
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 December 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1050070
This article is part of the Research Topic Recent Developments in Pancreatic Cancer Radiotherapy View all 11 articles
 Jae Hyup Jung1
Jae Hyup Jung1 Changhoon Song2
Changhoon Song2 In Ho Jung1
In Ho Jung1 Jinwoo Ahn1
Jinwoo Ahn1 Bomi Kim1
Bomi Kim1 Kwangrok Jung1
Kwangrok Jung1 Jong-Chan Lee1
Jong-Chan Lee1 Jaihwan Kim1
Jaihwan Kim1 Jin-Hyeok Hwang1*
Jin-Hyeok Hwang1*Introduction: FOLFIRINOX (the combination of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) is the preferred systemic regimen for locally advanced pancreatic cancer (LAPC). Furthermore, stereotactic body radiation therapy (SBRT) is a promising treatment option for achieving local control in these patients. However, clinical outcomes in patients with LAPC treated using FOLFIRINOX followed by SBRT have not been clarified. Therefore, we aimed to evaluate clinical outcomes of induction FOLFIRINOX treatment followed by SBRT in patients with LAPC.
Methods: To this end, we retrospectively reviewed the medical records of patients with LAPC treated with induction FOLFIRINOX followed by SBRT in a single tertiary hospital. We evaluated overall survival (OS), progression-free survival (PFS), resection rate, SBRT-related adverse events, and prognostic factors affecting survival.
Results: Fifty patients were treated with induction FOLFIRINOX for a median of 8 cycles (range: 3–28), which was followed by SBRT. The median OS and PFS were 26.4 (95% confidence interval [CI]: 22.4–30.3) and 16.7 months (95% CI: 13.0–20.3), respectively. Nine patients underwent conversion surgery (eight achieved R0) and showed better OS than those who did not (not reached vs. 24.1 months, p = 0.022). During a follow-up period of 23.6 months, three cases of grade 3 gastrointestinal bleeding at the pseudoaneurysm site were noted, which were managed successfully. Analysis of the factors affecting clinical outcomes revealed that a high radiation dose (≥ 35 Gy) resulted in a higher rate of conversion surgery (25% [8/32] vs. 5.6% [1/18], respectively) and was an independent favorable prognostic factor for OS in the adjusted analysis (hazard ratio: 2.024, 95% CI: 1.042–3.930, p = 0.037).
Conclusion: Our findings suggest that induction FOLFIRINOX followed by SBRT in patients with LAPC results in better survival with manageable toxicities. A high total SBRT dose was associated with a high rate of conversion surgery and could afford better survival.
Pancreatic cancer (PC) is the third leading cause of cancer-related deaths in the United States and it has been responsible for 49,830 deaths thus far in 2022. The death rate for PC has increased slightly since the mid-2000s (1, 2). Surgical resection is the only curative treatment for PC; however, only 10-15% of affected patients are considered suitable for surgical resection at the time of diagnosis. Approximately 30–35% of patients were diagnosed with locally advanced PC (LAPC), and the 5-year survival rate in LAPC was less than 15% (3). Conventionally, systemic chemotherapy with or without traditional fractionated external-beam radiotherapy (EBRT) was considered the standard of treatment for patients with LAPC (4–6). However, some randomized controlled trials (7, 8) investigating EBRT have reported unsatisfactory results in terms of efficacy, with considerable radiation-related adverse events (AEs). Moreover, conventional EBRT with concurrent chemotherapy may require quite a few weeks for completion (9).
Since the publication of a randomized trial by Conroy et al. in 2011 (10), the combination of folinic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) has become the standard of care for metastatic PC (11). Several studies have demonstrated that FOLFIRINOX is also effective in LAPC; thus, FOLFIRINOX is the preferred systemic chemotherapy regimen in patients with good performance status (12, 13). Furthermore, the 2022 National Comprehensive Cancer Network guidelines recommend FOLFIRINOX as the preferred systemic treatment for patients with LAPC (14).
However, over 70% of patients with LAPC are ineligible candidates for resection even after induction chemotherapy because either their lesions are not sufficiently reduced in size to be suitable for surgery or due to locoregional progression (15–18). Therefore, local ablative therapies have been explored as new therapeutic options for patients with LAPC, which could increase locoregional disease control rates (19–21). Among them, stereotactic body radiation therapy (SBRT) is a promising treatment that can overcome radio-resistance because it allows precise delivery of high-dose radiation while reducing radiation treatment-related AEs. The latter is achieved by decreasing the radiation dose delivered to adjacent healthy tissue compared to that associated with conventional EBRT (6, 22, 23).
In 2004, Koong et al. (24) conducted a dose-escalation study using SBRT for pancreatic cancer, which showed favorable results in terms of local disease control. In several retrospective (25–29) and single-arm prospective studies (LAPC-1 trial) (30, 31), sequential treatment with induction chemotherapy (FOLFIRINOX or other regimens) followed by SBRT yielded encouraging results in terms of local control in patients with LAPC. Moreover, SBRT was associated with a favorable rate of conversion surgery among patients with LAPC, which could result in better survival (32).
