
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Oncol. , 24 October 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1054399
This article is part of the Research Topic Lipids, Lipid Oxidation, and Cancer: From Biology to Therapeutics View all 7 articles
 Fahrul Nurkolis1*†‡§
Fahrul Nurkolis1*†‡§ Faqrizal Ria Qhabibi2†§
Faqrizal Ria Qhabibi2†§ Vincentius Mario Yusuf2§
Vincentius Mario Yusuf2§ Stanley Bulain2§
Stanley Bulain2§ Ghevira Naila Praditya2§
Ghevira Naila Praditya2§ Deogifta Graciani Lailossa2§
Deogifta Graciani Lailossa2§ Msy Firyal Nadya Al Mahira2§
Msy Firyal Nadya Al Mahira2§ Eka Nugraha Prima2§
Eka Nugraha Prima2§ Tony Arjuna3§
Tony Arjuna3§ Shilfiana Rahayu1§
Shilfiana Rahayu1§ William Ben Gunawan4§
William Ben Gunawan4§ Felicia Kartawidjajaputra5§
Felicia Kartawidjajaputra5§ Dionysius Subali6§
Dionysius Subali6§ Happy Kurnia Permatasari7‡§
Happy Kurnia Permatasari7‡§Cancer is in the second rank of leading causes of mortality around the world in 2020, with around 10 million death cases, just behind heart disease, as well as approximately 250 million disability-adjusted life years (DALY) due to cancers (1). The most common form of deadly cancer is tracheal, bronchus, and lung cancer generally, and the number is greater for males. However, women also possess the threat of female-specific cancer, including breast (higher in women, but also happens in men), cervical, ovarian, and uterine cancer. This is also burdened by low-middle socioeconomic status (1, 2). In 2019, the number of new cancer incidents reached around 23.6 million cases, which increased from the 2010 incidence (18.7 million cases) (1). The risk factor includes smoking, unhealthy diets, and pollution, which increases the risks of developing cancer in the future (3). There have been several cancer treatment options in recent years, such as surgery, radiation, and chemotherapy. Only a third of cancer patients are estimated to be cured with surgery or radiation therapy, while in cancers that have spread, chemotherapy is needed as a systemic treatment option (4). These cancer treatment options can also cause major side effects and are relatively expensive. Thus, it is necessary to develop and find new medicines derived from natural ingredients, especially plant-based functional food, so that the availability of these drugs is abundant and the price is also relatively cheaper (4).
A diet high in soy has been associated with a lower prevalence of various types of cancer as well as improved treatment outcomes and lower recurrence rates after cancer diagnosis (5, 6). Tempe, a soy-based food originating from Indonesia, is reported to be capable of inhibiting proliferation and angiogenesis as well as triggering apoptosis in cancer cells (7). Soybean’s anticancer activities are related to phenolic compounds, saponins, and phytic acid as well as enzyme inhibitors such as trypsin and the Bowman-Birk inhibitors, but the most notable compounds are isoflavones, phytochemicals found in soybeans that can act as antioxidants and protect human cells from oxidative stress linked to cancer (8, 9). Genistein, a predominant isoflavone in soy, has been shown to inhibit cancer development, growth, and metastasis in animal models, particularly by modulating the genes related to cell cycle control and apoptosis (5). The fermentation process has been shown to increase the number of bioactive compounds and their activity in a food ingredient (10, 11). This is an interest in functional food development technologies and cemented food-based medicines, especially as promising future anticancer functional food candidates. One preclinical study highlighted the chemopreventive and chemotherapeutic potential of tempe (7).
There have been many review articles discussing tempe, but only in general its health benefits. There has been no review or opinion that summarizes the findings of the latest research that provides clarity on the benefits of tempe as an anticancer functional food. Therefore, the main purpose of this opinion article is to elaborate on the recent finding on the anticancer potential and activities of soy-based tempe. This opinion paper presents updated evidence based on literature reviews about the anticancer potential of soy-based tempe. The author also believes that by sharing this viewpoint, researchers would reinvent the creation of soy-based tempe food products and, of course, carry out more studies on their anticancer effectiveness.
