of DI in TCA (slope, 0.69; p = 0.059), whereas an inverse association between GTR and occurrence of DI in EEA (slope, -0.12; p = 0.734) was observed (Figure 6B)
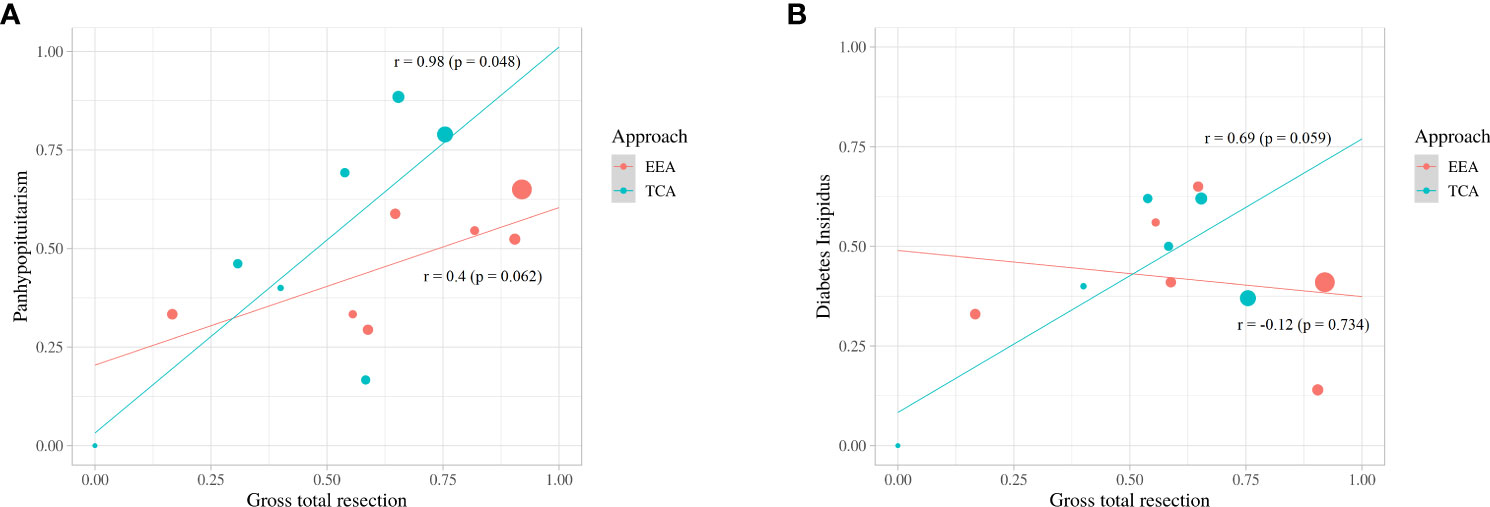
Figure 6 Scatter plot and linear-regression analysis between gross total resection and panhypopituitarism (A) and diabetes insipidus (B).
Using the Risk of Bias Assessment Tool for Non-randomized Studies system, the eight OS showed a low risk of bias in intervention measurement and blinding of outcome assessment and a high risk of bias in the selection of participants and confounding variables (Supplementary Figure S2A). Incomplete outcome data and selective outcome reporting were high risks of bias in two (20, 21) and four studies (Supplementary Figure S2B) (4, 16, 20, 21), respectively.
This systematic review and meta-analysis examined surgical outcomes of craniopharyngiomas treated with EEA and TCA. To the best of our knowledge, this is the first meta-analysis providing direct comparison based on comparative studies. We found that compared to TCA, EEA showed favorable EOR and visual outcomes. EEA also showed less likelihood of endocrine disorders, although this was not statistically significant. Compared to EEA, TCA showed less likelihood of CSF leakage, while the occurrence of meningitis was not significantly different between the approaches. These results suggest that when both approaches are feasible, EEA has favorable surgical outcomes.
Currently, the optimal management for treating patients with craniopharyngioma is controversial. GTR of craniopharyngioma was formerly considered to be challenging due to perioperative complications; therefore, sub-total resection (STR) followed by adjuvant radiotherapy was deemed as an alternative treatment option (7, 22). Although STR followed by adjuvant radiotherapy and GTR had comparable disease control rates, long-term complications after radiotherapy, such as hypopituitarism and cognitive impairment, have emerged (23). As a result, surgery remains the mainstay of treatment and offers radical resection, which maximizes the possibility of oncological cure (6, 8, 24, 25). The ability to accomplish GTR is an important factor in deciding surgical approaches. Liu et al. (6) emphasized the importance of a tailored approach for individual patients depending on the extent of the tumor and its proximity to neighboring structures in determining the optimal treatment strategy. TCA provides direct access to the parasellar compartments and is useful for tumors that extend laterally beyond the internal carotid artery bifurcation (3). However, EEA provides direct access to the anterior skull base and is appropriate for intrasellar lesions (26). In this study, EEA resulted in a significantly higher likelihood of GTR in lesions where both approaches are viable (77.2% vs 61.5%; OR, 2.24; p = 0.02). EEA allows for direct visualization and dissection of tumors and adhesive neurovascular structures, increasing the likelihood of complete resection.