The addition of SBRT is a promising treatment option for patients with LAPC; however, no consensus exists regarding the patients suitable for this treatment, when it should be administered, and the clinical factors that should be considered for better clinical outcomes (14, 33, 34). Therefore, in the present study, we aimed to evaluate clinical outcomes of induction FOLFIRINOX followed by SBRT in patients with LAPC at a single tertiary teaching hospital.
Electronic medical records of patients with LAPC who were treated between December 2015 and September 2020 at a single tertiary teaching hospital (Seoul National University Bundang Hospital, Seoungnam, South Korea) were retrospectively reviewed. The patients were treated with induction FOLFIRINOX regimen (oxaliplatin, 85 mg per m2 of the body-surface area; irinotecan, 180 mg per m2; leucovorin, 400 mg per m2; and fluorouracil, 400 mg per m2 delivered as a bolus followed by 2400 mg per square meter administered as a 46-hour continuous infusion, every 2 weeks) followed by SBRT (11). The inclusion criteria were as follows: (1) patients with LAPC diagnosed based on the results of radiological evaluations and a multidisciplinary conference, (2) patients who had received induction FOLFIRINOX (≥1 cycle) and were unsuitable candidates for conversion surgery despite induction FOLFIRINOX based on multidisciplinary discussion, (3) patients who revealed no evidence of metastatic disease or gastric or duodenal invasion at the time of SBRT, (4) patients who had not previously received abdominal radiotherapy, and (5) patients without a history of other malignancies within 5 years.
The patients’ baseline characteristics were assessed at diagnosis and before SBRT. Overall survival (OS), progression-free survival (PFS), resection rate, SBRT-related AEs, and prognostic factors were assessed. Furthermore, survival, disease progression, and resection data until 31 March 2022 were evaluated. OS was defined as the time from histological diagnosis to all-cause death or the last follow-up. PFS was defined as the time from histological diagnosis to radiological progression according to the Response Evaluation Criteria in Solid Tumors criteria version 1.1, all-cause death, or last follow-up. Locoregional progression was defined as disease progression within the primary tumor or peripancreatic lymph nodes, and distant progression was defined as distant metastasis. For those who underwent conversion surgery, T and N stages were assessed using resected specimens according to the eighth edition of the American Joint Committee on Cancer Staging System. The pathological response of the tumor to previous chemotherapy or radiotherapy was assessed according to the tumor regression scoring system of the College of American Pathologists (CAP) version 4.2. SBRT-related AEs were assessed according to the National Cancer Institute Common Terminology for Adverse Events version 5.0. SBRT-related acute and late AEs were defined as AEs occurring within 90 days and after 90 days from radiation therapy, respectively.
Patients were treated with five-fraction SBRT on 5 consecutive business days by using a Varian TruBeam linear accelerator (Varian Medical Systems Inc., Palo Alto, CA, USA). SBRT was initiated within 2 weeks after the completion of chemotherapy. At the time of simulation, a four-dimensional computed tomography (CT) (Philips Brilliance Big Bore CT scanner, Philips Medical Systems, Cleveland, OH, USA) simulation was performed during free breathing to determine the position variation of the pancreas and organ at risk (OAR). The respiratory cycle was recorded using an abdominal bellows strap (Philips Healthcare, Best, Netherlands). Thin-sliced CT scans with intravenous contrast were obtained, with patients positioned supine and arms above the head in a Body Pro-Lok ONE device (CIVCO Medical Solutions, Orange City, IA, USA) for immobilization. Pre-treatment diagnostic CT or magnetic resonance imaging (MRI) scans were matched if they provided better delineation of the tumor than did simulation CT images.
The Eclipse planning system was used for target and OAR delineation and treatment planning. Gross tumor volume (GTV) included the gross tumor and adjacent vessels, such as the common hepatic artery, celiac axis, and/or superior mesenteric vessels. The internal target volume (ITV) was obtained by summing the GTVs for all respiratory phases. The planning target volume (PTV) was generated by adding a 2-mm margin circumferentially and a 4- to 6-mm margin craniocaudally to the ITV. A 3-mm margin was added to the OAR volumes to obtain the planning OAR volume (PRV). The modified PTV was obtained from the PTV by subtracting the PRV. The desired prescribed dose was 40 Gy delivered in five fractions. Ninety-five percent of the modified PTV should be covered by the prescribed dose and at least 95% of the PTV should be covered by 30 Gy. If the desired prescribed dose violated the constraints of the OARs, the prescription dose was lowered from 40 to 35, 33, or 30 Gy. The OAR constraints were as follows: stomach and duodenum: Dmax ≤ 32 Gy, V20 < 3 cc, and V15 < 9 cc, and other small bowel intestine: Dmax: 35 Gy and V20 < 20 cc. Cone-beam CT was performed for positional validation before the delivery of each fraction. Daily cone beam CT 3-dimensional images without fiducial were registered to planning CT images. Patients were aligned to the spine and then shifted to align to great vessels, including the aorta, celiac axis, and/or superior mesenteric artery. Although the soft tissue is rarely visible on cone beam CT, soft tissue was sometimes used in alignment when visible.