Tempe is a fermented soybean food from Indonesia. Its distinctive taste and nutritional value make tempe one of the most consumed sources of protein for hundreds of years (12). Tempe consumption has the advantage of modulating the gut microbiota towards a healthier profile. This also shows that the consumption of tempe provides a healthier metabolic status. To maintain the composition of the beneficial gut microbiota, the consumption of dietary fiber, paraprobiotics, and probiotics from Soy-based tempe is highly recommended (13). Besides being the main protein source, soy-based tempe has been developed as a carbohydrate substitute in the form of flour. It has been found that tempe flour-based products have higher flavonoid content than wheat-based products (14). Because of its high glutamic acid and protein content, overripe tempe is utilized both as a source of umami flavor and protein in porridge for children under five years old to help overcome children’s malnutrition (15). Recent research has also proven that tempe application as yogurt has higher antioxidant, carbohydrate, protein, and lipid content compared with plain cow milk yogurt, thus it is being mass-produced (16). Furthermore, the bioactive peptides synthesized from soy-based tempe fermentation can be utilized as anti-hypertensive, antioxidant, anti-diabetic, and anticancer agents (17). Because of the various benefits of soy-based tempe, it leaves more room to explore the potential of tempe health properties, specifically as an anticancer agent.
Tempe is a soy-based fermented food that contains bioactive peptides such as calmodulin-dependent cyclic nucleotide phosphodiesterase (CaMPDE) inhibitors which are also rich in isoflavones and their derivatives such as genistein and daidzein (7, 18). Bio-functionality tests on bioactive chemicals derived from tempe have been shown to have anticancer or antitumor action. Anticancer mechanisms mediated by bioactive chemicals in tempe include cancer cell proliferation inhibition, angiogenesis inhibition, antioxidant properties, and induction of cancer cell apoptosis (7, 18). Inhibition of the cancer cell proliferation mechanism by isoflavones happened due to the inactivation of the phosphoinositol 3-kinase (PI3K)/Akt/mTOR pathway (19). When cancer cells are exposed to genistein, an isoflavone derivative, the expression level of the PI3K protein decreases, which has the key role of activating numerous proteins for cell growth and aberrant cell proliferation induction (18–20). Angiogenesis was also inhibited by the presence of genistein, which can reduce either the expression or excretion of vascular endothelial growth factor (VEGF) (7, 21). The VEGF protein stimulates cell proliferation by causing the development of new blood vessels, a process known as angiogenesis. Angiogenesis plays an important role in cancer cell proliferation and metastasis because it supplies adequate oxygen and nutrients (7). Therefore, inhibition of angiogenesis in cancer tissue is an important mechanism in suppressing cancer progression. The antioxidant activity of tempe is due to the presence of isoflavones and 3-hydroxyanthranilic acid which have excellent free radical and superoxide scavenging abilities which have been proven through the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay (22). Tempe’s antioxidant activity helps protect healthy cells from genetic material damage, which can develop into oncogenic genes. Genistein is also able to induce apoptosis through its effect on oxidative phosphorylation in mitochondria due to the inactivation of the anti-apoptotic Bcl-2 gene (7). Because the rise of Bcl-2 expression in cancer cells is not accompanied by an increase in Cas-3 protein, Bcl-2 acts as an anti-apoptotic protein. Meanwhile, genistein promotes the co-expression of Bcl-2 and Cas-3 proteins through the apoptotic protease activating factor (APAF) (Figure 1). An increase in the amount of Cas-3 protein which acts as an effector caspase will trigger apoptosis in cancer cells (23).