Endocrine dysfunction adversely affects health-related quality of life and seems inevitable after surgery (27–29). The pituitary stalk connects the pituitary gland to the hypothalamus and maintains the hypothalamic-pituitary function (2). The relationship between the tumor and stalk is critical for postoperative endocrine dysfunction, and the Kassam classification focused on this relationship (30). Dho et al. (2) reported that trans- and retro-infundibular tumors were associated more with endocrinological deterioration than pre-infundibular tumors according to the Kassam classification, and centrally located tumors were significantly associated with endocrinological deterioration than peripherally located tumors. A previous meta-analysis found that patients treated with GTR had a considerably higher incidence of panhypopituitarism and DI than those treated with STR (27). In this study, there was no significant difference in the incidence of panhypopituitarism and DI between TCA and EEA. In a linear regression, the incidence of panhypopituitarism and DI increased significantly with increasing GTR ratio in TCA, whereas the incidence of panhypopituitarism increased slightly and DI showed a tendency to decrease with increasing GTR ratio in EEA. Compared to TCA, EEA allows for a more direct view of the skull base, allowing for early identification of the pituitary stalk and GTR while preserving the stalk. Chen et al. (31) reported that when craniopharyngiomas were resected via EEA, stalk preservation significantly lowered endocrine dysfunction without decreasing the rate of GTR and without increasing the rate of tumor recurrence.
We found that EEA resulted in a significantly higher likelihood of visual improvement when compared to TCA (60.7% vs. 32.7%, p < 0.0001), whereas TCA resulted in a significantly higher likelihood of visual deterioration when compared to EEA (14.5% vs. 4.6%, p = 0.002), and the results were comparable to those reported in a previous meta-analysis (11). These results support the evidence that EEA has an advantage over TCA by increasing visual improvement but reducing visual deterioration. Stefko et al. also demonstrated that EEA improves the visual field as well as visual accuracy (32). This is because EEA allows for early decompression of the optic apparatus without retraction and superior visualization of superior hypophyseal arteries originating from the internal carotid artery.
CSF leakage was shown to be statistically more prevalent in EEA compared to TCA (9.9% vs. 1.2%, p = 0.008). When EEA was originally introduced, the increased possibility of postoperative CSF leakage was a major complication. To access the tumors, EEA penetrates through the nasal cavity and deconstructs the anterior skull base due to the pathway of the approach. However, with the introduction of skull base reconstruction techniques using a pedicled vascularized nasoseptal flap, first introduced in 2006, this risk has been considerably decreased to approximately 5% (33, 34). In our study, it was confirmed that the CSF leakage rate was as low as 5.5% in the studies with a study period after 2010 (33, 34). The development of multi-layer skull base reconstruction techniques, including gasket-seal, artificial collagen dura mater, and artificial bone substitute, and increased surgeon experience are expected to further reduce the rate of CSF leakage.
This meta-analysis adhered strictly to its selection criteria and the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. This study has several strengths. First, although direct comparative studies of craniopharyngioma resection using TCA vs. EEA are uncommon, we only incorporated this type of research to avoid intra-study variability which affects indirect comparisons, improve the validity of the results, and provide summary statistics. Second, we could reduce selection bias because most of the studies attempted to include tumors that were amenable to both approaches. Lei et al. (4) reported four types based on the location of the tumors, and we excluded the intrasellar type to avoid violating the inclusion criteria of other studies.
However, our study has some limitations. First, all included studies were retrospective in nature. Second, two of the eight studies reported incomplete outcome data and had selective outcome reporting, such as tumor size, pathology, and adjuvant radiotherapy. (Supplementary Figure 2B). However, missing data were not analyzed in this study and did not significantly impede the conclusions. Third, we were unable to analyze other complications such as hydrocephalus, nerve injury, cerebral infarction, cognitive dysfunction, and hemorrhage, as only a few studies have reported these parameters for their patients. Therefore, it is important to carefully interpret the results of this study, and a further well-designed study is warranted.