To compare the patients’ baseline characteristics, chi-square or Fisher’s exact test was used for categorical scales, and the t-test or Mann-Whitney U test was used for numerical scales. OS and PFS were evaluated using the Kaplan-Meier method, and differences in survival were analyzed using the log-rank test. The Cox proportional hazards model was used to analyze survival and other factors. The values of all continuous variables were dichotomized on the entire sample (< median vs. ≥ median) in univariate and multivariate cox proportional analyses. All tests were double-sided with a p-value of less than 0.05 being statistically significant. Multivariate analysis was performed using variables with p-values of less than 0.1 in the univariate analyses. All statistical analyses were performed using SPSS software version 25 (IBM Corporation, New York, USA) and R software version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Fifty patients were retrospectively evaluated, and the median follow-up period was 23.6 months. Among them, 39 (78.0%) patients died during the follow-up period and 11 (22.0%) were alive until March 31, 2022. The median age of the patients was 64.1 (range: 47.8–81.6) years. Twenty-eight (56.0%) patients were female, and 30 (60.0%) had pancreatic head or neck cancer. The median body mass index was 22.7 kg/m2 at diagnosis and before SBRT. The median serum albumin and CA 19-9 levels changed from 4.0 to 3.9 g/dL and from 106.0 to 48.5 U/mL, respectively, after induction FOLFIRINOX. The median tumor size changed from 3.2 to 2.9 cm after induction FOLFIRINOX. The median number of cycles and duration of FOLFIRINOX treatment was 8 (range: 3–28) and 4.9 (range: 1.4–21.7) months, respectively. Thirty-nine (78.0%) patients showed stable disease as the best response during induction FOLFIRINOX, while 11 (22.0%) showed partial response. The median time to SBRT from diagnosis, the total dose of SBRT, and SBRT dose per fraction was 6.1 (range: 2.8–22.3) months, 35 (range: 30–40) Gy in five fractions, and 7 (6–8) Gy, respectively. Nine (18.0%) patients underwent conversion surgery after SBRT during the follow-up period (median: 3.5 months, range: 0.8–11.7 months) (Table 1).
The patients’ median OS and PFS were 26.3 months (95% confidence interval [CI]: 22.4–30.3) and 16.7 months (95% CI: 13.0–20.3), respectively (Figures 1A, B). Nine patients (18%) who underwent conversion surgery showed longer OS than did those who did not (not reached vs. 24.1 months, p = 0.022). (Figure 2A) and longer median PFS (35.2 months vs. 16.0 months, p = 0.001) (Figure 2B). Among them, eight underwent margin-negative resection. The T and N stage distributions were as follows: five in T1 and four in T2 and seven in N0 and two in N1. One patient revealed a near-complete response (CAP grade 1), and eight exhibited a partial response (CAP grade 2) (Table 2). Among the 34 patients who exhibited disease progression after SBRT, 9 (26.5%) showed locoregional progression without distant metastasis (Table 3).
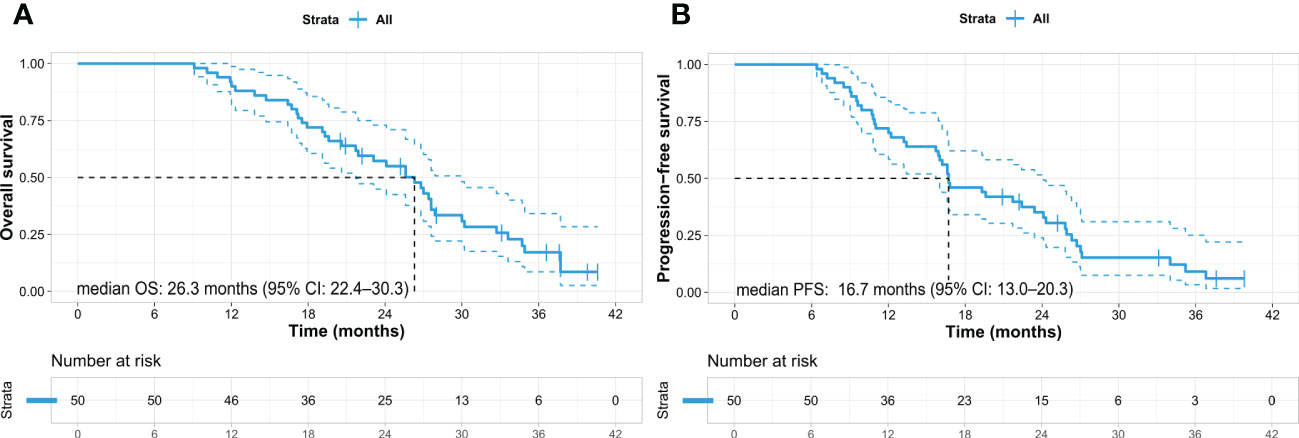
Figure 1 Kaplan–Meier survival curves in the entire cohort of pancreatic cancer patients. (A) Overall survival and (B) progression-free survival. OS, overall survival; PFS, progression-free survival; CI, confidence interval.
