
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 10 November 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1057560
This article is part of the Research TopicCombining Localised and Systemic Therapy Options for Advanced Hepatocellular CarcinomaView all 16 articles
 Di Zhu1†
Di Zhu1† Kun Ma2†
Kun Ma2† Wei Yang1
Wei Yang1 Hai-Feng Zhou1
Hai-Feng Zhou1 Qi Shi1
Qi Shi1 Jian-Wu Ren1
Jian-Wu Ren1 Yu-Guan Xie1
Yu-Guan Xie1 Sheng Liu1
Sheng Liu1 Hai-Bin Shi1
Hai-Bin Shi1 Wei-Zhong Zhou1*
Wei-Zhong Zhou1*Purpose: To compare the effectiveness and safety of transarterial chemoembolization (TACE) combined with apatinib and camrelizumab with those of TACE as well as apatinib among patients with unresectable hepatocellular carcinoma (HCC).
Materials and methods: The data of patients with unresectable HCC (uHCC) who received TACE-apatinib-camrelizumab combination (TACE + AC group) and TACE-apatinib combination (TACE + A group) were collected from two centers between January 2018 and January 2022. Propensity score matching (PSM) was conducted to diminish the bias between the two groups. The primary outcome measures of the study were overall survival (OS) and progression-free survival (PFS), and the secondary outcome measures were response rate (ORR), disease control rate (DCR), and adverse events (AEs).
Results: A total of 102 patients were enrolled in this study after PSM, with 34 patients in the TACE + AC group and 68 patients in the TACE + A group. Compared to the TACE + A group, TACE + AC had a significantly longer median OS (25.5 months, interquartile range [IQR], 23.5–33.0) than 18.5 months (IQR, 13.0–25.0; P = 0.001). Similarly, the PFS of the TACE + AC group was significantly improved (14.0 months, IQR, 9.0–NA) compared to that of the TACE + A group (5.0 months, IQR, 2.5–9.0; P = 0.001). The ORR rates (55.9% vs. 51.5%), and DCR rates (79.4% vs. 72.1%) were comparable between groups (P > 0.05). All treatment-related adverse events were tolerable and manageable, and no serious adverse events were observed.
Conclusion: TACE combined with apatinib plus camrelizumab demonstrated superior efficacy to TACE plus apatinib for patients with unresectable HCC. The two combination therapies showed similar safety profiles.
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related deaths worldwide and is often diagnosed at an advanced stage because of its insidious onset and nonspecific symptoms. Transarterial chemoembolization (TACE) and systematic therapy are considered standard therapeutic methods for patients with intermediate and advanced HCC, respectively (1–3). As a widely accepted and proven treatment strategy for HCC, TACE could effectively inhibit tumor progression. However, TACE could cause hypoxia in tumor tissue, which ultimately induces the expression of vascular endothelial growth factor (VEGF) and increases tumor angiogenesis (4), and consequently, mediates tumor growth and/or metastasis. Moreover, repeated TACE procedures can gradually impair liver function and aggravate liver cirrhosis.
Apatinib (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China), a novel targeted agent, has higher selectivity to VEGFR-2 than sorafenib. Qiu et al (5) proposed that TACE combined with apatinib can improve the efficacy of unresectable HCC compared to TACE alone. Meanwhile, camrelizumab (SHR-1210, Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) is a humanized monoclonal antibody against PD-1. According to the RESCUE trial (6), camrelizumab in combination with apatinib has an efficacy profile of 34.3% for advanced HCC. Moreover, with the continuation of the IMBrave150 study (7) and several clinical trials (8, 9), immunotherapy in combination with antiangiogenic drugs is known to significantly improve the outcome of patients with advanced HCC. Furthermore, evidence (10) shows that TACE is an inducer of immunogenic cell death, resulting in facilitating antigen presentation and priming of antitumour lymphocytes (11). Thus, there is an appealing rationale for the combination of TACE, tyrosine kinase inhibitors (TKIs), and immune checkpoint inhibitors (ICIs) (12).
Several studies (13, 14) have shown that TACE combined with anti-angiogenic therapy and immunotherapy can improve the treatment efficacy of patients with unresectable HCC, with an ORR of approximately 35%–59% and median overall survival (OS) of approximately 13–35 months. Few studies have been conducted using TACE along with apatinib and camrelizumab for patients with unresectable HCC. Therefore, we conducted this retrospective study to determine the efficacy and safety of TACE combined with apatinib and camrelizumab (TACE + AC) therapy compared to TACE combined with apatinib (TACE + A) therapy.
