
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 02 November 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1057631
This article is part of the Research TopicThe Role of Imaging in Gynecological MalignanciesView all 20 articles
 Stefania Rizzo1,2*
Stefania Rizzo1,2* Giorgio Raia1
Giorgio Raia1 Maria Del Grande3
Maria Del Grande3 Maria Luisa Gasparri4
Maria Luisa Gasparri4 Ilaria Colombo3
Ilaria Colombo3 Lucia Manganaro5
Lucia Manganaro5 Andrea Papadia2,4
Andrea Papadia2,4 Filippo Del Grande1,2
Filippo Del Grande1,2Objectives: The main objective of this systematic review was to examine the literature evaluating association of image-based body composition with chemotherapy-related toxicity in ovarian cancer patients. A secondary objective was to evaluate the different definitions of sarcopenia across studies.
Methods: This systematic review was conducted according to the PRISMA-DTA statement and the protocol was registered on Prospero. A comprehensive literature search of 3 electronic databases was performed by two authors. For each eligible article, information was collected concerning the clinical setting; basic study data; population characteristics; technical aspects; body composition features; chemotherapy drugs administered; association of body composition values and toxicities. The overall quality of the included studies was critically evaluated.
Results: After the initial retrieval of 812 articles, the systematic review included 6 articles (5/6 studies were retrospective; one was prospective). The number of patients ranged between 69 and 239; mean/median age ranged between 55 and 65 years; the percentage of sarcopenic patients ranged between 25% and 54%. The cut-off values to define sarcopenia and the vertebral levels for evaluation of body composition were different. Five studies included chemotherapy based on carboplatin and paclitaxel, 1 included chemotherapy based on pegylated liposomal doxorubicin. Among the studies including carboplatin and paclitaxel, 3/5 demonstrated an association with toxicity, whereas 2/5 did not. Altogether, 4/6 papers demonstrated an association between the body composition values and the development of chemotherapy-related toxicities.
Conclusions: There is a wide variability of results about the association of body composition and chemotherapy-related toxicity in ovarian cancer patients. Therefore further studies, possibly including a comprehensive assessment of body compartments and where the definition of body composition cut-offs is constant, are warranted to better understand this association.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022337753, identifier (CRD42022337753).
Ovarian cancer (OC) is the second most frequent cancer among gynecological malignancies, with 19.880 estimated new cases in the US in 2022, and the most lethal, with 12.810 estimated deaths (1). The current standard treatment for OC is primary cytoreductive surgery with complete resection of all macroscopic disease, followed by adjuvant platinum-based chemotherapy with or without the antiangiogenic agent bevacizumab (2, 3). When the patient is considered not operable or the disease is deemed not completely resectable, interval debulking surgery after neoadjuvant chemotherapy (NACT) is usually considered (4). In stage III-IV high grade epithelial ovarian cancer, maintenance treatment with poly-ADP-ribose inhibitors (PARPi) has been also incorporate in first line (5–7).
In both scenarios (primary surgery followed by adjuvant chemotherapy, neoadjuvant chemotherapy followed by interval debulking surgery), chemotherapy is dosed aiming at a balance between optimal efficacy and acceptable toxicity. Indeed, if severe toxicity occurs during chemotherapy, the standard chemotherapeutic regimen might not be administered or the dose and schedule adjusted and this might potentially lead to suboptimal treatment and decreased survival. Factors potentially predisposing to toxicity are age, previous chemotherapy, genetic characteristics, including toxicity-related polymorphisms or BRCA mutational status (8, 9). Many authors have hypothesized that body composition, indicating the amount and distribution of muscle and fat compartments, is one of the factors that may predict interpatient variation in toxicity profiles, accounting for different metabolism of chemotherapeutic drugs (10–13). In fact, there is substantial evidence of the variability in body composition in cancer populations (14–16), as well as emerging evidence suggesting that the size of body composition compartments relate to prognosis in many cancer subtypes, including ovarian (17), lung (18), bladder (19) and pancreatic malignancies (20). As demonstrated by some authors, sarcopenic patients may be prone to get higher doses of chemotherapy agents for a rather small amount of muscle mass and they may therefore encounter higher toxicity (21, 22).