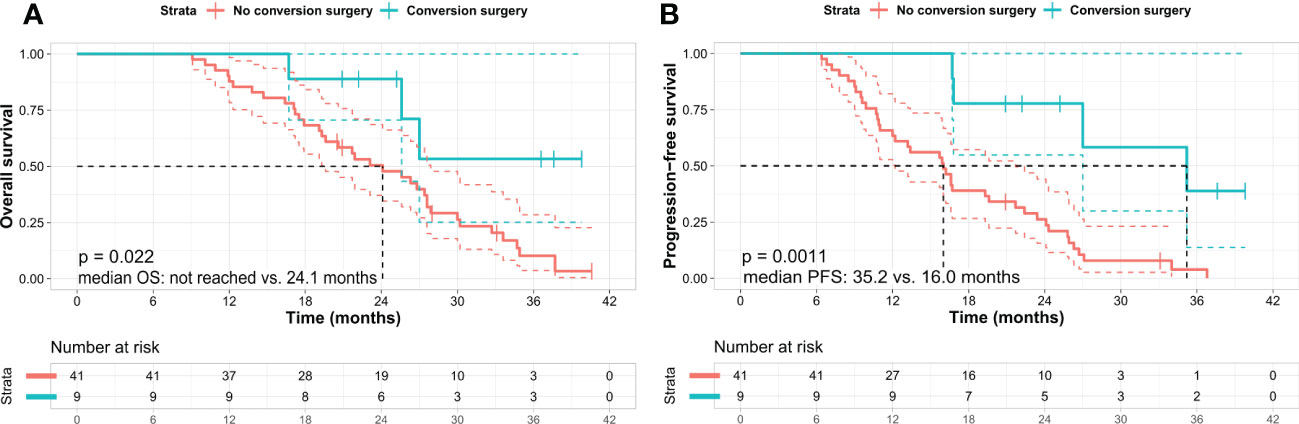
Figure 2 Kaplan–Meier survival curves in patients according to the conversion surgery. (A) Overall survival and (B) progression-free survival. Kaplan–Meier analysis shows that the patients who underwent conversion surgery exhibit better OS and PFS than those who did not. Log-rank test p-value was (A) 0.022 and (B) 0.001 between the two subgroups, respectively. OS, overall survival; PFS, progression-free survival.
SBRT-related AEs of grade 3 or higher occurred within 1 year of SBRT in three patients who had gastrointestinal (GI) bleeding at the pseudoaneurysm site. These bleeding events were controlled by supportive management and transarterial embolization (dose ≥ 35 Gy in two patients and < 35 Gy in one). SBRT-related acute AEs of grade 2 or lower included anorexia (three patients), fatigue (three patients), nausea (three patients), vomiting (one patient), and diarrhea (five patients). SBRT-related late AEs of grade 2 or lower were gastritis (two patients), GI ulcer (four patients), and non-significant GI bleeding (one patient). These complications were well-managed conservatively, and there were no deaths due to these complications (Table 4).
The two variables (Tumor size (pre-SBRT) and Total SBRT dose) with a p-value of less than 0.1 in univariate cox analysis were used for adjusted analysis in OS and PFS. Analysis of OS by using adjusted variables showed that a high total dose of SBRT was an independent and significant favorable prognostic factor (≥ 35 vs. < 35 Gy; 27.0 vs. 24.1 months; hazard ratio [HR] 2.024; 95% CI 1.042–3.930; p = 0.037) (Table 5, Figure 3A), although there were no statistically significant differences between the high and low total SBRT dose groups in terms of baseline characteristics (Supplementary Table). Moreover, a higher total dose of SBRT resulted in a higher resection rate than did a lower total dose of SBRT (25.0% vs. 5.6%, p = 0.086). Analysis of PFS using adjusted variables showed that a high total dose of SBRT (≥ 35 vs. < 35 Gy; 19.3 vs. 13.2 months; HR 2.364; 95% CI 1.218–4.588, p = 0.011) and small tumor size (< 2.9 vs. ≥ 2.9 cm; 23.4 vs. 15.9 months; HR: 1.853; 95% CI: 1.005–3.416, p = 0.048) were independent and significant favorable prognostic factors (Table 6 and Figure 3B).