This retrospective analysis was conducted between January 2018 and January 2022, on all patients with unresectable HCC from the First Affiliated Hospital of Nanjing Medical University and the Affiliated Hospital of Nanjing University of Chinese Medicine who received TACE plus apatinib with/without camrelizumab. The study was approved by the Institutional Ethics Review Boards of both hospitals, and the procedures followed in this study were conducted in accordance with the guidelines of the World Medical Association Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study. According to the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China, HCC was diagnosed pathologically or clinically. The inclusion criteria for the study were as follows: (1) Barcelona Clinic Liver Cancer (BCLC) stage B or C; (2) Child–Pugh class A5–B7; (3) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1; and (4) ≥ 1 cycle of TACE and apatinib with or without camrelizumab. The exclusion criteria were as follows: (1) < 1 month of apatinib or camrelizumab treatment; (2) appearance of secondary primary malignant tumors; (3) contraindication to camrelizumab (an allergy to the active ingredient and excipients of camrelizumab); and (4) incomplete data or loss to follow-up.
TACE was initiated before apatinib and camrelizumab administration. Under local anesthetic, TACE treatment was conducted through the femoral artery. To determine the number, size, location, and feeding arteries of the tumors, a 5-F catheter (COOK) was inserted and angiography was performed. Then, an emulsion of chemotherapeutic drugs (lobaplatin, 30–50 mg; epirubicin, 10–30 mg) mixed with lipiodol was administered through the hepatic artery. Thereafter, embolization via a microcatheter (2.7 F; Terumo Medical Corp., Tokyo, Japan; or 2.4 F; Merit Maestro, South Jordan, Utah, USA) was performed either selectively or superselectively. Selective embolization with 300 μm polyvinyl alcohol particles (Biosphere Medical, Paris, France; or Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) or gelatin sponge particles was performed to achieve blood flow stasis in the tumor-feeding artery. Post-TACE syndrome was recorded, and liver function indices were assessed within 1 week of each TACE session.
Apatinib was administered orally 250 mg once a day within 1 week of the initial TACE and was suspended 3 days before and after repeated TACE procedures. Camrelizumab was administered 200 mg intravenously within 1 week of the initial TACE and then every 3 weeks continuously (maximum of 24 months of camrelizumab treatment). The doses of camrelizumab and apatinib were reduced, suspended, or discontinued in patients who experienced severe adverse events (AEs).
All patients were followed up constantly until death or the end of the study (March 1, 2022). To track treatment-related adverse events (AEs), blood tests, including complete blood counts, liver, kidney, cardiac biomarkers, and thyroid functions, were conducted approximately every 3 weeks. Tumor markers and contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) were performed every 2 months to assess the treatment response. TACE was repeated in keeping with the tumor status, liver function, and patient’s general condition when residual viable tumors were detected or new lesions emerged after a multidisciplinary team discussion.
The primary measure outcomes were OS and progress-free survival (PFS). OS was defined from the date of the first TACE therapy to the date of death arising from any cause or the date of the last contact in both groups. PFS was defined as the time between the beginning of TACE treatment and the first sign of tumor progression or death. Secondary measure outcomes of this study included the objective response rate (ORR), disease control rate (DCR), and AEs. The tumor response was evaluated by two experienced radiologists using the modified Response Evaluation Criteria in Solid Tumors (mRECIST, version 1.1), including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The ORR was defined as CR + PR, and the DCR was defined as CR + PR + SD. AEs were assessed based on the Common Terminology Criteria for Adverse Events (CTCAE, version 4.03).
Propensity score matching (PSM) was performed to minimize the effects of selection bias and potential confounders. Categorical data are expressed as the number of patients (percentage). Quantitative data were expressed as mean ± standard deviation and median (range) for normally and nonnormally distributed variables. Categorical data between the two groups were compared using the c2 test or Fisher’s exact test, as appropriate. Quantitative data were compared using Student’s t-test or Mann–Whitney U test, as appropriate. Survival curves were analyzed by Kaplan–Meier method using the log-rank test. All statistical analyses were performed using SPSS Statistics version 26 (IBM, Armonk, New York, USA). All statistical tests were two-tailed, and P < 0.05 was considered statistically significant.