Since cancer patients routinely performs imaging examinations during their clinical management (23–25), imaging-based assessment of body composition might be added to the reading of imaging examinations (26, 27), so offering opportunistic clinical information that currently go unused. For instance, from Computed Tomography (CT) images it is possible to extract the areas of muscles at a pre-defined level, usually referred to as skeletal muscle area (SMA); psoas index (PI), indicating only the area of the psoas muscle; the area of visceral adipose tissue (VAT), indicating the fat within the abdomen outside the solid organs; the area of subcutaneous adipose tissue (SAT); the density of the skeletal muscle, as indirect sign of its adipose infiltration (SMD). Despite different definitions and a wide variability of cut-off values for the definition of sarcopenia, this is a condition that can be found in patients with OC and, although many studies have assessed its association with survival, only few studies have assessed the association with chemotherapy-related toxicity.
Therefore, the main objective of this systematic review was to collect and examine all the available literature evaluating association of image-based body composition with chemotherapy-related toxicity in patients with OC. A secondary objective was to evaluate the different definitions of sarcopenia across studies.
This systematic review was conducted according to the PRISMA-DTA (Preferred Reporting Items for Systematic Reviews and Meta-analysis for Diagnostic Test Accuracy) statement (28). The review protocol was registered on Prospero as CRD42022337753.
Two authors (SR and GR) performed a comprehensive literature search of the electronic databases PubMed, Cochrane and Web of Science to find primary publications evaluating association between body composition measures and chemotherapy-related toxicities in OC. No beginning date limit or language restrictions were used; the literature search was last updated on Aug 17th 2022; and the search was expanded by also screening the references of the retrieved articles for additional potentially eligible studies. The search terms consisted of ((ovarian cancer) OR (ovarian carcinoma)) AND ((sarcopenia) OR (body composition) OR (muscle) OR (fat) OR (adipose tissue)) AND ((complication) OR (complications) OR (chemotherapy-related) OR (adjuvant) OR (neo-adjuvant) OR (toxicity) OR (chemotoxicity) OR (chemo-toxicity)). Articles in which body composition assessment was based on CT were obtained in full for further independent evaluation by two authors (SR and GR). There was no exclusion for any type of toxicity and neither for the type or line of chemotherapy. Studies were excluded if they were case reports, conference abstracts, reviews or short communications because they do not provide sufficient information to assess the methodological quality. Uncertainties were resolved in consensus.
For each eligible article, information was collected by 3 authors (SR, GR, MDG) concerning the clinical setting (neo-adjuvant, adjuvant, further lines); basic study data (year of publication, country of origin, study design); population characteristics (number of patients, age, BMI, percentage of sarcopenic patients, cut-off values for sarcopenia used); technical aspects (axial level for evaluation of body composition); body composition features evaluated (SMA, SMI, VAT, SAT, SMD, PI, lean body mass (LBM), fat mass (FM)); chemotherapy drugs administered; association of body composition values and toxicities.
The overall quality of the included studies was critically evaluated based on the revised “Quality Assessment of Diagnostic Accuracy Studies” tool (QUADAS-2) (29). This tool comprises four domains for evaluation of risk of bias (patient selection, index test, reference standard, and flow and timing) and three domains for applicability concerns (patient selection, index test, reference standard). Each domain was assessed and graphs were constructed appropriately.
The initial search yielded 812 articles, all in English. According to inclusion and exclusion criteria, 6 full-text articles were included in this systematic review (17, 30–34). Details about the literature search results are reported in Figure 1.
Given the small number of papers included, the clear heterogeneity of the methods and, as a consequence, of the results, it was not possible to perform a meta-analysis for pooled data.
As shown in Table 1, among the 6 studies included, three were from the US (30, 32, 34); the other were from different countries (17, 31, 33). 5/6 studies were retrospective (17, 30–33); one was prospective (from a phase III clinical trial) (34). The number of patients included ranged between 69 (17) and 239 (31); mean/median age ranged between 55 (32, 34) and 65 years (17); the percentage of sarcopenic patients ranged between 25% (17) and 54% (32). The BMI ranged between 24.9 (17) and 28 (32). The cut-off values to define sarcopenia in different studies and the percentage of sarcopenic patients are summarized in Table 1.
As shown in Table 2, 4/6 articles evaluated the body composition values at the level of the 3rd lumbar vertebra (L3) (17, 30, 31, 34); 1/6 at the level of the 4th lumbar vertebra (L4) (32); 1/6 at the level of the 5th lumbar vertebra (L5) (33). The main body composition parameters evaluated were: SMI (derived from SMA) in 3/6 studies (17, 30, 31); psoas index (PI) in 2/6 studies (32, 33); SAT in 3/6 studies (17, 31, 32); SMD in 3/6 (17, 31, 34). Five studies included chemotherapy based on carboplatin and paclitaxel (17, 30– 33), 1 included chemotherapy based on pegylated liposomal doxorubicina (34).