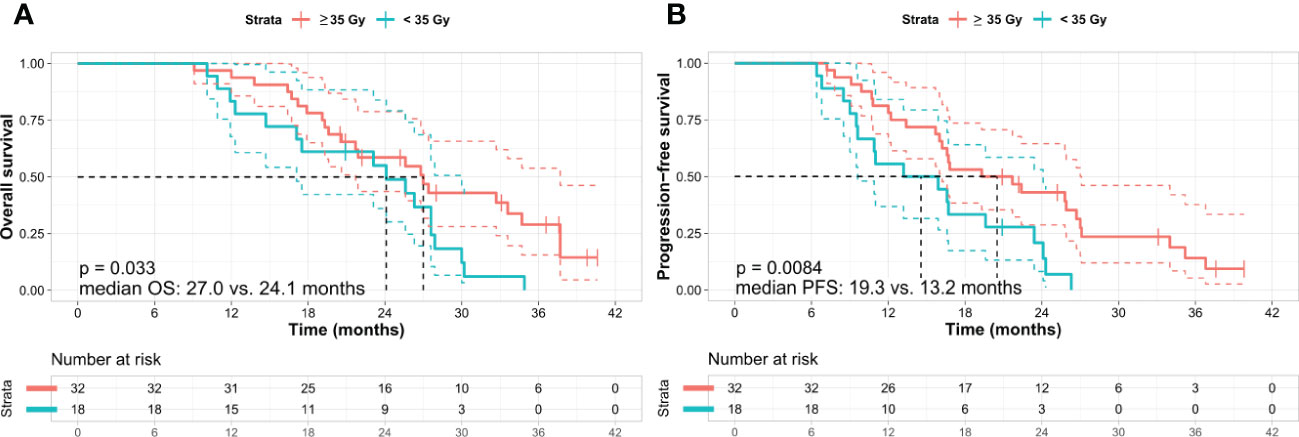
Figure 3 Kaplan–Meier survival curves according to the total dose of SBRT. (A) Overall survival and (B) progression-free survival. Kaplan–Meier analysis shows that the patients treated with a high total dose of SBRT (≥ 35 Gy in five fractions) exhibit better OS and PFS than those who received a low total dose of SBRT (< 35 Gy in five fractions). Log-rank test p-values were (A) 0.033 and (B) 0.008 between the two subgroups, respectively. OS, overall survival; PFS, progression-free survival; Gy, gray.
In the present study, we investigated the feasibility of induction FOLFIRINOX followed by SBRT as a strategy for local control and increased possibility of conversion surgery in patients with LAPC. Furthermore, we explored whether this strategy improves survival. After discussing multidisciplinary approach for one patient whose disease was stable but remained unresectable after sufficient FOLFIRINOX, sequential SBRT was conducted on patients who considered it helpful. Resultantly, 18.0% of patients who were considered unsuitable candidates for surgery despite induction FOLFIRINOX could undergo conversion surgery, and most patients achieved R0 resection. Moreover, the first recurrency occurred more often at the distant site than at the locoregional site, and SBRT-related AEs were rare and manageable. Therefore, induction FOLFIRINOX followed by SBRT may be a promising treatment strategy for patients who remained unresectable despite induction FOLFIRINOX, given its considerable efficacy (conversion rate and locoregional control rate) and acceptable SBRT-related AEs.
Several recent studies have investigated the issue of induction chemotherapy followed by SBRT. Mellon EA et al. (27) studied 49 patients with LAPC who received induction chemotherapy (43% of them were treated with FOLFIRINOX) followed by SBRT (30 Gy in five fractions), and their results showed a median OS of 15 months. Moningi S et al. (28) also reported similar results in 74 patients with LAPC who received induction chemotherapy (24% of them were treated with FOLFIRINOX) followed by SBRT (25–33 Gy in five fractions), with a median OS of 18.4 months. These results were worse than our results (median OS of 26.3 months), probably due to the reduced dose of SBRT and lower potency of induction chemotherapy. This suggestion is supported by a previous study (29) in which a combination of modified FOLFIRINOX and a higher SBRT dose (≥ 40 Gy in five fractions) reported results similar to ours (median OS of 24 months). Conversely, a small prospective trial (LAPC-1 trial) (30, 31) reported an OS of 18 months in 39 patients treated with induction FOLFIRINOX followed by SBRT (40 Gy in five fractions), which was worse than the OS (26 months) we identified.