Between January 2018 and January 2022, 147 patients with BCLC stage B or C were considered eligible for this study, including the TACE + AC group (n = 34) and TACE + A group (n = 113). The median follow-up time is 22.8 months in the TACE + AC group, while 29.3 months in the TACE + A group. The flow diagram is displayed in Figure 1. The baseline characteristics of the patients are summarized in Table 1. Before PSM, BCLC stage (B/C, P = 0.003) and tumor distribution (single/multiple, P = 0.02) showed statistically significant differences in the two groups. Groups were matched strictly in age, gender and grade of BCLC classification (caliper = 0.1). After PSM at a 1:2 radio, there were no statistically significant differences in the baseline characteristics between the two groups. A total of 102 patients were included after PSM, among whom, 34 were in the TACE + AC group and 68 were in the TACE + A group.
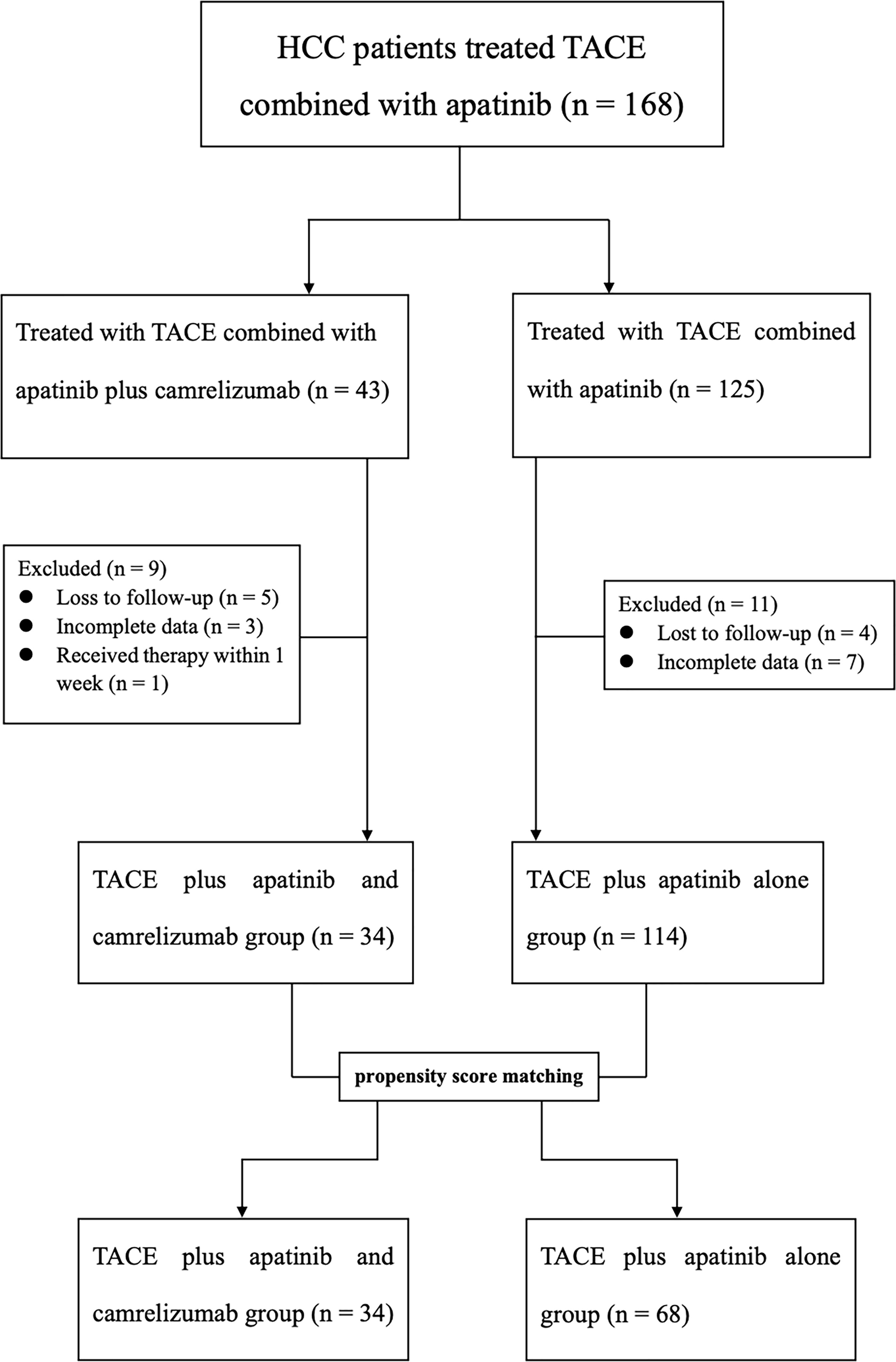
Figure 1 Flow diagram of patient enrollment. HCC, Hepatocellular carcinoma; TACE, Transarterial chemoembolization.