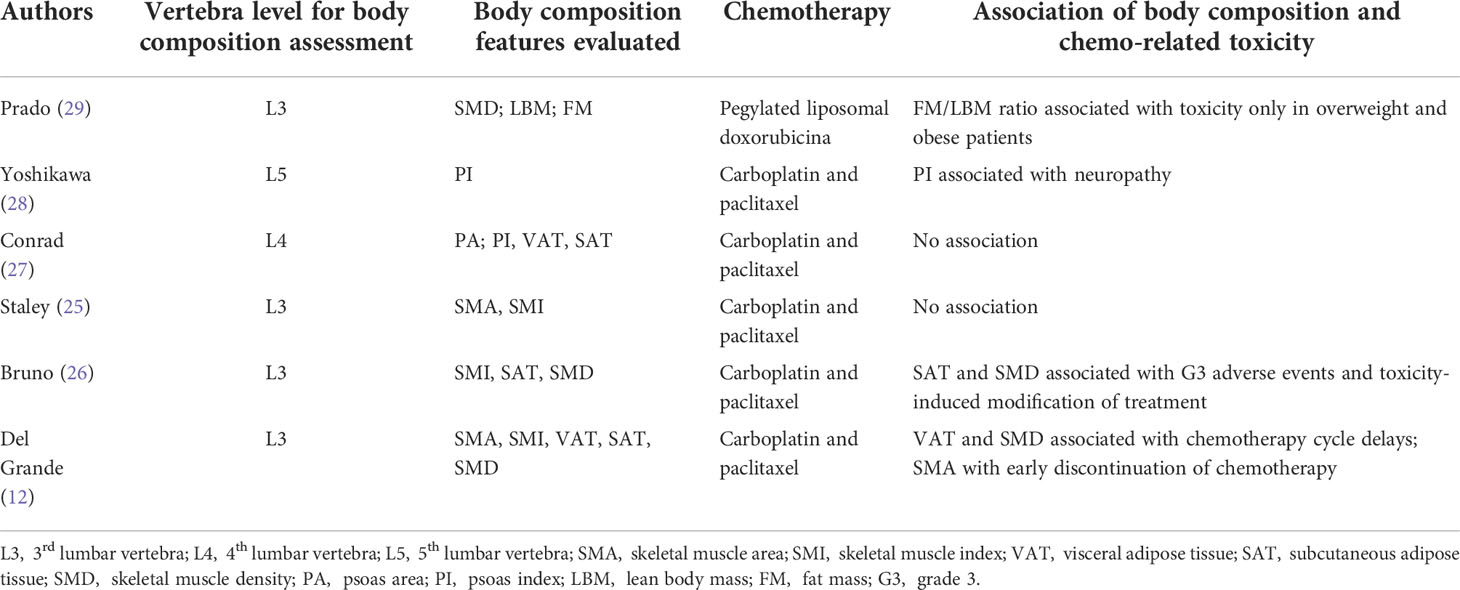
Table 2 Body composition evaluation details; chemotherapy administered and association of body composition to chemo-related toxicity (if any).
Two studies (12, 28) included both the neo-adjuvant and adjuvant settings; one declared only the first line setting (25); one included only patients treated with a further line treatment (29). Among the studies including carboplatin and paclitaxel, 3/5 demonstrated an association with toxicity (17, 31, 33), whereas 2/5 did not (30, 31). Altogether, 4/6 papers demonstrated an association between the body composition values and the development of chemotherapy-related toxicities (17, 31, 33, 35), with one showing association of VAT and SMD with chemotherapy cycle delays as well as of SMA and early discontinuation of chemotherapy (17); one showing an association of SAT and SMD with G3 adverse events and toxicity-induced modification of treatment (31); one showing association of the psoas index with neuropathy (33); one showing association of the FM/LBM ratio with toxicity only in overweight and obese patients (35).
The overall quality assessment of the studies is reported in Figure 2.