Not all patients in the present study could undergo resection despite prior induction FOLFIRINOX. However, after additional SBRT, nine patients (18%) could undergo curative resection (R0 resection in eight and N0 in seven); this finding was similar to that shown in other studies (27, 28, 30, 31). However, results of the present study cannot be explained solely based on SBRT because of selection bias due to the retrospective nature of the study. Nevertheless, considering that 18% of the patients who were unsuitable candidates for surgery after sufficient induction chemotherapy (median eight cycles of FOLFIRINOX) were able to undergo resection after continuing FOLFIRINOX with simultaneous SBRT, SBRT may arguably play a role in these patients. Recently, in a phase 2 randomized clinical trial (35), neoadjuvant FOLFIRINOX was used in patients with borderline resectable PC with or without hypofractionated radiation therapy. That trial showed that additional hypofractionated radiation therapy did not improve the 18-month OS and R0 resection rates. However, 12.5% of the patients in the study received a lower radiation dose (hypofractionated image-guided radiotherapy: 25 Gy in five fractions), which could have influenced the outcomes, since a higher radiation dose was associated with better outcomes in ours and other studies (29, 36).
SBRT-related AEs in the present study were well tolerated and managed, which was similar to that in previous studies (24–31). Furthermore, these AEs were less frequent than those associated with conventional EBRT (7–9). It is well known that GI bleeding is a severe late complication in patients and is more often observed in those who receive single-fraction SBRT compared with that in those who receive multi-fraction SBRT (37). In the present study, in which all patients received five fractions, three cases of grade 3 GI bleeding at the pseudoaneurysm site were noted and were well controlled by transarterial embolization, which was similar to the results of other studies (29, 30). One patient who died due to bowel perforation occurred sequentially superior mesenteric artery and superior mesenteric vein thrombosis, bowel infarction, and bowel perforation within five months after surgery. SBRT could contribute to the increased difficulty of surgery that resulted in severe surgical complications. Still, it is difficult to determine a direct causality and cannot be explained solely based on SBRT.
A higher total dose of SBRT (35 Gy) showed a trend toward better OS than did a dose of 30 Gy or less. This finding was similar to that in other studies (29, 36). Moreover, 25% (8 of 32) of the patients treated with a higher total dose of SBRT underwent surgery subsequently, compared with 5% (1 of 18) treated with a lower total dose of SBRT. However, the two groups were not significantly different in terms of radiation-related AEs. Taken together, these findings suggest that when additional SBRT is necessary and feasible for LAPC, a higher total dose of SBRT may be recommended, considering its efficacy and safety. More prospective studies are needed to determine the appropriate SBRT protocols and whether they have clinical benefits.
This study has some limitations. First, this was a retrospective study conducted in a single tertiary center, which may have resulted in selective bias. However, the enrolled patients had a uniform disease status and remained unsuitable candidates for resection after induction FOLFIRINOX and received radiation therapy using a uniform SBRT protocol, which provided informative results that were easy to apply in clinical practice. Second, we did not use fiducial marker placement to target tumors accurately during SBRT because this product was unavailable for clinical practice in Korea. However, SBRT without fiducial markers in our study was associated with manageable AEs compared with those associated with SBRT in other studies that used fiducial markers (38). Third, the interval and total cycles of FOLFIRINOX were not standardized because of this study’s retrospective design. FOLFIRINOX was continued until sequential SBRT was initiated, which was decided in a multidisciplinary discussion. However, except for one extreme case (28 cycles), the FOLFIRINOX cycles for the remaining patients ranged from 3 to 16. Moreover, the median cycle of FOLFIRINOX was similar to that used in other studies (30, 31).
The strategy of adding SBRT to LAPC patients who had received FOLFIRINOX was not standardized. LAPC-1 trial (30, 31) showed the advantage of SBRT followed by induction FOLFIRINOX in improving survival in patients initially inoperable at diagnosis. A large-sample size study (Gemenetzis et al.) (32) revealed that additional SBRT would contribute to an increased resection rate in patients with LAPC suitable for surgical exploration after FOLFIRINOX. However, there has yet to be a consensus on the role of SBRT in which clinical situations SBRT may be beneficial in LAPC patients who have received induction chemotherapy. Our study enrolled patients who remained unresectable (with reduced CA 19-9 but no significant change in tumor size) despite sufficient chemotherapy. Furthermore, among nine patients who underwent resection in our study, five patients received induction FOLFRINOX for more than eight cycles (range 10-15 cycles), unlike the LAPC-1 trial (induction FOLFIRINOX up to 8 cycles). SBRT may be helpful when curative resection is not possible despite sufficient induction chemotherapy in actual clinical practice. Our results may be valuable when making a decision (adding SBRT vs. continuing FOLFIRINOX) in the patients who remained unresectable despite sufficient chemotherapy. Furthermore, our study aimed to identify clinical factors influencing a better prognosis for these strategies and revealed that a higher total dose of SBRT could result in a better resection rate and OS.