Before PSM, Patients in the TACE + AC group had a median OS of 25.5 (IQR: 23.5–33) months and a median PFS of 14.0 (IQR: 9.0–NA) months, while 19.1 (IQR: 2.5–27.5) months and 5.1 (IQR: 2.7-8.1) for those in the TACE + A group, respectively. Patients in the TACE + AC group had a median OS of 25.5 (IQR: 23.5–33) months compared to 18.5 (IQR: 13.0–25.0) months for those in the TACE + A group (HR = 0.312, 95%CI = 0.162–0.602; Figure 2); and a median PFS of 14.0 (IQR: 9.0–NA) months compared to 5.0 (IQR: 2.5–9.0) months for those in the TACE + A group. The survival rates of the TACE + AC group were 91.1%, 63.2%, and 48.6% at 1, 2, and 3 years, while those of the TACE + A group were 76.3%, 27%, and 18.2%, respectively. Figure 3 is the representative MR imaging figures from 1 case of CR. All data and results are available in Supplementary Tables 1, 2.
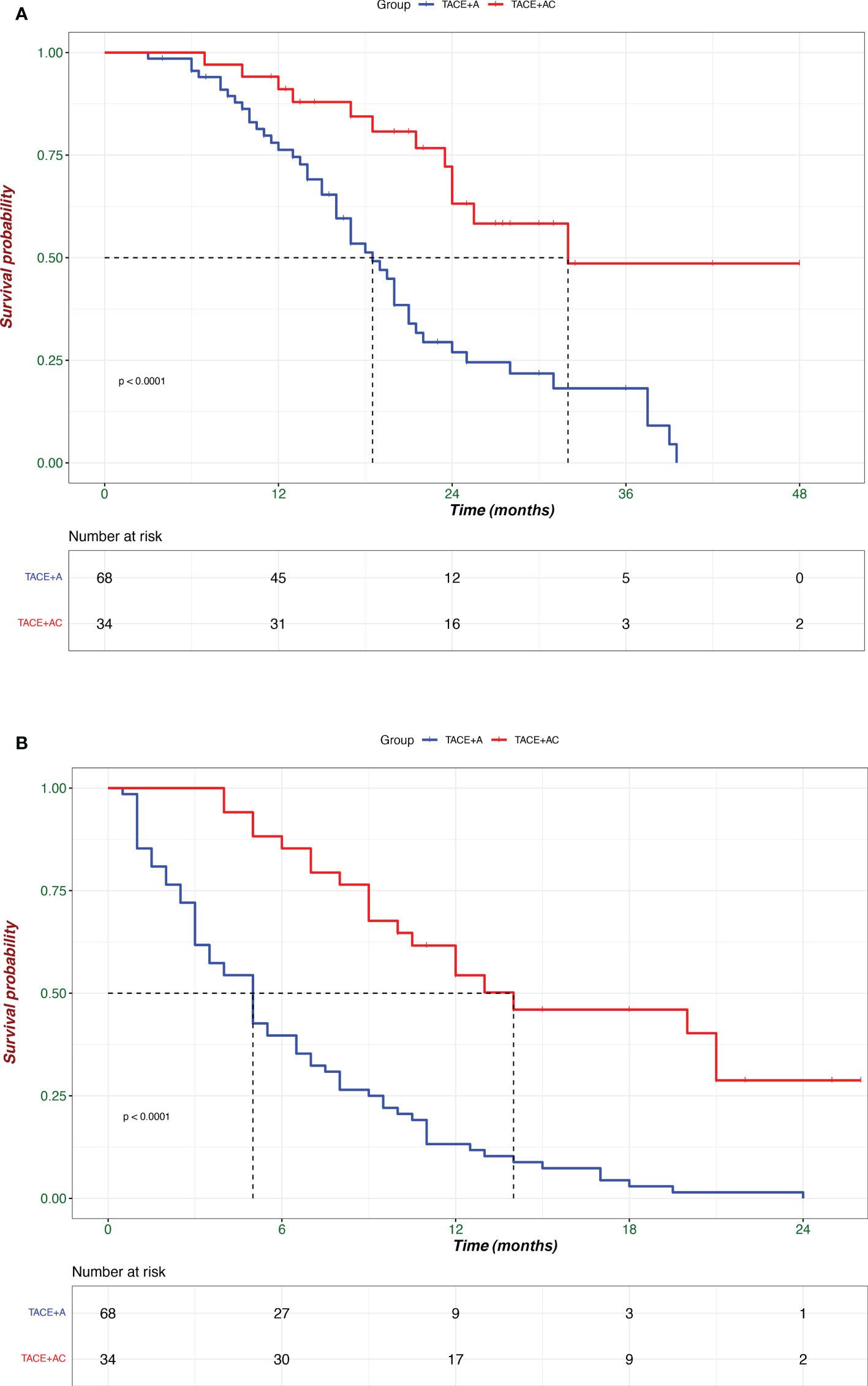
Figure 2 Kaplan–Meier analyses of overall survival (A) and progression-free survival (B) according to treatment groups. TACE + A, Transarterial chemoembolization combined with apatinib; TACE + AC, Transarterial chemoembolization combined with apatinib plus camrelizumab.
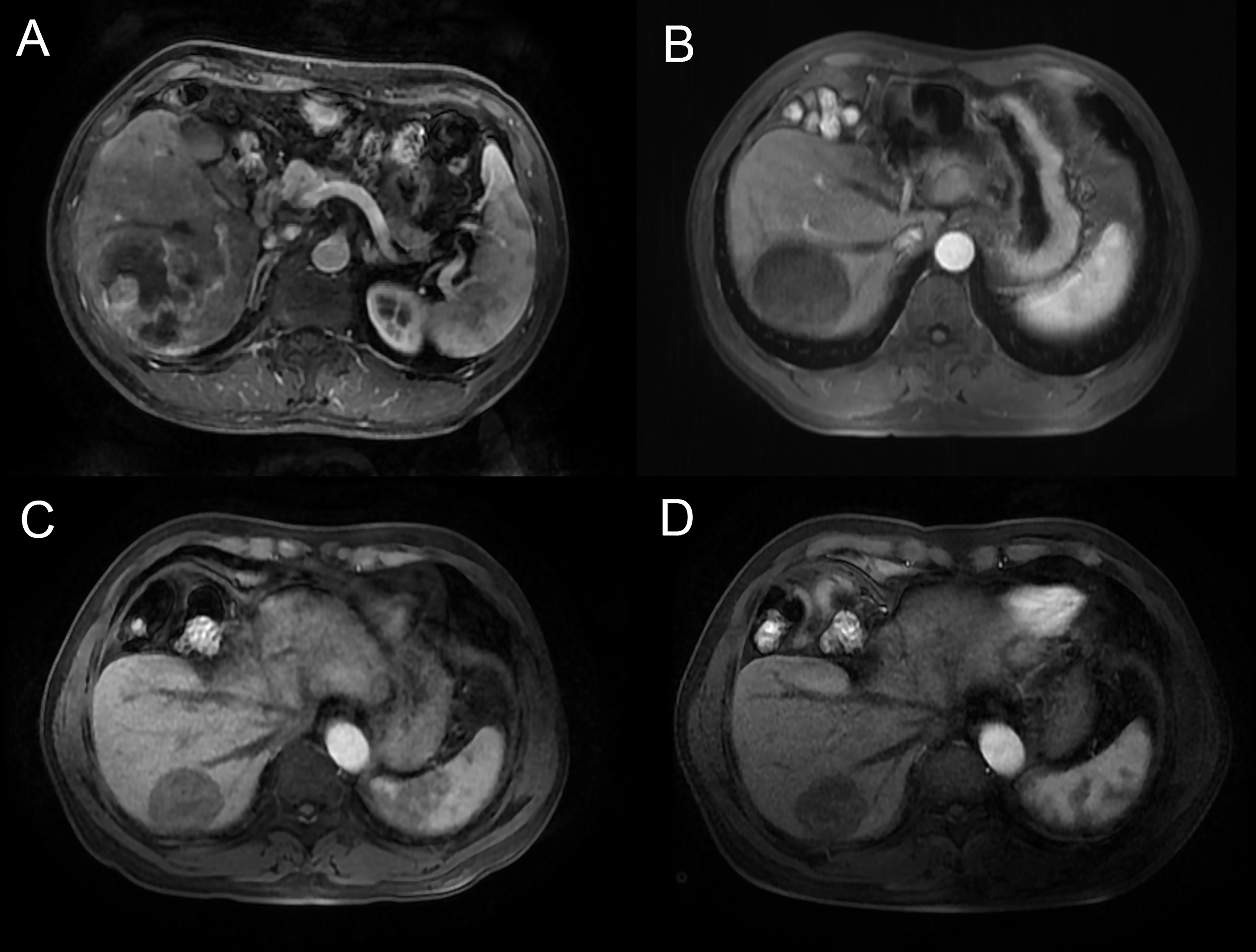
Figure 3 Male, 58y, BCLC B stage, Child-Pugh grade A6, massive and ruptured HCC. MR images (arterial phase) were acquired. (A) before TACE treatment, (B) 6 months after the combination treatment, (C) 12 months after the combination treatment, (D) 18 months after the combination treatment, demonstrating complete response, with a reduction in tumor size, the patient was classified as CR according to mRECIST. TACE, transarterial chemoembolization.