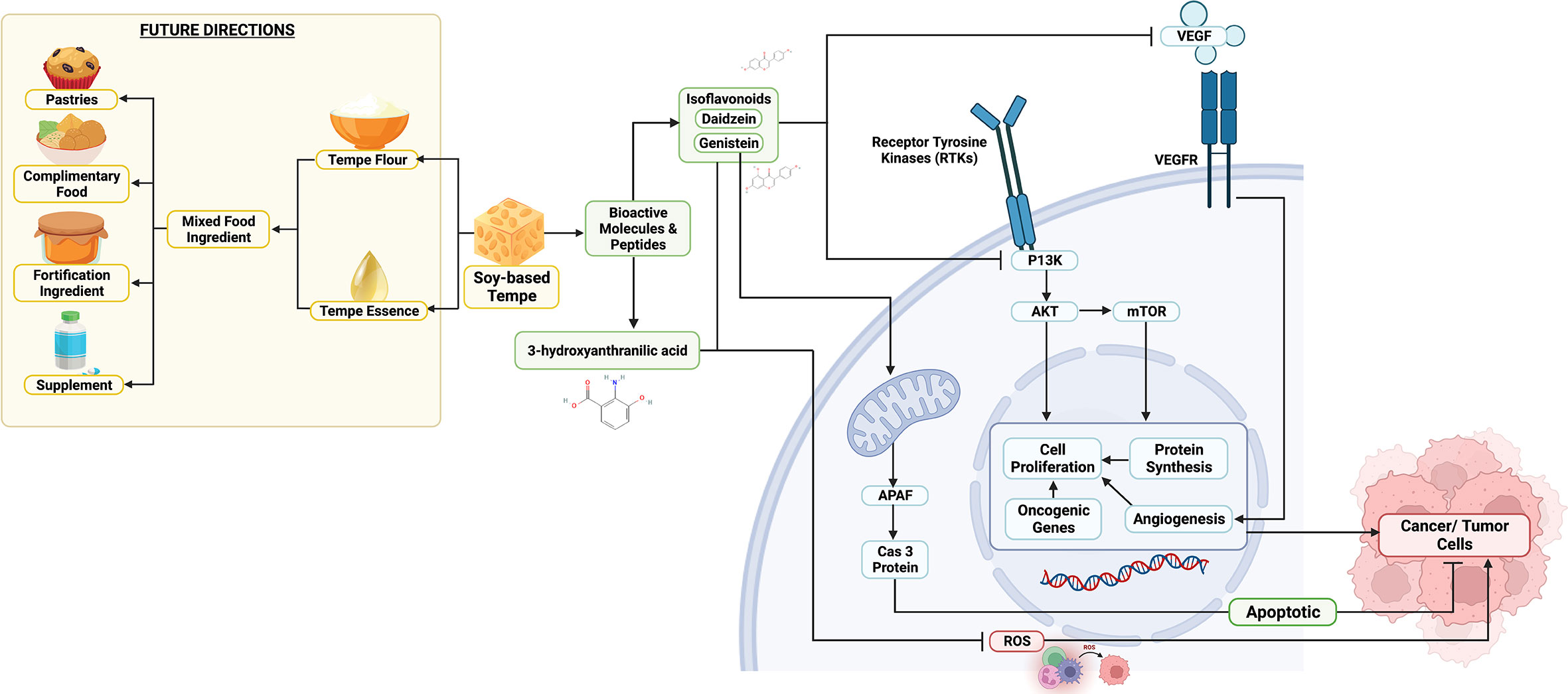
Figure 1 Predicted Mechanism of Anticancer Properties from Soy-Based Tempe and Their Future Meal Product Innovation.
Soy-based tempe – in addition to having promising potential as a functional food anticancer – also has the potential for product development into a varied and innovative healthy meal (Figure 1). Tempe can be used as a mixed food ingredient (MFI). The latest research combined tempe flour with eel flour which has bioactive peptides, nutrition content, and scavenging of potential anticancer free radicals (24, 25). Recent research has also combined tempe flour with algae and has succeeded in determining the potential antioxidant effects, one of which is inhibiting cancer-inducing free radicals (26, 27). Then, tempe flour can also be used as a healthy meal program for toddlers, children, and adults. An example of a food product with tempe essences is complementary food breast milk (breast milk) (28). This is one of the potential efforts from an early age to mediate the incidence of cancer. In addition, tempe can also be used as an ingredient to fortify biscuits for the prevention of anemia (29). This is a special interest for vegetarians or those who are on a plant-based diet because tempe is a vegetable superfood and source of Fe (Iron) which is useful for vegans who tend to have minimal Fe intake. In addition, a study shows that a vegetarian diet seems to protect against cancer (30). This reveals the importance of implementing a soy-based tempe-based healthy meal which can potentially minimize the prevalence of cancer through the mechanisms that have been described in the previous sub and Figure 1.