In conclusion, induction FOLFIRINOX followed by SBRT in LAPC results in favorable OS and PFS with manageable AEs related to SBRT. A high total dose of SBRT (≥ 35 Gy in five fractions) can improve survival with a higher resection rate.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the institutional review board of Seoul National University Bundang Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JJ, CS, and J-HH conceived and designed research. JJ, IJ, JA, BK, and KJ collected and assembled the data. JJ and J-HH performed or supervised analyses. JJ, J-CL, JK, CS, and J-HH interpreted the results. JJ and J-HH performed statistical expertise. JJ, CS, and J-HH wrote sections of the initial draft. J-CL and JK provided substantive suggestions for revision. J-CL, JK, CS, J-HH provided the provision of study materials or patients. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1050070/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open (2021) 4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708
3. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: A review. JAMA (2021) 326(9):851–62. doi: 10.1001/jama.2021.13027
4. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med (2004) 350(12):1200–10. doi: 10.1056/NEJMoa032295
5. Tempero MA, Malafa MP, Behrman SW, Benson AB 3rd, Casper ES, Chiorean EG, et al. Pancreatic adenocarcinoma, version 2.2014: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw (2014) 12(8):1083–93. doi: 10.6004/jnccn.2014.0106
6. de Geus SWL, Eskander MF, Kasumova GG, Ng SC, Kent TS, Mancias JD, et al. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer (2017) 123(21):4158–67. doi: 10.1002/cncr.30856
7. Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouche O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol (2008) 19(9):1592–9. doi: 10.1093/annonc/mdn281
8. Hammel P, Huguet F, van Laethem J-L, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA (2016) 315(17):1844–53. doi: 10.1001/jama.2016.4324
9. Sultana A, Smith CT, Cunningham D, Starling N, Tait D, Neoptolemos JP, et al. Systemic review, including meta-analyses, on the management of locally advanced pancreatic cancer using Radiation/Combined modality therapy. Br J Cancer (2007) 96(8):1183–90. doi: 10.1038/sj.bjc.6603719
10. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med (2011) 364(19):1817–25. doi: 10.1056/NEJMoa1011923
11. De Dosso S, Siebenhuner AR, Winder T, Meisel A, Fritsch R, Astaras C, et al. Treatment landscape of metastatic pancreatic cancer. Cancer Treat Rev (2021) 96:102180. doi: 10.1016/j.ctrv.2021.102180
12. Matsumoto I, Kamei K, Omae K, Suzuki S, Matsuoka H, Minzuno N, et al. FOLFIRINOX for locally advanced pancreatic cancer: Results and prognostic factors of subset analysis from a nation-wide multicenter observational study in Japan. Pancreatology (2019) 19(2):296–301. doi: 10.1016/j.pan.2019.01.001
13. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systemic review and patient-level meta-analysis. Lancet Oncol (2016) 17(6):801–10. doi: 10.1016/S1470-2045(16)00172-8
14. National Comprehensive Cancer Network. Pancreatic adenocarcinoma. version 1.2022 (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf (Accessed 06 July 2022).
15. Ushida Y, Inoue Y, Oba A, Mie T, Ito H, Ono Y, et al. Optimizing indications for conversion surgery based on analysis of 454 consecutive Japanese cases with unresectable pancreatic cancer who received modified FOLFIRINOX or gemcitabine plus nab-paclitaxel: A single-center retrospective study. Ann Surg Oncol (2022) 29(8):5038–50. doi: 10.1245/s10434-022-11503-6
16. Yoo C, Hwang I, Song TJ, Lee SS, Jeong JH, Park DH, et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther Adv Med Oncol (2020) 16(12):1758835920953294. doi: 10.1177/1758835920953294
17. Takano N, Yamada S, Sonohara F, Inokawa Y, Takami H, Hayashi M, et al. The impact of early tumor shrinkage on conversion surgery and the survival in patients with unresectable locally advanced pancreatic cancer. Surg Today (2021) 51(7):1099–107. doi: 10.1007/s00595-020-02220-2
18. Nitsche U, Wenzel P, Siveke JT, Braren R, Holzapfel K, Schlitter AM, et al. Resectability after first-line FOLFIRINOX in initially unresectable locally advanced pancreatic cancer: A single-center experience. Ann Surg Oncol (2015) 22 Suppl 3:S1212–20. doi: 10.1245/s10434-015-4851-2
19. Maxwell JE, Katz MHG. Radiotherapy for resectable and borderline resectable pancreas cancer: When and why? J Gastrointest Surg (2021) 25(3):843–8. doi: 10.1007/s11605-020-04838-6
20. Hahadevan A, Jain S, Goldstein M, Miksad R, Pleskow D, Sawhney M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2010) 78(3):735–42. doi: 10.1016/j.ijrobp.2009.08.046