The tumor responses at the 1-year rate of the two groups of patients are shown in Table 2. For the TACE + AC group, 4 (11.8%) patients achieved CR, 15 (44.1%) achieved PR, 8 (23.5%) patients were in the SD state, and 7 (20.6%) patients had PD. However, in the TACE + A group, 4 (5.9%) patients achieved CR, 31 (45.6%) patients achieved PR, 14 (20.6%) patients were in the SD state, and 19 (27.9%) had PD. The ORR rates (55.9% vs. 51.5%) and DCR rates (79.4% vs. 72.1%) of the TACE + AC group were numerically higher than those of the TACE + A group, and neither showed a statistically significant difference (P > 0.05).
No treatment-related deaths were observed and the treatment-related adverse events (TrAEs) are listed in Table 3. All toxicities were manageable. AEs of any grade during the TACE procedure included abdominal pain (65.1%), transaminitis (46.1%), fever (53.9%), lymphopenia (10.8%), decreased appetite (29.4%), nausea/vomiting (58.5%), diarrhea (25.5%), fatigue (13.7%), leukopenia (14.7%), neutropenia (11.8%), and anemia (13.7%). There were no significant differences in AEs resulting from TACE between the groups. In contrast, hand-foot syndrome (29.4%), hypertension (44.1%), and reactive cutaneous capillary endothelial proliferation (RCCEP) (23.5%) were the most common AEs in the period of apatinib and camrelizumab administration. In the TACE + AC group, apatinib administration was suspended in five patients and camrelizumab administration was suspended in one patient. In the TACE + A group, apatinib administration was suspended in 10 patients due to intolerance. Grade 4 myelosuppression occurred in one patient after the TACE procedure and recovered after symptomatic management.