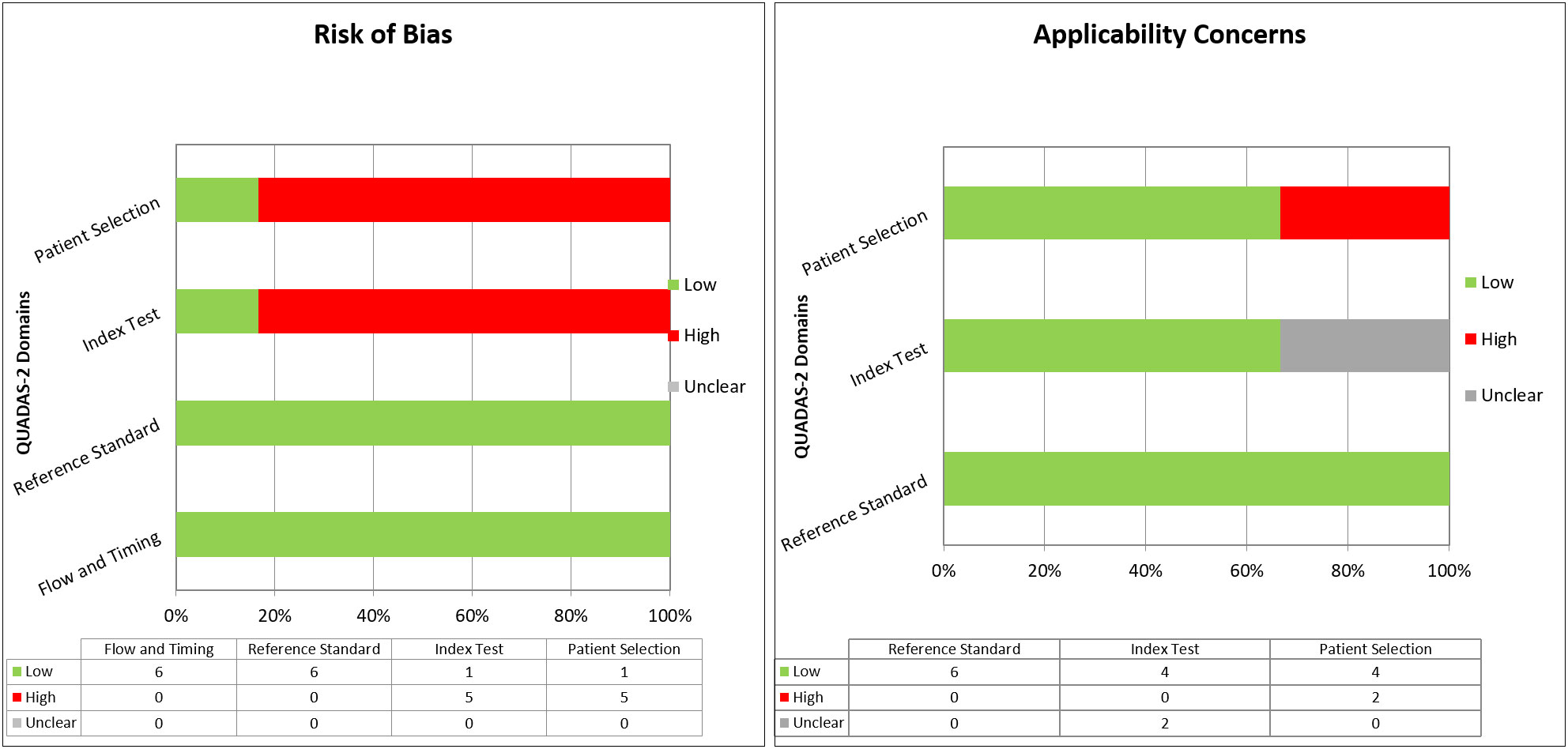
Figure 2 Overall quality assessment (risk of bias and applicability concerns) of the studies included in the Systematic Review, according to the QUADAS-2 Tool.
This systematic review demonstrates that the association between body composition and chemo-related toxicity in OC is still unclear. Indeed, 4/6 studies demonstrated the presence of a significant association, but 2/6 did not. Furthermore, the significant associations were not among the same covariates across studies.
Interestingly, the 2 studies showing no significant association of body composition and toxicity are from the same country (US) (30, 32), that is also known for its high percentage of overweight/obese patients. Indeed, the age-adjusted prevalence of obesity in the US in 2017–2018 was 42.4%, and the age-adjusted prevalence of severe obesity was as high as 9.2% among adults (>20 years), especially among women (35). This high prevalence of overweight/obese patients, confirmed by the high mean BMI in both studies, might have affected the results. Indeed, the low muscle mass may be underestimated in obese patients.