21. Sutera PA, Bernard ME, Gill BS, Harper KK, Quan K, Bahary N, et al. One- vs. three-fraction pancreatic sterotactic body radiation therapy for pancreatic carcinoma: Single institution retrospective review. Front Oncol (2017) 14(7):272. doi: 10.3389/fonc.2017.00272
22. Swaminath A, Chu W. Stereotactic body radiotherapy for the treatment of medically inoperable primary renal cell carcinoma: Current evidence and future directions. Can Urol Asso J (2015) 9(7-8):275–80. doi: 10.5489/cuaj.2900
23. Qiu B, Aili A, Xue L, Jiang P, Wang J. Advances in radiobiology of stereotactic ablative radiotherapy. Front Oncol (2020) 7(10):1165. doi: 10.3389/fonc.2020.01165
24. Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys (2004) 58(4):1017–21. doi: 10.1016/j.ijrobp.2003.11.004
25. Gurka MK, Collins SP, Slack R, Tse G, Charabaty A, Ley L, et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat Oncol (2013) 1(8):44. doi: 10.1186/1748-717X-8-44
26. Jung J, Yoon SM, Park JH, Seo DW, Lee SS, Kim MH, et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PloS One (2019) 14(4):e0214970. doi: 10.1371/journal.pone.0214970
27. Mellon EA, Hoffe SE, Springett GM, Frakes JM, Strom TJ, Hodul PJ, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotatic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol (2015) 54:979–85. doi: 10.3109/0284186X.2015.1004367
28. Moningi S, Dholakia AS, Raman SP, Blackford A, Cameron JL, Le DT, et al. The role of stereotactic body radiation therapy for pancreatic cancer: A single-institution experience. Ann Surg Oncol (2015) 22(7):2352–8. doi: 10.1245/s10434-014-4274-5
29. Toesca DAS, Ahmed F, Kashyap M, Baclay JRM, von Eyben R, Pollom EL, et al. Intensified systemic therapy and stereotactic ablative radiotherapy dose for patients with unresectable pancreatic adenocarcinoma. Radiother Oncol (2020) 152:63–9. doi: 10.1016/j.radonc.2020.07.053
30. Suker M, Nuyttens JJ, Eskens FALM, Haberkorn BCM, Coene P-PLO, van der Harst E, et al. Efficacy and feasibility of stereotactic radiotherapy after FOLFIRINOX in patients with locally advanced pancreatic cancer (LAPC-1 trial). EClinicalMedicine (2019) 19(17):100200. doi: 10.1016/j.eclinm.2019.10.013
31. Teriaca MA, Loi M, Suker M, Eskens FALM, van Eijck CHJ, Nuyttens JJ. A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): Long-term outcome. Radiother Oncol (2021) 155:232–6. doi: 10.1016/j.radonc.2020.11.006
32. Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, et al. Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg (2019) 270(2):340–7. doi: 10.1097/SLA.0000000000002753
33. Klaiber U, Hackert T. Conversion surgery for pancreatic cancer-the impact of neoadjuvant treatment. Front Oncol (2020) 14(9):1501. doi: 10.3389/fonc.2019.01501
34. Myrehaug S, Sahgal A, Russo SM, Lo SS, Rosati LM, Mayr NA, et al. Stereotactic body radiotherapy for pancreatic cancer: Recent progress and future directions. Expert Rev Anticancer Ther (2016) 16(5):523–30. doi: 10.1586/14737140.2016.1168698
35. Katz MHG, Shi Q, Meyers J, Herman JM, Chuong M, Wolpin BM, et al. Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocaricnoma of the pancreas: The A021501 phase 2 randomized clinical trial. JAMA Oncol (2022) 8(9):1263–70. doi: 10.1001/jamaconcol.2022.2319
36. Arcelli A, Guido A, Buwenge M, Simoni N, Mazzarotto R, Macchia G, et al. Higher biologically effective dose predicts survival in SBRT of pancreatic cancer: A multicentric analysis (PAULA-1). Anticancer Res (2020) 40(1):465–72. doi: 10.21873/anticanres.13975
37. Simoni N, Rossi G, Cellini F, Vitolo V, Orlandi VV, Valentini V, et al. Ablative radiotherapy (ART) for locally advanced pancreatic cancer (LAPC): Toward a new paradigm? Life (Basel) (2022) 12(4):465. doi: 10.3390/life12040465
Keywords: pancreatic cancer, locally advanced pancreatic cancer, FOLFIRINOX, stereotactic body radiation therapy (SBRT), conversion surgery, prognosis
Citation: Jung JH, Song C, Jung IH, Ahn J, Kim B, Jung K, Lee J-C, Kim J and Hwang J-H (2022) Induction FOLFIRINOX followed by stereotactic body radiation therapy in locally advanced pancreatic cancer. Front. Oncol. 12:1050070. doi: 10.3389/fonc.2022.1050070
Received: 21 September 2022; Accepted: 23 November 2022;
Published: 14 December 2022.
Edited by:
Antonio Pontoriero, University of Messina, ItalyReviewed by:
Xiaofei Zhu, Second Military Medical University, ChinaCopyright © 2022 Jung, Song, Jung, Ahn, Kim, Jung, Lee, Kim and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Hyeok Hwang, d29sdG9vbmdAc251LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.