Our study revealed that TACE + AC therapy was more effective than TACE + A therapy in patients with unresectable HCC. Patients who received the TACE + AC modality had a median OS of 25.5 (IQR: 23.5–33.0) months and a median PFS of 18.5 (IQR: 13.0–25.0) months, which yielded a sufficient edge over the TACE + A modality and was comparable to the results of previous studies (15, 16).
The combination of TACE with immunotherapy modalities has shown promising clinical efficacy. Regarding the survival time, several retrospective studies have shown that TACE combined with TKIs and ICIs demonstrated superior OS (18–24 months) and PFS (5.5–13.3 months) than TACE combined with TKIs or TKIs combined with ICIs, which were not better than our outcomes. In our study, the median OS and PFS in the TACE + AC group were numerically higher than those in the TACE + A group. Of note, Ju et al. (17, 18) reported comparable results of TACE combined with apatinib and camrelizumab; thus, our findings demonstrated a substantial and synergistic improvement in survival for patients with unresectable HCC treated with TACE + AC. Several possible explanations exist for this finding (1) TACE can induce the up-regulation of VEGF and neovascularization, and apatinib can inhibit tumor angiogenesis by targeting VEGFR-2 (12); (2) TACE can cause tumor cell necrosis and neoangiogenesis, while the immune tolerance induced by TACE can be attenuated by TKI and PD-1 inhibitors (19, 20); and (3) the combination of ICIs with TKIs can convert “cold tumors” into “hot tumor” by T cell activation (11), which may restore exhausted T cells and facilitate anti-tumor immunity (21). Therefore, patients with unresectable HCC may experience superior clinical results when applying TACE, apatinib, and camrelizumab in combination.