What emerges from the analysis of the results of the included studies, is that no study evaluated all the body compartments available, therefore some information is still missing. Indeed, for instance, Del Grande showed an association of SMA and early discontinuation of chemotherapy, but SMI was not significant (17). In the same study VAT and SMD were significantly associated with cycle delays, but SAT was not (17). Bruno et al. demonstrated the importance of SAT and SMD for G3 adverse events, but they did not evaluate the other compartments (31). Conrad and Yoshikawa analyzed only the PI, thus excluding all the other muscles in the same plane and even the other body compartments (32, 33).
Nevertheless, various methods are available for assessing body composition and they are based on an escalating level of complexity, from a two-compartment model (evaluating only fat mass and fat free mass), through a three-compartment model (including fat mass, lean tissue mass and bone mineral content), and a four-compartment model (including fat, mineral, total body water and proteins) to more complex multi-compartment models (including complex measurements of elements such as calcium, sodium, chloride, phosphorus, nitrogen, hydrogen, oxygen and carbon) (36). Therefore, other studies hypothesized that not only the quality and quantity of muscle are important in the metabolism of drugs, but also other compartments may contribute to the metabolism of chemotherapeutic agents (37). Indeed, the body proportions of lean and adipose tissues may be one of the phenotypic factors that affect the metabolism, clearance, and toxicity of antineoplastic agents (38). Accordingly, Schachar et al. analyzed a large number of body composition measures to assess predictors of toxicity in patients receiving chemotherapy for early stage breast cancer, and they demonstrated that body composition is extremely variable, demonstrating in their cohort that muscle metrics were clearly related to toxicity, whereas adipose metrics were not (39). Other studies tried to integrate the information of quality and quantity of muscle introducing a relatively new metric, a product of SMI and SMD (40), and demonstrated that this metric predicted G4 hematologic and G3/4 non-hematologic adverse event toxicity when eribulin was administered as a treatment in advanced soft tissue sarcoma (41).
As the technology advances, we may imagine that more comprehensive body composition quantifications will be possible as opportunistic assessments from imaging studies in patients with OC, including but not limited to assessment of bone mineral density, quantification of visceral and subcutaneous fat, assessment of muscle bulk and density, and quantification of liver fat (42).
This systematic review has some limitations. The first is the lack of a prospective cohort study evaluating the association of body composition and chemotherapy-related composition as primary objective. However, this type of study is difficult to obtain and usually have prognosis as primary outcome. Secondly, we included studies where the body composition was based on CT images and we do not know if other studies, based on DEXA or other techniques may show different results. However, CT (along with magnetic resonance) is currently considered as gold standard for assessment of body composition, therefore we may affirm that the data collected are reliable among the included studies. Lastly, the variability of definition of sarcopenia among the included studies, and the lack of reasons for the authors to choose different cut-offs, makes difficult an appropriate comparison. Indeed, we cannot know if the use of the same cut-off value for sarcopenia, would have led to more consisting results.
In conclusion, this systematic review of the literature demonstrated that there is a wide variability of results about the association of body composition and chemotherapy-related toxicity in patients with OC. Therefore further studies, possibly including a comprehensive assessment of body compartments and a constant definition of body composition cut-offs, are warranted to better understand this association.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conception and design: all authors. Data extraction from included studies: SR, GR, MDG, MLG. Analysis and interpretation of data: SR, GR, MDG, MLG. Manuscript writing: all authors. All authors contributed to the article and approved the submitted version.