Tempe primarily contains bioactive peptides and isoflavones which could promote health and prevent cancer due to their antioxidant activities, cancer cell apoptosis, and cancer cell proliferation inhibition activities (7, 18). The role of an antioxidant in cancer therapy is to counterbalance the production of reactive oxygen species (ROS) and their mediated injuries in the affected molecules (DNA, lipids, or proteins) (31). On the other side, a dietary intervention strategy that incorporates food with antioxidant properties also brings more beneficial outcomes on health status (32). Therefore, this opinion supports the consumption of tempe as a dietary antioxidant to promote health due to its anticancer potential, which was also highlighted by Wu et al., 2017 (31). Various applications and processing of tempe to be a functional food had also been described. The combinations of tempe with other ingredients brought more beneficial effects on health, nutrient composition, and more acceptable food products (24–27, 29). Utilizing tempe as a flour product also helps to increase the shelf-life period of the products (33). However, it is important to preserve the fresh probiotic properties of tempe, especially in larger industries or production scales since they have health-beneficial properties (34). Tempe supplementation was also known as a paraprobiotics agent which is capable of modulating gut microbiota, enhancing immunity, and alleviating inflammation (13, 35). Next to that, the importance of foodomics may be crucial for maximizing the health potential of tempe products. Foodomics is a new approach that utilizes the “omics” method in food and nutrition studies to bring forth the optimization of human health and well-being (36). As a fermentation product, tempe produces many bioactive compounds and secondary metabolites which could be further analyzed using the foodomics approach to find the specific compound needed for specific health issues. A metabolomic study found that using different starter cultures of tempe will result in different metabolite profiles of tempe products (12). On the other hand, a proteomic study dove deeper into the antihypertensive, antidiabetic, antioxidant, and antitumor activities of the bioactive peptides from tempe (18). Therefore, this opinion paper encourages more research that brings forth the innovation of tempe as a functional food product or many deeper types of research that utilize the foodomics to draw out tempe best potential as an anticancer, Indonesian functional food.
FN, FQ, VY, SB, GP, DL, MM, EP, TA, SR, HP, WG, FK, and DS, contributed to the conception and design of opinion studies and drafted manuscripts in advance. FN, FQ, and HP edited, revised, and approved the final version of the submitted manuscript. All authors contributed to the article and approved the submitted version.
We offer a great thank you to the Chairman of the Indonesian Association of Clinical Nutrition Physicians, namely Professor Nurpudji Astuti Taslim, MD., MPH., PhD., Sp.GK(K) and Professor Hardinsyah, Ph.D. [President of the Federation of Asian Nutrition Societies (FANS)] who have reviewed and provided suggestions, as well as input on the draft of this opinion article. We also thank Dr. Nelly Mayulu, MD., who has given her views on the nutrition dan functional food programs that are important to do now and in the future for anticancer treatments. We also thank the Nutrifood Research Center (PT. Nutrifood Indonesia) for providing support to the author in this publication. All of these mentioned have no conflict of interest.
Author FK was employed by the company PT. Nutrifood Indonesia.
The remaining authors declare that the study or opinion article was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol (2022) 8(3):420–44. doi: 10.1001/jamaoncol.2021.6987
2. Tran KB, Lang JJ, Compton K, Xu R, Acheson AR, Henrikson HJ, et al. The global burden of cancer attributable to risk factors, 2010–19: A systematic analysis for the global burden of disease study 2019. Lancet (2022) 400(10352):563–91. doi: 10.1016/S0140-6736(22)01438-6
3. Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J (2016) 48(3):889–902. doi: 10.1183/13993003.00359-2016
4. Wangchuk P. Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. J Biologically Active Products Nature (2018) 8:1–20. doi: 10.1080/22311866.2018.1426495
5. Sahin I, Bilir B, Ali S, Sahin K, Kucuk O. Soy isoflavones in integrative oncology: Increased efficacy and decreased toxicity of cancer therapy. Integr Cancer Therapies (2019) 18:1534735419835310. doi: 10.1177/1534735419835310
6. Kucuk O. Soy foods, isoflavones, and breast cancer. Cancer (2017) 123:1901–3. John Wiley & Sons, Ltd. doi: 10.1002/cncr.30614
7. Bintari SH, Nugraheni K. The potential of tempeh as a chemopreventive and chemotherapeutic agent targeting breast cancer cells. Pakistan J Nutr (2017) 16(10):743–9. doi: 10.3923/pjn.2017.743.749
8. Lima A, Oliveira J, Saúde F, Mota J, Ferreira RB. Proteins in soy might have a higher role in cancer prevention than previously expected: Soybean protein fractions are more effective MMP-9 inhibitors than non-protein fractions, even in cooked seeds. Nutrients (2017) 9(3):201. doi: 10.3390/nu9030201
9. Romulo A, Surya R. Tempe: A traditional fermented food of Indonesia and its health benefits. Int J Gastronomy Food Science (2021) 26:100413. doi: 10.1016/j.ijgfs.2021.100413
10. Limón RI, Peñas E, Torino MI, Martínez-Villaluenga C, Dueñas M, Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem (2015) 172:343–52. doi: 10.1016/j.foodchem.2014.09.084
11. Gumienna M, Szwengiel A, Górna B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur Food Res Technol (2016) 242:631–40. doi: 10.1007/s00217-015-2582-z
12. Kadar AD, Astawan M, Putri SP, Fukusaki E. Metabolomics based study of the effect of raw materials to the end product of tempe–an indonesian fermented soybean. Metabolites (2020) 10(9):1–11. doi: 10.3390/metabo10090367
13. Subandi ST, Kartawidjajaputra F, Silo W, Yogiara Y, Suwanto A. Tempeh consumption enhanced beneficial bacteria in the human gut. Food Res (2019) 3(1):57–63. doi: 10.26656/fr.2017.3(1).230
14. Bintari SH, Putri MF, Saputro DD, Parman S. Sunyoto. the potential effect of high flavonoid soybean diversification products through tempe flour substitution. In: Journal of physics: Conference series (2020) United Kingdom: IOP Publishing.