For the tumor response, the RESCUE trial (6) disclosed an ORR of 34.3% and a DCR of 77.1% following combined apatinib and camrelizumab therapy. The TACE + AC group had a greater ORR compared to those reported by the IMbrave150 trial (7) (atezolizumab plus bevacizumab: ORR = 33.2%), the phase 1b KEYNOTE-524 trial (22) (lenvatinib plus pembrolizumab: ORR = 46.0%), and the ORIENT-32 trial (sintilimab plus bevacizumab: ORR = 24%); this was likely because the addition of TACE is thought to be related to immune activation and can induce low expression of Tregs via modulating pro-inflammatory pathways. In our study, the CR, ORR, and DCR rates of the TACE + AC group were numerically greater than those of the TACE + A group (11.8% vs. 5.9%, 55.9% vs. 51.5%, and 79.4% vs. 72.1%, respectively), although the difference did not reach statistical significance. Simultaneously, Ju et al. reported an ORR of 58.8% and a DCR of 81.2% in the TACE + AC group, which is similar to our findings. However, our results did not achieve statistical significance, likely for the following reasons: (1) the intervals between TACE were believed to affect the results, (2) some patients in the TACE + AC group were newly included in the cohort and had comparatively fewer cycles of camrelizumab, and (3) PVTT and subsequent metastasis can induce tumor cells to spread, which may reduce the efficacy of immunotherapy in patients with PVTT. These findings and views were similarly shared by Cai et al. (23).
Regarding AEs, the most common AEs were hand-foot skin reaction and hypertension, which were predominantly related to apatinib. Moreover, the incidence of apatinib-related AEs (grade ≥ 3) was 11%–16%, whereas events such as increased AST/ALT and RCCEP were related to camrelizumab. According to previous studies, the most common AE in patients with HCC treated by TACE is embolization syndrome, including pain, fever, nausea, and vomiting. Altogether, TACE + AC therapy for patients with unresectable HCC presented a safe profile.
This study also has some limitations. First, this is a retrospective study with a small sample of enrolled patients and a short follow-up period. Therefore, the results may not be generalizable and should be interpreted with caution. Second, although we performed PSM to avoid selection bias, our analyses may still be influenced by some inherent biases, such as regional bias and population and tumor-related factors; indeed, the etiology of HCC, prevalence of cirrhosis, comorbidities, and overall treatment approach differs in some regions of the world. Thirdly, the subgroup analyses were lacked in this study. Lastly, some patients did not achieve endpoint events throughout the limited follow-up period.
In conclusion, this study showed that TACE combined with apatinib and camrelizumab therapy demonstrated superior efficacy to TACE combined with apatinib for patients with unresectable HCC. Although promising, our results need to be validated by more studies in the future.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Designing and instructing the study: WZZ. Collecting the data: DZ, YGX, JWR and QS. Analyses and interpretation of data: WY and HFZ. Dradting of manuscript: DZ and KM. Critical revision of manuscript: SL, HBS and WZZ. All authors contributed to the article and approved the submitted version. DZ and KM contributed equally this work.
This work was supported by the National Natural Science Foundation for Young Scholars of China (81701802).