We found that when both approaches can completely resect the tumor, EEA outperforms TCA in terms of GTR rate and visual outcomes, as well as favorable results in terms of complications other than CSF leakage, such as panhypopituitarism and DI, considering the meta-regression results. Although knowledge of and competence in traditional microsurgery and endoscopic surgery are essential in surgical decision-making for craniopharyngioma treatment, when both approaches are viable, EEA is associated with favorable surgical outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
K-SC contributed to the conceptualization, supervision, and project administration. MN, BJ, TL, WK, and YC contributed to the methodology, formal analysis, data curation, and writing the original draft. H-GS, CA, JK, JL, SK, and HL contributed to the validation, writing, review, and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (NRF-2019M3E5D1A01069356) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2022R1A5A1022977) to K-SC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1058329/full#supplementary-material
CSF, Cerebrospinal fluid; DI, Diabetes insipidus; EEA, Endoscopic endonasal approach; EOR, Extent of resection; GTR, Gross total resection; OR, Odds ratio; OS, Observational studies; PICO, Population, intervention, comparison, and outcome; RE, Random-effects; STR, Sub-total resection; TCA, Transcranial approach.
1. Karavitaki N, Cudlip S, Adams CB, Wass JA. Craniopharyngiomas. Endocr Rev (2006) 27:371–97. doi: 10.1210/er.2006-0002
2. Dho YS, Kim YH, Se YB, Han DH, Kim JH, Park CK, et al. Endoscopic endonasal approach for craniopharyngioma: the importance of the relationship between pituitary stalk and tumor. J Neurosurg (2018) 129:611–9. doi: 10.3171/2017.4.JNS162143
3. Moussazadeh N, Prabhu V, Bander ED, Cusic RC, Tsiouris AJ, Anand VK, et al. Endoscopic endonasal versus open transcranial resection of craniopharyngiomas: a case-matched single-institution analysis. Neurosurg Focus (2016) 41:E7. doi: 10.3171/2016.9.FOCUS16299
4. Lei C, Chuzhong L, Chunhui L, Peng Z, Jiwei B, Xinsheng W, et al. Approach selection and outcomes of craniopharyngioma resection: a single-institute study. Neurosurg Rev (2021) 44:1737–46. doi: 10.1007/s10143-020-01370-8
5. Ordóñez-Rubiano EG, Forbes JA, Morgenstern PF, Arko L, Dobri GA, Greenfield JP, et al. Preserve or sacrifice the stalk? endocrinological outcomes, extent of resection, and recurrence rates following endoscopic endonasal resection of craniopharyngiomas. J Neurol Surg (2018) 131:1–9. doi: 10.3171/2018.6.JNS18901
6. Liu JK, Sevak IA, Carmel PW, Eloy JA. Microscopic versus endoscopic approaches for craniopharyngiomas: choosing the optimal surgical corridor for maximizing extent of resection and complication avoidance using a personalized, tailored approach. Neurosurg Focus (2016) 41:E5. doi: 10.3171/2016.9.FOCUS16284
7. Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Strojan P, Suárez C, et al. Craniopharyngioma: A pathologic, clinical, and surgical review. Head Neck (2012) 34:1036–44. doi: 10.1002/hed.21771
8. Yaşargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. approaches and long-term results in 144 patients. J Neurosurg (1990) 73:3–11. doi: 10.3171/jns.1990.73.1.0003
9. Cossu G, Jouanneau E, Cavallo LM, Elbabaa SK, Giammattei L, Starnoni D, et al. Surgical management of craniopharyngiomas in adult patients: A systematic review and consensus statement on behalf of the EANS skull base section. Acta Neurochir (2020) 162:1159–77. doi: 10.1007/s00701-020-04265-1
10. Qiao N. Endocrine outcomes of endoscopic versus transcranial resection of craniopharyngiomas: A system review and meta-analysis. Clin Neurol Neurosurg (2018) 169:107–15. doi: 10.1016/j.clineuro.2018.04.009
11. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg (2012) 77:329–41. doi: 10.1016/j.wneu.2011.07.011
12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in EpidemiologyA proposal for reporting. JAMA (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
14. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol (2013) 66:408–14. doi: 10.1016/j.jclinepi.2012.09.016
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
16. Ozgural OMD, Kahilogullari GMDP, Dogan I, Al-Beyati ESM, Bozkurt M, Tetik B. Single-center surgical experience of the treatment of craniopharyngiomas with emphasis on the operative approach: endoscopic endonasal and open microscopic transcranial approaches. J Craniofac Surg (2018) 29:e572–8. doi: 10.1097/SCS.0000000000004592