15. Fortunata SA, Puteri MDPTG, Christli L, Permana T, Santoso F. Overcoming malnutrition problem by increasing nutritional awareness in desa pagedangan. Pros Konf Nas Pengabdi Kpd Masy dan Corp Soc Responsib (2019) 2:497–508. doi: 10.37695/pkmcsr.v2i0.454
16. Bintari SH, Parman S. Antioxidant capacity and nutritional value of tempe yogurt. In: Journal of physics: Conference series. Institute of Physics Publishing (2019).
17. Puteri NE, Astawan M, Palupi NS, Wresdiyati T, Takagi Y. Characterization of biochemical and functional properties of water-soluble tempe flour. Food Sci Technol (2018) 38:147–53. doi: 10.1590/fst.13017
18. Tamam B, Syah D, Suhartono MT, Kusuma WA, Tachibana S, Lioe HN. Proteomic study of bioactive peptides from tempe. J Biosci Bioeng (2019) 128(2):241–8. doi: 10.1016/j.jbiosc.2019.01.019
19. Zhu Y, Yao Y, Shi Z, Everaert N, Ren G. Synergistic effect of bioactive anticarcinogens from soybean on anti-proliferative activity in MDA-MB-231 and MCF-7 human breast cancer cells in vitro. Molecules (2018) 23(7):1557. doi: 10.3390/molecules23071557
20. Zhu S, Zhou J, Sun X, Zhou Z, Zhu Q. ROS accumulation contributes to abamectin-induced apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway in TM3 leydig cells. J Biochem Mol Toxicol (2020) 34(8):e22505. doi: 10.1002/jbt.22505
21. Sutrisno S, Aprina H, Simanungkalit HM, Andriyani A, Barlianto W, Sujuti H, et al. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J Tradit Complement Med (2018) 8(2):278–81. doi: 10.1016/j.jtcme.2017.03.002
22. Cao ZH, Green-Johnson JM, Buckley ND, Lin QY. Bioactivity of soy-based fermented foods: A review. Biotechnol Adv (2019) 37:223–38. Elsevier. doi: 10.1016/j.biotechadv.2018.12.001
23. Shafiee G, Saidijam M, Tayebinia H, Khodadadi I. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch Physiol Biochem (2022) 128(3):694–702. doi: 10.1080/13813455.2020.1717541
24. Ngadiarti I, Nurkolis F, Handoko MN, Perdana F, Muntikah M, Sabrina N, et al. Physicochemical properties and nutrient content of Tempe flour enriched eel flour. Open Access Maced J Med Sci (2022) 10(A):552–6. doi: 10.3889/oamjms.2022.8308
25. Sabrina N, Rizal M, Nurkolis F, Hardinsyah H, Tanner MJ, Ben GW, et al. Bioactive peptides identification and nutritional status ameliorating properties on malnourished rats of combined eel and soy-based Tempe flour. Front Nutr (2022) 1AD;0:2196. doi: 10.3389/fnut.2022.963065
26. Permatasari HK, Nurkolis F, Vivo CD, Noor SL, Rahmawati R, Radu S, et al. Sea Grapes powder with the addition of tempe rich in collagen: An anti-aging functional food. F1000Research (2022) 10:789. doi: 10.12688/f1000research.55307.3
27. Nurkolis F, Mantik KEK, Kuswari M, Perdana F, Mayulu N, Tanner MJ, et al. Sea Grape (Ceulerpa racemosa) cereal with addition of Tempe as an anti-aging functional food: In vitro study. Curr Dev Nutr (2021) 5(Supplement_2):41–1. doi: 10.1093/cdn/nzab033_041
28. Sukardi S, Wista ST, Wahyudi VA. Formulation and antioxidant test of baby instant porridge with kepok banana flour and Tempe. Food Technol Halal Sci J (2021) 4(2):142–55. doi: 10.22219/fths.v4i2.16502