We thank the nurses (Zhong-ling Pei, Yan Shen, Ling-ling Wu) for doing the follow-up work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1057560/full#supplementary-material
1. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis (1999) 19(3):329–38. doi: 10.1055/s-2007-1007122
2. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol (2022) 76(3):681–93. doi: 10.1016/j.jhep.2021.11.018
3. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(36):4317–45. doi: 10.1200/JCO.20.02672
4. Kong J, Kong J, Pan B, Ke S, Dong S, Li X, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PloS One (2012) 7(5):e37266. doi: 10.1371/journal.pone.0037266
5. Qiu Z, Shen L, Chen S, Qi H, Cao F, Xie L, et al. Efficacy of apatinib in transcatheter arterial chemoembolization (TACE) refractory intermediate and advanced-stage hepatocellular carcinoma: A propensity score matching analysis. Cancer Manag Res (2019) 11:9321–30. doi: 10.2147/CMAR.S223271
6. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res Off J Am Assoc Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
7. Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim T-Y, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(7):991–1001. doi: 10.1016/S1470-2045(21)00151-0
8. Meng X, Wu T, Hong Y, Fan Q, Ren Z, Guo Y, et al. Camrelizumab plus apatinib as second-line treatment for advanced oesophageal squamous cell carcinoma (CAP 02): A single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol (2022) 7(3):245–53. doi: 10.1016/S2468-1253(21)00378-2
9. Mei K, Qin S, Chen Z, Liu Y, Wang L, Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort a report in a multicenter phase Ib/II trial. J Immunother Cancer (2021) 9(3):e002191. doi: 10.1136/jitc-2020-002191
10. Pinato DJ, Murray SM, Forner A, Kaneko T, Fessas P, Toniutto P, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer (2021) 9(9):e003311. doi: 10.1136/jitc-2021-003311
11. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol (2022) 19(3):151–72. doi: 10.1038/s41571-021-00573-2
12. Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2021) 18(5):293–313. doi: 10.1038/s41575-020-00395-0
13. Qin J, Huang Y, Zhou H, Yi S. Efficacy of sorafenib combined with immunotherapy following transarterial chemoembolization for advanced hepatocellular carcinoma: A propensity score analysis. Front Oncol (2022) 12:807102. doi: 10.3389/fonc.2022.807102
14. Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: A retrospective study. Front Mol Biosci (2021) 7:609322. doi: 10.3389/fmolb.2020.609322
15. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: A multicenter retrospective study. Front Oncol (2021) 11:783480. doi: 10.3389/fonc.2021.783480
16. Liu J, Li Z, Zhang W, Lu H, Sun Z, Wang G, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol (2021) 12:709060. doi: 10.3389/fphar.2021.709060
17. Ju S, Zhou C, Yang C, Wang C, Liu J, Wang Y, et al. Apatinib plus camrelizumab with/without chemoembolization for hepatocellular carcinoma: A real-world experience of a single center. Front Oncol (2021) 11:835889. doi: 10.3389/fonc.2021.835889
18. Ju S, Zhou C, Hu J, Wang Y, Wang C, Liu J, et al. Late combination of transarterial chemoembolization with apatinib and camrelizumab for unresectable hepatocellular carcinoma is superior to early combination. BMC Cancer (2022) 22(1):335. doi: 10.1186/s12885-022-09451-1
19. You R, Xu Q, Wang Q, Zhang Q, Zhou W, Cao C, et al. Efficacy and safety of camrelizumab plus transarterial chemoembolization in intermediate to advanced hepatocellular carcinoma patients: A prospective, multi-center, real-world study. Front Oncol (2022) 12:816198. doi: 10.3389/fonc.2022.816198
20. Cheu JWS, Wong CCL. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology (2021) 74(4):2264–76. doi: 10.1002/hep.31840
21. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med (2010) 207(10):2187–94. doi: 10.1084/jem.20100643
22. Sun X, Zhang Q, Mei J, Yang Z, Chen M, Liang T. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: An exploration for expanded indications. BMC Cancer (2022) 22(1):293. doi: 10.1186/s12885-022-09405-7
Keywords: apatinib, immunotherapy, transarterial chemoembolization, hepatocellular carcinoma, PD-1, prognosis
Citation: Zhu D, Ma K, Yang W, Zhou H, Shi Q, Ren J, Xie Y, Liu S, Shi H and Zhou W (2022) Transarterial chemoembolization plus apatinib with or without camrelizumab for unresected hepatocellular carcinoma: A two-center propensity score matching study. Front. Oncol. 12:1057560. doi: 10.3389/fonc.2022.1057560
Received: 29 September 2022; Accepted: 24 October 2022;
Published: 10 November 2022.
Edited by:
Zhongmin Wang, Shanghai Jiao Tong University, ChinaReviewed by:
Bin-Yan Zhong, The First Affiliated Hospital of Soochow University, ChinaCopyright © 2022 Zhu, Ma, Yang, Zhou, Shi, Ren, Xie, Liu, Shi and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Zhong Zhou, eG1qYnEwMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.