17. Wannemuehler TJ, Rubel KE, Hendricks BK, Ting JY, Payner TD, Shah MV, et al. Outcomes in transcranial microsurgery versus extended endoscopic endonasal approach for primary resection of adult craniopharyngiomas. Neurosurg Focus (2016) 41:E6. doi: 10.3171/2016.9.FOCUS16314
18. Jeswani S, Nuño M, Wu A, Bonert V, Carmichael JD, Black KL, et al. Comparative analysis of outcomes following craniotomy and expanded endoscopic endonasal transsphenoidal resection of craniopharyngioma and related tumors: A single-institution study. J Neurosurg (2016) 124:627–38. doi: 10.3171/2015.3.JNS142254
19. Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Oper Neurosurg (2009) 64:269–84; discussion 284-6. doi: 10.1227/01.NEU.0000327857.22221.53
20. Li X, Wu W, Miao Q, He M, Zhang S, Zhang Z, et al. Endocrine and metabolic outcomes after transcranial and endoscopic endonasal approaches for primary resection of craniopharyngiomas. World Neurosurg (2019) 121:e8–e14. doi: 10.1016/j.wneu.2018.08.092
21. Marx S, Tsavdaridou I, Paul S, Steveling A, Schirmer C, Eördögh M, et al. Quality of life and olfactory function after suprasellar craniopharyngioma surgery-a single-center experience comparing transcranial and endoscopic endonasal approaches. Neurosurg Rev (2021) 44:1569–82. doi: 10.1007/s10143-020-01343-x
22. Sughrue ME, Yang I, Kane AJ, Fang S, Clark AJ, Aranda D, et al. Endocrinologic, neurologic, and visual morbidity after treatment for craniopharyngioma. J Neurooncol (2011) 101:463–76. doi: 10.1007/s11060-010-0265-y
23. Kiehna EN, Merchant TE. Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus (2010) 28:E10. doi: 10.3171/2010.1.FOCUS09297
24. Okada T, Fujitsu K, Ichikawa T, Miyahara K, Tanino S, Uriu Y, et al. Radical resection of craniopharyngioma: Discussions based on long-term clinical course and histopathology of the dissection plane. Asian J Neurosurg (2018) 13:640–6. doi: 10.4103/ajns.AJNS_258_16
25. Elliott RE, Hsieh K, Hochm T, Belitskaya-Levy I, Wisoff J, Wisoff JH. Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J Neurosurg Pediatr (2010) 5:30–48. doi: 10.3171/2009.7.PEDS09215
26. Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH. The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg (2008) 108:1043–7. doi: 10.3171/JNS/2008/108/5/1043
27. Akinduro OO, Izzo A, Lu VM, Ricciardi L, Trifiletti D, Peterson JL, et al. Endocrine and visual outcomes following gross total resection and subtotal resection of adult craniopharyngioma: Systematic review and meta-analysis. World Neurosurg (2019) 127:e656–68. doi: 10.1016/j.wneu.2019.03.239
28. Crespo I, Santos A, Webb SM. Quality of life in patients with hypopituitarism. Curr Opin Endocrinol Diabetes Obes (2015) 22:306–12. doi: 10.1097/MED.0000000000000169
29. Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, et al. The endoscopic endonasal approach for the management of craniopharyngiomas: A series of 103 patients. J Neurosurg (2014) 121:100–13. doi: 10.3171/2014.3.JNS131521
30. Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: A new classification based on the infundibulum. J Neurosurg (2008) 108:715–28. doi: 10.3171/JNS/2008/108/4/0715
31. Chen Z, Ma Z, He W, Shou X, Ye Z, Zhang Y, et al. Impact of pituitary stalk preservation on tumor recurrence/progression and surgically induced endocrinopathy after endoscopic endonasal resection of suprasellar craniopharyngiomas. Front Neurol (2021) 12:753944. doi: 10.3389/fneur.2021.753944
32. Stefko ST, Snyderman C, Fernandez-Miranda J, Tyler-Kabara E, Wang E, Bodily L, et al. Visual outcomes after endoscopic endonasal approach for craniopharyngioma: the Pittsburgh experience. J Neurol Surg B Skull Base (2016) 77:326–32. doi: 10.1055/s-0036-1571333
33. Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery (2008) 63:ONS44–52; discussion ONS52-3. doi: 10.1227/01.neu.0000297074.13423.f5
Keywords: craniopharyngioma, endoscopic endonasal approach, transcranial approach, metaanalysis, systematic review
Citation: Na MK, Jang B, Choi K-S, Lim TH, Kim W, Cho Y, Shin H-G, Ahn C, Kim JG, Lee J, Kwon SM and Lee H (2022) Craniopharyngioma resection by endoscopic endonasal approach versus transcranial approach: A systematic review and meta-analysis of comparative studies. Front. Oncol. 12:1058329. doi: 10.3389/fonc.2022.1058329
Received: 30 September 2022; Accepted: 02 November 2022;
Published: 30 November 2022.
Edited by:
Arianna Rustici, University of Bologna, ItalyReviewed by:
Alessandro Carretta, University of Bologna, ItalyCopyright © 2022 Na, Jang, Choi, Lim, Kim, Cho, Shin, Ahn, Kim, Lee, Kwon and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyu-Sun Choi, dmVydGV4LTA5QGhhbm1haWwubmV0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.