The reviewer FT declared a shared affiliation with the author LM to the handling editor at the time of the review.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Oza AM. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol (2015) 16(8):928–36. doi: 10.1016/S1470-2045(15)00086-8
3. Tewari KS. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol (2019) 37(26):2317–28. doi: 10.1200/JCO.19.01009
4. Colombo N, Sessa C, Bois AD, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol (2019) 30(5):672–705. doi: 10.1093/annonc/mdz062
5. DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: The SOLO1/GOG 3004 trial. J Clin Oncol (2022) JCO2201549. doi: 10.1200/JCO.22.01549
6. González-Martín A. PRIMA/ENGOT-OV26/GOG-3012 investigators. niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med (2019) 381(25):2391–402. doi: 10.1056/NEJMoa1910962
7. Ray-Coquard I, PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med (2019) 381(25):2416–28. doi: 10.1056/NEJMoa1911361
8. Ferracini AC, Lopes-Aguiar L, Lourenço GJ, Yoshida A, Lima CSP, Sarian LO, et al. GSTP1 and ABCB1 polymorphisms predicting toxicities and clinical management on carboplatin and paclitaxel-based chemotherapy in ovarian cancer. Clin Transl Sci (2021) 14(2):720–8. doi: 10.1111/cts.12937
9. Tomao F, Bardhi E, Di Pinto A, Sassu CM, Biagioli E, Petrella MC, et al. Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat Rev (2019) 80:101909. doi: 10.1016/j.ctrv.2019.101909
10. Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. implications for drug therapy. Clin Pharmacokinet (1994) 26(4):292–307. doi: 10.2165/00003088-199426040-00005
11. Aslani A, Smith RC, Allen BJ, Pavlakis N, Levi JA. The predictive value of body protein for chemotherapy-induced toxicity. Cancer (2000) 88(4):796–803. doi: 10.1002/(SICI)1097-0142(20000215)88:4<796::AID-CNCR10>3.0.CO;2-P
12. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res (2009) 15(8):2920–6. doi: 10.1158/1078-0432
13. Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol (2010) 21(8):1594–8. doi: 10.1093/annonc/mdp605
14. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol (2008) 9(7):629–35. doi: 10.1016/S1470-2045(08)70153-0
15. Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr (2010) 91(4):1133S–7S. doi: 10.3945/ajcn.2010.28608C
16. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol (2013) . 31(12):1539–47. doi: 10.1200/JCO.2012.45.2722
17. Del Grande M, Rizzo S, Nicolino GM, Colombo I, Rossi L, Manganaro L, et al. Computed tomography-based body composition in patients with ovarian cancer: Association with chemotoxicity and prognosis. Front Oncol (2021) 11:718815. doi: 10.3389/fonc.2021.718815
18. Rizzo S, Petrella F, Bardoni C, Bramati L, Cara A, Mohamed S, et al. CT-derived body composition values and complications after pneumonectomy in lung cancer patients: Time for a sex-related analysis? Front Oncol (2022) 12:826058. doi: 10.3389/fonc.2022.826058
19. Sanchez A, Kissel S, Coletta A, Scott J, Furberg H. Impact of body size and body composition on bladder cancer outcomes: Risk stratification and opportunity for novel interventions. Urol Oncol (2020) 38(9):713–8. doi: 10.1016/j.urolonc.2020.03.017
20. Rizzo S, Scala I, Robayo AR, Cefalì M, De Dosso S, Cappio S, et al. Del grande f body composition as a predictor of chemotherapy-related toxicity in pancreatic cancer patients: A systematic review. Front Oncol (2022) 12:974116. doi: 10.3389/fonc.2022.974116
21. Sjøblom B, Grønberg BH, Benth JŠVerifytat, Baracos VE, Fløtten Ø, Hjermstad MJ, et al. Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer (2015) 90:85–91. doi: 10.1016/j.lungcan.2015.07.001
22. Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res (2009) 15:6973–9. doi: 10.1158/1078-0432.CCR-09-1525
23. Bellomi M, Rizzo S, Travaini LL, Bazzi L, Trifirò G, Zampino MG, et al. Role of multidetector CT and FDG-PET/CT in the diagnosis of local and distant recurrence of resected rectal cancer. Radiol Med (2007) 112(5):681–90. doi: 10.1007/s11547-007-0172-2
24. Rietjens M, Villa G, Toesca A, Rizzo S, Raimondi S, Rossetto F, et al. Appropriate use of magnetic resonance imaging and ultrasound to detect early silicone gel breast implant rupture in postmastectomy reconstruction. Plast Reconstr Surg (2014) 134(1):13e–20e. doi: 10.1097/PRS.0000000000000291