29. Bolang ASL, Rizal M, Nurkolis F, Mayulu N, Taslim NA, Radu S, et al. Cookies rich in iron (Fe), folic acid, cobalamin (vitamin B12), and antioxidants: a novel functional food potential for adolescent with anemia. F1000Research (2021) 10:1075. doi: 10.12688/f1000research.74045.2
30. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr (2017) 57(17):3640–9. doi: 10.1080/10408398.2016.1138447
31. Wu X, Cheng J, Wang X. Dietary antioxidants: Potential anticancer agents. Nutr Cancer (2017) 69(4):521–33. doi: 10.1080/01635581.2017.1299872
32. Medina-Vera I, Gómez-De-regil L, Gutiérrez-Solis AL, Lugo R, Guevara-Cruz M, Pedraza-Chaverri J, et al. Dietary strategies by foods with antioxidant effect on nutritional management of dyslipidemias: A systematic review. Antioxidants (2021) Vol. 10:1–19. doi: 10.3390/antiox10020225
33. Prabakaran M, Lee KJ, An Y, Kwon C, Kim S, Yang Y, et al. Changes in soybean (Glycine max l.) flour fatty-acid content based on storage temperature and duration. Molecules (2018) 23(10):2713. doi: 10.3390/molecules23102713
34. Huang YC, Wu BH, Chu YL, Chang WC, Wu MC. Effects of tempeh fermentation with lactobacillus plantarum and rhizopus oligosporus on streptozotocin-induced type II diabetes mellitus in rats. Nutrients (2018) 10(9) 1143. doi: 10.3390/nu10091143
35. Siciliano RA, Reale A, Mazzeo MF, Morandi S, Silvetti T, Brasca M. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients. (2021) 13:1225. Multidisciplinary Digital Publishing Institute. doi: 10.3390/nu13041225
Keywords: tempe, anticancer, soy, molecular and cellular oncology, antioxidant, phytochemicals, peptides, functional food
Citation: Nurkolis F, Qhabibi FR, Yusuf VM, Bulain S, Praditya GN, Lailossa DG, Mahira MFNA, Prima EN, Arjuna T, Rahayu S, Gunawan WB, Kartawidjajaputra F, Subali D and Permatasari HK (2022) Anticancer properties of soy-based tempe: A proposed opinion for future meal. Front. Oncol. 12:1054399. doi: 10.3389/fonc.2022.1054399
Received: 26 September 2022; Accepted: 11 October 2022;
Published: 24 October 2022.
Edited by:
Marc Poirot, INSERM U1037 Centre de Recherche en Cancérologie de Toulouse, FranceReviewed by:
Abdelbaset Mohamed Elasbali, Al Jouf University, Saudi ArabiaCopyright © 2022 Nurkolis, Qhabibi, Yusuf, Bulain, Praditya, Lailossa, Mahira, Prima, Arjuna, Rahayu, Gunawan, Kartawidjajaputra, Subali and Permatasari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahrul Nurkolis, ZmFocnVsLm51cmtvbGlzLm1haWxAZ21haWwuY29t
†These authors have contributed equally to this work
‡These authors share senior authorship
§ORCID: Fahrul Nurkolis, orcid.org/0000-0003-2151-0854
Faqrizal Ria Qhabibi, orcid.org/0000-0001-5320-358X
Vincentius Mario Yusuf, orcid.org/0000-0002-7645-731X
Stanley Bulain, orcid.org/0000-0002-3779-4663
Ghevira Naila Praditya, orcid.org/0000-0002-5464-2645
Deogifta Graciani Lailossa, orcid.org/0000-0003-2335-2466
Msy Firyal Nadya Al Mahira, orcid.org/0000-0002-7272-8135
Eka Nugraha Prima, orcid.org/0000-0001-6380-6050
Tony Arjuna, orcid.org/0000-0001-7357-3529
Shilfiana Rahayu, orcid.org/0000-0003-3666-2519
William Ben Gunawan, orcid.org/0000-0003-0633-4477
Felicia Kartawidjajaputra, orcid.org/0000-0002-9110-2584
Dionysius Subali, orcid.org/0000-0003-0889-2827
Happy Kurnia Permatasari, orcid.org/0000-0002-4777-624X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.