25. Genovese E, Canì A, Rizzo S, Angeretti MG, Leonardi A, Fugazzola C. Comparison between MRI with spin-echo echo-planar diffusion-weighted sequence (DWI) and histology in the diagnosis of soft-tissue tumours. Radiol Med (2011) 116(4):644–56. doi: 10.1007/s11547-011-0666-9
26. Huber FA, Del Grande F, Rizzo S, Guglielmi G, Guggenberger R. MRI In the assessment of adipose tissues and muscle composition: how to use it. Quant Imaging Med Surg (2020) 10(8):1636–49. doi: 10.21037/qims.2020.02.06
27. Zaffina C, Wyttenbach R, Pagnamenta A, Grasso RF, Biroli M, Del Grande F, et al. Body composition assessment: comparison of quantitative values between magnetic resonance imaging and computed tomography. Quant Imaging Med Surg (2022) 12(2):1450–66. doi: 10.21037/qims-21-619
28. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accu-racy studies: The PRISMA-DTA statement. JAMA (2018) 319(4):388–96. doi: 10.1001/jama.2017.19163
29. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
30. Staley SA, Tucker K, Newton M, Ertel M, Oldan J, Doherty I, et al. Sarcopenia as a predictor of survival and chemotoxicity in patients with epithelial ovarian cancer receiving platinum and taxane-based chemotherapy. Gynecol Oncol (2020) 156(3):695–700. doi: 10.1016/j.ygyno.2020.01.003
31. Bruno KA, Sobreira da Silva MJ, Chaves GV. Association of body composition with toxicity to first-line chemotherapy and three-year survival in women with ovarian adenocarcinoma. Acta Oncol (2021) 60(12):1611–20. doi: 10.1080/0284186X.2021.1983210
32. Conrad LB, Awdeh H, Acosta-Torres S, Conrad SA, Bailey AA, Miller DS, et al. Pre-operative core muscle index in combination with hypoalbuminemia is associated with poor prognosis in advanced ovarian cancer. J Surg Oncol (2018) 117(5):1020–8. doi: 10.1002/jso.24990
33. Yoshikawa T, Takano M, Miyamoto M, Yajima I, Shimizu Y, Aizawa Y, et al. Psoas muscle volume as a predictor of peripheral neurotoxicity induced by primary chemotherapy in ovarian cancers. Cancer Chemother Pharmacol (2017) 80(3):555–61. doi: 10.1007/s00280-017-3395-5
34. Prado CM, Baracos VE, Xiao J, Birdsell L, Stuyckens K, Park YC, et al. The association between body composition and toxicities from the combination of doxil and trabectedin in patients with advanced relapsed ovarian cancer. Appl Physiol Nutr Metab (2014) 39(6):693–8. doi: 10.1139/apnm-2013-0403
35. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United states, 2017–2018. NCHS data brief, no 360. Hyattsville, MD: National Center for Health Statistics (2020).
36. Kuriyan R. Body composition techniques. Indian J Med Res (2018) 148(5):648–58. doi: 10.4103/ijmr.IJMR_1777_18
37. Sandini M, Patino M, Ferrone CR, Alvarez-Pérez CA, Honselmann KC, Paiella S, et al. Association between changes in body composition and neoadjuvant treatment for pancreatic cancer. JAMA Surg (2018) 153(9):809–15. doi: 10.1001/jamasurg.2018.0979
38. Cushen SJ, Power DG, Teo MY, MacEneaney P, Maher MM, McDermott R, et al. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am J Clin Oncol (2017) 40(1):47–52. doi: 10.1097/COC.0000000000000061
39. Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res (2017) 23(14):3537–43. doi: 10.1158/1078-0432.CCR-16-2266
40. Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, et al. Characterization of skeletal muscle and body mass indices in younger and older women with stage II and III breast cancer. J Am Geriatr Soc (2016) 24(3):278–284. doi: 10.1111/tbj.12952
41. Kobayashi H, Okuma T, Oka H, Okajima K, Ishibashi Y, Zhang L, et al. Body composition as a predictor of toxicity after treatment with eribulin for advanced soft tissue sarcoma. Int J Clin Oncol (2019) 24(4):437–44. doi: 10.1007/s10147-018-1370-8
Keywords: ovarian cancer, chemotherapy, body composition, sarcopenia, toxicity
Citation: Rizzo S, Raia G, Del Grande M, Gasparri ML, Colombo I, Manganaro L, Papadia A and Del Grande F (2022) Body composition as a predictor of chemotherapy-related toxicity in ovarian cancer patients: A systematic review. Front. Oncol. 12:1057631. doi: 10.3389/fonc.2022.1057631
Received: 29 September 2022; Accepted: 21 October 2022;
Published: 02 November 2022.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Milly Buwenge, University of Bologna, ItalyCopyright © 2022 Rizzo, Raia, Del Grande, Gasparri, Colombo, Manganaro, Papadia and Del Grande. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Rizzo, c3RlZmFuaWEucml6em9AZW9jLmNo; cml6em9zQHVzaS5jaA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.